Abstract
Background
The surgical efficacy and prognostic outcomes of patients with unspecific malignant bone tumors (UMBTs) remain unclear. The study is to address: 1) What are the clinicopathological features and prognostic determinants for patients with UMBTs? 2) Can a nomogram be developed for clinicians to predict the short and long-term outcomes for individuals with UMBTs? 3) Does surgery improve outcomes for UMBT patients who received radiotherapy or chemotherapy after balancing the confounding bias?
Methods
400 UMBT patients were filtrated from the Surveillance, Epidemiology, and End Results database to assess the clinicopathological features, treatments, and factors affecting prognosis. The optimal cutoff values of continuous variables were identified by the x-tile software. Kaplan-Meier method and multivariate Cox proportional hazard modeling were performed to evaluate the independent prognostic factors. Nomogram was further developed by using R software with rms package. The surgical efficacy was further assessed for patients receiving radiotherapy or chemotherapy after performing propensity score matching.
Results
The enrolled cohort included 195 (48.8%) female and 205 (51.2%) male patients. The 2- and 5-year cancer-specific survival (CSS) and overall survival (OS) rate were 58.2 ± 3.0%, 46.8 ± 3.2%, and 46.5 ± 2.6%, 34.4 ± 2.5%, respectively. Nomogram was finally developed for CSS and OS according to the identified independent factors: age, tumor extent, primary tumor surgery, tumor size, and pathology grade. For UMBT patients who received radiotherapy or chemotherapy, surgical intervention was associated with better CSS (pr = 0.003, pc = 0.002) and OS (pr = 0.035, pc = 0.002), respectively.
Conclusions
Nomogram was developed for individual UMBT patient to predict short and long-term CSS and OS rate, and more external patient cohorts are warranted for validation. Surgery improves outcomes for UMBT patients who received either radiotherapy or chemotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-10153-x.
Keywords: Unspecific malignant bone tumor, Surgery, Nomogram, Propensity score matching, Prognosis
Background
The unspecific malignant bone tumors (UMBTs) are a group of bone tumors in addition to the specific bone tumors (e.g. osteosarcoma, chondrosarcoma, Ewing sarcoma, etc). The definition of UMBTs are provided by the International Classification of Childhood Cancer, Third Edition (ICCC-3) based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [1]. The UMBT is relatively rare, which only accounts for 1.2% among all the bone malignancies [2]. Compared to the histology-specific bone tumors, limited information was published regarding the clinical characteristics, treatments, and prognosis of UMBTs.
The Surveillance, Epidemiology, and End Results (SEER) database (http://seer.cancer.gov) provides a nationwide, population-based patients to help study the epidemic features, efficacy of treatments, and prognostic outcomes of different cancer patients [3–5]. Therefore, we utilized the SEER database to possibly address the following clinical questions: 1) What are the clinicopathological features and prognostic determinants for patients with UMBTs? 2) Can a nomogram be developed for clinicians to predict the preliminary and long-term outcomes for individuals with UMBTs? 3) Does surgery improve outcomes for UMBT patients who received radiotherapy or chemotherapy after balancing the data bias?
Methods
Study population
UMBTs were defined as a group of bone tumors in addition to osteosarcoma, chondrosarcoma, Ewing sarcoma and other specific bone tumors, which were provided by the ICCC-3 and encoded in the SEER database. By using the SEER*Stat software (Version 8.3.5, National Cancer Institute, Bethesda, MD, USA), the clinical information of UMBT patients from 1973 to 2016 was obtained from SEER database, which covers 30% US population [6, 7]. According to the ICD-O-3, the codes for selected histologic types were 8000–8005, 8800–8801, and 8803–8805, and the primary site codes were C40.0–40.3, C40.9–41.4, and C41.8–41.9. The anatomic site record was “bone and joints”. This study was a retrospective design with duration of over 40 years (1973–2016), and all the enrolled patients were from the USA population.
Inclusion and exclusion
In our study, the inclusion criteria were as follows: 1) Confirmed diagnosis of UMBT with positive histology; 2) the diagnosis was acquired at a living status; 3) active follow up was confirmed for each patient; 4) definite survival months. While the enrolled patients were further excluded for at least one of the following reasons: 1) incomplete information of tumor sites; 2) lack of surgical information; 3) patients under 18 years old; 4) the survival month was 0; 5) number of patients from Alaska < 11(please note we did not give the exact number to protect patient privacy). Notably, according to the provided information of the SEER database, tumor sites were divided into the following three subgroups for further analysis: limbs (including upper and lower limbs, and associated joints), and the spine (including vertebral column/pelvis, sacrum, coccyx, and associated joints), and others (including mandible/skull, face and associated joints/rib, sternum, clavicle and associated joints).
Baseline information
The collected clinicopathological factors included: age, race, sex, year of diagnosis, region (southwest/pacific coast/northern plains/east), primary tumor site, pathological grade, tumor laterality, tumor extent (localized/regional/distant), primary tumor surgery, radiotherapy and chemotherapy, tumor size, initial tumor diagnosed, hispanic/non-hispanic patients, marital status, and annual family income. The primary endpoints were cancer-specific survival (CSS) and overall survival (OS). CSS was defined as the interval from the first day to the death date due to UMBT or the end of follow-up, while OS was defined as the duration from the first day to the date of all-cause death or the end day of follow-up. Figure 1 showed the flow chart of patients collecting process: in short, 545 UMBT patients were initially screened out from the case information list via SEER*Stat software, then 145 patients were further excluded for the following reasons: Age < 18 years old(n = 54), unknown tumor site (n = 27), incomplete surgical information (n = 17), 0 survival month of follow up (n = 7), and the patient from Alaska (n = 1) was also removed because of the limited number. Finally, 400 UMBT cases were enrolled in our study for further analysis.
Fig. 1.
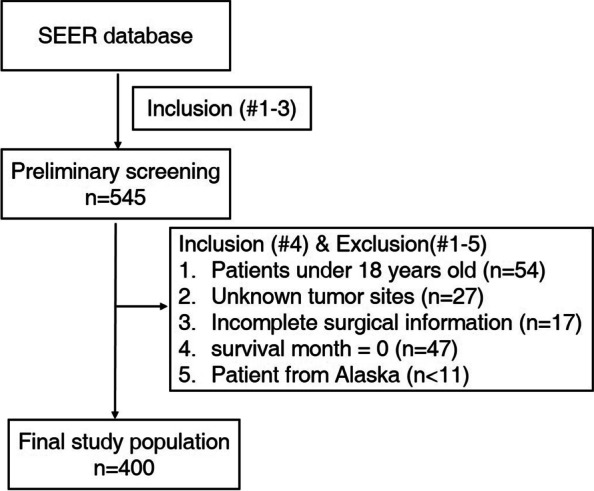
The flowchart of step-by-step screening process of eligible patients (n = 400)
Statistical analysis
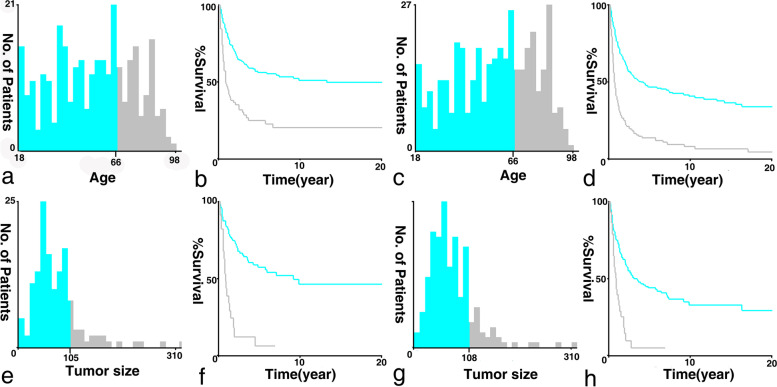
The mean with standard deviation (SD) and/or range were used to describe the quantitative data, while counts and percentages for qualitative data. The cutoff values of continuous factors were identified optimally by using the x-tile software (Yale University, New Haven, CT, USA) [8]. Theoretically, the optimal cutoff value was generated based on the highest chi-square values calculated upon the division. Notably, the results showed that the binary cutoff values of CSS and OS were described as follows: 66 years old for age, and 105/108 mm for tumor size, respectively (Fig. 2).
Fig. 2.
The histogram and survival curves of UMBT patients based on the identified optimal cutoff values. The x-tile histograms and Kaplan-Meier curves were illustrated for cancer-specific and overall survival when (A-D) Age cutoff value was 66 years old; E-H Tumor size cutoff values were 105 and 108 mm, respectively
Univariate analysis was then performed by the SPSS 22.0 (SPSS Inc., Chicago, IL, USA) to figure out the potential significant factors by log-rank test, and parameters with p < 0.05 were further subjected to multivariate Cox proportional hazard model to identify the independent determinants with estimated hazard ratios. Notably, since the information of patients who did not receive radiotherapy and chemotherapy was incomplete in the SEER database (“None/Unknown” for radiotherapy, and “No/Unknown” for chemotherapy), these two factors were not subjected into survival analysis. Kaplan-Meier curve of independent factors was drawn by the GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA).
After identifying independent contributors by multivariate analysis, Nomogram was further developed by using R software (version 3.5.1) with rms package (available at: http://CRAN.R-project.org/package=rms). The developed nomogram can be used to predict the 2 and 5-year CSS and OS, respectively. Harrell’s concordance index (C-index) and calibration curves were generated to assess the predictive value of the nomogram by comparing with the actual proportions.
Besides, the clinical features between surgery and non-surgery groups were assessed by using student t and Pearson χ2 test or Fisher exact test. Propensity score matching (PSM) method (R with MatchIt package, available at: http://CRAN.R-project.org/package=MatchIt) was utilized to balance the impact caused by the unmatched bias. The matching ratio was designated as 1:1 with caliper of 0.05 for surgery and non-surgery groups. After PSM, the effects of surgical treatments on CSS and OS were further evaluated among patients receiving either radiotherapy or chemotherapy, respectively. The related death rate curves were analyzed and depicted by using the GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA), with p < 0.05 being regarded statistically significant.
Results
The baseline features of 400 UMBT patients were summarized in Table S1. The study population consisted of 195 (48.8%) female and 205 (51.2%) male patients, with the average age of 59.6 ± 19.7 and 55.2 ± 20.3 years old, respectively. 194 UMBTs originated from limbs, and 132 cases occurred in mandible/skull, face and associated joints/rib, sternum, clavicle and associated joints, while UMBTs of the spine accounted for 18.5%. Two hundred ninety-two patients had confirmed CSS status, while OS status was obtained from all patients (n = 400, 100%). During the mean follow-up of 49.4 ± 76.8 months (range 1–437), the 2, 5-year CSS and OS rate were 58.2 ± 3.0%, 46.8 ± 3.2%, and 46.5 ± 2.6%, 34.4 ± 2.5%, respectively.
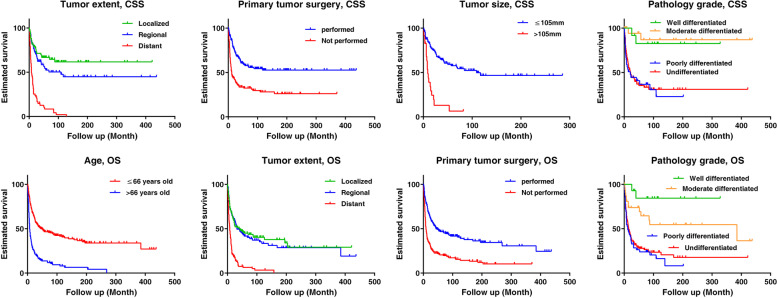
The univariate analysis (Table 1) indicated that the potential significant factors for CSS and OS were age (p < 0.001 for both), diagnosis year (p = 0.048 & p = 0.011), tumor site (p < 0.001 for both), tumor extent (p < 0.001 for both), tumor size (p < 0.001 for both), primary tumor surgery (p < 0.001 for both), pathology grade (p < 0.001 for both), first tumor diagnosed (p < 0.001 for OS), marital status (p < 0.001 for both). The related 5-year survival rates for subgroups were also shown in Table 1. The potential factors were then subjected to multivariate Cox proportional hazard model, and the results (Table 2) revealed tumor extent (p < 0.001), primary tumor surgery (p = 0.018), tumor size (p = 0.027), and pathology grade (p = 0.005) were independently associated with CSS. While the contributors for OS were age (p = 0.001), tumor extent (p = 0.002), primary tumor surgery (p < 0.001), and pathology grade (p = 0.010). The Kaplan-Meier curves regarding the independent factors were illustrated in Fig. 3.
Table 1.
Univariate analysis of preoperative factors and surgery affecting cancer-specific and overall survival
| Factors | Cancer-specific survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| n | 5-year Survival rate (%, mean ± SE) | p value | n | 5-year Survival rate (%, mean ± SE) | p value | |
| Age:18–66/> 66 yrs | 201/91 | 56.1 ± 3.8/24.8 ± 5.2 | < 0.001 | 254/146 | 46.5 ± 3.4/13.7 ± 3.0 | < 0.001 |
| Race: Black/white/othersa | 37/235/13 | 56.5 ± 8.6/44.7 ± 3.6 /27.7 ± 15.0 | 0.532 | 48/323/23 | 45.7 ± 7.8/32.7 ± 2.7 /17.2 ± 8.9 | 0.408 |
| Gender: Female/male | 138/154 | 46.6 ± 4.5/46.7 ± 4.5 | 0.434 | 195/205 | 35.5 ± 3.6/33.3 ± 3.5 | 0.895 |
| Diagnosis year: ≤2000/> 2000 | 77/215 | 53.0 ± 5.8/44.2 ± 3.9 | 0.048 | 94/306 | 43.6 ± 5.1/31.1 ± 2.9 | 0.011 |
| Region: Southeast/pacific coast/ northern plains/east | 28/118/ 28/118 | 23.6 ± 11.9/42.3 ± 5.2/ 36.3 ± 9.4/53.7 ± 4.9 | 0.533 | 34/176/ 38/152 | 32.5 ± 9.2/31.2 ± 3.8/ 25.2 ± 7.2/40.3 ± 4.1 | 0.516 |
| Site: Limbs/spine/othersb | 161/46/85 | 49.1 ± 4.3/67.3 ± 7.5 /31.2 ± 5.6 | < 0.001 | 194/74/132 | 40.3 ± 3.7/48.5 ± 6.3 /17.8 ± 3.6 | < 0.001 |
| Tumor laterality: Left/right | 111/95 | 51.6 ± 5.1/40.6 ± 5.6 | 0.145 | 139/139 | 41.2 ± 4.3/30.3 ± 4.2 | 0.070 |
| Tumor extent: Localized/regional/distant | 84/84/79 | 66.7 ± 5.5/52.4 ± 6.4 /8.5 ± 3.6 | < 0.001 | 123/112/108 | 44.9 ± 4.7/41.6 ± 5.2 /6.3 ± 2.5 | < 0.001 |
| Tumor size: ≤105(108)/105(108) mm | 116/23 | 57.6 ± 5.2/6.5 ± 6.0 | < 0.001 | 167/29 | 44.0 ± 4.2/4.8 ± 4.5 | < 0.001 |
| Primary tumor surgery: Yes/no | 164/128 | 57.7 ± 4.3/32.4 ± 4.4 | < 0.001 | 213/187 | 46.7 ± 3.7/20.5 ± 3.1 | < 0.001 |
| Pathology grade: I/II/III/IV | 12/21/39/100 | 82.5 ± 11.3/86.9 ± 8.7/ 36.7 ± 8.4/35.3 ± 5.3 | < 0.001 | 14/34/55/137 | 84.4 ± 10.2/64.5 ± 9.3 /23.8 ± 6.1/27.8 ± 4.1 | < 0.001 |
| First tumor diagnosed: Yes/no | 292/0 | \ | \ | 293/107 | 40.7 ± 3.0/16.7 ± 4.0 | < 0.001 |
| Annual income aggregate level: Low/High (Cutoff: 75,000USD) | 162/130 | 43.5 ± 4.3/50.7 ± 4.7 | 0.185 | 210/190 | 32.4 ± 3.5/36.4 ± 3.6 | 0.338 |
| Hispanic: Yes/no | 43/249 | 42.2 ± 7.9/47.7 ± 3.5 | 0.422 | 52/348 | 40.0 ± 7.2/33.7 ± 2.7 | 0.516 |
| Marital status: Single/married/othersc | 135/66/68 | 48.1.1 ± 4.8/51.0 ± 6.6 /32.8 ± 6.1 | < 0.001 | 191/82/97 | 34.2 ± 3.7/43.8 ± 5.8 /21.9 ± 4.4 | < 0.001 |
SE standard error, yrs years old, USD US dollar
aincluding: American Indian/AK Native, Asian/Pacific Islander
bincluding: mandible/skull, face and associated joints/rib, sternum, clavicle and associated joints
cincluding: divorced, separated, widowed
Table 2.
Multivariate analysis of clinicopathological factors affecting cancer-specific and overall survival
| Factors | Cancer-specific survival | Overall survival | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | \ | \ | 2.255(1.415–3.593) | 0.001 |
| Tumor extent | 3.186(1.827–5.556) | < 0.001 | 1.652(1.194–2.285) | 0.002 |
| Primary tumor surgery | 0.438(0.221–0.869) | 0.018 | 0.309(0.191–0.501) | < 0.001 |
| Tumor size | 2.121(1.087–4.139) | 0.027 | \ | \ |
| Pathology grade | 2.028(1.237–3.325) | 0.005 | 1.526(1.106–2.105) | 0.010 |
HR hazard ratio, CI confidence interval
Fig. 3.
The Kaplan-Meier curves were delineated according to the identified independent prognostic factors, including (A-D) Tumor extent (p < 0.001), primary tumor surgery (p < 0.001), tumor size (p < 0.001) and pathology grade (p < 0.001) for CSS; E-H Age, tumor extent, primary tumor surgery (p < 0.001), and pathology grade (p < 0.001) for OS. CSS = Cancer-specific survival; OS=Overall survival
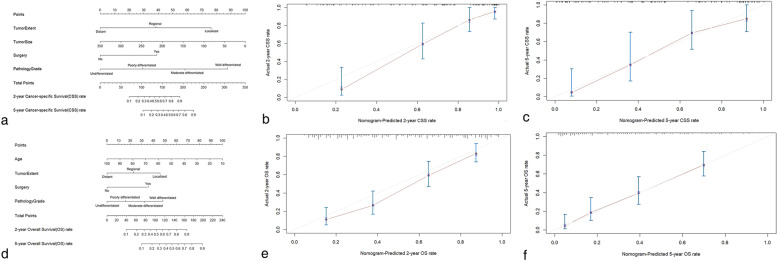
The nomograms were further developed to predict CSS and OS rate for individual UMBT patient by analyzing the above factors (Fig. 4). The developed nomograms can be used conveniently in the clinical practice. For example, when a 60-year-old patient with regional, poorly differentiated UMBT (tumor size = 100 mm, no previous primary tumor surgery) were presented to the institution, total points of 150 and 86 were assessed on this patient for CSS and OS, respectively. The estimated 2, 5-year CSS and OS rates were 65, 38 and 35%, 16%, respectively. The C-index of CSS and OS prediction for UMBTs were 0.829 ± 0.054 and 0.752 ± 0.043, respectively, and the calibration plots (Fig. 4b-c & e-f) showed favorable consistence between nomogram-based prediction and actual outcomes of 2, 5-year CSS and OS for UMBT patients, respectively.
Fig. 4.
The graphs showed nomograms and related calibration curves for 2, 5-year (A-C) cancer-specific survival; D-F overall survival, respectively. For example, when a 60-year-old patient (CSS points = N/A, OS points = 45) with regional (CSS points = 38, OS points = 23), poorly differentiated (CSS points = 30, OS points = 18) UMBT (tumor size = 100 mm [CSS points = 72, OS points = N/A], no previous primary tumor surgery [CSS points = 0, OS points = 0]) were presented to the institution, total points of 140 and 86 were assessed on this patient for CSS and OS estimation, respectively. The estimated 2, 5-year CSS and OS rates were 58, 32 and 35%, 16%, respectively
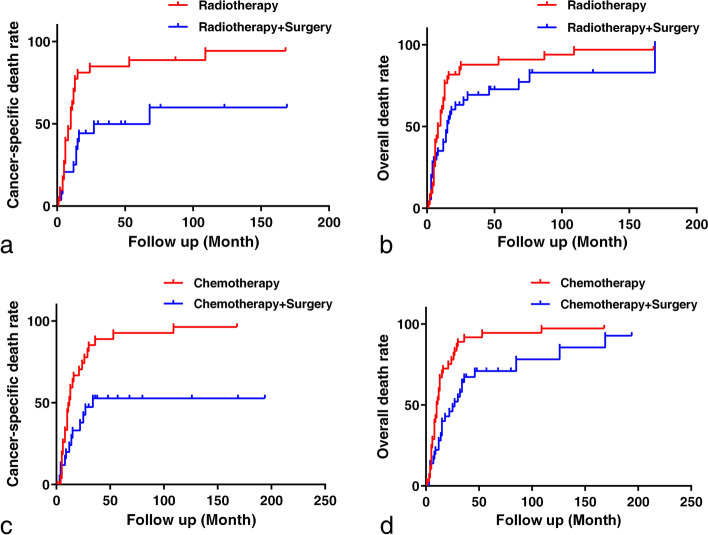
Meanwhile, the effect of surgery on UMBT patients who underwent radiation and chemotherapy were further explored after using the PSM method for imbalance mitigation. The distributions of propensity score after performing PSM were delineated in Fig. S1. As shown in Table 3, the baseline bias for unmatched patients existed among the following factors: age (p < 0.001), sex (p = 0.010), tumor site (p < 0.001), tumor extent (p < 0.001), pathology grade (p = 0.041), first tumor diagnosed (p = 0.042), marital status (p = 0.001), and family income (p = 0.001). However, after performing PSM, the bias was mitigated for age (p = 0.533), sex (p = 0.879), tumor site (p = 0.070), pathology grade (p = 0.568), first tumor diagnosed (p = 0.308), marital status (p = 0.520), and family income (p = 0.570). One hundred seventy-two cases (86:86) were finally matched to explore the surgical effect for patients who received either radiotherapy or chemotherapy, respectively. According to the results (Fig. 5), compared with patients who only received radiotherapy, surgery plus radiation increased CSS (p = 0.003) and OS rates (p = 0.035). Likewise, lower cancer-specific (p = 0.002) and overall death rates (p = 0.002) were observed for patients who underwent combined surgery and chemotherapy.
Table 3.
Baseline characteristics between surgery and non-surgery groups
| Factors | Primary tumor surgery (Unmatched) | p value | Primary tumor surgery (Propensity score matching) | p value | ||
|---|---|---|---|---|---|---|
| Yes (n = 213) | No (n = 187) | Yes (n = 86) | No (n = 86) | |||
| Age (yrs, mean ± SD) | 52.6 ± 19.5 | 62.8 ± 19.3 | < 0.001 | 56.3 ± 18.7 | 58.1 ± 19.3 | 0.533 |
| Race (n,%) | 0.107 | 0.142 | ||||
| Black | 20 (9.4) | 28 (15.0) | 6 (7.0) | 14 (16.3) | ||
| White | 181 (85.0) | 142 (75.9) | 77 (89.5) | 68 (79.1) | ||
| Othersa | 10 (4.7) | 13 (7.0) | 3 (3.5) | 4 (4.6) | ||
| Sex (n,%) | 0.010 | 0.879 | ||||
| Female | 91 (42.7) | 104 (55.6) | 41 (47.7) | 42 (48.8) | ||
| Male | 122 (57.3) | 83 (44.4) | 45 (52.3) | 44 (51.2) | ||
| Diagnosis year (n,%) | 0.160 | 0.578 | ||||
| ≤ 2000 | 56 (26.3) | 38 (20.3) | 20 (23.3) | 17 (19.8) | ||
| > 2000 | 157 (73.7) | 149 (79.7) | 66 (76.7) | 69 (80.2) | ||
| Region (n,%) | 0.935 | 0.852 | ||||
| Southeast | 17 (8.0) | 17 (9.1) | 6 (6.9) | 8 (9.3) | ||
| Pacific coast | 96 (45.1) | 80 (42.8) | 36 (41.9) | 39 (45.3) | ||
| Northern plains | 19 (8.9) | 19 (10.2) | 9 (10.5) | 9 (10.5) | ||
| East | 81 (38.0) | 71 (38.1) | 35 (40.7) | 30 (34.9) | ||
| Tumor site (n,%) | < 0.001 | 0.070 | ||||
| Limbs | 128 (60.1) | 66 (35.3) | 34 (39.5) | 39 (45.3) | ||
| Spine | 45 (21.1) | 29 (15.5) | 23 (26.8) | 11 (12.8) | ||
| Othersb | 40 (18.8) | 92 (49.2) | 29 (33.7) | 36 (41.9) | ||
| Tumor laterality (n,%) | 0.277 | 0.097 | ||||
| Left | 82 (38.5) | 57 (30.8) | 27 (31.4) | 32 (37.2) | ||
| Right | 73 (34.3) | 66 (35.3) | 25 (29.1) | 32 (37.2) | ||
| Tumor extent | < 0.001 | 0.032 | ||||
| Localized | 76 (35.7) | 47 (25.1) | 21 (24.4) | 20 (23.3) | ||
| Regional | 84 (39.4) | 28 (15.0) | 36 (41.9) | 20 (23.3) | ||
| Distant | 36 (16.9) | 72 (38.5) | 29 (33.7) | 36 (41.9) | ||
| Tumor size (mm, mean ± SD) | 71.5 ± 44.9 | 80.5 ± 46.8 | 0.204 | 77.6 ± 52.0 | 80.1 ± 46.5 | 0.812 |
| Pathology grade (n,%) | 0.041 | 0.568 | ||||
| I: Well differentiated | 11 (51.6) | 3 (1.6) | 3 (3.5) | 1 (1.2) | ||
| II: Moderately differentiated | 27 (12.7) | 7 (3.7) | 8 (9.3) | 5 (5.8) | ||
| III: Poorly differentiated | 30 (14.1) | 25 (13.4) | 11 (12.8) | 14 (16.3) | ||
| IV: Undifferentiated | 79 (37.1) | 58 (31.0) | 35 (40.7) | 35 (40.7) | ||
| First tumor diagnosis (n,%) | 0.042 | 0.308 | ||||
| Yes | 165 (77.5) | 128 (68.5) | 21 (24.4) | 27 (31.4) | ||
| No | 48 (22.5) | 59 (31.6) | 65 (75.6) | 59 (68.6) | ||
| Hispanic (n,%) | 0.813 | 0.670 | ||||
| No | 184 (86.4) | 164 (87.7) | 74 (86.0) | 72 (83.7) | ||
| Yes | 29 (13.6) | 23 (12.3) | 12 (14.0) | 14 (16.3) | ||
| Marital status (n,%) | 0.001 | 0.520 | ||||
| Single | 111 | 80 | 17 (19.8) | 17 (19.8) | ||
| Married | 53 | 29 | 53 (61.6) | 47 (54.7) | ||
| Othersc | 38 | 59 | 16 (18.6) | 22 (25.5) | ||
| Family income (USD, mean ± SD) | 80,780 ± 20,320 | 74,620 ± 19,630 | 0.002 | 74,140 ± 19,590 | 75,830 ± 20,110 | 0.570 |
aincluding: American Indian/AK Native, Asian/Pacific Islander
bincluding: mandible/skull, face and associated joints/rib, sternum, clavicle and associated joints
cincluding: divorced, separated, widowed
Fig. 5.
The graphs revealed Kaplan-Meier curves between the following groups (data from the propensity score sample): A-B surgery + radiotherapy vs. radiotherapy for cancer-specific (p = 0.003) and overall death rate (p = 0.002); C-D surgery + chemotherapy vs. chemotherapy for cancer-specific (p = 0.035) and overall death rate (p = 0.002)
Discussion
UMBTs are much less common than the specific bone malignancies, including osteosarcoma, chondrosarcoma, and Ewing sarcoma, etc. To our knowledge, due to the rarity of UMBTs, no study has been reported regarding the clinical features, related treatments, and contributors for survival towards such patients. Meanwhile, the application of nomogram has been validated as an effective mean for clinicians to anticipate prognosis in different malignant bone tumors [6, 9, 10], thus a well-defined nomogram based on the prognostic factors is urgently required to predict survivals for UMBT patients. Moreover, although surgical resection was reported to be effective in treating various specific malignant bone tumors regardless of metastatic status [6, 11–14], the surgical efficacy for UMBTs remains to be elucidated. The SEER database is the most common used clinical database which consists of about 30% of population from 18 registries that represent all regions of US [3]. It contains various sociodemographic and clinicopathological factors for oncologists to analyze the relevance between risks and tumorigenesis [15, 16], and the prognostic factors affecting CSS and OS [17, 18].
In our study, the estimated 5-year CSS rate for non-metastatic (localized and regional status) and metastatic UMBT patients were 53.2 ± 4.7% and 6.4 ± 3.3%, which were much worse than those for relevant osteosarcoma (71.8 and 30.4%) [19] and Ewing sarcoma patients (72.3 and 35.7%) [20]. Because of the dismal survival outcomes, it is essential to identify the prognostic determinants and effective therapeutics by assessing the large-scale, population-based UMBT patients from SEER database. We firstly identified the survival contributors for UMBTs, including age (only for OS), tumor extent, primary tumor surgery, tumor size (only for CSS), and pathology grade, the nomogram was then developed for UMBTs patients based on the independent factors. The C-index (0.829 ± 0.054 and 0.752 ± 0.043) and calibration curves (Fig. 4) indicated possible feasibilities in predicting the individual CSS and OS rate in the clinical practice.
These clinicopathological factors were also confirmed in assessing other specific malignant bone tumors [6, 10, 13]. Although this is the first-reported nomogram for UMBTs, limitation does exist as follows: 1) The information of radiotherapy and chemotherapy is incomplete in the database, we could only obtain the definite number of patients receiving radiation or chemotherapy, but failed to get the definite numbers for those who did not underwent radiotherapy or chemotherapy due to the “None/Unknown” labeling. Therefore, the prognostic role of radiotherapy and chemotherapy cannot be evaluated completely and independently. 2) Despite favorable C-index and calibration curves, external cohorts are necessary for nomogram validation. 3) As noted in the previous study [6], the SEER database is lack of some well-defined prognostic factors, such as pathologic fracture and other imagelogical parameters. 4) Last but not the least, our study excluded 145 UMBT patients because of age < 18 years old and incomplete information, and nearly 1/4 of the rest patients failed to provide definite cancer-specific status, which may pose an impact on the nomogram development.
In consideration of importance of both surgical and non-surgical treatments, by accessing the available information from SEER database, we further studied the role of primary tumor resection among UMBT patients who received radiotherapy or chemotherapy. PSM was regarded as a useful method to generate similar cases between comparative groups after maximally balancing the bias [21]. Our findings indicated surgery could bring a survival advantage for UMBTs who received radiotherapy or chemotherapy. In other words, the combination of primary tumor resection and radiotherapy/chemotherapy is superior to sole adjuvant therapy for both CSS and OS status. However, we admitted that, due to the limited information of SEER database, we failed to clearly separate “before surgery” from “after surgery” regarding the administration of radiation or chemotherapy. From our own experience, we routinely collected the detailed medical history and assessed imagelogical findings of such patients, including age, tumor site, tumor size, previous treatments, etc. Positron Emission Tomography-Computed Tomography was applied to detect the potential distant metastasis if necessary. Surgical strategy was then conducted to remove the tumor integrally, and chemotherapy (general regimen for sarcoma) was applied if the pathology report indicated high degree of malignancy or metastatic risk. Besides, if the tumor size was large enough, which put high risk for operation, we might conduct radiotherapy before surgery. Last but not least, the rigorous postoperative follow-up was applied to monitor the patient’s recovery, usually monthly for the first 3 months and at 3-month intervals for the next 12 months on the outpatient basis.
Conclusions
The nomograms are preliminarily developed for UMBT patients after identify independent prognostic factors, and it warrants more external large-scale cohort to validate their feasibilities. The combination of surgery and radiotherapy/chemotherapy for UMBTs is associated with better CSS and OS than those who solely received radiotherapy/chemotherapy.
Supplementary Information
Additional file 2: Fig. S1. The graphs showed the distributions of propensity score after performing PSM in bubble and bar forms. PSM = Propensity score matching.
Additional file 3: Table S1. Baseline characteristics of patients with UMBT (n = 400).
Acknowledgements
Not applicable.
Abbreviations
- UMBT
Unspecific malignant bone tumor
- SEER
The Surveillance, Epidemiology, and End Results
- CSS
Cancer-specific survival
- OS
Overall survival
- C-index
Concordance index
- PSM
Propensity score matching
- HR
Hazard ratio
- CI
Confidence interval
Authors’ contributions
JX, XY, XC and SH designed and wrote the manuscript; SH, RJ, HS, JY, CY, and WL participated in literature search, data acquisition, data analysis, or data interpretation; JX, XY, XC and SH contributed to the revision of manuscript. All authors approved the final version to be published.
Funding
This study was funded by the National Natural Science Foundation of China (SH, 82203401) and the key project funding in the basic research field of the Shanghai Municipal Science and Technology Commission (JX, 17JC1400903). The funding sources had no role in the study design, data gathering, analysis, and interpretation, writing of the report, or the decision to submit the report for publication. The corresponding authors had full access to all the data and the final responsibility to submit for publication.
Availability of data and materials
All data generated or analyzed during this study are included as supplemental material (Additional file 1) in this published article.
Declarations
Ethics approval and consent to participate
As all the data of this study were derived from the SEER database, institutional review board approval and consent to participate were not demanded.
Consent for publication
Not applicable.
Competing interests
All the authors did not report conflicts of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaohui He, Runyi Jiang and Haitao Sun contributed equally to this study.
Contributor Information
Shaohui He, Email: heshaohui1025@163.com.
Xinghai Yang, Email: cnspineyang@163.com.
Xiaopan Cai, Email: caipan1982@163.com.
Jianru Xiao, Email: jianruxiao83@smmu.edu.cn.
References
- 1.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, Third Edition. Cancer. 2005;103:1457–67. [DOI] [PubMed]
- 2.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::AID-CNCR2820751308>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Doll KM, Rademaker A, Sosa JA. Practical guide to surgical data sets: surveillance, epidemiology, and end results (SEER) database. JAMA Surg. 2018;153(6):588–589. doi: 10.1001/jamasurg.2018.0501. [DOI] [PubMed] [Google Scholar]
- 4.Lewis DR, Chen HS, Cockburn MG, Wu XC, Stroup AM, Midthune DN, Zou Z, Krapcho MF, Miller DG, Feuer EJ. Early estimates of SEER cancer incidence, 2014. Cancer. 2017;123(13):2524–2534. doi: 10.1002/cncr.30630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute. Cancer. 2014;120(Suppl 23):3755–3757. doi: 10.1002/cncr.29049. [DOI] [PubMed] [Google Scholar]
- 6.Song K, Shi X, Wang H, Zou F, Lu F, Ma X, Xia X, Jiang J. Can a Nomogram help to predict the overall and Cancer-specific survival of patients with Chondrosarcoma? Clin Orthop Relat Res. 2018;476(5):987–996. doi: 10.1007/s11999.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Li H, Liu C, Chen C, Yan J. Solitary Plasmacytoma of bone of the spine: results from surveillance, epidemiology, and end results (SEER) registry. Spine (Phila Pa 1976) 2019;44(2):E117–Ee25. doi: 10.1097/BRS.0000000000002777. [DOI] [PubMed] [Google Scholar]
- 8.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Lee SY, Lee TR, Cho WH, Song WS, Koh JS, Lee JA, Yoo JY, Jeon DG. Prognostic nomogram for predicting the 5-year probability of developing metastasis after neo-adjuvant chemotherapy and definitive surgery for AJCC stage II extremity osteosarcoma. Ann Oncol. 2009;20(5):955–960. doi: 10.1093/annonc/mdn723. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Wu ZY, Lin ZQ. A nomogram to predict prognosis in Ewing sarcoma of bone. J Bone Oncol. 2019;15:100223. doi: 10.1016/j.jbo.2019.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song K, Song J, Chen F, Lin K, Ma X, Jiang J. Does resection of the primary tumor improve survival in patients with metastatic Chondrosarcoma? Clin Orthop Relat Res. 2019;477(3):573–583. doi: 10.1097/CORR.0000000000000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Zhao J, Bai J, Shen H, Zhang B, Deng L, Sun C, Liu Y, Zhang J, Zheng J. Risk and clinicopathological features of osteosarcoma metastasis to the lung: a population-based study. J Bone Oncol. 2019;16:100230. doi: 10.1016/j.jbo.2019.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song K, Song J, Chen F, Lin K, Ma X, Jiang J. Prognostic nomograms for predicting overall and cancer-specific survival of high-grade osteosarcoma patients. J Bone Oncol. 2018;13:106–113. doi: 10.1016/j.jbo.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song K, Song J, Lin K, Chen F, Ma X, Jiang J, Li F. Survival analysis of patients with metastatic osteosarcoma: a surveillance, epidemiology, and end results population-based study. Int Orthop. 2019;43(8):1983–1991. doi: 10.1007/s00264-019-04348-4. [DOI] [PubMed] [Google Scholar]
- 15.Molenaar RJ, Sidana S, Radivoyevitch T, Advani AS, Gerds AT, Carraway HE, Angelini D, Kalaycio M, Nazha A, Adelstein DJ, et al. Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid Cancer. J Clin Oncol. 2018;36(18):1831–1839. doi: 10.1200/JCO.2017.75.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green AL, Furutani E, Ribeiro KB, Rodriguez Galindo C. Death within 1 month of diagnosis in childhood Cancer: an analysis of risk factors and scope of the problem. J Clin Oncol. 2017;35(12):1320–1327. doi: 10.1200/JCO.2016.70.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W, He J, Shen Y, Shen J, He Q, Zhang J, Jiang G, Wang Q, Liu L, Gao S, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung Cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. 2017;35(11):1162–1170. doi: 10.1200/JCO.2016.67.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and Cancer-specific survival in papillary thyroid Cancer: rethinking current staging systems. J Clin Oncol. 2016;34(36):4415–4420. doi: 10.1200/JCO.2016.68.9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with high-grade osteosarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39(4):593–599. doi: 10.1016/j.canep.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Duchman KR, Gao Y, Miller BJ. Prognostic factors for survival in patients with Ewing's sarcoma using the surveillance, epidemiology, and end results (SEER) program database. Cancer Epidemiol. 2015;39(2):189–195. doi: 10.1016/j.canep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151–161. doi: 10.1162/003465302317331982. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Fig. S1. The graphs showed the distributions of propensity score after performing PSM in bubble and bar forms. PSM = Propensity score matching.
Additional file 3: Table S1. Baseline characteristics of patients with UMBT (n = 400).
Data Availability Statement
All data generated or analyzed during this study are included as supplemental material (Additional file 1) in this published article.