Abstract
The aim of our work was to study whether taurine administration has neuroprotective effects in dystrophic Royal College of Surgeons (RCS) rats, suffering retinal degeneration secondary to impaired retinal pigment epithelium phagocytosis caused by a MERTK mutation. Dystrophic RCS-p + female rats (n = 36) were divided into a non-treated group (n = 16) and a treated group (n = 20) that received taurine (0.2 M) in drinking water from postnatal day (P)21 to P45, when they were processed. Retinal function was assessed with electroretinogram. Retinal morphology was assessed in cross-sections using immunohistochemical techniques to label photoreceptors, retinal microglial and macroglial cells, active zones of conventional and ribbon synaptic connections, and oxidative stress. Retinal pigment epithelium function was examined using intraocular fluorogold injections. Our results document that taurine treatment increases taurine plasma levels and photoreceptor survival in dystrophic rats. The number of photoreceptor nuclei rows at P45 was 3–5 and 6–11 in untreated and treated animals, respectively. Electroretinograms showed increases of 70% in the rod response, 400% in the a-wave amplitude, 30% in the b-wave amplitude and 75% in the photopic b-wave response in treated animals. Treated animals also showed decreased numbers of microglial cells in the outer retinal layers, decreased glial fibrillary acidic protein (GFAP) expression in Müller cells, decreased oxidative stress in the outer and inner nuclear layers and improved maintenance of synaptic connections. Treated animals showed increased FG phagocytosis in the retinal pigment epithelium cells. In conclusion, systemic taurine treatment decreases photoreceptor degeneration and increases electroretinographic responses in dystrophic RCS rats and these effects may be mediated through various neuroprotective mechanisms.
Keywords: Taurine, Retinal degeneration, RCS, Merkt, RPE, Microglia, Müller cells, Neuroprotection, Photoreceptor degeneration
1. Introduction
Retinal degenerative diseases comprise a large group of heterogeneous retinal degenerations caused by environmental and/or genetic factors that lead to untreatable blindness. Among them, there are two common diseases that initially affect the outer retina: retinitis pigmentosa (RP) and age-related macular degeneration (AMD). These diseases are initiated by the loss of photoreceptors and/or retinal pigment epithelial cells and may culminate retinal remodeling that produces loss of the output neurons of the retina, the retinal ganglion cells [[1], [2], [3], [4], [5], [6]]. RP and AMD are currently leading causes of irreversible blindness worldwide [[7], [8]], affecting severely the patient's quality of life [9]. Furthermore, increased life expectancy, among other factors, has caused a rise in the incidence of visual impairment and blindness in the world, mainly through an increase in age-related diseases such as AMD [10]. Because both RP and AMD are common causes of visual loss and there is currently no effective treatment for them, they pose a major challenge for retinal research [[11], [12], [13], [14]]. However, the heterogeneity of these diseases [[14], [15], [16], [17]], further complicates the identification of a universal treatment for all of them [11,18].
Taurine is an amino sulfonic acid, considered a non-essential amino acid, found in high concentrations in several body tissues and, particularly, in the retina [19], the body structure that contains more taurine [20]. Although taurine can be synthesized in the liver of most mammals, the main source of taurine comes from the diet, particularly from seafood, fish and meat [19,21]. A variety of functions have been attributed to taurine in the retina [[20], [21], [22], [23]], however, its exact role is still unknown. Our group has shown that taurine is necessary for photoreceptor [[24], [25], [26]] and retinal ganglion cell [[24], [25], [26]] survival in the normal retina, and that taurine depletion increases the susceptibility of the retina to light damage [25,27]. Recently, we have also shown that taurine deficiency increases retinal gliosis and oxidative stress and impairs the phagocytic capacity of the retinal pigment epithelium [27]. Other groups have shown that taurine deficiency causes retinal degeneration in animal models [28,29,30] and in humans [31,32]. On the other hand, taurine has shown beneficial effects in several animal models of retinal degeneration, such as animal models of Usher syndrome [33], diabetic retinopathy [34], experimental photoreceptor degeneration [35,36], and retinal and optic nerve damage [37,38]. Recently, a mutation of the taurine transporter has been found to cause retinal degeneration and cardiomyopathy in humans and dietary taurine supplementation has been found to be protective [39].
The Royal College of Surgeons (RCS) rat is a well-known used animal model for RP. These rats suffer an impairment of the phagocytic capacity of the retinal pigment epithelium [[40], [41], [42], [43]] due to an autosomal recessive mutation in the MERTK gene [44]. This mutation has also been observed in human patients with a form of early-onset RP [[45], [46], [47]]. In RCS rats, it causes progressive photoreceptor degeneration [[2], [48], [49], [50], [51], [52]], increased retinal gliosis [50] and alteration of the retrograde axonal transport in retinal ganglion cells and retinal ganglion cell loss [1,2,4,53]. A previous work has claimed that the first sign of retinal degeneration in the RCS rat is decreased taurine levels [54]. Since taurine is provided to the retina by the retinal pigment epithelial and Müller cells [20,55], it is tempting to speculate that taurine deficiency may be one of the causes of retinal pigment epithelium impairment and that taurine supplementation may be used to halt them.
In this work, we have studied whether taurine supplementation in RCS rats diminishes retinal degeneration.
2. Material & methods
2.1. Animal handling
Dystrophic RCS-p + female rats (n = 36) were obtained from the breeding colony of the University of Murcia (Murcia, Spain). The animals were divided in two groups: a non-treated group (n = 16) and a treated group that received taurine (0.2 M) in the drinking water [37] during 24 days, from postnatal day (P)21 (n = 20) just after weaning and up to P45 when they were processed. Only female rats were used as they have homogeneous sizes according to age and also to be able to compare with our previous studies.
Animals were housed in light- and temperature-controlled rooms with a 12 h light/dark cycle (light from 08:00 to 20:00; intensity within the cages from 5 to 30 lux) with access to water and food ad libitum. Animal manipulations were previously approved by the Ethics and Animal Studies Committee of the University of Murcia and were carried out following the Spanish and European Union regulations for the use of animals in research (Council Directive 86/609/EEC) and the ARVO statement for the use of animals in ophthalmic and vision research.
For electrophysiology and intravitreal injection procedures, general anesthesia was induced with an intraperitoneal injection of a mixture of ketamine (70 mg/kg, Ketolar, Parke-Davies, S.L., Barcelona, Spain) and xylazine (10 mg/kg, Rompún, Bayer, S.A., Barcelona, Spain). To prevent corneal desiccation in the recovery from anesthesia an ointment containing tobramycin (Tobrex, Alcon S.A., Barcelona, Spain) was applied on the cornea. Animals were sacrificed by an intraperitoneal injection of an overdose of sodium pentobarbital (Dolethal Vetoquinol, Especialidades Veterinarias, S.A., Alcobendas, Madrid, Spain).
2.2. ERG
Longitudinal electroretinographic recordings were carried out in untreated (n = 5) and taurine treated (n = 5) animals at P24 and P45, following previously described methods [56,57]. Briefly, in dark-adapted animals, retinal responses were recorded simultaneously from both eyes with bipolar Brian-Allen electrodes placed on the corneas. A reference electrode was placed inside the mouth and a 30G needle placed subcutaneously at the base of the tail as a ground electrode. Electrical signals were digitized at 20khZ using a Power Lab digitizer card (AD Instruments, Chalgrove, UK). In scotopic conditions, rod response and mixed response were recorded with a light intensity of −2,5 log cd·s/m2 and 0.5 log cd·s/m2, respectively. After adapting the animals to light for 5 min (30 cd/m2), we registered the photopic b-wave was recorded with a light intensity of 0.5 log cd·s/m2. The stimulation and recording protocols were done following the recommendations of the International Society for Clinical Electrophysiology of Vision (ISCEV).
2.3. Retinal pigment epithelium labeling
Intravitreal injections of Fluorogold (FG) were carried out in some non-treated (n = 6) and taurine treated (n = 6) animals one day prior to processing to label the retinal pigment epithelium following previously described methods developed by our group [27,43]. Briefly, intravitreal injections of 1.5 μL of 3% FG (Fluorochrome Inc., Engelwood, CO, USA) diluted in saline were done through the superotemporal sclera using a Hamilton microsyringe (30 G; Hamilton 701 N, Esslab, Benfleet, UK) [[27], [43], [51], [58]].
2.4. Tissue processing and taurine measurement
All animals were sacrificed at P45. Before sacrifice, animals were sedated with an intraperitoneal injection of sodium pentobarbital (Dolethal Vetoquinol, S.A., Lure, France) and were subsequently euthanized with a lethal dose of sodium pentobarbital. Just before euthanasia, blood samples were collected from the heart of each experimental and control animal, and then the plasma was collected by centrifugal separation and frozen for posterior taurine plasma levels analysis using an HPLC-MS system [24,25,27]. Plasma taurine levels were measured in 5 randomly selected control (untreated) RCS rats and 5 randomly selected taurine-treated RCS rats. Following euthanasia, the animals were perfused transcardially with saline and with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The eyes were enucleated and the eyecups immersed in Tissue-TEK, frozen and sectioned in the cryostat to obtain 15-microns-thick sagittal sections [[49], [50], [51]]. In the eyes that received an intravitreal injection of FG, the retinal pigment epithelium was dissected from the neural retina and flat-mounted following described methods [27,43].
2.5. Immunohistofluorescence and image analysis
For morphological analysis, three retinal cross-sections spanning the optic disc were selected per eye and animal and processed for immunohistofluorescence following standard procedures [[3], [27], [49], [50], [59]]. Briefly, the sections were permeated, washed in phosphate-buffered saline (PBS) containing 0.5% Triton X-100 (Tx) and incubated overnight at 4 °C with a mixture of primary antibodies to detect: i) Microglial cells using a rabbit monoclonal anti-Iba1 antibody (1:1000–500; ab178846: Abcam, Cambridge, UK); ii) Astrocytes and Müller cells with a goat monoclonal anti- Glial Fibrillary Acidic Protein (GFAP) antibody (1:500; 019–19741: Abcam, Cambridge, UK); iii) L-cones with a rabbit monoclonal anti-L/M-opsin antibody (1:1200; ab5405; Chemicon-Millipore Iberica, Madrid, Spain); iv) S-cones with a goat monoclonal anti-S-opsin antibody (1:1000; N-20; anti-OPN1SW; Santa Cruz Biotechnology, Heidelberg, Germany); v) rods outer segments with a rat monoclonal anti-rhodopsin antibody (1:1200, 1D4; Sigma-Aldrich, Madrid, Spain); vi) Signs of Oxidative stress with a monoclonal antibody against mouse α-8-hydroxy-2′-deoxyguanosine (8-OHdG; 1:1000, sc-66036; Santa Cruz Biotechnology, Heidelberg, Germany); vii) active zones of conventional and ribbon synaptic connections with a mouse monoclonal anti-Bassoon antibody (1:750; ADI-VAM-PS003; Enzo life Science, Lausen, Switzerland); viii) cone photoreceptors with a rabbit monoclonal anti-cone arrestin antibody (1:1000; AB15282, Merck, Germany). The next morning, the sections were washed in PBS and incubated for 2 h at room temperature with a mixture of the secondary antibodies: i) donkey anti-goat Alexa 594 (1:500; Molecular Probes, Invitrogen, ThermoFisher, Madrid, Spain) and ii) goat anti-mouse IgG1 Alexa 488 (1:500; Molecular Probes, Invitrogen, ThermoFisher, Madrid, Spain) diluted in 2% Tx PBS. Finally, sections were washed in PBS and mounted with a mounting media containing DAPI (4′,6-diamidino-2-phenylindole; Vectashield Mounting Medium con DAPI, Vector Atom, Alicante, España) to counterstain all retinal nuclei. Some additional sections of some animals were also processed for TdT-mediated dUTP nick-end labeling (TUNEL) to label apoptotic nuclei [50,60,61].
The sections were examined and photographed under a fluorescence microscope Leica DM6 B (Leica) microscope equipped with various filters and magnifications (20X, 40X or 63X, Leica Microsytems, Wetzlar, Germany) and with a confocal microscope Leica SP8 (Leica Microsytems, Wetzlar, Germany) as previously described in detail [[27], [49], [50], [51], [62], [63]]. When needed, images were further processed using Adobe Photoshop CS 6 (Adobe Systems, Inc., San Jose, CA, USA). The flat-mounted retinal pigment epitheliums were photographed using a confocal microscope Leica SP8 (20 × , 40 × or 63 × , Leica Microsystems, Wetzlar, Germany).
2.6. Retinal cell quantification
In each selected retinal section four photomicrographs were taken both in the dorsal and the ventral retina at distances representing 25, 50, 75 and 95% of the length between the optic disc and the retinal periphery. The number of nuclei rows in the outer nuclear layer (ONL) was quantified in three representative regions of each of these photomicrographs and averaged, obtaining a mean number or nuclei rows per picture, per retinal region analyzed and per animal [[3], [27], [49], [50], [51], [60], [61]]. Therefore, a total of 24 microphotographs (8 photos x 3 sections) were analyzed per animal [[27], [50]]. The same photomicrographs used to measure ONL thickness were used to quantify the numbers of microglial cells in the different retinal layers [27] and the photoreceptor outer segment (OS) . The numbers of 8-OHdG and TUNEL positive cells were quantified in different photomicrographs from the same retinal area.
For GFAP fluorescence quantification, additional pictures of the same retinal regions (24 per animal) were photographed again but with fixed gain and exposure times that corresponded to the mean automatic exposure time necessary to take the same retinal regions in control animals. In these pictures, the Relative Fluorescence Units (RFU) of the photographs were quantified as a measure of GFAP expression by using the tool “Histogram Analysis” of the Image Pro Plus software (IPP 5.1 for Windows; Media Cybernetics, Silver Spring, MD, USA) following previously described methods [[27], [49]]. This tool provides for each picture a plot of the various GFAP RFU and their average and area under the curve (AUC), which indicates the total amount of fluorescence in the eight regions analyzed per retina.
2.7. Statistical analysis
Statistical analysis was performed by using the GraphPad Prism® program (GraphPad Prism 6, GraphPad Software, LaJolla, CA, USA). All quantitative data obtained is presented as the mean ± standard deviation (SD). For comparisons of quantitative variables between two subgroups, we used the Student's t-test and Two Way-ANOVA. Differences were considered statistically significant when p ≤ 0.05.
3. Results
3.1. Taurine plasma levels
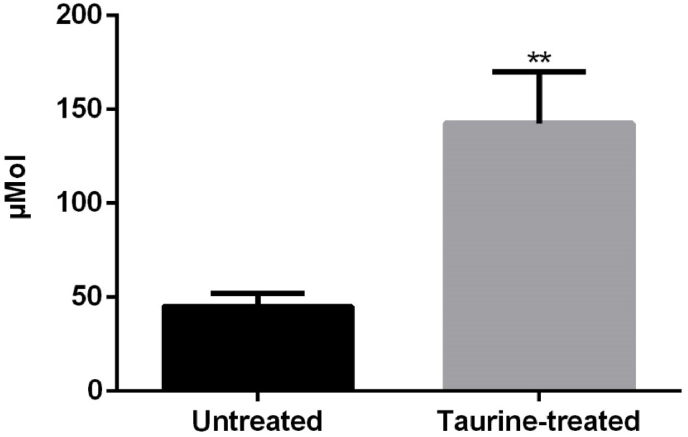
Taurine treatment in drinking water increased significantly the plasma taurine levels (Fig. 1; t-test p < 0.001). Approximately, a threefold increase was found in plasma taurine levels in treated animals when compared to non-treated animals (Fig. 1).
Fig. 1.
Taurine plasma levels in untreated (n = 5) and taurine treated (n = 5) RCS rats just before processing at P45. Twenty-four days of taurine treatment achieves a significant increase of the taurine plasma levels. ** = Statistically significant difference when compared to untreated animals (t-test, p < 0.001).
3.2. Effect of systemic taurine treatment in photoreceptor survival and function
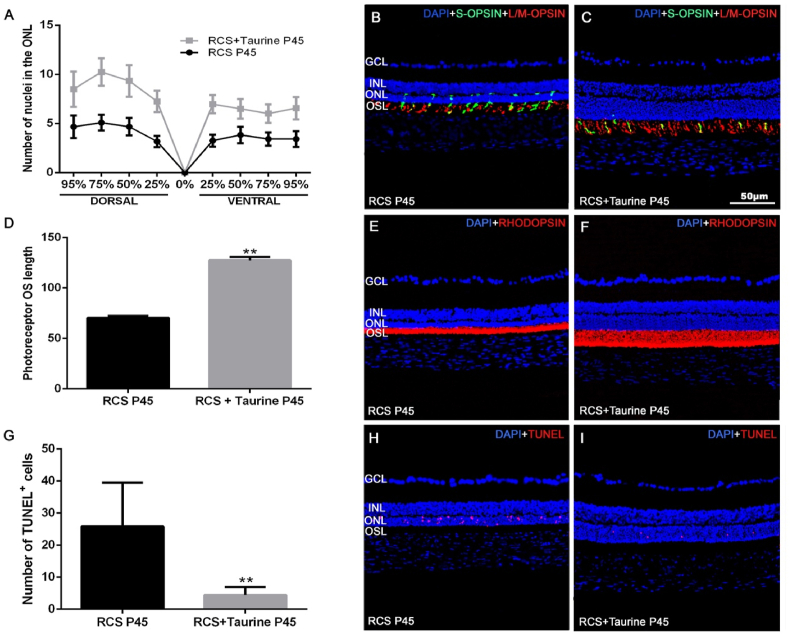
The thickness of the ONL in non-treated RCS rats ranged from 3 to 5 nuclei rows, depending on the retinal area analyzed at P45 (Fig. 2 A, B), and this thickness decreased from the optic nerve to the retinal periphery. However, at P45 in the taurine-treated group, the thickness of the ONL ranged from 6 to 11 nuclei rows (Fig. 2 A, C). Taurine treatment increased significantly the thickness of the ONL in all the regions analyzed (p < 0.0001; t-test).
Fig. 2.
Photoreceptor outer segment morphology and apoptosis in the ONL. Graphs showing the mean ± SD number of nuclei rows in the ONL of the dorsal and ventral areas analyzed in the two subgroups (A), the mean photoreceptor outer segment length (D) and the number of TUNEL positive cells (G). Photomicrographs of retinal cross-sections from representative retinas of the untreated (B, E, H) and taurine treated (C, F, I) RCS rats showing S- (red, C, D) and L/M − (green, C, D) opsin immunoreactivity, rhodopsin (red, E, F), TUNEL positive cells in the ONL (red, H, I) and nuclear DAPI counterstaining (blue). Rod and cone outer segments (OS) show reduced length in untreated rats. n = 10 per subgroup. Scale bar: 50 μm. GCL: Ganglion cell layer. INL: Inner nuclear layer. ONL: Outer nuclear layer. OSL: Outer segments layer. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The photoreceptor OS layer was significantly thinner at P45 in untreated RCS rats when compared to taurine-treated RCS rats (Fig. 2D), and both rods and L/M − and S- cones outer segments were longer in the taurine-treated rats (Fig. 2 B, C, E, F).
Quantification of the TUNEL-positive nuclei showed that the retinas of untreated RCS rats contain significantly higher numbers of apoptotic nuclei in the ONL than the taurine-treated RCS rats (Fig. 2G–I), indicating that taurine treatment inhibited partially photoreceptor apoptosis.
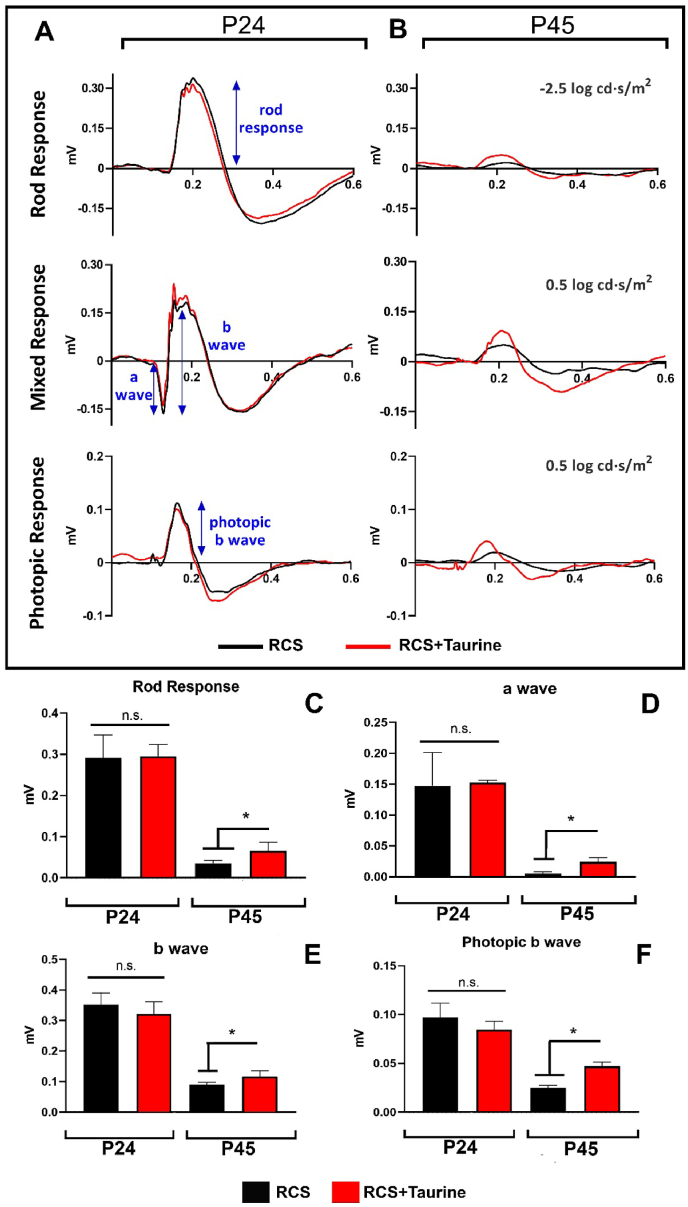
Electroretinographic recordings did not show significant differences between untreated and taurine treated RCS rats at the first postnatal age analyzed, P24, four days after initiation of the treatment. However, significant differences were observed between them at P45, 24 days after systemic taurine treatment.
At P24, the rod response, the mixed response (formed by the a-wave and b-wave) and the photopic b-wave were similar between both groups (p > 0.05 Mann-Whitney test; Fig. 3A, C-F). However, at P45, the rod response was approximately 70% greater in taurine treated than in untreated RCS rats (p < 0.001 Mann-Whitney test; Fig. 3B and C). At this age, the amplitude of the a-wave was approximately 400% greater in taurine treated RCS rats, compared to an almost null response in untreated RCS rats (p < 0.001 Mann-Whitney test; Fig. 3B, D) and the amplitude of the b-wave was 30% greater in taurine treated RCS rats (p < 0.001 Mann-Whitney test; Fig. 3B, E). The photopic b-wave response was also approximately 75% higher in taurine treated than in untreated RCS rats (p < 0.001 Mann-Whitney test; Fig. 3B, F).
Fig. 3.
(A, B) Electroretinographic recordings. Representative traces of the rod response (upper row), mixed response (a-wave and b-wave; middle row) and photopic b wave (lower row) recorded in RCS rats at P24 (A) and P45 (B). The black trace corresponds to the untreated animals and the red trace to the taurine treated RCS animals. (C–F) Bar histograms showing the amplitude of the rod response (C), a wave (D), b wave (E), and photopic b wave (F). The black bar corresponds to the untreated animals and the red bar to the taurine treated animals. n.s.: not significant differences between groups. *significant differences between groups (p < 0.05; Mann-Whitney test). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Effects of taurine treatment on retinal microglial activation
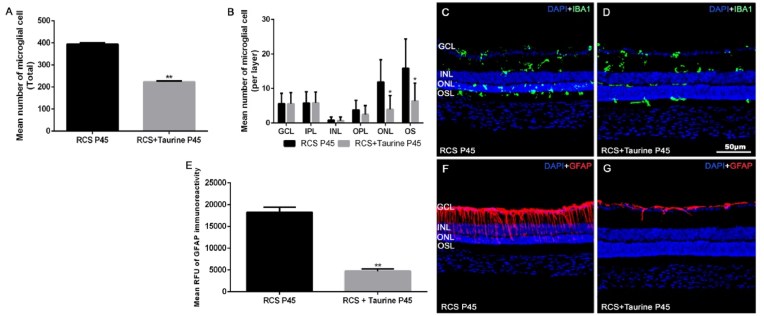
In untreated RCS rats, at P45 the mean total number of Iba-1+ cells per retina in the 24 regions analyzed was 394 ± 7.0 (Fig. 4) and in the taurine treated RCS rats there were only 223.6 ± 4.0 Iba-1+ cells per retina and this difference was significant (t-test; p < 0.0001 Fig. 4A).
Fig. 4.
Microglial cell quantification and GFAP immunoreactivity. Bar graphs showing the mean ± SD numbers of microglial cells per retina (A) and per retinal layer (B) in untreated (black bars) and taurine treated (grey bars) RCS. Microglial cells were more abundant in the outer retinal layers in untreated animals. Photomicrographs from cross-sections of representative retinas from the untreated (C) and taurine treated (D) RCS rats at P45 showing Iba-1+ immunoreactive cells (green) and DAPI nuclear counterstaining (blue). Bar graphs showing mean ± SD RFU of GFAP immunoreactivity (AUC) in untreated (black) and taurine treated (grey) RCS rats (E) n = 10 per subgroup. Photomicrographs from cross-sections of representative retinas from the untreated (F) and taurine treated (G) RCS rats at P45 showing GFAP immunoreactivity (red) and DAPI nuclear counterstaining (blue). Scale bar: 50 μm * Statiscally significant differences with untreated group in the same retinal layer (p ≤ 0.05; t-test). GCL: Ganglion cell layer. INL: Inner nuclear layer. ONL: Outer nuclear layer. OSL: Outer segments layer. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In untreated RCS rats at P45, the mean number of Iba-1+ cells per retinal layer was: 5.6 ± 3.0 in the ganglion cell layer (GCL), 5.8 ± 3.2 in the inner plexiform layer (IPL), 0.9 ± 0.8 in the inner nuclear layer (INL) and 3.8 ± 2.8 in the outer plexiform layer (OPL), 11.9 ± 6.5 in the ONL and 15.9 ± 8.5 in the photoreceptor outer segments layer (OSL) (Fig. 4B and C).
The mean number of Iba-1+ cells in taurine-treated RCS rats at P45 was: 5.6 ± 3.3 in the GCL, 5.8 ± 3.1 in the IPL, 0.7 ± 1.1 in the INL and 2.5 ± 2.6 in the OPL, 3.9 ± 4.0 in the ONL and 6.3 ± 5.2 in the photoreceptor OSL (Fig. 4B, D).
When we compared the numbers of Iba-1+ cells in the different layers between the untreated and the taurine treated RCS rats (Fig. 4A), we found that taurine treatment decreased significantly the numbers of Iba-1+ cells in the ONL (p < 0.05; Two Way ANOVA) and in the OSL (p < 0.05; Two Way ANOVA), but not in the GCL (p = 0.9928; Two Way ANOVA), the IPL (p = 0.9893; Two Way ANOVA), the INL (p = 0.7828; Two Way ANOVA) and the OPL (p = 0.4790; Two Way ANOVA).
3.4. Effects of taurine treatment on retinal macroglial activation
The mean RFU of GFAP immunoreactivity was 18,230.56 ± 1,180.26 in untreated RCS rats (n = 11) and 4,729.72 ± 535.17 in taurine-treated RCS rats (n = 8) and this difference was highly significant (p = 0.0001, t-test; Fig. 4E). Therefore, taurine treatment decreases significantly GFAP immunoreactivity in RCS rats.
Qualitatively, the macroglial cell reaction was different in the treated and untreated groups at P45. In untreated RCS rats, the GFAP signal was observed not only in astrocytes of the GCL and nerve fiber layer but also in the inner processes of Müller cells (Fig. 4F). In the taurine-treated RCS rats, the GFAP signal was restricted to the astrocytes of the GCL and nerve fiber layer (Fig. 4G).
3.5. Effect of taurine treatment on retinal pigment epithelium function and morphology
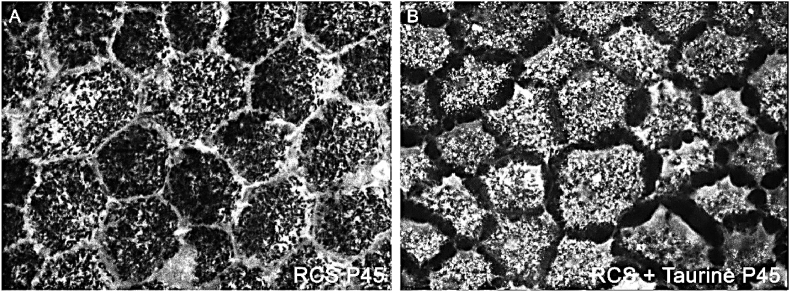
The retinal pigment epithelium of the untreated RCS rats showed at P45 an inverted FG-labeling at P45, namely we found FG labeling mostly in the membrane of the retinal pigment epithelial cells (Fig. 5A). We have shown previously that FG labeling accumulates in the cytoplasm in non-dystrophic animals and in the plasma membrane in dystrophic RCS rats [43]. In P45 taurine treated RCS rats, FG accumulated mostly in the cytoplasm of the retinal pigment epithelial cells (Fig. 5B), as in non-dystrophic animals. However, FG fluorescence in P45 taurine treated dystrophic RCS rats was different from that found in control non dystrophic animals [43] because it was obscured by melanin granules. No FG fluorescence was observed in the cell membrane in taurine treated RCS rats.
Fig. 5.
Confocal fluorescence photomicrographs from representative regions of the flat mounted retinal pigment epithelium in untreated (A) and taurine treated (B) P45 RCS rats showing FG-labelling. Untreated RCS rats show an inverted pattern of labelling (FG more abundant in the plasma membrane) and taurine treated rats showed FG accumulations mostly within the cytoplasm.
3.6. Effect of taurine treatment on oxidative stress
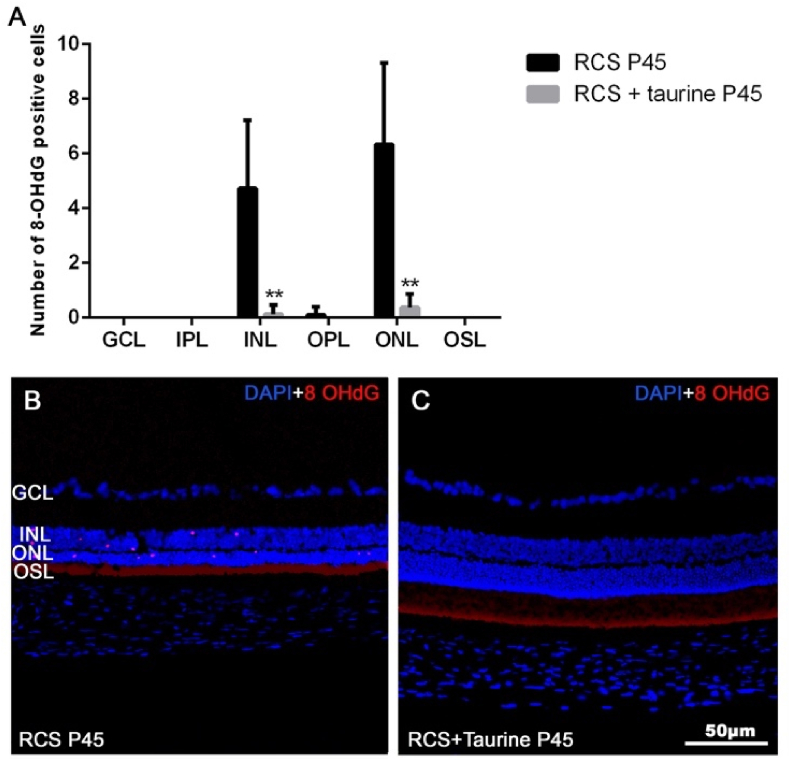
In the retinas of untreated RCS rats, at P45, there were 4.7 ± 2.5 8-OHdG + cells in the ONL and 6.3 ± 3.0 INL (Fig. 6 A, B). However, in the retinas of taurine-treated RCS rats, at P45, we did not find 8-OHdG + cells in most retinal sections (Fig. 6 A, C), although we found some isolated 8-OHdG + cells in a few sections, 0.1 ± 0.3 in the ONL and 0.4 ± 0.5 in the INL. These results document that retinal degeneration in the RCS rat courses with oxidative damage that is alleviated by taurine treatment.
Fig. 6.
Oxidative stress. Graph showing the mean number of 8-OHdG positive cells per retinal layer in untreated (black bars) and taurine treated (grey bars) RCS rats (A). Photomicrographs of retinal cross sections from representative retinas of untreated (B) and taurine treated (C) P45 RCS rats, showing 8-OHdG + cells (red), and DAPI nuclear counterstaining (blue). Some 8-OHdG + immunoreactive cells are observed in the INL and ONL (A) in untreated RCS rats. ** Statiscally significant differences between groups in this layer (p ≤ 0.0001; Two Way ANOVA). GCL: Ganglion cell layer. IPL: Inner Plexiform Layer. INL: Inner nuclear layer. OPL: Outer Plexiform Layer. ONL: Outer nuclear layer. OSL: Outer segments layer. Scale Bar: 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.7. Effect of taurine treatment on synaptic connections
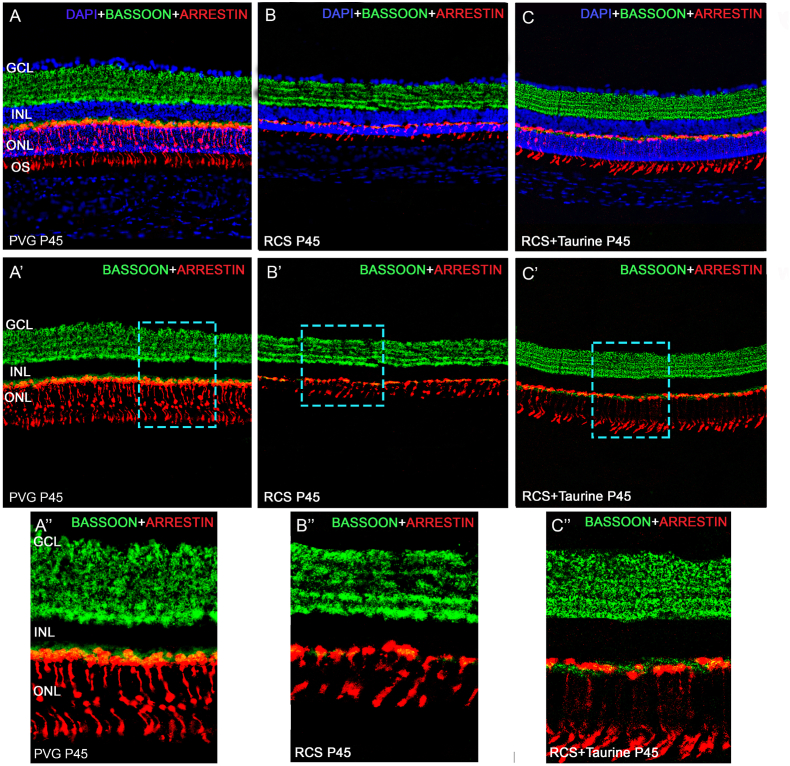
The typical appearance of the bassoon immunoreactivity in the rat retina (Fig. 7A, A′, A″), i.e., punctated and homogeneous immunofluorescence in the OPL and several immunofluorescent sublayers in the IPL [27], was lost in untreated RCS rats at P45, particularly in the OPL (Fig. 7B, B′, B″), where only some spots of fluorescence could be seen. In the IPL, at P45, both the immunofluorescent sublayers and the homogeneous fluorescence decreased, and as a consequence, some sublayers could not be clearly defined (Fig. 7B, B′, B″). However, in the retinas of taurine-treated RCS rats, at P45, bassoon immunoreactivity had a close to normal appearance (Fig. 7A, A′, A″) both in the OPL and the IPL. Specifically, bassoon staining in the OPL showed a punctate and somewhat homogeneous immunofluorescence (Fig. 7C, C′, C″) and in the IPL the immunofluorescent sublayers were well defined (Fig. 7C, C′, C″), resembling the immunoreactivity found in healthy retinas [27].
Fig. 7.
Synaptic connections. Photomicrographs of retinal cross-sections taken from representative retinas of healthy (A, A′, A″), untreated (B, B′, B″) and taurine treated (C, C′, C″) P45 RCS rats showing bassoon (green) and arrestin (red) immunoreactivity. Figures A, B, C are the same shown in A′, B′, C′ but include DAPI counterstaining (blue). Figures A″, B″, C″ show higher power magnifications of the microphotographs shown in A′, B′ and C′. Untreated rats show scarce and disorganized bassoon labelling, while taurine-treated rats show a close to normal bassoon immunoreactivity. Scale Bar: 50 μm. GCL: Ganglion cell layer. IPL: Inner Plexiform layer. INL: Inner nuclear layer. OPL: Outer Plexiform layer. ONL: Outer nuclear layer. OSL: Outer segments layer. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In this work, we have studied the neuroprotective effect of systemic taurine treatment in the RCS rat, an animal model of inherited photoreceptor degeneration due to a mutation in the MERTK gene. This defect impairs the phagocytic capacity of the retinal pigment epithelium and causes rapid and progressive photoreceptor loss.
We have distinguished two groups of animals: untreated and taurine-treated animals. The treated group received taurine (0.2 M) in the drinking water from P21 to P45, when they were processed. We have chosen these study ages because previous works from our lab have showed that at P21, coinciding with weaning, retinal degeneration has not yet started in the RCS rat [50] and is, therefore, an appropriate time point to start neuroprotection [[49], [50], [51]], and that between P33 and P45 photoreceptor loss is maximal in the RCS rat [[49], [50], [51]].
In treated animals, we have used a 0.2 M concentration in the drinking water, the same concentration of taurine used in a previous work that showed a two-fold increase of the taurine plasma concentration and neuroprotective effects in the rat retina [37]. However, in this study we find that the administration of taurine during 24 days increases three-fold the taurine plasma levels. Other studies have also shown two to threefold increases in plasma taurine levels after oral administration in rodents. A study found that administration of a smaller concentration (0.1 M) of taurine to mice for 25 days produced a three-fold increase of taurine plasma levels [30], whereas another study performed also in mice showed that four months of taurine supplementation at the same dose increased the taurine levels by only two-fold [37]. A three-fold increase in taurine plasma concentration has been found in humans treated with taurine at a daily dose of 1.5 g divided into two capsules of each 0.75 g during 8 weeks [64]. We think that these differences may be due to a peak in taurine plasma levels at the beginning of the treatment.
We document here, by quantifying the thickness of the ONL and qualitatively analyzing photoreceptor apoptosis that taurine treatment diminishes significantly photoreceptor death in RCS rats. We also document that taurine treatment has a preservation effect on the outer segments of both rods and cones. We thus conclude that taurine has photoreceptor neuroprotective effects in dystrophic RCS rats. Our results are thus in accordance with other studies documenting neuroprotective effects of taurine in animal models of induced photoreceptor degeneration [36], diabetic retinopathy [34], Usher syndrome type 1 [33], in endothelin-induced retinal and optic nerve damage [38], and also in human retinal degeneration due to taurine transporter deficiency [39] or vigabatrin therapy [31]. In the rat model of diabetic retinopathy [34] and in the mice model of Usher syndrome type 1 [33], taurine has also been shown to reduce apoptosis of retinal cells [[33], [34]]. Thus, taurine may alleviate photoreceptor degeneration due to its anti-apoptotic properties [20,21,23] and thus it may be neuroprotective in retinal degenerations coursing with apoptosis, regardless of their etiology.
We also document in this work that taurine treatment improves retinal function in dystrophic RCS rats because we show that various ERG parameters ameliorate with taurine treatment. Specifically, we show augmented rod response, a-wave and b wave amplitude, and photopic b-wave in the RCS rats that received taurine treatment. Other authors have also shown improvement of retinal function with taurine treatment in various animal models of retinal degeneration. In a mice model of Usher syndrome type 1 taurine treatment augmented ERG amplitudes under dark-adapted, photopic and flicker conditions [33]. Also, in a rat model of induced photoreceptor degeneration it achieved larger scotopic and photopic a- and b-waves [36]. Finally, in a rat model of induced diabetic retinopathy, taurine prevented the reduction of the amplitude of the photopic b-wave [34].
We have also studied the influence of the taurine treatment on the retinal glial cells and we document, for the first time, that taurine treatment decreases the microglial and macroglial cell reaction in dystrophic RCS rats. We document that taurine treatment decreases significantly the total numbers of IBA-1+ microglial cells in the retina, and the numbers of microglial cells in ONL and OSL. We also document that taurine treatment decreases the macroglial cell reaction in the RCS rat retina because in the taurine treated animals the Müller cells did not show GFAP immunoreactivity. Microglial cell activation, migration and proliferation [[27], [50], [65]], increased expression of GFAP by Müller cells [65,67,68] and hypertrophy of Müller cells [65,[67], [68], [69], [70]], are usually found in photoreceptor degenerative diseases and have been related to secondary retinal remodeling [[1], [2], [5], [65]] and subsequent retinal ganglion cell death [[2], [3], [4], [53], [60]]. Taurine treatment has been previously documented to reduce the microglia-dependent inflammation in a mouse model of Parkinson's Disease [71] and to decrease the accumulation of GFAP in the brain in a rat model of traumatic brain injury [72]. In the retina, taurine has been shown to reduce reactive gliosis and GFAP overexpression in a rat model of diabetic retinopathy [34]. Thus, taurine could be useful as a therapeutic agent in inflammatory central nervous system diseases.
An interesting finding of this study is the documentation of an improvement of the phagocytosis of the retinal pigment epithelium with taurine administration in dystrophic RCS rats, demonstrated with intravitreal administration of FG [43]. In untreated RCS rats, FG was observed to accumulate in the plasma membrane, while in taurine-treated RCS rats, FG accumulated in the cytoplasm through phagocytosis, as shown previously in control animals [43]. A previous study has documented that taurine stimulates retinal pigment epithelium phagocytosis in cultured chick embryo retinal pigment epithelial cell explants [73]. Furthermore, a relationship between taurine deficiency and retinal degeneration was documented in dystrophic RCS rats [54]. A recent work has suggested that taurine may be a potential candidate to treat the damaged retinal pigment epithelium [74]. Finally, it has been shown in human age-related macular degeneration that taurine together with cone opsin accumulates between the photoreceptors and the retinal pigment epithelium and this may reflect the inability of the epithelial cells to process taurine and other molecules originating from cone photoreceptor phagocytosis [70]. Our results showing increased fluorogold labeling of the retinal pigment epithelial cells with taurine treatment demonstrate that this amino acid ameliorates the defective phagocytosis of these cells in the dystrophic RCS rats. Thus, taurine processing by the retinal pigment epithelium may influence its ability to phagocytose photoreceptor.
We also document using immunohistochemistry to 8-OHdG that taurine treatment decreases oxidative damage in the ONL and INL of the RCS rat retina. Oxidative stress has an important pathogenic potential in neurodegenerative diseases such as RP [66], being one of the main contributors to photoreceptor death [66,75]. Taurine is a well-known antioxidant [20,33] and its neuroprotective effects may be mediated through its antioxidant effects [[20], [33], [38]]. However, to the best of our knowledge, this is the first study analyzing the presence of 8-OHdG positive cells in the degenerating retina following taurine treatment.
Lastly, we document using bassoon, one of the ubiquitously expressed proteins at active zones of conventional and ribbon-type synapses [76,77] that labels synaptic ribbons in the OPL and conventional synapses in the IPL [[27], [78]], immunohistochemistry that taurine treatment decreases the synaptic deterioration in RCS rats. This is in accordance with a previous work showing that taurine treatment achieves restoration of synaptic integrity of bipolar cell terminals in the OPL using metabotropic glutamate receptor subtype 6 (mGluR6) in an induced rat model of diabetic retinopathy [34]. Importantly, in this work, we show for the first time that taurine treatment improves ribbon synapses in both the OPL and the IPL of the RCS rat retina. The reported neuroprotection to the synaptic ribbons of the photoreceptors indicates rescued connections to second-order neurons [[76], [79]], that would prevent degenerative changes (sprouting) in bipolar and horizontal cells [[76], [80], [81]]. This fact could be key for preventing retinal remodeling and, therefore, secondary cell loss in the inner retina [[1], [2], [3], [4], [5], [6], [60]]. Moreover, a recent work has proposed that taurine plays an important role in synaptogenesis, neurite outgrowth, and synaptic transmission during the early stages of neuronal development [82]. Indeed, taurine is very abundant in mammalian milk [[83], [84], [85]]. and in human milk, it accounts for approximately 50% of the total free amino acid content [84]. Thus, taurine may be necessary for healthy neurodevelopment, including retinal development [85]. Moreover, neonatal screening of taurine plasma levels has been proposed for the identification of children at risk of retinal degeneration [86] and decreased taurine plasmatic levels have been related to certain retinal degenerations such as central serous chorioretinopathy [87] and Leber hereditary optic neuropathy [88].
5. Conclusion
In summary, our study provides strong evidence that systemic taurine treatment decreases retinal degeneration in dystrophic RCS rats. Taurine treatment decreases photoreceptor loss, oxidative damage in the outer and inner nuclear layers and glial cell activation, and improves the synaptic structure, retinal function and retinal pigment epithelium phagocytosis. Thus, we document that taurine shows various neuroprotective effects in dystrophic RCS rats. Although these rats suffer retinal degeneration due to retinal pigment epithelium dysfunction, it is possible that the neuroprotective properties of taurine may also be employed successfully in retinal diseases coursing with apoptosis, oxidative damage, inflammation or synaptic impairment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Funding:Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia: 19881/GERM/15 to M.V.-S.; Instituto de Salud Carlos III (ISCIII): PI19/00203, co-funded by European Regional Development Fund (ERDF), “A way to make Europe” to M.P.V.-P. and D.G.-A., and PI22/00900, co-funded by ERDF, "A way to make Europe" to M.P.V.-P. and D.G.-A., and RD16/0008/0026 co-funded by ERDF, “A way to make Europe” to M.P.V.-P and RD21/0002/0014 financiado por la Unión Europea – NextGenerationEU; Fundación Robles Chillida to D.G-.A.; RED2018-102499-T and PID2019-106498 GB-I00 funded by MCIN/AEI/ 10.13039/501100011033 to M.V.-S.; and the RHU LIGHT4DEAF [ANR-15-RHU-0001] and IHU FOReSIGHT [ANR-18-IAHU-0001] to S.P.
Data availability
Data will be made available on request.
References
- 1.García-Ayuso D., Di Pierdomenico J., Agudo-Barriuso M., Vidal-Sanz M., Villegas-Pérez M. Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen. Res. 2018;13:1885–1886. doi: 10.4103/1673-5374.239436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Ayuso D., Di Pierdomenico J., Vidal-Sanz M., Villegas-Pérez M.P. Retinal ganglion cell death as a late remodeling effect of photoreceptor degeneration. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20184649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Ayuso D., Salinas-Navarro M., Agudo M., Cuenca N., Pinilla I., Vidal-Sanz M., Villegas-Pérez M.P. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp. Eye Res. 2010;91:800–810. doi: 10.1016/j.exer.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.García-Ayuso D., Salinas-Navarro M., Nadal-Nicolás F.M., Ortín-Martínez A., Agudo-Barriuso M., Vidal-Sanz M., Villegas-Pérez M.P. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br. J. Ophthalmol. 2014;98:396–401. doi: 10.1136/bjophthalmol-2013-303958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer R.L., Marc R.E., Jones B.W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin. Eye Res. 2020;74 doi: 10.1016/j.preteyeres.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vugler A., Semo M., Ortín-Martínez A., Rojanasakul A., Nommiste B., Valiente-Soriano F.J., García-Ayuso D., Coffey P., Vidal-Sanz M., Gias C. A role for the outer retina in development of the intrinsic pupillary light reflex in mice. Neuroscience. 2015;286:60–78. doi: 10.1016/j.neuroscience.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 7.Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., Leasher J., Limburg H., Naidoo K., Pesudovs K., Silvester A., Stevens G.A., Tahhan N., Wong T.Y., Taylor H.R., Bourne R., Ackland P., Arditi A., Barkana Y., Bozkurt B., Braithwaite T., Bron A., Budenz D., Cai F., Casson R., Chakravarthy U., Choi J., Cicinelli M.V., Congdon N., Dana R., Dandona R., Dandona L., Das A., Dekaris I., Del Monte M., Deva J., Dreer L., Ellwein L., Frazier M., Frick K., Friedman D., Furtado J., Gao H., Gazzard G., George R., Gichuhi S., Gonzalez V., Hammond B., Hartnett M.E., He M., Hejtmancik J., Hirai F., Huang J., Ingram A., Javitt J., Jonas J., Joslin C., Keeffe J., Kempen J., Khairallah M., Khanna R., Kim J., Lambrou G., Lansingh V.C., Lanzetta P., Leasher J., Lim J., Limburg H., Mansouri K., Mathew A., Morse A., Munoz B., Musch D., Naidoo K., Nangia V., Palaiou M., Parodi M.B., Pena F.Y., Pesudovs K., Peto T., Quigley H., Raju M., Ramulu P., Rankin Z., Resnikoff S., Reza D., Robin A., Rossetti L., Saaddine J., Sandar M., Serle J., Shen T., Shetty R., Sieving P., Silva J.C., Silvester A., Sitorus R.S., Stambolian D., Stevens G., Taylor H., Tejedor J., Tielsch J., Tsilimbaris M., van Meurs J., Varma R., Virgili G., Wang Y.X., Wang N.-L., West S., Wiedemann P., Wong T., Wormald R., Zheng Y. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 8.Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C.J., Ma Y., Jin Z.B. The road to restore vision with photoreceptor regeneration. Exp. Eye Res. 2021;202 doi: 10.1016/j.exer.2020.108283. [DOI] [PubMed] [Google Scholar]
- 10.Bourne R., Steinmetz J.D., Flaxman S., Briant P.S., Taylor H.R., Resnikoff S., Casson R.J., Abdoli A., Abu-Gharbieh E., Afshin A., Ahmadieh H., Akalu Y., Alamneh A.A., Alemayehu W., Alfaar A.S., Alipour V., Anbesu E.W., Androudi S., Arabloo J., Arditi A., Asaad M., Bagli E., Baig A.A., Bärnighausen T.W., Battaglia Parodi M., Bhagavathula A.S., Bhardwaj N., Bhardwaj P., Bhattacharyya K., Bijani A., Bikbov M., Bottone M., Braithwaite T., Bron A.M., Butt Z.A., Cheng C.-Y., Chu D.-T., Cicinelli M.V., Coelho J.M., Dagnew B., Dai X., Dana R., Dandona L., Dandona R., Del Monte M.A., Deva J.P., Diaz D., Djalalinia S., Dreer L.E., Ehrlich J.R., Ellwein L.B., Emamian M.H., Fernandes A.G., Fischer F., Friedman D.S., Furtado J.M., Gaidhane A.M., Gaidhane S., Gazzard G., Gebremichael B., George R., Ghashghaee A., Golechha M., Hamidi S., Hammond B.R., Hartnett M.E.R., Hartono R.K., Hay S.I., Heidari G., Ho H.C., Hoang C.L., Househ M., Ibitoye S.E., Ilic I.M., Ilic M.D., Ingram A.D., Irvani S.S.N., Jha R.P., Kahloun R., Kandel H., Kasa A.S., Kempen J.H., Keramati M., Khairallah M., Khan E.A., Khanna R.C., Khatib M.N., Kim J.E., Kim Y.J., Kisa S., Kisa A., Koyanagi A., Kurmi O.P., Lansingh V.C., Leasher J.L., Leveziel N., Limburg H., Majdan M., Manafi N., Mansouri K., McAlinden C., Mohammadi S.F., Mohammadian-Hafshejani A., Mohammadpourhodki R., Mokdad A.H., Moosavi D., Morse A.R., Naderi M., Naidoo K.S., Nangia V., Nguyen C.T., Nguyen H.L.T., Ogundimu K., Olagunju A.T., Ostroff S.M., Panda-Jonas S., Pesudovs K., Peto T., Quazi Syed Z., Rahman M.H.U., Ramulu P.Y., Rawaf S., Rawaf D.L., Reinig N., Robin A.L., Rossetti L., Safi S., Sahebkar A., Samy A.M., Saxena D., Serle J.B., Shaikh M.A., Shen T.T., Shibuya K., Shin J. Il, Silva J.C., Silvester A., Singh J.A., Singhal D., Sitorus R.S., Skiadaresi E., Skirbekk V., Soheili A., Sousa R.A.R.C., Spurlock E.E., Stambolian D., Taddele B.W., Tadesse E.G., Tahhan N., Tareque M.I., Topouzis F., Tran B.X., Travillian R.S., Tsilimbaris M.K., Varma R., Virgili G., Wang Y.X., Wang N., West S.K., Wong T.Y., Zaidi Z., Zewdie K.A., Jonas J.B., Vos T. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Global Health. 2021;9:e130–e143. doi: 10.1016/S2214-109X(20)30425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias M.F., Joo K., Kemp J.A., Fialho S.L., da Silva Cunha A., Woo S.J., Kwon Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: basic research and clinical perspectives. Prog. Retin. Eye Res. 2018 doi: 10.1016/j.preteyeres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Gagliardi G., Ben M'Barek K., Goureau O. Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: a pluripotent stem cell-based approach. Prog. Retin. Eye Res. 2019;71:1–25. doi: 10.1016/j.preteyeres.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Garafalo A.V., Cideciyan A.V., Héon E., Sheplock R., Pearson A., WeiYang Yu C., Sumaroka A., Aguirre G.D., Jacobson S.G. Progress in treating inherited retinal diseases: early subretinal gene therapy clinical trials and candidates for future initiatives. Prog. Retin. Eye Res. 2020;77 doi: 10.1016/j.preteyeres.2019.100827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadziahmetovic M., Malek G. Age-related macular degeneration revisited: from pathology and cellular stress to potential therapies. Front. Cell Dev. Biol. 2021;8 doi: 10.3389/fcell.2020.612812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleckenstein M., Keenan T.D.L., Guymer R.H., Chakravarthy U., Schmitz-Valckenberg S., Klaver C.C., Wong W.T., Chew E.Y. Age-related macular degeneration. Nat. Rev. Dis. Prim. 2021;7:31. doi: 10.1038/s41572-021-00265-2. [DOI] [PubMed] [Google Scholar]
- 16.Tatour Y., Ben-Yosef T. Syndromic inherited retinal diseases: genetic, clinical and diagnostic aspects. Diagnostics. 2020;10:779. doi: 10.3390/diagnostics10100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verbakel S.K., van Huet R.A.C., Boon C.J.F., den Hollander A.I., Collin R.W.J., Klaver C.C.W., Hoyng C.B., Roepman R., Klevering B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Ayuso D., Di Pierdomenico J., García-Bernal D., Vidal-Sanz M., Villegas-Pérez M. Bone marrow-derived mononuclear stem cells in the treatment of retinal degenerations. Neural Regen. Res. 2022;17:1937. doi: 10.4103/1673-5374.335692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Froger N., Moutsimilli L., Cadetti L., Jammoul F., Wang Q.-P., Fan Y., Gaucher D., Rosolen S.G., Neveux N., Cynober L., Sahel J.-A., Picaud S. Taurine: the comeback of a neutraceutical in the prevention of retinal degenerations. Prog. Retin. Eye Res. 2014;41:44–63. doi: 10.1016/j.preteyeres.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Castelli V., Paladini A., D'Angelo M., Allegretti M., Mantelli F., Brandolini L., Cocchiaro P., Cimini A., Varrassi G. Taurine and oxidative stress in retinal health and disease. CNS Neurosci. Ther. 2021;27:403–412. doi: 10.1111/cns.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakaria M., Azam S., Haque M.E., Jo S.H., Uddin M.S., Kim I.S., Choi D.K. Taurine and its analogs in neurological disorders: focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jong C.J., Sandal P., Schaffer S.W. The role of taurine in mitochondria health: more than just an antioxidant. Molecules. 2021;26:4913. doi: 10.3390/molecules26164913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffer S., Kim H.W. Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. (Seoul). 2018;26:225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García-Ayuso D., Di Pierdomenico J., Valiente-Soriano F.J., Martínez-Vacas A., Agudo-Barriuso M., Vidal-Sanz M., Picaud S., Villegas-Pérez M.P. Β-alanine supplementation induces taurine depletion and causes alterations of the retinal nerve fiber layer and axonal transport by retinal ganglion cells. Exp. Eye Res. 2019;188 doi: 10.1016/j.exer.2019.107781. [DOI] [PubMed] [Google Scholar]
- 25.García-Ayuso D., Pierdomenico J., Di, Hadj-Said W., Marie M., Agudo-Barriuso M., Vidal-Sanz M., Picaud S., Villegas-Pérez M.P. Taurine depletion causes iprgc loss and increases light- induced photoreceptor degeneration. Investig. Ophthalmol. Vis. Sci. 2018;59:1396–1409. doi: 10.1167/iovs.17-23258. [DOI] [PubMed] [Google Scholar]
- 26.Hadj-Saïd W., Froger N., Ivkovic I., Jiménez-López M., Dubus É., Dégardin-Chicaud J., Simonutti M., Quénol C., Neveux N., Villegas-Pérez M.P., Agudo-Barriuso M., Vidal-Sanz M., Sahel J.A., Picaud S., García-Ayuso D. Quantitative and topographical analysis of the losses of cone photoreceptors and retinal ganglion cells under taurine depletion. Investig. Ophthalmol. Vis. Sci. 2016;57:4692–4703. doi: 10.1167/iovs.16-19535. [DOI] [PubMed] [Google Scholar]
- 27.Martínez-Vacas A., Di Pierdomenico J., Valiente-Soriano F.J., Vidal-Sanz M., Picaud S., Villegas-Pérez M.P., García-Ayuso D. Glial cell activation and oxidative stress in retinal degeneration induced by β-alanine caused taurine depletion and light exposure. Int. J. Mol. Sci. 2021;23:346. doi: 10.3390/ijms23010346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaucher D., Arnault E., Husson Z., Froger N., Dubus E., Gondouin P., Dherbécourt D., Degardin J., Simonutti M., Fouquet S., Benahmed M.A., Elbayed K., Namer I.-J., Massin P., Sahel J.-A., Picaud S. Taurine deficiency damages retinal neurones: cone photoreceptors and retinal ganglion cells. Amino Acids. 2012;43:1979–1993. doi: 10.1007/s00726-012-1273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jammoul F., Dégardin J., Pain D., Gondouin P., Simonutti M., Dubus E., Caplette R., Fouquet S., Craft C.M., Sahel J.A., Picaud S. Taurine deficiency damages photoreceptors and retinal ganglion cells in vigabatrin-treated neonatal rats. Mol. Cell. Neurosci. 2010;43:414–421. doi: 10.1016/j.mcn.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao Y., Yang J., Ma Z., Yan Z., Liu C., Ma J., Wang Y., Yang Z., Huang Y.F. The vigabatrin induced retinal toxicity is associated with photopic exposure and taurine deficiency: an in vivo study. Cell. Physiol. Biochem. 2016;40:831–846. doi: 10.1159/000453143. [DOI] [PubMed] [Google Scholar]
- 31.Horvath G.-A., Hukin J., Stockler-Ipsiroglu S., Aroichane M. Eye findings on vigabatrin and taurine treatment in two patients with succinic semialdehyde dehydrogenase deficiency. Neuropediatrics. 2016;47:263–267. doi: 10.1055/s-0036-1583183. [DOI] [PubMed] [Google Scholar]
- 32.Preising M.N., Görg B., Friedburg C., Qvartskhava N., Budde B.S., Bonus M., Toliat M.R., Pfleger C., Altmüller J., Herebian D., Beyer M., Zöllner H.J., Wittsack H.J., Schaper J., Klee D., Zechner U., Nürnberg P., Schipper J., Schnitzler A., Gohlke H., Lorenz B., Häussinger D., Bolz H.J. Biallelic mutation of human SLC6A6 encoding the taurine transporter TAUT is linked to early retinal degeneration. Faseb. J. 2019;33:11507–11527. doi: 10.1096/fj.201900914RR. [DOI] [PubMed] [Google Scholar]
- 33.Trouillet A., Dubus E., Dégardin J., Estivalet A., Ivkovic I., Godefroy D., García-Ayuso D., Simonutti M., Sahly I., Sahel J.A., El-Amraoui A., Petit C., Picaud S. Cone degeneration is triggered by the absence of USH1 proteins but prevented by antioxidant treatments. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-20171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan Y., Lai J., Yuan Y., Wang L., Wang Q., Yuan F. Taurine protects retinal cells and improves synaptic connections in early diabetic rats. Curr. Eye Res. 2020;45:52–63. doi: 10.1080/02713683.2019.1653927. [DOI] [PubMed] [Google Scholar]
- 35.Lambuk L., Iezhitsa I., Agarwal R., Bakar N.S., Agarwal P., Ismail N.M. Antiapoptotic effect of taurine against NMDA-induced retinal excitotoxicity in rats. Neurotoxicology. 2019;70:62–71. doi: 10.1016/j.neuro.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Tao Y., He M., Yang Q., Ma Z., Qu Y., Chen W., Peng G., Teng D. Systemic taurine treatment provides neuroprotection against retinal photoreceptor degeneration and visual function impairments. Drug Des. Dev. Ther. 2019;13:2689–2702. doi: 10.2147/DDDT.S194169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froger N., Cadetti L., Lorach H., Martins J., Bemelmans A.-P., Dubus E., Degardin J., Pain D., Forster V., Chicaud L., Ivkovic I., Simonutti M., Fouquet S., Jammoul F., Léveillard T., Benosman R., Sahel J.-A., Picaud S. Taurine provides neuroprotection against retinal ganglion cell degeneration. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nor Arfuzir N., Agarwal R., Iezhitsa I., Agarwal P., Sidek S., Ismail N. Taurine protects against retinal and optic nerve damage induced by endothelin-1 in rats via antioxidant effects. Neural Regen. Res. 2018;13 doi: 10.4103/1673-5374.239450. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansar M., Ranza E., Shetty M., Paracha S.A., Azam M., Kern I., Iwaszkiewicz J., Farooq O., Pournaras C.J., Malcles A., Kecik M., Rivolta C., Muzaffar W., Qurban A., Ali L., Aggoun Y., Santoni F.A., Makrythanasis P., Ahmed J., Qamar R., Sarwar M.T., Henry L.K., Antonarakis S.E. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2020;29:618–623. doi: 10.1093/hmg/ddz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Audo I., Mohand-Said S., Boulanger-Scemama E., Zanlonghi X., Condroyer C., Démontant V., Boyard F., Antonio A., Méjécase C., El Shamieh S., Sahel J.-A., Zeitz C. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018;39:887–913. doi: 10.1002/humu.23431. [DOI] [PubMed] [Google Scholar]
- 41.Edwards R.B., Szamier R.B. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science (80-.) 1977;197:1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- 42.Li L., Turner J.E. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp. Eye Res. 1988;47:911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 43.Valiente-Soriano F.J., Salinas-Navarro M., Di Pierdomenico J., García-Ayuso D., Lucas-Ruiz F., Pinilla I., Cuenca N., Vidal-Sanz M., Villegas-Pérez M.P., Agudo-Barriuso M. Tracing the retina to analyze the integrity and phagocytic capacity of the retinal pigment epithelium. Sci. Rep. 2020;10:7273. doi: 10.1038/s41598-020-64131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Cruz P.M. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 45.Gal A., Li Y., Thompson D.A., Weir J., Orth U., Jacobson S.G., Apfelstedt-Sylla E., Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- 46.Jespersgaard C., Bertelsen M., Arif F., Gellert-Kristensen H.G., Fang M., Jensen H., Rosenberg T., Tümer Z., Møller L.B., Brøndum-Nielsen K., Grønskov K. Bi-allelic pathogenic variations in mertk including deletions are associated with an early onset progressive form of retinitis pigmentosa. Genes (Basel) 2020;11:1–10. doi: 10.3390/genes11121517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakti D.H., Cornish E.E., Mustafic N., Zaheer A., Retsas S., Rajagopalan S., Chung C.W.T., Ewans L., McCluskey P., Nash B.M., Jamieson R.V., Grigg J.R. MERTK retinopathy: biomarkers assessing vision loss. Ophthalmic Genet. 2021;42:706–716. doi: 10.1080/13816810.2021.1955278. [DOI] [PubMed] [Google Scholar]
- 48.Di Pierdomenico J., García-Ayuso D., Agudo-Barriuso M., Vidal-Sanz M., Villegas-Pérez M.P. Role of microglial cells in photoreceptor degeneration. Neural Regen. Res. 2019;14:1186–1190. doi: 10.4103/1673-5374.251204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Pierdomenico J., García-Ayuso D., González-Herrero M.E.R., García-Bernal D., Blanquer M., Bernal-Garro J.M., García-Hernández A.M., Vidal-Sanz M., Villegas-Pérez M.P. Bone marrow-derived mononuclear cell transplants decrease retinal gliosis in two animal models of inherited photoreceptor degeneration. Int. J. Mol. Sci. 2020;21:1–21. doi: 10.3390/ijms21197252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Pierdomenico J., García-Ayuso D., Pinilla I., Cuenca N., Vidal-Sanz M., Agudo-Barriuso M., Villegas-Pérez M.P. Early events in retinal degeneration caused by rhodopsin mutation or pigment epithelium malfunction: differences and similarities. Front. Neuroanat. 2017;11 doi: 10.3389/fnana.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Pierdomenico J., Scholz R., Valiente-Soriano F.J., Sánchez-Migallón M.C., Vidal-Sanz M., Langmann T., Agudo-Barriuso M., García-Ayuso D., Villegas-Pérez M.P. Neuroprotective effects of FGF2 and minocycline in two animal models of inherited retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2018;59:4392–4403. doi: 10.1167/iovs.18-24621. [DOI] [PubMed] [Google Scholar]
- 52.Dowling J.E., Sidman R.L. Inherited retinal dystrophy in the rat. J. Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villegas-Pérez M.P., Lawrence J.M., Vidal-Sanz M., Lavail M.M., Lund R.D. Ganglion cell loss in RCS rat retina: a result of compression of axons by contracting intraretinal vessels linked to the pigment epithelium. J. Comp. Neurol. 1998;392:58–77. [PubMed] [Google Scholar]
- 54.Okada M., Okuma Y., Osumi Y., Nishihara M., Yokotani K., Ueno H. Neurotransmitter contents in the retina of RCS rat. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000;238:998–1001. doi: 10.1007/s004170000215. [DOI] [PubMed] [Google Scholar]
- 55.Rascher K., Servos G., Berthold G., Hartwig H.-G., Warskulat U., Heller-Stilb B., Häussinger D. Light deprivation slows but does not prevent the loss of photoreceptors in taurine transporter knockout mice. Vision Res. 2004;44:2091–2100. doi: 10.1016/j.visres.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 56.Di Pierdomenico J., Gallego‐Ortega A., Martínez‐Vacas A., García‐Bernal D., Vidal‐Sanz M., Villegas‐Pérez M.P., García‐Ayuso D. Intravitreal and subretinal syngeneic bone marrow mononuclear stem cell transplantation improves photoreceptor survival but does not ameliorate retinal function in two rat models of retinal degeneration. Acta Ophthalmol. 2022 doi: 10.1111/aos.15165. [DOI] [PubMed] [Google Scholar]
- 57.Gallego-Ortega A., Vidal-Villegas B., Norte-Muñoz M., Salinas-Navarro M., Avilés-Trigueros M., Villegas-Pérez M.P., Vidal-Sanz M. 7,8-Dihydroxiflavone maintains retinal functionality and protects various types of Rgcs in adult rats with optic nerve transection. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Pierdomenico J., García-Ayuso D., Jiménez-López M., Agudo-Barriuso M., Vidal-Sanz M., Villegas-Pérez M.P. Different ipsi- and contralateral glial responses to anti- VEGF and triamcinolone intravitreal injections in rats. Investig. Ophthalmol. Vis. Sci. 2016;57:3533–3544. doi: 10.1167/iovs.16-19618. [DOI] [PubMed] [Google Scholar]
- 59.García-Ayuso D., Ortín-Martínez A., Jiménez-López M., Galindo-Romero C., Cuenca N., Pinilla I., Vidal-Sanz M., Agudo-Barriuso M., Villegas-Pérez M.P. Changes in the photoreceptor mosaic of P23H-1 rats during retinal degeneration: implications for rod-cone dependent survival. Investig. Ophthalmol. Vis. Sci. 2013;54:5888–5900. doi: 10.1167/iovs.13-12643. [DOI] [PubMed] [Google Scholar]
- 60.García-Ayuso D., Salinas-Navarro M., Agudo-Barriuso M., Alarcón-Martínez L., Vidal-Sanz M., Villegas-Pérez M.P. Retinal ganglion cell axonal compression by retinal vessels in light induced retinal degeneration. Mol. Vis. 2011;17:1716–1733. [PMC free article] [PubMed] [Google Scholar]
- 61.Montalbán-Soler L., Alarcón-Martínez L., Jiménez-López M., Salinas-Navarro M., Galindo-Romero C., de Sá F.B., García-Ayuso D., Avilés-Trigueros M., Vidal-Sanz M., Agudo-Barriuso M., Villegas-Pérez M.P. Retinal compensatory changes after light damage in albino mice. Mol. Vis. 2012;18:675–693. [PMC free article] [PubMed] [Google Scholar]
- 62.Valiente-Soriano F.J., Di Pierdomenico J., García-Ayuso D., Ortín-Martínez A., de Imperial-Ollero J.A.M., Gallego-Ortega A., Jiménez-López M., Villegas-Pérez M.P., Patricia Becerra S., Vidal-Sanz M. Pigment epithelium-derived factor (PEDF) fragments prevent mouse cone photoreceptor cell loss induced by focal phototoxicity in vivo. Int. J. Mol. Sci. 2020;21:1–11. doi: 10.3390/ijms21197242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valiente-Soriano F.J., Ortín-Martínez A., Pierdomenico J.D., García-Ayuso D., Gallego-Ortega A., Miralles de Imperial-Ollero J.A., Jiménez-López M., Villegas-Pérez M.P., Wheeler L.A., Vidal-Sanz M. Topical brimonidine or intravitreal bdnf, cntf, or bfgf protect cones against phototoxicity. Transl. Vis. Sci. Technol. 2019;8 doi: 10.1167/tvst.8.6.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brøns C., Spohr C., Storgaard H., Dyerberg J., Vaag A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes. Eur. J. Clin. Nutr. 2004;58:1239–1247. doi: 10.1038/sj.ejcn.1601955. [DOI] [PubMed] [Google Scholar]
- 65.Di Pierdomenico J., Martínez-Vacas A., Hernández-Muñoz D., Gómez-Ramírez A.M., Valiente-Soriano F.J., Agudo-Barriuso M., Vidal-Sanz M., Villegas-Pérez M.P., García-Ayuso D. Coordinated intervention of microglial and Müller cells in light-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2020;61 doi: 10.1167/iovs.61.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Narayan D.S., Wood J.P.M., Chidlow G., Casson R.J. A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol. 2016;94:748–754. doi: 10.1111/aos.13141. [DOI] [PubMed] [Google Scholar]
- 67.Fernández-Sánchez L., Lax P., Campello L., Pinilla I., Cuenca N. Astrocytes and Müller cell alterations during retinal degeneration in a transgenic rat model of retinitis pigmentosa. Front. Cell. Neurosci. 2015;9 doi: 10.3389/fncel.2015.00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hippert C., Graca A.B., Barber A.C., West E.L., Smith A.J., Ali R.R., Pearson R.A. Müller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher S.K., Lewis G.P. Müller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003;43:887–897. doi: 10.1016/S0042-6989(02)00680-6. [DOI] [PubMed] [Google Scholar]
- 70.Jones B.W., Pfeiffer R.L., Ferrell W.D., Watt C.B., Tucker J., Marc R.E. Retinal remodeling and metabolic alterations in human AMD. Front. Cell. Neurosci. 2016;10 doi: 10.3389/fncel.2016.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Che Y., Hou L., Sun F., Zhang C., Liu X., Piao F., Zhang D., Li H., Wang Q. Taurine protects dopaminergic neurons in a mouse Parkinson's disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018;9:435. doi: 10.1038/s41419-018-0468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su Y., Fan W., Ma Z., Wen X., Wang W., Wu Q., Huang H. Taurine improves functional and histological outcomes and reduces inflammation in traumatic brain injury. Neuroscience. 2014;266:56–65. doi: 10.1016/j.neuroscience.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 73.Ogino N., Matsumura M., Shirakawa H., Tsukahara I. Phagocytic activity of cultured retinal pigment epithelial cells from chick embryo: inhibition by melatonin and cyclic AMP, and its reversal by taurine and cyclic GMP. Ophthalmic Res. 1983;15:72–89. doi: 10.1159/000265239. [DOI] [PubMed] [Google Scholar]
- 74.Shin E.Y., Park J.H., Shin M.E., Song J.E., Thangavelu M., Carlomagno C., Motta A., Migliaresi C., Khang G. Injectable taurine-loaded alginate hydrogels for retinal pigment epithelium (RPE) regeneration. Mater. Sci. Eng. C. 2019;103 doi: 10.1016/j.msec.2019.109787. [DOI] [PubMed] [Google Scholar]
- 75.Pinilla I., Maneu V., Campello L., Fernández-Sánchez L., Martínez-Gil N., Kutsyr O., Sánchez-Sáez X., Sánchez-Castillo C., Lax P., Cuenca N. Inherited retinal dystrophies: role of oxidative stress and inflammation in their physiopathology and therapeutic implications. Antioxidants. 2022;11:1086. doi: 10.3390/antiox11061086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dick O., Tom Dieck S., Altrock W.D., Ammermüller J., Weiler R., Garner C.C., Gundelfinger E.D., Brandstätter J.H. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/S0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 77.Ryl M., Urbasik A., Gierke K., Babai N., Joachimsthaler A., Feigenspan A., Frischknecht R., Stallwitz N., Fejtová A., Kremers J., von Wittgenstein J., Brandstätter J.H. Genetic disruption of bassoon in two mutant mouse lines causes divergent retinal phenotypes. Faseb. J. 2021;35 doi: 10.1096/fj.202001962R. [DOI] [PubMed] [Google Scholar]
- 78.Brandstätter J.H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur. J. Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- 79.Babai N., von Wittgenstein J., Gierke K., Brandstätter J.H., Feigenspan A. The absence of functional bassoon at cone photoreceptor ribbon synapses affects signal transmission at off cone bipolar cell contacts in mouse retina. Acta Physiol. 2021;231 doi: 10.1111/apha.13584. [DOI] [PubMed] [Google Scholar]
- 80.Cuenca N., Pinilla I., Sauvé Y., Lu B., Wang S., Lund R.D. Regressive and reactive changes in the connectivity patterns of rod and cone pathways of P23H transgenic rat retina. Neuroscience. 2004;127:301–317. doi: 10.1016/j.neuroscience.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 81.Cuenca N., Pinilla I., Sauvé Y., Lund R. Early changes in synaptic connectivity following progressive photoreceptor degeneration in RCS rats. Eur. J. Neurosci. 2005;22:1057–1072. doi: 10.1111/j.1460-9568.2005.04300.x. [DOI] [PubMed] [Google Scholar]
- 82.Mersman B., Zaidi W., Syed N.I., Xu F. Taurine promotes neurite outgrowth and synapse development of both vertebrate and invertebrate central neurons. Front. Synaptic Neurosci. 2020;12 doi: 10.3389/fnsyn.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almeida C.C., Mendonça Pereira B.F., Leandro K.C., Costa M.P., Spisso B.F., Conte-Junior C.A. Bioactive compounds in infant formula and their effects on infant nutrition and health: a systematic literature review. Int. J. Food Sci. 2021;2021:1–31. doi: 10.1155/2021/8850080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chuang C.K., Lin S.P., Lee H.C., Wang T.J., Shih Y.S., Huang F.Y., Yeung C.Y. Free amino acids in full-term and pre-term human milk and infant formula. J. Pediatr. Gastroenterol. Nutr. 2005;40:496–500. doi: 10.1097/01.MPG.0000150407.30058.47. [DOI] [PubMed] [Google Scholar]
- 85.Tochitani S. Taurine: a maternally derived nutrient linking mother and offspring. Metabolites. 2022;12:228. doi: 10.3390/metabo12030228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Antonarakis S.E. Taurine newborn screening to prevent one form of retinal degeneration and cardiomyopathy. Eur. J. Hum. Genet. 2020;28:1479–1480. doi: 10.1038/s41431-020-0671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu H., Huang L., Jin E., Liang Z., Zhao M. Plasma metabolomic profiling of central serous chorioretinopathy. Exp. Eye Res. 2021;203 doi: 10.1016/j.exer.2020.108401. [DOI] [PubMed] [Google Scholar]
- 88.Bocca C., Le Paih V., Chao De La Barca J.M., Kouassy Nzoughet J., Amati-Bonneau P., Blanchet O., Védie B., Géromin D., Simard G., Procaccio V., Bonneau D., Lenaers G., Orssaud C., Reynier P. A plasma metabolomic signature of Leber hereditary optic neuropathy showing taurine and nicotinamide deficiencies. Hum. Mol. Genet. 2021;30:21–29. doi: 10.1093/hmg/ddab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.