Abstract
Cigarette smoking is undoubtedly the single most important risk factor and trigger for vasospastic angina, a condition also known as Prinzmetal angina secondary to coronary artery vasospasm. Even decades before vasospastic angina was first described by Dr. Myron Prinzmetal and his colleagues in 1959, there had been suspected connections between smoking and coronary artery vasospasm in what was alluded to then as “tobacco angina.” The intimate relationship between smoking and vasospastic angina has since been extensively researched and validated through decades of epidemiological and clinical studies. The fact that smoking would aggravate vasospastic angina comes with very little surprise, as it has been shown to adversely impact many of the disease processes thought to underlie vasospastic angina, including autonomic dysfunction, endothelial dysfunction, smooth muscle hyperactivity, and genetic susceptibility. While avoidance of smoking is the first logical step in managing smokers with vasospastic angina, there have been reported cases of vasospastic angina paradoxically triggered by smoking cessation or relieved with smoking resumption or nicotine replacement therapy. Thus, there appears to be patient-specific factors that could significantly alter the close connection between smoking and vasospastic angina, warranting further mechanistic investigations. In this review, we will examine this complicated relationship between smoking and vasospastic angina from multiple perspectives (historical, mechanistic, and clinical) and call attention to the “smoking paradox,” which, with further elucidation, may provide additional insight into the complex mechanisms of VSA and potentially new strategies to treat medically refractory VSA, at least in selected individuals.
Keywords: Smoking, vasospastic angina, coronary artery vasospasm, smoking paradox
INTRODUCTION
Vasospastic angina (VSA), historically referred to as Prinzmetal or variant angina, is characterized by angina occurring mostly at rest due to coronary artery vasospasm. Dr. Myron Prinzmetal first described this “variant form of angina pectoris” in 1959 as rest angina associated with transient ST segment elevation that responded to sublingual nitrates.1 This syndrome was referred to as “variant” in contrast to Dr. William Heberden’s classical description of effort angina with ST segment changes. Dr. Prinzmetal’s team hypothesized that the variant type of angina likely resulted from “temporary occlusion of large diseased artery with a narrow lumen due to normal increase in tonus of vessel wall.” With the introduction of coronary angiography years later, Dr. Prinzmetal’s suspicion that variant angina is attributable to coronary artery vasospasm would be confirmed, and thus, the term “vasospastic angina” has since evolved.2, 3 However, little is known that Dr. Eli Moschcowitz might have described the same condition about thirty years earlier, which he then called “tobacco angina pectoris.”4 Even before his time, Dr. Henri Huchard in 1899 had described a form of angina associated with tobacco as “angine spasmo-tabagique,” which he speculatively attributed to spams of the coronary arteries.5 Thus, the intimate connection between tobacco smoking and VSA had long been known before Dr. Prinzmetal’s time. While smoking is undoubtedly the most well-known risk factor and trigger for VSA, there have been reports to suggest that smoking cessation could paradoxically trigger angina and resumption of smoking could provide relief. In this article, we will review the current evidence on this intimate and yet increasingly complicated relationship between smoking and VSA and highlight areas of further opportunities to advance the management of VSA.
EPIDEMIOLOGICAL IMPORTANCE OF SMOKING IN VASOSPASTIC ANGINA
It has been reported that nearly 50% of patients presenting with acute coronary syndrome could have coronary artery vasospasm confirmed by coronary angiography with provocative testing.6 However, the actual prevalence of VSA has not been comprehensively studied and appears to depend on the populations being studied, with some reports suggesting about 20% in Caucasian patients7 and about 40% in Japanese patients presenting with angina.8 There is a male predominance to this condition, but significant sex-specific differences in cardiovascular outcomes have not been consistently observed.9, 10 Because of significant heterogeneity in the clinical presentations, with some patients being asymptomatic, VSA is underdiagnosed.11 The typical risk factors for coronary artery disease such as diabetes12 and hypertension13 do not fully extend to VSA, and smoking remains the single most important acquired risk factor from numerous studies.14–17 Other known triggers of VSA include emotional stress, cold, stimulants, magnesium deficiency, hyperventilation, alcohol, and medications such as parasympathomimetics, ergot alkaloids, and nonselective beta-blockers.18
DIAGNOSIS OF VASOSPASTIC ANGINA
VSA is a condition that results primarily from the abnormal vasoreactivity of the epicardial coronary arteries and is associated with a heterogeneous angiographic presentation of coronary artery vasospasm, from diffuse to focal and involving one or more coronary arteries. The latest guidelines advocate for the standardized use of the following criteria for a definitive diagnosis of VSA: 1) nitrate-responsive angina (i.e., rest angina, marked diurnal variation in exercise tolerance, hyperventilation as a precipitation factor, or response to calcium channel blockers but not beta-blockers), 2) transient ischemic electrocardiogram changes, and 3) documented coronary artery vasospasm (i.e., >90% spontaneous or provoked constriction on coronary angiography [Figure 1] with concurrent angina and ischemic electrocardiographic changes).3 The choice of provocative stimulus (i.e., acetylcholine, ergonovine, or hyperventilation) can potentially affect the test readout, with acetylcholine more likely to provoke diffuse spasm distally and ergonovine more likely to provoke focal spasm proximally.19 Provocation with acetylcholine, the most commonly used agent, is based on the fact that it normally causes vasodilation by acting on the functional endothelial cells to release nitric oxide (NO), whereas it will directly activate the vascular smooth muscle cells (VSMCs) to cause vasoconstriction when there is endothelial dysfunction, such as in VSA.
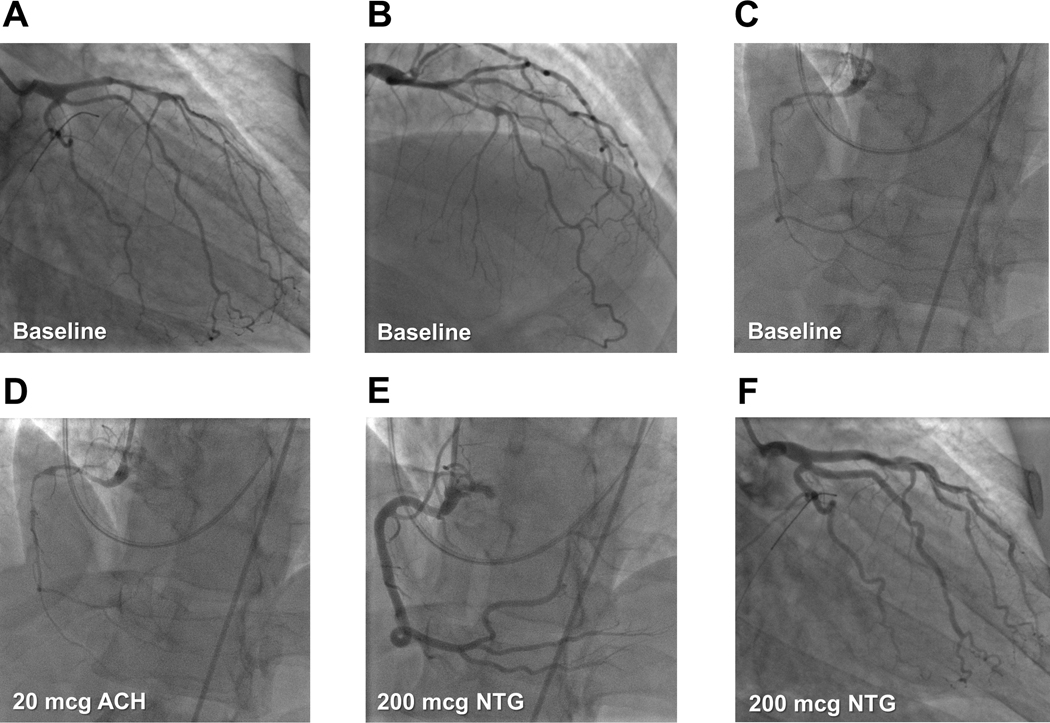
Figure 1. Coronary angiographic features of vasospastic angina.
Selective left coronary angiography of a patient with refractory vasospastic angina in the setting of chronic smoking shows diffuse narrowing of (A) the left circumflex artery, (B) the left anterior descending artery, and their respective branches. Selective right coronary angiography of the same patient shows (C) severe diffuse vasospasm of the right coronary artery, which (D) worsens after intracoronary acetylcholine (ACH) and (E) resolves after intracoronary nitroglycerin (NTG). NTG also vasodilates all major vessels in the left coronary circulation. Adapted from reference [58], with permission from Elsevier.
MECHANISMS OF VASOSPASTIC ANGINA IN RELATION TO SMOKING
The disease mechanisms of VSA are multifaceted and could involve microvascular dysfunction as a co-existing condition, which has been well-reviewed elsewhere.20, 21 Here, we will focus our review on the most essential mechanisms relevant to smoking that mainly impact the epicardial coronary arteries, which constitute the main location of vasomotion abnormality in VSA.
Autonomic dysfunction and smoking in vasospastic angina
Autonomic dysfunction in both arms of the autonomic nervous system is known to contribute to VSA (Figure 2). Some early studies have suggested parasympathetic derangement in VSA based on the observation of frequent anginal episodes from midnight to early morning, a period of heightened vagal tone.22 The fact that intracoronary infusion of acetylcholine, the main neurotransmitter of the parasympathetic nervous system, causes coronary artery vasospasm in patients with VSA further supports this assumption.23, 24 There has also been evidence to suggest sympathetic overactivity in VSA based on findings of frequent anginal episodes during the rapid eye movement stages of sleep with sympathetic outbursts.25, 26 Further supporting this hypothesis is the finding of abnormal myocardial sympathetic activity in the vascular territories that correspond to vasospastic arteries in patients.27 Thus, both arms of the autonomic nervous system contribute to VSA such that sympathovagal imbalance often precedes anginal attacks.28
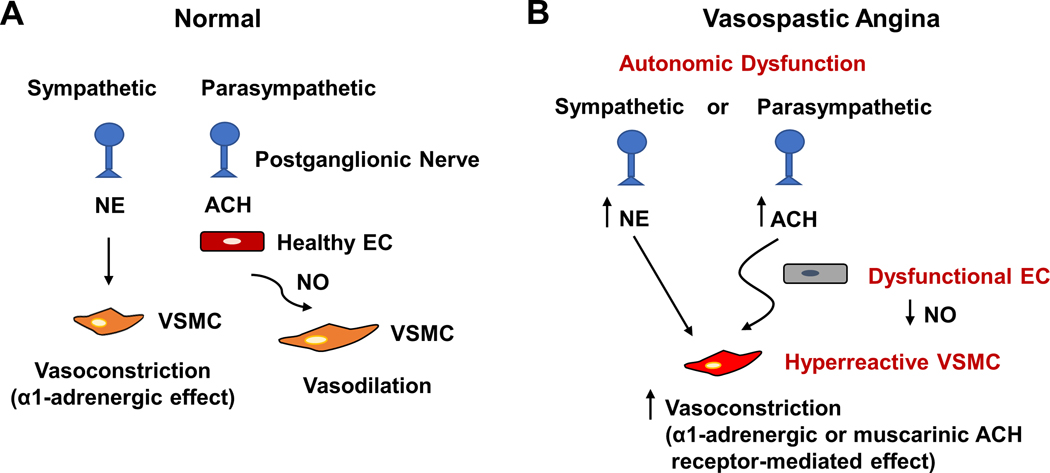
Figure 2. Schematics showing mechanisms of vasospastic angina.
(A) In normal epicardial coronary arteries, activation of the sympathetic nervous system leads to an increased release of norepinephrine (NE), which causes vasoconstriction via activation of the α1-adrenoreceptors on the vascular smooth muscle cells (VSMCs). The parasympathetic control of vessel caliber is generally weak, but the release of acetylcholine (ACH) can act on healthy endothelial cells (ECs) to produce nitric oxide (NO), which subsequently causes vasodilation (relaxation of VSMCs). (B) Vasospastic angina can be triggered by either sympathetic or parasympathetic activation. The former leads to α1-adrenorecptor-mediated vasoconstriction, whereas the latter leads to muscarinic acetylcholine receptor-mediated vasoconstriction. The endothelial cells in vasospastic angina are dysfunctional and deficient in NO production, and the VSMCs are hyperreactive.
Both cigarette smoking and nicotine consumption have significant impact on the autonomic nervous system innervating the heart. Numerous human studies have shown that cardiac sympathetic activity, as assessed by heart rate variability, increases after cigarette smoking29 and nicotine use.30 The increased norepinephrine release from the sympathetic nerves innervating the coronary vasculature can then cause epicardial vasoconstriction by activating the α1-adrenoreceptors on the VSMCs, the degree of which depends on the extent that norepinephrine also increases cardiac output and the subsequent flow-mediated dilation (FMD).31 Overall, smoking has the tendency to promote vasoconstriction by increasing the sympathetic tone, and acute smoking has been shown to trigger VSA in patients undergoing coronary angiography.32
Endothelial dysfunction and smoking in vasospastic angina
Endothelial cells lining the epicardial coronary arteries contribute to the regulation of vessel caliber by sensing coronary flow and releasing factors (e.g., NO) to cause relaxation of the underlying VSMCs during FMD. Endothelial cells also release NO to favor vasodilation in response to various endogenous substances (acetylcholine, serotonin, histamine) or endothelin-1 to favor vasoconstriction by activating the underlying VSMCs. Thus, endothelial dysfunction of the epicardial arteries typically manifests as either reduced FMD or acetylcholine-induced vasoconstriction, the latter of which forms the basis of provocative testing for VSA.24 Indeed, multiple studies have found FMD to be reduced in patients with VSA, more so in coronary segments exhibiting vasospasm than those without.33, 34 Other studies have shown that intracoronary acetylcholine leads to vasospasm only in patients with VSA but not in those without.23, 24 The endothelial dysfunction underlying coronary artery vasospasm has been associated with NO deficiency,35 which is the reason for using nitrates (NO donors) to treat VSA.
Cigarette smoking can aggravate VSA by causing further endothelial dysfunction. Many preclinical studies have shown that both cigarette smoking and nicotine can cause oxidative stress to impair endothelial function and reduce NO bioavailability,36 and these results appear to extend to humans. Both acute and chronic cigarette smoking have been shown to cause endothelial dysfunction and reduced FMD in the brachial arteries,37, 38 which can be ameliorated with vitamin C (an antioxidant), suggesting the essential role of oxidative stress in smoking-related endothelial dysfunction.39 Chronic cigarette smoking has also been shown to cause endothelial dysfunction in the epicardial coronary arteries, manifesting as either reduced FMD40 or vasoconstriction in response to acetylcholine.41 The smoking-induced impairment is at least partly reversible, as smoking cessation has been shown to reduce coronary vasoconstriction during acetylcholine challenge in patients with recent myocardial infarction.42
Smooth muscle hyperreactivity and smoking in vasospastic angina
There appears to be some racial differences in the angiographic presentation of VSA, such that Japanese patients tend to have more diffuse or multi-vessel vasospasm, whereas Caucasians tend to present with focal vasospasm.43 In contrast to the diffuse form, the focal form of coronary artery vasospasm cannot be explained by generalized endothelial dysfunction alone and likely involves hypercontraction of VSMCs, which can be inferred from intracoronary optical coherence tomography (OCT) of patients with VSA (Figure 3). It has been shown in a porcine model of coronary artery vasospasm that only coronary segments treated with interleukin-1β, but not those untreated, show inducible hypercontraction with intracoronary serotonin and histamine.44 A molecular study using the same animal model has further implicated the activation of both protein kinase C and Rho-kinase pathways to cause VSMC hyperreactivity in the treated segments.45 The fact that endothelium-dependent vasodilating responses can be preserved at these segments in this animal model46, as well as at the vasospastic sites of patients,47 further supports smooth muscle hyperreactivity as an important disease mechanism of VSA.
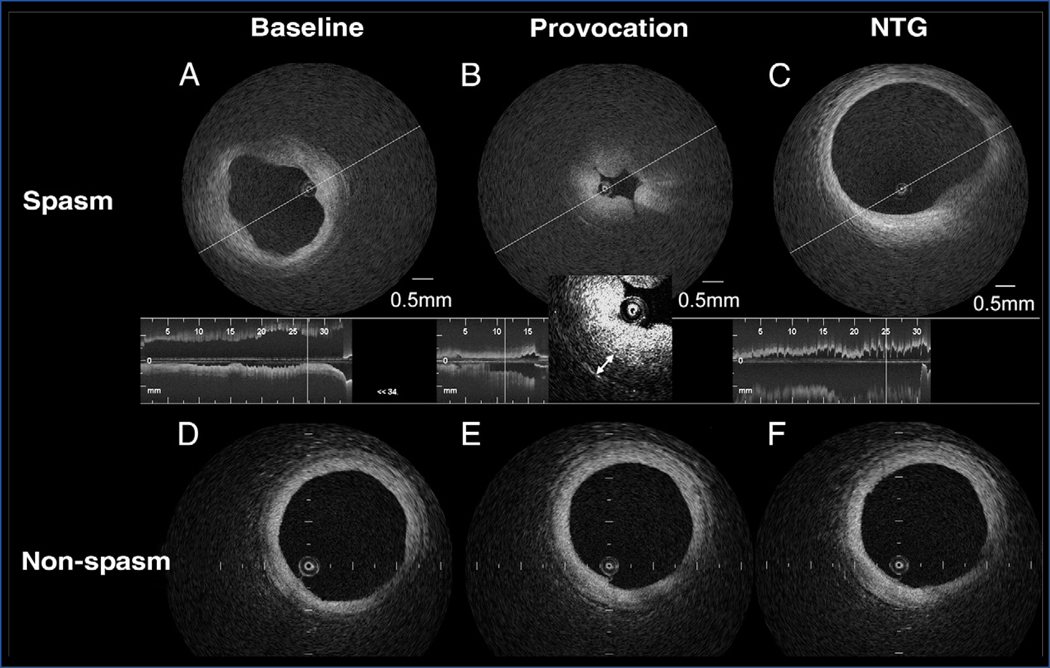
Figure 3. Optical coherence tomography features of vasospastic angina.
Representative intracoronary optical coherence tomography images of a patient with vasospastic angina at a site of coronary vasospasm (top row) versus a site without coronary vasospasm (bottom row). (A) Both intimal bumps around the lumen and a thickened medial layer can be seen at baseline. (B) With provocation, intimal gathering appears, with a further increase in the medial layer (double headed arrows). (C) Both intimal bumps and gathering disappear with intracoronary nitroglycerin (NTG). Compared to the morphology at (D) baseline, no changes are seen at the non-spasm site with either (E) acetylcholine or (F) nitroglycerin administration. Reproduced from reference [75], with permission from Elsevier.
Smoking appears to have a moderate effect on smooth muscle hypercontraction, which can occur with either reduced sensitivity to vasodilators or increased sensitivity to vasoconstrictors. Although some earlier studies have suggested otherwise,48 a more recent study using increasing doses of sublingual nitroglycerin has found that cigarette smoking can reduce nitroglycerin-mediated endothelium-independent vasodilation in human brachial artery and thus VSMCs’ sensitivity to NO.49 This observation is consistent with a prior study showing that chronic cigarette smoking can cause a mildly impaired nitroglycerin-mediated endothelium-independent vasodilation in the epicardial coronary arteries, compared to much greater impairment on endothelium-dependent vasodilation in the same subjects.40 Nicotine alone also has been shown to enhance both norepinephrine-mediated vasoconstriction in human skin50 and the contractile response to endothelin-1 in rat coronaries.51 Thus, cigarette smoking and nicotine have the ability to alter VSMCs’ sensitivity to multiple vasoactive substances to favor vasoconstriction, although its effect is likely overall smaller compared to its impact on the endothelium.
Genetic influences of vasospastic angina in relation to smoking
Significant progress has been made over the past two decades to unravel the genetics of VSA. One of the most well-known genetic variants associated with VSA is the T786C variant of the nitric oxide synthase (eNOS) gene, which has been shown in vitro to confer reduced eNOS activity,15, 52 thus affirming NO deficiency as a critical contributor to VSA. Worth noting are also the C242T variant of the NADH/NADPH oxidase 22 phox gene (also known as cytochrome B-245 alpha chain gene; CYBA) in men and the C634G variant of the interleukin-6 (IL-6) gene in women.16 The former variant is associated with reduced superoxide production53 and thus a protective variant less seen in VSA patients. These variants implicate both oxidative stress and inflammation in VSA and reveal important sex-specific differences in genetic susceptibility to VSA. The Ala370Ser variant of the Rho GTPase activating protein 9 (ARHGAP9) gene is another interesting variant implicated in VSA, which in mouse models causes increased vascular infiltration of hematopoietic cells (inflammation) and endothelial dysfunction,54 suggesting that perturbation of the extravascular compartment can also contribute to VSA. Most recently, the aldehyde dehydrogenase 2 (ALDH2) genotype, ALDH2*2, that underlies alcohol flush syndrome in East Asians55 was found to be associated with VSA, providing a rationale for the greater prevalence of VSA in East Asians than Caucasians.56 Compared to the various genetic variants identified, smoking is by far the single most consistent risk factor for VSA.15, 16
Smoking also affects many of the disease pathways related to the VSA-associated genetic variants and can cause significant gene-environment interactions. For instance, smoking can cause oxidative stress in endothelial cells and lead to NO deficiency and potentially worsening of angina in patients already with one or more of the pathogenic variants of the eNOS, CYBA, or ALDH2 genes. This is possible because the products of CYBA (p22-phox) and ALDH2 are involved in the generation of reactive oxygen species and the metabolism of toxic aldehydes from cigarette smoke, respectively. Smoking has also been shown to cause increased blood levels of inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β, and IL-6, and can potentially worsen angina in patients with variants related to cytokines (IL-6) or their potential cell targets (ARHGAP9). In fact, inflammatory cytokines have been shown to cause increased reactive oxygen species and endothelial dysfunction in animal models of smoking-induced vasculopathy.57 Thus, although smokers have varying tendencies to experience VSA, those with a severe course could very well have significant underlying genetic predispositions worth investigating.58
PARADOXICAL EFFECTS OF SMOKING ON VASOSPASTIC ANGINA
There is little dispute that smoking is a strong trigger and contributor of VSA, but some case reports have ironically suggested the possibility of developing VSA after smoking cessation. The first case report of this phenomenon described a middle-aged female chronic cigarette smoker who experienced VSA one week after stopping cigarette smoking, with subsequent angiogram confirming coronary artery vasospasm and esophageal manometry revealing diffuse esophageal vasospasm.59 It was deduced that nicotine likely exerted “acetylcholine antagonistic effects” to protect her from concurrent VSA (Figure 4A) and esophageal vasospasm, as acetylcholine is the main neurotransmitter of the parasympathetic nervous system innervating both the coronary artery and esophageal smooth muscle cells. The site of nicotine’s action was presumed to be on the postganglionic nerves, which contain nicotinic, rather than muscarinic, acetylcholine receptors. The patient’s angina was eventually controlled with calcium channel blockers alone.
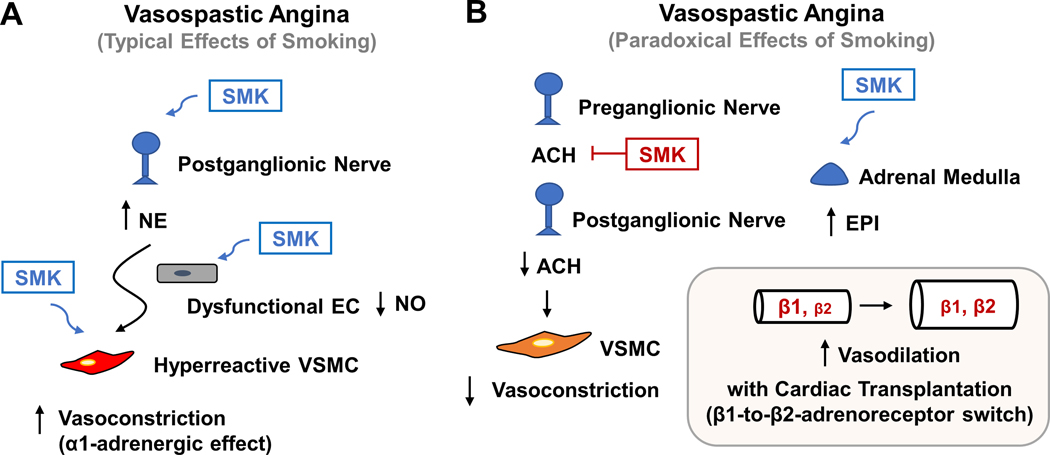
Figure 4. Schematics showing typical and paradoxical effects of smoking on vasospastic angina.
(A) Smoking (SMK) can predispose to vasospastic angina by activating the sympathetic nervous system, impair endothelial function, and make vascular smooth muscle cells (VSMCs) more prone to contraction. (B) Very rarely, smoking can paradoxically provide relief for vasospastic angina by potentially antagonizing actetylcholine (ACH) action on the postganglionic nerve. Additionally, in the setting of cardiac transplantation, smoking or nicotine can cause an increase in the adrenal release of epinephrine (EPI), which may favor vasodilation, and thus less vasospasm, due to a β1-to-β2-adrenoreceptor switch in the transplanted heart.
Recently we reported a similar case in which a middle-aged male chronic smoker with multiple known genetic variants of VSA developed severe VSA after quitting cigarettes because of myocardial infarction.58 His angina was resistant to all sorts of medical therapy such that he eventually underwent cardiac autotransplantation for complete denervation and symptom relief. Unfortunately, his angina recurred after nine months due to autonomic re-innervation and could only be controlled paradoxically with nicotine patch or cigars but not antianginals. As with the first case, it would be reasonable to speculate that nicotine could have provided relief in this patient by antagonizing parasympathetic overactivity, assuming it was driving his VSA. Additionally, because heart transplant is known to cause a β1-to-β2-adrenoreceptor switch in the transplanted heart, it is possible that nicotine could have minimized epicardial vasospasm by increasing epinephrine release to favor β2-mediated coronary vasodilation (Figure 4B). Although the mechanisms underlying this “smoking paradox” remain mostly speculative, these cases underscore the complicated relationship between cigarette smoking and VSA, which can be affected by patient-specific factors (e.g., concurrent diffuse esophageal spasm, heart transplant, and genetic susceptibility). Further research into how these patient-specific factors interact with smoking may provide insight on more effective strategies to treat resistant cases of VSA.
TREATMENTS FOR VASOSPASTIC ANGINA AND IMPLICATIONS FOR SMOKERS
The treatment of VSA starts with lifestyle modifications, which, in concert with medical therapy, typically provide adequate symptomatic control. Only rarely would non-medical or surgical intervention be needed to address medically refractory VSA.
Lifestyle modifications
Smoking cessation is the single most important intervention in smokers with VSA because those who continue to smoke are more likely to experience angina than those who successfully quit.60 Concurrent abstinence from alcohol is also essential in smokers with the ALDH2*2* genotype (or alcohol flush reaction), which has been associated with reduced metabolism of harmful aldehydes from both alcohol and cigarette smoke.55 Other substances that are known to trigger VSA, such as cocaine, methamphetamine, and marijuana, should also be avoided.61
Medical management
Some medications can precipitate VSA and should be first withdrawn if clinically feasible. Nonselective beta-blockers can exacerbate VSA by blocking β2-adrenoreceptor-mediated vasodilation and leaving α1-adrenoreceptor-mediated vasoconstrictive effects unopposed. Sumatriptan, a serotonin receptor agonist, is contraindicated in patients with VSA due to its ability to directly provoke coronary artery vasospasm. Aspirin, which inhibits prostacyclin synthesis, has been reported to exacerbate VSA at high doses and should be judiciously used if otherwise indicated in patients with VSA.62
For drug treatments, calcium channel blockers (CCBs) are first-line agents, which work by suppressing the calcium influx needed for VSMC contraction. CCB therapy is associated with reduced myocardial infarction in patients with VSA, with the second-generation CCBs more effective at preventing acute coronary syndrome than the first-generation agents.63 When angina cannot be completely controlled with a single agent, dual CCB therapy can be considered for additional relief.64 Long-acting nitrates are also frequently prescribed early in the management of VSA for symptomatic control. These agents are metabolized to NO in vivo, which directly causes VSMC relaxation. When used concomitantly with CCBs, however, they do not provide additional mortality benefit.65 Nitrate tolerance is a major limitation of chronic nitrate therapy, and the use of multiple nitrates (including nicorandil) may be associated with adverse outcomes.65
Patients with refractory VSA despite being on a combination of CCBs and nitrates could be trialed on other less conventional agents. Statins can be effective at reducing VSA because of their pleiotropic effects, including enhancement of NO activity, reduction of VSMC hyperactivity, and attenuation of vascular inflammation.66 Their long-term mortality benefits in patients with VSA, however, are subjects of ongoing debate that remain to be further elucidated.67, 68 The Rho-kinase inhibitor fasudil works by inhibiting Rho-kinase, an enzyme that normally reduces myosin phosphatase activity to promote vasoconstriction. It specifically targets the VSMC hyperactivity underlying VSA and can suppress inducible vasospasm in patients with VSA.69 However, it is only available in an intravenous form, which limits its use in the outpatient population. Cilostazol is a phosphodiesterase-3 inhibitor that promotes VSMC relaxation by increasing intracellular cyclic adenosine monophosphate. When used in combination with a CCB, cilostazol has been shown to significantly reduce the incidence of angina.70 Magnesium supplementation has also been shown to cause coronary vasodilation and prevent acetylcholine-induced coronary artery vasospasm in patients with VSA.71
In smokers who develop refractory VSA only after smoking cessation, the possibility of a “smoking paradox” should be recognized after ruling out all other potential causes of VSA. If a paradoxical case is deemed probable, nicotine replacement therapy (NRT) could be considered for symptomatic relief with significantly less cardiovascular risks than smoking resumption.72 Compared to placebo control and other smoking cessation medications such as varenicline and bupropion, NRT does not appear to increase the risk of serious cardiovascular events.73 Compared to cigarettes, NRT is deemed safer because it contains less nicotine and is devoid of other cardiotoxic substances (e.g., carbon monoxide, oxidant gases, and polycyclic aromatic hydrocarbons) that can contribute to an increased risk of cardiovascular events.31
In all medically refractory cases, strong genetic susceptibility to VSA should be suspected, and its characterization may point to specific disease pathways of coronary artery vasospasm that can be potentially remedied with specific drug treatments.58 Such form of genetics-based personalized medicine, although not in current practice, should be studied and encouraged more if validated.
Non-medical Interventions
In medically refractory cases, percutaneous coronary intervention could be attempted if an obstructive lesion is thought to be the persistent trigger of localized vasospasm. Left stellate ganglion block could be considered for short-term relief, and if effective, followed by bilateral thoracoscopic sympathectomy, for more sustaining effects.74 In very rare cases, cardiac autotransplantation could also provide short-term relief, but its long-term efficacy is likely limited by autonomic reinnervation, a process that can vary significantly among patients.58
CONCLUSION
Since the initial description of tobacco angina followed by Prinzmetal angina and then VSA, there has been significant strides made to elucidate the intimate relationship between smoking and VSA. As the single most important risk factor for VSA, smoking contributes to many pathological processes underlying VSA (e.g., autonomic dysfunction, endothelial dysfunction, smooth muscle hyperreactivity) and can expose those patients with strong genetic predispositions. While generally considered harmful, smoking and nicotine could have rare paradoxical effects on certain individuals, raising the possibility that there are yet unfamiliar patient-specific factors that may influence patients’ vasoactive responses to smoking and nicotine. Although nicotine replacement therapy could provide symptomatic relief as a safer alternative to smoking for these individuals, further research into the mechanisms underpinning this “smoking paradox” of VSA could potentially open the door for the development of more novel therapeutics for these individuals and those with medically refractory VSA.
ACKNOWLEDGMENTS
We thank Dr. David Liang of Stanford University School of Medicine for kindly providing some of the angiographic images included in this article.
SOURCES OF FUNDING
This work was supported in part by the AHA 18CDA34110047 (IYC).
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Prinzmetal M, Kennamer R, Merliss R, Wada T and Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–88. [DOI] [PubMed] [Google Scholar]
- 2.Oliva PB, Potts DE and Pluss RG. Coronary arterial spasm in Prinzmetal angina. Documentation by coronary arteriography. N Engl J Med. 1973;288:745–51. [DOI] [PubMed] [Google Scholar]
- 3.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN and Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 4.Moschcowitz E.Tobacco angina pectoris. Journal of the American Medical Association. 1928;90:733–737. [Google Scholar]
- 5.Oram S and Sowton E. Tobacco angina. Q J Med. 1963;32:115–143. [PubMed] [Google Scholar]
- 6.Ong P, Athanasiadis A, Hill S, Vogelsberg H, Voehringer M and Sechtem U. Coronary artery spasm as a frequent cause of acute coronary syndrome: The CASPAR (Coronary Artery Spasm in Patients With Acute Coronary Syndrome) Study. J Am Coll Cardiol. 2008;52:523–7. [DOI] [PubMed] [Google Scholar]
- 7.Bertrand ME, LaBlanche JM, Tilmant PY, Thieuleux FA, Delforge MR, Carre AG, Asseman P, Berzin B, Libersa C and Laurent JM. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65:1299–306. [DOI] [PubMed] [Google Scholar]
- 8.Group JCSJW. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014;78:2779–801. [DOI] [PubMed] [Google Scholar]
- 9.Kim HL, Jo SH, Kim HJ, Lee MH, Seo WW and Baek SH. Sex differences in clinical characteristics and long-term outcomes in patients with vasospastic angina: results from the VA-Korea registry, a prospective multi-center cohort. Biol Sex Differ. 2020;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pikkarainen E, Blomster J, Sipila J, Rautava P and Kyto V. Occurrence and mortality of vasospastic angina pectoris hospitalised patients in Finland: a population-based registry cohort study. BMJ Open. 2019;9:e030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishida H, Tada Y, Fukuma N, Saitoh T, Kusama Y and Sano J. Significant characteristics of variant angina patients with associated syncope. Jpn Heart J. 1996;37:317–26. [DOI] [PubMed] [Google Scholar]
- 12.Li YJ, Hyun MH, Rha SW, Chen KY, Jin Z, Dang Q, Park CM, Lee JE, Park JY, Choi CU, Na JO, Lim HE, Kim JW, Kim EJ, Park CG, Seo HS and Oh DJ. Diabetes mellitus is not a risk factor for coronary artery spasm as assessed by an intracoronary acetylcholine provocation test: angiographic and clinical characteristics of 986 patients. J Invasive Cardiol. 2014;26:234–9. [PubMed] [Google Scholar]
- 13.Chen KY, Rha SW, Li YJ, Poddar KL, Jin Z, Minami Y, Saito S, Park JH, Na JO, Choi CU, Lim HE, Kim JW, Kim EJ, Park CG, Seo HS and Oh DJ. Impact of hypertension on coronary artery spasm as assessed with intracoronary acetylcholine provocation test. J Hum Hypertens. 2010;24:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nobuyoshi M, Abe M, Nosaka H, Kimura T, Yokoi H, Hamasaki N, Shindo T, Kimura K, Nakamura T, Nakagawa Y and et al. Statistical analysis of clinical risk factors for coronary artery spasm: identification of the most important determinant. Am Heart J. 1992;124:32–8. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama M, Yoshimura M, Sakamoto T, Shimasaki Y, Nakamura S, Ito T, Abe K, Yamamuro M, Miyamoto Y, Saito Y, Nakao K, Yasue H and Ogawa H. Synergistic interaction of T-786-->C polymorphism in the endothelial nitric oxide synthase gene and smoking for an enhanced risk for coronary spasm. Pharmacogenetics. 2003;13:683–8. [DOI] [PubMed] [Google Scholar]
- 16.Murase Y, Yamada Y, Hirashiki A, Ichihara S, Kanda H, Watarai M, Takatsu F, Murohara T and Yokota M. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur Heart J. 2004;25:970–7. [DOI] [PubMed] [Google Scholar]
- 17.Takaoka K, Yoshimura M, Ogawa H, Kugiyama K, Nakayama M, Shimasaki Y, Mizuno Y, Sakamoto T and Yasue H. Comparison of the risk factors for coronary artery spasm with those for organic stenosis in a Japanese population: role of cigarette smoking. Int J Cardiol. 2000;72:121–6. [DOI] [PubMed] [Google Scholar]
- 18.Picard F, Sayah N, Spagnoli V, Adjedj J and Varenne O. Vasospastic angina: A literature review of current evidence. Arch Cardiovasc Dis. 2019;112:44–55. [DOI] [PubMed] [Google Scholar]
- 19.Sueda S, Kohno H, Fukuda H, Ochi N, Kawada H, Hayashi Y and Uraoka T. Induction of coronary artery spasm by two pharmacological agents: comparison between intracoronary injection of acetylcholine and ergonovine. Coron Artery Dis. 2003;14:451–7. [DOI] [PubMed] [Google Scholar]
- 20.Tjoe B, Barsky L, Wei J, Samuels B, Azarbal B, Merz CNB and Shufelt C. Coronary microvascular dysfunction: Considerations for diagnosis and treatment. Cleve Clin J Med. 2021;88:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Cheng S and Bairey Merz CN. Coronary microvascular dysfunction causing cardiac ischemia in women. JAMA. 2019;322:2334–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasue H and Kugiyama K. Coronary spasm: clinical features and pathogenesis. Intern Med. 1997;36:760–5. [DOI] [PubMed] [Google Scholar]
- 23.Kugiyama K, Ohgushi M, Motoyama T, Sugiyama S, Soejima H, Matsumura T, Yoshimura M, Ogawa H and Yasue H. Enhancement of constrictor response of spastic coronary arteries to acetylcholine but not to phenylephrine in patients with coronary spastic angina. J Cardiovasc Pharmacol. 1999;33:414–9. [DOI] [PubMed] [Google Scholar]
- 24.Yasue H, Horio Y, Nakamura N, Fujii H, Imoto N, Sonoda R, Kugiyama K, Obata K, Morikami Y and Kimura T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74:955–63. [DOI] [PubMed] [Google Scholar]
- 25.King MJ, Zir LM, Kaltman AJ and Fox AC. Variant angina associated with angiographically demonstrated coronary artery spasm and REM sleep. Am J Med Sci. 1973;265:419–22. [DOI] [PubMed] [Google Scholar]
- 26.Verrier RL, Muller JE and Hobson JA. Sleep, dreams, and sudden death: the case for sleep as an autonomic stress test for the heart. Cardiovasc Res. 1996;31:181–211. [PubMed] [Google Scholar]
- 27.Inobe Y, Kugiyama K, Miyagi H, Ohgushi M, Tomiguchi S, Takahashi M and Yasue H. Long-lasting abnormalities in cardiac sympathetic nervous system in patients with coronary spastic angina: quantitative analysis with iodine 123 metaiodobenzylguanidine myocardial scintigraphy. Am Heart J. 1997;134:112–8. [DOI] [PubMed] [Google Scholar]
- 28.Tan BH, Shimizu H, Hiromoto K, Furukawa Y, Ohyanagi M and Iwasaki T. Wavelet transform analysis of heart rate variability to assess the autonomic changes associated with spontaneous coronary spasm of variant angina. J Electrocardiol. 2003;36:117–24. [DOI] [PubMed] [Google Scholar]
- 29.Garcia PD, Gornbein JA and Middlekauff HR. Cardiovascular autonomic effects of electronic cigarette use: a systematic review. Clin Auton Res. 2020;30:507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjoberg N and Saint DA. A single 4 mg dose of nicotine decreases heart rate variability in healthy nonsmokers: implications for smoking cessation programs. Nicotine Tob Res. 2011;13:369–72. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL and Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26:515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quillen JE, Rossen JD, Oskarsson HJ, Minor RL Jr, Lopez AG and Winniford MD.Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22:642–7. [DOI] [PubMed] [Google Scholar]
- 33.Kugiyama K, Ohgushi M, Motoyama T, Sugiyama S, Ogawa H, Yoshimura M, Inobe Y, Hirashima O, Kawano H, Soejima H and Yasue H. Nitric oxide-mediated flow-dependent dilation is impaired in coronary arteries in patients with coronary spastic angina. J Am Coll Cardiol. 1997;30:920–6. [DOI] [PubMed] [Google Scholar]
- 34.Kang SH, Park HK, Lee CW, Kim JJ, Hong MK, Park SW and Park SJ. Impaired flow-mediated vasodilation of epicardial coronary artery in vasospastic angina. J Korean Med Sci. 1998;13:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, Yoshimura M, Motoyama T, Inobe Y and Kawano H. Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation. 1996;94:266–71. [DOI] [PubMed] [Google Scholar]
- 36.Grassi D, Desideri G, Ferri L, Aggio A, Tiberti S and Ferri C. Oxidative stress and endothelial dysfunction: say NO to cigarette smoking! Curr Pharm Des. 2010;16:2539–50. [DOI] [PubMed] [Google Scholar]
- 37.Ozaki K, Hori T, Ishibashi T, Nishio M and Aizawa Y. Effects of chronic cigarette smoking on endothelial function in young men. J Cardiol. 2010;56:307–13. [DOI] [PubMed] [Google Scholar]
- 38.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A and Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4. [DOI] [PubMed] [Google Scholar]
- 39.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, Ogawa H and Yasue H. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: effect of vitamin C. Am J Physiol. 1997;273:H1644–50. [DOI] [PubMed] [Google Scholar]
- 40.Zeiher AM, Schachinger V and Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–100. [DOI] [PubMed] [Google Scholar]
- 41.Lavi S, Prasad A, Yang EH, Mathew V, Simari RD, Rihal CS, Lerman LO and Lerman A. Smoking is associated with epicardial coronary endothelial dysfunction and elevated white blood cell count in patients with chest pain and early coronary artery disease. Circulation. 2007;115:2621–7. [DOI] [PubMed] [Google Scholar]
- 42.Hosokawa S, Hiasa Y, Miyazaki S, Ogura R, Miyajima H, Ohara Y, Yuba K, Suzuki N, Takahashi T, Kishi K and Ohtani R. Effects of smoking cessation on coronary endothelial function in patients with recent myocardial infarction. Int J Cardiol. 2008;128:48–52. [DOI] [PubMed] [Google Scholar]
- 43.Beltrame JF, Sasayama S and Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33:1442–52. [DOI] [PubMed] [Google Scholar]
- 44.Shimokawa H, Ito A, Fukumoto Y, Kadokami T, Nakaike R, Sakata M, Takayanagi T, Egashira K and Takeshita A. Chronic treatment with interleukin-1 beta induces coronary intimal lesions and vasospastic responses in pigs in vivo. The role of platelet-derived growth factor. J Clin Invest. 1996;97:769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S and Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol. 2003;23:2209–14. [DOI] [PubMed] [Google Scholar]
- 46.Miyata K, Shimokawa H, Yamawaki T, Kunihiro I, Zhou X, Higo T, Tanaka E, Katsumata N, Egashira K and Takeshita A. Endothelial vasodilator function is preserved at the spastic/inflammatory coronary lesions in pigs. Circulation. 1999;100:1432–7. [DOI] [PubMed] [Google Scholar]
- 47.Egashira K, Inou T, Yamada A, Hirooka Y and Takeshita A. Preserved endothelium-dependent vasodilation at the vasospastic site in patients with variant angina. J Clin Invest. 1992;89:1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esen AM, Barutcu I, Acar M, Degirmenci B, Kaya D, Turkmen M, Melek M, Onrat E, Esen OB and Kirma C. Effect of smoking on endothelial function and wall thickness of brachial artery. Circ J. 2004;68:1123–6. [DOI] [PubMed] [Google Scholar]
- 49.Lanza GA, Spera FR, Villano A, Russo G, Di Franco A, Lamendola P and Crea F. Effect of smoking on endothelium-independent vasodilatation. Atherosclerosis. 2015;240:330–2. [DOI] [PubMed] [Google Scholar]
- 50.Black CE, Huang N, Neligan PC, Levine RH, Lipa JE, Lintlop S, Forrest CR and Pang CY. Effect of nicotine on vasoconstrictor and vasodilator responses in human skin vasculature. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1097–104. [DOI] [PubMed] [Google Scholar]
- 51.Cao L, Zhang Y, Cao YX, Edvinsson L and Xu CB. Cigarette smoke upregulates rat coronary artery endothelin receptors in vivo. PLoS One. 2012;7:e33008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glueck CJ, Munjal J, Khan A, Umar M and Wang P. Endothelial nitric oxide synthase T-786C mutation, a reversible etiology of Prinzmetal’s angina pectoris. Am J Cardio l. 2010;105:792–6. [DOI] [PubMed] [Google Scholar]
- 53.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R and Channon KM. Functional effect of the C242T polymorphism in the NAD(P)H oxidase p22phox gene on vascular superoxide production in atherosclerosis. Circulation. 2000;102:1744–7. [DOI] [PubMed] [Google Scholar]
- 54.Takefuji M, Asano H, Mori K, Amano M, Kato K, Watanabe T, Morita Y, Katsumi A, Itoh T, Takenawa T, Hirashiki A, Izawa H, Nagata K, Hirayama H, Takatsu F, Naoe T, Yokota M and Kaibuchi K. Mutation of ARHGAP9 in patients with coronary spastic angina. J Hum Genet. 2010;55:42–9. [DOI] [PubMed] [Google Scholar]
- 55.Chen CH, Ferreira JC, Gross ER and Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizuno Y, Harada E, Morita S, Kinoshita K, Hayashida M, Shono M, Morikawa Y, Murohara T, Nakayama M, Yoshimura M and Yasue H. East asian variant of aldehyde dehydrogenase 2 is associated with coronary spastic angina: possible roles of reactive aldehydes and implications of alcohol flushing syndrome. Circulation. 2015;131:1665–73. [DOI] [PubMed] [Google Scholar]
- 57.Messner B and Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–15. [DOI] [PubMed] [Google Scholar]
- 58.Tran MV, Marceau E, Liu Y, Sallam K, Medina P, Liu C, Sayed N, Muller MD, Liang DH and Chen IY. Coronary artery vasospasm requiring cardiac autotransplantation yet controlled with tobacco. JACC Case Rep. 2021;3:1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipton SA, Markis JE, Pine MB, Paulin S and Lindsay HE. Cessation of smoking followed by Prinzmetal’s variant angina and diffuse esophageal spasm. N Engl J Med. 1978;299:775–6. [PubMed] [Google Scholar]
- 60.Miwa K, Fujita M and Miyagi Y. Beneficial effects of smoking cessation on the short-term prognosis for variant angina--validation of the smoking status by urinary cotinine measurements. Int J Cardiol. 1994;44:151–6. [DOI] [PubMed] [Google Scholar]
- 61.Slavich M and Patel RS. Coronary artery spasm: Current knowledge and residual uncertainties. Int J Cardiol Heart Vasc. 2016;10:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin Y, Chen Y, Yuan J, Qin H, Dong S and Chen Q. Impact of aspirin use on clinical outcomes in patients with vasospastic angina: a systematic review and meta-analysis. BMJ Open. 2021;11:e048719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SE, Jo SH, Han SH, Lee KY, Her SH, Lee MH, Seo WW, Cho SS and Baek SH. Comparison of calcium-channel blockers for long-term clinical outcomes in patients with vasospastic angina. Korean J Intern Med. 2021;36:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tandon V, Mosebach CM, Kumar M and Joshi S. Refractory vasospastic angina: when typical medications don’t work. Cureus. 2019;11:e4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takahashi J, Nihei T, Takagi Y, Miyata S, Odaka Y, Tsunoda R, Seki A, Sumiyoshi T, Matsui M, Goto T, Tanabe Y, Sueda S, Momomura S, Yasuda S, Ogawa H, Shimokawa H and Japanese Coronary Spasm A. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J. 2015;36:228–37. [DOI] [PubMed] [Google Scholar]
- 66.Yasue H, Mizuno Y, Harada E, Itoh T, Nakagawa H, Nakayama M, Ogawa H, Tayama S, Honda T, Hokimoto S, Ohshima S, Hokamura Y, Kugiyama K, Horie M, Yoshimura M, Harada M, Uemura S, Saito Y and Investigators S. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J Am Coll Cardiol. 2008;51:1742–8. [DOI] [PubMed] [Google Scholar]
- 67.Kim SR, Choi KH, Song YB, Lee JM, Park TK, Yang JH, Hahn JY, Choi JH, Choi SH and Gwon HC. Effect of sarpogrelate and high-dose statin on the reduction of coronary spasm in vasospastic angina: A two by two factorial, pilot randomized study. Clin Cardiol. 2019;42:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ishii M, Kaikita K, Sato K, Yamanaga K, Miyazaki T, Akasaka T, Tabata N, Arima Y, Sueta D, Sakamoto K, Yamamoto E, Tsujita K, Yamamuro M, Kojima S, Soejima H, Hokimoto S, Matsui K and Ogawa H. Impact of statin therapy on clinical outcome in patients with coronary spasm. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M and Takeshita A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation. 2002;105:1545–7. [DOI] [PubMed] [Google Scholar]
- 70.Shin ES, Lee JH, Yoo SY, Park Y, Hong YJ, Kim MH, Lee JY, Nam CW, Tahk SJ, Kim JS, Jeong YH, Lee CW, Shin HK and Kim JH. A randomised, multicentre, double blind, placebo controlled trial to evaluate the efficacy and safety of cilostazol in patients with vasospastic angina. Heart. 2014;100:1531–6. [DOI] [PubMed] [Google Scholar]
- 71.Teragawa H, Kato M, Yamagata T, Matsuura H and Kajiyama G. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest. 2000;118:1690–5. [DOI] [PubMed] [Google Scholar]
- 72.Ford CL and Zlabek JA. Nicotine replacement therapy and cardiovascular disease. Mayo Clin Proc. 2005;80:652–6. [DOI] [PubMed] [Google Scholar]
- 73.Benowitz NL, Pipe A, West R, Hays JT, Tonstad S, McRae T, Lawrence D, St Aubin L and Anthenelli RM. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Intern Med. 2018;178:622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cardona-Guarache R, Pozen J, Jahangiri A, Koneru J, Shepard R, Roberts C, Abbate A and Cassano A. Thoracic sympathectomy for severe refractory multivessel coronary artery spasm. Am J Cardiol. 2016;117:159–61. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka A, Shimada K, Tearney GJ, Kitabata H, Taguchi H, Fukuda S, Kashiwagi M, Kubo T, Takarada S, Hirata K, Mizukoshi M, Yoshikawa J, Bouma BE and Akasaka T. Conformational change in coronary artery structure assessed by optical coherence tomography in patients with vasospastic angina. J Am Coll Cardiol. 2011;58:1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]