Abstract
During a nematological survey in Iraq, in the Bashika area, Ninevah province, an anguinid nematode population was isolated from galls of infected barley plants. The morphological characteristics indicated that the recovered species is identical to Anguina tritici. The barley population of A. tritici was molecularly characterized by sequencing two ribosomal regions (ITS and 18S rRNA genes), and their phylogenetic analyses revealed the newly generated sequences are in sister relation to corresponding sequences of A. tritici from wheat in the Bayesian tree, providing further evidence that the host plant can contribute to the separation of new isolates of plant parasitic nematodes.
Keywords: 18S rRNA gene, Anguina tritici, barley, identification, ITS, phylogeny, systematics, molecular biology, interaction
Anguina tritici (Steinbuch, 1799) Filipjev, 1936 is a highly specialized nematode with a narrow host range. It is commonly called wheat seed gall nematode as multiplication only occurs on wheat or closely related plants. It is worldwide distributed in all wheat-growing areas (Ozberk et al., 2011; EPPO, 2015). The nematode is considered a low-level risk pest because it is easy to detect and control and has been eliminated from many grain-growing areas. It has caused yield losses up to 70% to Secale cereale L. and 50% to Triticum aestivum L. (Anwar et al., 2001). However, the adoption of modern seed cleaning methods has contributed to almost elimination of this species from commercial wheat production in developed countries (Nicol and Rivoal, 2008; CABI, 2014). But its occurrence is still reported from North Africa, India, Iran, Turkey, Iraq, Bulgaria, and very recently, has known as a re-emergence pest in Pakistan (Bonjar et al., 2004; Nicol and Rivoal, 2008; Kausar and Khan, 2009; Mohamedova and Piperkova, 2013; Mukhtar et al., 2018). In Iraq, it has already reported from wheat-growing areas (Rao, 1921; Al-Bedawi et al., 1977; Ami and Taher, 2013; Ami et al., 2019), and its infection was estimated around 22.9%, causing 30% crop losses (Al-Beldawi et al., 1977; Stephan and Antoon, 1990). In the last decades, the wheat seed nematode was also found in Iraq, infecting barley with an infection rate up to 90%, suggesting the occurrence of a tentative new biotype of the nematode (Al-Talib et al., 1986; Mustafa, 2009; Ami et al., 2019; Qassem et al., 2021). The present study aims to characterize the recently recovered population of A. tritici from barley fields in Iraq by sequencing the ribosomal ITS and the partial 18S rRNA genes.
Materials and Methods
Sampling and nematode extraction
During 2019, six barley ears of the local barley cultivar were collected from two infected fields located in Shakhan and Bashika, Ninevah province (northern Iraq). Infected plants showed basal swelling of the stem, leaf distortion, thin and deformed ears, and spindle-shaped galls. Green and mature galls from barely spikes were dissected in petri dishes containing distilled water under a stereomicroscope. Different stages of the nematode were picked up on slides containing a drop of lactophenol, and then, measurements of eggs, J2, females, and males were taken under a light microscope.
Infected seeds were left in sterile nuclease-free water for 2 hr and then crashed to release emerging juveniles. From the crashed Bashika sample, three 25 ml aliquots containing emerged juveniles were transferred into 1.5-ml sterile tubes and conserved at -20°C. Molecular identification was carried out at the laboratory of IPSP-CNR in Bari, Italy.
DNA extraction, amplification, and sequencing
Ten individual specimens of the Bashika population were handpicked and placed on a glass slide in 3 ml of lysis buffer (10 mM Tris–HCl, pH 8.8, 50 mM KCl, 15 mM MgCl2, 0.1% Triton X100, 0.01% gelatine with 90 mg/ml proteinase K) and then cut into small pieces by using a sterilized syringe needle under a dissecting microscope (De Luca et al., 2004). The samples were incubated at 65°C for 1 hr and then at 95°C for 15 min to deactivate proteinase K. DNA purity and concentration were quantified using a NanoDropÔ spectrophotometer (ThermoFisher Scientific, MA, USA) and then was used for conventional PCR.
The two ribosomal regions were amplified by using the following sets of primers: (i) 18S ext (5'-TTGATTACGTCCCTGCCCTTT-3') and 28S ext (5'-TTTCACTCGCCGTTACTAAGG-3') for the amplification of the ITS region (Vrain et al., 1992); (ii) and 18SnF (5'-TGGATAACTGTGGTAATTCTAGAGC-3') and 18SnR (5'-TTACGACTTTTGCCCGGTTC-3') for the partial amplification of 18S rRNA gene (Kanzaki and Futai, 2002).
The size of amplification products was determined by comparison with the molecular marker Ladder 100 (Fermentas, St. Leon-Rot, Germany) following electrophoresis of 10 ml on 1.5% agarose gel. Purified ITS and 18S rRNA gene fragments were cloned in a TA cloning vector (Invitrogen, MA, USA), and two clones from each molecular marker were sequenced by MWG Eurofins (Germany). The newly obtained sequences were submitted to the GenBank database with the following accession numbers: ON146309-ON146310 for the ITS region and ON146324 for the 18S rRNA gene.
Phylogenetic analyses
Both ITS and 18 rRNA gene sequences obtained in this study were aligned with the corresponding ITS and 18S sequences (57 and six sequences, respectively) of anguinids deposited in the database. Sequence alignments were performed using MAFFT V. 7 software (Katoh et al., 2019) with default parameters and were manually edited using BioEdit (Hall, 1999) in order to remove the poorly aligned or ambiguous regions. Some species of the genus Ditylenchus Filipjev, 1936 were used as outgroup taxa (Subbotin et al., 2005; Mobasseri et al., 2017).
Phylogenetic analyses of both sequence datasets were based on Bayesian inference (BI) using MrBayes 3.1.2 (Ronquist and Huelsenbeck, 2003). The best-fit model of DNA evolution, a general time reversible model including among site heterogeneity (GTR + G + I), was selected using the Akaike information criterion (AIC) using JModelTest V.2.1.10 (Darriba et al., 2012) for both ITS and 18S datasets. The Markov chain Monte Carlo search was run with two default chains for 1,000,000 generations, sampled at every 100 trees. After discarding burn-in samples and evaluating convergence, the remaining samples were retained for in-depth analyses. Trees from all analyses were visualized using Dendroscope v3.2.8 (Huson and Scornavacca, 2012) and digitally drawn in CorelDRAW software version 2020.
Results and Discussion
Close similarities were observed among juveniles and females collected from barley in the northern Iraq with A. tritici attacking wheat in Iraq (Ami and Taher, 2013). The infected barley fields showed typical swelling of the stem, deformation of leaves, thin and deformed ears, and spindle-shaped galls. The harvested seeds were also infected with the nematode, appearing darker, spotted on the surface, and smaller in size. Ami et al. (2019) already described the presence of three biotypes of A. tritici in three legume plants with high infection rates: (i) durum wheat biotype, (ii) the bread wheat biotype, and (iii) barley biotype. In the current study, nematodes collected from infected seeds were molecularly identified in order to investigate the affinities of the recovered tentative barley biotype.
The amplification of the ITS and 18S rRNA genes of this population yielded single fragments of 964 bp and 1,621 bp, respectively. Intraspecific variability of two ITS sequences of the barley population was 0.5% (5 mismatches). BLAST search at the NCBI revealed 95%–96% (36–47 mismatches and seven gaps) identity with the corresponding sequences of A. tritici available in the database.
Phylogenetic relationships of A. tritici from barley from Iraq based on 57 ITS1-5.8S-ITS2 sequences are presented in Figure 1. In this tree, the newly generated sequences have formed a maximally supported clade with previously deposited sequences of A. tritici from wheat. Anguina agropyronifloris Norton, 1965 formed a clade with A. tritici sequences from wheat and barley, a similar relationship that was already resolved (Barrantes-Infante et al., 2018). The current phylogenetic analysis also confirmed the monophyly of Anguina and polyphyly of Ditylenchus (Aliverdi et al., 2021).
Figure 1.
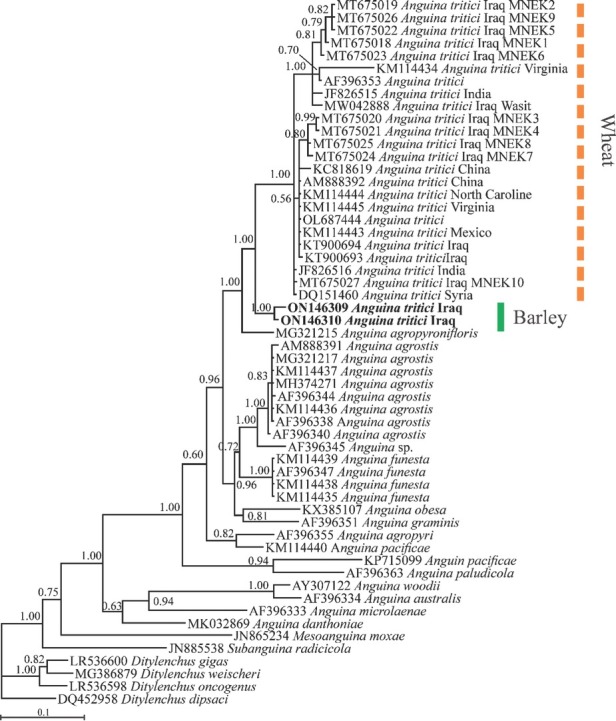
Bayesian phylogenetic tree of Anguina tritici (Steinbuch, 1799) Filipjev, 1936 from barley in Iraq, reconstructed using ITS rDNA sequences under the GTR + G + I model. Posterior probability values higher than 0.50 are given for appropriate clades. The newly obtained sequences are in bold font.
The phylogenetic tree reconstructed using 18S data is presented in Figure 2. In this tree, the newly generated sequence of A. tritici from barley has formed a clade with sequences of A. tritici from wheat currently available in GenBank.
Figure 2.
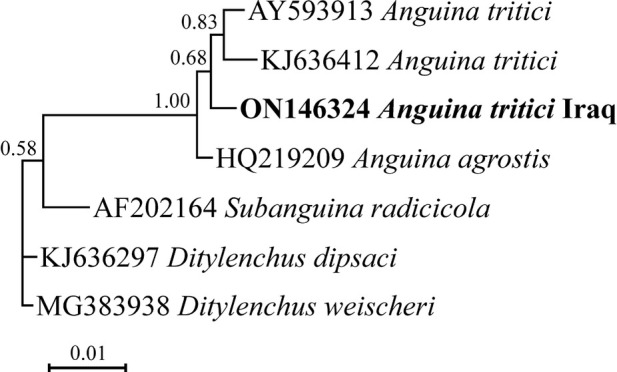
Bayesian phylogenetic tree of Anguina tritici (Steinbuch, 1799) Filipjev, 1936 from barley in Iraq, reconstructed using 18S rDNA sequences under the GTR + G + I model. Posterior probability values higher than 0.50 are given for appropriate clades. The newly obtained sequence is in bold font.
The results of the current study confirm, for the first time, the existence of a barley biotype of A. tritici showing sister relationships with wheat biotypes using both ITS and 18S rRNA gene data. This finding could suggest that infection of different host plants can contribute to isolated nematode populations and has roles in their evolution (Powers et al., 2001; Subbotin et al., 2004; Mobasseri et al., 2017). These findings are in agreement with recent studies on Meloidogyne incognita (Kofoid and White, 1919) Chitwood, 1949 biotypes (Koutsovoulos et al., 2019) showing adaptative potential to different environments, geographical distribution, and host plants. This study revealed that M. incognita pathotypes do not share an evolutionary common ancestor; instead, it is likely that the same pathotypes have arisen independently multiple times in different regions. Furthermore, our results suggest the existence of A. tritici species complex, as reported in Ditylenchus dipsaci (Kühn, 1857) Filipjev 1936 (Yavuzaslanoglu et al., 2018; Storelli et al., 2021). Different populations of D. dipsaci showed variations in virulence and pathogenicity on sugar beet compared to onion, and only one of these populations/biotypes was highly adapted to sugar beet (Storelli et al., 2021). In conclusion, the two forms of A. tritici adapted to different host plants were genetically heterogenous and occupied different placements in presently resolved phylogenies.
References
- Al-Beldawi A., Stephan Z. A., Lwaa N. H., Shali R. A.. Studies on wheat gall nematode in Iraq. Yearbook of Plant Protect. Research, Baghdad. 1977;1:268–283. [Google Scholar]
- Aliverdi S., Pourjam E., Pedram M.. Description of Ditylenchus acantholimonis n. sp. (Rhabditida: Anguinidae) from Iran, a morphological and molecular phylogenetic study. Nematology. 2021;24:109–118. doi: 10.1163/15685411-bja10115. [DOI] [Google Scholar]
- Al-Talib N. Y., Al-Taae A. K. M., Nimer S. M., Stephan Z. A., Al-Beldawi A. S.. New record of Anguina tritici on barley from Iraq. International Nematology Network Newsletter. 1986;3:25–27. [Google Scholar]
- Ami S. N., Taher I. E.. Wheat seed gall nematode Anguina tritici in Duhok province, Kurdistan Region-Iraq and its biology. Journal of University of Zakho. 2013;1:674–685. [Google Scholar]
- Ami S. N., Taher I. E., Hussen F. S., Ahmed A. I.. First molecular identification of wheat seed gall nematode Anguina tritici races parasitized on wheat in Iraq. Acta Universitatis Sapientiae Agriculture and Environment. 2019;11:5–15. doi: 10.2478/ausae-2019-0001. [DOI] [Google Scholar]
- Anwar S. A., Mc Kenry M. V., Riaz A., Khan M. S. A.. Evaluation of wheat cultivars for Anguina tritici resistance, development and influence of nematode on wheat growth. International Journal of Nematology. 2001;11:150–156. [Google Scholar]
- Barrantes-Infante B. L., Schroeder B. K., Subbotin S. A., Murray T. D.. Afrina sporoboliae sp. n. (Nematoda: Anguinidae) associated with Sporobolus cryptandrus from Idaho, USA: Phylogenetic relationships and population structure. Phytopathology. 2018;108:768–779. doi: 10.1094/PHYTO-12-17-0395-R. [DOI] [PubMed] [Google Scholar]
- Bonjar G. H. S., Hassani H. S., Pakgohar N., Barkhorder B.. Investigation for resistance traits in three hexaploid amphidiploids (Ttitiipyrum, Triticales and Wheats) to seed gall nematode and covered smut diseases. Asian Journal of Plant Science. 2004;3(3):325–329. doi: 10.3923/ajps.2004.325.329. [DOI] [Google Scholar]
- Datasheet for Anguina tritici. Crop Protection Compendium. Wallingford, UK: CABI; 2014. CABI. [Google Scholar]
- Darriba D., Taboada G. L., Doallo R., Posada D.. jModelTest 2: More models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca F., Fanelli E., Di Vito M., Reyes A., De Giorgi C.. Comparison of the sequences of the D3 expansion of the 26S ribosomal genes reveals different degrees of heterogeneity in different populations and species of Pratylenchus from the Mediterranean region. European Journal of Plant Pathology. 2004;110:949–957. doi: 10.1007/s10658-004-0813-4. [DOI] [Google Scholar]
- EPPO Global Database. Paris: EPPO; 2015. https://gd.eppo.int. EPPO (European and Mediterranean Plant Protection Organization) [Google Scholar]
- Filipiev I. N.. On the classification of the Tylenchinae. Proceedings of the Helminthological Society of Washington. 1936;3:80–82. [Google Scholar]
- Hall T. A.. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98NT. Nucleic Acids Symposium Serial. 1999;41:95–98. [Google Scholar]
- Huson D. H., Scornavacca C.. Dendroscope 3: An interactive tool for rooted phylogenetic trees and networks. Systematic Biology. 2012;61:1061–1067. doi: 10.1093/sysbio/sys062. [DOI] [PubMed] [Google Scholar]
- Kanzaki N., Futai K.. A PCR primer set for determination of phylogenetic relationships of Bursaphelenchus species within xylophilus group. Nematology. 2002;4:35–41. doi: 10.1163/156854102760082186. [DOI] [Google Scholar]
- Katoh K., Rozewicki J., Yamada K. D.. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 2019;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausar S., Khan A. A.. Interaction of simulated acid rain and seed gall nematode Anguina tritici on wheat. Biology and Medicine. 2009;1(2):100–106. [Google Scholar]
- Koutsovoulos G. D., Marques E., Arguel M. J., Duret L., Machado A. C. Z., Carneiro R. M. D. G., Kozlowski D. K., Bailly-Bechet M., Castagnone-Sereno P., Albuquerque E. V. S., Danchin E. G. J.. Population genomics supports clonal reproduction and multiple independent gains and losses of parasitic abilities in the most devastating nematode pest. Evolutionary Applications. 2019;13:442–457. doi: 10.1111/eva.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasseri M., Pedram M., Pourjam E., Bertozzi T.. Description of a new species of seed-gall nematode, Anguina obesa n. sp. (Nematoda: Anguinidae) from northern Iran, and its phylogenetic relations with other species and genera. European Journal of Plant Pathology. 2017;148:423–434. doi: 10.1007/s10658-016-1101-9. [DOI] [Google Scholar]
- Mohamedova M., Piperkova N.. Seed gall nematode Anguina tritici in Bulgaria: Nematode impact on wheat growth and grain yield. AgroLife Scientific Journal. 2013;2:15–19. [Google Scholar]
- Mukhtar T., Jabbar A., Raja M. U., Javed H.. Re-emergence of wheat seed gall nematode (Anguina tritici) in Punjab, Pakistan. Pakistan Journal Zoology. 2018;50:1195–1198. doi: 10.17582/journal.pjz/2018.50.3.sc4. [DOI] [Google Scholar]
- Mustafa S. A. Study on wheat and barley ear-cockle disease caused by nematode Anguina tritici in Erbil province. M.Sc. thesis, College of Agriculture. University of Salahaddin-Erbil; 2009. [Google Scholar]
- Nicol J. M., Rivoal R. Ciancio A., Mukerji K. G. Integrated management and biocontrol of vegetable and grain crops nematodes. The Netherlands: Springer; 2008. Global knowledge and its application for the integrated control and management of nematodes on wheat; pp. 243–287. [Google Scholar]
- Ozberk I., Yolcu S., Yucel A., Koten M., Nicol J. M.. The impact of seed gall nematode on grain yield, quality and marketing prices on durum wheat in Anatolia, Turkey. African Journal of Agricultural Research. 2011;6:3891–3896. doi: 10.5897/AJAR10.503. [DOI] [Google Scholar]
- Powers T. O., Szalansky A. L., Mullin P. G., Harris T. S., Bertozzi T., Griesbach J. A.. Identification of seed gall nematodes of agronomic and regulatory concern with PCR-RFLP of ITS1. Journal of Nematology. 2001;33:191–194. [PMC free article] [PubMed] [Google Scholar]
- Qassem N. E., Al-Taae H. H., Thanoon A. H.. Screening of some varieties of wheat for the infestation by the seed gall nematode Anguina tritici. Plant Cell Biotechnology and Molecular Biology. 2021;22:94–105. [Google Scholar]
- Rao R. S. R. A preliminary list of insect pest of Iraq. Department of Agriculture Iraq. Printed at the Time Press; 1921. Bull. 7. [Google Scholar]
- Ronquist F., Huelsenbeck J. P.. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Stephan Z. A., Antoon B. G.. Biotypes of earcockle nematode Anguina tritici in Iraq. Current Nematology. 1990;1:85–88. [Google Scholar]
- Storelli A., Kiewnick S., Daub M., Mahlein A. K., Schumann M., Beyer W., Keiser A.. Virulence and pathogenicity of four Ditylenchus dipsaci populations on sugar beet. European Journal of Plant Pathology. 2021;161:63–71. doi: 10.1007/s10658-021-02304-w. [DOI] [Google Scholar]
- Subbotin S. A., Krall E. L., Riley I. T., Chizhov V. N., Staelens A., De Loose M., Moens M.. Evolution of the gall-forming plant parasitic nematodes (Tylenchida: Anguinidae) and their relationships with hosts as inferred from internal transcribed spacer sequences of nuclear ribosomal DNA. Molecular Phylogenetic and Evolution. 2004;30:226–235. doi: 10.1016/S1055-7903(03)00188-X. [DOI] [PubMed] [Google Scholar]
- Subbotin S. A., Madani M., Krall E., Sturhan D., Moens M.. Molecular diagnostics, taxonomy and phylogeny of the stem nematode Ditylenchus dipsaci species complex based on the sequences of the intertranscribed spacer rDNA. Phytopathology. 2005;95:1308–1315. doi: 10.1094/PHYTO-95-1308. [DOI] [PubMed] [Google Scholar]
- Vrain T. C., Wakarchuk D. A., Levesque A. C., Hamilton R. I.. Intraspecific rDNA restriction fragment length polymorphism the Xiphinema americanum group. Fundamental and Applied Nematology. 1992;15:563–573. [Google Scholar]
- Yavuzaslanoglu E. Ates Sonmezoglu, O., Genc N., Akar Z., Terzi B.. Molecular characterization of Ditylenchus dipsaci on onion in Turkey. European Journal of Plant Pathology. 2018;151:195–200. doi: 10.1007/s10658-017-1366-7. [DOI] [Google Scholar]


