Abstract
Purpose of Review:
Rituximab is increasingly prescribed for glomerular diseases. However, the recently published Kidney Disease Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases lacks details on recommended dosing regimens for most individual glomerular diseases. We performed this scoping review summarizing the evidence for rituximab dosing in glomerular disease.
Sources of Information:
PubMed database.
Methods:
The PubMed search methodology was developed with a medical librarian and performed by the first, with review by a second, author. Randomized controlled trials (RCTs) and prospective cohort studies (PCSs) examining rituximab efficacy and/or safety in antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV), membranous nephropathy (MN), lupus nephritis (LN), or podocytopathies (minimal change disease or focal segmental glomerulosclerosis [FSGS]) were included. Fifty-three studies (14 RCTs and 39 PCSs) were included.
Key Findings:
We identified 16 different rituximab dosing regimens studied as induction therapy for one or more of the 5 glomerular diseases of interest. The most frequently studied rituximab induction regimens were 1000 mg as 2 doses 2 weeks apart (17 studies, 32%) and 4 doses of 375 mg/m2/week (18 studies, 33.9%). Twenty-six studies (49%) examined rituximab as monotherapy or in conjunction with corticosteroids alone, while the remaining studies examined rituximab as part of combination immunosuppression. Adapting treatment to achieve B-cell depletion, with frequent evaluation of disease-specific biomarkers, might prove the optimal approach to achieving and maintaining remission. Rituximab might also enable steroid minimization or avoidance.
Limitations:
Restriction of the search to a single database and to studies published in the English language, and with an accompanying abstract, could have led to selection bias. While the search was limited to prospective observational studies and RCTs, no formal assessment of study quality was performed.
Keywords: antineutrophil cytoplasmic antibody-associated vasculitis, glomerulonephritis, kidney disease, nephrotic syndrome, rituximab
Abrégé
Motif de la revue:
Le rituximab est de plus en plus prescrit pour traiter les maladies glomérulaires. Les lignes directrices de pratique clinique 2021 pour la prise en charge des maladies glomérulaires, publiées récemment par KDIGO, ne contiennent cependant aucun détail sur les schémas posologiques recommandés pour la plupart des maladies glomérulaires. Cette étude de portée résume les données concernant l’administration de rituximab pour le traitement des maladies glomérulaires.
Sources:
Base de données PubMed
Méthodologie:
La méthodologie de recherche PubMed a été élaborée avec un bibliothécaire médical, réalisée par le premier auteur et révisée par le deuxième auteur. Ont été inclus des essais contrôlés randomisés (ECR) et des études de cohortes prospectives (ÉCP) portant sur l’efficacité et/ou l’innocuité du rituximab dans le traitement des vascularites associés aux ANCA (VAA), de la néphropathie membraneuse (NM), de la néphrite lupique (NL) ou des podocytopathies (maladie à changement minimal ou hyalinose segmentaire et focale (HSF). Cinquante-trois études (14 ECR et 39 ÉCP) ont été incluses.
Principaux résultats:
Nous avons répertorié 16 différents schémas posologiques de rituximab étudiés comme traitement d’induction pour une ou plusieurs des cinq maladies glomérulaires d’intérêt. Les traitements d’induction avec rituximab les plus fréquemment étudiés étaient l’administration de 1 000 mg à raison de deux doses à deux semaines d’intervalle (17 études; 32 %) et de quatre doses de 375 mg/m2/semaine (18 études; 33,9 %). Vingt-six études (49 %) avaient examiné le rituximab en monothérapie ou en association avec des corticostéroïdes seuls; les autres études avaient examiné le rituximab dans le cadre d’un traitement immunosuppresseur combiné. Adapter le traitement pour atteindre l’épuisement des cellules B, avec évaluation fréquente des biomarqueurs spécifiques de la maladie, pourrait s’avérer l’approche optimale pour atteindre et maintenir la rémission. Le rituximab pourrait également permettre de minimiser ou d’éviter les stéroïdes.
Limites:
La restriction de la recherche à une seule base de données et à des études publiées en anglais accompagnées d’un résumé pourrait avoir entraîné un biais de sélection. Bien que la recherche se limitait aux études observationnelles prospectives et aux ECR, aucune évaluation formelle de la qualité des études n’a été effectuée.
Introduction
Rituximab is a chimeric murine/human monoclonal antibody targeted against the pan-B-cell marker CD20.1 Its use is approved by the United States Food and Drug Administration for the treatment of indolent B-cell non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL), rheumatoid arthritis (RA), and antineutrophil cytoplasm antibody (ANCA)-associated vasculitis (AAV).2 There is also mounting evidence for its use in the treatment of other glomerular kidney diseases, as reflected in the Kidney Disease Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases.3
Rituximab is typically well tolerated and has an acceptable safety profile. Infusion reactions and infections are the most frequent short-term adverse effects.4 However, longer-term safety concerns are emerging: prolonged use has been associated with hypogammaglobulinaemia and neutropenia, and a black box warning highlights the risk for hepatitis B reactivation and the rare complication of progressive multifocal leukoencephalopathy. Malignancy risk does not appear to be significantly increased.
Patients with impaired kidney function frequently require medication dose adjustments, due to impaired renal drug clearance and/or more frequent reported adverse events.5 Patients with glomerular diseases as a cause of kidney disease might also require adjunct therapies to achieve disease control, which introduce additional toxicity risk.3 Determining the optimal dose of Rituximab in glomerular disease should consider not only the dose required to induce and maintain remission but should also minimize risk for development of treatment-emergent side effects. However, the optimal dose and frequency of administration of rituximab for the treatment of specific glomerular disease subtypes have not been established. To fill this knowledge gap, this scoping review aims to summarize rituximab dosing regimens studied for the treatment of glomerular disease in an effort to guide safe and effective prescribing in this setting.
Methods
Data Source and Search Strategy
One author (H.A.) independently searched the PubMed database after developing a search methodology with a medical librarian. Selected articles were reviewed for relevance and data extraction in conjunction with a second author (K.S.). Search terms (Table 1) included more common glomerular disease subtypes for which rituximab is prescribed AND randomized controlled trials (RCTs) OR prospective cohort studies (PCSs) examining rituximab efficacy or safety. Results were limited to the English language, from inception to December 2021.
Table 1.
Summary of Search Terms Used for Literature Review (See Search Strategy as Footnote).
| Section | Search term |
|---|---|
| Rituximab | “Rituximab,” “RTX,” “b-cell depletion,” “Anti-CD20” |
| ANCA vasculitis | “ANCA,” “ANCA associated vasculitis,” “vasculitis” “MPO,” “PR3,” “anti-MPO,” “anti-PR3” |
| Membranous nephropathy | “nephrotic syndrome,” “Membranous Nephropathy,” “Membranous,” “MN,” “membranous glomerulonephritis,” “MGN,” |
| Minimal change disease | “nephrotic syndrome,” “Minimal change disease,” “minimal change,” “MCD,” “steroid resistant,” “steroid responsive” |
| Focal segmental glomerulosclerosis | “nephrotic syndrome,” “Focal Segmental Glomerulosclerosis,” “FSGS,” “steroid resistant,” “steroid responsive” |
| Lupus nephritis | “Lupus,” “Lupus Nephritis,” “SLE” |
Note. Search strategy: (“Rituximab,” OR “RTX,” OR “b-cell depletion,” OR “Anti-CD20”) AND (“ANCA,” OR “ANCA associated vasculitis,” OR “vasculitis” OR “MPO,” OR “PR3,” OR “anti-MPO,” OR “anti-PR3”) OR (“nephrotic syndrome,” OR “Membranous Nephropathy,” OR “Membranous,” OR “MN,” OR “membranous glomerulonephritis,” OR “MGN”) OR (“Minimal change disease,” OR “minimal change,” OR “MCD”) OR (“Focal Segmental Glomerulosclerosis,” OR “FSGS,” OR “steroid resistant,” OR “steroid responsive”) OR (“Lupus,” OR “Lupus Nephritis,” OR “SLE”); Filters: Abstract, English.
Study Selection
All RCTs and PCSs including adults (≥18 years) receiving rituximab as an experimental drug for the treatment of one or more of the following glomerular diseases were included: AAV, primary membranous nephropathy (MN), lupus nephritis (LN), minimal change disease (MCD), or focal segmental glomerulosclerosis (FSGS). We excluded studies in languages other than English and those lacking a freely available abstract in PubMed, by setting English language and abstract filters. Following manual review of study titles and abstracts, we excluded systematic reviews, review articles, retrospective studies, case series, case reports, and studies not detailing rituximab dosing. We also excluded studies with less than 10 participants and those that failed to report clinically meaningful outcomes (remission, relapse, patient or kidney survival). Studies examining rituximab use for systemic lupus erythematosus were included only if there were at least 10 adult participants with LN who received rituximab. Cited references from any identified systematic review articles were manually reviewed to identify additional potentially eligible studies.
Data Extraction and Analysis
The following study characteristics were extracted by the first author (H.A.) and confirmed by a second author (K.S.): first author, year of publication, country, study aim, study design, number of adult subjects, clinical characteristics (glomerular disease subtype, sex, age), rituximab indication and dose, concomitant immunosuppressive therapy, follow-up duration, outcome definitions (complete and partial remission, relapse), and primary outcome and adverse event findings. Data interpretation discrepancies were resolved by consensus discussion. All data were stored in Microsoft Excel, which was also used to generate descriptive summary statistics, tables, and graphs.
Results
Study Characteristics
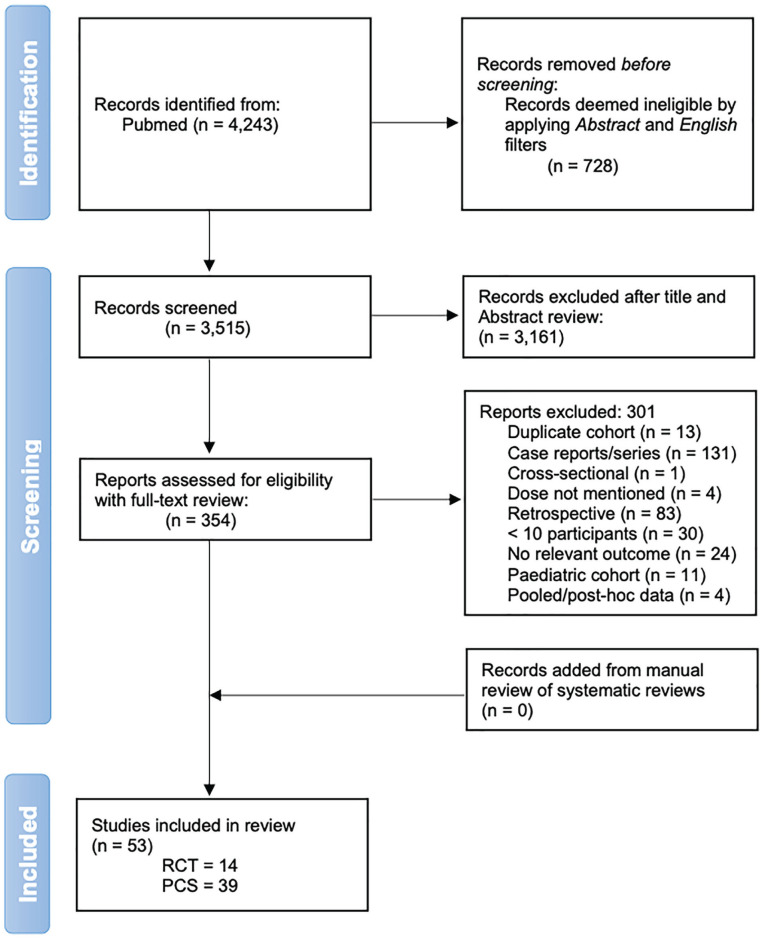
After removal of duplicates and manuscripts lacking an abstract or not in English, our initial search strategy returned 3515 results. Manual screening of the titles and abstracts of these manuscripts resulted in 354 full-text articles selected for further review for potential eligibility (Figure 1). Fifty-three of these studies were ultimately included in this review: 14 RCTs and 39 PCSs (Tables 2-6). Forty-seven studies evaluated rituximab as an induction treatment, 2 as a maintenance treatment, and 4 as both an induction and maintenance treatment. The studies included 2972 adult participants: 1215 (40.8%) with AAV, 856 (28.8%) with primary MN, 661 (22.2%) with LN, and 240 (8%) with MCD or FSGS. The most frequently studied rituximab induction regimens were 1000 mg as 2 doses 2 weeks apart (17 studies, 32%) and 4 doses of 375 mg/m2/week (18 studies, 33.9%). Twenty-six studies (49%) examined rituximab as monotherapy or in conjunction with steroids alone, while the remaining studies examined rituximab as part of combination immunosuppression.
Figure 1.
Flow diagram of study identification and selection.
Note. RCT = randomized controlled trials; PCS = prospective cohort studies.
Table 2.
Summary of Included Studies of ANCA-Associated Vasculitis (Listed in Chronological Order According to Type of Study).
| Study | Type | Intervention/aim | Number of participants | RTX regimen | Concomitant immunosuppression | B-cell depletion reported | Study duration | Primary findings | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Induction regimen | |||||||||
| Stone et al6
USA & Europe |
RCT | RTX vs CYC for the induction of CR for severe AAV | 197 | 4 doses of 375 mg/m2/wk | GC for induction and maintenance AZA for maintenance |
Yes | 6 months | CR: RTX group: 63 (64%) Control: 52 (53%) P < .001 for noninferiority |
AE: RTX: 1035, Control: 1061 SAE: RTX: 79, Control: 78 |
| Jones et al7
Europe and Australia |
RCT | RTX vs CYC-based regimens for renal AAV | 44 | 4 doses of 375 mg/m2/wk | Two doses of IV CYC for induction and GC for induction
and maintenance PLEX use was allowed before enrolment |
Yes | 12 months | Sustained remission: RTX group: 25/33 (76%) Control: 9/11 (82%) (P = .68) |
AE: RTX: 68 Control: 26 SAE: RTX: 31 Control: 12 |
| Furuta et al8
Japan |
RCT | Compare efficacy and safety of reduced-dose vs standard-dose GC, plus RTX (both groups), in remission induction of AAV | 140 | 4 doses of 375 mg/m2/wk | GC (low vs high dose) | Yes | 6 months | Remission: Reduced GC group: 49/69 patients (71%) High GC group: 45/65 patients (69%) P = .003 for noninferiority |
AE: Not mentioned SAE: Low GC: 21 High GC: 41 |
| Mansfield et al9
UK |
PCS | RTX and CYC for induction of remission for renal AAV | 23 | 1000 mg on days 0, 14 | Induction: IV CYC and oral GC Maintenance: AZA and low-dose GC |
Yes | Median: 39 months |
Remission: 100% within 6 week Relapse: 3 major and 2 minor in 5 patients (21%) at a median of 30 months, treated by redosing with RTX for major relapses and GC increase alone for minor relapses |
AE: 21 SAE: 3 |
| Turner-Stokes et al10
UK |
PCS | Evaluate single dose of RTX for induction treatment of AAV | 19 | Single dose of 375 mg/m2 | GC 8 (42%) were also receiving CYC or MMF |
Yes (<0.005 cells/ml) |
Median: 11.5 months |
Satisfactory BCD in 89% of patients after a median of 13
days; 3-month probability of BCD was 89%. Median time to
CR was 38 days; 3-month probability of CR was
80% Median time to B-cell repopulation 9.2 months and to relapse/re-dose was 27 months. |
Not mentioned |
| McGregor et al11
USA |
PCS | Describe outcomes and adverse events following RTX use in AAV | 120 | 1000 mg on days 0, 14 Or 4 doses of 375 mg/m2/wk |
GC 83% were exposed to CYC 75% receiving MMF 53% receiving AZA |
Yes | Median: 19 months |
RTX resulted in 86% achieving remission and 41% having a subsequent relapse after a median of 19 months (range, 9-29) | AE: not mentioned SAE: not mentioned |
| Pepper et al12
UK & Ireland |
PCS | Early rapid GC withdrawal for induction therapy in patients with severe AAV, using RTX and low-dose CYC | 49 | 1000 mg on days 0, 14 | GC for 2 weeks and CYC Maintenance: from week 12 (first line AZA, Alternatives MMF, MTX, RTX) | Yes | 12 months | Remission: 44/49 (90%) without addition of further GC | AE: 69 SAE: not mentioned |
| Miyazaki et al13
Japan |
PCS | Identify abnormalities in lymphocyte differentiation, analyze its clinical significance, & investigate its effect on using RTX and CYC | 54 | 4 doses of 375 mg/m2/wk | GC | Yes | 6 months | BVAS remission rate: RTX group = 61.8% IV- CYC group = 40.0 [P = .16] No significant differences in the rate of clinical improvement, or relapses between patients receiving RTX and IV-CYC |
AE: RTX: 19 CYC: 14 SAE: not mentioned |
| Smith et al14 | PCS | The use of RTX as therapy to induce remission after relapse in AAV | 188 | 4 doses of 375 mg/m2/wk | GC | Yes | 4 months | 171/188 (90%) achieved remission | AE: 41 SAE: 27 |
| Maintenance regimen | |||||||||
| Guillevin et al15
France |
RCT | RTX vs AZA for remission maintenance in AAV after induction with CYC-based regimen | 115 | 6 months after induction: RTX 500 mg on days 0 and 14 then at months 6, 12, & 18 | Low-dose oral GC | Patient were B-cell depleted on recruitment | 28 months | Major relapse: AZA group: 17 patients (29%) RTX group: 3 patients (5%) HR 6.61 (1.56-27.96; P = .002) |
AE: not mentioned SAE: RTX: 39 AZA: 44 |
| Charles et al16
France |
RCT | Evaluate the efficacy of prolonged RTX therapy vs placebo in preventing AAV relapses after achieving CR | 97 | 500 mg every 6 months (4 doses) |
GC | Yes | 28 months | Relapse-free survival: RTX group: 96% (95% CI, 91%-100%) Placebo group: 74% (63%-88%) in the RTX HR of 7.5 (CI, 1.67-33.7) (P = .008) Major relapse-free survival at month 28 100% (93%-100%) vs 87% (78%-97%) (P = .009) |
AE: not mentioned SAE: RTX: 21 placebo: 18 |
| Induction and maintenance regimen | |||||||||
| Charles et al17
France |
RCT | Compare tailored vs fixed-schedule RTX regimen to maintain remission | 162 | Tailored arm: 500 mg; then further 500 mg doses based on
ANCA and CD19+ count every 3 months Control-arm: 500 mg on days 0 and 14, & at months 6, 12, 18 |
GC | Yes | 28 months | 21 patients suffered 22 relapses: 14/81 (17.3%) in
tailored-arm and 8/81 (9.9%) in fixed-arm
(P = .22). The tailored vs fixed-schedule groups, respectively, received 248 vs 381 infusions, with medians (IQR) of 3 (2-4) vs 5 (5-5) per patient |
AE: not mentioned SAE: Tailored: 37 Control: 53 |
Note. ANCA = antineutrophil cytoplasm antibody; RTX = rituximab; RCT = randomized controlled trial; CYC = cyclophosphamide; CR = complete remission; AAV = ANCA associated vasculitis; wk = week; GC = glucocorticoids; AZA = azathioprine; AE = adverse events; SAE = severe adverse events; PCS = prospective cohort study; MMF = mycophenolate mofetil; BCD = B-cell depletion; CI = confidence interval; IQR = interquartile range; PLEX = plasma exchange; MTX = methotrexate; BVAS = Birmingham vasculitis activity score; HR = hazard ratio.
Table 3.
Summary of Included Studies of Membranous Nephropathy (Listed in Chronological Order and According to Type of Study—All Are Induction Regimens).
| Study | Type | Intervention/aim | Number of participants | RTX regimen | Concomitant immunosuppression | B-cell depletion reported | Study duration | Primary findings | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Dahan et al18
France |
RCT | Evaluate the efficacy of RTX added to supportive therapy compared with supportive therapy alone | 77 | 2 doses of RTX (375 mg/m2/wk) | None | Yes | 6 months | 6 months CR: NIAT-RTX group: 13/37 (35.1%); 95% CI, 19.7-50.5 NIAT group: 8/38 (21.1%) [8.1-34.0, 19.7-50.5] (P = .21) Observational phase follow-up: Remission rates before change of assigned treatment were NIAT-RTX: 24/37 (64.9%) NIAT: 13/38 (34.2%) (P = .01) |
AE: not mentioned SAE: RTX: 6 NIAT: 5 |
| Fervenza et al19
USA |
RCT | Investigate whether RTX is non-inferior to cyclosporine in inducing & maintaining remission | 130 | 1000 mg on days 0, 14 followed by single 1000 mg in case of PR | None | Yes | 24 months | CR or PR: RTX group: 39 (60%) Cyclosporine group: 13 (20%) Risk difference, 40%; 95% CI, 25%-55%; P < .001 for both noninferiority and superiority |
AE: RTX: 179 Cyclosporine: 218 SAE: RTX: 13 Cyclosporine: 22 |
| Scolari et al20
Italy & Switzerland |
RCT | Obtain estimates of RTX efficacy relative to cyclical CYC/GC and assess RCT recruitment potential using a multisite design | 74 | 1000 mg on days 0, 14 | None | No | 24 months | Probability of CR or PR: RTX group: 0.83 (95% CI, 0.65-0.95) CYC/GC group: 0.82 (95% CI, 0.68-0.93) |
AE: RTX: 2 CYC: 30 SAE: RTX: 7 CYC: 5 |
| Fernández-Juárez21
Spain & Netherlands |
RCT | Investigate if sequential therapy with TAC & RTX is superior to cyclical alternating treatment with GC and CYC in inducing persistent remission in MN | 86 | 1000 mg, 6 months after starting TAC | TAC | No | 24 months | The composite outcome: GC-CYC group: 36
(83.7%) TAC-RTX group: 25 (58.1%) (RR: 1.44; 95% CI, 1.08-1.92) CR: GC-CYC group: 26 (60%) TAC-RTX group: 11 (26%) (RR: 2.36; 95% CI, 1.34-4.16) |
AE: RTX: 170 CYC/GC: 239 SAE: RTX: 7 CYC/GC: 10 |
| Cravedi et al22
Italy |
PCS | Whether titrating RTX to circulating CD20 B-cell counts improves safety and reduces costs | 36 | 4 doses of 375
mg/m2/wk vs targeted B-cell approach: single 375 mg/m2 with monthly evaluation |
None | Yes | 12 months | Only one patient needed a second dose to achieve full
CD20 cell depletion At 1 year, disease remission was identical in both groups (25%) Persistent CD20 cell depletion was achieved in all patients |
AE: 8 SAE: 0 |
| Fervenza et al23
USA & Canada |
PCS | A pilot trial of RTX treatment in severe NS refractory to ACEi and/or ARB but with adequately controlled blood pressure | 15 | 1000 mg on days 0, 14 and 1000 mg after 6 months based on B-cell count | None | Yes | 12 months | 14 completed follow-up: CR: 2/14 (14%) PR: 6/14 (42%) CR or PR: 8/14 (56%) |
AE: 14 SAE: 2 |
| Fervenza et al24
USA & Canada |
PCS | To investigate the efficacy and safety of 4 weekly RTX regimen, with re-treatment at 6 months | 20 | 4 doses of 375 mg/m2/wk and repeated after 6 months | None | Yes | 24 months | 18 completed 24-month follow-up: CR: 4/18 (22%) PR: 12/18 (66%) 1 had a limited response 1 relapse |
AE: 12 SAE: 1 |
| Cravedi et al25
Italy |
PCS | Evaluate whether RTX is equally effective in patients who failed to respond to previous immunosuppressive treatment | 22 | Pre-October 2005, 4 doses of 375
mg/m2/wk. Thereafter, 375 mg/m2 once with redosing based on circulating B cells |
None | Yes | 24 months | CR: RTX: 3/11 Reference: 2/11 PR: RTX: 5/11 Reference: 5/11 |
AE: RTX: 1 Control: 1 SAE: 0 |
| Ruggenenti et al26
Italy |
PCS | Describe the experience of using RTX in persistent NS | 100 | Pre-October 2005, 4 doses of 375
mg/m2/wk. Thereafter, 375 mg/m2 once with redosing based on circulating B cells |
None | Yes | Median: 29 months |
CR or PR: 65/100 (65%) Median time to remission was 7.1 months All 24 patients who had at least 4 years of follow-up achieved CR or PR |
AE: 28 SAE: 0 |
| Lionaki et al27
Greece |
PCS | Assess the long-term benefit of RTX & search for potential predictors of response | 12 | 4 doses of 375 mg/m2/wk (maximum 700 mg) |
None | Yes | Median: 48 months |
CR: 7/12 (58.3%) PR: 4/12 (33.3%) CR or PR: 11/12 (91.6%) |
AE: 0 SAE: 0 |
| Busch et al28
Germany |
PCS | Evaluate the effect of 4 monthly RTX doses on relapse rates | 14 | 375 mg/m2 (maximum 750 mg) monthly for 4 months (4-doses) | None | No | Median: 3 years |
After 1 year: CR: 3/14 (21.4%) PR: 7/14 (50.0%) 2 relapses |
AE: 3 SAE: 2 |
| Waldman et al29
USA |
PCS | Investigate induction treatment with RTX plus a 6-month course of CYC followed by a maintenance course of RTX vs either agent alone | 13 | 1000 mg on days 0, 14 repeated after 6 months | Cyclosporine tapered after 6 months over 9-21 weeks | Yes | 24 months | CR: 54% at 12 months CR or PR: 92% by 9 months 2 relapses |
AE: 45 SAE: 5 |
| Fiorentino et al30
Italy |
PCS | Describe the efficacy and safety of RTX | 38 | 4 doses of 375 mg/m2/wk | None | Yes | Median: 15 months |
CR: 15/38 (39.5%) PR: 14/38 (36.8%) CR or PR: 29/38 (76.3%) |
AE: 2 SAE: 1 |
| Moroni et al3
Italy |
PCS | Evaluate the efficacy and safety of low-dose RTX | 34 | 1-2 doses of RTX (375 mg/m2) | None | Yes | 12 months | CR: 5/34 (14.7%) PR: 10/34 (29.4%) No response: 19/34 (55.8%) |
AE: 5 SAE: 2 |
| Wang et al32
China |
PCS | Examine the efficacy and safety of RTX in non-responsive MN and monitor anti-PLA2R antibodies | 36 | 4 doses of 375 mg/m2/wk or B-cell-based frequency dosing: at discretion of the treating physicians | None | Yes | Median: 12 months |
CR: 2/36 (5%) PR: 13/36 (36%) CR or PR: 15/36 (41.7%) |
AE: 1 SAE: 0 |
| Boyer-Suavet et al33
France |
PCS | Monitor development of anti-RTX Ab in primary MN and assess whether resistance/relapse of MN after RTX is associated with development of anti-RTX Ab | 44 | 1000 mg on days 0, 14 Course was repeated in case of resistance or relapse at 6 months |
None | Yes | Median: 30 months |
CR or PR: 35/44 (79%) after a median of 3 months (range,
3-9) 9 patients were resistant to a first RTX course Remission did not differ by anti-RTX Ab status (8/10 [80%] vs 27/34 [79%] P > .99) but relapses were associated with anti-RTX Ab presence (5/10 [50%] vs 3/34 [9%] P = .009). |
AE: 2 SAE: 0 |
| Ramachandran et al34
India |
PCS | Report clinical outcomes using CYC or RTX in PMN with renal dysfunction | 64 | 4 doses of 375 mg/m2/wk, 1000 mg on days 0, 14, or CD19 targeted treatment | None | No | Median:24 months | 28/64 (44%) and 30/64 (47%) were in remission at 12 months and end of the study, respectively. | AE: 22 SAE: 8 |
RTX = rituximab; RCT = randomized controlled trial; wk = week; CR = complete remission; NIAT = non-immunosuppressive anti-proteinuric treatment; CI = confidence interval; AE = adverse events; SAE = severe adverse events; ACEi = angiotensin-converting-enzyme inhibitors; PR = partial remission; CYC = cyclophosphamide; GC = glucocorticoids; MN = membranous nephropathy; TAC = tacrolimus; PCS = prospective cohort study; NS = nephrotic syndrome; ARB = angiotensin II receptor blockers; RR = relative risk.
Table 4.
Summary of Included Studies of Lupus Nephritis (Listed in Chronological Order and According to Type of Study—All Are Induction Regimens).
| Study | Type | Intervention/aim | Number of participants | RTX regimen | Concomitant immunosuppression | B-cell depletion reported | Study duration | Primary findings | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Li et al35
Hong Kong |
RCT | Assess whether combination RTX & CYC is more effective than RTX monotherapy for induction therapy for proliferative LN | 19 | 1000 mg (single dose) | GC | Yes | 48 weeks | CR: 4/19 (21%) PR: 11/19 (58%) 2/19 (11%) remained the same or stable and 2/19 (11%) worsened No statistical differences in CR or PR between the 2 groups |
AE: RTX: 18 CYC/RTX: 27 SAE: 3 |
| Rovin et al36
US & Latin America |
RCT | Evaluate the efficacy and safety of RTX in patients with LN treated concomitantly with MMF & GC | 144 | 1000 mg on days 1, 15, 168, and 182 | MMF for 52 weeks and GC until week 16 | Yes | 54 weeks | The overall (CR and PR) renal response rates were 45.8% among the 72 patients receiving placebo and 56.9% among the 72 patients receiving RTX (P = .18); partial responses accounted for most of the difference. Primary end point (RTX superiority) not achieved. | AE: RTX: 72 placebo: 68 SAE: RTX: 29 placebo: 31 |
| Zhang et al37
China |
RCT | Compare the effects of RTX & CYC on serum levels of anti-C1q antibodies and ANCA in assessing the prognosis of severe and refractory LN | 84 | 4 doses of 375 mg/m2/wk | GC and 2 doses of 800 mg of IV CYC with oral GC at weeks 1 and 3 | Yes | 12 months | RTX+CYC group: CR: 27/42 (64.3%) PR: 8/42 (19.0%) CR or PR: 35/42 (83.3%) CYC group: CR: 9/42 (21.4%) PR: 15/42 (35.7%) CR or PR: 24/42 (57.1%) Anti-C1q antibodies and ANCA were decreased to 12 and 26% in the RTX-treated group, which was significantly lower than in CYC-treated patients (21 and 69 % respectively, P < .05). |
Not mentioned |
| Atisha-Fregoso et al38
USA |
RCT | Assess the safety, mechanism of action, and preliminary efficacy of RTX followed by belimumab in the treatment of refractory LN | 43 | 1000 mg Single dose |
RTX in both groups: Belimumab weekly, CYC, and GC CYC, and GC only |
Yes | 48 weeks | Belimumab group: CR or PR: 11/21 (52%) Control: CR or PR: 9/22 (41%) (P = .452) |
AE: RC: 287 RCB: 202 SAE: RC: 40 RCB: 7 |
| Sfikakis et al39
Greece |
PCS | Evaluate the efficacy of RTX in the treatment of LN | 10 | 4 doses of 375 mg/m2/wk | GC – CYC was given to 1 patient with relapse | Yes | 12 months | CR: 5/10 (median 3 months) PR: 3/10 (median 2 months) Sustained remission: 4/5 at 12 months |
AE: 5 SAE: 1 |
| Vigna-Perez et al40
Mexico |
PCS | Evaluate the clinical and immunological effects of RTX in refractory LN | 22 | 500 mg – 1000 mg on days 0, 14 | Immunosuppression was allowed: GC, CYC, AZA, MMF, MTX | Yes | 90 days | CR: 5/22 (22%) PR: 7/22 (31%) CR or PR: 12/22 (53%) |
AE: 2 SAE: 2 |
| Pepper et al41
UK |
PCS | Assess the use of RTX added to MMF in LN | 18 | 1000 mg on days 0, 14 | MMF and GC | Yes | 1 year | CR or PR: 14/18 (78%) Sustained response at 1 year: 12/18 (67%) Relapse: 2/18 with proteinuria |
AE: 6 SAE: 4 |
| Boletis et al42
Greece |
PCS | Assess the use of RTX added to MMF in relapsing proliferative LN | 10 | 4 doses of 375 mg/m2/wk | MMF and GC | Yes | Mean: 38 months |
CR: 6/10 (sustained by 38 months) PR: 8/10 (median of 3.5 months) |
AE: 0 SAE: 0 |
| Pinto et al43
2011 Columbia |
PCS | Report the results of severe and refractory SLE treated with RTX in Colombian patients | 42 (LN: 32) | 1000 mg on days 0, 14 | Immunosuppression was allowed: GC, CYC, AZA, MMF, HCQ | No | 12 months (min. 3 months) | After 3 months (LN results) CR: 28% PR: 36% persisted at 12 months |
AE: 28 SAE: 5 |
| Davies et al44
UK |
PCS | Report the clinical outcome of RTX in refractory LN | 18 | 1000 mg on days 0, 14 | IV CYC and 500 mg IV methylprednisolone, 2 weeks apart. Along with HCQ and oral steroids. | Yes | 6 months | CR: 11/18 (61%) PR: 2/18 (11%) CR or PR: 13/18 (72%) |
AE: 4 SAE: 3 |
| Condon et al45
UK |
PCS | Evaluate the effectiveness of treating LN with RTX and MMF but no oral GC | 50 | 1000 mg on days 0, 14 | Methylprednisolone 500 mg with RTX
infusion MMF |
Yes | 12 months | CR: 26/50 (52%) PR: 17/50 (34%) 12 relapses occurred in 11 patients, at a median time of 65 weeks (20-112) from remission |
AE: 11 SAE: 11 |
| Moroni et al46
Italy |
PCS | Compare RTX and either MMF or CYC pulses in the treatment of active LN | 54 | 1000 mg on days 3, 18 | HCQ and GC AZA, MMF, or cyclosporine from the beginning of the fourth month as maintenance therapy |
No | 12 months | CR: RTX group: 71% MMF group: 53% CYC group: 65% PR: RTX group: 29% MMF group: 41% CYC group: 25% |
AE: RTX: 6 MMF: 6 CYC: 11 SAE: RTX: 1 MMF: 2 CYC: 3 |
| Tanaka et al47
Japan |
PCS | Evaluate the efficacy and safety of RTX in Japanese patients with refractory SLE & LN | 34 (LN: 17) | 1000 mg on days 1, 15, 169, and 183 | Continued to receive pre-study enrolment immunosuppressants at the same dose (cyclosporine/MMF/AZA) | Yes | 53 weeks | Decrease in disease activity was achieved in 16/34 (76.5%). In 17 patients with LN, response rates of 58.8% and 52.9% by ACR and LUNAR criteria, respectively. Successful GC tapering was achieved in association with disease remission. | AE: 154 SAE: 10 |
| Kotagiri et al48
Australia |
PCS | Investigate the response to single-dose RTX, added to standard GC plus additional immunosuppressive agent, in refractory LN | 14 | 375 mg/m2
Single dose |
CYC, MMF, AZA, and GC were all allowed and tapered at the physician’s discretion according to clinical response | Yes | Median 18 months (IQR: 9-24) | CR: 2/14 (14%) PR: 9/14 (64%) CR or PR: 11/14 (79%) Median time to response 5 months 6-month probability of renal response of 43% Five patients (45%) relapsed at a median time of 17 months |
AE: 3 SAE: 3 |
| Kraaij et al49
Netherland |
PCS | Investigate the immunological effects and feasibility of combining RTX and belimumab | 15 (LN: 12) | 1000 mg on days 0, 14 | Induction and maintenance: Belimumab and steroids (MMF was started but quickly tapered to avoid cumulative over-immunosuppression) | Yes | 24 months | 10/15 (67%) achieved a lupus low disease activity state,
of which 8 (53%) continued treatment (BLM + ≤7.5 mg
prednisolone) for the complete 2 years of
follow-up Of 12 patients with LN: Renal response: 9 (75%) CR: 8 (67%) |
AE: 15 SAE: 4 |
| Roccatello et al50
Italy |
PCS | Investigate the safety and efficacy of an intensified B-cell depletion induction therapy without immunosuppressive maintenance regimen compared with standard of care in LN | 60 | 4 doses of 375 mg/m2/wk and 2 more doses 1 and 2 months from last dose | CYC: 2 infusions of 10 mg/kg at days 4 and
17 Pulse GC followed by oral regimen tapered to 5 mg/d by 3 months |
Yes | 12 months Mean post Rx, months: IBCDT 44.5 MMF 48.6 CYC 45.3 |
CR at 12 months: IBCDT group: 93% MMF group: 62.7% CYC group: 75% (P = .03) The dose of oral GC was lower in the IBCDT group (mean ± SD, 2.9 ± 5.0 mg/dl) than MMF (10.5 ± 8.0 mg/d, P < .01) or CYC group (7.5 ± 9.0 mg/d, P < .01) At their last follow-up visit, there was no significant differences in proteinuria and serum creatinine, nor in new flares frequency |
AE: IBCDT: 3 MMF: 5 CYC: 4 SAE: 0 |
Note. RTX = rituximab; RCT = randomized controlled trial; CYC = cyclophosphamide; wk = week; LN = lupus nephritis; GC = glucocorticoids; CR = complete remission; PR = partial remission; AE = adverse events; SAE = severe adverse events; MMF = mycophenolate mofetil; ANCA = antineutrophil cytoplasm antibody; PCS = prospective cohort study; AZA = azathioprine; SLE = systemic lupus erythematosus; HCQ = hydroxychloroquine; IQR = interquartile range; IBCDT = intensified B-cell depletion induction therapy; SD = steroid dependent; RC = rituximab and cyclophosphamide; RCB = rituximab, cyclophosphamide, and belimumab; ACR = American college of rheumatology; LUNAR = lupus nephritis assessment with rituximab study; MTX = methotrexate; BLM = belimumab.
Table 5.
Summary of Included Studies of Podocytopathies (Listed in Chronological Order and Type of Study—All Are Induction Regimens).
| Study | Type | Intervention/aim | Number of participants | RTX regimen | Concomitant immunosuppression | B-cell depletion reported | Study duration | Primary findings | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Minimal change disease | |||||||||
| Takei et al51
Japan |
PCS | Assess the therapeutic effects of RTX in adult patients with GC dependent MCD | 25 | 375 mg/m2 (maximum, 500 mg) ×2, 6 months apart | Tapering cyclosporine | Yes | 12 months | A significant reduction in the number of relapses and
the total/maintenance dose of GC when compared with the
findings during the prior 12-month period (25 [100%] vs
4 [16%], P < .001; 8.2 vs 3.3 g,
P < .001; 26.4 mg/day at
baseline vs 1.1 mg/day at 12-month, P
< .0001) CR achieved/maintained in 17/25 and 4/17 developed relapse |
AE: 5 SAE: 0 |
| Papakrivopoulou et al52
UK |
PCS | Evaluate the efficacy and safety of RTX in maintaining remission, reducing relapse frequency and enabling withdrawal of immunosuppression, in frequently relapsing and steroid-dependent MCD | 15 | 1000 mg ×2, 6 months apart | GC tapered by 3 month and CNI tapered after 12 months by 25% every 6 months | Yes | 36 months | Median GC-free survival after RTX was 25 months (range,
4-34) Mean relapse frequency decreased from 2.60 ± 0.28 to 0.4 ± 0.19 (P < .001) after RTX Seven relapses occurred, 5 of which (71%) when CD19 counts were greater than 100 per μl. |
AE: 9 SAE: 0 |
| Minimal change disease and focal segmental glomerulosclerosis | |||||||||
| Ruggenenti et al53
Italy |
PCS | Evaluate the efficacy of RTX in reducing relapse and steroid exposure in children and adults with steroid-dependent or frequently relapsing NS due to MCD, MesGN, or FSGS | 30 (adults: 20) | 1-2 doses of rituximab (375 mg/m2/wk) | GC, CNI, MMF, CYC | Yes | 1 year | At 1 year, all patients were in remission 18/30 (60%) were treatment-free 15/30 (50%) never relapsed Compared with the year pre-rituximab, total relapses decreased from 88 to 22 and per-patient median number of relapses decreased from 2.5 (IQR, 2–4) to 0.5 (IQR, 0–1; P = .001) per year |
AE: 8 SAE: 8 |
| Ren et al54
China |
PCS | Investigate the therapeutic effects of RTX in patients with refractory MCD or FSGS | 15 | 4 doses of rituximab (375 mg/m2/wk) | GC and other immunosuppressive medications allowed. All were tapered during the study | Yes | Median: 8 months (range, 3-36 months) | At 3 months: CR: 13/15 (87%) PR: 2/15 (13%) Relapses approximately 30-fold less compared with the year pre-RTX |
AE: 0 SAE: 0 |
| Ramachandran et al55
India |
PCS | Describe the clinical outcome of adults with SD/SR NS treated with RTX | 53 | 375 mg/m2 followed by 100 mg based on CD19 level after 2-3 days | GC and CNI | Yes | Median: 36 months (IQR 19-48) | CR: 44/53 (83%) PR: 6/53 (11%) 33/53 (62%) did not require steroid or CNI during the follow-up period |
AE: 27 SAE: 0 |
| Post-kidney transplant focal segmental glomerulosclerosis | |||||||||
| Alasfar et al56
USA |
PCS | Evaluate risk factors for posttransplant FSGS recurrence, describes its course, and determine the efficacy of RTX and TPE in its prevention and treatment | 66 | 1 or 2 doses (375 mg/m2 per dose) | Perioperative TPE sessions were started anytime between day 7 before transplant to postoperative day 2 (3–10 sessions) | No | Median 29.5 months | 23 /37 (62%) who received preventative therapy developed
recurrence compared with 14/27 (51%) who did not receive
any therapy (P = .21) There was a trend for less relapse when rituximab was used as a therapy for recurrent FSGS, (6/22 vs 9/18, P = .066) |
Not mentioned |
Note. RTX = rituximab; PCS = prospective cohort study; GC = glucocorticoids; wk = week; MCD = minimal change disease; CR = complete remission; AE = adverse events; SAE = severe adverse events; CNI = calcineurin inhibitor; NS = nephrotic syndrome; FSGS = focal segmental glomerulosclerosis; MMF = mycophenolate mofetil; CYC = cyclophosphamide; IQR = interquartile range; PR = partial remission; SD = steroid dependent; SR = steroid resistant; TPE = plasma exchange.
Table 6.
Summary of Included Studies of Multiple Disease (Listed in Chronological Order and Type of Study—All Are Induction Regimens).
| Study | Type | Intervention/aim | Number of participants | RTX regimen | Concomitant immunosuppression | B-cell depletion reported | Study duration | Primary findings | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| El-Reshaid et al57
Kuwait Idiopathic nephrotic syndrome |
PCS | Assess the role of RTX in treatment of refractory idiopathic NS | 78 ≤14 y: 11 Adults: 67 MCD: 32 FSGS: 18 MN: 28 |
4 doses of 500 mg/wk | None | Yes | 12 months | MCD (32): CR 29/32, PR 2/32 FSGS (18): CR 0/18, PR 17/18 MN (38): CR 12/38, PR 14/38 |
AE: 8 SAE: 0 |
| Xu et al58
China Refractory nephrotic syndrome |
PCS | Explore the efficacy and safety of RTX in refractory NS | 60 MN: 13 MCD: 6 FSGS: 1 SLE: 24 AAV: 7 |
4 doses of RTX (375 mg/m2/wk) followed by 375 mg/m2 based on CD19+B lymphocyte count during follow-up | None | Yes | 16 ± 10 months (at least 6 months) | RTX was effective in 27/54 (50%) with complete
follow-up CR: 7/54 (13%) PR: 20/54 (37%) In the PN, SN-1, SN-2 groupsa: CR: 25%, 8.3%, & 4.5% PR: 40%, 58.3%, & 22.7% CR or PR:65%, 66.7%, & 27.3% (P = .022) |
AE: 47 SAE: 24 |
Note. RTX = rituximab; PCS = prospective cohort study; wk = week; NS = nephrotic syndrome; MCD = minimal change disease; FSGS = focal segmental glomerulosclerosis; MN = membranous nephropathy; CR = complete remission; PR = partial remission; AE = adverse events; SAE = severe adverse events; SLE = systemic lupus erythematosus; AAV = ANCA associated vasculitis; PN = primary nephropathy; SN = secondary nephropathy; GFR = glomerular filtration rate.
The SN group was then divided into 2 subgroups based on the estimated glomerular filtration rate (eGFR) level before RTX treatment, namely SN-1 group (eGFR ≥ 30 ml/min), and SN-2 group (eGFR < 30 ml/min).
Rituximab Dosing for Glomerular Diseases:
Antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV)
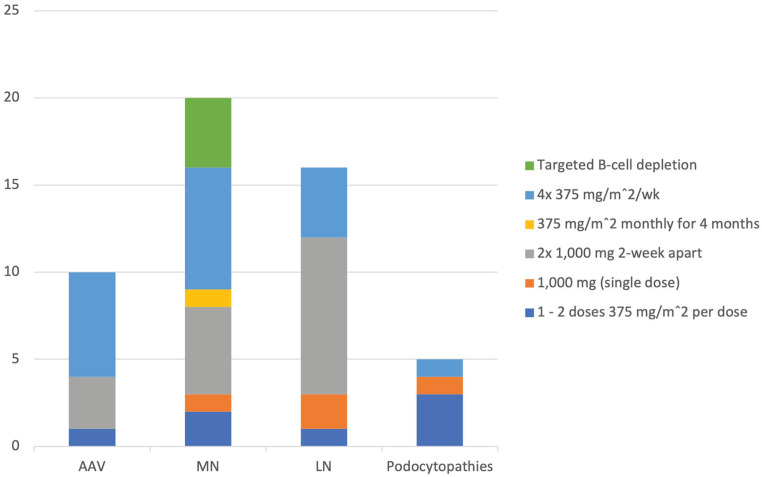
We identified 12 studies (6 RCTs and 6 PCSs) evaluating the use of rituximab in AAV (Table 2). Studied regimens for induction therapy included 4 doses of 375 mg/m2/week (3 RCTs and 2 PCSs);6-8,13,14 1000 mg as 2 doses 2 weeks apart (2 PCSs)9,12; and one dose of 375 mg/m2 (1 PCS)10 (Figure 2). One PCS allowed either of 2 dosing regimens based on physician preference: 1000 mg as 2 doses 2 weeks apart (101 patients) or 4 doses of 375 mg/m2/week (15 patients).11
Figure 2.
Most frequently studied rituximab induction regimens, by the number of studies. (Some studies included more than one regimen)
Note. AAV = ANCA associated vasculitis; MN = membranous nephropathy; LN = lupus nephritis; Podocytopathies = focal segmental glomerulosclerosis and minimal change disease.
The rituximab dosing regimen most frequently studied for maintenance therapy in AAV was 500 mg every 6 months for a total of 4 doses (2 RCTs).15,16 One RCT evaluated a tailored approach: the control group received 500 mg on days 0 and 14 and at months 6, 12, and 18, while the experimental group received identical treatment at baseline and thereafter based on trends in ANCA and CD19+ B lymphocyte counts measured every 3 months.17
No head-to-head studies comparing the efficacy and/or safety of different rituximab dosing regimens for induction of remission in AAV were identified. One study17 compared individually tailored to fixed-schedule rituximab for maintenance of remission in AAV and found no significant difference in relapse rate (14/81 [17.3%] vs 8/81 [9.9%], P = 0.22).
Key findings from each of the individual studies we identified are summarized in Table 2 but—due to differences in study populations, concomitant treatments, outcome definitions, and follow-up durations—these data are insufficient to allow definitive conclusions regarding an optimal dosing regimen to be drawn.
Membranous nephropathy
We identified 17 studies (4 RCTs and 13 PCSs) evaluating the use of rituximab in primary MN (Table 3). Studied regimens for induction therapy in MN were highly variable (Figure 2): 1 or 2 (RCT),18 or 2 (PCS),31 weekly doses of 375 mg/m2/week; 4 weekly doses of 375 mg/m2/week (2 PCS)27,30 that could be repeated after 6 months (1 PCS)24; 4 monthly doses of 375 mg/m2/month (1 PCS)28; 4 weekly doses of 375 mg/m2/week or based on B-cell count (2 PCS);22,32 or 1000 mg as 2 doses 2 weeks apart (1 RCT and 1 PCS)20,33 that could be followed after 6 months by 1 or 2 further doses of 1000 mg 2 weeks apart (1 RCT and 2 PCSs).19,23,29 Two PCSs initially used 4 doses of 375 mg/m2/week but later changed to a B-cell based approach to guide redosing.25,26 Another PCS included patients receiving 3 different regimens: 4 doses of 375 mg/m2/week; 1000 mg as 2 doses 2 weeks apart; and a CD19 targeted treatment approach.34 The remaining RCT evaluated a single dose of 1000 mg after 6 months of tacrolimus.21 We did not identify any RCT or PCS examining use of rituximab to maintain remission in MN.
A single PCS22 evaluated whether titrating rituximab to circulating CD20 B-cell counts improves safety and reduces costs. Twelve patients with incident primary MN who received a single 375 mg/m2 followed by monthly re-evaluation of B-cell counts were compared with 24 historical reference patients who received 4 weekly doses of 375 mg/m2 and were followed for 12 months with comparable response rates (CR n = 2 [17%] vs 2 [8%] and PR n = 6 [50%] vs 14 [58%]).
Key findings from each of the individual studies we identified are summarized in Table 3 but—due to differences in study populations, concomitant treatments, outcome definitions, and follow-up durations—these data are insufficient to allow definitive conclusions regarding an optimal dosing regimen to be drawn.
Lupus nephritis
We identified 16 studies (4 RCTs and 12 PCSs) evaluating the use of rituximab in Lupus Nephritis (LN) (Table 4). Studied regimens for induction therapy in LN were variable (Figure 2): 1 dose of 375 mg/m2 (1 PCS)48; 4 doses of 375 mg/m2/week (1 RCT and 2 PCSs),37,39,42 that could be followed by 2 more monthly doses of 375 mg/m2 (1 PCS)50; a single dose of 1000 mg (2 RCT)35,38; 500 to 1000 mg as 2 doses 2 weeks apart (1 PCS)40; and 1000 mg as 2 doses 2 weeks apart (6 PCS)41,43-46,49 that could be followed after 6 months by 2 additional doses of 1000 mg 2 weeks apart (1 RCT and 1 PCS).36,47 We did not identify any RCT or PCS examining use of rituximab to maintain remission in LN.
No head-to-head studies comparing the efficacy and/or safety of different rituximab dosing regimens for the treatment of LN were identified.
Key findings from each of the individual studies we identified are summarized in Table 4 but—due to differences in study populations, concomitant treatments, outcome definitions, and follow-up durations—these data are insufficient to allow definitive conclusions regarding an optimal dosing regimen to be drawn.
Minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS)
We identified 6 studies (6 PCSs) evaluating the use of rituximab in MCD and/or FSGS (Table 5). In adults with MCD, we identified 2 PCSs that evaluated rituximab as an induction and/or maintenance regimen: 2 doses of 375 mg/m2 (maximum, 500 mg) 6 months apart,51 and 2 doses of 1000 mg 6 months apart.52 We identified 3 PCSs that included patients with either MCD or FSGS that examined 3 different rituximab regimens for induction therapy: 4 doses of 375 mg/m2/week54; 375 mg/m2 followed by 100 mg after 2-3 days based on CD19 level;55 and 1 or 2 doses of rituximab 375 mg/m2/wk.53 Finally, one PCS evaluating the efficacy of rituximab in reducing the risk of post-kidney transplant FSGS56 used 2 doses of 375 mg/m2 with or without plasma-exchange.
No head-to-head studies comparing the efficacy and/or safety of different rituximab dosing regimens for the treatment of MCD/FSGS were identified.
Key findings from each of the individual studies we identified are summarized in Table 5 but—due to differences in study populations, concomitant treatments, outcome definitions, and follow-up durations—these data are insufficient to allow definitive conclusions regarding an optimal dosing regimen to be drawn.
Miscellaneous
We identified 2 PCSs evaluating rituximab in the treatment of multiple glomerular diseases (Table 6). The first included patients with MCD, FSGS, or MN who received rituximab 500 mg weekly for 4 doses.57 The second included patients with refractory nephrotic syndrome due to MN, LN, MCD, FSGS, or AAV and used the following regimen: 4 doses of 375 mg/m2/week followed by 375 mg/m2 based on CD19+ B lymphocyte count during follow-up.58
Measurement of B-Cell Depletion as a Rituximab Treatment Target
Of the 53 studies we evaluated, 46 measured and/or reported data on B-cell depletion, of which 31 provided a definition for B-cell depletion. The most frequent definition was a B-cell count of less than 5 per mm3 (18 studies), followed by a count less than 10 per mm3 (5 studies). Other studies reported “complete B-cell depletion” or a B-cell concentration below the normal lab reference range: see online supplement for further details.
Adverse Event (AE) Reporting
AEs were described in 50 of the 53 studies, variably reported as total number of overall and/or serious AEs, total number of patients affected by an AE, and/or descriptions of serious AEs. In total, 2717 AEs among the 2803 patients included in these 50 studies were identified, with at least 561 defined as serious AEs. Most of the serious AEs were reported in studies using rituximab for treatment of systemic diseases (ie, 352 in AAV and 123 in LN), for which rituximab was always prescribed along with concomitant corticosteroids and/or other immunosuppressive therapies (12 of 12 studies in AAV and 16 of 16 studies in LN). Fewer serious AEs were reported in renal-limited diseases (ie, 54 in MN and 8 in MCD/FSGS), for which rituximab was more commonly prescribed as monotherapy (15 of 17 studies in MN) or along with tapering immunosuppression doses (4 of 6 studies in MCD/FSGS). Details of AEs for each of the individual studies are summarized in Tables 2 to 6.
Discussion
Rituximab Dosing for Glomerular Diseases
This scoping review reveals a lack of consensus with respect to dosing of rituximab for the treatment of glomerular disease and insufficient data to compare the efficacy and/or safety of individual dosing regimens. Across the 53 studies we reviewed, we identified 16 different rituximab dosing regimens studied as induction therapy for one or more of 5 glomerular diseases (AAV, MN, LN, MCD, FSGS) for which rituximab is included in a treatment algorithm in the KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases.3
In the first 4 months of treatment (Figure 2), the most frequently studied induction regimens were
AAV: 375 mg/m2 weekly for 4 doses (6 of 12 studies);
LN: 2 doses of 1000 mg 2 weeks apart (9 of 16 studies);
MN: either of the above 2 regimens (8 and 6 of 17 studies, respectively); and
Podocytopathies: a lower dose regimen, eg, 1 to 2 doses of 375 mg/m2 (3 of 6 studies).
Studies examining rituximab to maintain remission were fewer and no predominant dosing approaches were identified. In some cases, additional dosing was tailored to measures of B-cell depletion/reconstitution or, less frequently, to markers of disease activity,59 eg, degree of proteinuria, and/or evidence of immunological disease activity (anti-PLA2R or ANCA).
Head-to-head studies comparing the efficacy and/or safety of different rituximab dosing regimens for the treatment of glomerular disease were generally lacking and differences in study populations, concomitant treatments, outcome definitions, and follow-up durations in the individual studies we examined precluded our ability to draw definitive conclusions regarding optimal dosing regimens for each of the glomerular disease subtypes we examined.
The Importance of Achieving B-Cell Depletion
Rituximab results in prolonged B-cell depletion. This is a dose-dependent effect, and response to induction treatment appears dependent on achieving this target.60,61 Of the 46 studies that measured and/or reported data on B-cell depletion, only 31 provided a definition for B-cell depletion, which varied across studies. This non-standardized approach to measuring, reporting, and defining B-cell depletion adds to the challenge of evaluating and comparing the efficacy and safety of different dosing regimens. While not a head-to-head comparison, Seitz-Polski et al60 compared the results of 2 PCSs in MN using different rituximab regimens: participants with primary MN from the Department of Nephrology at Pasteur Hospital in Nice, the (NICE) cohort, received 2 doses of 1000 mg 2 weeks apart, while the GEMRITUX cohort received 2 doses of 375 mg/m2 at a 1-week interval. There were significant differences in treatment outcomes, favoring the higher dose NICE cohort, in terms of achievement of remission at 6 months, median time to achieve remission, and nadir CD19 counts. However, in the absence of a large representative head-to-head clinical trial, it is not possible to attribute these differences in outcome to differences in drug dosing or achievement of B-cell depletion (vs differences in study population, treatment setting, concomitant therapies etc.). A similar observation was made by Takei et al51 in a study of 17 patients with steroid-dependent MCD: complete remission was observed in all those who achieved B-cell depletion, with 4 relapses associated with B-cell repletion. In LN, Gomez Mendez et al61 described substantial variability in achievement of B-cell depletion in the LUNAR study: those achieving this target were more likely to obtain a complete response. A more recent PCS in LN by Roccatello et al50 used an intensive B-cell depletion approach with six 375 mg/m2 doses of rituximab and 2 doses of IV cyclophosphamide without maintenance therapy: complete remission was achieved in 93% of patients. Individualizing rituximab dosing to achieve B-cell depletion is likely an important therapeutic target.
Adverse Events Associated With Rituximab Prescribing in Glomerular Disease
An important consideration when prescribing rituximab is not only achievement and maintenance of remission but also the avoidance of treatment-related toxicity. In this review, we identified 2717 AEs, including 561 serious AEs, in the 50 studies that reported adverse events. The risk for AEs appeared to be greater when rituximab was prescribed for the treatment of systemic (ie, AAV or LN) vs renal-limited (ie, MN or MCD/FSGS) glomerular disease. This could reflect the systemic disease burden and/or greater exposure to additional immunosuppression in systemic diseases rather than a direct effect of rituximab dosing. Most of the studies reported a comparable safety profile with rituximab vs comparator immunosuppressive therapies for the outcomes of death, severe infection, or cancer. However, there appears to be a heightened risk for hypogammaglobulinemia, which can develop many years following treatment and beyond the observation window of many clinical trials.62 Severe or prolonged hypogammaglobulinemia can cause complicated infections that require treatment withdrawal and/or immunoglobulin replacement therapy.63,64
A Role for Rituximab to Achieve Steroid Minimization or Avoidance
Glucocorticoids are a cornerstone in the management of glomerular diseases but are often associated with unacceptable toxicity. This has motivated the development of novel steroid-sparing regimens to minimize glucocorticoid associated morbidity and mortality. Rituximab has been used successfully as part of steroid-sparing regimens in glomerular disease.
In AAV, Furuta et al8 conducted an RCT in Japanese patients with AAV comparing the efficacy of a reduced-dose glucocorticoid (0.5 mg/kg/day) vs a standard dose (1 mg/kg/day) in a 6-month study while using rituximab as an induction agent in both arms. This study showed comparable remission rates (71% vs 69%; P = 0.003 for noninferiority). Another 12-month PCS by Pepper et al,12 also in patients with AAV, was conducted in the United Kingdom and Ireland. The investigators evaluated a novel induction regimen of rituximab and low-dose cyclophosphamide for 3 months and an early rapid steroid withdrawal over 2 weeks. Most patients (90%) achieved sustained remission without the need for additional steroids.
In MN, the modified Ponticelli protocol had become standard of care for the treatment of MN. This regimen includes a cyclical monthly regimen of cyclophosphamide alternating with high-dose steroids for a total of 6 months.65 The RI-CYCLO20 RCT showed that rituximab had similar efficacy to the modified Ponticelli protocol in the treatment of MN and could substantially reduce glucocorticoid exposure in these patients.
In LN, steroid avoidance was best examined in a PCS (n = 50) by Condon et al,45 in which 90% of participants who received rituximab along with mycophenolate as induction therapy achieved complete or partial remission. This cohort of patients received only 2 doses of methylprednisolone 500 mg 2 weeks apart with no oral steroids. However, a planned RCT from the same group was terminated early due to insufficient recruitment.
Finally, in MCD and FSGS, rituximab is typically reserved for steroid-dependent and refractory cases. Most study participants who received rituximab could completely discontinue steroids and/or other immunosuppressive treatments, after achieving remission, with a markedly reduced subsequent relapse rate.51-55 There are several ongoing studies evaluating rituximab vs steroids in MCD/FSGS for which results are eagerly awaited.
Limitations
This scoping review was performed with a structured methodology: nevertheless, study selection and data abstraction likely involved some subjectivity on the part of the investigators. Restriction of the search to a single database (PubMed) and to studies published in the English language and with an accompanying abstract could have led to selection bias, eg, exclusion of research letters or studies from non-English speaking investigators. This work was initially intended to inform the feasibility and design of a systematic review and meta-analysis of rituximab dosing in glomerular diseases: however, heterogeneity with respect to dosing, study populations, and study outcomes precluded this. Accordingly, while we limited our review to prospective observational studies and RCTs to ensure higher-quality data, we did not formally assess the quality of individual studies.
Conclusion
Numerous rituximab dosing regimens have been studied for the treatment of glomerular diseases, either as monotherapy or as an adjunct treatment, with heterogeneous treatment responses. Individualizing treatment (dose, frequency) based on the extent of B-cell depletion and the level and trajectory of disease-specific biomarkers might be key to achieving and maintaining disease control. More studies are needed to establish the optimal rituximab dosing regimen, and factors impacting treatment response, for individual glomerular disease subtypes and diverse disease states (eg, active nephrotic syndrome, remission). In the absence of a head-to-head clinical trial comparing dosing regimens, restriction to a limited set of standardized dosing regimens along with standardized reporting of efficacy and safety outcomes (including B-cell depletion) will at least enable valid indirect inferences to be made.
Supplemental Material
Supplemental material, sj-xlsx-1-cjk-10.1177_20543581221129959 for Rituximab Dosing in Glomerular Diseases: A Scoping Review by Husam Alzayer, Kuruvilla K. Sebastian and Michelle M. O’Shaughnessy in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors thank Ms Shauna Barrett, Cork University Hospital librarian, for her help in developing the search methodology for this work.
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: All authors have provided consent for publication.
Availability of Data and Materials: All available data and materials are attached in the article’s supplement.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Husam Alzayer  https://orcid.org/0000-0003-2851-4604
https://orcid.org/0000-0003-2851-4604
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359-7368. [DOI] [PubMed] [Google Scholar]
- 2. Sanz I. Indications of rituximab in autoimmune diseases. Drug Discov Today Ther Strateg. 2009;6(1):13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1-S276. [DOI] [PubMed] [Google Scholar]
- 4. Chauhan K, Mehta AA. Rituximab in kidney disease and transplant. Anim Model Exp Med. 2019;2(2):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65(8):757-773. [DOI] [PubMed] [Google Scholar]
- 6. Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones RB, Tervaert JWC, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211-220. [DOI] [PubMed] [Google Scholar]
- 8. Furuta S, Nakagomi D, Kobayashi Y, et al. Effect of reduced-dose vs high-dose glucocorticoids added to rituximab on remission induction in ANCA-associated vasculitis: a randomized clinical trial. JAMA. 2021;325(21):2178-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mansfield N, Hamour S, Habib AM, et al. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal ANCA-associated vasculitis. Nephrol Dial Transplant. 2011;26(10):3280-3286. [DOI] [PubMed] [Google Scholar]
- 10. Turner-Stokes T, Sandhu E, Pepper RJ, et al. Induction treatment of ANCA-associated vasculitis with a single dose of rituximab. Rheumatology (Oxford). 2014;53(8):1395-1403. [DOI] [PubMed] [Google Scholar]
- 11. McGregor JG, Hogan SL, Kotzen ES, et al. Rituximab as an immunosuppressant in antineutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2015;30 suppl 1(suppl 1):i123-i131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pepper RJ, McAdoo SP, Moran SM, et al. A novel glucocorticoid-free maintenance regimen for anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford). 2019;58(2):260-268. [DOI] [PubMed] [Google Scholar]
- 13. Miyazaki Y, Nakayamada S, Kubo S, et al. Favorable efficacy of rituximab in ANCA-associated vasculitis patients with excessive B cell differentiation. Arthritis Res Ther. 2020;22(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith RM, Jones RB, Specks U, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis. 2020;79(9):1243-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771-1780. [DOI] [PubMed] [Google Scholar]
- 16. Charles P, Perrodeau É, Samson M, et al. Long-term rituximab use to maintain remission of antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2020;173(3):179-187. [DOI] [PubMed] [Google Scholar]
- 17. Charles P, Terrier B, Perrodeau É, et al. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial (MAINRITSAN2). Ann Rheum Dis. 2018;77(8):1143-1149. [DOI] [PubMed] [Google Scholar]
- 18. Dahan K, Debiec H, Plaisier E, et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J Am Soc Nephrol. 2017;28:348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36-46. [DOI] [PubMed] [Google Scholar]
- 20. Scolari F, Delbarba E, Santoro D, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32(4):972-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernández-Juárez G, Rojas-Rivera J, Logt AV, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99(4):986-998. [DOI] [PubMed] [Google Scholar]
- 22. Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2(5):932-937. [DOI] [PubMed] [Google Scholar]
- 23. Fervenza FC, Appel GB, Barbour SJ, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73(1):117-125. [DOI] [PubMed] [Google Scholar]
- 24. Fervenza FC, Abraham RS, Erickson SB, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5(12):2188-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cravedi P, Sghirlanzoni MC, Marasà M, Salerno A, Remuzzi G, Ruggenenti P. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol. 2011;33(5):461-468. [DOI] [PubMed] [Google Scholar]
- 26. Ruggenenti P, Cravedi P, Chianca A, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(8):1416-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lionaki S, Marinaki S, Nakopoulou L, et al. Depletion of B lymphocytes in idiopathic membranous glomerulopathy: results from patients with extended follow-up. Nephron Extra. 2013;3(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Busch M, Rüster C, Schinköthe C, Gerth J, Wolf G. Rituximab for the second- and third-line therapy of idiopathic membranous nephropathy: a prospective single center study using a new treatment strategy. Clin Nephrol. 2013;80(2):105-113. [DOI] [PubMed] [Google Scholar]
- 29. Waldman M, Beck LH, Jr, Braun M, Wilkins K, Balow JE, Austin HA, III. Membranous nephropathy: pilot study of a novel regimen combining cyclosporine and rituximab. Kidney Int Rep. 2016;1(2):73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fiorentino M, Tondolo F, Bruno F, et al. Treatment with rituximab in idiopathic membranous nephropathy. Clin Kidney J. 2016;9(6):788-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moroni G, Depetri F, Del Vecchio L, et al. Low-dose rituximab is poorly effective in patients with primary membranous nephropathy. Nephrol Dial Transplant. 2017;32(10):1691-1696. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Cui Z, Zhang YM, et al. Rituximab for non-responsive idiopathic membranous nephropathy in a Chinese cohort. Nephrol Dial Transplant. 2018;33(9):1558-1563. [DOI] [PubMed] [Google Scholar]
- 33. Boyer-Suavet S, Andreani M, Lateb M, et al. Neutralizing anti-rituximab antibodies and relapse in membranous nephropathy treated with rituximab. Front Immunol. 2019;10:3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ramachandran R, Prabakaran R, Priya G, et al. Immunosuppressive therapy in primary membranous nephropathy with compromised renal function. Nephron. 2022;146:138-145. [DOI] [PubMed] [Google Scholar]
- 35. Li EK, Tam LS, Zhu TY, et al. Is combination rituximab with cyclophosphamide better than rituximab alone in the treatment of lupus nephritis. Rheumatology (Oxford). 2009;48(8):892-898. [DOI] [PubMed] [Google Scholar]
- 36. Rovin BH, Furie R, Latinis K, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 2012;64(4):1215-1226. [DOI] [PubMed] [Google Scholar]
- 37. Zhang J, Zhao Z, Hu X. Effect of rituximab on serum levels of anti-C1q and antineutrophil cytoplasmic autoantibodies in refractory severe lupus nephritis. Cell Biochem Biophys. 2015;72(1):197-201. [DOI] [PubMed] [Google Scholar]
- 38. Atisha-Fregoso Y, Malkiel S, Harris KM, et al. Phase II randomized trial of rituximab plus cyclophosphamide followed by belimumab for the treatment of lupus nephritis. Arthritis Rheumatol. 2021;73(1):121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501-513. [DOI] [PubMed] [Google Scholar]
- 40. Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, et al. Clinical and immunological effects of rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8(3):R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pepper R, Griffith M, Kirwan C, et al. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant. 2009;24(12):3717-3723. [DOI] [PubMed] [Google Scholar]
- 42. Boletis JN, Marinaki S, Skalioti C, Lionaki SS, Iniotaki A, Sfikakis PP. Rituximab and mycophenolate mofetil for relapsing proliferative lupus nephritis: a long-term prospective study. Nephrol Dial Transplant. 2009;24(7):2157-2160. [DOI] [PubMed] [Google Scholar]
- 43. Pinto LF, Velásquez CJ, Prieto C, Mestra L, Forero E, Márquez JD. Rituximab induces a rapid and sustained remission in Colombian patients with severe and refractory systemic lupus erythematosus. Lupus. 2011;20(11):1219-1226. [DOI] [PubMed] [Google Scholar]
- 44. Davies RJ, Sangle SR, Jordan NP, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly progressive crescentic lupus nephritis. Lupus. 2013;22(6):574-582. [DOI] [PubMed] [Google Scholar]
- 45. Condon MB, Ashby D, Pepper RJ, et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis. 2013;72(8):1280-1286. [DOI] [PubMed] [Google Scholar]
- 46. Moroni G, Raffiotta F, Trezzi B, et al. Rituximab vs mycophenolate and vs cyclophosphamide pulses for induction therapy of active lupus nephritis: a clinical observational study. Rheumatology (Oxford). 2014;53(9):1570-1577. [DOI] [PubMed] [Google Scholar]
- 47. Tanaka Y, Takeuchi T, Miyasaka N, et al. Efficacy and safety of rituximab in Japanese patients with systemic lupus erythematosus and lupus nephritis who are refractory to conventional therapy. Mod Rheumatol. 2016;26(1):80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kotagiri P, Martin A, Hughes P, Becker G, Nicholls K. Single-dose rituximab in refractory lupus nephritis. Intern Med J. 2016;46(8):899-901. [DOI] [PubMed] [Google Scholar]
- 49. Kraaij T, Arends EJ, van Dam LS, et al. Long-term effects of combined B-cell immunomodulation with rituximab and belimumab in severe, refractory systemic lupus erythematosus: 2-year results. Nephrol Dial Transplant. 2021;36:1474-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roccatello D, Sciascia S, Naretto C, et al. A prospective study on long-term clinical outcomes of patients with lupus nephritis treated with an intensified B-cell depletion protocol without maintenance therapy. Kidney Int Rep. 2021;6(4):1081-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takei T, Itabashi M, Moriyama T, et al. Effect of single-dose rituximab on steroid-dependent minimal-change nephrotic syndrome in adults. Nephrol Dial Transplant. 2012;28(5):1225-1232. [DOI] [PubMed] [Google Scholar]
- 52. Papakrivopoulou E, Shendi AM, Salama AD, Khosravi M, Connolly JO, Trompeter R. Effective treatment with rituximab for the maintenance of remission in frequently relapsing minimal change disease. Nephrology (Carlton). 2016;21(10):893-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruggenenti P, Ruggiero B, Cravedi P, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25(4):850-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ren H, Lin L, Shen P, et al. Rituximab treatment in adults with refractory minimal change disease or focal segmental glomerulosclerosis. Oncotarget. 2017;8(55):93438-93443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramachandran R, Bharati J, Nada R, Minz R, Kohli HS. Rituximab in maintaining remission in adults with podocytopathy. Nephrology (Carlton). 2020;25(8):616-624. [DOI] [PubMed] [Google Scholar]
- 56. Alasfar S, Matar D, Montgomery RA, et al. Rituximab and therapeutic plasma exchange in recurrent focal segmental glomerulosclerosis postkidney transplantation. Transplantation. 2018;102(3):e115-e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. El-Reshaid K, Sallam HT, Hakim AA, Al-Attiyah R. Rituximab in treatment of idiopathic glomerulopathy. Saudi J Kidney Dis Transpl. 2012;23(5):973-978. [DOI] [PubMed] [Google Scholar]
- 58. Xu J, Ding Y, Wan L, Yang Q, Qu Z. A prospective cohort study of rituximab in the treatment of refractory nephrotic syndrome. Int Urol Nephrol. 2022;54:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ruggenenti P, Debiec H, Ruggiero B, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26(10):2545-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Seitz-Polski B, Dahan K, Debiec H, et al. High-dose rituximab and early remission in pla2r1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14(8):1173-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gomez Mendez LM, Cascino MD, Garg J, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. 2018;13(10):1502-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barmettler S, Ong MS, Farmer JR, et al. Association of Immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Network Open. 2018;1(7):e184169-e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tieu J, Smith RM, Gopaluni S, et al. Rituximab associated hypogammaglobulinemia in autoimmune disease. Fron Immunol. 2021;12:671503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jha V, Ganguli A, Saha TK, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18(6):1899-1904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-xlsx-1-cjk-10.1177_20543581221129959 for Rituximab Dosing in Glomerular Diseases: A Scoping Review by Husam Alzayer, Kuruvilla K. Sebastian and Michelle M. O’Shaughnessy in Canadian Journal of Kidney Health and Disease