Abstract
Background
Peri-implantitis is a usual finding but estimates of its prevalence fluctuate very much. This may be due to the wide variety of disease definitions. This systematic review aims to estimate the overall prevalence of peri-implantitis and the effect of different study designs, function times and use of probing depth on prevalence rate.
Methods
Following electronic and manual searches of the literature published from January 2005 to December 2021, data were extracted from the studies fitting the study criteria. Fifty-seven articles were included in this study.
Results
Prevalence of peri-implantitis was 19.53% (95% CI 12.87–26.19) at the patient-level, and 12.53% (95% CI 11.67–13.39) at the implant-level and it remains highly variable even following restriction to the clinical case definition. The use of probing depth like diagnostic criteria affected the prevalence data.
Conclusion
The results indicate that it remains essential the identification of the diagnostic markers for more accurate disease classification.
Keywords: Dental implants, Peri-implantitis, Epidemiology
Background
Dental implants are currently one of the safest alternatives for the replacement of missing teeth, regardless of their cause. This treatment has shown a high degree of predictability, with a survival rate in the range of 90–95% for more than 5 years [1].
It is important to discriminate between survival and success rates of treatment. An implant with enough insertion and no mobility (positive survival) can be a failure (negative success) if it exhibits any coil or constant inflammation of the peri-implant soft tissue. The incidence of technical and biological complications appears to be common [2–4], and these complications can have substantial economic implications and effects on the perception of treatment of the patient [5–8]. As the number of patients receiving dental implants is continually growing, the prevention and treatment of associated complications represents a serious and relevant challenge.
Within the biological complications, peri-implant diseases are considered the most relevant. They have an infectious cause and two entities have been described: mucositis and peri-implantitis [9]. Peri-implantitis is characterized by a destructive inflammatory lesion of polymicrobial etiology that affects both soft and hard tissues leading to progressive peri-implant bone loss, along with the formation of a pocket and inflammation in peri-implant tissues [2, 10]. Thus, the pathognomonic clinical sign of peri-implantitis will be the increase in pocket depth accompanied by bleeding and sometimes suppuration [11].
In order to better understand the magnitude of peri-implant diseases, it is mandatory to understand their epidemiology. It has therefore been suggested that epidemiological studies with a cross-sectional design, adequate sample sizes, and clinical and radiographic records are necessary to study the prevalence and risk indicators of peri-implant diseases [12]. Previous study reported that the prevalence of peri-implantitis ranged from 14.38 to 24.27% [13]. The reported variability may depend on different factors, including the follow-up period or disease definition. The definition is quite controversial and many different definitions have been proposed [14], until the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions [15] proposed a new classification of periodontal and peri-implant diseases, where, in the absence of a previous examination, the diagnosis of peri-implantitis may be based on the combination of bleeding on probing (BOP) and/or suppuration, probing depth ≥ 6 mm, and loss of supporting bone ≥ 3 mm. Another relevant factor is the use of convenience samples instead of randomized samples, which ultimately results in a potential selection bias [16].
The variability in the prevalence of peri-implantitis can be also explained by the different clinical parameters used to define the disease in the different studies, especially in terms of the magnitude of loss of supporting bone and the probing depth, the heterogeneity of the groups evaluated, or the individual risk factors of each population. Individual risk factors significantly increase the prevalence of peri-implantitis and may include the patient's previous history of periodontal disease, smoking habit, poor oral hygiene, diabetes and genetic factors [17].
Due to the great heterogeneity in peri-implantitis prevalence data, it is necessary to evaluate the currently data to approach the knowledge of its epidemiology and provide the clinicians relevant information to evaluate, for example, new complementary therapies to mechanical debridement treatment, such as probiotic or postbiotic gels [18, 19]. Therefore, the aim of this systematic review is to estimate the prevalence of peri-implantitis and its variations according to the applied definition and the elapsed time.
Methods
The present study was registered in PROSPERO with ID CRD42022313472. The practice-oriented research question was: “What is the current state of knowledge regarding the prevalence of peri-implantitis in patients treated with titanium dental implants?”.
Search strategy and search terms
A thorough search for literature was conducted from 1 December 2005 to 31 December 2021, using the following electronic databases: MEDLINE/PubMed, Web of Science, Science Direct and the Cochrane Library. The main key search terms used, alone or in combination with Boolean operators, for different searches were: "dental implants", "peri-implantitis" and "epidemiology”. The first combination was “dental implants and peri-implantitis” and the second option was “peri-implantitis epidemiology”. This search strategy was adapted for use in the various databases. Table 1 shows the results.
Table 1.
Number of articles found according to search strategy
| Database | “Dental implants” + “peri-implantitis” | “Peri-implantitis” + “epidemiology” | Total |
|---|---|---|---|
| PubMed | 681 | 53 | 734 |
| Web Of Science | 764 | 56 | 820 |
| Cochrane | 1054 | 19 | 1073 |
| Science Direct | 1241 | 68 | 1309 |
| TOTAL | 3740 | 196 | 3936 |
Screening and selection: eligibility criteria
The inclusion criteria were as follow: original studies describing the diagnosis of peri-implantitis (BOP, probing depth, loss of supporting bone); observational and experimental studies (cross-sectional, longitudinal, cohort or randomized controlled trial) with original prevalence data published over the past 16 years; studies published in peer review system journals; articles published in English language; and technical possibility to access the full text. The exclusion criteria were: studies where the number of subjects treated with implants were less than 10; or if the minimum time of function of implants was less than 5 years; and studies including subjects with clotting disorders.
The titles and summaries identified in the initial search were evaluated by three authors (PD, LJG-V and EG) for eligibility after removing duplicate items. Studies that appeared to meet the inclusion criteria were recovered in their full-text version and evaluated. A manual search of additional relevant titles was also carried out in the references section of each article. Any disagreement among the reviewers was resolved by discussion with all authors until consensus was reached.
Quality assessment of the risk of bias
The Cochrane Collaboration tool for assessing risk of bias was applied to the pre-selected papers [20]. Articles with ‘high risk’ were rejected.
Data extraction and collection
Once the articles meeting the inclusion criteria were identified, the following data for each article was collected using a specific form: surname and first author's name, geographic scope, sample size type of study design, type of peri-implantitis diagnosis, year and type of publication, and data on peri-implantitis for the calculation of prevalence at both, the patient level (number of patients with peri-implantitis/total number of patients × 100) and the implant level (number of implants with peri-implantitis/total number of implants × 100). This information was felt in different sections. These tasks were performed by the same three authors (PD, LJG-V and EG).
Statistical analysis
In order to reduce the heterogeneity of the results and to facilitate their interpretation, the studies were grouped according to diagnostic criteria into four groups: Group 1 (BOP, PD ≥ 6 mm and Loss of supporting Bone ≥ 3 mm), Group 2 (BOP, PD ≥ 6 mm and BL ≥ 2 mm), Group 3 (Progressive Bone Loss), and Group 4 (Other Criteria). A meta-analysis of Group 2 was performed due to its large specific weight (31 articles). The program used was MetaXL, tool for meta-analysis in Microsoft Excel for Windows. Sensitivity analyzes were performed, replicating the results after the exclusion of a study, to observe the robustness of the analysis and the influence of the eliminated study. Heterogeneity was evaluated using the I2 test which analyzes the proportion of total variability between studies explained by heterogeneity [21]. To prevent the presence of publication bias, we used the Egger´s regression test (p ≥ 0.1) complemented with Doi plot [22].
Results
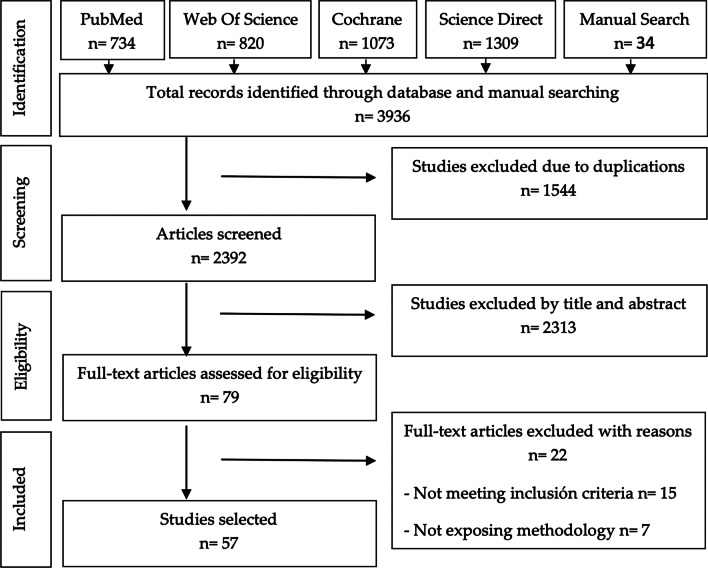
This initial electronic search produced 3902 articles and the manual search 34 articles. After eliminating duplication, examining, and applying inclusion criteria, 79 articles were included for data extraction and full-text evaluation. However, 22 articles were excluded because they did not meet the objectives of the review, or they did not have a clear methodology. Therefore, a total of 57 articles [23–79], were selected as relevant to the objectives of the review. The PRISMA flowchart in Fig. 1 synthesizes the screening and selection processes. The study designs were as follow: 18 cross-sectional studies, 18 longitudinal studies, 1 case–control study, 17 cohort studies, and 3 randomized controlled trials (Tables 2, 3, 4, 5, 6, 7, 8, 9). The study of Rodrigo et al. [66] was included in Groups 1 and 2.
Fig. 1.
PRISMA flowchart
Table 2.
Characteristics of studies Group 1 (BOP + probing depth ≥ 6 mm + bone loss ≥ 3 mm)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Costa [29] | SA | 80 | 221 | Longitudinal | 5 ± 0.5 | BOP/suppuration + PD ≥ 5 mm + BL ≥ 3 mm | Missing | 31.2217 |
| Rodrigo [66] | EU | 275 | 474 | Cohort | 9 ± 1.7 | BOP + BL ≥ 3 mm | 14.1818 | 11.3924 |
| Rocuzzo (2012) | EU | 101 | 228 | Cohort | 10 | BOP + PD ≥ 6 mm + BL ≥ 3 mm | 29.7029 | 17.1053 |
| Rocuzzo (2014) | EU | 123 | 246 | Longitudinal | 10 | BOP + PD ≥ 6 mm + BL ≥ 3 mm | Missing | 7.7236 |
| Shimchuk [71] | USA | 95 | 220 | Cross-sectional | 10.9 | BOP/suppuration + PD ≥ 6 mm + BL ≥ 3 mm | 6.3158 | 3.6363 |
| Tenenbam (2017) | EU | 52 | 108 | Cohort | 10.8 ± 1.7 | BOP/suppuration + PD ≥ 5 mm + BL ≥ 4.5 mm | 15.3846 | 12.037 |
| Trullenke-Eriksson (2015) | EU | 105 | 342 | Longitudinal | 13.19 ± 3.7 | BOP/suppuration + PD ≥ 5 mm + BL > 3 mm | Missing | 1.7544 |
Table 3.
Characteristics of studies Group 2 (BOP + probing depth ≥ 6 mm + bone loss ≥ 2 mm)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up |
Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Adler [23] | EU | 376 | 1095 | Cohort | 11 (9–15) | BOP/suppuration + PD > 5 mm + BL ≥ 2 mm | 21.0106 | Missing |
| Ahn [25] | Korea | 111 | 209 | Longitudinal | > 7 | BOP + PD > 5 mm + BL > 2 mm | Missing | 16.7464 |
| Bäumer [26] | EU | 100 | 242 | Longitudinal | 10 ± 0.31 (9.5–10.7) | BOP/suppuration + BL > 2 mm | 16 | 10.3306 |
| Becker [27] | EU | 92 | 328 | Longitudinal | 14 ± 1.9 | BOP/suppuration + PD ≥ 5 mm + BL ≥ 2.5 mm | Missing | 9.7561 |
| Dalago [30] | SA | 183 | 916 | Cross-sectional | > 5 | BOP/suppuration + PD > 5 mm + BL > 2 mm | 16.3934 | 7.3144 |
| Daubert [31] | USA | 96 | 225 | Cross-sectional | 10.9 ± 1.5 (8.9–14.8) | BOP/suppuration + PD ≥ 4 mm + BL ≥ 2 mm | 26.0417 | 16 |
| Den Hartog [33] | EU | 93 | 93 | Randomized Controlled Trial | 5 | BOP/suppuration + BL ≥ 2 mm | 15.0538 | 15.0538 |
| Derks[11] | EU | 427 | 1578 | Cross-sectional | 9 | BOP/suppuration + BL > 2 mm | 14.5199 | 7.9848 |
| Fransson [36] | EU | 182 | 1070 | Cross-sectional | 5 to 20 | BOP/suppuration + PD > 6 mm + BL > 2 mm | Missing | 39.1589 |
Table 4.
Characteristics of studies Group 2 (BOP + probing depth ≥ 6 mm + bone loss ≥ 2 mm)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate(DI) (%) | |
| Gamper [38] | EU | 56 | 143 | Randomized Controlled Trial | 5 | BOP/suppuration + PD ≥ 5 mm + BL ≥ 2 mm | 10.7143 | 7.6923 |
| Gatti [39] | EU | 56 | 227 | Cohort | 5 | BOP/suppuration + PD > 5 mm + BL > 2 mm | 3.5714 | 1.7621 |
| Gonzalez-Glez (2020) | EU | 65 | 558 | Longitudinal | 5 | BOP/suppuration + PD ≥ 5 mm + BL > 2 mm | 16.9231 | 1.9713 |
| Guarneri (2018) | EU | 74 | 166 | Longitudinal | 5 | BOP/suppuration + PD > 5 mm + BL > 2 mm | 13.5135 | 7.8313 |
| Hu [42] | Singapore | 200 | 284 | Cohort | 6.8 | BOP/ + incr PD + BL > 2 | 13 | 10.2113 |
| Ioannidis[43] | EU | 64 | 103 | Randomized Controlled Trial | 5 | BOP + BL > 2 mm | Missing | 6.7961 |
| Karlsson[44] | EU | 596 | Missing | Cohort | 9 | BOP/suppuration + BL > 2 mm | 18.4564 | Missing |
| Kosdsland (2010) | EU | 104 | 295 | Cross-sectional | 8.4 + 4.6 | BOP/suppuration + PD ≥ 4 mm + BL ≥ 2 mm | 47.1154 | 36.6102 |
| Konstantinidis [46] | EU | 90 | 226 | Cross-sectional | 5.5 | BOP + PD > 5 mm + BL > 2 mm | 13.3333 | 6.1947 |
Table 5.
Characteristics of studies Group 2 (BOP + probing depth ≥ 6 mm + bone loss ≥ 2 mm)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Lee [47] | Australia | 60 | 117 | Case–control | 8 (5–13.46) | BOP + PD ≥ 5 mm + BL > 2 mm | 26.6666 | 19.6581 |
| Marrone [49] | EU | 103 | 266 | Cross-sectional | > 5 | BOP/suppuration + PD > 5 mm + BL > 2 mm | 36.8932 | 22.9323 |
| Meijer [50] | EU | 140 | 276 | Cohort | 5 | BOP/suppuration + BL ≥ 2 mm | 17.1428 | 11.5942 |
| Nobre [53] | EU | 353 | 1238 | Cohort | 5 | BOP/suppuration + PD ≥ 5 mm + BL ≥ 2 mm | 24.0793 | Missing |
| Papaspyridakos (2019) | USA | 41 | 359 | Cohort | 5 | BOP/suppuration + BL > 2 mm | Missing | 8.0779 |
| Pimentel [56] | SA | 147 | 490 | Cross-sectional | > 5 | BOP/suppuration + PD > 4 mm + BL > 2 mm | 19.0476 | 9.1837 |
| Ravald [57] | EU | 46 | 371 | Longitudinal | 12 to 15 | BOP/suppuration + PD ≥ 4 mm + BL ≥ 2 mm | 21.7391 | 3.7786 |
| Ravidá [58] | USA | 145 | 382 | Longitudinal | 5.2–6.5 | BOP + BL > 2 mm | 16.5517 | 9.9476 |
| Rodrigo [66] | EU | 275 | 474 | Cohort | 9 ± 1.7 | BOP + BL ≥ 2 mm | 24 | 19.6202 |
Table 6.
Characteristics of studies Group 2 (BOP + probing depth ≥ 6 mm + bone loss ≥ 2 mm)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Rokn [67] | Iran | 134 | 478 | Cross-sectional | 5 | BOP/suppuration + BL > 2 mm | 20.1492 | 8.7866 |
| Romandini (2020) | EU | 99 | 458 | Cross-sectional | 7.8 | BOP/suppuration + BL ≥ 2 mm | 56.5656 | 27.9476 |
| Tey [75] | Singapore | 194 | 266 | Longitudinal | 5.2 ± 1.5 | BOP + PD ≥ 6 mm + BL ≥ 2.5 mm | 8.2474 | 7.1428 |
| Vandeweghe [77] | EU | 33 | 197 | Longitudinal | 14.3(10–21) | BOP/suppuration + PD > 6 mm + BL ≥ 2.5 mm | Missing | 4.0609 |
Table 7.
Characteristics of studies Group 3 (Progressive bone loss)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Chappuis [28] | EU | 67 | 95 | Cohort | 20 | BOP + infection + BL progressive | Missing | 13.6842 |
| Gurgel [32] | SA | 155 | Missing | Cross-sectional | 5 | BOP/supp + PD > 5 mm + BL Rx visibl | 28.3871 | Missing |
| Francetti [35] | EU | 46 | 56 | Longitudinal | 5 | BOP/supp + increPD + BL Rx visible | 0 | 0 |
| French [37] | EU-USA | 2060 | 4591 | Cohort | 6–7 | BOP/suppuration + PD > 2 mm + BL > 1 mm least year | 11.6990 | 4.7048 |
| Pandolfi [54] | EU | 475 | 1991 | Cohort | 10 | BOP/supp + BL changes | 9.6842 | 12.09081 |
| Ravidá[59] | USA | 99 | 221 | Cohort | 10.6 ± 4.5 | BOP/supp + increPD + BL progressiv | 20.4 | 15 |
| Rinke [61] | EU | 89 | Missing | Cross-sectional | 5.5 ± 2 | BOP/supp + PD ≥ 4 mm + BL progress | 11.2359 | Missing |
| Rinke[62] | EU | 65 | 112 | Longitudinal | 6.8 + 1.96 | BOP/supp + PD ≥ 5 mm + BL progress | 9.2308 | Missing |
| Rodrigo [65] | EU | 22 | 68 | Cohort | 5 | BOP/supp + PD ≥ 4 mm + BL significa | Missing | 5.8823 |
| Simonis [72] | EU | 55 | 124 | Longitudinal | 10 to 16 | BOP + PD ≥ 5 mm + BL > 0.2 mm/year | Missing | 16.9355 |
Table 8.
Characteristics of studies Group 3 (progressive bone loss)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Swierkot [73] | EU | 53 | 179 | Longitudinal | 5 to 16 | BOP + PD ≥ 5 mm + BL > 0.2 mm/year | 32.0755 | 23.4637 |
Table 9.
Characteristics of studies Group 4 (other criteria)
| Studies | Characteristics | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Population | Sample (N)(P) | Sample (N)(DI) | Design follow-up | Time load (Y) | Diagnostic | Rate (P) (%) | Rate (DI) (%) | |
| Aguirre-Zorzamo [24] | EU | 239 | 786 | Cross-sectional | 5.25 ± 3.4 | BOP/sup + incre PD + BL ≥ 1.5 mm | 15.0637 | 9.7964 |
| Mameno [48] | Japan | 477 | 1420 | Cohort | 5 to 10 | BOP/sup + BL ≥ 1 mm | 15.3040 | 9.2253 |
| Menini [51] | EU | 72 | 331 | Longitudinal | 5.8 | BOP/sup + BL | 6.9444 | 1.5106 |
| Mir-Mari [52] | EU | 245 | 964 | Cross-sectional | 6.3 ± 4.3 | BOP/sup + BL ≥ 2thread | 16.3265 | 9.1286 |
| Renvert [60] | EU | 213 | 976 | Cross-sectional | 10.8 ± 1.5 | BOP/sup + BL > 3 exposed threads | 15.0235 | Missing |
| Roos-Jansàker [69] | EU | 216 | 987 | Cross-sectional | 9 to14 | BOP/sup + BL > 1.8 mm | 16.2037 | 6.5856 |
| Serino [70] | EU | 23 | 109 | Cross-sectional | 5 to 10 | BOP/sup + PD ≥ 6 mm | 100 | 53.2110 |
| Van Velzen [78] | EU | 169 | 356 | Cohort | 10 | BOP + BL ≥ 1.5 mm | 14.7929 | 7.0225 |
| Wada [79] | Japan | 543 | 1613 | Longitudinal | 5.8 ± 2.5 | BOP/sup + BL > 1 mm | 15.8379 | 9.2374 |
The peri-implantitis mean prevalence obtained was 19.53% (95% CI, 12.87 to 26.19%) at the patient-level and 12.53% (11.67 to 13.39%) at the implant-level. Table 10 shows all the results of the study. Given the high specific weight of group 2 compared to the other groups (53.45%), the total results were calculated by weighted average.
Table 10.
Means of peri-implantitis prevalence (%), with confidence interval (CI-95%) in parenthesis
| Group | Patient-level | Implant-level |
|---|---|---|
| 1 | 16.4 (0.9–31.89) | 12.12 (2.96–21.29) |
| 2 | 20.67 (15.89–25.44) | 12.65 (8.98–16.31) |
| 3 | 14.68 (4.13–25.23) | 12.04 (4.71–19.37) |
| 4 | 23.94 (1.91–45.98) | 13.21 (0.45–25.98) |
| Total | 19.53 (12.87–26.19) | 12.53 (11.67–13.39) |
In addition, an analysis of the influence of the load time or time variable was carried out based on implants on the registered peri-implantitis prevalence at the patient level and at the implant level, that is displayed in Table 11. No significant differences were observed in prevalence among studies with follow-up period of 5 to 9 years and studies with greater longevity, both at patient-level (17.1% vs. 18.63%, p = 0.82) as at implant-level (10.98% vs. 9.76%, p = 0.8).
Table 11.
Prevalence of peri-implantitis (%) at patient-level and implant-level, in function of load time (CI-95%)
| Group | Patient-level | Implant-level | ||
|---|---|---|---|---|
| 5–9 y | > 9 y | 5–9 y | > 9 y | |
| 1 | 14.18 | 17.13 (− 12.15 to 46.4) | 21.3 (104.67 to 147.28) | 8.45 (1.51–15.39) |
| 2 | 20.57 (14.88 to 26.29) | 21.2 (14.65 to 27.74) | 12.32 (8.53 to 16.11) | 8.78 (2.49–15,08) |
| 3 | 12.11 (− 0.62 to 24.84) | 15.04 (− 53.06 to83.14) | 3.53 (− 4.2 to 11.25) | 15.78 (12.07–19.48) |
| 4 | 13.54 (6.49 to 20.59) | 15.34 (13.46 to 17.21) | 7.42 (1.13 to 13.7) | 6.8 (4–9.6) |
| Total | 17.1 | 18.63 | 10.98 | 9.76 |
Considering the Consensus report of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions [15] recommending that probing depth should not be included as a diagnostic criterion, studies have been divided according to this variable to study its impact on peri-implantitis prevalence (Table 12). Prevalence in studies that used probing depth, as one more diagnostic criterion was higher than those that did not used it, both at patient-level (24.69% and 17.56% respectively) and at implant-level (15.21% and 11.99% respectively). However, no significant differences were observed (p = 0.27 and p = 0.31 respectively).
Table 12.
Peri-implantitis prevalence with/without probing depth inclusion (CI-95%)
| PI with probing depth | PI without probing depth | ||||
|---|---|---|---|---|---|
| Group | Patient-level | Implant-level | Group | Patient-level | Implant-level |
| 1 | 17.13 (− 12.1 to 46.41) | 12.25 (0.86 to 23.62) | 1 | 14.18 | 11.39 |
| 2 | 19.89 (14.09 to 25.69) | 12.78 (7.24 to 18.08) | 2 | 22.05 (11.85–32.24) | 13.07 (7.87–17.34) |
| 3 | 16.15 (5.03 to 25.75) | 10.99 (1.82 to 21.95) | 3 | 9.68 | 13.29 (8.4–18.18) |
| 4 | 57.53(− 482.1 to 597.16) | 31.05 (− 244.3 to 307.3) | 4 | 14.35 (11.27–17.41) | 7.12 (2.68–9.61) |
| Total | 24.69 | 15.21 | Total | 17.56 | 11.99 |
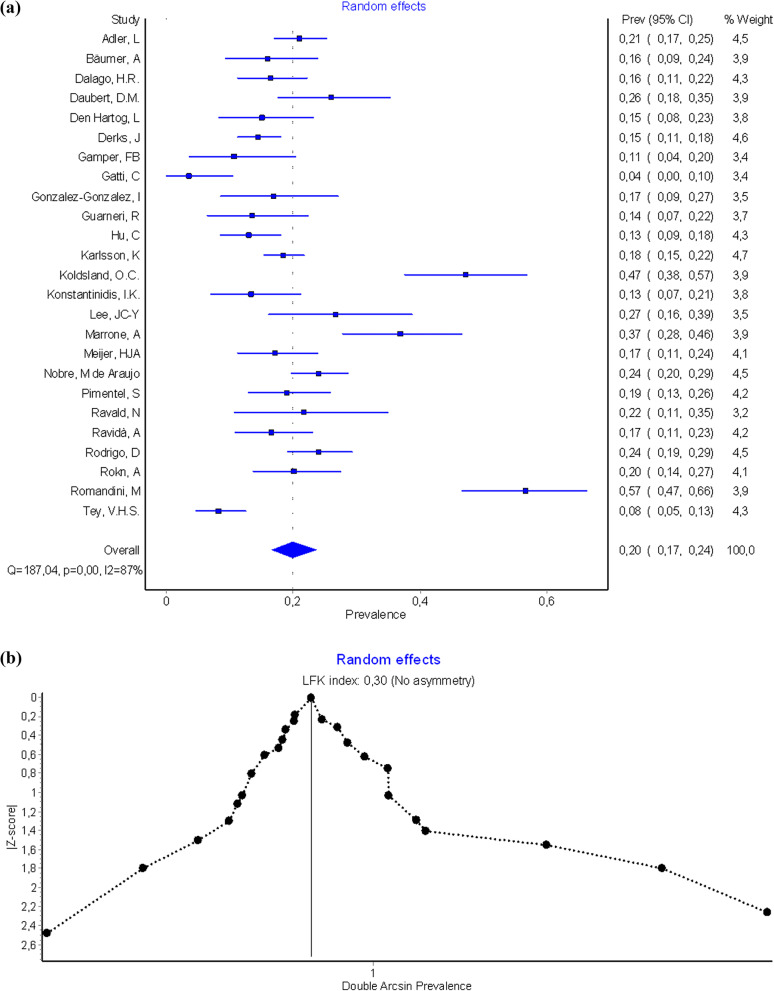
The results of meta-analysis indicated a prevalence of peri-implantitis at patient level of 19.6% (CI-95%, 18.4–20.8) for the fixed effects model and 20% (CI-95%, 16.6–23.7) for the random effects model. Results of meta-analysis for the quality effects by income (studies with high effect size -Pimentel [56], Ravald [57], Rokn [67]—versus low/middle effects size -Romandini [68], Konstantinidis [46], Tey [75] -), were 19.2% (CI-95%, 15.2–23.6). Heterogeneity analysis in both models was high (I2 = 87.169%). (Fig. 2a, b shows Forest plot and Doi plot-LFK index). After performing the sensitivity analysis excluding studies of Gatti [39], Koldsland [45], Marrone [49], Romandini [68], and Tey [75] (Fig. 2c), the prevalence at the patient level for the random effects was 18.1% (CI-95%, 16.2–19.9%), with moderate heterogeneity (I2 = 44.3%). The Egger’s intercept test was 0.17 (CI-95%, 0.12–0.23; t = 6.70; df = 19; p = 0.846) and LFK index was 0.30 indicating no small-study effects.
Fig. 2.
a Forest plot prevalence of peri-implantitis at patient-level. b Doi plot prevalence peri-implantitis at patient-level and LFK index analysis of publication bias. c Funnel plot prevalence of peri-implantitis at patient-level
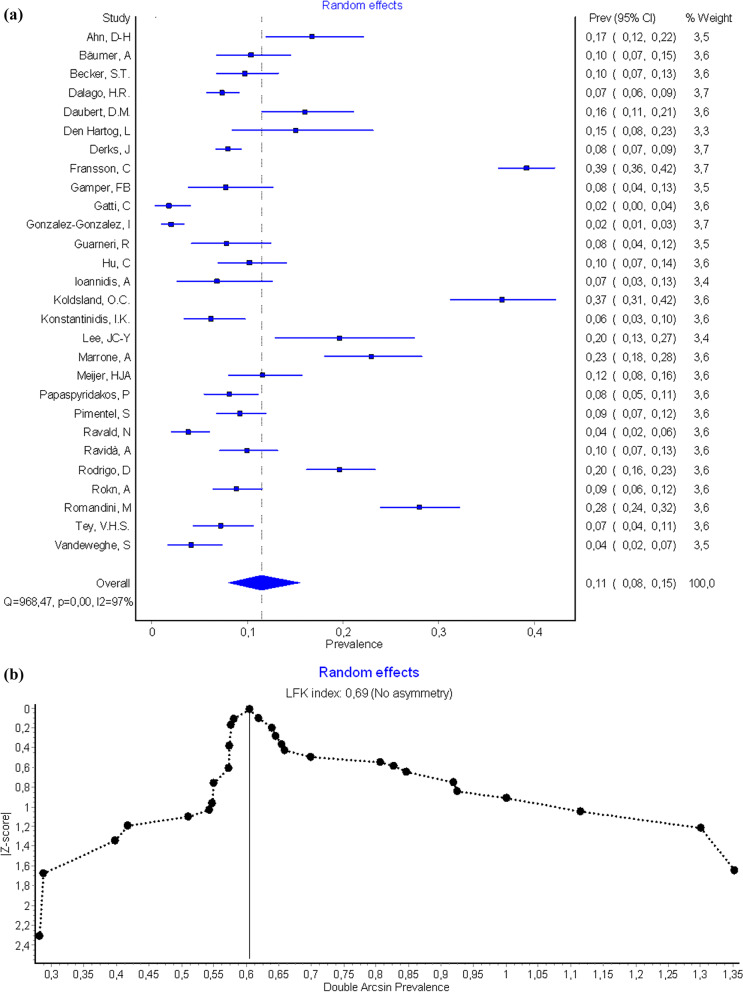
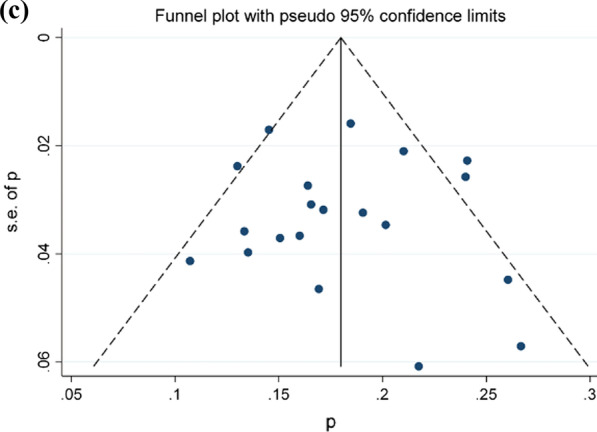
The prevalence of peri-implantitis at implant-level obtained was 12.3% (CI-95%, 11.7–12.9%) for the fixed effects model and 11.5% (CI-95%, 8–15.4%) for the random effects model. Results of meta-analysis for the quality effects by income (studies with high effect size-Meijer [50], Den Hartog [33], Bäumer [26]- vs. low/middle effects size -Fransson [36], Gonzalez-Gonzalez [40], Koldsland [45]-), was 11.1% (CI-95%, 7–15.9). High heterogeneity in both models was also observed (I2 = 97,21%). (Fig. 3a, b shows Forest plot and Doi plot-LFK index). After performing the sensitivity analysis excluding studies of Fransson [36], Gatti [39], González-González [40], Koldsland [45], Lee [47], Marrone [49], Ravald [57], Rodrigo [66], Romandini [68] and Vandeweghe [77] (Fig. 3c), the prevalence at the implant level was 9.1% (95% CI, 8.1–10.2%). Meta-analysis found heterogeneity among the studies (I2 = 46.2%). The Egger’s test was 0.06 (CI-95%, 0.00–0.39; t = 5.96; df = 17; p = 0.01) and LFK index was 0.69 indicating the presence of small-study effects (Fig. 3a, b).
Fig. 3.
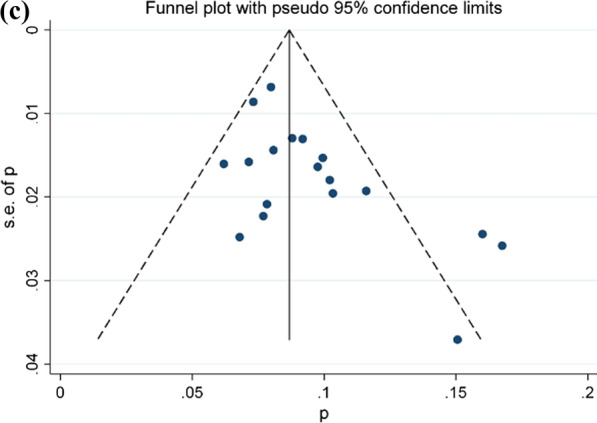
a Forest plot prevalence of peri-implantitis at implant-level. b Doi plot prevalence of peri-implantitis at implant-level and LFK index analysis of publication bias. c Funnel plot prevalence of peri-implantitis at implant-level
Discussion
The present systematic review highlighted some limitations of the definition, severity, and prevalence of peri-implantitis. Peri-implant health can exist around implants with reduced bone support. Peri-implantitis occurring in sites with clinical signs of inflammation, bleeding on probing and/or suppuration, increased probing depths and/or recession of the mucosal margin in addition to radiographic bone loss [15].
The case definition of peri-implantitis is affected by the different criteria used to define a “case” in studies investigating the prevalence of peri-implant diseases [80]. Discordance in disease definition among published studies makes the prevalence range highly variable and illustrates the lack of consensus in research, making it difficult to globally estimate the real elementary epidemiological parameters such as prevalence [13, 81]. In fact, there is currently a difference in how the peri-implantitis is defined in daily clinical practice and in epidemiological studies. Zitzmann and Berglundh [2] suggested that epidemiological research on peri-implant diseases should report not only on the prevalence or incidence of such but also on extent and severity. To determine the prevalence and incidence of peri-implantitis correctly, more prospective studies with adequate sample size and sampling method would be needed. In addition, baseline radiographic and probing measurements before and after loading the implant supported prosthesis must be performed to establish a bone level reference of physiological remodeling. Currently not many studies of this type are available. Most of the studies included in this research provided data from convenience samples, and most data were cross-sectional or collected retrospectively, rather than using randomized samples, resulting in a potential selection bias.
In the present systematic review, because a direct comparison was not possible, the referent case definition of peri-implantitis was subdivided into 4 groups with various thresholds for bone loss or exposed implant threads, and values for included peri-implant pocket depths, because only a few study protocols have applied the new classification of periodontal diseases of World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions [15]. The design was thoroughly done to review the published literature and to retrieve as much data as possible from the filtered papers.
Revised studies reported a mean prevalence for peri-implantitis of 19.53% at patient-level and 12.53% at implant-level. The global values reported at patient-level were similar to those previously reported by Ting et al. [82] (18.8%), Atieh et al. [83] (18.8%), and Lee et al. [84] (19.83%). However, the same authors reported lower prevalence values than in the present review at implant-level (9.25–9.6%). The differences may be because a small-study effect was found in the present study. Derks and Tomasi [13] also showed similar results at patient-level (21.7%). Conversely, Salvi et al. [85] reported lower prevalence values both at patient-level (10.3%) and at implant-level (7.5%).
Follow-up time and the evaluation in a convenience population may have influenced the prevalence values since peri-implantitis represents rather a chronic form of disease implying time for the osseous destruction [86]. Analyzing the influence of the period of functional loading, the results showed no differences in prevalence among studies with a follow-up period of 5 to 9 years and studies over 9 years of function, both at patient-level (17.19 and 17.75% respectively) and at implant-level (11.11 and 9.43% respectively). Conversely, Derks and Tomasi [13] meta-regression showed a significant positive relationship between the prevalence of peri-implantitis and mean function time, in a follow-up period of 3 to 9 years. Consistent with the present systematic review, Dreyer et al. [87] have reported that there is not an increase in the prevalence of peri-implantitis at patient-level due to longer functional loading period.
The authors are unaware of previous studies analyzing the influence of probing depth measurement and how it affects the prevalence of peri-implantitis. In this systematic review it was observed that the prevalence of peri-implantitis was higher when probing depth was used as one of the diagnostic criteria, but without significant differences. Hence the controversy of changes in the definition of peri-implantitis.
The meta-analysis of the prevalence of peri-implantitis should be interpreted with caution, due to the high heterogeneity found in the group 2. Muñoz Giraldo et al. [88] reported a prevalence of peri-implantitis of 18% at patient-level similar to the results of the study (20%). However, their I2 index of 95.7% was higher than in the present study (87.169%). At implant-level, the present study reported a prevalence of peri-implantitis of 11.5% with I2 index of 97.21%, and Muñoz Giraldo et al. [88] obtained a prevalence of 10% consistent with this data, also with a high heterogeneity (I2 = 95.0%). The differences in heterogeneity observed between both systematic reviews and meta-analysis, may be due to the greater number of studies (33) included in this study for the group analyzed (probing depth ≥ 6 mm).
Limitations of the study included that the methodology used in data collection did not record the ethnic differences in the populations of the selected studies. But the main limitation of this study was the small number of articles found with original prevalence data of peri-implantitis (number of cases and number of patients/implants). The strength of this study is its novelty when analyzing the prevalence by four diagnostic criteria.
Conclusions
Within the limitations of this study, it can be concluded that prevalence of peri-implantitis, using 4 different definitions, was found to be approximately 20% at patient-level and 11.5% at implant-level. The results indicate that the identification of the peri-implantitis diagnostic criteria is essential to achieve greater accuracy in the disease classification, and for correct estimation of the true prevalence value of peri-implantitis. Further studies should use more consistent periodontal measurements, and using only the definition proposed in the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions.
Acknowledgements
The authors thank Dr. Carmen Bravo, Centre of Data Processing, Computing Service for Research Support, Complutense University of Madrid for her assistance with the statistical analysis and Prof. Ignacio Sanz-Sánchez, Complutense University of Madrid for his assistance with the methodology.
Abbreviations
- CI
Confidence Interval
- BOP
Bleeding on Probe
- PD
Probing Depth
Author contributions
PD contributed to the conceptualization, literature search, data acquisition and interpretation, first drafted and critically revised the manuscript; EG contributed to the conceptualization, literature search, analysis interpretation, writing, editing and critical review of the article; LJGV contributed to the literature search, data acquisition, writing, editing and critical review of the article; BM contributed to data acquisition, and critical review of the article; MJS contributed to the conceptualization, supervision, project administration and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the manuscript.
Funding
None.
Availability of data and materials
All data analyzed during this study are included in this manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berglundh T, Persson LG, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J Clin Periodontol. 2008;3:197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. [DOI] [PubMed] [Google Scholar]
- 2.Zitzmann NU, Berglundh T. Definition and Prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8):286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T, Donos N. Implant survival and complications. The third EAO consensus conference 2012. Clin Oral Implants Res. 2012;23(6):63–65. doi: 10.1111/j.1600-0501.2012.02557.x. [DOI] [PubMed] [Google Scholar]
- 4.Pjetursson BE, Karoussis I, Burgin W, Bragger U, Lang NP. Patients’ satisfaction following implant therapy. A 10-year prospective cohort study. Clin Oral Implants Res. 2005;16:185–193. doi: 10.1111/j.1600-0501.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 5.Fardal O, Grytten J. A comparison of teeth and implants during maintenance therapy in terms of the number of disease-free years and costs: an in vivo internal control study. J Clin Periodontol. 2013;40:645–651. doi: 10.1111/jcpe.12101. [DOI] [PubMed] [Google Scholar]
- 6.Listl S, Fischer L, Giannakopoulos NN. An economic evaluation of maxillary implant overdentures based on six vs. four implants. BMC Oral Health. 2014;14:105. doi: 10.1186/1472-6831-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derks J, Hakansson J, Wennstrom JL, Klinge B, Berglundh T. Patient-reported outcomes of dental implant therapy in a large randomly selected sample. Clin Oral Implants Res. 2015;26:586–591. doi: 10.1111/clr.12464. [DOI] [PubMed] [Google Scholar]
- 8.Vogel R, Smith-Palmer J, Valentine W. Evaluating the health economic implications and cost-effectiveness of dental implants: a literature review. Int J Oral Maxillofac Implants. 2013;28:343–356. doi: 10.11607/jomi.2921. [DOI] [PubMed] [Google Scholar]
- 9.Lindhe J, Meyle J. Group of European Workshop on Periodontology. Peri-implant diseases: consensus of the sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:282–285. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 10.Charalampakis G, Rabe P, Leonhardt A, Dahlén G. A follow-up study of peri-implantitis cases after treatment. J Clin Periodontol. 2011;38:864–871. doi: 10.1111/j.1600-051X.2011.01759.x. [DOI] [PubMed] [Google Scholar]
- 11.Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, Berglundh T. Peri-implantitis-onset and patter of progression. J Clin Periodontol. 2016;43:383–388. doi: 10.1111/jcpe.12535. [DOI] [PubMed] [Google Scholar]
- 12.Mombelli A, Mueller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23:66–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 13.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42:158–171. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 14.Tomasi C, Derks J. Clinical research of peri-implant diseases–quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J Clin Periodontol. 2012;39(12):207–223. doi: 10.1111/j.1600-051X.2011.01831.x. [DOI] [PubMed] [Google Scholar]
- 15.Berglundh T, Armitage G, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 2018;45(20):S286–S291. doi: 10.1111/jcpe.12957. [DOI] [PubMed] [Google Scholar]
- 16.Patten SB. Selection bias in studies of major depression using clinical subjects. J Clin Epidemiol. 2000;53:351–357. doi: 10.1016/S0895-4356(99)00215-2. [DOI] [PubMed] [Google Scholar]
- 17.Heitz-Mayfield LJA. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 18.Butera A, Pascadopoli M, Pellegrini M, Gallo S, Zampetti P, Cuggia G, Scribante A. Domiciliary use of chlorhexidine vs. postbiotic gels in patients with peri-implant mucositis: a split-mouth randomized clinical trial. Appl Sci. 2022;12:2800. doi: 10.3390/app12062800. [DOI] [Google Scholar]
- 19.Alqahtani F, Alshaikh M, Mehmood A, Alqhtani N, Alkhtani F, Alenazi A. Efficacy of antibiotic versus probiotics as adjuncts to mechanical debridement for the treatment of peri-implant mucositis. J Oral Implantol. 2022;48(2):99–104. doi: 10.1563/aaid-joi-D-20-00259. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions V.5.1.0. The Cochrane Library; 2011. [Accessed 26.10.16] http://www.cochrane-handbook.org.
- 21.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester UK; 2009.
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler L, Buhlin K, Jansson L. Survival and complications: a 9- to 15-year retrospective follow-up of dental implant therapy. J Oral Rehabil. 2020;47:67–77. doi: 10.1111/joor.12866. [DOI] [PubMed] [Google Scholar]
- 24.Aguirre-Zorzano LA, Estefania-Fresco R, Telletxea O, Bravo M. Prevalence of peri-implant inflammatory disease in patients with a history of periodontal disease who receive supportive periodontal therapy. Clin Oral Impl Res. 2015;26:1338–1344. doi: 10.1111/clr.12462. [DOI] [PubMed] [Google Scholar]
- 25.Ahn D-H, Kim H-J, Joo J-Y, Lee J-Y. Prevalence and risk factors of peri-implant mucositis and peri-implantitis after at least 7 years of loading. J Periodontol Implant Sci. 2019;49:397–405. doi: 10.5051/jpis.2019.49.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baeumer A, Toekan S, Saure D, Koerner G. Survival and success of implants in a private periodontal practice: a 10 year retrospective study. BMC Oral Health. 2020;20:92. doi: 10.1186/s12903-020-01064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker ST, Beck-Broichsitter BE, Rossmann CM, Behrens E, Jochens A, Wiltfang J. Long-term survival of Straumann dental implants with TPS surfaces: a retrospective study with a follow-up of 12 to 23 years. Clin Implant Dent Relat Res. 2016;18:480–488. doi: 10.1111/cid.12334. [DOI] [PubMed] [Google Scholar]
- 28.Chappuis V, Buser R, Brägger U, Bornstein MM, Salvi GE, Buser D. Long-term outcomes of dental implants plasma-sprayed Surface: a 20-year prospective case series study in partially edentulous patients. Clin Implant Dent Relat Res. 2013;15:780–790. doi: 10.1111/cid.12056. [DOI] [PubMed] [Google Scholar]
- 29.Costa FO, Takenaka-Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39:173–181. doi: 10.1111/j.1600-051X.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- 30.Dalago HR, Schuldt Filho G, Rodrigues MA, Renvert S, Bianchini MA. Risk indicators for peri-implantitis. A cross-sectional study with 916 implants. Clin Oral Implants Res. 2017;28:144–150. doi: 10.1111/clr.12772. [DOI] [PubMed] [Google Scholar]
- 31.Daubert DM, Weinstein BF, Bordin S, Leroux BG, Flemmig TF. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015;86:337–347. doi: 10.1902/jop.2014.140438. [DOI] [PubMed] [Google Scholar]
- 32.Gurgel BCDV, Montenegro SCL, Dantas PMC, Pascoal ALDB, Lima KC, Calderon PDS. Frequency of peri-implant diseases and associated factors. Clin Oral Implants Res. 2017;28:1211–1217. doi: 10.1111/clr.12944. [DOI] [PubMed] [Google Scholar]
- 33.den Hartog L, Meijer HJA, Vissink A, Raghoebar GM. Anterior single implants with different neck designs: 5 Year results of a randomized clinical trial. Clin Implant Dent Relat Res. 2017;19:717–724. doi: 10.1111/cid.12498. [DOI] [PubMed] [Google Scholar]
- 34.Derks J, Schaller D, Hakansson J, Wennstrom JL, Tomasi C, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95:43–49. doi: 10.1177/0022034515608832. [DOI] [PubMed] [Google Scholar]
- 35.Francetti L, Rodolfi A, Barbaro B, Taschieri S, Cavali N, Corbella S. Implant success rates in full-arch rehabilitations supported by upright and tilted implants: a retrospective investigation with up to five years of follow-up. J Periodontol Implants Sci. 2015;45:210–215. doi: 10.5051/jpis.2015.45.6.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fransson C, Wennström J, Tomasi C, Berglundh T. Extent of peri-implantitis associated bone loss. J Clin Periodontol. 2009;36:357–363. doi: 10.1111/j.1600-051X.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 37.French D, Grandin HM, Ofec R. Retrospective cohort study of 4,591 dental implants: analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J Periodontol. 2019;90:691–700. doi: 10.1002/JPER.18-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gamper FB, Benic GI, Sanz-Martín I, Asgeirson AG, Hämmerle CHF, Thoma DS. Randomized controlled clinical trial comparing one-piece and two-piece dental implants supporting fixed and removable dental prostheses: 4- to 6-year observations. Clin Oral Implants Res. 2017;28:1553–1559. doi: 10.1111/clr.13025. [DOI] [PubMed] [Google Scholar]
- 39.Gatti C, Gatti F, Chiapasco M, Esposito M. Outcome of dental implants in partially edentulous patients with and without a history of periodontitis: a 5-year interim analysis of a cohort study. Eur J Oral Implantol. 2008;1:45–51. [PubMed] [Google Scholar]
- 40.González-González I, de Llanos-Lanchares H, Brizuela-Velasco A, Alvarez-Riego JA, Llorente-Pendas S, Herrero-Climent M, Alvarez-Arenal A. Complications of fixed full-arch implant-supported metal-ceramic prostheses. Int J Environ Res Public Health. 2020;17:4250. doi: 10.3390/ijerph17124250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarnieri R, Grande M, Zuffetti F, Testori T. Incidence of peri-implant diseases on implants with and without laser-microgrooved collar: a 5-year retrospective study carried out in private practice patients. Int Oral and Maxillofac Implants. 2018;33:457–465. doi: 10.11607/jomi.6178. [DOI] [PubMed] [Google Scholar]
- 42.Hu C, Lang NP, Ong MM-A, Lim LP, Tan WC. Influence of periodontal maintenance and periodontitis susceptibility on implant success: a 5-year retrospective cohort on moderately rough surfaced implants. Clin Oral Implants Res. 2020;31:727–736. doi: 10.1111/clr.13621. [DOI] [PubMed] [Google Scholar]
- 43.Ioannidis A, Heierle L, Hämmerle CHF, Hüsler J, Jung RE, Thoma DS. Prospective randomized controlled clinical study comparing two types of two-piece dental implants supporting fixed reconstructions—results at 5 years of loading. Clin Oral Implants Res. 2019;30:1126–1133. doi: 10.1111/clr.13526. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson K, Derks J, Wennstrom JL, Petzold M, Berglundh T. Occurrence and clustering of complications in implant dentistry. Clin Oral Implants Res. 2020;31:1002–1009. doi: 10.1111/clr.13647. [DOI] [PubMed] [Google Scholar]
- 45.Koldsland OC, Schei AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;8:231–238. doi: 10.1902/jop.2009.090269. [DOI] [PubMed] [Google Scholar]
- 46.Konstantinidis IK, Kotsakis GA, Gerdes S, Walter MH. Cross-sectional study on the prevalence and risk indicators of periimplant diseases. Eur J Oral Implantol. 2015;8:75–88. [PubMed] [Google Scholar]
- 47.Lee JC-Y, Mattheos N, Nixon KC, Ivanovski S. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin Oral Implants Res. 2012;23:325–333. doi: 10.1111/j.1600-0501.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 48.Mameno T, Wada M, Onodera Y, Fujita D, Sato H, Ikebe K. Longitudinal study on risk indicators for peri-implantitis using survival-times analysis. J Prosthodont Res. 2019;63:216–220. doi: 10.1016/j.jpor.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Marrone A, Lasserre J, Bercy P, Brecx MC. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin Oral Implants Res. 2013;24:934–940. doi: 10.1111/j.1600-0501.2012.02476.x. [DOI] [PubMed] [Google Scholar]
- 50.Meijer HJA, Raghoebar GM, de Waal YCM, Vissink A. Incidence of peri-implant mucositis and peri-implantitis in edentulous patients with an implant-retained mandibular overdenture during a 10-year follow-up period. J Clin Periodontol. 2014;41:1178–1183. doi: 10.1111/jcpe.12311. [DOI] [PubMed] [Google Scholar]
- 51.Menini M, Setti P, Pera P, Pera F, Pesce P. Peri-implant tissue health and bone resorption in patients with immediately loaded, implant-supported, full-arch prostheses. Int J Prosthodont. 2018;31:327–333. doi: 10.11607/ijp.5567. [DOI] [PubMed] [Google Scholar]
- 52.Mir-Mari J, Mir-Orfila P, Figueiredo R, Valmaseda-Castellon E, Gay-Escoda C. Prevalence of peri-implant diseases. A cross-sectional study based on a private practice environment. J Clin Periodontol. 2012;39:490–494. doi: 10.1111/j.1600-051X.2012.01872.x. [DOI] [PubMed] [Google Scholar]
- 53.Nobre MA, Salvado F, Nogueira P, Rocha E, Ilg P, Maló P. A peri-implant disease risk score for patients with dental implants: validation and the influence of the Interval between maintenance appointments. J Clin Med. 2019;8:252. doi: 10.3390/jcm8020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandolfi A, Rinaldo F, Pasqualotto D, Sorrentino F, La Torre G, Guerra F. A retrospective cohort study on peri-implant complications in implants up to 10 years of functional loading in periodontally compromised patients. J Periodontol. 2020;91:995–1002. doi: 10.1002/JPER.18-0715. [DOI] [PubMed] [Google Scholar]
- 55.Papaspiridakos P, Bordin TB, Natto ZS, El-Rafie K, Pagni SE, Cochlidakis K, Ercoli C, Weber HP. Complications and survival rates of 55 metal-ceramic implant-supported fixed complete-arch prostheses: a cohort study with mean 5-year follow-up. J Prosthet Dent. 2019;122:441–449. doi: 10.1016/j.prosdent.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Pimentel SP, Shiota R, Cirano FR, et al. Occurrence of peri-implant diseases and risk indicators at the patient and implant levels: a multilevel cross-sectional study. J Periodontol. 2018;89:1091–1100. doi: 10.1002/JPER.17-0599. [DOI] [PubMed] [Google Scholar]
- 57.Ravald N, Dahlgren S, Teiwik A, Grondahl K. Long-term evaluation of Astra Tech and Brånemark implants in patients treated with full-arch bridges. Results after 12–15years. Clin Oral Implants Res. 2013;24:1144–1151. doi: 10.1111/j.1600-0501.2012.02524.x. [DOI] [PubMed] [Google Scholar]
- 58.Ravidà A, Tattan M, Askar H, Barootchi S, Tavelli L, Wang H-L. Comparison of three different types of implant-supported fixed dental prostheses: a long-term retrospective study of clinical outcomes and cost-effectiveness. Clin Oral Implants Res. 2019;30:295–305. doi: 10.1111/clr.13415. [DOI] [PubMed] [Google Scholar]
- 59.Ravidà A, Vera Rodríguez M, Saleh HAM, Galli M, Qazi M, Troiano G, Wang H-L, Galindo Moreno P. The correlation between history of periodontitis according to staging and grading and the prevalence/severity of peri-implantitis in patients enrolled in maintenance therapy. J Periodontol. 2021;92:1522–1535. doi: 10.1002/JPER.21-0012. [DOI] [PubMed] [Google Scholar]
- 60.Renvert S, Roos-Jansaker A-M, Lindahl C, Renvert H, Persson GR. Infection at titanium implants with or without a clinical diagnosis of inflammation. Clin Oral Implants Res. 2007;18:509–516. doi: 10.1111/j.1600-0501.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 61.Rinke S, Ohl S, Ziebolz D, Lange K, Eickholz P. Prevalence of periimplant disease in partially edentulous patients: a practice-based cross-sectional study. Clin Oral Implants Res. 2011;22:826–833. doi: 10.1111/j.1600-0501.2010.02061.x. [DOI] [PubMed] [Google Scholar]
- 62.Rinke S, Roediger M, Eickholz P, Lange K, Ziebolz D. Technical and biological complications of single-molar implant restorations. Clin Oral Implants Res. 2015;26:1024–1030. doi: 10.1111/clr.12382. [DOI] [PubMed] [Google Scholar]
- 63.Roccuzzo M, Bonino F, Aglietta M, Dalmasso P. Ten-year results of a three arms prospective cohort study on implants in periodontally compromised patients. Part 2: clinical results. Clin Oral Implants Res. 2012;23:389–395. doi: 10.1111/j.1600-0501.2011.02309.x. [DOI] [PubMed] [Google Scholar]
- 64.Roccuzzo M, Bonino L, Dalmasso P, Aglietta M. Long-term results of a three arms prospective cohort study on implants in periodontally compromised patients: 10-year data around sandblasted and acid-etched (SLA) surface. Clin Oral Implants Res. 2014;25:1105–1112. doi: 10.1111/clr.12227. [DOI] [PubMed] [Google Scholar]
- 65.Rodrigo D, Martin C, Sanz M. Biological complications and peri-implant clinical and radiographic changes at immediately placed dental implants. A prospective 5-year cohort study. Clin Oral Implants Res. 2012;23:1224–1231. doi: 10.1111/j.1600-0501.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- 66.Rodrigo D, Sanz-Sánchez I, Figuero E, et al. Prevalence and risk indicators of peri-implant diseases in Spain. J Clin Periodontol. 2018;45:1510–1520. doi: 10.1111/jcpe.13017. [DOI] [PubMed] [Google Scholar]
- 67.Rokn A, Akbari S, Roosta HA, Najafi H, Zayeri F, Hashemi K. Prevalence of peri-implantitis in patients not participating in well-designed supportive periodontal treatments: a cross sectional study. Clin Oral Implants Res. 2017;28:314–319. doi: 10.1111/clr.12800. [DOI] [PubMed] [Google Scholar]
- 68.Romandini M, Lima C, Pedrinaci I, Araoz A, Costanza Soldini M, Sanz M. Clinical signs, symptoms, perceptions, and impact on quality of life in patients suffering from peri-implant diseases: a university representative cross-sectional study. Clin Oral Implants Res. 2021;32:100–111. doi: 10.1111/clr.13683. [DOI] [PubMed] [Google Scholar]
- 69.Roos-Jansaker AM, Renvert H, Lindahl C, Renvert S. Nine to four teen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33:296–301. doi: 10.1111/j.1600-051X.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 70.Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20:169–174. doi: 10.1111/j.1600-0501.2008.01627.x. [DOI] [PubMed] [Google Scholar]
- 71.Shimchuk AA, Weinstein BF, Daubert DM. The impact of a change in classification criteria on the prevalence of peri-implantitis: a cross-sectional analysis. J Periodontol. 2020;1–8 [DOI] [PubMed]
- 72.Simonis P, Dufour T, Tenenbaum H. Long-term implant survival and success: a 10–16-year follow-up of non-submerged dental implants. Clin Oral Implants Res. 2010;21:772–777. doi: 10.1111/j.1600-0501.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 73.Swierkot K, Lottholz P, Flores-de-Jacoby L, Mengel R. Mucositis, peri-implantitis, implant success, and survival of implants in patients with treated generalized aggressive periodontitis. 3- to 16-year results of a prospective long-term cohort study. J Periodontol. 2012;83:1213–1225. doi: 10.1902/jop.2012.110603. [DOI] [PubMed] [Google Scholar]
- 74.Tenenbaum H, Bogen O, Séverac F, Elkaim R, Davideau J-L, Huck O. Long-term prospective cohort study on dental implants: clinical and microbiological parameters. Clin Oral Implants Res. 2017;28:86–94. doi: 10.1111/clr.12764. [DOI] [PubMed] [Google Scholar]
- 75.Tey VHS, Phillips R, Tan K. Five-year retrospective study on success, survival and incidence of complications of single crowns supported by dental implants. Clin Oral Implants Res. 2017;28:620–625. doi: 10.1111/clr.12843. [DOI] [PubMed] [Google Scholar]
- 76.Trullenque-Eriksson A, Guisado MB. Retrospective long-term evaluation of dental implants in totally and partially edentulous patients: part II: periimplant disease. Implant Dent. 2015;24:217–221. doi: 10.1097/ID.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 77.Vandeweghe S, Ferreira D, Vermeersch L, Mariën M, De Bruyn H. Long-term retrospective follow-up of turned and moderately rough implants in the edentulous jaw. Clin Oral Implants Res. 2016;27:421–426. doi: 10.1111/clr.12602. [DOI] [PubMed] [Google Scholar]
- 78.Van Velzen FJJ, Ofec R, Schulten EAJM, ten Bruggenkate CM. 10-year survival rate and the incidence of peri-implant disease of 374 titanium dental implants with a SLA surface: a prospective cohort study in 177 fully and partially edentulous patients. Clin Oral Implants Res. 2015;26:1121–1128. doi: 10.1111/clr.12499. [DOI] [PubMed] [Google Scholar]
- 79.Wada M, Mameno T, Onodera Y, Matsuda H, Daimon K, Ikebe K. Prevalence of peri-implant disease and risk indicators in a Japanese population with at least 3 years in function: a multicentre retrospective study. Clin Oral Implants Res. 2019;30:111–120. doi: 10.1111/clr.13397. [DOI] [PubMed] [Google Scholar]
- 80.Sanz M, Chapple IL, on behalf of Working Group 4 of the VIII European Workshop on Periodontology Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol. 2012;39(12):202–206. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- 81.Rakic M, Galindo-Moreno P, Monje A, Radovanovic S, Wang H-L, Cochran D, Sculean A, Canullo L. How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin Oral Investig. 2018;22:1805–1816. doi: 10.1007/s00784-017-2276-y. [DOI] [PubMed] [Google Scholar]
- 82.Ting M, Craig J, Balkin BE, Suzuki JB. Peri-implantitis: a comprehensive overview of systematic reviews. J Oral Implantol. 2018;29:225–247. doi: 10.1563/aaid-joi-D-16-00122. [DOI] [PubMed] [Google Scholar]
- 83.Atieh MA, Alsabeeha NHM, Faggion CM, Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84:1586–1598. doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 84.Lee C-T, Huang Y-W, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017;62:1–12. doi: 10.1016/j.jdent.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Salvi GE, Monje A, Tomasi C. Long-term biological complications of dental implants placed either in pristine or in augmented sites: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(16):294–310. doi: 10.1111/clr.13123. [DOI] [PubMed] [Google Scholar]
- 86.Cosgarea R, Sculean A, Shibli JA, Salvi GE. Prevalence of peri-implant diseases: a critical review on the current evidence. Braz Oral Res. 2019;33(1):e063. doi: 10.1590/1807-3107bor-2019.vol33.0063. [DOI] [PubMed] [Google Scholar]
- 87.Dreyer H, Grischke J, Tiede C, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodont Res. 2018;53:657–681. doi: 10.1111/jre.12562. [DOI] [PubMed] [Google Scholar]
- 88.Muñoz Giraldo V, Duque A, Giraldo Aristizabal A, Manrique Hernández RD. Prevalence of peri-implantitis disease according to periodontal probing depth and bleeding on probing: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2018;33:e89–e105. doi: 10.11607/jomi.5940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this manuscript.