Correction to: AIDS Res Ther (2020) 17:62 https://doi.org/10.1186/s12981-020-00318-8
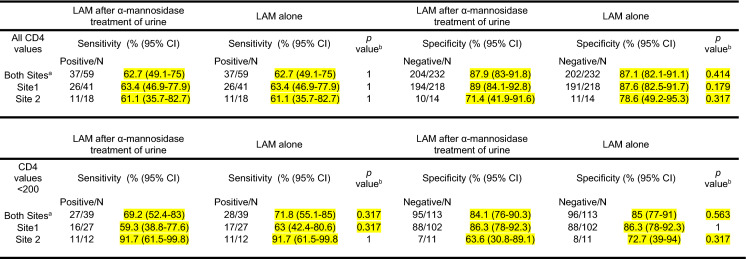
In the original publication of this article [1], the authors were notified that there were some errors in Table 2 that were also present in the main text, specifically in the results section of the abstract, the overall sensitivity of the LAM-test of 56.1% with 95% CI of (43.3–68.3) should have been 62.7 with 95% CI of (49.1–75); the LAM-test sensitivity in PLWH with < 200 CD4 T cells/µl of 62.2% (95% CI 46.5–76.2) should have been of 71.8% with 95% CI of (55.1–85), and the differences in sensitivity when comparing LAM-test results obtained from untreated vs. α-mannosidase treated urine had been deleted.
The sentence “the two participating UAIs using the Fischer exact test” in the statistical analysis sub-heading should have been “the LAM tests using the McNemar exact test”.
In the results section, the LAM-test sensitivity of 56.1% with 95% CI of (43.3–68.3) should have been 62.7 with 95% CI of (49.1–75), and the LAM-test sensitivity in PLWH with < 200 CD4 T cells/µl of 62.2% (95% CI 46.5–76.2) should have been of 71.8% with 95% CI of (55.1–85), the acronym “UIA” should have been “UAI”; the sentence “the defined gold standard composite” should have been “the defined reference standard”; the word “gold” has been eliminated; the sentence “composite gold referenced standard” should have been “composite reference standard”; the acronym “IAUs” should have been “UAI”, and the term “composite gold standard” should have been “composite reference standard”.
As a result, the authors have corrected Table 2. All sensitivity and specificity values have been corrected; the McNemar exact test has replaced the Fischer exact test for p value calculations, and these p values have been corrected; the footnote has been modified to reflect these changes.
Table 2.
Sensitivity and specificity comparisons of LAM test after α-mannosidase treatment of urine vs. LAM test by Site and CD4 values using a composite reference standard
aSite = Site 1 includes UAI 1 = "Dr. Isaac Cohen Alcahé" UAI and Rodolfo Robles Hospital. Site 2 includes UAI 2 = "Dr. Carlos Rodolfo Mejía" UAI and Roosevelt Hospital
bMcNemar exact test was used when comparing LAM test alone and LAM test after α-mannosidase treatment
The authors apologize for any inconvenience.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juan Ignacio García, Johanna Meléndez and Rosa Álvarez: co-authors contributed equally to this work
Contributor Information
Juan Ignacio García, Email: juangarcia@txbiomed.org.
Jordi B. Torrelles, Email: JTorrelles@txbiomed.org
Janet Ikeda, Email: janet.m.ikeda@gmail.com.
Reference
- 1.García JI, Meléndez J, Álvarez R, Mejía-Chew C, Kelley HV, Sidiki S, Castillo A, Mazariegos C, López-Téllez C, Forno D, Ayala N, Balada-Llasat J-M, Mejía-Villatoro CR, Wang S-H, Torrelles JB, Ikeda J. Accuracy of the tuberculosis point-of-care Alere determine lipoarabinomannan antigen diagnostic test using α-mannosidase treated and untreated urine in a cohort of people living with HIV in Guatemala. AIDS Res Therapy. 2020;17:62. doi: 10.1186/s12981-020-00318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]