Abstract
Background and Objectives
Immune checkpoint inhibitors (ICIs) are effective in various types of cancer and cause immune-related adverse events (irAEs). The occurrence of irAEs is associated with improved survival outcome. We investigated the association between the occurrence of irAEs and overall survival (OS) and progression free survival (PFS), and the risk factors for the development of irAEs, in patients with non–small-cell lung cancer (NSCLC), gastric cancer (GC) and melanoma (MM) treated with ICIs.
Methods
This was a retrospective observational cohort study, and the data were taken from inpatients in a hospital. OS and PFS were compared among patients with different numbers of irAEs. Log-rank test and Cox regression and logistic regression analysis were applied, and details of irAEs characteristics were summarized.
Results
We obtained data from 200 patients. The major tumor types were NSCLC, GC, and MM. Median OS and PFS in all patients were 9.3 and 3.5 months, respectively. Patients without irAEs tended to have shorter OS or PFS compared with those with a single irAE or multi-system irAEs. Covariate analysis suggested that age (≥75 years), albumin (≥3.5 g/dL) and smoking history were significant for increased occurrence of irAEs. Pneumonitis and thyroiditis tended to occur frequently in patients with NSCLC and MM. The irAE grade was ≤2 in 67.3% of all irAEs, and days of irAEs onset varied.
Conclusion
We observed patients with irAEs tended to have better OS or PFS in patients with various types of cancers treated with ICIs. We suggest that ICIs should be used appropriately by continuously monitoring the irAEs.
Keywords: multiple immune-related adverse events, immune checkpoint inhibitors, clinical outcomes, long-term immune-related adverse events
Key Point
This was a retrospective observational cohort study to investigate the association between the occurrence of irAEs and overall survival (OS) and progression free survival (PFS), and the risk factors for the development of immune-related adverse events (irAEs), in patients with non–small-cell lung cancer, gastric cancer and melanoma treated with ICIs. Details of irAEs characteristics were also summarized.
In conclusion, patients with irAEs tended to have better OS or PFS with various types of cancers treated with ICIs. The irAE grade was ≤2 in 67.3% of all irAEs, and days of irAEs onset varied. We suggest that ICIs should be used appropriately by continuously monitoring the irAEs.
Introduction
The use of immune checkpoint inhibitors (ICIs) has transformed cancer treatment in clinical practice and has been reported to be effective in various types of cancer, including malignant melanoma (MM),1 non-small-cell lung cancer (NSCLC),2 gastric cancer (GC),3 and other types of cancers.4-11 ICIs, including anti-programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), are checkpoints inhibitors that have been successfully targeted with antagonist antibodies.12-14
ICIs are known to cause inflammatory side effects referred to as immune-related adverse events (irAEs), which appear in nearly every organ system.15-18 Various types of irAEs have been reported, including gastrointestinal, hepatic, skin, endocrine, neurological, renal, and interstitial lung diseases.15 Several studies targeted to MM and NSCLC have shown that the development of irAEs is associated with improved survival outcome.19-26 On the other hand, some reports indicated that interstitial lung disease caused by ICIs is associated with poor prognosis in NSCLC.27-29 In addition, data from patients with MM treated with anti–PD-1 monotherapy indicated that the number of irAEs, and not the grade of irAE, is correlated with the response rate.30
In our previous retrospective study, we focused on interstitial pneumonia, which is a frequent irAEs, and investigated the risk factors for interstitial pneumonia in patients with advanced NSCLC and Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1 during nivolumab monotherapy.31 Shanker et al have reported that the development of multi-system irAEs is associated with improving survival in patients with NSCLC treated with ICIs.32 In the previous study, we could not obtain enoughr data for patients (i) with cancers other than NSCLC, (ii) with poor ECOG PS, and (iii) patients treated with combination therapy of ICIs and cytotoxic anticancer agents, and the risk factors in such conditions remain unclear.
In this study, we investigated the association between the occurrence of irAEs and clinical efficacy given by such indices as overall survival (OS) and progression free survival (PFS), and the risk factors for the development of irAEs, in patients with NSCLC, GC and MM treated with ICIs. Details of irAEs characteristics were also summarized.
Methods
Patients, Data Collection, and Study Design
This was a retrospective observational cohort clinical study including patients who underwent treatment with ICIs at the National Hospital Organization Osaka National Hospital (Osaka, Japan). Patients were enrolled from September 2014 to December 2020 and followed up until March 31, 2021. The reporting of this study conformed to STROBE (cohort study) guideline.33 We included patients treated with the following ICIs: nivolumab, pembrolizumab, atezolizumab, durvalumab, and ipilimumab. We consecutively chose the eligible patients according to our criteria. Patients with completely missing baseline data were excluded from the analysis, and other each missing data were ignored in the data analysis. The dose and dosing schedules of the ICIs were at the clinicians’ discretion.
We collected basic patient data from the medical records of the hospital at the time of initiation of ICI treatment (ie, baseline), including age (years), sex, ECOG PS, body mass index (BMI), tumor type, metastasis site, name of ICIs used, number of prior chemotherapy regimens, percentage of PD-L1 expression, existence of baseline corticosteroid treatment, smoking history, laboratory data obtained from peripheral blood (ie, absolute neutrophil count [ANC (/mm3)], absolute lymphocyte count [ALC (/mm3)], and platelet count [PLT (/mm3)]) and serum biochemistry (ie, levels of C-reactive protein [CRP (mg/dL)], albumin [ALB (g/dL)], and lactate dehydrogenase [LDH (IU/L)]).
Data regarding irAEs during ICI treatments were collected, which were the onset date of the irAEs, severity grade of the irAEs obtained during ICIs treatment, the irAEs that required steroid treatment, and the clinical outcome of the irAEs. In the present study, the occurrence of irAEs was based on the medical records that the physicians routinely assessed and recorded. The physicians confirmed the irAEs whether they were related to ICIs based on pathologic diagnosis, by consultation to a specialist, or by laboratory tests for definitive diagnosis, and recorded their final judgement on the medical chart. We graded the severity of the irAEs using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 5.0). The largest grade during the treatment was defined in this study as “max grade” of each irAE in individual patients.
OS was defined as the time between the beginning of ICI treatment to the day of death from any causes, where the dates of death were obtained from the medical record. PFS was defined as the time between the beginning of the ICI treatment to the day of progressive disease (PD) or death from any causes, where PD was defined according to the Response Evaluation Criteria in Solid Tumor (version 1.1). The types of irAEs were summarized for each grade and each major cancer type, median days of onset from the baseline, number of patients with long-term irAEs and receiving corticosteroid therapy, and the information for the clinical outcomes. Long-term irAEs were defined as immune-related side effects lasting for at least 12 weeks after the patient stopped taking the ICI.34 Clinical outcomes were classified as “improved”, “resolved”, “refractory,” “dead” or “unknown” according to the medical records. More precisely, we focused on cases in which the max grade was ≥2 and improved and “resolved” were defined as cases in which the grade was decreased to 0 and 1, respectively. Otherwise, we defined cases as not improved, except for cases of death or unknown.
This study was carried out according to the Declaration of Helsinki, and the protocol of this retrospective observational study was approved by the ethics committees of both the National Hospital Organization Osaka National Hospital (No. ONH 21067, approved on November 11, 2021) and Kyoto Pharmaceutical University (No. E21-018, approved on August 2, 2021). No informed consents were obtained from individual patients in the study because this was a retrospective observational study and an “opt-out approach” written in the Japanese “Ethical Guideline for Clinical Study” was applied. Instead, we published information of this research on the Website of the hospital, and we guarantee the opportunity of patient rejection. We have de-identified patient detailed information so that the identity of any person may not be ascertained.
Statistical Analysis
Statistics regarding patients’ basic characteristics and other information taken from the electronic charts were summarized as mean and standard deviation (SD) or the number of patients and their percentages of the total patients. No statistical estimation of the sample size was performed prior to the study, and we collected all the available data from the electronical files in our hospital according to our criteria.
The Kaplan–Meier plots for OS and PFS were created with stratification according to the number of irAEs (0: absence, 1: single irAE, or >1: multi-system irAEs) in the patients with the major tumor types in this study, NSCLC, GC, or MM. We also created Kaplan–Meier plots for OS and PFS with binary stratification according to the absence (0) or presence (single or multi-system) of irAEs. To examine possible relationship between irAEs and OS, Kaplan-Meier plots for some major irAEs in these tumor types were created. Statistical differences in the OS or PFS profiles between the groups were tested by log-rank test with Bonferroni correction in case of a comparison among more than two groups.
We examined the risk factors for OS and PFS as well as the risk factors for the occurrence of irAEs using Cox regression analysis and binary logistic regression analysis, respectively. For each regression analysis, we first adopted a univariate analysis followed by a multivariate analysis for the covariates with P–values <.2 in the univariate analysis. Correlations among the covariates were not considered in the univariate analysis. The possible affecting factors included in the analyzes are listed in the tables. Some of the values of the clinical laboratory tests were divided into two categories; the cutoff values of ALB, CRP and LDH were referenced from the literatures.35,36 We did not include the irAEs information for the regression analysis of OS or PFS because we wanted to know the factors on these survival data at the time of ICIs treatment, ie, no information of irAEs is available at that time.
Detailed information of the irAEs occurring in the patients, including grades, median days of onset of irAEs after ICIs treatments, presence of long-term irAEs, number of patients who required corticosteroid therapy for irAE treatment, and clinical outcomes, were summarized and stratified by the major cancer types.
All analyzes were carried out using BellCurve for Excel (Social Survey Research Information Co., Ltd. Tokyo, Japan). The level of statistical significance was set at .05 in all cases, except for the cases individually cited.
Results
Patient Characteristics
Table 1 summarizes the patient characteristics at the beginning of ICI treatment. The details of each boundary (cut-off value) of the independent variables are given. Data from 207 patients were collected retrospectively, and data from 7 patients were excluded because of missing baseline data. Finally, data from 200 patients were used for the analysis in this study. The mean age of the patients was 66.9 years, and about 64% of the patients were male. Most patients had an ECOG PS of 0 (53.5%) or 1 (30.0%), and the major tumor types were NSCLC (25%), GC (21.5%), and MM (19.0%). Patients with other tumors were, renal cell carcinoma (n = 17, 8.5%), esophageal cancer (13, 6.5%), bladder cancer (14, 7.0%), head and neck cancer (10, 5.0%), breast cancer (8, 4.0%), hepatocellular cancer (4, 2.0%), and Microsatellite Instability-High (3, 1.5%). Here we focused on three major cancers, GC and MM for which the numbers of patients were larger in this study. Nivolumab (57.5%) or pembrolizumab (23.0%) was mainly used as the ICIs. The number of patients with ICI alone was 175 (87.5%), and 19 patients (9.5%) received cytotoxic agents with ICIs, 4 patients (2%) received molecular target drugs with ICIs.
Table 1.
Patient Characteristics.
| All cancers n = 200 | NSCLC n = 50 | Gastric cancer n = 43 | Melanoma n = 38 | ||
|---|---|---|---|---|---|
| Age (years, mean ± SD) | 66.9 ± 10.4 | 66.0 ± 8.7 | 70.2 ± 9.3 | 66.5 ± 12.3 | |
| Sex | Male/Female | 127 (63.5)/73 (36.5) | 34 (68.0)/16 (32.0) | 25 (58.1)/18 (41.9) | 21 (55.3)/17 (44.7) |
| ECOG PS | 0/1/2/3 | 107 (53.5)/60 (30.0)/25 (12.5)/8 (4.0) | 18 (36.0)/21 (42.0)/7 (14.0)/4 (8.0) | 17 (39.5)/18 (41.9)/8 (18.6)/0 (.0) | 23 (60.5)/7 (18.4)/6 (15.8)/2 (5.3) |
| BMI | <18.5 | 47 (23.5) | 10 (20.0) | 19 (44.2) | 4 (10.5) |
| ≥18.5, <25 | 113 (56.5) | 30 (60.0) | 22 (51.2) | 21 (55.3) | |
| ≥25 | 40 (20.0) | 10 (20.0) | 2 (4.7) | 13 (34.2) | |
| Metastasis site | Brain/lung/liver/bone | 22 (11.0)/86 (43.0)/61 (30.5)/43 (21.5) | 13 (26.0)/20 (40.0)/10 (20.0)/14 (28.0) | 0 (.0)/5 (11.6)/18 (41.9)/3 (7.0) | 7 (18.4)/23 (60.5)/19 (50.0)/11 (28.9) |
| Therapy | Nivolumab | 115 (57.5) | 8 (16.0) | 43 (100) | 25 (65.8) |
| Pembrolizumab | 46 (23.0) | 28 (.56) | 0 (.0) | 1 (2.6) | |
| Atezolizumab | 18 (9.0) | 7 (14.0) | 0 (.0) | 0 (.0) | |
| Durvalumab | 7 (3.5) | 7 (14.0) | 0 (.0) | 0 (.0) | |
| Ipilimumab | 11 (5.5) | 0 (.0) | 0 (.0) | 11 (28.9) | |
| Nivolumab plus ipilimumab | 3 (1.5) | 0 (.0) | 0 (.0) | 1 (2.6) | |
| Number of prior chemotherapy regimens | <2/≥3 | 105 (52.5)/95 (47.5) | 33 (66.0)/17 (34.0) | 1 (2.3)/42 (97.7) | 32 (84.2)/6 (15.8) |
| PD-L1 expression | 0-49%/50% or more | 24 (12.0)/13 (6.5) | 20 (40.0)/13 (26.0) | 0 (.0)/0 (.0) | 0 (.0)/0 (.0) |
| Unknown | 163 (81.5) | 17 (34.0) | 43 (100) | 38 (100) | |
| Baseline corticosteroids | Yes/no | 5 (2.5)/195 (97.5) | 0 (.0)/50 (100) | 0 (.0)/43 (100) | 5 (13.2)/33 (86.8) |
| Smoking status | Yes/no/unknown | 139 (69.5)/60 (30.0)/1 (.5) | 42 (84.0)/8 (16.0)/0 (.0) | 27 (62.8)/16 (37.2)/0 (.0) | 19 (50.0)/18 (47.4)/1 (2.6) |
| History of pneumonia | Yes/no | 7 (3.5)/193 (96.5) | 2 (4.0)/48 (96.0) | 1 (2.3)/42 (97.7) | 3 (7.9)/35 (92.1) |
| History of autoimmune disease | Yes/no | 27 (13.5)/173 (86.5) | 1 (2.0)/49 (98.0) | 5 (11.6)/38 (88.4) | 7 (18.4)/31 (81.6) |
| Baseline ANC (mean ± SD) | 4.51 ± 3.18* | 5.06 ± 4.41** | 4.01 ± 3.08 | 4.87 ± 2.30 | |
| Baseline ALC (mean ± SD) | 1.32 ± .58* | 1.33 ± .59*** | 1.36 ± .57 | 1.60 ± .65 | |
| Baseline PLT (mean ± SD) | 260 ± 126* | 281 ± 123*** | 209 ± 109 | 297 ± 116 | |
| Baseline CRP | <1.0/≥1.0/Unknown | 121 (60.5)/75 (37.5)/4 (2.0) | 23 (46.0)/26 (52.0)/1 (2.0) | 30 (69.8)/13 (30.2)/0 (.0) | 27 (71.1)/9 (23.7)/2 (5.3) |
| Baseline ALB | <3.5/≥3.5/Unknown | 86 (43.0)/108 (54.0)/6 (3.0) | 21 (42.0)/27 (54.0)/2 (4.0) | 29 (67.4)/14 (32.6)/0 (.0) | 9 (23.7)/26 (68.4)/3 (7.9) |
| Baseline LDH | <400/≥ 400/Unknown | 172 (86.0)/23 (11.5)/5 (2.5) | 42 (84.0)/7 (14.0)/1 (2.0) | 34 (79.1)/7 (16.3)/2 (4.7) | 32 (84.2)/6 (15.8)/0 (.0) |
Total number of eligible patients was 200.
*n = 198, **n = 48, ***n = 49, because the baseline data were partly missing.
The median OS for patients with NSCLC, GC and MM were 12.2 months (m) (95% confidence interval [CI]: 6.1-18.4), 4.6 m (2.4-6.8 m) and 7.5 m (3.6-11.4 m), respectively. The median PFS for patients with NSCLC, GC and MM were 5.3 m (3.7-7.3 m), 2.2 m (1.6-2.8 m) and 2.8 m (1.9-3.7 m), respectively. In most patients (81.5%), the expression of PD-L1 was unclear, and almost all patients (97.5%) did not use corticosteroids at baseline and had no history of pneumonia (96.5%) nor autoimmune diseases (86.5%). About 70% were smokers. The values of the actual data used for the regression analyzes are provided in the corresponding tables.
Comparison of Survival Curves According to irAE Occurrence
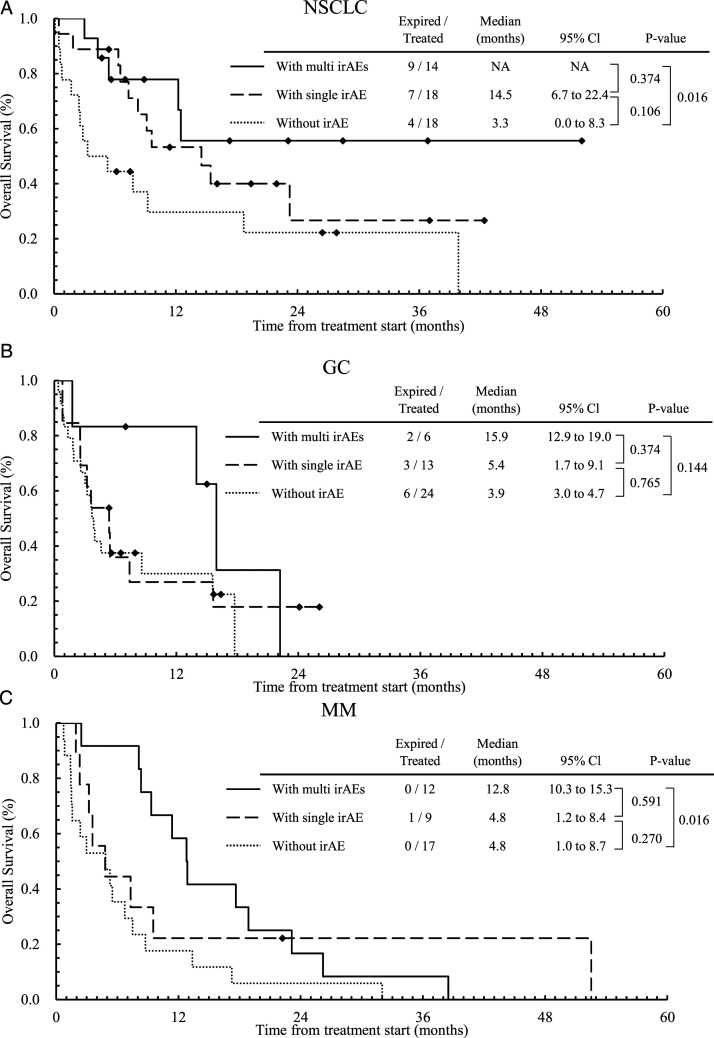
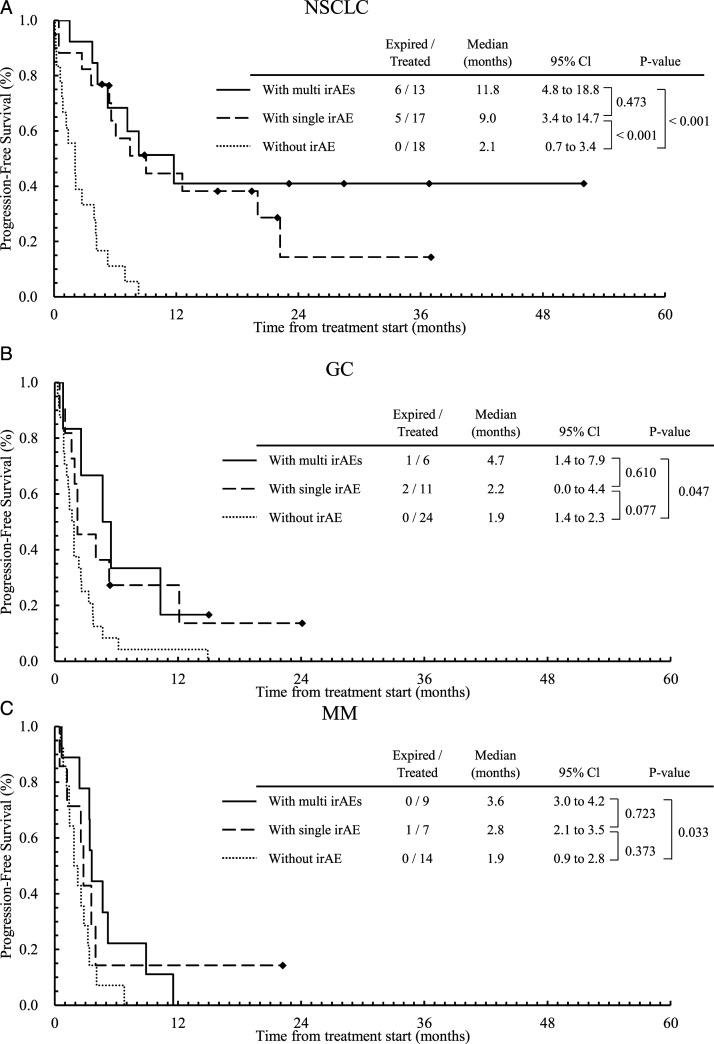
Figure 1 and 2 shows Kaplan–Meier plot for OS (Figure 1) and PFS (Figure 2) stratified by the number of irAEs (0, 1, or multi-system irAEs), for patients with NSCLC (a), GC (b), and MM (c), respectively. We applied the Bonferroni correction, ie, the P–values less than .0167 (=.05/3) are considered statistically significant for comparison among three groups. Other plots for OS and PFS stratified by the existence or presence of irAEs are given in Supplementary Figures A1 and A2, respectively. The calculated P–values for all cases are given in the figures. In patients with NSCLC (Figure 1A), OS with multi-system irAEs (median: NA (Not Applicable), 95% CI: NA) tended to be longer than those with no irAE (median: 3.3 m, 95% CI: .0-8.3 m, P = .016). In patients with MM (Figure 1C), OS with multi-system irAEs (median: 12.8 m, 95% CI: 10.3-15.3 m) tended to be longer than those with no irAE (median: 4.8 m, 95% CI: 1.0-8.7 m, P = .016). For PFS, we found similar results to those for OS. Among patients with NSCLC (Figure 2B), those without irAEs had a shorter PFS (median: 2.1 m, 95% CI: .7-3.4 m) than those with a single irAE (median: 9.0 m, 95% CI: 3.4-14.7 m, P = .001), or with multi-system irAEs (median: 11.8 m, 95% CI: 4.8-18.8 m, P < .001). In patients with GC and MM, similar profiles were obtained but not statistically significant.
Figure 1.
Kaplan–Meier curves for OS stratified by the number of irAEs (0, 1, or more [multi-system irAEs]) for patients with NSCLC (a), GC (b), and MM (c), respectively.
Figure 2.
Kaplan–Meier curves for PFS stratified by the number of irAEs (0, 1, or more [multi-system irAEs]) for patients with NSCLC (a), GC (b), and MM (c), respectively.
Covariate Analysis for OS and PFS by Cox Regression
Table 2 summarizes the results of the Cox regression analysis for OS. Some variables, including the number of metastatic sites, liver metastasis, PS, some laboratory test values, and cancer type, were significant in the univariate analysis. The results by the multivariate analysis suggested that the covariate significantly associated with longer OS (ie, hazard ratio [HR] <1.0) was baseline ALB (HR = .55, 95% CI: .37-.81, P = .003 for ALB ≥3.5 g/dL), and the significant covariates associated with shorter OS (ie, HR > 1.0) were age (HR = 1.61, 95% CI: 1.05-2.45, P = .03 for age ≥75 years), number of metastatic sites (HR = 1.25, 95% CI: 1.07-1.46, P = .005, for one difference of the number), liver metastasis (HR = 1.77, 95% CI: 1.16-2.73, P = .01), PS (HR = 2.69, 95% CI: 1.68-4.32, P < .001 for PS ≥ 2), and baseline LDH (HR = 5.05, 95% CI: 2.87-8.88, P < .001 for LDH ≥400 IU/L).
Table 2.
Univariate and Multivariate Analyses of OS With Cox Regression Models.
| Covariates (n for univariate analysis*) | Univariate analysis (n = 200) | Multivariate analysis (n = 191) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender (female (73) vs male (127)) | 1.13 | .79-1.62 | .50 | — | — | — |
| Age (≥75 (44) vs <75 (156)) | 1.38 | .92-2.06 | .12 | 1.61 | 1.05-2.45 | .03 |
| Smoking (yes (139) vs no (60)) | .85 | .58-1.23 | .38 | — | — | — |
| BMI (≥18.5 (153) vs <18.5 (47)) | .69 | .47-1.02 | .07 | — | — | — |
| Number of metastatic sites | 1.34 | 1.18-1.52 | <.001 | 1.25 | 1.07-1.46 | .005 |
| Metastasis | ||||||
| Brain (with (22) vs without (178)) | 1.41 | .86-2.33 | .18 | — | — | — |
| Lung (with (86) vs without (114)) | 1.12 | .79-1.59 | .52 | — | — | — |
| Liver (with (61) vs without (139)) | 2.05 | 1.45-2.92 | <.001 | 1.77 | 1.16-2.73 | .01 |
| PS (≥2 (33) vs <2 (167)) | 3.42 | 2.26-5.17 | <.001 | 2.69 | 1.68-4.32 | <.001 |
| Therapy line (≥3 (95) vs <3 (105)) | 1.17 | .83-1.65 | .38 | — | — | — |
| Baseline ANC (198) | 1.06 | 1.02-1.11 | .004 | — | — | — |
| Baseline ALC (198) | .67 | .48-.92 | .01 | — | — | — |
| Baseline PLT (198) | 1.00 | 1.00-1.00 | .15 | — | — | — |
| Baseline ALB (≥3.5 (108) vs <3.5 (86)) | .46 | .33-.66 | <.001 | .55 | .37-.81 | .003 |
| Baseline CRP (≥1.0 (75) vs <1.0 (121)) | 2.04 | 1.43-2.91 | <.001 | — | — | — |
| Baseline LDH (≥400 vs <400) (195) | 7.22 | 4.46-11.69 | <.001 | 5.05 | 2.87-8.88 | <.001 |
| NSCLC (vs others) (50) | .76 | .50-1.15 | .19 | — | — | — |
| Gastric cancer (vs others) (43) | 1.68 | 1.12-2.52 | .01 | — | — | — |
| Melanoma (vs others) (38) | 1.66 | 1.13-2.43 | .01 | — | — | — |
HR: hazard ratio, 95% CI: 95% confidence interval, P-value: P-value by regression analysis. *Sums of n are not necessarily 200 because of missing data.
Table 3 shows the results of the Cox regression analysis for PFS. PFS data were available for 184 patients. Similar to OS, liver metastasis, PS, and some laboratory test values were significant in the univariate analysis. The results of the multivariate analysis suggested that NSCLC was significantly associated with a longer PFS (HR = .64, 95% CI: .43-.95, P = .03), and the significant covariates associated with shorter PFS were liver metastasis (HR = 1.61, 95% CI: 1.10-2.35, P = .01 with liver metastasis), PS (HR = 3.64, 95% CI: 2.31-5.72, P < .001 for PS ≥ 2), and baseline LDH (HR = 3.44, 95% CI: 2.03-5.83, P < .001 for LDH ≥400 IU/L).
Table 3.
Univariate and Multivariate Analyses of PFS With Cox Regression Models.
| Covariates (n for univariate analysis*) | Univariate analysis (n = 184) | Multivariate analysis (n = 179) | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender (female (65) vs male (119)) | 1.10 | .79-1.54 | .58 | — | — | — |
| Age (≥75 (37) vs <75 (147)) | 1.11 | .75-1.66 | .59 | — | — | — |
| Smoking (Yes (128) vs No (55)) | .72 | .51-1.02 | .06 | — | — | — |
| BMI (≥18.5 (140) vs <18.5 (44)) | .61 | .42-.88 | .01 | — | — | — |
| Number of metastatic sites | 1.24 | 1.09-1.41 | <.001 | — | — | — |
| Metastasis | ||||||
| Brain (with (19) vs without (165)) | 1.12 | .67-1.89 | .66 | — | — | — |
| Lung (with (74) vs without (110)) | .96 | .69-1.33 | .81 | — | — | — |
| Liver (with (57) vs without (127)) | 1.98 | 1.42-2.77 | <.001 | 1.61 | 1.10-2.35 | .01 |
| PS (≥2 (29) vs <2 (155)) | 3.67 | 2.41-5.59 | <.001 | 3.64 | 2.31-5.72 | <.001 |
| Therapy line (≥3 (92) vs <3 (92)) | 1.14 | .83-1.57 | .42 | — | — | — |
| Baseline ANC (182) | 1.02 | .98-1.07 | .28 | — | — | — |
| Baseline ALC (182) | .74 | .55-.99 | .04 | — | — | — |
| Baseline PLT (182) | 1.00 | 1.00-1.00 | .39 | — | — | — |
| Baseline ALB (≥3.5 (100) vs <3.5 (79)) | .63 | .45-.87 | .01 | — | — | — |
| Baseline CRP (≥1.0 (69) vs <1.0 (111)) | 1.41 | 1.01-1.96 | .04 | — | — | — |
| Baseline LDH (≥400 (21) vs <400 (158)) | 3.88 | 2.42-6.21 | <.001 | 3.44 | 2.03-5.83 | <.001 |
| NSCLC (vs others) (n = 48) | .70 | .48-1.01 | .06 | .64 | .43-.95 | .03 |
| Gastric cancer (vs others) (n = 41) | 1.73 | 1.19-2.50 | .004 | — | — | — |
| Melanoma (vs others) (n = 30) | 1.69 | 1.12-2.56 | .01 | — | — | — |
HR: Hazard ratio, 95% CI: 95% confidence interval, P-value: P-value by regression analysis. *Sums of n are not necessarily 200 because of missing data.
Covariate Analysis for Occurrence of irAEs or Multi-system irAEs by Logistic Regression
Table 4 shows the results of logistic regression analysis for detecting affecting factors on occurrence of any irAEs (single or multi-system) compared with the absence of irAEs. The results of the multivariate analysis suggested that the significant risk factors (odds ratio [OR] >1.0) were age (OR = 3.55, 95% CI: 1.55-8.14, P = .003, for age ≥75 years), smoking habit (OR = 2.75, 95% CI: 1.41-5.36, P = .003 for smoker), and baseline ALB (OR = 2.47, 95% CI: 1.32-4.60, P = .004 for ALB ≥3.5 g/dL).
Table 4.
Univariate and Multivariate Analyses for irAE (Existence of irAEs vs Absence) With Binary Logistic Regression Analysis.
| Covariates (n for univariate analysis*) | Univariate analysis (n = 200) | Multivariate analysis (n = 194) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Gender (female (73) vs male (127)) | .63 | .35-1.13 | .12 | --- | --- | --- |
| Age (≥75 (44) vs <75 (156)) | 2.70 | 1.25-5.84 | .01 | 3.55 | 1.55-8.14 | .003 |
| Smoking (yes (139) vs no (60)) | 2.31 | 1.25-4.29 | .01 | 2.75 | 1.41-5.36 | .003 |
| BMI (≥18.5 (153) vs <18.5 (47)) | 1.66 | .86-3.21 | .13 | --- | --- | --- |
| Number of metastatic sites | .83 | .67-1.04 | .11 | --- | --- | --- |
| Metastasis | ||||||
| Brain (with (22) vs without (178)) | .76 | .31-1.85 | .55 | --- | --- | --- |
| Lung (with (86) vs without (114)) | 1.54 | .86-2.74 | .15 | --- | --- | --- |
| Liver (with (61) vs without (139)) | .51 | .28-.94 | .03 | --- | --- | --- |
| PS (≥2 (33) vs <2 (167)) | .36 | .16-.76 | .01 | --- | --- | --- |
| Therapy line (≥3 (95) vs <3 (105)) | .58 | .33-1.03 | .06 | --- | --- | --- |
| Baseline ANC (198) | .99 | .90-1.08 | .74 | --- | --- | --- |
| Baseline ALC (198) | 1.32 | .80-2.18 | .28 | --- | --- | --- |
| Baseline PLT (198) | 1.00 | 1.00-1.00 | .59 | --- | --- | --- |
| Baseline ALB (≥3.5 (108) vs <3.5 (86)) | 2.18 | 1.21-3.91 | .01 | 2.47 | 1.32-4.60 | .004 |
| Baseline CRP (≥1.0 (75) vs <1.0 (121)) | .75 | .42-1.35 | .34 | --- | --- | --- |
| Baseline LDH (≥400 (23) vs <400 (172)) | .46 | .19-1.10 | .08 | --- | --- | --- |
| NSCLC (vs others) (50) | 1.54 | .78-3.03 | .21 | --- | --- | --- |
| Gastric cancer (vs others) (43) | .48 | .24-.95 | .04 | --- | --- | --- |
| Melanoma (vs others) (38) | .87 | .43-1.79 | .72 | --- | --- | --- |
OR: odds ratio, 95% CI: 95% confidence interval, P-value: P-value by regression analysis. *Sums of n are not necessarily 200 because of missing data.
Details of the irAE Characteristics
Table 5 presents detailed information on the irAEs, including the grade, median days of onset, number of the cases with long-term irAEs, and corticosteroid therapy, with the clinical outcomes are given separately for each irAE. For major irAEs that occurred in more than five cases, the data are also given for each major type of cancer, such as NSCLC, GC, MM, and other cancers. In total, 113 (56.5%) patients experienced any irAE, and in all patients, the major irAEs were dermatitis (23.8% of all irAEs [n = 193]), hepatitis (19.2%), and fever (11.9%). The grades were mostly 1 or 2 in all irAEs. Multi-system irAEs were observed in 46 (23.0%) patients, and the irAEs with the grade ≥3 were observed in 37 (18.5%) patients (data not shown). Fever and fatigue may not be necessarily irAEs for their definitions, but in this study, we included them in Table 5 in case the physicians diagnosed these symptoms as irAEs.
Table 5.
Details of irAE Characteristics With Their Grades and Clinical Outcomes.
| irAEs (n = 193 in 113 patients) | Grade | Median days to onset | Long-term irAEs | Corticosteroid therapy | Clinical outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (NSCLC/GC/MM/others) | All | 1 | 2 | 3 | 4 | 5 | Improved | Resolved | Refractory | Dead | |||
| Dermatitis (outcome unknown; 2) | 46 (23.8) 10/5/10/21 | 20 (10.4) 4/4/4/8 | 21 (10.9) 6/1/3/11 | 5 (2.6) 0/0/3/2 | 0 | 0 | 42 30/42/109/42 | 7 (3.6) 1/0/3/3 | 6 (3.1) 1/0/5/0 | 13 (6.7) 3/0/1/9 | 6 (3.1) 0/0/4/2 | 1 (.5) 1/0/0/0 | 4 (2.1) 2/1/1/0 |
| Hepatitis* | 37 (19.2) 12/6/11/8 | 13 (6.7) 3/4/4/2 | 9 (4.7) 3/1/2/3 | 12 (6.2) 4/1/4/3 | 2 (1.0) 2/0/0/0 | 1 (.5) 0/0/1/0 | 34 56/24/27/28 | 5 (2.6) 1/0/1/3 | 12 (6.2) 3/2/5/2 | 11 (5.7) 6/2/2/1 | 7 (3.6) 3/0/2/2 | 0 | 6 (3.1) 0/0/3/3 |
| Fever | 23 (11.9) 6/4/4/9 | 22 (11.4) 6/4/3/9 | 0 | 1 (.5) 0/0/1/0 | 0 | 0 | 2 8/1/30/1 | 0 | 0 | 1 (.5) 0/0/1/0 | 0 | 0 | 0 |
| Pneumonitis (outcome unknown; 1) | 16 (8.3) 10/0/3/3 | 3 (1.6) 3/0/0/0 | 8 (4.1) 6/0/2/0 | 5 (2.6) 1/0/1/3 | 0 | 0 | 49 54/0/27/140 | 7 (3.6) 5/0/1/1 | 9 (4.7) 3/0/3/3 | 3 (1.6) 3/0/0/0 | 7 (3.6) 2/0/3/2 | 1 (.5) 1/0/0/0 | 1 (.5) 0/0/0/1 |
| Fatigue | 13 (6.7) 3/5/3/2 | 11 (5.7) 3/4/2/2 | 1 (.5) 0/0/1/0 | 1 (.5) 0/1/0/0 | 0 | 0 | 14 5/4/21/49 | 2 (1.0) 1/0/0/1 | 0 | 0 | 2 (1.0) 0/1/1/0 | 0 | 0 |
| Thyroiditis (hyper hypo) | 13 (6.7) 3/1/6/3 | 3 (1.6) 1/0/1/1 | 10 (5.2) 2/1/5/2 | 0 | 0 | 0 | 70 56/30/84/89 | 8 (4.1) 2/0/5/1 | 1 (.5) 0/0/0/1 | 2 (1.0) 1/1/0/0 | 0 | 2 (1.0) 1/0/0/1 | 6 (3.1) 0/0/5/1 |
| Diarrhea/colitis | 12 (6.2) 3/2/2/5 | 4 (2.1) 1/0/1/2 | 3 (1.6) 1/2/0/0 | 5 (2.6) 1/0/1/3 | 0 | 0 | 82 315/102/32/29 | 4 (2.1) 3/0/0/1 | 6 (3.1) 2/0/2/2 | 6 (3.1) 1/2/0/3 | 1 (.5) 0/0/1/0 | 1 (.5) 1/0/0/0 | 0 |
| Hypoadrenocorticism | 7 (3.6) 1/3/0/3 | 0 | 5 (5.2) 1/2/0/2 | 2 (1.0) 0/1/0/1 | 0 | 0 | 132 210/154/0/112 | 3 (1.6) 0/0/0/3 | 7 (3.6) 1/3/0/3 | 1 (.5) 0/1/0/0 | 0 | 5 (2.6) 1/2/0/2 | 1 (.5) 0/0/0/1 |
| Oral mucosal toxicities | 5 (2.6) 1/1/1/2 | 1 (.5) 0/0/1/0 | 2 (1.0) 0/1/0/1 | 2 (1.0) 1/0/0/1 | 0 | 0 | 78 135/7/42/176 | 2 (1.0) 0/0/0/2 | 0 | 2 (1.0) 1/1/0/0 | 0 | 1 (.5) 0/0/0/1 | 1 (.5) 0/0/0/1 |
| Nervous system disorder (grade unknown; 2) | 4 (2.1) 1/0/1/2 | 0 | 2 (1.0) 1/0/1/0 | 0 | 0 | 0 | 29 32/0/19/42 | 0 | 4 (2.1) 1/0/1/2 | 0 | 0 | 0 | 1 (.5) 0/0/1/0 |
| Musculoskeletal disorders | 4 (2.1) 0/0/3/1 | 1 (.5) 0/0/0/1 | 0 | 3 (1.6) 0/0/3/0 | 0 | 0 | 99 0/0/23/175 | 1 (.5) 0/0/0/1 | 3 (1.6) 0/0/3/0 | 0 | 1 (.5) 0/0/1/0 | 0 | 2 (1.0) 0/0/2/0 |
| Gastrointestinal disorder* (except diarrhea/hepatitis) | 3 (1.6) 1/0/1/1 | 0 | 2 (1.0) 1/0/0/1 | 0 | 0 | 1 (.5) 0/0/1/0 | 420 64/0/936/420 | 1 (.5) 0/0/1/0 | 1 (.5) 0/0/1/0 | 2 (1.0) 1/0/0/1 | 0 | 0 | 1 (.5) 0/0/1/0 |
| Renal toxicity | 3 (1.6) 0/0/0/3 | 0 | 1 (.5) 0/0/0/1 | 2 (1.0) 0/0/0/2 | 0 | 0 | 92 0/0/0/92 | 3 (1.6) 0/0/0/3 | 0 | 0 | 1 (.5) 0/0/0/1 | 2 (1.0) 0/0/0/2 | 0 |
| Electrolyte abnormality | 2 (1.0) 0/0/1/1 | 0 | 1 (.5) 0/0/1/0 | 1 (.5) 0/0/0/1 | 0 | 0 | 372 0/0/617/126 | 0 | 0 | 1 (.5) 0/0/1/0 | 1 (.5) 0/0/0/1 | 0 | 0 |
| Eye disorder | 2 (1.0) 1/0/1/0 | 0 | 2 (1.0) 1/0/1/0 | 0 | 0 | 0 | 835 772/0/898/0 | 0 | 0 | 2 (1.0) 1/0/1/0 | 0 | 0 | 0 |
| Type 1 diabetes mellitus (Grade unknown; 2) | 2 (1.0) 1/1/0/0 | 0 | 0 | 0 | 0 | 0 | 142 112/172/0/0 | 2 (1.0) 1/1/0/0 | 0 | 0 | 0 | 0 | 0 |
| Hypopituitarism | 1 (.5) 0/0/1/0 | 0 | 0 | 1 (.5) 0/0/1/0 | 0 | 0 | 13 0/0/13/0 | 1 (.5) 0/0/1/0 | 1 (.5) 0/0/1/0 | 0 | 0 | 0 | 1 (.5) 0/0/1/0 |
1) NSCLC: non-small-cell lung cancer, GC: gastric cancer, MM: melanoma, Other: other cancers.
2) Long-term irAEs: The immune-related side effect that lasted for at least 12 weeks after the patient finished taking the immune checkpoint inhibitor. Values are the number of irAEs, and their percentages are given in parentheses.
*: One case each of hepatitis and gastrointestinal disorders was reported to have a causal relationship with death.
The median days of onset ranged widely among the irAEs (from 2 to 835 days). Fever was observed on median day 2, and major irAEs such as dermatitis and hepatitis occurred on median days 42 and 34, respectively. There were 46 cases (46/193 = 23.8%) in 35 patients (35/113 = 31.0%) treated with irAEs that continued for more than 12 weeks (long-term irAEs) after the end of ICI therapy. In particular, patients with thyroiditis (8 long-term irAEs within 13 all irAEs, 8/13 = 61.5%) and hypoadrenocorticism (3 long-term irAEs within 7 all irAEs, 3/7 = 42.9%) showed a relatively higher rate of long-term irAEs. Thirty-five patients (17.5%) used steroids (including hydrocortisone) to treat irAEs. In terms of clinical outcome, some of the irAEs showed equally or more frequently showed “refractory” rather than “improved” and “resolved” for example, in the case of pneumonitis, thyroiditis, hyperadrenocorticism, and nervous system. One case each of hepatitis and gastrointestinal disorders was reported to have a causal relationship with death.
Supplementary Figures 3A and 4A show the OS profiles in patients with major irAEs such as dermatitis (Supplementary Figure 3A) and hepatitis (Supplementary Figure 4A) for NSCLC, GC and MM. OS in patients with irAEs tended to be longer but no clear conclusion was obtained due to small numbers of data.
Discussion
In our previous retrospective study among patients with NSCLC,31 we examined the possible factors affecting the efficacy and safety of nivolumab. We found that the ECOG PS before ICI therapy was associated with OS and that history of interstitial pneumonia was associated with nivolumab-related pneumonitis. We also found that a decreased albumin level during nivolumab treatment might be associated with disease progression and nivolumab-related pneumonitis. Our previous study was limited to cases of NSCLC receiving nivolumab, and thus, other possible factors that might be associated with OS, PFS and irAEs, in patients with various types of tumors including NSCLC and under ICIs therapies other than nivolumab should be examined.
For this purpose, we conducted this retrospective study in a single hospital and collected the data in patients treated with five ICIs, with major cancers of NSCLC, GC, and MM. Kaplan–Meier plots suggested that, although not necessarily significant, OS and PFS tended to be larger in patients with any irAEs with NSCLC, GC, or MM. These results coincide with the findings of some other studies19,30 that the occurrence of irAEs was associated with the efficacy of ICIs in terms of OS or PFS. We did not precisely examine the effect of the grade of irAEs on OS or PFS because a report32 suggested no effects of the grade, and the number patients with each irAEs grade for each cancer was not enough large for statistical consideration as given in Table 5. Instead, we summarized the individual data regarding irAEs grade and clinical outcomes in Table 5. The NSCLC is a major cancer treated with ICIs and the relationship between the efficacy and irAE occurrences were reported. In a previous report,32 OS and PFS were longer in patients with irAEs than those without irAEs. It was also shown that OS and PFS were longer with multi-system irAEs than with single irAE. The similar trend was obtained in our study and we could confirm the relationship in our patients. In a previous report of hepatocellular, colorectal, GC etc.,37 no clear efficacy difference was found between patients with single irAE and multi-system irAEs. For MM, a review article noted that the association between irAEs and the efficacy were with mixed results.38 Regarding GC and MM in the present study, OS and PFS tended to be longer in patients with irAEs than without irAEs, although no clear difference was found between patients with single and multi-system irAEs.
According to the results of the Cox regression analysis, the significant covariate for longer OS was baseline ALB ≥3.5 g/dL. For shorter OS, age (≥75 years), number of metastatic sites, liver metastasis, PS ≥ 2, and baseline LDH (≥400 IU/L) were significant (Table 2). For PFS, the significant covariates for shorter PFS were BMI, number of metastatic sites, liver metastasis, PS ≥ 2, and baseline LDH ≥400 IU/L (Table 3). These results are generally acceptable in that patients with a more severe stage of cancer tend to show a shorter OS or PFS. The results of the logistic regression analysis showed that age (≥75 years), smoking, and baseline ALB (≥3.5 g/dL) were associated with the occurrence of irAEs (Table 4).
The result of baseline ALB suggests that patients with higher efficacy more likely have irAEs, because higher baseline ALB was associated with longer OS in Table 2. Regarding age, no effect of age on irAEs were shown inn some previous studies.39-42 This may due to insufficient data for the frequency and severity of irAE in the elderly as large-scale clinical trials had not been conducted in the elderly.43 A post-marketing surveillance of nivolumab in NSCLC showed that smoking history was associated with higher risks of pneumonitis and hepatitis.44 In our present study, a high incidence of pneumonitis and hepatitis with Grade 3 or higher was observed (5 patients with ≥ Grade 3 pneumonitis; 15 patients with ≥ Grade 3 hepatitis including 1 death). As the number of patients was small in this study, we could not clearly specify risk factors for irAEs, patients with higher ALB, elderly and smoking history might be careful of developing irAEs when starting ICI therapy.
A limitation of the regression analysis was that we did not separately examine the effect of cancer types because the number of patients in each group became small for reliable regression results.
In this study (Table 5), pneumonitis occurred most frequently in NSCLC (10 of 16 cases) as compared with other cancer types, and the same findings were reported previously.45,46 Thyroiditis seems to have occurred often (in 6 of 13 patients). Long-term irAEs generally occurred less frequently in patients with GC (zero cases in most irAEs) compared with NSCLC and MM. Such precise information regarding irAEs by ICIs are valuable for individual pharmacotherapeutic care using ICIs by especially carefully monitoring patients with irAEs who have a long-term irAE and a higher rate of “refractory” as given in Table 5. In some previous studies, higher grade of irAEs occurred in about 50% of patients received a combination of anti-PD-1 and anti-CTLA-4 antibodies, but in 10-15% of patients with ICI monotherapy. In this study, most of the irAE grade were 1 or 2 probably, this was because most of the patients (n = 175, 87.5%) were treated with ICI alone and irAEs were generally mild.
We examined the relationship of dermatitis and hepatitis with OS (Supplementary Figures A3 and A4). It was reported that patients with dermatitis showed prolonged OS in patients with MM.47 In case of NSCLC and GC, there are few reports on the relationships. For hepatitis, discontinuation of ICI due to hospitalization by hepatitis was reported,48 and discontinuation of ICI may affect prognosis.49-51 In the present study, there were some patients with hepatitis including patients with 3 or higher grade (15/37), but no clear difference of OS was found. Further investigation of the impact of ICI discontinuation on prognosis is necessary.
In the present study, based on the data in a single hospital, we examined the effect of the ‘numbers’ of irAEs on efficacy for 3 major cancers. We also followed up of irAEs after ICIs treatment has ended (as shown in Table 5) which few studies have ever examined. The finding of this study would be useful for ICI use more safely and effectively.
This study has some limitations. First, clinical outcomes were classified into four categories (improved, relieved, refractory, and death) using our own method defined by grading changes, and this is not necessarily an established evaluation method. Second, this was a retrospective observational study that used medical records from a single clinical site and the sample size may not be enough for reliable statistical results in this study, therefore the generality of the results is not ensured and the findings of this study should be confirmed in a larger sample size study. We examined the relationships between the types of irAEs and prognosis impact only the limited cases of irAEs because of small sample size.
Conclusion
We examined the efficacy in terms of OS and PFS and their association with the occurrence of some irAEs during ICIs therapy in patients with NSCLC, GC or MM in our hospital and we found patients with irAEs tended to have better OS or PFS, although the number of patients were limited. We suggest ICIs should be used appropriately by continuously monitoring irAEs.
Supplemental Material
Supplemental Material for A Retrospective Cohort Study of Multiple Immune-Related Adverse Events and Clinical Outcomes Among Patients With Cancer Receiving Immune Checkpoint Inhibitors by Hiroki Hata, Chikako Matsumura, Yugo Chisaki, Kae Nishioka, Misaki Tokuda, Kazuyo Miyagi, Tomoki Suizu, and Yoshitaka Yano in Cancer Control
Acknowledgments
We would like to thank MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing.
Author Contributions: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by HH, CM, YC, and YY. The first draft of the manuscript was written by HH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: This study was carried out according to the Declaration of Helsinki, and the protocol of this retrospective observational study was approved by the ethics committees of both the National Hospital Organization Osaka National Hospital (No. ONH 21067, approved on November 11, 2021) and Kyoto Pharmaceutical University (No. E21-018, approved on August 2, 2021).
Informed consent: We had not obtained Informed consent from individual participants included in the study, because this study was a retrospective observational study. However, we published information on the implementation of research on the National Hospital Organization Osaka National Hospital website, and we guarantee the opportunity of patient rejection. It was based on the Ethical Guidelines for Medical Research on Humans established by Japan Ministry of Health, Labour, and Welfare.
Data availability: The datasets generated during and analyses during the current study are available from the corresponding author on reasonable request.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Yugo Chisaki https://orcid.org/0000-0002-3492-8291
Yoshitaka Yano https://orcid.org/0000-0001-7787-883X
References
- 1.Coit DG, Thompson JA, Albertini MR, et al. Cutaneous melanoma, Ver. 2. NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2019;17:367-402. [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: Non–small cell lung cancer, Ver. 1.2020: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. 2019;17:1464-1472. [DOI] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, Ver. 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2020;18:329-354. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Jonasch E, Boyle S, et al. NCCN guidelines insights: Kidney cancer, Ver. 1.2021. J Natl Compr Cancer Netw. 2020;18:1160-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 2. Esophagus. 2019;16:25-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn L, Mansfield AS, Szczęsna A, et al. First–line atezolizumab plus chemotherapy in extensive–stage small–cell lung cancer. N Engl J Med. 2018;379:2220-2229. [DOI] [PubMed] [Google Scholar]
- 8.Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab–paclitaxel as first–line treatment for unresectable, locally advanced or metastatic triple–negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double–blind, placebo–controlled, phase 3 trial. Lancet Oncol. 2020;21:44-59. [DOI] [PubMed] [Google Scholar]
- 9.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894-1905. [DOI] [PubMed] [Google Scholar]
- 10.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous–cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishima S, Taniguchi H, Akagi K, et al. Japan Society of Clinical Oncology provisional clinical opinion for the diagnosis and use of immunotherapy in patients with deficient DNA mismatch repair tumors, cooperated by Japanese Society of Medical Oncology, First Edition. Int J Clin Oncol. 2020;25:217-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [DOI] [PubMed] [Google Scholar]
- 14.Chen DS, Mellman I. Oncology meets immunology: The cancer–immunity cycle. Immunity. 2013;39:1-10. [DOI] [PubMed] [Google Scholar]
- 15.Medina P, Jeffers KD, Trinh VA, Harvey RD. The role of pharmacists in managing adverse events related to immune checkpoint inhibitor therapy. J Pharm Pract. 2020;33:338-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michot JM, Bigenwald C, Champiat S, et al. Immune–related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [DOI] [PubMed] [Google Scholar]
- 17.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow–up. Ann Oncol. 2018;29:264-266. [DOI] [PubMed] [Google Scholar]
- 18.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman–Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune–related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hussaini S, Chehade R, Boldt RG, et al. Association between immune–related side effects and efficacy and benefit of immune checkpoint inhibitors – A systematic review and meta–analysis. Cancer Treat Rev. 2011;92:102134. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Akamatsu H, Murakami E, et al. Correlation between immune–related adverse events and efficacy in non–small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71-74. [DOI] [PubMed] [Google Scholar]
- 22.Haratani K, Hayashi H, Chiba Y, et al. Association of immune–related adverse events with nivolumab efficacy in non–small–cell lung cancer. JAMA Oncol. 2018;4:374-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akamatsu H, Murakami E, Oyanagi J, et al. Immune–related adverse events by immune checkpoint inhibitors significantly predict durable efficacy even in responders with advanced non–small cell lung cancer. Oncologist. 2020;25:e679-e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugisaka J, Toi Y, Taguri M, et al. Relationship between programmed cell death protein ligand 1 expression and immune–related adverse events in non–small–cell lung cancer patients treated with pembrolizumab. JAMA J. 2020;3:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuda K, Shoji H, Nagashima K, et al. Correlation between immune–related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer. 2019;19:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi K, Suzuki K, Hiraide M, et al. Association of immune–related adverse events with pembrolizumab efficacy in the treatment of advanced urothelial carcinoma. Oncology. 2020;98:237-242. [DOI] [PubMed] [Google Scholar]
- 27.Suresh K, Psoter KJ, Voong KR, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14:494-502. [DOI] [PubMed] [Google Scholar]
- 28.El Majzoub I, Qdaisat A, Thein KZ, et al. Adverse effects of immune checkpoint therapy in cancer patients visiting the emergency department of a comprehensive cancer center. Ann Emerg Med. 2019;73:79-87. [DOI] [PubMed] [Google Scholar]
- 29.Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non–small cell lung cancer. Thorac Cancer. 2019;10:2006-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35:785-792. [DOI] [PubMed] [Google Scholar]
- 31.Hata H, Mio T, Yamashita D, et al. Factors associated with efficacy and nivolumab–related interstitial pneumonia in non–small cell lung cancer: A retrospective survey. Cancer Control. 2020;27:1073274820977200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6:1952-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 34.Patrinely JR, Johnson R, Lawless AR, et al. Chronic immune–related adverse events following adjuvant anti–PD–1 therapy for high–risk resected melanoma. JAMA Oncol. 2021;7:744-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534-540. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z, Li Y, Yan X, et al. Pretreatment lactate dehydrogenase may predict outcome of advanced non-small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med. 2019;8:1467-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das S, Ciombor KK, Haraldsdottir S, et al. Immune-related adverse events and immune checkpoint inhibitor efficacy in patients with gastrointestinal cancer with food and drug administration-approved indications for immunotherapy. Oncologist. 2020;25:669-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattar J, Kartolo A, Hopman WM, Lakoff JM, Baetz T. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population. J Geriatr Oncol. 2019;10:411-414. [DOI] [PubMed] [Google Scholar]
- 40.Muchnik E, Loh KP, Strawderman M, et al. Immune checkpoint inhibitors in real-world treatment of older adults with non-small cell lung cancer. J Am Geriatr Soc. 2019;67:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leroy V, Gerard E, Dutriaux C, et al. Adverse events need for hospitalization and systemic immunosuppression in very elderly patients (over 80 years) treated with ipilimumab for metastatic melanoma. Cancer Immunol Immunother. 2019;68:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Archibald WJ, Victor AI, Strawderman MS, Maggiore RJ. Immune checkpoint inhibitors in older adults with melanoma or cutaneous malignancies: The Wilmot Cancer Institute experience. J Geriatr Oncol. 2020;11:496-502. [DOI] [PubMed] [Google Scholar]
- 43.Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer. 2017;82:155-166. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto N, Nakanishi Y, Gemma A, et al. Real-world safety of nivolumab in patients with non-small-cell lung cancer in Japan: Post marketing surveillance. Cancer Sci. 2021;112:4692-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer: A systematic review and meta–analysis. JAMA Oncol. 2016;2:1607-1616. [DOI] [PubMed] [Google Scholar]
- 46.Zhang S, Liang F, Zhu J, Chen Q. Risk of pneumonitis associated with programmed cell death 1 inhibitors in cancer patients: A meta–analysis. Mol Cancer Therapeut. 2017;16:1588-1595. [DOI] [PubMed] [Google Scholar]
- 47.Quach HT, Dewan AK, Davis EJ, et al. Association of anti-programmed cell death 1 cutaneous toxic effects with outcomes in patients with advanced melanoma. JAMA Oncol. 2019;5:906-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molina GE, Zubiri L, Cohen JV, et al. Temporal trends and outcomes among patients admitted for immune-related adverse events: A single-center retrospective cohort study from 2011 to 2018. Oncologist. 2021;26:514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol. 2017;35:3807-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warner AB, Palmer JS, Shoushtari AN, et al. Long-term outcomes and responses to retreatment in patients with melanoma treated with PD-1 blockade. J Clin Oncol. 2020;38:1655-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for A Retrospective Cohort Study of Multiple Immune-Related Adverse Events and Clinical Outcomes Among Patients With Cancer Receiving Immune Checkpoint Inhibitors by Hiroki Hata, Chikako Matsumura, Yugo Chisaki, Kae Nishioka, Misaki Tokuda, Kazuyo Miyagi, Tomoki Suizu, and Yoshitaka Yano in Cancer Control