Abstract
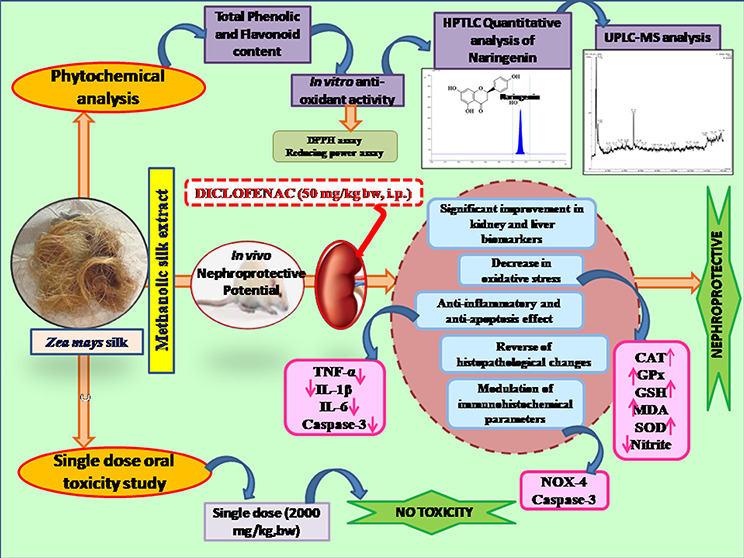
The lack of sufficient scientific evidence prompted the analytical investigation of nephroprotective potential of the silk extract of Zea mays L., which is traditionally and ethnomedicinally used for various disorders including kidney dysfunction. The present study was conducted to investigate the phytochemical analysis and demonstrate the nephroprotective potential of the methanolic silk extract of Z. mays L. using a rodent model. High-performance thin-layer chromatography (HPTLC) analysis was carried out to standardize the methanolic silk extract of Z. mays (ZME) using naringenin as a marker. The metabolite profiling of the ZME was carried out using ultrahigh-performance liquid chromatography mass spectrometry (UPLC-MS) on a monolithic capillary silica-based C18 column to identify bioactive compounds and for confirmation of the identified markers. Furthermore, for acute toxicity study, a single dose (2000 mg/kg bw) of the ZME was administered orally to Wistar rats. Also, nephrotoxicity was induced in Wistar rats by injecting diclofenac (DC) (50 mg/kg, bw, i.p.) at a single dose. The efficacy of the ZME as a nephroprotective agent was then evaluated at doses of 100, 200, and 400 mg/kg/day, bw, p.o. Furthermore, the kidney, liver, antioxidant, inflammatory, and apoptotic biochemical markers and histopathological and immunohistochemical alterations (caspase-3 and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-4 (NOX-4)) were evaluated. Phytochemical analysis by HPTLC and UPLC-MS revealed the presence of naringenin, vanillic acid, ferulic acid, gallic acid (GA), ellagic acid, quercetin, and morin, along with other bioactive constituents exhibiting multiple pharmacological properties. The acute toxicity study of the ZME showed no mortality or any clinical signs of toxicity through all the 14 days of the toxicity study at a dose of 2000 mg/kg. Also, administration of DC caused a significant elevation (P < 0.001) in kidney biochemical parameters and also caused oxidative, inflammatory, and apoptotic stress. Furthermore, DC also caused histopathological and immunohistochemical changes. Pretreatment with the ZME attenuated the elevated biochemical markers significantly at medium and high doses along with improvement in histopathological and immunohistochemical damages and showing comparable results to those of α-ketoanalogue. The present study verifies the traditional claims of Z. mays silk alleviating various kidney and related disorders by concluding the nephroprotective potential of the ZME. The nephroprotective activity of the ZME is attributed to the phytoconstituents present, acting as potent restoring antioxidants and preventing inflammatory and apoptotic cellular damages in rats. Thus, it holds promising potential in the management of nephrotoxicity.
1. Introduction
Herbs play a supreme role in the current drug development, which have been traditionally utilized in the treatment of various disorders. Presently, the popularity of herbal remedies is growing due to increasing side effects related to chemical agents administered for the treatment of diseases. Among various herbs, corn silk which is a famous traditional Chinese medicine and identified functional food is one of them.1Zea mays, an annual crop, ranks third highest grown in the world after wheat and rice. It is considered a major cereal in various parts of the world mainly Southern and Eastern Africa, Central America, and Mexico.2 It contains various minerals, vitamins, carbohydrates, proteins, saponins, tannins, alkaloids, volatile oils, steroids (sitosterol and stigmasterol), and flavonoids.3 Corn silk, the elongated stigma of the female flower of Z. mays, is a waste byproduct abundantly available in the world. Traditionally, corn silk extracts have been widely used in several parts of the world, mainly China, France, Turkey, and the United states. Various studies have reported the pleiotropic bioactivities of corn silk such as antioxidant, antidiabetic, antibacterial, antifatigue, antidepressant, anti-coagulant, anti-inflammatory, anti-obesity, and anticancer.4 Additionally, targeting corn silk for its nephroprotective potential will promote the use and development of this agricultural waste product.
Diclofenac (DC) is a potent nonsteroidal anti-inflammatory drug (NSAID) widely prescribed for anti-inflammatory, antirheumatic, antipyretic, and analgesic properties, mainly acting through cyclooxygenase (COX) inhibition.5 COX-1 and COX-2 are particularly expressed in the kidney, and inhibition of these was reported to cause oxidative stress and renal damage. DC (chemical formula: C14H10C12NO2) is a derivative of phenyl acetic acid and is lipophilic in nature.6 It produces the reactive metabolites N, 5-dihyrdoxy DC, and 5-hydroxy DC, which behave as reactive oxygen and nitrogen species, causes accumulation of reactive oxygen species (ROS) and oxidative stress in tissues, and further leads to oxidation of lipids, nucleic acids, and proteins.7 According to the World Health Organization (WHO) statistics, NSAIDs are the second most widely used drugs after acetaminophen.8 The increase in consumption leads to an increase in the public concern on the usage of NSAIDs such as aspirin, ibuprofen, and DC. At high concentrations, NSAIDs cause several kidney, liver, and colon cell-related adverse effects, which have been reported in multiple studies.9
Natural products, particularly medicinal plants, are considered a major source of new medicines. Herbal medicines prevent complications related to kidney disorders (KDs) and offer a potential alternative, since synthetic and semisynthetic molecules pose problems. Presently, phytochemicals have gained attention for their protective activity against nephrotoxicity. Medicinal plants provide a potential source of novel drug compounds, which is a sequel to the evidence that drugs derived from medicinal plants have made huge contributions to the health and well-being of humans. Medicinal plants have various types of phytoconstituents and are safe and highly effective in reducing nephrotoxic effects.10
These herbs are reported to contain phytocompounds, which have antioxidant, anti-inflammatory, and antiapoptotic properties. Recently, the study conducted by Wans and colleagues reported the nephroprotective activity of the methanolic extract of corn silk in acetaminophen-induced nephrotoxicity by normalizing the levels of creatinine, urea, malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in renal tissue. Also, the expressions of nuclear factor-κ B (NF-κb), caspase-3, proliferating cell nuclear antigen, and transforming growth factor β 1 have been modulated.
Thus, the corn silk extract reversed renal toxicity through its antioxidant, anti-inflammatory, and antiapoptotic protective potential.11 The aqueous corn silk extract, reported to contain phenols, flavonoids, alkaloids, saponins, tannins, and phytosterols, exhibits inhibiting potential against α-amylase and α-glucosidase activities, and by controlling the starch metabolism, it modulates the levels of postprandial hyperglycemia.12 Hetero-polysaccharide PSC2, obtained from corn silk, is reported to exhibit antidiabetic activity by reducing blood glucose and serum insulin levels.13 Another study reported the amelioration of acute systemic inflammation induced through bacterial endotoxin in mice via inhibition of NF-κB activation by peptide obtained from hydrolysis of trypsin of corn silk, FK2.14
Medicinal plants offer potential alternatives for the limited currently available therapeutic options in the treatment of kidney disorders (KDs). Various phytoconstituents including phenols, flavonoids, alkaloids, etc. showed nephroprotective characteristics, which becomes a major turning point. Z. mays possesses a wide spectrum of pharmacological activities particularly due to its potential in reversing oxidative, inflammatory, and apoptotic stress. However, limited investigations are available on the nephroprotective potential of corn silk. Focusing on this, the present study aimed toward the identification and metabolite profiling of bioactive compounds using high-performance thin-layer chromatography (HPTLC) and ultrahigh-performance liquid chromatography mass spectrometry (UPLC-MS) and its protective potential against DC-induced nephrotoxicity and elucidation of molecular mechanism for its nephroprotective potential.
2. Results
2.1. Total Phenolic (TPC) and Flavonoid Contents (TFC)
Phytochemicals, specifically phenols and flavonoids, are the most abundant and explored natural compounds due to the potential pharmacological activities as reported in many studies. The TPC and TFC were found to be 52.31 ± 1.4 mg of gallic acid (GA)/g dried weight and 23.09 ± 2.9 mg of rutin/g dried weight of the Z. mays silk extract (ZME), respectively.
2.2. In Vitro Antioxidant Activity
2.2.1. 2,2- Diphenyl-1-picrylhyrazyl (DPPH) Radical Scavenging Potential
The methanolic Z. mays silk extract (ZME) exhibited strong antioxidant activity comparable to that of ascorbic acid, indicated by its capacity to scavenge DPPH radicals. ZME exhibited significant inhibition of DPPH in a dose-dependent manner, and the scavenging potential of the ZME and ascorbic acid at 500 μg/mL was 79.32 ± 3.83 and 93.40 ± 4.61%, respectively, whereas IC50 of the ZME was found to be 325.8 ± 5.32 μg/mL. Comparison of the antioxidant potential of the ZME and standard is shown in Figure 1.
Figure 1.
In vitro antioxidant activity (n = 3). (A) Percentage DPPH free radical scavenging potential of the ZME and ascorbic acid (standard). (B) Reducing capacity of teh ZME and ascorbic acid (standard).
2.2.2. Reducing Power Activity
The ZME showed concentration-dependent activity in reducing ferricyanide (Fe3+) transformation to ferrocyanide (Fe2+) at a concentration range of 50–250 μg/mL indicated by the increase in the green color absorbance. A similar effect was recorded with ascorbic acid at the same concentration range. The reducing potential absorbance of the ZME and standard at a concentration of 1000 μg/mL was found to be 0.83 and 0.95, respectively, whereas the ZME showed an IC50 value of 335.1 ± 4.32 μg/mL. The graph (Figure 1) represents Fe3+ transformation to Fe2+ in the presence of the ZME and standard to illustrate reductive capability.
2.3. Quantitative Estimation of Naringenin in the ZME
Quantitative estimation of naringenin in the ZME was performed using HPTLC. The quantification estimation showed that the method selected for development and validation was linear, accurate, and precise in different concentrations of naringenin ranging from 50 to 4000 ng/mL. The linear regression calibration curve between area vs concentration was plotted and found to be linear for naringenin (R2 = 0.997). For the developed method, the average limit of detection (LOD) and limit of quantitation (LOQ) were found to be 27.37 ± 3.42 and 82.91 ± 5.38 ng/spot, respectively. The intraday and interday precision was quantified as percentage relative standard deviation (%RSD) and found to be 0.22–1.36 and 0.08–0.92, respectively. Furthermore, to determine the accuracy of the developed method, the percentage drug recovery by a percentage spiking of 0, 50, 100, and 150% of the standard to the sample was calculated. The percentage recovery for naringenin was found in the 71.9–111.8% range. The content of naringenin was found to be 5.55 ± 1.23 μg/mg of the sample. Figure S1 summarizes the HPTLC plate and the chromatogram.
2.4. Identification of Metabolites in the ZME by UPLC-MS
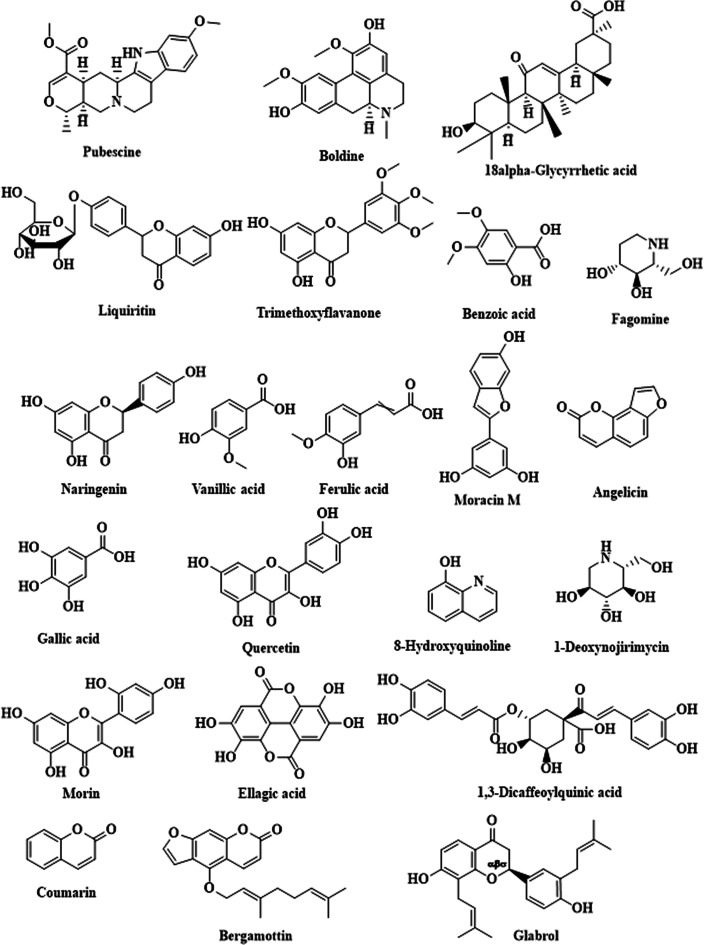
The bioactive compounds present in the ZME were identified using UPLC-MS. The tentatively identified components are summarized in Table 1 with the structures shown in Figure 2. The chromatogram of the ZME is given in Figure S2. The analysis revealed the presence of 22 phytochemicals that belong to different subclasses including phenols, flavonoids, alkaloids, etc. Major metabolites, namely naringenin, quercetin, morin, benzoic acid, ferulic acid, vanillic acid, gallic acid (GA), and ellagic acid, are enlisted based on the data of the observed peaks.
Table 1. Identified Metabolites in the Z. mays Extract Using UPLC-MS.
| compound no. | Rt | m/z theoretical | m/z practical | compound name | PubChem CID |
|---|---|---|---|---|---|
| 1 | 0.607 | 382.5 | 382.11 | pubescine | 72313 |
| 2 | 0.893 | 327.4 | 327.0448 | boldine | 10154 |
| 3 | 0.893 | 470.7 | 471.97 | 18α-glycyrrhetic acid | 73398 |
| 4 | 2.410 | 418.398 | 417.21 | liquiritin | 503737 |
| 5 | 4.247 | 314.34 | 314.00 | trimethoxyflavanone | 90690372 |
| 6 | 5.141 | 272.25 | 273.16 | naringenin | 439246 |
| 7 | 9.978 | 122.12 | 122.41 | benzoic acid | 243 |
| 8 | 9.978 | 168.04 | 169.93 | vanillic acid | 8468 |
| 9 | 9.978 | 194.18 | 195.14 | ferulic acid | 445858 |
| 10 | 11.529 | 170.12 | 170.12 | gallic acid | 370 |
| 11 | 11.529 | 186.168 | 186.95 | angelicin | 10658 |
| 12 | 11.529 | 302.23 | 301.20 | quercetin | 5280343 |
| 13 | 12.67 | 147.17 | 148.95 | fagomine | 72259 |
| 14 | 14.091 | 145.16 | 146.93 | 8-hydroxyquinoline | 1923 |
| 15 | 14.091 | 163.17 | 164.13 | 1-deoxynojirimycin | 29435 |
| 16 | 14.024 | 302.23 | 301.01 | morin | 5281670 |
| 17 | 14.024 | 302.19 | 301.01 | ellagic acid | 5281855 |
| 18 | 14.091 | 310.3 | 311.16 | moracin N | 641376 |
| 19 | 14.024 | 516.4 | 515.44 | 1,3-dicaffeoylquinic acid | 6474640 |
| 20 | 15.777 | 146.14 | 147.05 | coumarin | 323 |
| 21 | 15.777 | 338.4 | 338.45 | bergamottin | 5471349 |
| 22 | 15.777 | 392.495 | 393.39 | glabrol | 11596309 |
Figure 2.
Structures of metabolites identified in the ZME using UPLC-MS.
2.5. In Vivo Evaluation of the ZME
2.5.1. Acute Toxicity Study
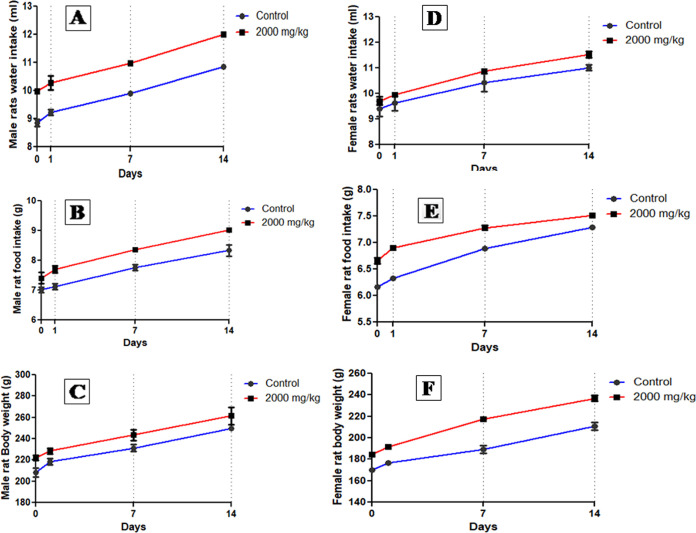
No mortality or adverse clinical signs were observed during the acute toxicity studies in rats. Also, no behavioral changes were recorded throughout the two-week study (Table 2). No significant differences were observed in the body weight of the control and treatment groups. The food and water consumption of female and male animals of the treatment group was found to be similar to that of the control group (Figure 3). Furthermore, Table 3 shows the absolute and relative organ weights of rats of both groups. Thus, the ZME is considered to be nontoxic by administration through the oral route.
Table 2. Cage Side Behavioral Observations for 14 days of Both Groups of Female and Male Rats (n = 10).
| observations
on different days |
|||||||
|---|---|---|---|---|---|---|---|
| female rats | male rats | ||||||
| s. no. | parameters | 0 | 7 | 14 | 0 | 7 | 14 |
| 1 | fur condition | normal | normal | normal | normal | normal | normal |
| 2 | skin change | no change | no change | no change | no change | no change | no change |
| 3 | color of the eye | normal | normal | normal | normal | normal | normal |
| 4 | posture of the body | normal | normal | normal | normal | normal | normal |
| 5 | respiration | normal | normal | normal | normal | normal | normal |
| 6 | sedation | no effect | no effect | no effect | no effect | no effect | no effect |
| 7 | drowsiness | not present | not present | not present | not present | not present | not present |
| 8 | mortality | alive | alive | alive | alive | alive | alive |
Figure 3.
Water intake, food intake, and body weight of male and female rats of control and ZME (2000 mg/kg bw)-administered rats (n = 10 rats/group).
Table 3. Absolute and Relative Organ Weights of Female and Male Rats (n = 10).
| Female
Rats | |||||
|---|---|---|---|---|---|
| liver | heart | lung | intestine | kidney | |
| Absolute Organ Weight (g) | |||||
| group 1 | 6.7 ± 0.3 | 0.3 ± 0.2 | 1.3 ± 0.2 | 6.1 ± 0.1 | 0.7 ± 0.3 |
| group 2 | 7.1 ± 0.2 | 0.5 ± 0.3 | 1.4 ± 0.3 | 6.4 ± 0.3 | 1.1 ± 0.1 |
| Relative Organ Weight (g/100 g, bw) | |||||
| group 1 | 3.1 ± 0.2 | 0.3 ± 0.3 | 1.0 ± 0.2 | 5.1 ± 0.1 | 0.6 ± 0.2 |
| group 2 | 3.5 ± 0.2 | 0.4 ± 0.2 | 1.2 ± 0.1 | 5.3 ± 0.4 | 1.2 ± 0.4 |
| Male Rats | |||||
|---|---|---|---|---|---|
| liver | heart | lung | intestine | kidney | |
| Absolute Organ Weight (g) | |||||
| group 1 | 7.3 ± 0.2 | 0.2 ± 0.2 | 1.2 ± 0.2 | 6.3 ± 0.1 | 0.8 ± 0.2 |
| group 2 | 7.5 ± 0.5 | 0.3 ± 0.3 | 1.3 ± 0.3 | 6.5 ± 0.3 | 1.2 ± 0.2 |
| Relative Organ Weight (g/100 g, bw) | |||||
| group 1 | 3.3 ± 0.1 | 0.3 ± 0.3 | 1.3 ± 0.4 | 5.4 ± 0.2 | 0.8 ± 0.4 |
| group 2 | 3.8 ± 0.2 | 0.4 ± 0.2 | 1.4 ± 0.4 | 5.5 ± 0.1 | 1.3 ± 0.1 |
2.5.2. Nephroprotective Potential of the ZME on Kidney and Liver Markers
DC-induced nephrotoxicity rats showed a significant elevation in kidney biomarkers in creatinine, uric acid, urea, and blood urea nitrogen (BUN) levels (P < 0.001). The ZME at different doses, specifically high dose (P < 0.001), was found to restore the levels of kidney markers, which were comparable to those of the toxic control (TC) group. Positive control (PC) groups and treated α-ketoanalogue (P < 0.001) rats also showed a normal range of kidney markers. Similar results were obtained for liver biomarkers, i.e., total protein, serum glutamic-oxalacetic transaminase (sGOT), serum glutamic–pyruvic transaminase (sGPT), albumin, globulin, direct bilirubin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) as shown in Table 4.
Table 4. Biochemical Parameters in Serum and Urine for Nephroprotective Activity of ZME against DC-Induced Nephrotoxicity (n = 8)a,b.
| animal
grouping |
||||||||
|---|---|---|---|---|---|---|---|---|
| parameters | NC | TC | T-LD | T-MD | T-HD | PC | ||
| Kidney Function Test | ||||||||
| creatinine (mg/dL) | serum | 0.83 ± 0.10 | 2.75 ± 0.20### | 2.55 ± 0.18NS | 1.73 ± 0.18*** | 0.97 ± 0.08** | 0.86 ± 0.13*** | |
| urine | 2.80 ± 0.19 | 0.5 ± 0.18## | 0.94 ± 0.04NS | 1.66 ± 0.30* | 2.60 ± 0.17** | 2.65 ± 0.12** | ||
| blood urea nitrogen (mg/dL) | serum | 32.92 ± 1.6 | 50.67 ± 2.5## | 42.26 ± 0.9NS | 37.50 ± 2.1* | 35.34 ± 1.1** | 34.47 ± 0.8** | |
| urea (mg/dL) | serum | 31.59 ± 1.0 | 52.30 ± 1.9### | 47.45 ± 0.8NS | 43.39 ± 1.1* | 33.04 ± 0.4*** | 31.96 ± 2.6*** | |
| urine | 67.55 ± 2.4 | 29.79 ± 1.4### | 37.69 ± 1.4NS | 50.59 ± 1.7** | 59.40 ± 2.2*** | 65.85 ± 1.5*** | ||
| uric acid (mg/dL) | serum | 2.44 ± 0.09 | 4.47 ± 0.3### | 3.60 ± 0.07* | 3.24 ± 0.08** | 2.89 ± 0.03*** | 2.63 ± 0.07*** | |
| urine | 5.72 ± 0.1 | 2.40 ± 0.12### | 2.87 ± 0.02NS | 3.86 ± 0.08** | 4.82 ± 0.15*** | 5.39 ± 0.27** | ||
| Liver Function Test | ||||||||
| total protein (g/dL) | serum | 6.08 ± 0.14 | 3.51 ± 0.13### | 4.07 ± 0.09NS | 5.05 ± 0.07** | 5.37 ± 0.25*** | 5.84 ± 0.10*** | |
| sGOT (U/L) | serum | 143.7 ± 2.8 | 276.4 ± 5.3### | 250.1 ± 9.0* | 228.2 ± 3.3*** | 152.8 ± 5.6*** | 137.1 ± 3.5*** | |
| sGPT (U/L) | serum | 43.87 ± 2.6 | 135.1 ± 3.4### | 109.1 ± 5.7* | 100.2 ± 6.1** | 41.52 ± 3.1*** | 37.15 ± 3.5*** | |
| albumin (g/dL) | serum | 9.54 ± 0.35 | 5.28 ± 0.05### | 6.43 ± 0.45 NS | 7.47 ± 0.20** | 8.29 ± 0.17** | 9.06 ± 0.08*** | |
| globulin (g/dL) | serum | 5.04 ± 0.18 | 8.34 ± 0.2### | 6.84 ± 0.27* | 6.17 ± 0.20** | 5.53 ± 0.19** | 5.55 ± 0.28** | |
| direct bilirubin (mg/dL) | serum | 0.02 ± 0.009 | 0.11 ± 0.008### | 0.09 ± 0.04NS | 0.06 ± 0.004* | 0.03 ± 0.001*** | 0.02 ± 0.04*** | |
| total bilirubin (mg/dL) | serum | 0.14 ± 0.02 | 0.30 ± 0.008## | 0.20 ± 0.07* | 0.17 ± 0.6** | 0.15 ± 0.01** | 0.14 ± 0.05** | |
| ALP (U/L) | serum | 141.7 ± 1.4 | 185.4 ± 2.2### | 172.7 ± 1.9NS | 162.6 ± 1.8*** | 150.8 ± 3.2*** | 142.0 ± 2.5*** | |
| ASAT (U/L) | serum | 173.8 ± 2.3 | 216.0 ± 2.4### | 201.8 ± 3.7 * | 195.9 ± 2.6** | 175.3 ± 1.7*** | 177 ± 1.4*** | |
| ALAT (U/L) | serum | 26.97 ± 1.3 | 71.74 ± 1.1### | 52.68 ± 2.9*** | 40.22 ± 2.9*** | 30.74 ± 1.8*** | 30.25 ± 0.7*** | |
Data are expressed as a means ± standard means error (n = 8 rats/group). One-way analysis of variance (ANOVA) was used for statistical analysis. [Normal control (NC), toxic control (TC), treatment with a low dose (T-LD), treatment with a medium dose (T-MD), treatment with a high dose (T-HD), and positive control (PC).]
TC was compared with NC, ###P < 0.001, ##P < 0.01, and #P < 0.05 and all the other groups were compared with TC ***P < 0.001, **P < 0.01, and *P < 0.05 considered significant and NS: nonsignificant.
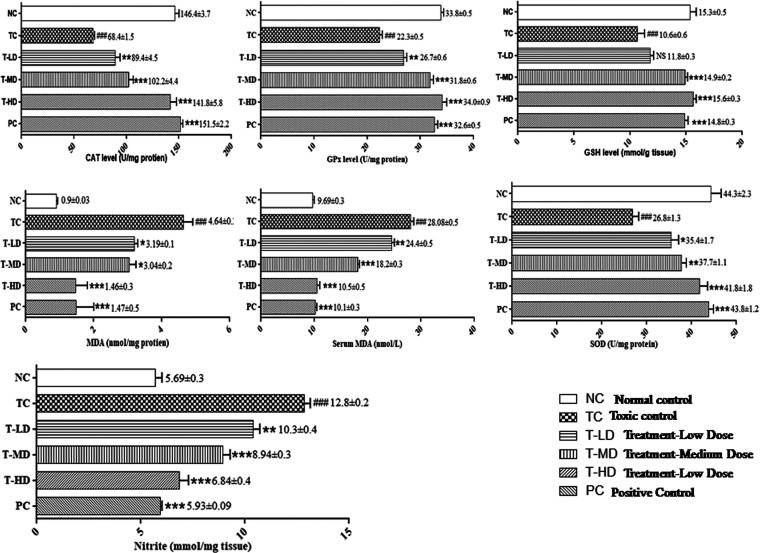
2.5.3. Protective Activity of the ZME in Renal Antioxidants of DC-Administered Rats
A significant alteration (P < 0.001) in the levels of renal antioxidants such as catalase (CAT), Gpx, glutathione (GSH), MDA, SOD, and nitrite was observed in DC-administered rats. The ZME at all doses, specifically at medium and high doses, was observed to normalize the elevated levels caused by DC. Also, the PC group was observed to possess a normal range of renal antioxidant parameters as shown in Figure 4 and Table S1.
Figure 4.
Antioxidant potential of the ZME in DC-induced nephrotoxicity. Data are expressed as a means ± standard means error (n = 8 rats/group). One-way ANOVA was used for statistical analysis. [TC was compared with NC, ###P < 0.001, ##P < 0.01, #P < 0.05 and all the other groups were compared with TC ***P < 0.001, **P < 0.01, and *P < 0.05 were considered significant and NS: nonsignificant].
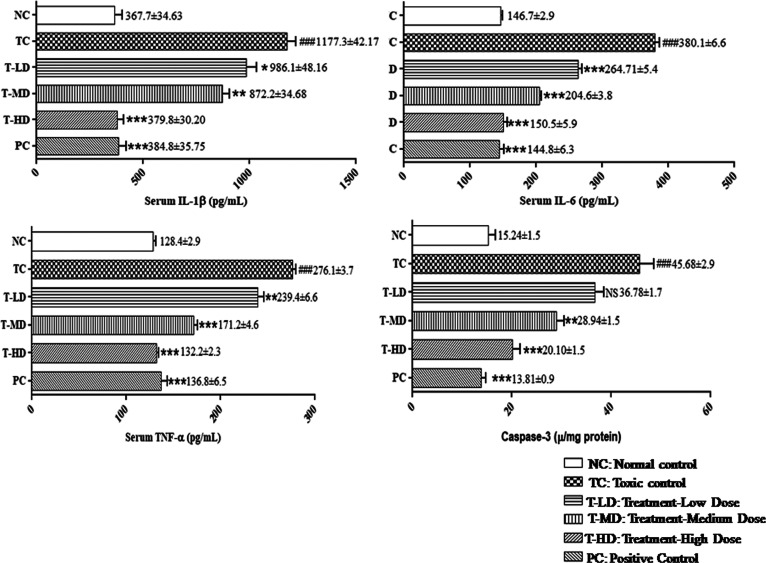
2.5.4. Nephroprotective Activity of the ZME in Cytokine Levels in DC-Administered Rats
DC-induced renal toxicity rats showed a significant elevation (P < 0.001) in the serum levels of tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β), interleukin-6 (IL-6), and caspase-3 levels. Rats with nephrotoxicity treated with the ZME were observed to have a normal range of cytokine markers, which was comparable to that of the normal control (NC) group. Furthermore, PC group rats possessed strong nephroprotective potentials shown in Figure 5 and Table S1.
Figure 5.
Protective potential of the ZME in DC-induced nephrotoxicity in IL-1β, IL-6, TNF-α, and caspase-3. Data are expressed as means ± standard mean error (n = 8 rats/group). One-way ANOVA was used for statistical analysis. [TC was compared with NC, ###P < 0.001, ##P < 0.01, and #P < 0.05 and all the other groups were compared with TC ***P < 0.001, **P < 0.01, and *P < 0.05 were considered significant and NS: nonsignificant].
2.5.5. Histopathological Evaluation
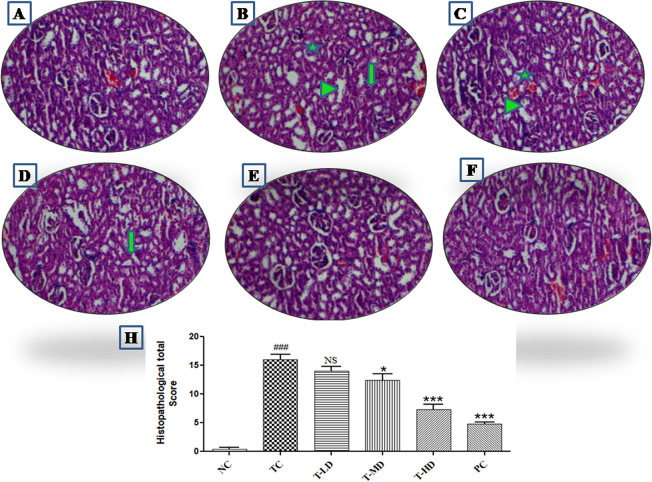
The section of the kidney in the NC group showed normal glomeruli and tubules along with no tissue necrosis and other deformities. DC-induced nephrotoxicity causes glomerulus shrinkage along with Bowman’s widening and remarkable cortical blood vessel congestion. Also, degeneration and vacuolation of tubules were recorded. The treatment group exhibited an improved pattern in glomeruli with improvement in cellularity and narrowing of Bowman’s space. Proximal and distal convoluted tubules were recorded to have a fine epithelial lining. Also, blood vessels are recorded to have mild congestion. In the treatment group, treatment with a low dose (T-LD) and treatment with a medium dose (T-MD) kidney sections showed mild tubular damage along with irregular contour and tubular vacuolization. Also, no abnormalities in the glomeruli were observed. Furthermore, T-HD and PC groups showed that the kidney sections regained the normal histoarchitecture as shown in Figure 6.
Figure 6.
Histopathological photomicrographs of kidney tissue (n = 8). (A) Kidney section showing the normal architecture of kidney in NC group rats. (B) Kidney sections of the TC group showing degeneration of the glomerulus (star), infiltration of the mononuclear cell or inflammation (arrow) and necrotic and degenerative tubular changes (arrow head). (C, D) Kidney sections of T-LD and T-MD depicting moderate lesions as compared to the TC group, i.e., (E)–(G) lesser intensities of changes in glomerular degeneration, inflammation, and necrotic and degenerative tubular changes as compared to the TC group i.e., (B, H) histopathological total score for kidney damage by semiquantitative estimation (NC: normal control, TC: toxic control, T-LD: treatment-low dose, T-MD: treatment-medium dose, T-HD: treatment-high dose, PC: positive control). [TC was compared with NC, ###P < 0.001, ##P < 0.01, #P < 0.05 and all the other groups were compared with TC ***P < 0.001, **P < 0.01, and *P < 0.05 considered significant and NS: nonsignificant].
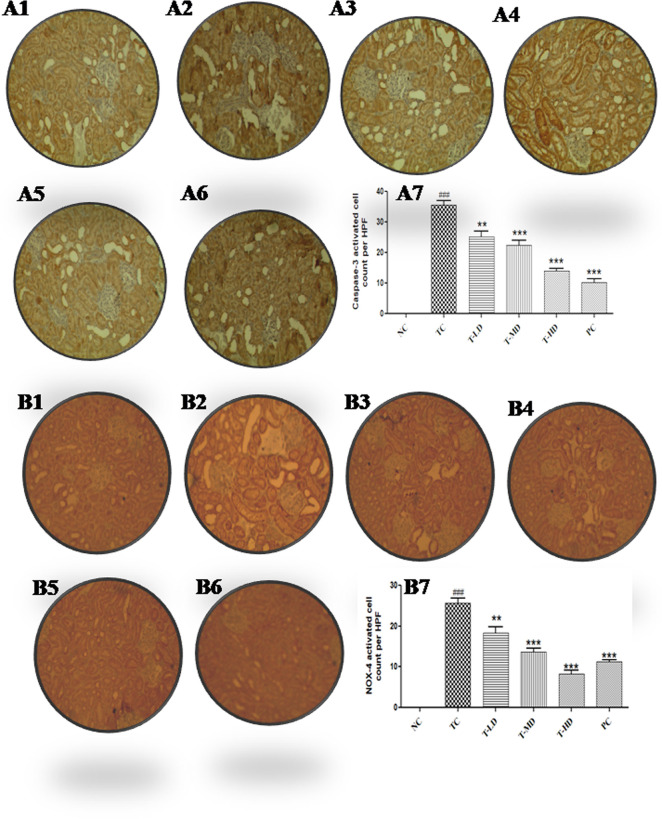
2.5.6. Immunohistochemistry Evaluation
IHC of the renal tissue of NC showed negative immunostaining for caspase-3 and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-4 (NOX-4) reaction, whereas TC strong immune staining of caspase-3 and NOX-4 as compared to that of group I was recorded. On the contrary, treatment with low and medium dose groups, i.e., T-LD and T-MD, displayed moderate reduction in caspase-3 immune reactivity in the rat renal tissue. Moreover, T-HD showed a significantly reduced immune reactivity of caspase-3 and NOX-4. The PC group showed a strong negative expression of caspase-3 and NOX-4 (Figure 7).
Figure 7.
Immunohistochemical staining of the renal tissue by caspase-3 and NOX-4 (n = 8). NC groups showing negative caspase-3 (A1) and NOX-4 (B1) reaction in the kidney sections. (A2) and (B2) showed higher reaction levels in caspase-3, NOX-4 expressions in TC as compared to the NC group. (A3, A4, B3) Moderate decrease in caspase-3, NOX-4 immune staining at T-LD and T-MD groups as compared to the TC group. (A5, B4, B5) Significant reduction in immune staining of caspase-3 and NOX-4 in T-MD and T-HD as compared to TC groups. (A6, B6) Strong negative caspase-3 and NOX-4 reaction in PC-1 and PC-2 groups relative to rats treated with DC alone. (A7, B7) Graphs representing the caspase-3 and NOX-4 activated cell counts in high-power field (NC: normal control, TC: toxic control, T-LD: treatment with a low dose, T-MD: treatment with a medium dose, T-HD: treatment with a high dose, PC: positive control). [TC was compared with NC, ###P < 0.001, ##P < 0.01, #P < 0.05 and all the other groups were compared with TC ***P < 0.001, **P < 0.01, and *P < 0.05 were considered significant and NS: nonsignificant].
3. Discussion
Medicinal plants have been used traditionally for centuries in the treatment of various diseases, playing a vital role in modern medicines. Medicinal plants are thought to act through multifunctional mechanisms because of the presence of various phytoconstituents that have multiple pharmacological properties. The major mechanism responsible for DC-induced nephrotoxicity is through deterioration of kidney functions evidently by oxidative, inflammatory, and apoptotic stress.15 Thus, it is presupposed that the use of medicinal plants with reported antioxidant and reversal of inflammatory and apoptotic stress minimize the DC-induced nephrotoxicity.16
The present study revealed the nephroprotective effect of the ZME against DC-induced nephrotoxicity in vitro and in vivo. Although Z. mays has been consumed by humans, no scientific studies have systematically examined its nephroprotective potential. In this study, the ZME was analyzed through HPTLC and UPLC-MS followed by demonstration of its nephroprotective activity and mechanism involved. Specifically, among different parts, silk has been reported to exert the best potential in reversing inflammatory stress.11 Also, the presence of different bioactive compounds lends a plethora of pharmacological actions such as antioxidant and antidiabetic activities.17
Natural products are known to be more complex compared to synthetic compounds and exhibit chemical diversity, thus occupying larger chemical space regions than synthetic origin drugs.18 In our study, the phytoconstituents have been analyzed that may be responsible for the nephroprotective potential of the ZME. The HPTLC fingerprint showed the presence of naringenin in the ZME. Furthermore, UPLC-MS analysis revealed the presence of bioactive compounds in the extract including naringenin, vanillic acid, ferulic acid, GA, ellagic acid, quercetin, morin, etc., which are reported to elicit strong nephroprotective properties through antioxidant, anti-inflammatory, and antiapoptotic effects. For instance, Ramos-Escudero and colleagues identifies eight phenolic compounds by high-performance liquid chromatography (HPLC) including naringenin, kaempferol, quercetin, rutin, ferulic acid, morin, caffeic acid, and cholorogenic acid in Z. mays L. by HPLC.19 Also, compounds including naringenin, kaempferol, apigenin, luteolin, aesculin, orientin, hyperoside, diosmin, etc. were identified in the ethanolic extract of corn silk by hybrid-quadrupole-TOF LC/MS/MS, which may be responsible for its hypouricemic effect.20 A plethora of research studies illustrated the protective action of various phytocompounds that are identified by UPLC-MS in kidney-related disorders.
Naringenin was reported to be present in the silk of Z. mays, which may be accountable for the nephroprotective activity of the ZME.21 Naringenin at doses of 50 and 100 mg/kg mitigated the streptozotocin-induced nephrotoxicity by modulating renal function biomarkers and antioxidant enzymes.22 Naringenin also showed hepato- and nephroprotective effects against cisplatin-induced toxicity by normalizing the levels of lipid peroxidation, BUN, creatinine, uric acid, and antioxidant enzymes namely SOD, CAT, and GPx.23
Similarly, vanillic acid, another identified compound, at doses of 50 and 100 mg/kg was reported to exhibit nephroprotective property through reversing oxido-inflammatory stress and necroptosis in the kidney tissue mainly due antioxidant and anti-inflammatory effects, thus ameliorateing kidney dysfunction in streptozotocin-induced diabetic nephropathy in in vivo.(24) Another study highlighted the nephroprotective action of vanillic acid in cisplatin-induced nephrotoxicity through depletion of the antioxidant defense system.25 Also, ferulic acid which is found in various medicinal plants reduces the inflammation in gentamicin-induced nephrotoxicity, causing reduction in oxidative stress, eliciting protective potential for kidneys. The ferulic acid significantly modulated the levels of serum creatinine, urea, and uric acid, and oxidative and inflammatory markers including MDA, GSH, SOD, CAT, advanced oxidized protein products (AOPP), IL-6, and TNF-α.26 Ferulic acid in in vitro studies reduces cisplatin-induced apoptosis and increases the overall cell viability of human kidney-2 cells. Also, improvement in the renal proximal tubular epithelial cell regeneration capacity by stimulation of proliferation and motility and lowering the amount of collagen synthesis has been reported. Thus, ferulic acid showed promising nephroprotective potential mainly via prevention of dedifferentiation processes through the β-catenin pathway.27
Various studies approved the effective potential of GA in nephrotoxicity. The ameliorative effect of GA against paraquat-induced kidney injury and oxidative stress at doses of 25, 50, and 100 mg/kg has been reported. It modulated the levels of vitamin C, SOD, CAT, MDA, protein carbonyl, creatinine, urea, uric acid, sGPT, sGOT, and IL-1β, thus alleviating the paraquat noxious effect.28 Another recent study conducted by Moradi and colleagues reported the nephroprotective potential of GA through antioxidative and -inflammatory stress against DC-induced kidney injury in rodents.15
Quercetin, a flavonoid ubiquitously present in medicinal plants, has been reported to exhibit a strong antioxidant potential. Pretreatment of kidney tissue with quercetin against valproic acid-induced nephrotoxicity reported renoprotective and antioxidant action.29 Ellagic acid has also been reported to exhibit a strong renoprotective potential through free radical scavenging, anti-inflammatory, and antiapoptotic activities.30,31 Recently, Neamatallah and colleagues elucidated the possible nephroprotective mechanism of ellagic acid through downregulation of OAT (1 and 3) in cisplatin-induced nephrotoxicity.
Morin, a flavonoid with properties of reversing oxidative, inflammatory, and apoptotic stress, is a constituent present in many medicinal plants reported to exhibit nephroprotective potential against gentamicin-, imipenem-, and streptozotocin-induced nephrotoxicity.32 Other phytochemicals including benzoic acid, glabrol, liquiritin, boldine, angelicin, and coumarin, present in various medicinal plants and herbs, have been reported to exhibit nephroprotective potential in in vitro, in vivo, and in silico studies. Thus, the nephroprotective activity of ZME is attributed to properties and mechanism of action of the bioactive compounds present.
Due to the escalating use of plant products, toxicity studies are essential to collect data regarding the safety of their use for further development as phytopharmaceutical products. An acute toxicity study which is exposure to the chemical for <24 h is performed to assess the qualitative property of the plant extract.33 Based on the result of acute toxicity studies, the as-observed adverse effect level of the ZME was found to be 2000 mg/kg/day. The liver, heart, lung, intestine, and kidney are the primary organs that are affected by any toxicant. In gross observation, no changes were observed in systemic organs in control and treatment groups. It helps in identifying the level of dose at which rats are expected to survive. The extract showed no acute toxicity. The previous study has reported that the corn silk extract showed no significant toxic effects on rodents in both acute and subacute toxicity, and LD50 was estimated to be more than 2000 mg/kg.34
Z. mays L. silk is used traditionally in treating kidney diseases and exhibits strong free radical scavenging and antioxidant activities.35 It is also used as a herbal medicine in China and other countries and exhibits multiple pharmacological activities. DC is considered a good NSAID and one of the most frequently prescribed drugs. At therapeutic doses, DC is considered safe, but when administered for a longer duration or at high doses, it damages the kidney and causes nephrotoxicity.
In the present study, DC administration at a dose of 50 mg/kg, bw, i.p. for seven days to Wistar rats induced impairment in kidney and liver functions. However, levels of kidney and liver marker concentrations significantly fluctuated compared with the values of the corresponding control group, which is in agreement with the previously reported data.15 Creatinine, BUN, urea, and uric acid are kidney enzyme markers, which are evaluated to examine the renal function and other related diseases. In our study, elevation in the levels of kidney biomarkers caused by DC-induced toxicity confirms a KD and other disorders such as urinary obstruction, arthritis, etc. It was proven that renal markers that are elevated are normalized using the ZME. Furthermore, the obtained data declared significant improvement in the levels of total protein, albumin, and globulin in T-LD, T-MD, T-HD group as compared to that in the TC and NC groups. Similar to our results, DC administration was associated with fluctuated transaminases (ALP, ASAT, ALAT), widely used liver injury markers.15 DC-induced liver injury may be correlated to metabolic digression and hypersensitivity, which produces acute liver damage.36
Also, DC administration evoked an increase in the levels of MDA and a significant decrease in the concentrations of GSH and CAT. These results are supported by previous studies, which suggested that DC-caused nephrotoxicity which results from ROS production leads to oxidative stress. Our results are in line with the previously reported outcome, which stated that DC metabolism induces ROS, which leads to oxidative reactions, further resulting in renal tissue damage. The increase in SOD and MDA levels in the kidney tissue indicates peroxidative damage, which causes cell damage in the renal tissue.37 Thus, the current study examined the renoprotective effect of the ZME against DC-induced renal damage.
In our study, the ZME reversed the nephrotoxicity produced by DC, which could be due to the antioxidant effect represented by modulation of MDA, GSH, CAT, SOD, and ROS levels. The obtained results are in parallel with those of the previous study conducted.38 The antioxidant activity of the ZME may be attributed to the bioactive compounds, mainly phenols and flavonoid present (Table 1), as stated by Liu and colleagues that phenols and flavonoid are responsible for the main antioxidant activity of corn silk.39
Lastly, in addition to the antioxidant activity of the ZME, it also exhibits potential for maintaining the levels of IL-6, IL-1β, TNF-α, and caspase-3. DC-induced nephrotoxicity rats showed an increase in the levels of IL-6, which is known to actively mediate the responses of the acute phase and controls the level of IL-10 and anti-inflammatory ILs. Also, an elevated level of IL-6 indicates increased regeneration and proliferation of cells; similar effects were seen in the elevated levels of IL-1β, and TNF-α. The ZME was observed to maintain IL-6 levels, which prove its potential in downplaying the regeneration and proliferation of cells. Similar to our observation, elevated levels of IL-6 were observed in previous studies.11 IL-1β mediates cellular inflammation, such as apoptosis, proliferation, and differentiation. ZME-treated rats were able to decrease the elevated levels of TNF-α and IL-1β, which evidences nephroprotective activity. Thus, the reversal of the effect of DC on IL-6, IL-1β, and TNF-α may evidence the potential of the ZME, which is parallel to the previous results.11 On the other hand, the ZME potential in reversing the inflammation can be explained from polyphenolic constituents and the tannin content present.40
Further, caspases are the most important apoptosis mediators. Caspase-3 is considered a hallmark of apoptosis, which leads to the activation of death protease and stimulates specific cleavage of cellular proteins. Thus, modulation of it is considered important in many ways. The study showed that DC caused an increased caspase-3 expression in renal tubules as compared to that in the NC group, which matches with the previous study, which proved that DC-induced nephrotoxicity leads to significant cell apoptosis in the kidney tubules.16 In contrast, the ZME reverse this action by reducing the caspase-3 expression in the renal tubules, which is in agreement with Wans and colleagues.11
Biochemical results are in line and explain the histopathological and IHC observations of DC-induced renal damage. Histopathological figures depicted glomerular outline distortion, expansion of Bowman’s space, and mislaying of brush borders of renal tubules (both proximal and distal) which is similar to the previous histopathological observation.41 The ZME reversed the histopathological abnormalities and brought it near normal characteristics.11
Furthermore, IHC study was conducted to detect the oxidant and apoptotic markers in the renal tissue. NOX4, a NADPH oxidase, has been reported to contribute in the redox processes of multiple kidney diseases including kidney injury, obstructive nephropathy, renal cell carcinoma, and other renal diseases through activation of multiple signaling pathways.42 As an effective nephroprotective agent, ZME administration markedly alleviated DC-induced nephrotoxicity by modulating the levels of NOX4, and caspase-3. The examination of DC-treated sections using NOX4 and caspase-3 showed positive immunoreactivity with dense cytoplasmic expression in the renal tubules, which is in agreement with previous studies.43,44 Thus, the present investigation showed that rats treated with ZME regained its normal biochemical levels and pathological changes induced by administration of DC.
4. Conclusions
In conclusion, the silk extract of Z. mays exhibited a beneficial effect on DC-induced nephrotoxicity and validated its traditional use in kidney and related disorders. Also, the presence of phytoconstituents with the potential to improve oxidative and inflammatory stress confirmed by HPTLC and UPLC-MS may attribute to the nephroprotective effect of the ZME. No toxicity was observed with the ZME. The study recorded that biochemical parameters and histopathological and ICH pictures of the renal tissue of Wistar rats treated with ZME regained almost its normal levels. Thus, the study suggests that the ZME possesses a strong nephroprotective effect, which may be attributed to its antioxidative defense mechanism and its ability to modulate inflammatory and apoptotic properties. The overall results of the present work lend the baseline data for the possible nephroprotective potential of Z. mays. However, studies are required to confirm its efficacy through safety and clinical evaluation, which will further enhance its beneficial therapeutic role for human health. Furthermore, investigations are needed to evaluate the molecular mechanism responsible for its nephroprotective role.
5. Materials and Methods
5.1. Chemical and Reagents
DC was purchased from Troikaa Pharmaceuticals Ltd., enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-1β, and caspase-3 were obtained from Abbkine (China), GA and naringenin were procured from Sigma-Aldrich, and other solvents of analytical grade were used.
5.2. Preparation of the Extract
The plant material was collected from a local market in New Delhi, India, and authentication was carried out. The authenticated plant material was further deposited to Bioactive Natural Product Laboratory, Jamia Hamdard, New Delhi, with the specimen number BNPLJH/PhD/10/19/02 for further reference. For extraction, the dried silk material was crushed into powder followed by overnight maceration in methanol in 1:10 (w/v) ratio. The macerated material was sonicated for 30 min at a temperature of 50 °C. The extract using muslin cloth and Whatman filter paper no-1 was filtered, and the remnants were evaporated under reduced pressure to dryness to prepare a fine ZME. The percentage yield of the extract was calculated and stored at 4 °C for further analysis.
5.3. Total Phenolic (TPC) and Flavonoid Contents (TFC)
The TPC in the ZME was determined using the Folin–Ciocalteu method with slight modifications.45 The stock solution (5 mg/mL) of the ZME was prepared. Furthermore, 0.2 mL of the test sample and Folin–Ciocalteu’s phenol reagent was mixed followed by addition of 0.6 mL of water and kept for 5 min. Saturated sodium carbonate (8% w/v) at 1 mL was added to the mixture, and the volume was made up to 3 mL using distilled water and kept for 30 min in the dark for reaction to proceed. Furthermore, the optical density was measured at 765 nm. The assay was performed in triplicate, and mean values were determined, The TPC was expressed as μg gallic acid equivalent per mg of PCE and CZE (μg of GAE/mg of extract).
The TFC in the ZME was determined using the aluminum chloride method with slight modifications.45 Initially, 5 mg/mL stock solution of the ZME was prepared. Furthermore, 0.6 mL of the sample and aluminum chloride (2%) were mixed and incubated at room temperature for an hour. The absorbance was measured at 420 nm against the blank. The assay was performed in triplicate, and mean values were determined. The TFC was expressed as μg rutin equivalent per mg of ZME (μg of RUT/mg of extract).
5.4. In Vitro Antioxidant Activity
5.4.1. DPPH Radical Scavenging Assay
The free radical scavenging potential of the ZME on DPPH radicals was performed as per the modified method previously determined.46 Ascorbic acid was adopted as a standard. Different concentrations of both the ZME and ascorbic acid were prepared. Then, 1 mL of the ZME sample or ascorbic acid followed by 3 mL of methanol and 0.5 mL of DPPH (1 mM) in methanol was added and kept for 10 min. A blank solution was prepared by adding 3 mL of methanol and 0.5 mL of DPPH. The absorption was observed using an UV spectrophotometer at 517 nm. The assay was performed in triplicate, and mean values were determined. The IC50 was calculated using GraphPad Prism Software, version 5.00, and also, the results were expressed in percentage inhibition of DPPH using the following equation
5.4.2. Determination of the Reducing Potential
The reducing potential of the ZME was evaluated by the previously performed method.47 Various different concentrations of the ZME were prepared, and equal volumes of the ZME samples were mixed followed by addition of 1.5 mL of 0.2 M phosphate buffer (pH 6.6) and 1.5 mL of 1% potassium ferricyanide. After 20 min of incubation at 50 °C, 1.5 mL of trichloroacetic acid (10% in distilled water) was added and centrifuged for 10 min at 3000 rpm. Furthermore, the upper layer was diluted with distilled water, and ferric chloride was added and centrifuged for 5 min at 3000 rpm. The assay was performed in triplicate, and mean values were determined. The absorbance was measured at 700 nm. The IC50 was calculated using GraphPad Prism Software, version 5.00.
5.5. Quantitative Estimation of Naringenin in the ZME
Naringenin was used as a marker compound for Z. mays and quantified in the ZME using HPTLC. ZME solution (30 mg/mL) and naringenin solution (1 mg/mL) were prepared in HPLC-grade methanol. The stock solution was then filtered, and ZME (6 μL) and naringenin (50–4000 ng/mL) were applied to precoated HPTLC plates, 10 × 10 cm2 (Merck, Germany), which were prewashed and activated with silica gel 60 F254, simultaneously with 6 mm band length with the help of an applicator Camag Linomat V (Camag, Switzerland). The spots were applied under a flow of nitrogen gas with automated pressure providing a speed of 150 nL/s from the syringe. HPTLC plates were allowed to develop vertically in a Camag twin trough glass chamber, which was previously saturated in the mobile phase for 30 min at room temperature in the linear ascending mode up to 80 mm. Multiple mobile phases with different ratios were employed for better metabolite separation present in the extract using the hit-and-trial method. However, the solvent system toluene, ethyl acetate, glacial acetic acid (6:3.5:0.5; v/v/v) as a mobile phase showed maximum separation of metabolites on the basis of optimal bands with compactness. The chamber was presaturated for the development of the TLC plate at room temperature and a relative humidity of 60 ± 5%. Furthermore, the quantification of naringenin was carried out at 540 nm using a Camag TLC scanner III (Camag, Switzerland) using software Wincats 1.2.3 with 6 × 0.45 mm2 slit dimension and 10 mm/s scanning speed.48
5.6. Metabolite Profiling of the ZME by UPLC-MS
The ZME (50 mg) was dissolved in methanol of LC-MS grade (5 mL) in a volumetric flask to obtain 10 mg/mL solution followed by filtration using a membrane filter of 0.2 μm. The solution was then diluted in a ratio of 1:10 using methanol. Then, high-throughput profiling of the extract was carried out using UPLC-MS to detect metabolites. The analysis was performed on an ACQUITY UPLC system of Waters equipped with an autosampler, binary solvent delivery system, column manager, and tunable MS detector, which is installed and controlled using Mass Lynx (Waters). All the data acquisition has been obtained in the positive mode. Chromatography was performed on a monolithic capillary silica-based C18 column using (A) acetonitrile and (B) water as the mobile phase at ambient temperature with a precolumn split ratio 1:5. Separation was obtained by the gradient elution mode, and 16 min was the total run time. The voltages of the cone and capillary were set to 40 and 3.0 kV, respectively. Argon was employed for collision at 5.3 × 10–5 Torr pressure. Lastly, accurate mass and composition for precursor ions were figured using software Mass Lynx v.4.1, which was incorporated in the instrument.48
5.7. In Vivo Studies
5.7.1. Acute Toxicity Study of Z. mays
The evaluation of acute toxicity of the extract is conducted according to the protocol of the Organization for Economic Cooperation and Development guidelines no. 420 on Wistar rats with slight modifications.49 All the animals were kept under fasting for 3–4 h, and to determine the orally administered dose expressed as mg/kg, the body weight of each animal is measured. Ten animals (five males and five females) were arbitrarily divided into two groups, namely control and experimental. Group I (control group) received distilled water orally, and group II received a single dose of ZME at 2000 mg/kg. After the administrations, the animals were observed systematically within the first four hours for any toxicity, and neither water nor food was given. Furthermore, for 14 days, the animals were observed once in a day for signs of toxicity including general behavioral changes, changes in the physical appearance, ingestion of food and water, respiration, and mortality. After 14 days, animals were fasted for 12 h and sacrificed. Organs including the liver, heart, lungs, intestine, and kidneys were removed and weighed, and the relative organ weight was calculated as per the following formula.50
5.7.2. Nephroprotective Study
The animals were procured from the Central Animal House of Jamia Hamdard for in vivo experimentation approved by the Institutional Animal Ethics Committee (IAEC), Jamia Hamdard, New Delhi (registration no. and date of registration 173/GO/RE/S/2000/CPCSEA and 28th January, 2000). The committee follows the guidelines of the Indian CPCSEA. 48 female Wistar Albino rats were kept under acclimatization housed in polypropylene cages for about a week under standard laboratory conditions with free access to commercial pellet diets and water ad libitium. The animals were maintained under controlled room temperature of 25 ± 2 °C and relative humidity of 55 ± 5%.
Albino Wistar female rats of 6–7 weeks weighing 175–200 g were allocated into six groups (Figure 8). The dose and time schedule of DC administration were adopted based on the previous report in the literature.15 The rats of group I received normal saline and served as a normal control (NC) group. Remaining groups received DC at 50 mg/kg, i.p., for 7 consecutive days. Group II served as a TC group. Groups III, IV, and V received (100, 200, and 400 mg/kg, p.o.) for all 7 days and served as a treatment group (T-LD, T-MD, and T-HD). Also, group VI served as a PC group and received α-ketoanalogue at 10 mg/kg p.o. (PC) on all 7 days. After 24 h of completion of dosing, the animals were anesthetized for the collection of blood followed by centrifugation to obtain serum at 2000 rpm for 10 min, which was used for various biochemical analyses. Furthermore, for oxidative parameter and histopathological and ICH examination, the animals were sacrificed for collection of the kidney.
Figure 8.
In vivo experimental design and protocol divided into six groups (n = 8) for 7 days to evaluate the nephroprotective activity of the ZME in DC-induced nephrotoxicity. [DC: diclofenac, NC: normal control, TC: toxic control, T-LD: treatment with a low dose, T-MD: treatment with a medium dose, T-HD: treatment with a high dose, PC: positive control, ZME: Z. mays silk extract].
5.7.3. Evaluation of Biochemical Analysis and Antioxidant Activities
The nephroprotective potential of the experimental Wistar rats was analyzed by evaluating the kidney function markers including creatinine, BUN, uric acid, and urea. The blood samples were collected and centrifuged at 2000 rpm for 15 min to obtain serum for analysis of creatinine, BUN, uric acid, and urea. Also, a urine sample was collected through metabolic cages from Wistar rats before sacrifice using commercial kits (Shanghai Korain Biotech Co., Ltd., Shanghai, China) according to the mentioned procedure for analysis of creatinine, urea, and uric acid. Also, liver function markers such as total protein, sGOT, sGPT, albumin, globulin, direct bilirubin, total bilirubin, AST, ALT, and ALP were analyzed from serum for evaluation of the protective activity of the ZME using commercial kits. For antioxidant assays, a small portion of the kidney tissue was homogenized in ice-cold phosphate-buffered saline (PBS) (0.1 M). CAT, GPx, MDA, and SOD were evaluated and expressed as protein (μm/mg protein), whereas GSH and nitrite were analyzed using the tissue sample and expressed as mmol/g tissue.
5.7.4. Nephroprotective Activity of the ZME on TNF-α, IL-1β, IL-6, and Caspase-3 on DC-Induced Nephrotoxicity
Serum samples were used for analysis of TNF-α, IL-1β, and IL-6 according to the standard protocols of commercial ELISA kits. Furthermore, from the homogenized renal sample, the supernatant was separated and used for evaluation of caspase-3 with the help of commercially available ELISA kits, expressed as μm/mg protein (Shanghai Korain Biotech Co., Ltd., Shanghai, China).
5.7.5. Histopathological Examination
Renal tissue obtained from rats was rinsed using PBS buffer and fixed in 10% formalin and Later stained with dye hematoxylin–eosin (H&E), and photographs were taken under a light microscope. The histopathological changes of the renal tissue were analyzed at 10 high power. The morphological changes in glomerular pathology, epithelial tubular cell damages, and brush border in cortical and medullar zone impairment were examined. Also, vacuoles present in the tubular cells were examined, and renal injury on the basis of complete morphological changes was allocated on a scale of 0–6 (0: normal, 0–2: mild, 2–4: moderate, 4–6: severe). Histopathological scores of all groups are illustrated and the results are depicted.51
5.7.6. Immunohistochemical (IHC) Examination
Caspase-3 and NOX4 IHC staining of renal sections was carried out as illustrated in an earlier method.43,44 Renal sections were allowed to be deparaffinized followed by rehydration by serial washes with graded alcohol. Furthermore, 3% hydrogen peroxide in methanol was used for inactivation of endogenous peroxidase for 30 min at 4 °C followed by washing using PBS. The slides were then blocked at 25 °C for 1 h using normal blocking serum. Thereafter, the slides were incubated with the primary antibody of anti-caspase-3. Later, the slides were exposed for 60 min to biotinylated goat antirabbit IgG anti-serum and washed with PBS. Finally, the slides were treated with streptavidin peroxidase conjugate for 30 min. The visualization of the reaction was done by treating the slides with 3,3′-diaminobenzidine tetrahydrochloride hydrogen peroxide solution at pH 7.0 for 3 min followed by washing in distilled water. The slides were counterstained using hematoxylin. Similarly, the rabbit polyclonal anti-NOX4 antibody are used for staining of renal sections
5.7.7. Statistical Analysis
The statistical analyses were performed using GraphPad Prism Software, version 5.00, software via two-way ANOVA using Tukey’s test. All the data were expressed as a mean ± standard error mean (SEM). The significance at P < 0.05 was considered =statistically significant.
Acknowledgments
The authors would like to acknowledge the Ministry of AYUSH, Govt of India, for providing the infrastructure facility to the Centre of Excellence in Unani Medicine (Pharmacognosy and Pharmacology), Jamia Hamdard, New Delhi, India, and University Grant Commission, New Delhi, India, for providing fellowship to Parakh Basist [Candidate ID: NFO-2018-19- OBC-DEL-79804].
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04396.
HPTLC plate view and chromatograms of the ZME; UPLC-MS chromatogram of the ZME; and protective potential of the ZME in DC-induced nephrotoxicity on antioxidant and IL-1β, IL-6, TNF-α, and caspase-3 parameters (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liu J.; Wang C.; Zhang T.; Liu J.; Lu S.; Zhang C.; Wang E.; Wang Z.; Zhang Y. Subchronic Toxicity Study of Corn Silk with Rats. J. Ethnopharmacol. 2011, 137, 36–43. 10.1016/j.jep.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Ranum P.; Peña-Rosas J. P.; Garcia-Casal M. N. Global Maize Production, Utilization, and Consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. 10.1111/nyas.12396. [DOI] [PubMed] [Google Scholar]
- Velazquez D. V. O.; Xavier H. S.; Batista J. E. M.; De Castro-Chaves C. Zea mays L. Extracts Modify Glomerular Function and Potassium Urinary Excretion in Conscious Rats. Phytomedicine 2005, 12, 363–369. 10.1016/j.phymed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Al-Oqail M. M.; Al-Sheddi E. S.; Farshori N. N.; Al-Massarani S. M.; Al-Turki E. A.; Ahmad J.; Al-Khedhairy A. A.; Siddiqui M. A. Corn Silk (Zea mays L.) Induced Apoptosis in Human Breast Cancer (MCF-7) Cells via the ROS-Mediated Mitochondrial Pathway. Oxid. Med. Cell. Longevity 2019, 2019, 9789241 10.1155/2019/9789241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaie M.; Forootanfar H.; Ghaseminejad A.; Salimi A.; Ameri A.; Doostmohammadi M.; Jafari E.; Rahimi H. R. Ondansetron Enhanced Diclofenac-Induced Nephrotoxicity in Mice. J. Biochem. Mol. Toxicol. 2019, 33, e22378 10.1002/jbt.22378. [DOI] [PubMed] [Google Scholar]
- Altman R.; Bosch B.; Brune K.; Patrignani P.; Young C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R.; Ponsoda X.; Jover R.; Gómez-Lechón M. J.; Castell J. V. Diclofenac Toxicity to Hepatocytes: A Role for Drug Metabolism in Cell Toxicity. J. Pharmacol. Exp. Ther. 1999, 288, 65–72. [PubMed] [Google Scholar]
- Soleimanpour M.; Imani F.; Safari S.; Sanaie S.; Soleimanpour H.; Ameli H.; Alavian S. M. The Role of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in the Treatment of Patients with Hepatic Disease: A Review Article. Anesthesiol. Pain Med. 2016, 6, e37822 10.5812/aapm.37822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. P.; Evan Prince S. Natural Remedies for Non-Steroidal Anti-Inflammatory Drug-Induced Toxicity. J. Appl. Toxicol. 2017, 37, 71–83. 10.1002/jat.3391. [DOI] [PubMed] [Google Scholar]
- Kpemissi M.; Eklu-Gadegbeku K.; Veerapur V. P.; Potârniche A. V.; Adi K.; Vijayakumar S.; Banakar S. M.; Thimmaiah N. V.; Metowogo K.; Aklikokou K. Antioxidant and Nephroprotection Activities of Combretum Micranthum: A Phytochemical, in-Vitro and Ex-Vivo Studies. Heliyon 2019, 5, e01365 10.1016/j.heliyon.2019.e01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wans E. M.; Ahmed M. M.; Mousa A. A.; Tahoun E. A.; Orabi S. H. Ameliorative Effects of Corn Silk Extract on Acetaminophen-Induced Renal Toxicity in Rats. Environ. Sci. Pollut. Res. 2021, 28, 1762–1774. 10.1007/s11356-020-10588-4. [DOI] [PubMed] [Google Scholar]
- Sabiu S.; O’Neill F. H.; Ashafa A. O. T. Kinetics of α-Amylase and α-Glucosidase Inhibitory Potential of Zea mays Linnaeus (Poaceae), Stigma maydis Aqueous Extract: An in Vitro Assessment. J. Ethnopharmacol. 2016, 183, 1–8. 10.1016/j.jep.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Pan Y.; Wang C.; Chen Z.; Li W.; Yuan G.; Chen H. Physicochemical Properties and Antidiabetic Effects of a Polysaccharide from Corn Silk in High-Fat Diet and Streptozotocin-Induced Diabetic Mice. Carbohydr. Polym. 2017, 164, 370–378. 10.1016/j.carbpol.2017.01.092. [DOI] [PubMed] [Google Scholar]
- Ho T. Y.; Li C. C.; Lo H. Y.; Chen F. Y.; Hsiang C. Y. Corn Silk Extract and Its Bioactive Peptide Ameliorated Lipopolysaccharide-Induced Inflammation in Mice via the Nuclear Factor-KB Signaling Pathway. J. Agric. Food Chem. 2017, 65, 759–768. 10.1021/acs.jafc.6b03327. [DOI] [PubMed] [Google Scholar]
- Moradi A.; Abolfathi M.; Javadian M.; Heidarian E.; Roshanmehr H.; Khaledi M.; Nouri A. Gallic Acid Exerts Nephroprotective, Anti-Oxidative Stress, and Anti-Inflammatory Effects Against Diclofenac-Induced Renal Injury in Malerats. Arch. Med. Res. 2021, 52, 380–388. 10.1016/j.arcmed.2020.12.005. [DOI] [PubMed] [Google Scholar]
- Jerine Peter S.; Evan Prince S. Diclofenac-Induced Renal Toxicity in Female Wistar Albino Rats Is Protected by the Pre-Treatment of Aqueous Leaves Extract of Madhuca longifolia through Suppression of Inflammation, Oxidative Stress and Cytokine Formation. Biomed. Pharmacother. 2018, 98, 45–51. 10.1016/j.biopha.2017.12.028. [DOI] [PubMed] [Google Scholar]
- Riaz M.; Nawaz S.; Ilyas I.; ur Rehman M. M.; Qadir R.; Mehmood T.; Afzal M.; Rehman N. A.; Ali A. Evaluation of Antidiabetic, Antioxidant, and Cytotoxic Potential of Maize (Zea mays L.) Husk Leaf Extracts. Cell. Mol. Biol. 2021, 67, 165–170. 10.14715/cmb/2021.67.1.25. [DOI] [PubMed] [Google Scholar]
- Akinyede K. A.; Oyewusi H. A.; Hughes G. D.; Ekpo O. E.; Oguntibeju O. O. In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum Petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus. Molecules 2022, 27, 155 10.3390/molecules27010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Escudero F.; Muñoz A. M.; Alvarado-Ortíz C.; Alvarado Á.; Yáñez J. A. Purple Corn (Zea mays L.) Phenolic Compounds Profile and Its Assessment as an Agent against Oxidative Stress in Isolated Mouse Organs. J. Med. Food 2012, 15, 206–215. 10.1089/jmf.2010.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L.; Bao Z.; Ma T.; Lin S. Hypouricemia Effects of Corn Silk Flavonoids in a Mouse Model of Potassium Oxonated-Induced Hyperuricemia. J. Food Biochem. 2021, 45, e13856 10.1111/jfbc.13856. [DOI] [PubMed] [Google Scholar]
- Duru C. E. Mineral and Phytochemical Evaluation of Zea mays Husk. Sci. Afr. 2020, 7, e00224 10.1016/j.sciaf.2019.e00224. [DOI] [Google Scholar]
- Rajappa R.; Magesh S. B.; Sarvajayakesavulu S.; Madhunapantula S. V. Nephroprotective Effect of Naringenin against Multiple Low Dose Streptozotocin (MLDSTZ) Induced Renal Damage in Mice. Biomed. Pharmacol. J. 2017, 10, 583–593. 10.13005/bpj/1145. [DOI] [Google Scholar]
- Rajamani S.; Kalyanasundaram G.; Sengodan T.; Thangavelu S.; Shanmukhan N. K.; Radhakrishnan A. Hepato & Nephro Protective Effects of Naringenin-Loaded Tpgs Polymeric Nanosuspension against Cisplatin-Induced Toxicity. Int. J. Res. Pharm. Sci. 2019, 10, 2755. 10.26452/ijrps.v10i4.1544. [DOI] [Google Scholar]
- Kumari S.; Kamboj A.; Wanjari M.; Sharma A. K. Nephroprotective Effect of Vanillic Acid in STZ-Induced Diabetic Rats. J. Diabetes Metab. Disord. 2021, 20, 571–582. 10.1007/s40200-021-00782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhu G.; Nishanthi E.; Sharmila R. Nephroprotective Effect of Vanillic Acid against Cisplatin Induced Nephrotoxicity in Wistar Rats: A Biochemical and Molecular Study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. 10.1016/j.etap.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Erseçkin V.; Mert H.; İrak K.; Yildirim S.; Mert N. Nephroprotective Effect of Ferulic Acid on Gentamicin-Induced Nephrotoxicity in Female Rats. Drug Chem. Toxicol. 2022, 45, 663–669. 10.1080/01480545.2020.1759620. [DOI] [PubMed] [Google Scholar]
- Bunel V.; Antoine M. H.; Nortier J.; Duez P.; Stévigny C. Nephroprotective Effects of Ferulic Acid, Z-Ligustilide and E-Ligustilide Isolated from Angelica sinensis against Cisplatin Toxicity in Vitro. Toxicol. In Vitro 2015, 29, 458–467. 10.1016/j.tiv.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Nouri A.; Heibati F.; Heidarian E. Gallic Acid Exerts Anti-Inflammatory, Anti-Oxidative Stress, and Nephroprotective Effects against Paraquat-Induced Renal Injury in Male Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1–9. 10.1007/s00210-020-01931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S.; Ganjoo P.; Raiusddin S.; Parvez S. Nephroprotective Activities of Quercetin with Potential Relevance to Oxidative Stress Induced by Valproic Acid. Protoplasma 2015, 252, 209–217. 10.1007/s00709-014-0670-8. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A.; Kulkarni V. H.; Chakraborty M.; Habbu P. V.; Ray A. Ellagic Acid Restored Lead-Induced Nephrotoxicity by Anti-Inflammatory, Anti-Apoptotic and Free Radical Scavenging Activities. Heliyon 2021, 7, e05921 10.1016/j.heliyon.2021.e05921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesan G.; Bharathi E. In Vivo Restoration of Hepatic and Nephro Protective Potential of Hesperidin and Ellagic Acid against Mercuric Chloride Intoxicated Rats. Biomed. Aging Pathol. 2014, 4, 219–222. 10.1016/j.biomag.2014.01.008. [DOI] [Google Scholar]
- Thakur K.; Zhu Y. Y.; Feng J. Y.; Zhang J. G.; Hu F.; Prasad C.; Wei Z. J. Morin as an Imminent Functional Food Ingredient: An Update on Its Enhanced Efficacy in the Treatment and Prevention of Metabolic Syndromes. Food Funct. 2020, 11, 8424–8443. 10.1039/d0fo01444c. [DOI] [PubMed] [Google Scholar]
- Kim H. Y.; Zuo G.; Lee S. K.; Lim S. S. Acute and Subchronic Toxicity Study of Nonpolar Extract of Licorice Roots in Mice. Food Sci. Nutr. 2020, 8, 2242–2250. 10.1002/fsn3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha A. W.; Kang H. J.; Kim S. L.; Kim M. H.; Kim W. K. Acute and Subacute Toxicity Evaluation of Corn Silk Extract. Prev. Nutr. Food Sci. 2018, 23, 70. 10.3746/pnf.2018.23.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehri G.; Derakhshanfar A.; Zadeh F. Y. Protective Effects of Corn Silk Extract Administration on Gentamicin-Induced Nephrotoxicity in Rat. Comp. Clin. Pathol. 2011, 20, 89–94. 10.1007/s00580-009-0943-3. [DOI] [Google Scholar]
- Hamza A. A. Curcuma longa, Glycyrrhiza glabra and Moringa oleifera Ameliorate Diclofenac-Induced Hepatoxicity in Rats. Am. J. Pharmacol. Toxicol. 2007, 2, 80–88. 10.3844/ajptsp.2007.80.88. [DOI] [Google Scholar]
- Hassan R. A.; Hozayen W. G.; Abo Sree H. T.; Al-Muzafar H. M.; Amin K. A.; Ahmed O. M. Naringin and Hesperidin Counteract Diclofenac-Induced Hepatotoxicity in Male Wistar Rats via Their Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Oxid. Med. Cell. Longevity 2021, 2021, 9990091 10.1155/2021/9990091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanideh N.; Zarifi F.; Rafiee S.; Khastkhodaei M.; Koohi Hosseinabadi O.; Tarkesh F.; Kherad Z.; Mojahed Taghi M.; Kamali M.; Shekarkhar G.; Jahromi M.; Zarifi F. Effect of Methanolic Extract of Corn Silk on Cisplatin-Induced Nephrotoxicity in Rats. Galen Med. J. 2018, 7, e1258 10.31661/gmj.v7i0.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Wang C.; Wang Z.; Zhang C.; Lu S.; Liu J. The Antioxidant and Free-Radical Scavenging Activities of Extract and Fractions from Corn Silk (Zea mays L.) and Related Flavone Glycosides. Food Chem. 2011, 126, 261–269. 10.1016/j.foodchem.2010.11.014. [DOI] [Google Scholar]
- Wang G. Q.; Xu T.; Bu X. M.; Liu B. Y. Anti-Inflammation Effects of Corn Silk in a Rat Model of Carrageenin-Induced Pleurisy. Inflammation 2012, 35, 822–827. 10.1007/s10753-011-9382-9. [DOI] [PubMed] [Google Scholar]
- Ahmed A. Y.; Gad A. M.; El-Raouf O. M. A. Curcumin Ameliorates Diclofenac Sodium-Induced Nephrotoxicity in Male Albino Rats. J. Biochem. Mol. Toxicol. 2017, 31, e21951 10.1002/jbt.21951. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Wu F.-r.; Wang J.-n.; Gao L.; Jiang L.; Li H. D.; Ma Q.; Liu X.-q.; Wei B.; Zhou L.; Wen J.; Ma T. t.; Li J.; Meng X. m. Nox4 in Renal Diseases: An Update. Free Radical Biol. Med. 2018, 124, 466–472. 10.1016/j.freeradbiomed.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Hashem K. S.; Abdelazem A. Z.; Mohammed M. A.; Nagi A. M.; Aboulhoda B. E.; Mohammed E. T.; Abdel-Daim M. M. Thymoquinone Alleviates Mitochondrial Viability and Apoptosis in Diclofenac-Induced Acute Kidney Injury (AKI) via Regulating Mfn2 and MiR-34a MRNA Expressions. Environ. Sci. Pollut. Res. 2021, 28, 10100–10113. 10.1007/s11356-020-11313-x. [DOI] [PubMed] [Google Scholar]
- Elshopakey G. E.; Elazab S. T. Cinnamon Aqueous Extract Attenuates Diclofenac Sodium and Oxytetracycline Mediated Hepato-Renal Toxicity and Modulates Oxidative Stress, Cell Apoptosis, and Inflammation in Male Albino Rats. Vet. Sci. 2021, 8, 9 10.3390/vetsci8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A. M. A.; El-Nour M. E. A. M.; Yagi S. M. Total Phenolic and Flavonoid Contents and Antioxidant Activity of Ginger (Zingiber Officinale Rosc.) Rhizome, Callus and Callus Treated with Some Elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. 10.1016/j.jgeb.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbe R. J.; Luka C. D.; Adoga G. I. Effect of Aqueous Ethanol Extract of Dialium Guineense Leaf on Diclofenac-Induced Oxidative Stress and Hepatorenal Injuries in Wistar Rats. Comp. Clin. Pathol. 2019, 28, 241–248. 10.1007/s00580-018-2822-2. [DOI] [Google Scholar]
- Sherikar A.; Mahanthesh M. Evaluation of Aqueous and Methanolic Extract of Leaves of Epipremnum Aureum for Radical Scavenging Activity by DPPH Method, Total Phenolic Content, Reducing Capacity Assay and FRAP Assay. J. Pharmacogn. Phytochem. 2015, 4, 36. [Google Scholar]
- Gaurav; Zahiruddin S.; Parveen B.; Ibrahim M.; Sharma I.; Sharma S.; Sharma A. K.; Parveen R.; Ahmad S. TLC-MS Bioautography-Based Identification of Free-Radical Scavenging, A-amylase, and A-glucosidase Inhibitor Compounds of Antidiabetic Tablet BGR-34. ACS Omega 2020, 5, 29688–29697. 10.1021/acsomega.0c02995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD . Test No. 420: Acute Oral Toxicity—Fixed Dose Procedure, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publications: Paris, 2001.
- Manaharan T.; Chakravarthi S.; Radhakrishnan A. K.; Palanisamy U. D. In Vivo Toxicity Evaluation of a Standardized Extract of Syzygium Aqueum Leaf. Toxicol. Rep. 2014, 1, 718–725. 10.1016/j.toxrep.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdou R. M.; El-Maadawy W. H.; Hassan M.; El-Dine R. S.; Aboushousha T.; El-Tanbouly N. D.; El-Sayed A. M. Nephroprotective Activity of Aframomum melegueta Seeds Extract against Diclofenac-Induced Acute Kidney Injury: A Mechanistic Study. J. Ethnopharmacol. 2021, 273, 113939 10.1016/j.jep.2021.113939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.