Abstract
Introduction: This retrospective study aimed to compare the efficacy and safety of transarterial chemoembolization plus lenvatinib and programmed death 1 (PD-1) inhibitors versus transarterial chemoembolization plus lenvatinib or sorafenib in patients with unresectable hepatocellular carcinoma. Methods: Consecutive patients with unresectable hepatocellular carcinoma who received transarterial chemoembolization plus lenvatinib and PD-1 inhibitors, lenvatinib, or sorafenib were retrospectively identified in our institution between January 2018 and August 2020. The primary endpoint was overall survival. Results: A total of 84 patients were included in this analysis. The median overall survival was significantly improved in the transarterial chemoembolization plus lenvatinib and PD-1 inhibitor group compared with the transarterial chemoembolization plus sorafenib group (26.7 months [95% confidence interval 25.2-31.6] vs 14.4 months [95% confidence interval 9.5-18.9]; hazard ratio 0.39 [95% confidence interval 0.17-0.72]; P = .007) or the transarterial chemoembolization plus lenvatinib group (26.7 months [95% confidence interval 25.2-31.6] vs 17.9 [95% confidence interval 13.4-22.2] months; hazard ratio 0.45 [95% confidence interval 0.17-0.87]; P = .031). Transarterial chemoembolization plus lenvatinib and PD-1 inhibitor also significantly prolonged median progression-free survival compared with transarterial chemoembolization plus sorafenib group (8.2 months [95% confidence interval 3.3-13.0] vs 6.0 months [95% confidence interval 4.2-7.8]; hazard ratio 0.47 [95% confidence interval 0.24-0.74]; P = .005) or the transarterial chemoembolization plus lenvatinib group (8.2 months [95% confidence interval 3.3-13.0] vs 6.6 [95% confidence interval 4.3-7.9] months; hazard ratio 0.58 [95% confidence interval 0.31-0.96]; P = .047). No significant difference was seen between groups in the incidence of an adverse event or grade 3 or higher adverse event. Conclusion: Transarterial chemoembolization plus lenvatinib, and PD-1 inhibitor was associated with better survival benefits and acceptable toxicities, which may provide an additional therapeutic option for unresectable hepatocellular carcinoma.
Keywords: hepatocellular carcinoma, lenvatinib, nivolumab, programmed death 1 inhibitors, sorafenib, toripalimab, transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is a major cause of cancer-related death and the most frequently diagnosed primary liver tumor worldwide and in China.1,2 Despite improved survival owing to potentially curative approaches, including surgical resection, liver transplantation, and some locoregional treatments, in early-stage disease, however, most of the patients present with advanced HCC which has a limited chance for radical treatment.3
The Barcelona Clinic Liver Cancer (BCLC) staging system is the most widely validated staging system for HCC, which integrates tumor burden, liver function, and general health status to provide a clinical algorithm for treatment decision-making according to disease stages. The BCLC system allocates management choices based on the following 5 different disease categories: very early, early, intermediate, advanced, and terminal. Transarterial chemoembolization (TACE) is the standard of care for intermediate-stage HCC in asymptomatic patients with preserved liver function. It is also incorporated in the treatment of patients with early-stage diseases who are not feasible for or have failed the recommended treatments. TACE induces tumor necrosis by the delivery of drugs and embolic particles into tumor-nourishing arterial vessels.4 However, this approach is associated with deterioration of liver function and tumor hypoxia, resulting in poor prognosis and increased tumor angiogenesis.5–7
Emerging novel agents have modified the therapeutic landscape of advanced and metastatic HCC in recent years. In addition to sorafenib, lenvatinib has been approved in the first-line treatment setting for patients with advanced HCC with improved progression-free survival (PFS) and similar overall survival (OS) benefits to sorafenib.8 Immune checkpoint inhibitors, especially programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors also demonstrated promising activities in patients with advanced HCC.9,10 Despite failures in phase 3 randomized studies, PD-1 inhibitors showed a trend of prolonged survival and durable response in different treatment settings.11,12 In the recently reported KEYNOTE-394 study, pembrolizumab plus best supportive care significantly prolonged OS (hazard ratio [HR] 0.79, 95% confidence interval [CI] 0.63-0.99, P = .0180) and PFS (HR 0.74, 95% CI 0.60-0.92, P = .0032) as second-line treatment in Asian patients with advanced HCC.13 Immune-based combinations were also being investigated. In the open-label, randomized, phase III IMbrave150 study in which atezolizumab plus bevacizumab was compared with sorafenib in 501 treatment-naïve patients with advanced HCC, both primary co-endpoints PFS and OS were met, leading to the US Food and Drug Administration approval of this combination as first-line treatments.14 In the phase Ib study of pembrolizumab plus lenvatinib demonstrated that this combination had promising survival benefits, with an objective response rate (ORR) of 46%, a median duration of response of 8.6 months, a median PFS of 9.3 months, and a median OS of 22 months, as well as acceptable toxicity profiles without severe adverse events (AEs) without new safety signals.15 These findings suggested that a combination of antiangiogenic drugs and PD-1 inhibitors may represent a novel treatment option in this setting. Moreover, emerging novel therapeutics focus on other major mechanisms of HCC immune escape, including cytotoxic T-lymphocyte antigen 4, lymphocyte activating gene 3 protein, and mucin-domain molecule 3 (T cell immunoglobulin and mucin-domain containing molecule 3), which will bring new combination strategies.16
Some evidence indicates the therapeutic potential of combining antiangiogenic drugs with TACE in selected subpopulations and the combination of PD-1 inhibitors with TACE is being investigated in ongoing studies (for instance NCT03143270 and NCT03397654). However, limited data is available for a combination of TACE, antiangiogenic drugs, and immune checkpoint inhibitors. Thus, here we present a retrospective analysis of the efficacy and safety of TACE combined with sorafenib, levatinib, or lenvatinib plus PD-1 inhibitors in patients with unresectable HCC.
Method
Study Design and Patients
This retrospective study included all adult patients (18 years or older) with intermediate-stage and advanced-stage HCC who were consecutively admitted to our department from January 2018 to August 2020. Patients included in our study met the following criteria: an age of 18 years or older; histologically or cytologically confirmed HCC or clinically confirmed HCC according to the practice guidelines of the American Association for the Study of Liver Disease17; TACE-eligible patients; patients treated with a combination of TACE and sorafenib, lenvatinib, or lenvatinib plus PD-1 inhibitors; BCLC stage B or C; Child-Pugh class A or B; Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0 or 1; at least 1 measurable target nodule per modified Response Evaluation Criteria in Solid Tumors (mRECIST, version 1.1); and acceptable heart, kidney, and bone marrow functions. Microsatellite instability status or PD-L1 expression analyses of tumor tissue samples were not mandatory. Patients were excluded if they had any of the following reasons: missing or unevaluable data; symptomatic brain metastases; a complete obstructive invasion of the bile duct; tumor burden over 50% of the total liver volume; or had received any previous systemic therapy.
Our study did not require an ethical board approval or informed consent from patients because the institutional review board does not require ethical board approval for a retrospective study using anonymous data. All patient details have been deidentified in this study. The reporting of this study conforms to Strengthening the Reporting of Observational Studies in Epidemiology guidelines.18
Treatment Procedure
This is a retrospective study to investigate the efficacy and safety of sorafenib, lenvatinib, or lenvatinib combining PD-1 inhibitors plus TACE in unresectable HCC. Patients were selected consecutively, for whom treatment was assigned based on the disease, patient status, availability of drug, as well as the physician's preference.
TACE consisted of intraarterial injection of chemotherapy drug, followed by embolization to interrupt blood flow. The tip of the catheter was inserted into the artery branches for tumor feeding according to tumor size, location, and arterial supply. Embolization was initially conducted using different diameter microspheres or drug-eluting beads, and the trunk was ultimately embolized with an absorbable gelatin sponge until the bleeding stopped. Pharmorubicin was used as the chemotherapy drug.
Systemic treatment was administered within 3 months following TACE until disease progression or intolerable toxicity. Sorafenib was administered orally at 400 mg twice daily. Lenvatinib was administered orally at 8 mg (body weight<60 kg) or 12 mg (body weight>60 kg) once per day. Nivolumab was administered intravenously at 3 mg/kg once every 2 weeks. Toripalimab was administered intravenously at 3 mg/kg once every 2 weeks.
Data Collection
Clinical information was retrieved from the medical records. Patient characteristics were retrospectively examined, which included sex, age, ECOG-PS score, body mass index, BCLC staging, hepatitis virus status, presence of liver cirrhosis, tumor size, tumor number, presence of tumor embolus, presence of extrahepatic metastasis, Child-Pugh status, and history of previous treatment. Survival, tumor response, AE, and other related treatment data were also analyzed.
The primary endpoint was OS, defined as the time from the date of receiving the first TACE therapy to the date of death. The secondary endpoints were PFS, defined as the interval between the date of receiving the first TACE therapy and the date of disease progression according to the mRECIST or death from any cause, whichever occurred first, the ORR, defined as the proportion of patients with complete response or partial response, and the disease control rate (DCR), defined as the proportion of patients with ORR plus stable disease. The ORR and DCR were evaluated by the investigator according to the mRECIST criteria.
Statistics Analysis
Continuous variables were presented as mean ± standard deviation and compared using a t-test, or Mann–Whitney U-test for nonnormally distributed continuous variables. Categorical variables were reported as frequency and compared using the chi-square test and Fisher's exact test. Kaplan–Meier method was used to evaluate the OS and PFS, and the difference between groups was analyzed with a log-rank test. P values < .05 were considered to be statistically significant. All data were statistically analyzed using Statistical Package for the Social Sciences version 21.0.
Results
Between January 1, 2018, and August 1, 2020, a total of 84 patients were included in this analysis: 23 patients received a triple combination with TACE plus lenvatinib and PD-1 inhibitor (including nivolumab [n = 13] and toripalimab [n = 10]), 32 received TACE plus lenvatinib, and 29 received TACE plus sorafenib. Table 1 summarized the baseline characteristics, and no significant difference was observed between groups except for the presence of liver cirrhosis and tumor number. The lowest percentage of liver cirrhosis was noted in the TACE plus sorafenib group (86.96% vs 75% vs 55.17%, P = .036).
Table 1.
Baseline Patient Demographic and Disease Characteristics.
| TACE + lenvatinib + PD-1 inhibitors (n = 23) | TACE + lenvatinib (n = 32) | TACE + sorafenib (n = 29) | P-value | |
|---|---|---|---|---|
| Sex | .095 | |||
| Male | 23 (100.00) | 31 (96.88) | 25 (86.21) | |
| Female | 0 (0.00) | 1 (3.13) | 4 (13.79) | |
| Age (mean ± SD, y) | 52.83 ± 7.14 | 57.38 ± 9.44 | 55.90 ± 8.18 | .146 |
| BMI (mean ± SD) | 23.00 ± 2.96 | 23.96 ± 3.47 | 23.03 ± 3.40 | .450 |
| ECOG-PS | .928 | |||
| 0 | 12 (52.17) | 18 (56.25) | 15 (51.72) | |
| 1 | 11 (47.83) | 14 (43.75) | 14 (48.28) | |
| Hypertension | .450 | |||
| No | 17 (73.91) | 19 (59.38) | 17 (58.62) | |
| Yes | 6 (26.09) | 13 (40.63) | 12 (41.38) | |
| BCLC stage | .319 | |||
| B | 6 (26.09) | 13 (40.63) | 7 (24.14) | |
| C | 17 (73.91) | 19 (59.38) | 22 (75.86) | |
| Tumor embolus | .629 | |||
| No | 10 (43.48) | 18 (56.25) | 14 (48.28) | |
| Yes | 13 (56.52) | 14 (43.75) | 15 (51.72) | |
| Longest diameter of liver tumor (median, Q1, Q3) | 8.00(3.40,11.30) | 7.80(3.80,11.65) | 9.00(4.90,10.90) | .921 |
| Liver cirrhosis | .036 | |||
| No | 3 (13.04) | 8 (25.00) | 13 (44.83) | |
| Yes | 20 (86.96) | 24 (75.00) | 16 (55.17) | |
| Extrahepatic metastasis | .294 | |||
| No | 11 (47.83) | 22 (68.75) | 17 (58.62) | |
| Yes | 12 (52.17) | 10 (31.25) | 12 (41.38) | |
| Etiology | .116 | |||
| No | 1 (4.35) | 7 (21.88) | 4 (13.79) | |
| HBV | 22 (95.65) | 21 (65.63) | 23 (79.31) | |
| HCV | 0 (0.00) | 4 (12.50) | 2 (6.90) | |
| Child-Pugh class | .999 | |||
| A | 19 (82.61) | 27 (84.38) | 24 (82.76) | |
| B | 4 (17.39) | 5 (15.63) | 5 (17.24) | |
| Tumor node | .344 | |||
| Single | 8 (34.78) | 6 (18.75) | 6 (20.69) | |
| Multiple | 15 (65.22) | 26 (81.25) | 23 (79.31) | |
| Surgery | .722 | |||
| No | 18 (81.82) | 24 (75.00) | 24 (82.76) | |
| Yes | 4 (18.18) | 8 (25.00) | 5 (17.24) | |
| Radiotherapy | .999 | |||
| No | 21 (91.30) | 30 (93.75) | 27 (93.10) | |
| Yes | 2 (8.70) | 2 (6.25) | 2 (6.90) | |
| Ablation | .248 | |||
| No | 21 (95.45) | 27 (84.38) | 28 (96.55) | |
| Yes | 1 (4.55) | 5 (15.63) | 1 (3.45) |
Abbreviations: BCLC, Barcelona clinic liver cancer; BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; PD-1, programmed death 1; SD, standard deviation; TACE, transarterial chemoembolization.
Efficacy
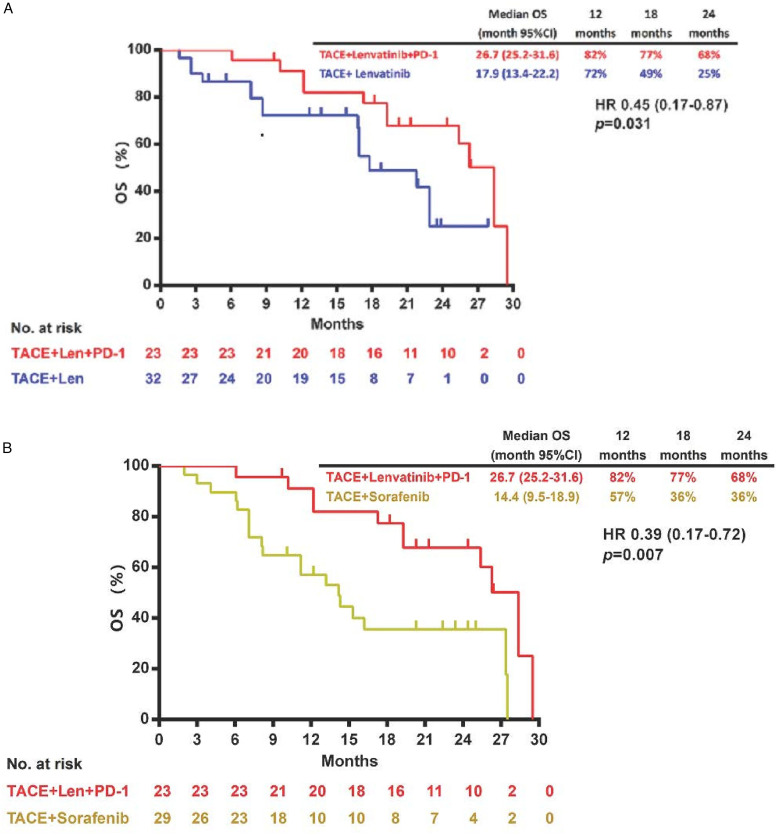
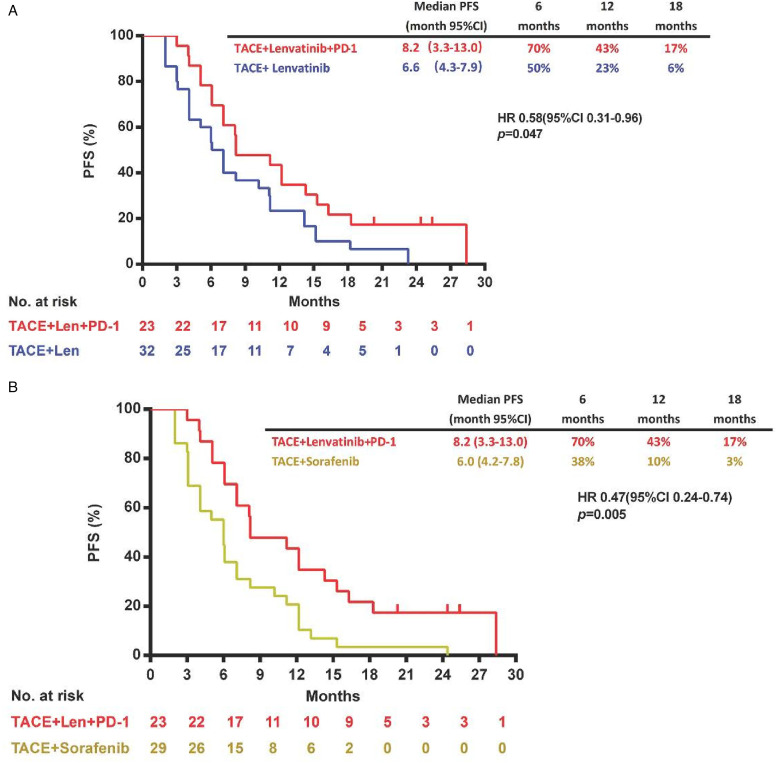
By data cut-off (May 16, 2021), the median OS was significantly improved in the TACE plus lenvatinib and PD-1 inhibitor group compared with the TACE plus lenvatinib group (26.7 months [95% CI 25.2-31.6] vs 17.9 [95% CI 13.4-22.2] months; HR 0.45 [95% CI 0.17-0.87]; P = .031; Figure 1A) or the TACE plus sorafenib group (26.7 months [95% CI 25.2-31.6] vs 14.4 months [95% CI 9.5-18.9]; HR 0.39 [95% CI 0.17-0.72]; P = .007; Figure 1B). TACE plus lenvatinib and PD-1 inhibitor also significantly prolonged median PFS compared with the TACE plus lenvatinib group (8.2 months [95% CI 3.3-13.0] vs 6.6 [95% CI 4.3-7.9] months; HR 0.58 [95% CI 0.31-0.96]; P = .047; Figure 2A) or the TACE plus sorafenib group (8.2 months [95% CI 3.3-13.0] vs 6.0 months [95% CI 4.2-7.8]; HR 0.47 [95%CI 0.24-0.74]; P = .005; Figure 2B).
Figure 1.
Kaplan–Meier curves showing OS of comparison of TACE plus lenvatinib and PD-1 inhibitor with TACE plus lenvatinib (A), and TACE plus lenvatinib and PD-1 inhibitor with TACE plus sorafenib (B).
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PD-1, programmed death 1; TACE, transarterial chemoembolization.
Figure 2.
Kaplan–Meier curves showing PFS of comparison of TACE plus lenvatinib and PD-1 inhibitor with TACE plus lenvatinib (A), and TACE plus lenvatinib and PD-1 inhibitor with TACE plus sorafenib (B).
Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression-free survival; PD-1, programmed death 1; TACE, transarterial chemoembolization.
The evaluation of the best response in each group was shown in Table 2 according to the mRECIST criteria. The TACE plus lenvatinib and PD-1 inhibitors were associated with significantly higher ORR and DCR than TACE plus lenvatinib (ORR: 86.96% vs 46.88%; DCR: 100% vs 75%) and TACE plus sorafenib (ORR: 86.96% vs 34.48%; DCR: 100% vs 48.28%).
Table 2.
Best Objective Response According to mRECIST.
| TACE + lenvatinib + PD-1 inhibitors (n = 23) | TACE + lenvatinib (n = 32) | TACE + sorafenib (n = 29) | P-value | |
|---|---|---|---|---|
| Complete response | 1 (4.35) | 1 (3.13) | 0 (0.00) | |
| Partial response | 19 (82.61) | 14 (43.75) | 10 (34.48) | |
| Stable disease | 3 (13.04) | 9 (28.13) | 4 (13.79) | |
| Progressive disease | 0 (0.00) | 8 (25.00) | 15 (51.72) | |
| ORR | 20 (86.96) | 15 (46.88) | 10 (34.48) | .0005 |
| Disease control rate | 23 (100) | 24 (75) | 14 (48.28) | .0002 |
Abbreviations: mRECIST, modified response evaluation criteria in solid tumors; PD-1, programmed death 1; TACE, transarterial chemoembolization; ORR, objective response rate.
Safety
AEs were summarized in Tables 3 and 4. AE occurred in 79% of patients, with fever, elevated alanine transaminase (ALT), and elevated aspartate transaminase (AST) being the most common ones. Fever, nausea, and fatigue were more frequently seen in patients treated with TACE plus lenvatinib and PD-1; hypertension and diarrhea were more common in patients treated with TACE plus lenvatinib; while elevated ALT, elevated AST, abdominal pain, loss of appetite, diarrhea, rash, and hand-foot syndrome were more common in patients treated with TACE plus sorafenib. However, these differences were not significant. With respect to grade 3 or higher AE, the incidence was similar in all groups. Table 5 summarized liver function in terms of elevated ALT and elevated AST of enrolled patients at enrollment and after treatment discontinuation, showing no difference was found between the 3 groups at enrollment or after treatment discontinuation. We also found no difference in liver function after treatment discontinuation as compared with that at enrollment in each group (Table 5).
Table 3.
Summary of All-Grade Adverse Events.
| TACE + lenvatinib + PD-1 inhibitors (n = 23) | TACE + lenvatinib (n = 32) | TACE + sorafenib (n = 29) | P-value | |
|---|---|---|---|---|
| All-grade | 18 (78.26) | 24 (75) | 24 (82.75) | .761 |
| Fever | 18 (78.26) | 23 (71.88) | 22 (75.86) | .857 |
| Elevated ALT | 17 (73.91) | 20 (62.5) | 22 (75.86) | .471 |
| Elevated AST | 16 (69.57) | 21 (65.63) | 21 (72.41) | .847 |
| Abdominal pain | 11 (47.82) | 13 (40.63) | 15 (51.72) | .678 |
| Nausea | 5 (21.74) | 3 (9.38) | 1 (3.45) | .101 |
| Hypertension | 5 (21.74) | 8 (25.00) | 2 (6.90) | .155 |
| Loss of appetite | 3 (13.04) | 3 (9.38) | 4 (13.79) | .851 |
| Diarrhea | 3 (13.04) | 2 (20.00) | 5 (17.24) | .408 |
| Fatigue | 3 (13.04) | 3 (9.38) | 3 (10.34) | .907 |
| Rash | 3 (13.04) | 3 (9.38) | 6 (20.69) | .443 |
| Hypothyroidism | 2 (8.70) | 1 (3.13) | 0 | .241 |
| Hand-foot syndrome | 1 (4.35) | 2 (0.00) | 15 (51.72) | 0 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; RECIST, modified response evaluation criteria in solid tumors; PD-1, programmed death 1; TACE, transarterial chemoembolization.
Table 4.
Grade 3 or Higher Adverse Events.
| TACE + lenvatinib + PD-1 inhibitors (n = 23) | TACE + lenvatinib (n = 32) | TACE + sorafenib (n = 29) | P-value | |
|---|---|---|---|---|
| Elevated ALT | 5 (21.74) | 6 (18.75) | 6 (20.69) | .961 |
| Elevated AST | 4 (17.39) | 7 (21.88) | 4 (13.79) | .711 |
| Hypertension | 1 (4.35) | 1 (3.13) | 0 (0.00) | .558 |
| Nausea | 1 (4.35) | 2 (6.25) | 0 (0.00) | .411 |
| Diarrhea | 1 (4.35) | 1 (3.13) | 1 (3.45) | .970 |
| Fatigue | 1 (4.35) | 1 (3.13) | 1 (3.45) | .970 |
| Rash | 0 (0.00) | 0 (0.00) | 1 (3.45) | .383 |
| Hand-foot syndrome | 0 (0.00) | 0 (0.00) | 2 (6.70) | .143 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; RECIST, modified response evaluation criteria in solid tumors; PD-1, programmed death 1; TACE, transarterial chemoembolization.
Table 5.
Liver Function of Enrolled Patients at Enrollment and After Treatment Discontinuation.
| TACE + lenvatinib + PD-1 inhibitors (n = 23) | TACE + lenvatinib (n = 32) | TACE + sorafenib (n = 29) | P-value | |
|---|---|---|---|---|
| Elevated ALT | ||||
| At enrolment | 3 (13.04) | 3 (9.38) | 4 (13.79) | .837 |
| After treatment discontinuation | 3 (13.04) | 5 (15.63) | 5 (17.24) | .925 |
| P-value | 1.000 | .708 | 1.000 | |
| Elevated AST | ||||
| At enrolment | 3 (13.04) | 4 (12.50) | 5 (17.24) | 1.000 |
| After treatment discontinuation | 6 (26.09) | 8 (25.00) | 8 (27.59) | .728 |
| P value | .459 | .337 | .530 |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; RECIST, modified response evaluation criteria in solid tumors; PD-1, programmed death 1; TACE, transarterial chemoembolization.
Discussion
The current study found that the combination of TACE plus lenvatinib and PD-1 inhibitor resulted in significantly longer OS as well as PFS than TACE plus lenvatinib (OS: 26.7 [95% CI 25.2-31.6] vs 17.9 [95% CI 13.4-22.2] months; HR 0.45 [95% CI 0.17-0.87], P = .031; PFS: 8.2 [95% CI 3.3-13.0] vs 6.6 [95% CI 4.3-7.9] months; HR 0.58 [95% CI 0.31-0.96]; P = .047) or TACE plus sorafenib (OS: 26.7 [95% CI 25.2-31.6] vs 14.4 months [95% CI 9.5-18.9]; HR 0.39 [95% CI 0.17-0.72], P = .007; PFS: 8.2 [95% CI 3.3-13.0] vs 6.0 months [95% CI 4.2-7.8]; HR 0.47 [95% CI 0.24-0.74]; P = .005). Moreover, a higher ORR and DCR were also seen in the TACE plus lenvatinib and PD-1 inhibitor group (ORR: 86.96%; DCR: 100%) than that in the TACE plus lenvatinib group (ORR: 46.88%; DCR: 75%) or in the TACE plus sorafenib group (ORR: 34.48%; DCR: 48.28%). With respect to safety, no significant difference was observed in all-grade AE or grade 3 or higher AE among groups. The TACE plus lenvatinib and PD-1 inhibitor may provide an effective and safe option for patients with unresectable HCC.
Vascular endothelial growth factor (VEGF) is a key biomarker of tumor angiogenesis which is directly involved in tumor growth and metastasis in HCC.19 TACE-associated acute hypoxia can cause the upregulation of VEGF, leading to tumor revascularization and local recurrence.20,21 This evidence establishes the rationale of combined antiangiogenic systemic therapies with TACE. Additionally, TACE can cause immune activation and immunogenic cell death that promotes the release of tumor antigens, which partly owing to chemotherapy-induced apoptosis.22 TACE has also been shown to be associated with a change in the immune microenvironment that could be modulated by immunotherapy.22,23 Furthermore, a synergistic effect from the combination of VEGF receptor (VEGFR) tyrosine kinase inhibitors (TKIs) and checkpoint inhibitors was also reported in several preclinical studies. Cabozantinib can enhance sensitivity to T cell-mediated killing by upregulating the expression of major histocompatibility complex class 1 antigens on tumor cells.24 The PD-1 inhibitors plus lenvatinib are able to improve anti-PD-1 efficacy by decreasing the number of tumor-associated macrophages and reducing tumor PD-L1 level and regulatory T (Treg) cell differentiation.25,26 Thus, the combination of TACE, antiangiogenic drugs and checkpoint inhibitors may be a potential therapy for unresectable HCC.
Studies have demonstrated the TACE combined with antiangiogenic agents is safe, effective, and feasible in patients with intermediate and advanced HCC. The efficacy and safety of subsequent sorafenib after TACE has been evaluated in 2 randomized studies with a time-to-progression (TTP) of 5.4 and 5.6 months, and an OS of 29.7 and not reached, respectively.27,28 Different from the aforementioned studies, in the TACTICS trial, however, sorafenib was administered 2 to 3 weeks prior to first TACE, and disease progression was defined as untreatable (UnTACEable) progression.29 In this study, TACE plus sorafenib significantly prolonged PFS over TACE alone (25.2 vs 13.5 months; P = .006) with increased but manageable AE.29 Furthermore, TACE plus sorafenib improved TACE-associated deterioration of liver function with a prolonged interval between pairs of TACE sessions. Lenvatinib is a multikinase inhibitor that targets VEGFRs 1-3, fibroblast growth factor receptors 1-4, platelet-derived growth factor receptor alpha, rearranged during transfection, and TKI. The phase III REFLECT study confirmed its noninferiority to sorafenib in terms of OS, based on which lenvatinib was approved as first-line treatment for advanced and metastatic HCC.8 Ding et al. reported a randomized study that compared TACE plus lenvatinib with TACE plus sorafenib in patients with HCC and portal vein tumor thrombus, showing a significantly longer TTP of TACE plus lenvatinib than TACE plus sorafenib (4.7 vs 3.1 months; HR, 0.55; 95% CI 0.32-0.95; P = .029). The TACE plus lenvatinib was also associated with a numerically longer OS (14.5 vs 10.8 months), however, the difference was not statistically significant (P = .17).30 In a retrospective study, a combination of TACE, sorafenib, and PD-1 inhibitors (pembrolizumab or nivolumab) showed promising outcomes with increased disease control rate (81.8% vs 55.2%, P = .046), and prolonged PFS (16.26 vs 7.30 months, P < .001) and OS (23.3 vs 13.8, P = .012) as compared with TACE and sorafenib.31 Another study also found that TACE plus lenvatinib and pembrolizumab was associated with improvement of OS (18.1 vs 14.1 months; P = .004) and PFS (9.2 vs 5.5 months; P = .006) when compared with TACE plus lenvatinib.32 Remarkable tumor shrinkage was seen in both combinations while enhanced antitumor activity was achieved by TACE plus lenvatinib and pembrolizumab (90% vs 72.2%, P = .007).32 The current study found that in patients with unresectable HCC, TACE plus lenvatinib and PD-1 inhibitor resulted in significantly longer OS and PFS than did TACE plus lenvatinib or TACE plus sorafenib. The median OS and PFS in the TACE plus lenvatinib group were consistent with that in the previous study. The PFS of the TACE plus sorafenib group was 6.0 months, which was also comparable with previous reports, while the median OS was 14.4 months, which is numerically shorter than that reported in other studies. Possible explanations included distinct populations and a small sample size. The clinical benefit of TACE plus lenvatinib and PD-1 inhibitor over TACE plus lenvatinib or TACE plus sorafenib was also supported by the higher ORR and DCR, which was 86.96% versus 46.88% versus 34.48% (P = .0005) and 100% versus 75% versus 48.28% (P = .0002), respectively. Overall, ORR and DCR for the 3 groups seem similar to previous studies, although somewhat different patient populations and methods of assessment limit comparison.
The combination of TACE plus lenvatinib and PD-1 inhibitor was clinically feasible and safe. The incidence of AE and grade 3 or higher AE was similar among the 3 groups. The safety profile of each combination was in line with that reported in previous studies. None of the AEs in either group was unexpected. However, we should note that the potential effect of immunotherapies in the liver microenvironment and immunosuppressive cells such as Treg cells, which was associated with the occurrence of immune-related AE.33 Treg cells were found to be specifically distributed in the tumor microenvironment of HCC. Several immune checkpoints, including tumor necrosis factor receptor 4, T cell immunoreceptor with immunoglobulin and ITIM domain, and cytotoxic T-lymphocyte-associated protein 4, were found to be uniquely overexpressed in Treg cells.34 CD4 + CD25 + Tregs are thought to contribute to the impaired immune response during chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, while HBV and HCV infection is a major cause of HCC in China.
A major limitation of this study was the retrospective nature with inherent bias. Moreover, the small sample size limited the reliability, however, the aim of this study was exploration more than proof of concept. We also failed to collect data on biomarkers including PD-L1 status since PD-L1 expression was not mandatory in the clinical practice; in addition, the study has demonstrated that baseline PD-L1 expression did not have an impact on the objective response rates to anti-PD-1 therapy.10 Finally, we did not conduct subgroup analysis to further identify the effect of baseline characteristics on the clinical outcome due to the small sample size, further study with a prospective design and large population was warranted.
Conclusion
In summary, our study indicated that, compared with TACE plus lenvatinib or sorafenib, triple combination therapy with TACE, lenvatinib, and PD-1 inhibitor was associated with better survival benefits and acceptable toxicities in patients with unresectable HCC. TACE plus lenvatinib and PD-1 inhibitor may provide an additional therapeutic option for unresectable HCC.
Abbreviations
- AE
adverse event
- BCLC
Barcelona clinic liver cancer
- BMI
body mass index
- CI
confidence interval
- DCR
disease control rate
- ECOG-PS
Eastern Cooperative Oncology Group performance status
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- mRECIST
modified response evaluation criteria in solid tumor
- ORR
objective response rate
- OS
overall survival
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- PFS
progression-free survival
- TACE
transarterial chemoembolization
- TTP
time-to-progression
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Peng Song https://orcid.org/0000-0003-3580-3631
Reference
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7‐33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Meyer T, Palmer DH, Cheng AL, Hocke J, Loembe AB, Yen CJ. mRECIST to predict survival in advanced hepatocellular carcinoma: analysis of two randomised phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017;37(7):1047‐1055. doi: 10.1111/liv.13359. [DOI] [PubMed] [Google Scholar]
- 4.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52(2):762‐773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 5.Hiraoka A, Kumada T, Kudo M, et al. Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: multicenter analysis. Dig Dis. 2017;35(6):602‐610. doi: 10.1159/000480256. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10(19):2878‐2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523‐529. doi: 10.1080/02841850801958890. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163‐1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhu AX, Finn RS, Edeline J, et al. ; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940‐952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 10.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492‐2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn RS, Ryoo BY, Merle P, et al. ; KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193‐202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 12.Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77‐90. doi: 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 13.Qin S, Chen Z, Fang W, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40(4):383s‐383s. doi: 10.1200/JCO.2022.40.4_suppl.383. [DOI] [Google Scholar]
- 14.Finn RS, Qin S, Ikeda M, et al. ; IMbrave150 investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894‐1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 15.Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960‐2970. doi: 10.1200/JCO.20.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone P, Solimando AG, Fasano R, et al. The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines (Basel). 2021;9(5):532. doi: 10.3390/vaccines9050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723‐750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 19.Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11(8):1000‐1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahm JH, Rhee H, Kim H, et al. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: a biopsy and resection matched study. Oncotarget. 2017;8(59):99359‐99371. doi: 10.18632/oncotarget.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Y, Yang H, Xu X, et al. Effect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasion. Cancer Sci. 2017;108(4):753‐762. doi: 10.1111/cas.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohles N, Nagel D, Jüngst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour Biol. 2012;33(6):2401‐2409. doi: 10.1007/s13277-012-0504-2. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690‐714. doi: 10.1016/j.ccell.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72(2):307‐319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185‐204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi C, Chen L, Lin Z, et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology. 2021;74(5):2544‐2560. doi: 10.1002/hep.31921. [DOI] [PubMed] [Google Scholar]
- 27.Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47(14):2117‐2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090‐1098. doi: 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492‐1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127(20):3782‐3793. doi: 10.1002/cncr.33677. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L, Fang S, Wu F, et al. Efficacy and safety of TACE combined with sorafenib plus immune checkpoint inhibitors for the treatment of intermediate and advanced TACE-refractory hepatocellular carcinoma: a retrospective study. Front Mol Biosci. 2021;7:609322. doi: 10.3389/fmolb.2020.609322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Wu Z, Shi F, et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol. 2021;148(8):2115-2125. doi: 10.1007/s00432-021-03767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granito A, Muratori L, Lalanne C, et al. Hepatocellular carcinoma in viral and autoimmune liver diseases: role of CD4+CD25+Foxp3+regulatory T cells in the immune microenvironment. World J Gastroenterol. 2021;27(22):2994‐3009. doi: 10.3748/wjg.v27.i22.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai Y, Chen D, Cheng C, et al. Immunosuppressive landscape in hepatocellular carcinoma revealed by single-cell sequencing. Front Immunol. 2022;13:950536. doi: 10.3389/fimmu.2022.950536. [DOI] [PMC free article] [PubMed] [Google Scholar]