Abstract
In some countries, excessive non-measles-related mortality has been observed among female recipients of high-titer measles vaccines. We determined if differences in the immune response to measles vaccines underlie the excessive female mortality by measuring the measles virus (MV)-specific antibody-dependent cellular cytotoxicity (ADCC) antibody response in 65 3-year-old Gambian children immunized with Edmonston-Zagreb medium-titer (EZ) or Schwarz standard vaccines during infancy. Among the 20 females and 22 males with undetectable anti-MV antibodies at the time of immunization, females had significantly lower ADCC than males (median cytotoxicities of 1/100 serum dilutions = 8.4 and 12%, respectively; P = 0.04). This sex-associated difference was present only among the six female and seven male recipients of EZ vaccine (median cytotoxicities = 5.1 and 19.0%, respectively; P = 0.02). There were no significant sex-associated differences in neutralizing antibody activity. Decreased ADCC antibody activity may contribute to the lower survival rate observed in females receiving high-titer measles vaccination.
High-titer measles vaccines (≥104.7 PFU/dose) are more immunogenic than standard vaccines when given to 4- to 6-month-old infants, even in the presence of maternal antibodies (13). Since measles case fatality rates in developing countries are highest between 4 and 12 months of age, a strategy of early vaccination with high-titer vaccines could prevent measles-associated deaths (6). However, excessive non-measles-related mortality among female recipients of high-titer vaccines in Senegal, Guinea Bissau, and Haiti (1, 2, 8) has caused concern among public health experts and has led to a moratorium on the further use of these vaccines (15).
Although the mechanism of sex-related mortality following high-titer immunization is unknown, it has been postulated that vaccine-induced, prolonged immunosuppression leads to increased susceptibility to disease. Both measles infection and immunization cause transient immunosuppression (9, 12), and measles case fatality rates may be highest among females (7). Thus, it is possible that the degree or length of immunosuppression resulting from either vaccine or wild-type infection may differ by sex and may account for the sex-related differences in mortality.
In acute measles infection, antibody-dependent cellular cytotoxicity (ADCC) antibody titers have been correlated with a reduction in viremia and, along with virus neutralization, may play a role in recovery from measles (3). Additionally, young females have lower ADCC antibody titers during acute infection than either young males or older females (4).
In view of the potential role of ADCC in controlling viremia and the sex-specific differences in ADCC antibody responses to acute measles, we postulated that similar differences in the ADCC response to high-titer vaccines might occur and that such differences might account for the reduced survival among female recipients of high-titer vaccines. We therefore determined the measles virus (MV)-specific ADCC activity in the sera of Gambian children participating in a trial comparing measles vaccines of different titers.
(This work was presented in part at the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, September 1996 [S. Atabani, M. Steward, H. Whittle, and D. Forthal, abstr. H60].)
Sera were obtained from 65 of 183 children (28%) who had participated in a measles vaccine trial in the Gambia from January 1985 to July 1987 (13). Children were randomly assigned to receive either medium-titer (104.6-PFU/dose) Edmonston-Zagreb (EZ) vaccine subcutaneously at 4 months of age or standard (103.7-PFU/dose) Schwarz vaccine at 9 months of age. Frozen serum samples of appropriate quantity, obtained prior to immunization and at 36 months of age from 33 EZ and 32 Schwarz recipients, were chosen for further analysis. Thirty-five children were male and 30 were female.
A 4-h chromium-51 release assay was used to measure MV-specific ADCC-mediating antibodies (5). Raji cells persistently infected with a clinical strain of MV served as target cells; both F and H glycoproteins are expressed on the surfaces of these cells, as determined by live-cell immunofluorescent-antibody staining. Peripheral blood mononuclear cells, provided by a single healthy donor, were used as effector cells. Assays were performed at an effector/target ratio of 100:1. All serum samples were tested in triplicate at a dilution of 1:100 and the percent cytotoxicity was determined as described previously (5). MV-specific ADCC was expressed as the percent cytotoxicity obtained by subtracting the percent cytotoxicity with effector cells alone from that with serum and effector cells. MV-seronegative and -seropositive control sera were included in each run.
Neutralizing-antibody titers were measured by plaque neutralization assays of samples from 53 of the 65 study patients at 36 months of age (14). A hemagglutination inhibition assay (HIA) was used to measure preimmunization and postimmunization antibody titers (14). Data were analyzed by the Mann-Whitney U test and by calculation of Spearman's correlation coefficients.
MV-specific ADCC was similar in the 32 Schwarz and the 33 EZ vaccinees (Table 1) (P = 0.4). Furthermore, ADCC did not differ by sex, whether all subjects were analyzed together or recipients of each vaccine were analyzed separately (Table 1); when subjects of each sex were analyzed separately, ADCC activity did not differ according to vaccine type (Table 1).
TABLE 1.
Antibody levels in 3-year-old Gambian children immunized with EZ medium-titer (104.6-PFU/dose) vaccine at 4 months of age or Schwarz standard-titer (103.7-PFU/dose) vaccine at 9 months of age
| Vaccine and group | Median (range)
|
||
|---|---|---|---|
| % ADCCa | Reciprocal titer
|
||
| PNTb | HIAc | ||
| EZ | |||
| Males | 13.2 | 160 | 32 |
| Females | 9.9 | 160 | 96 |
| Total | 12.4 (4.1–37.1) | 160 (40–1,280) | 64 (8–4,096) |
| Schwarz | |||
| Males | 11.0 | 640 | 96 |
| Females | 10.9 | 1,280 | 128 |
| Total | 11.0 (5.8–21.7) | 640 (80–1,280) | 128 (8–4,096) |
65 samples were tested (15 from EZ females, 18 from EZ males, 15 from Schwarz females, and 17 from Schwarz males). Values reflect cytotoxicity obtained with sera at a 1:100 dilution.
53 samples were tested (13 from EZ females, 15 from EZ males, 11 from Schwarz females, and 14 from Schwarz males). PNT titers are significantly higher for Schwarz recipients than for EZ recipients (P < 0.001).
60 samples were tested (12 from EZ females, 17 from EZ males, 15 from Schwarz females, and 16 from Schwarz males).
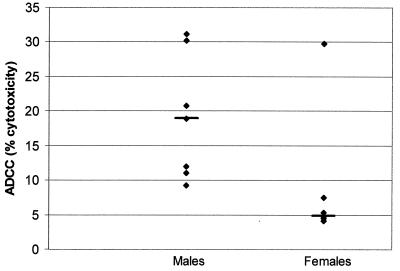
Since children with lower levels of preimmunization antibodies are potentially at greater risk for prolonged vaccine replication and hence more profound immunosuppression, particularly following high-titer immunization, a separate analysis was performed on serum samples from children with undetectable levels of preimmunization anti-MV antibodies. A significant difference in ADCC was observed between the 22 male and 20 female children with undetectable (<1:2) preimmunization HIA titers (median ADCC = 12.0% for males versus 8.4% for females; P = 0.04). On further analysis, it was found that this sex-associated difference was present only among the 7 males and 6 females who had undetectable preimmunization titers and who had received EZ vaccine (median = 19.0 and 5.1% for males and females, respectively; P = 0.02) (Fig. 1). On the other hand, no difference was noted between the 15 males and 14 females who had undetectable preimmunization anti-MV antibody titers and received Schwarz vaccine (median = 11.5 and 10.6% for males and females, respectively; P = 0.7).
FIG. 1.
MV-specific ADCC antibody in 3-year-old Gambian children with undetectable HAI antibodies at the time of vaccination with EZ vaccine at 4 months of age. ADCC antibody activity was measured in serum at a dilution of 1/100. Median values are depicted by short horizontal lines.
Fifty-three children were tested for MV-specific neutralizing antibody at 36 months of age by a plaque neutralization test (PNT). PNT titers correlated poorly with ADCC (r = 0.1; P = 0.4). As was the case with ADCC, there was no association between neutralizing-antibody titer and sex (Table 1). However, Schwarz vaccine recipients had higher PNT titers than EZ recipients (median titers = 160 and 640, respectively; P < 0.001; (Table 1). Among 11 EZ-immunized children with undetectable prevaccine antibody and a sufficient amount of serum (4 girls and 7 boys), neutralizing-antibody titers did not differ by sex (median titer = 1:160 for both boys and girls; P = 0.4).
MV-specific antibody levels measured by HIA in 60 post-immunization serum samples demonstrated no statistically significant differences either by vaccine type or by sex (Table 1). These 60 samples included 10 (4 girls and 6 boys) from EZ-immunized children with undetectable prevaccine antibody; among these 10, there was also no difference in HIA antibody levels by sex (median titer = 1:48 for boys and 1:96 for girls; P = 0.6).
This study demonstrates that among children with undetectable measles antibody at the time of immunization, females respond to EZ vaccine with a significantly lower ADCC antibody response than males. This sex-related difference in the ADCC response occurs uniquely among EZ vaccine recipients. However, since EZ vaccine was given at an earlier age than Schwarz vaccine, it is not possible to determine whether vaccine type, age at vaccination, or both contributed to the attenuated ADCC antibody response among girls.
Vaccine trials in Guinea-Bissau, Senegal, and Haiti have revealed lower survival rates among girls given high-titer measles vaccines than girls given standard vaccines, although not compared with unvaccinated children (1, 2, 8). Although vaccine strain-induced immunosuppression has been suggested as the underlying cause, only subtle, long-term sex-associated differences in cellular immune responses have been detected (10, 11). Although differences in survival were not observed in the Gambia, the factors associated with a low ADCC antibody response observed in our study—namely, female sex, high-titer vaccine, and early age—are the same as those associated with lower survival rates in other vaccine trials (1, 2, 8). Thus, differences in the ADCC response could underlie the observed difference in postimmunization mortality between females and males.
A similar reduction in ADCC antibody was demonstrated for children with acute measles under the age of 24 months compared with older children (4); in that study, the ADCC response was also lower in young girls than in boys of the same age. In addition, lower ADCC was associated with lymphopenia, a known risk factor for severe measles. The parallels between risk factors for low ADCC responses and risk factors for severe measles and, as shown in this study, for mortality following measles immunization strengthen the likelihood of an important role for ADCC in MV infection.
ADCC, like cytotoxic T lymphocyte activity, results in lysis of virus-infected cells and is thus a means of clearing intracellular infection. Deaths among female recipients of high-titer measles vaccines generally occurred months or years following immunization and were due to diarrheal and respiratory diseases (11). One explanation for this phenomenon is that female recipients of high-titer vaccines had longer or more profound periods of vaccine-induced immunosuppression than did recipients of standard vaccines or male recipients of high-titer vaccines. It is possible (though it is only a speculation) that a depressed ADCC antibody response to vaccination with a high dose of virus could result in a higher level or greater persistence of intracellular infection because of the reduced ability to lyse infected cells. The greater magnitude or duration of infection might then prolong or augment immunosuppression.
The ADCC antibody response was associated with sex only among EZ recipients who had undetectable measles antibody prior to immunization. An absence of maternal antibody may result in greater MV replication following immunization, putting these infants at greater risk for events associated with immunosuppression: an inadequate immune response to vaccine might exacerbate the immunosuppression. It is also possible that a large inoculum of vaccine virus, in the absence of maternal antibody, is responsible for a global immunosuppression that includes a depressed ADCC response and that differs between males and females. It should be noted, however, that MV-specific neutralizing and HIA antibody responses were not significantly different between male and female EZ recipients.
Although our findings suggest a link between ADCC antibody response and the lower survival rate among female high-titer vaccine recipients, a causal relationship cannot be established from this study. In addition to antibody, functioning effector cells are required for ADCC to occur, and effector function was not measured in this retrospective study. Furthermore, as ADCC was measured at a single time point at 3 years of age, we do not know how ADCC antibody levels evolved over time or whether during the period of greatest risk for excessive female mortality (up to 2 years postimmunization), ADCC antibody levels differed according to sex or vaccine type. Finally, the number of patients in this cross-sectional study was small; a larger, prospective study of ADCC and other immune functions—a study which will not be possible in view of the suspension of testing high-titer vaccines—would be necessary to establish the role of different immune parameters in vaccine-associated mortality.
REFERENCES
- 1.Aaby P, Knudsen K, Whittle H, et al. Long-term survival after Edmonston-Zagreb measles vaccination in Guinea-Bissau: increased female mortality rate. J Pediatr. 1993;122:904–908. doi: 10.1016/s0022-3476(09)90015-4. [DOI] [PubMed] [Google Scholar]
- 2.Aaby P, Samb B, Simondon F, et al. Sex-specific mortality after high-titre measles vaccines in rural Senegal. Bull W H O. 1994;72:761–770. [PMC free article] [PubMed] [Google Scholar]
- 3.Forthal D N, Landucci G, Habis A, Zartarian M, Katz J, Tilles J G. Measles-virus specific functional antibody responses and viremia during acute measles. J Infect Dis. 1994;169:1377–1380. doi: 10.1093/infdis/169.6.1377. [DOI] [PubMed] [Google Scholar]
- 4.Forthal D N, Landucci G, Habis A, Laxer M, Javato-Laxer M, Tilles J G, Janoff E N. Age, sex and household exposure are associated with the acute measles-specific antibody-dependent cellular cytotoxicity antibody response. J Infect Dis. 1995;172:1587–1591. doi: 10.1093/infdis/172.6.1587. [DOI] [PubMed] [Google Scholar]
- 5.Forthal D N, Landucci G, Katz J, Tilles J G. Comparison of measles virus-specific antibodies with antibody-dependent cellular cytotoxicity and neutralizing functions. J Infect Dis. 1993;168:1020–1023. doi: 10.1093/infdis/168.4.1020. [DOI] [PubMed] [Google Scholar]
- 6.Garenne M, Aaby P. Pattern of exposure and measles mortality in Senegal. J Infect Dis. 1990;161:1088–1094. doi: 10.1093/infdis/161.6.1088. [DOI] [PubMed] [Google Scholar]
- 7.Garenne M. Sex differences in measles mortality: a world review. Int J Epidemiol. 1994;23:632–642. doi: 10.1093/ije/23.3.632. [DOI] [PubMed] [Google Scholar]
- 8.Holt E A, Moulton L H, Siberry G K, Halsey N A. Differential mortality by measles vaccine titer and sex. J Infect Dis. 1993;168:1087–1096. doi: 10.1093/infdis/168.5.1087. [DOI] [PubMed] [Google Scholar]
- 9.Hussey G D, Goddard E A, Hughes J, et al. The effect of Edmonston-Zagreb and Schwarz measles vaccines on immune responses in infants. J Infect Dis. 1996;173:370–376. doi: 10.1093/infdis/173.6.1320. [DOI] [PubMed] [Google Scholar]
- 10.Leon M E, Ward B, Kanashiro R, et al. Immunologic parameters 2 years after high-titer measles immunisation in Peruvian children. J Infect Dis. 1993;168:1097–1104. doi: 10.1093/infdis/168.5.1097. [DOI] [PubMed] [Google Scholar]
- 11.Lisse I M, Aaby P, Knudsen K, Whittle H, Andersen H. Long-term impact of high titre EZ measles vaccine on T lymphocyte subsets. Pediatr Infect Dis J. 1994;13:109–112. doi: 10.1097/00006454-199402000-00006. [DOI] [PubMed] [Google Scholar]
- 12.McChesney M B, Oldstone M B A. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- 13.Whittle H, Hanlon P, O'Neill K, et al. Trial of high-dose Edmonston-Zagreb measles vaccine in the Gambia: antibody responses and side-effects. Lancet. 1988;ii:811–814. doi: 10.1016/s0140-6736(88)92781-x. [DOI] [PubMed] [Google Scholar]
- 14.Whittle H C, Campbell H, Rahman S, Armstrong J R. Antibody persistence in Gambian children after high-dose Edmonston-Zagreb measles vaccine. Lancet. 1990;336:1046–1048. doi: 10.1016/0140-6736(90)92501-8. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation Expanded Programme of Immunisation. Safety of high-titre measles vaccines. Wkly Epidemiol Rec. 1992;67:357–361. [PubMed] [Google Scholar]