Abstract
Purpose: The present retrospective study aimed to evaluate the efficacy and safety of camrelizumab addition to transarterial chemoembolization (TACE) in the treatment of hepatocellular carcinoma (HCC) with TACE-related untreatable progression (UP). Methods: Patients with HCC who received addition of camrelizumab due to UP after initial TACE treatment were enrolled at our institution between May 2019 and January 2021. Patients were assessed for tumor response, progression-free survival (PFS), and adverse events (AEs). Risk factors for PFS were evaluated with logistic regression analysis. Results: A total of 41 patients were included. The objective response rates (ORR) and disease control rates (DCR) were 24.4% and 61.0% at 2 to 3 months, and 12.2% and 58.5% at 6 months, respectively. The median PFS of the patients were 6 months (95% confidence interval [CI]: 3.8 months, 8.2 months). Of the 41 patients, 23 received camrelizumab combined with TACE (hereafter, camrelizumab–TACE) on whom 52 combined TACE procedures were performed, with a median of 2 procedures (range: 1-6) per patient. The remaining 18 patients received camrelizumab alone due to TACE contraindications. Multivariable analysis indicated that camrelizumab–TACE was an independent prognostic factor for PFS. Subgroup analysis showed a median PFS of 8 months in the camrelizumab–TACE group and 3 months in the camrelizumab monotherapy group (P < .001). No treatment-related mortalities occurred. Seventeen patients (41.5%) developed at least 1 type of AE after treatment with camrelizumab, with reactive cutaneous capillary endothelial proliferation (RCCEP) (n = 14, 34.1%) being the most common AE. Conclusion: Addition of camrelizumab to TACE offered an effective and safe treatment for HCC with UP.
Keywords: camrelizumab, transarterial chemoembolization, untreatable progression, progression-free survival, adverse events
Introduction
Globally, and in particular China, the prognosis of patients with hepatocellular carcinoma (HCC) remains depressed. It is currently one of the most frequent tumors in the world and the second commonest cause of cancer-related death.1 Due to insufficient liver reserve and donor shortage, some curative methods, such as hepatectomy and liver transplantation, are only suitable for a small number of patients.2 As a result, only <30% of HCC patients can benefit from curative therapies.3 Currently, transarterial chemoembolization (TACE) as a palliative therapy has been recognized as the most commonly used treatment for unresectable HCC.4 However, some tumor cells may still survive after a session of TACE, and low rates of complete response (CR) after TACE have been reported, ranging from 23% to 27%.5, 6 Hence, repeated TACE procedure is the most commonly used method for local or intrahepatic residual HCC.7
However, the efficacy of TACE decreases significantly as the number of TACE procedures increases, with rates of progressive disease (PD) after the first, second, third, and fourth TACE procedures reported to be 18%, 21%, 25%, and 27%, respectively.8 Based on this phenomenon, the Japan Society of Hepatology (JSH) first proposed the concept of TACE failure/refractoriness in 2010,9 and updated it in 2014.10 It should be noted, however, that the JSH standard appears to be problematic because new intrahepatic tumors are far from the target territory, and while new intrahepatic tumors represent PD, additional TACE therapy is not contraindicated. Thus, a new concept, termed “untreatable progression (UP)” was proposed to determine TACE discontinuation,1,11,12 which includes both major progression (such as extensive hepatic involvement, extrahepatic metastasis, or vascular invasion) and minor intrahepatic progression associated with impaired liver function and performance status. Currently, for those patients who have UP after TACE, treatment is challenging and there is still no consensus, which may create an incentive to try other therapeutic approaches.
Immune checkpoint inhibitors (ICIs) that reverse immune exhaustion have been shown to be effective in HCC, when used alone and in combination with TACE in the treatment of intermediate and advanced HCC.13–15 Recently, camrelizumab, an anti-programmed cell death protein 1 monoclonal antibody, was approved in China as a second-line treatment for unresectable HCC. Camrelizumab has been shown to be comparable to the efficacy of nivolumab and pembrolizumab in the treatment of advanced HCC.16 Accordingly, we hypothesized that camrelizumab may be effective in treating UP HCC after TACE, but no studies have been reported so far. Meanwhile, tumors with a low mutation burden and fewer neoantigens are generally less immunogenic, so they have little response to immunotherapy. TACE has been reported to promote tumor-specific CD8+ T cell response by killing HCC cells and causing the release of tumor-associated antigens.17 Thus, the application of camrelizumab after TACE treatment may attain promising outcomes in UP HCC. However, to our knowledge, no studies have been reported on the treatment of UP HCC with camrelizumab, therefore, this study reports the safety and efficacy of camrelizumab in the treatment of UP HCC patients.
Material and Methods
Patients
A retrospective analysis was conducted of all consecutive HCC patients who received TACE plus camrelizumab at our institution between May 2019 and January 2021. The criteria for patient selection included (1) patients aged > 18 years with HCC confirmed by pathological or clinical diagnosis according to European Association for the Study of the Liver criteria1; (2) patients who received additional camrelizumab due to UP after initial TACE treatment1,11,12; (3) Child–Pugh class A or B without ascites; and (4) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Exclusion criteria were (1) concurrent ablation, resection, radiotherapy, or systemic therapies for HCC; (2) discontinuation of camrelizumab due to serious adverse events (AEs); (3) patients with main portal vein obstruction; and (4) loss to follow up.
All patient details in this study have been de-identified. The retrospective single-center study was conducted in accordance with the principles of the Declaration of Helsinki and all procedures performed in this study were approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (UHCT-IEC-SOP-016-03-01). The need for informed consent was waived by the local ethics committee and the institutional review board of the Huazhong University of Science and Technology because clinical data were analyzed retrospectively and anonymously. The reporting of this study conforms to STROBE guidelines.18
TACE Procedure
TACE was performed according to institutional standard protocol.19 In summary, after superselective catheterization of tumor-feeding arteries with coaxial microcatheter (Progreat, Terumo), a solution of multiple chemotherapeutic agents (epirubicin 40-60 mg, cisplatin, oxaliplatin, or lobaplatin 50-100 mg, and 5-Fu or floxuridine 1.0 g) or single chemotherapeutic agent (epirubicin 40-60 mg) with ethiodized oil was subsequently injected. This was followed by injection of gelatin sponge particles (350-560 or 560-710 μm, Alicon). Embolization was performed under fluoroscopic guidance until there was stasis of arterial flow. Hepatic artery angiography was then subsequently performed to confirm success of the embolization procedure.
UP after TACE was defined as progression associated with a clinical profile that prevents retreatment, which includes at least one of the following situations: Ib—targeted tumor failed to achieve objective response after at least 2 initial TACE treatments; II—local tumor progression or new intrahepatic tumor did not achieve objective response after another TACE session; III—presence of significant progression, including substantial liver involvement, vascular invasion, or extrahepatic metastasis; and then IV—presence of hepatic dysfunction (Child–Pugh C) or ECOG performance status > 2 that contraindicates TACE therapy.11
Camrelizumab Therapy
The administration of camrelizumab was initiated after UP in HCC patients. Camrelizumab was administered intravenously at a dose of 200 mg every 3 weeks. If patients developed serious AEs, the drug was interrupted or discontinued and symptomatic treatment such as glucocorticoids or immunosuppressant agents were administered, depending on the severity and the affected organs. For patients who required multiple TACE procedures, the camrelizumab was administered within 2 weeks after TACE.
Follow up and Evaluation
All patients were followed up until June 2021. Laboratory examinations, abdominal contrast-enhanced computed tomography (CT) or MRI) were performed every 6 to 8 weeks after initial camrelizumab treatment. Laboratory tests mainly included routine blood tests, cardiac, liver, and renal function tests. Follow-up CT or MRI at 2 to 3 and 6 months after initial camrelizumab treatment were compared with pretreatment imaging to determine objective response rate (ORR) and disease control rate (DCR) according to Modified Response Evaluation Criteria in Solid Tumors (mRECIST).20 ORR was defined as a CR or partial response (PR). DCR represented CR, PR, or stable disease (SD).
Progression-free survival (PFS), defined as the time interval from initial camrelizumab treatment to the date of progression for patients who displayed radiological evidence of disease progression or the date of death or last follow up, was the primary endpoint of this study. AEs were recorded and assessed by the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. In addition, postembolization syndrome, such as fever, pain, nausea, and vomiting, is not considered as an AE in itself, but rather as an expected outcome of embolization therapy.21
Statistical Analyses
All analyses were performed using SPSS software, Version 24.0 (IBM). Discrete variables were presented as numbers with percentages, and quantitative data were presented as mean ± standard deviation. PFS were calculated using Kaplan–Meier method. The 95% confidence interval (CI) was calculated for median PFS and hazard ratio (HR). Risk factors for PFS were evaluated with logistic regression analysis. All statistical tests were two-tailed and P < .05 indicated statistical significance.
Results
Patient Characteristics
A total of 89 HCC patients received additional camrelizumab due to UP after initial TACE treatment in our hospital between May 2019 and January 2021. Of those, 28 were excluded due to concurrent ablation, resection, radiotherapy, or systemic therapies, 11 were excluded due to camrelizumab discontinuation, and 9 patients were excluded because they were lost to follow up. Eventually, 41 patients were included in this study (Figure 1). Situations I, II, and III of UP occurred in 11, 13, and 17 patients, respectively. A total of 150 TACE sessions were performed before the addition of camrelizumab, with a median TACE procedure of 3 (range: 1-19) per patient. The overall patient characteristics are shown in Table 1. In addition, 15 patients (36.6%) died during the observation period of the study.
Figure 1.
Flowchart of the patients who were included in this study.
Abbreviations: HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; UP, untreatable progression.
Table 1.
Patient Demographics and Clinical Status at Camrelizumab Initiation (n = 41) and Univariate Analysis of Prognostic Factors for Progression-Free Survival (PFS).
| Characteristic | Patients with UP after TACE (No, %; mean ± SD) | HR (95% CI) | P value |
|---|---|---|---|
| Gender | |||
| Male | 32 (78.0) | 1 | |
| Female | 9 (22.0) | 0.945 (0.404, 2.213) | .897 |
| Age (years) | 54.2 ± 11.4 | 1.011 (0.979, 1.044) | .503 |
| ECOG | |||
| 1 | 23 (56.1) | 1 | |
| 0 | 18 (43.9) | 0.886 (0.442, 1.775) | .732 |
| BCLC stage | |||
| C | 30 (73.2) | 1 | |
| B | 11 (26.8) | 0.682 (0.310, 1.501) | .342 |
| Largest diameter of tumor (cm) | 8.0 ± 4.9 | 1.009 (0.941, 1.082) | 799 |
| Hepatitis | |||
| Hepatitis B | 35 (85.4) | 1 | |
| Other | 6 (14.6) | 1.240 (0.474, 3.243) | .661 |
| α-fetoprotein level (ng/mL) | |||
| >400 | 20 (48.8) | 1 | |
| ≤400 | 21 (51.2) | 0.780 (0.390, 1.560) | .483 |
| Child–Pugh score | |||
| B | 5 (12.2) | 1 | |
| A | 36 (87.8) | 1.213 (0.367, 4.004) | .751 |
| Combination therapy | |||
| Yes | 23 (56.1) | 1 | |
| No | 18 (43.9) | 3.899 (1.818, 8.364) | .000 |
| TACE sessions before UP | 3.7 ± 3.4 | 1.036 (0.934, 1.150) | .500 |
| Tumor number | |||
| ≥3 | 36 (87.8) | 1 | |
| <3 | 5 (12.2) | 1.361 (0.469, 3.949) | .570 |
| Ascites | |||
| Present | 4 (9.8) | 1 | |
| Absent | 37 (90.2) | 1.620 (0.384, 6.827) | .511 |
| Extrahepatic spread | |||
| Present | 13 (31.7) | 1 | |
| Absent | 28 (68.3) | 1.247 (0.579, 2.689) | .573 |
| Vascular invasion | |||
| Present | 18 (43.9) | 1 | |
| Absent | 23 (56.1) | 0.933 (0.469, 1.856) | .844 |
| Interval time (weeks)a | |||
| >2 | 16 (39.0) | 1 | |
| <2 | 25 (61.0) | 0.570 (0.284, 1.146) | .115 |
| Baseline laboratory test result | |||
| TB (µmol/L) | 15.9 ± 8.4 | 1.031 (0.990, 1.072) | .138 |
| Albumin (g/L) | 35.1 ± 4.0 | 0.957 (0.883, 1.038) | .292 |
| PT (s) | 14.0 ± 1.0 | 1.025 (0.739, 1.421) | .883 |
| AST (µmol/L) | 53.1 ± 42.1 | 1.002 (0.997, 1.008) | .394 |
| ALT (µmol/L) | 38 ± 19.6 | 1.000 (0.983, 1.018) | .992 |
| Creatinine (µmol/L) | 63.8 ± 18.4 | 0.999 (0.979, 1.020) | .950 |
| PLR | 181.9 ± 117.6 | 1.003 (1.000, 1.005) | .032 |
| NLR | 4.0 ± 3.9 | 1.057 (0.975, 1.146) | .178 |
Interval time refers to the time interval between TACE and camrelizumab initiation.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PT, prothrombin time; SD, standard deviation; TACE, transcatheter arterial chemoembolization; TB, total bilirubin; UP, untreatable progression.
Addition of Camrelizumab to TACE
Forty-one patients received 280 camrelizumab therapy sessions, with a median of 5 sessions (range: 1-17) per patient. Twenty-three patients received camrelizumab combined with TACE (hereafter, camrelizumab–TACE), on whom 52 combined TACE procedures were performed, with a median of 2 procedures (range: 1-6) per patient. The remaining 18 patients received camrelizumab alone due to TACE contraindications.
Tumor Response
Imaging results 2-3 months after intervention indicated that 1 patient (2.4%) achieved CR, 9 patients (22.0%) achieved PR, and 15 patients (36.6%) achieved SD. Thus, the ORR is 24.4% and the DCR is 61.0%. Imaging results 6 months after intervention showed CR in 1 patient (2.4%), PR in 4 patients (9.8%), and SD in 19 patients (46.3%). Therefore, ORR was 12.2% and DCR was 58.5%.
PFS
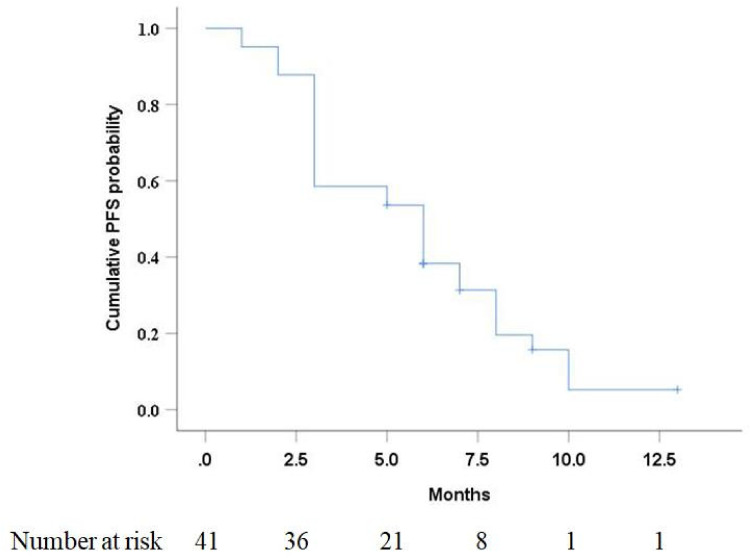
The median follow-up period from camrelizumab initiation to the study’s endpoint was 7 months (range: 4-14 months). Of the 41 patients, 33 (80.5%) developed disease progression after addition of camrelizumab. The median PFS in this study was 6 months (95% CI: 3.8 months, 8.2 months) (Figure 2). Univariate analysis (Table 1) indicated that combination therapy and platelet-to-lymphocyte ratio (PLR) were significantly associated with PFS (P < .05). At multivariable analysis (Table 2), combination therapy (camrelizumab–TACE) was significantly associated with better PFS (P = .003).
Figure 2.
Kaplan–Meier curve of progression-free survival (PFS) in HCC patients with untreatable progression (UP) after TACE.
Abbreviations: HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization.
Table 2.
Multivariate Analysis of Prognostic Factors for Progression-Free Survival (PFS).
| Variables | HR (95% CI) | P value |
|---|---|---|
| Combination therapy | ||
| Yes | 1 | |
| No | 3.636 (1.565, 8.447) | .003 |
| PLR | 1.001 (0.998, 1.004) | .690 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PLR, platelet-to-lymphocyte ratio.
AEs
Twenty-one (51.2%) patients developed postembolization syndrome, including fever (n = 18), abdominal pain (n = 15), nausea, and vomiting (n = 10) within 1 week after TACE. After symptomatic treatment during hospitalization, the symptoms of all patients were relieved or significantly improved. In addition, there were no TACE-related severe AEs such as liver abscess and biloma, and no allergic events occurred during camrelizumab injection.
During the follow-up period, 17 (41.5%) patients developed at least 1 type of AE after treatment with camrelizumab (Table 3), and no patients developed severe AEs (more than grade III). Grade I/II AEs included reactive cutaneous capillary endothelial proliferation (RCCEP) (n = 14), hypothyroidism (n = 6), asthenia (n = 2), rash (n = 1), myositis (n = 1), and pneumonitis (n = 1). Of note, 2 patients who developed pneumonia and myositis who received glucocorticoids and were temporarily interrupted from camrelizumab demonstrated significant improvement in symptoms, and then resumed camrelizumab therapy. No treatment-related mortalities occurred.
Table 3.
Adverse Events (AEs).
| AE | All events | CTCAE grade | ||
|---|---|---|---|---|
| 1 | 2 | ≥3 | ||
| RCCEP | 14 (34.1%) | 10 (24.4%) | 4 (9.8%) | 0 (0%) |
| Hypothyroidism | 6 (14.6%) | 6 (14.6%) | 0 (0%) | 0 (0%) |
| Asthenia | 2 (4.9%) | 1 (2.4%) | 1 (2.4%) | 0(0%) |
| Rash | 1 (2.4%) | 1 (2.4%) | 0 (0%) | 0 (0%) |
| Myositis | 1 (2.4%) | 0 (0%) | 1 (2.4%) | 0 (0%) |
| Pneumonitis | 1 (2.4%) | 0 (0%) | 1 (2.4%) | 0 (0%) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; RCCEP, reactive cutaneous capillary endothelial proliferation.
In addition, 3 patients were discontinued due to grade III AEs, including 2 patients who developed pneumonia and 1 patient who developed myocarditis.
Discussion
ICIs have made great progress in the field of HCC in recent years. The tumor microenvironment of HCC is infiltrated by different types of immune cells, including T cells, natural killer cells, etc.22 Currently, the treatment of HCC patients with UP after TACE remains a crucial issue. Changes in T cell populations after TACE have been demonstrated, which provides impetus for the exploration of immunotherapy after TACE.23 The present study indicated that additional camrelizumab therapy is effective and safe in the treatment of HCC patients with UP after initial TACE treatment.
Patients with HCC who develop UP after TACE treatment have a poor prognosis, and these patients are reported to be good candidates for tyrosine kinase inhibitors.24–26 Lee et al investigated 54 patients receiving sorafenib who met the criteria of TACE failure as defined by the European and Japanese International Guidelines and showed a median PFS of 3.2 months.24 Similarly, another study25 comparing sorafenib with hepatic arterial infusion of cisplatin in HCC patients who were refractory to TACE showed a median time to progression of 3.9 months for sorafenib and 2 months for cisplatin. In addition, the efficacy of lenvatinib in patients with intermediate-stage HCC refractory to TACE was reported by Shimose et al.26 The median PFS in the lenvatinib group was 5.8 months, higher than that in the sorafenib group (3.2 months) and TACE group (2.4 months). However, the study only included patients with intermediate-stage HCC and lacked results for patients with advanced HCC. In this study, additional camrelizumab therapy achieved a median PFS of 6 months in patients with UP after TACE, which seems to imply that ICIs represent effective systemic therapy for patients with TACE failure.
In terms of tumor response, camrelizumab also had comparable outcomes for HCC with UP after TACE treatment. Previous studies have shown that nivolumab as the first-line and second-line treatments for advanced HCC patients has an ORR of 15%27 and 20%,13 respectively. Similarly, pembrolizumab as the first-line and second-line treatments for advanced HCC patients has an ORR of 17%28 and 18.4%,29 respectively. Meanwhile, the ORR of camrelizumab in advanced HCC patients who had failed chemotherapy or sorafenib treatment was 14.7%.16 The results of this study showed that ORR and DCR were 24.4% and 61.0%, respectively, suggesting that camrelizumab therapy may achieve comparable tumor control in HCC patients with TACE failure.
In this study, multivariable analysis indicated that camrelizumab–TACE was an independent prognostic factor for PFS. Additional subgroup analysis demonstrated that the camrelizumab–TACE therapy significantly improved median PFS when compared with camrelizumab monotherapy. It is reported that TACE can promote tumor-specific CD8+ T cell response by killing HCC cells and causing the release of tumor-associated antigens.17 As suggested by the theoretical advantages, both Zhan et al15 and Marinelli et al14 demonstrated the efficacy of transarterial embolization combined with ICIs in the treatment of HCC patients. Similarly, this study also confirmed that TACE combined with ICI was superior to ICI monotherapy. These outcomes should promote further prospective studies evaluating combination TACE and systemic immunotherapy for the treatment of HCC patients.
Recent studies have shown that the inflammation ratio of PLR may potentially serve as a quantitative biomarker for individual tumor characteristics.30,31 Schobert et al32 investigated inflammatory biomarkers in patients with HCC treated with TACE and found that high baseline PLR predicted poorer tumor response and shorter PFS. Shen et al33 and Fan et al34 also reported that high PLR is associated with poorer overall survival (OS) and metastasis in HCC patients treated with TACE. In this study, although the univariate analysis showed that PLR was associated with PFS, multivariable analysis did not indicate that it was an independent prognostic factor affecting PFS. This may be related to the small sample size of this study. An additional study with larger sample size is warranted to validate risk factors to UP after TACE.
This study found that 39.6% of patients had AEs, but no patients of grade III or higher had AEs. Meanwhile, camrelizumab had a lower incidence of AEs in HCC patients with UP after TACE compared with sorafenib.25,35 Unlike nivolumab and pembrolizumab, RCCEP is more common after camrelizumab treatment.36 RCCEP incidence after camrelizumab monotherapy has been reported in 76.7% to 97.3% in other tumors, but no grade III RCCEP was reported.29,37 Similar to the study by Qin et al,16 the most common AE was RCCEP. However, the incidence of grade III or higher grade AEs with camrelizumab seemed to be lower than with other ICIs,14,15 suggesting that camrelizumab is safe for the treatment of HCC patients with UP after TACE.
The retrospective study had several limitations. First, the present study was conducted in a single institution with a small sample size, and therefore, a multicenter prospective randomized trial is needed. Second, most patients were still alive at the time of data collection and were followed up for a relatively short period of time and did not obtain the median OS for this study. Thus, it is necessary to explore the long-term efficacy on patients. Lastly, we did not include a control group of HCC patients with UP after TACE who received sorafenib or other forms of systemic therapies. Therefore, further comparative studies are needed to elucidate the clinical efficacy of camrelizumab in the treatment of HCC with UP.
Conclusions
The present study first reported camrelizumab in the treatment of HCC patients with UP after TACE, and the results indicated that camrelizumab has an acceptable safety profile and promising tumor control. Meanwhile, this study also demonstrated the superiority of camrelizumab–TACE verse camrelizumab monotherapy. The results of this study may prompt further prospective studies to evaluate the combination of TACE and ICIs in the treatment of HCC patients.
Acknowledgement
The author(s) are very grateful to Ms. Tingting Qin for her help in the statistical analysis of this study.
Abbreviations
- AEs
adverse events
- CI
confidence interval
- CR
complete response
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse events
- DCR
disease control rate
- ECOG
Eastern Cooperative Oncology Group
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- ICIs
immune checkpoint inhibitors
- JSH
Japan Society of Hepatology
- mRECIST
Modified Response Evaluation Criteria in Solid Tumors
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progression-free survival
- PLR
platelet-to-lymphocyte ratio
- PR
partial response
- RCCEP
reactive cutaneous capillary endothelial proliferation
- SD
stable disease
- TACE
transarterial chemoembolization
- UP
untreatable progression.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grant from National Nature Science Foundation of China (grant numbers 81771950, 81471765, and 81873919) and Fundamental Research Funds for the Central Universities (grant number 2021yjsCXCY101).
Ethics Approval: The retrospective single-center study was conducted in accordance with the principles of the Declaration of Helsinki and all procedures performed in this study were approved by the Ethics Committee of the local hospital (approval number UHCT-IEC-SOP-016-03-01). The need of informed consent was waived by the local ethics committee and the institutional review board of the Huazhong University of Science and Technology because clinical data were analyzed retrospectively and anonymously.
ORCID iD: Yanqiao Ren https://orcid.org/0000-0001-8086-4527
References
- 1.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. [DOI] [PubMed] [Google Scholar]
- 2.Kim JW, Kim JH, Sung KB, et al. Transarterial chemoembolization vs. radiofrequency ablation for the treatment of single hepatocellular carcinoma 2 cm or smaller. Am J Gastroenterol. 2014;109(8):1234-1240. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429-442. [DOI] [PubMed] [Google Scholar]
- 4.Sheta E, El-Kalla F, El-Gharib M, et al. Comparison of single-session transarterial chemoembolization combined with microwave ablation or radiofrequency ablation in the treatment of hepatocellular carcinoma: a randomized-controlled study. Eur J Gastroenterol Hepatol. 2016;28(10):1198-1203. [DOI] [PubMed] [Google Scholar]
- 5.Song MJ, Chun HJ, Song DS, et al. Comparative study between doxorubicineluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57(6):1244-1250. [DOI] [PubMed] [Google Scholar]
- 6.Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(3):327-332. [DOI] [PubMed] [Google Scholar]
- 7.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461-469. [DOI] [PubMed] [Google Scholar]
- 8.Peck-Radosavljevic M, Kudo M, Raoul JL, Han CL, Cheng AL. Outcomes of patients (pts) with hepatocellular carcinoma (HCC) treated with transarterial chemoembolization (TACE): Global OPTIMIS final analysis. J Clin Oncol. 2018;36(15_suppl):4018. [Google Scholar]
- 9.Kudo M, Izumi N, Kokudo N, et al. HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339-364. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Matsui O, Izumi N, et al. Liver Cancer Study Group of Japan . Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl1):22-31. [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11(9):525-535. [DOI] [PubMed] [Google Scholar]
- 12.Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3(2):119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open- label, non- comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marinelli B, Cedillo M, Pasik SD, et al. Safety and efficacy of locoregional treatment during immunotherapy with nivolumab for hepatocellular carcinoma: a retrospective study of 41 interventions in 29 patients. J Vasc Interv Radiol. 2020;31(11):1729-1738. e1. [DOI] [PubMed] [Google Scholar]
- 15.Zhan C, Ruohoniemi D, Shanbhogue KP, et al. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2020;31(1):25-34. [DOI] [PubMed] [Google Scholar]
- 16.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571-580. [DOI] [PubMed] [Google Scholar]
- 17.Chao J, Zhu Q, Chen D, et al. Case report: transarterial chemoembolization in combination with tislelizumab downstages unresectable hepatocellular carcinoma followed by radical salvage resection. Front Oncol. 2021;11:667555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. [DOI] [PubMed] [Google Scholar]
- 19.Ren Y, Cao Y, Ma H, et al. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study. BMC Cancer. 2019;19(1):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52-60. [DOI] [PubMed] [Google Scholar]
- 21.Gaba RC, Lokken RP, Hickey RM, et al. Quality improvement guidelines for transarterial chemoembolization and embolization of hepatic malignancy. J Vasc Interv Radiol. 2017;28(9):1210-1223. [DOI] [PubMed] [Google Scholar]
- 22.Abd El Aziz MA, Facciorusso A, Nayfeh T, et al. Immune checkpoint inhibitors for unresectable hepatocellular carcinoma. Vaccines (Basel). 2020;8(4):616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaki H, Imai N, Contessa TT, et al. Peripheral blood regulatory T-cell and type 1 helper T-cell population decrease after hepatic artery embolization. J Vasc Interv Radiol. 2016;27(10):1561-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Kang JH, Kim DY, et al. Prognostic factors of sorafenib therapy in hepatocellular carcinoma patients with failure of transarterial chemoembolization. Hepatol Int. 2017;11(3):292-299. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda M, Mitsunaga S, Shimizu S, et al. Efficacy of sorafenib in patients with hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. J Gastroenterol. 2014;49(5):932-940. [DOI] [PubMed] [Google Scholar]
- 26.Shimose S, Kawaguchi T, Tanaka M, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett. 2020;20(3):2257-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau T, Park JW, Finn RS, et al. Checkmate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30(supplement 5):v874-v875. [Google Scholar]
- 28.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952. [DOI] [PubMed] [Google Scholar]
- 29.Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193-202. [DOI] [PubMed] [Google Scholar]
- 30.Kuang DM, Zhao Q, Wu Y, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54(5):948-955. [DOI] [PubMed] [Google Scholar]
- 31.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9(2):237-249. [DOI] [PubMed] [Google Scholar]
- 32.Schobert IT, Savic LJ, Chapiro J, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of tumor response in hepatocellular carcinoma after DEB-TACE. Eur Radiol. 2020;30(10):5663-5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Y, Wang H, Chen X, et al. Prognostic significance of lymphocyte-to-monocyte ratio and platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma undergoing transcatheter arterial chemoembolization and radiofrequency ablation. Onco Targets Ther. 2019;12:7129-7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan W, Zhang Y, Wang Y, Yao X, Yang J, Li J. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One. 2015;10(3):e0119312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin PT, Teng W, Jeng WJ, et al. Add-on sorafenib is beneficial for hepatocellular carcinoma patients with transarterial chemoembolization refractoriness: a real-world experience. Eur J Gastroenterol Hepatol. 2020;32(9):1192-1199. [DOI] [PubMed] [Google Scholar]
- 36.Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455-461. [DOI] [PubMed] [Google Scholar]
- 37.Huang J, Xu B, Mo H, et al. Safety, activity, and biomarkers of SHR-1210, an anti-pd-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24(6):1296-1304. [DOI] [PubMed] [Google Scholar]