Abstract
In this present study, Lavandula angustifolia, Lavandula stoechas, and Lavandula × heterophylla essential oils and their main compounds linalool and camphor were evaluated in vitro for lipoxygenase enzyme (LOX) and for angiotensin converting enzyme 2 (ACE2) inhibition potential. The chemical compositions of L. angustifolia, L. stoechas, and L. heterophylla essential oils were confirmed both by gas chromatography–mass spectrometry and gas chromatography–flame ionization detection, where 22.4, 0.9, and 30.6% linalool and 17.8, 54.7, and 15% camphor were identified for each oil among other components, respectively. Enzyme inhibitory activity studies were performed at 20 μg/mL for the tested essential oils, whereas for linalool and camphor concentrations, 5 μg/mL was used. The ACE2 inhibitions of L. angustifolia, L. stoechas, and L. heterophylla essential oils were 25.4, 34.1, and 27.1%, while the LOX inhibitions were observed as 79, 49.1, and 86.7%, respectively. In addition, linalool and camphor showed remarkable ACE2 inhibition with 77.1 and 85.1%, whereas the LOX inhibition was observed at 92 and 67.2%, respectively. In conclusion of the initial findings, further detailed in vivo studies are needed to confirm the safe use.
1. Introduction
Lavender essential oils generally consist of mixtures of mono- and sesquiterpenes and their esters, alcohols, ketones, and oxides along with other volatile compounds. The major components are mostly linalyl acetate, linalool, 1,8-cineole, terpinen-4-ol, β-ocimene, and camphor among others.1 Commercial essential oils are mainly produced by distillation from Lavandula latifolia Medik., Lavandula angustifolia Mill., or Lavandula hybrid cultivars. Other species, especially L. stoechas L., are recorded to be used ethnobotanically.2 However, L. angustifolia essential oil and extract are preferred by the cosmetic and food industry due to their low camphor and high linalool contents. Lavandin (L. x intermedia) is not the first preference of the industry due to the relatively high camphor content. However hybrid species are used successfully in antifungal, antibacterial, and antiseptic applications.3−5
L. angustifolia is used for its antimicrobial potential against human pathogenic fungi and bacteria.6,7 According to a recent work, L. angustifolia essential oil was evaluated in vitro against the H1N1 virus and showed remarkable antiviral effects.8 Also, L. x heterophylla Viv. (syn. L. x hybrida) showed strong antibacterial effects against human pathogenic strains.4 In addition, L. stoechas essential oil is more known with its antifungal effect.9,10 Due to its anti-inflammatory properties, L. angustifolia essential oil was studied for pathologies and associated diseases.11 The major component linalool showed also in vivo inhibition of inflammatory mediator production.12Lavandula species are known and used for their anxiolytic potential, and L. angustifolia essential oil medications are available in pharmaceutical form.13
In this present study, the potential biological effects of different Lavandula essential oils and their major compounds, namely, linalool and camphor, were evaluated by in vitro ACE2 and LOX enzyme assays comparatively. To the best of our knowledge, this study is the first work that uses L. angustifolia, L. stoechas, and L. heterophylla essential oils and linalool and camphor for their in vitro LOX and ACE2 enzyme inhibitory potential.
2. Results and Discussion
2.1. GC/MS and GC-FID Analyses
The essential oil compositions (as relative percentages, %) of the tested Lavandula sp. are reported in Table 1 with the details up to 97%. When compared, L. angustifolia and L. heterophylla essential oils showed a relatively high linalool percentage, while camphor percentage was found in high relative amounts in L. stoechas essential oil as shown also in the table. The major components of L. angustifolia essential oil were identified as 22.4% linalool, 19.2% linalyl acetate, 17.9% camphor, 12.3%1,8-cineole, and 3.8% borneol. The major components of L. stoechas oil were characterized and confirmed as 54.7% camphor, 19.2% α-fenchone, 5.4% bornyl acetate, 2.5% 1,8-cineole, and 2.5% camphene. The analyses showed that L. × heterophylla essential oil major volatile components were 30.6% linalool, 19.6% linalyl acetate, 15% camphor, 11.3% 1,8-cineole, and 4.2% borneol. According to the analytical results, the linalool content was found relatively high in L. angustifolia and L. heterophylla oils, while the camphor content was found high in L. stoechas essential oil. It was observed that the L. angustifolia and L. heterophylla oils are in compliance with the European Pharmacopeia in terms of their linalyl acetate and linalool contents. The essential oils analyzed in terms of linalyl acetate, linalool, camphor, borneol, and 1,8-cineol, which are among the important metabolites of Lavandula species, were also found to be rich.1,14
Table 1. Chemical Composition (%) Data of the Tested Lavandula Essential Oils.
| RRI | compound | L. angustifolia | L. heterophylla | L. stoechas |
|---|---|---|---|---|
| 1014 | tricyclene | 0.3 | ||
| 1032 | α-pinene | 0.3 | 0.3 | 1.3 |
| 1076 | camphene | 0.7 | 0.5 | 2.5 |
| 1118 | β-pinene | 0.2 | 0.4 | 0.1 |
| 1132 | sabinene | 0.1 | ||
| 1174 | myrcene | 0.2 | 0.2 | |
| 1203 | limonene | 0.4 | 0.7 | 0.4 |
| 1213 | 1,8-cineole | 12.3 | 11.3 | 2.5 |
| 1246 | (Z)-β-ocimene | tr | 0.4 | |
| 1266 | (E)-β-ocimene | tr | 1.3 | |
| 1271 | 3-octanone | tr | ||
| 1280 | p-cymene | 0.4 | 0.3 | 0.5 |
| 1290 | terpiolene | 0.1 | ||
| 1353 | hexyl isobutyrate | 0.1 | ||
| 1386 | octenyl acetate | 0.4 | 0.2 | |
| 1406 | α-fenchone | 19.2 | ||
| 1424 | hexyl butyrate | 0.2 | 0.1 | |
| 1450 | trans-linalool oxide (furanoid) | 3.0 | 0.7 | |
| 1474 | camphenilone | 0.5 | ||
| 1478 | cis-linalool oxide (furanoid) | 2.5 | 0.5 | |
| 1532 | camphor | 17.9 | 15.0 | 54.7 |
| 1553 | linalool | 22.4 | 30.6 | 0.9 |
| 1565 | linalyl acetate | 19.2 | 19.6 | 0.6 |
| 1583 | α-santalene | 0.7 | 0.8 | |
| 1591 | bornyl acetate | 0.2 | 0.3 | 5.4 |
| 1611 | terpinen-4-ol | 0.8 | ||
| 1612 | β-caryophyllene | 1.4 | 2.2 | |
| 1617 | lavandulyl acetate | 1.9 | 1.8 | |
| 1616 | hotrienol | 1.6 | 0.8 | 0.1 |
| 1648 | myrtenal | 0.5 | ||
| 1662 | pulegone | 0.1 | ||
| 1670 | trans-pinocarveol | 0.5 | ||
| 1683 | trans-verbenol | 0.5 | ||
| 1686 | lavandulol | 1.4 | 1.7 | |
| 1690 | lavender lactone | 0.2 | ||
| 1704 | myrtenyl acetate | 0.1 | ||
| 1706 | α-terpineol | 1.0 | 1.2 | |
| 1719 | borneol | 3.8 | 3.0 | 1.5 |
| 1725 | verbenone | 1.4 | ||
| 1733 | neryl acetate | 0.2 | ||
| 1750 | cis-linalool oxide (pyranoid) | 0.3 | ||
| 1751 | carvone | 0.3 | ||
| 1765 | geranyl acetate | 0.4 | 0.4 | |
| 1770 | trans-linalool oxide (pyranoid) | 0.3 | ||
| 1786 | ar-curcumene | 0.3 | ||
| 1804 | myrtenol | tr | 0.3 | |
| 1808 | nerol | tr | 1.4 | |
| 1845 | trans-carveol | 0.3 | ||
| 1857 | geraniol | 0.2 | 0.3 | |
| 1864 | p-cymen-8-ol | 0.3 | 0.3 | 1.0 |
| 1961 | 3,7-dimethyl-1,5-octadien-3,7-diol | 0.3 | tr | |
| 2008 | caryophyllene oxide | 0.9 | 0.6 | |
| 2104 | viridiflorol | 0.1 | tr | |
| 2238 | carvacrol | 0.1 | ||
| 2255 | α-cadinol | 0.4 | ||
| total | 95.7 | 93.8 | 97.3 |
2.2. Enzyme Inhibitory Activity
The in vitro LOX enzyme and ACE2 inhibitory activities of the L. angustifolia, L. stoechas, and L. × heterophylla at 20 μg/mL concentration and their major components linalool and camphor at 5 μg/mL concentrations were evaluated.
2.2.1. ACE2 Inhibition
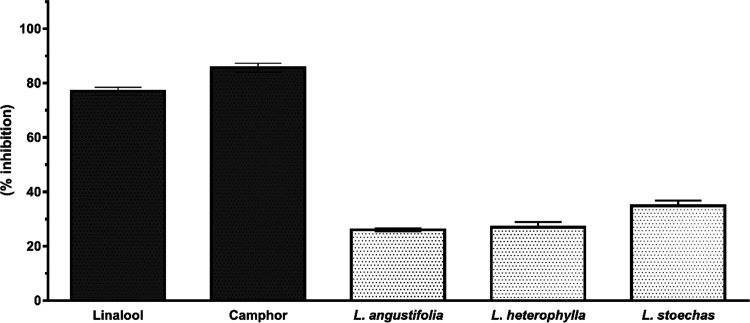
Camphor showed relatively higher ACE2 inhibitory activity than linalool and the tested Lavandula essential oils. L. angustifolia,L. stoechas, and L. heterophylla essential oils showed 25.4 ± 0.88%, 34.1 ± 0.9%, and 27.1 ± 0.98% ACE2 enzyme inhibitory activity, respectively, as shown in Figure 1. The pure major compounds camphor and linalool showed 85.1 ± 0.95% and 77.1 ± 0.4% ACE2 enzyme inhibitions, respectively.
Figure 1.
ACE2 enzyme inhibition of tested essential oils and major components (vertical bars indicate standard deviation values).
Lavandula essential oils (primarily L. angustifolia) were found inhibitory against a broad spectrum of fungi and bacteria.14−16 The antimicrobial effect of many essential oils, where linalool was the major component, was remarkable in several studies.17,18 Essential oils are also known for their antiviral effects against human herpes viruses, human immunodeficiency virus, influenza virus, and yellow fever virus among others.19 Their modes of action are mainly through protease inhibition, genome replication inhibition, or by inhibiting the COVID-19 receptor ACE2 via Pi-H bonding.8,20
Essential oils and their main components have lipophilic properties, and some studies show that SARS-CoV-2 virus membrane integrity is disrupted and they have the potential to penetrate the membrane due to such structural features.20,21 In a previous study by our group, it was demonstrated that linalool inhibited the ACE2 enzyme also by docking.22 Essential oils and their volatile major components also disrupt viral replication, benefiting the host respiratory system through mucus lysis and bronchodilation.23 The major compound linalool of Lavandula sp. is an important active volatile component present in many essential oils, including Origanum, Mentha, and Laurus sp. oils, which are also remarkable for antiviral as well as antimicrobial properties. In addition to the broad antimicrobial effects of essential oils, anti-inflammatory and bronchodilator activities are also prominent and well-known.
ACE2, a zinc metallopeptidase, is the only known human homolog of the particular enzyme. It was discovered in early 2000 and is associated mainly with heart function, hypertension, and diabetes. ACE2 is an exopeptidase that catalyzes the conversion of angiotensin 2 to angiotensin 1–7 and l-phenylalanine.24,25 ACE2 is a type-I integral membrane glycoprotein that acts as a carboxypeptidase rather than a dipeptidase.26,27 The main locations of the receptors of this enzyme, which is active and expressed in most tissues, are cells in contact externally, such as enterocytes of the small intestine and alveolar epithelial cells of the lung.28 ACE2 is also found in venous and arterial cells, renal and cardiovascular tissue, and smooth muscle cells.29 ACE2 was also one of the receptors for the SARS-CoV, the human respiratory coronavirus NL63, and the novel coronavirus 2019 nCoV/SARS-CoV-2.30 Previous studies reported also that ACE2 is one of the essential receptors for various coronavirus types for cell entry.31,32 Cardiovascular and lung involvement as complications of coronavirus are the two well-known main causes for death.30 Recent studies on essential oils and their active ingredients demonstrated effects on the SARS-CoV virus, such as reducing lethal symptoms, lowering inflammatory responses, inhibiting viral replication, inhibiting viral attachment, and easily penetrating the virus followed by its membrane disruption.8 As it was mentioned earlier, ACE2 expression is increased in some regions with the coronavirus. The SARS-CoV-2 virus infects the respiratory epithelium and alveolar macrophages via ACE2 receptors in the heart, lungs, and gastrointestinal tract.33 Based on the ACE2 enzyme inhibition potentials of essential oils and volatile components, it can be proposed that such substances may be effective in the treatment and prevention of coronavirus cases. In addition, the results of previous antiviral and antimicrobial activity studies with other tested Lavandula essential oils, especially L. angustifolia, also support the antiviral findings and potential.3,4,6−8,34
2.2.2. LOX Inhibition
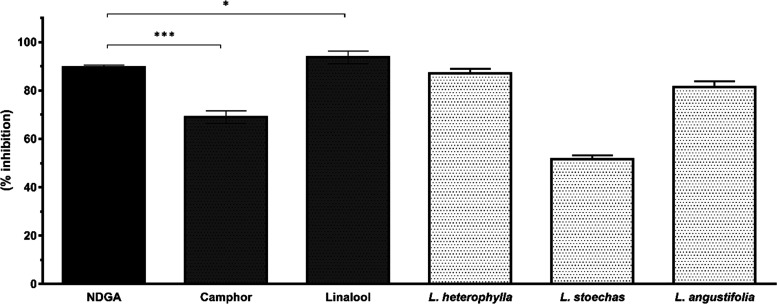
The inhibition results of L. angustifolia, L. stoechas, and L. × heterophylla essential oils and linalool and camphor were 79, 49.1, 86.7, 92, and 62.2%, respectively, as illustrated in Figure 2. Also, the anti-inflammatory standard NDGA was compared in the experiments as a positive control, which showed 90.3% LOX enzyme inhibitory activity.
Figure 2.
LOX enzyme inhibition of tested essential oils and components (*p < 0.05, ***p < 0.001, vertical bars indicate standard deviation).
The anti-inflammatory effect of essential oils can be linked to many signal cascade mechanisms, including cytokines, in addition to their antioxidant effects, also thought to be associated with proinflammatory gene expression. It was reported that L. angustifolia essential oil showed a significantly higher LOX enzyme inhibition, which may be associated to the combination of linalool and camphor, which are known to have high LOX enzyme inhibition levels.35 In another study, TNF-α and IL-6 cytokine levels were measured to test the in vitro anti-inflammatory effect of linalool-rich essential oils, and it was claimed that the reason for the significant decrease in cytokine levels may be due to the linalool content along with other components, which is one of the main components.36 In another in vivo study conducted with a camphor-rich essential oil, a decrease in IL-6 cytokine level was observed.37
As it is well-known, the LOX enzyme is effective on the free arachidonic acid (AA) cascade. This enzyme is responsible for proinflammatory leukotrienes (LTs) and leukotriene receptors, which are also increased in expression in many cells such as vascular endothelial and smooth muscle cells related to SARS-CoV-2.38,39 It is proposed that LOX inhibitors may be as important as antiviral drugs for modulating SARS-CoV-2 infections as leukotrienes enhance local inflammation in various diseases.35
In a previous study, the high LOX inhibition of Salvia officinalis was evaluated, and it was stated that this effect may be caused by the high content of camphor.40In vivo experiments showed recently that linalool has a relatively high anti-inflammatory effect by inhibiting inflammatory mediator production.12 Due to its anti-inflammatory properties, L. angustifolia essential oil is also traditionally used in aromatherapy.11
In this present study, the relatively high LOX inhibition percentages for L. angustifolia and L. heterophylla essential oils and linalool and camphor, which are the main components, showed potential for anti-inflammatory-related diseases.
As a conclusion of this present study, as Lavandula essential oils rich in linalool have a relatively high ACE2 and LOX inhibition, they can be further experimented against coronaviruses using various formulations. Detailed in vivo study data for safety and efficacy, however, are needed before antiviral applications.
3. Methods
3.1. Materials
Linalool, camphor, lipoxygenase enzyme, and other chemicals were acquired from Sigma Aldrich. “Angiotensin II Converting Enzyme (ACE2) Inhibitor Screening Kit” enzyme kit was obtained from BioVision (K310). Commercial Lavandula essential oils were supplied by Doallin, İstanbul, Turkey. Voucher samples are deposited at the IMEF Herbarium (herbarium no.: IMEF 1188-1189-1190).
3.2. GC-FID and GC/MS Analysis
For gas chromatography–flame ionization detection (GC-FID) analyses, the FID was used at 300 °C (Agilent 6890N GC system). Simultaneous automatic injection was carried out using the same conditions in two identical columns in the gas chromatography–mass spectrometry (GC/MS) system (Agilent 5975 GC-MSD). Relative percentages (%) of the volatiles were calculated using the FID chromatograms. This process was performed by GC/MS Library, MassFinder 3 Library, and in-house “Baser Library of Essential Oil Constituents” by analyzing either authentic samples or the relative retention index (RRI) of n-alkanes.41,42
3.3. Enzyme Inhibitory Activity
3.3.1. ACE2 Inhibition
The standard instructions of “Angiotensin II Converting Enzyme (ACE2) Inhibitor Screening Kit” (BioVision, catalog number: K310) were applied. Stock solutions of the test substances were prepared using DMSO (1%, v/v) and 20 μg/mL each oil; the pure compounds (5 μg/mL) were transferred to the well. The prepared ACE2 enzyme solution was added to all wells except the blank. The substrate solution was prepared and added as 40 μL to each well. The reaction mixture was measured with Ex/Em = 320/420 nm wavelength using a microplate reader (SpectraMax i3) at fluorescence mode after the incubation period. The results were reported as % inhibition values, which were obtained for all samples resulting from triplicate analyses.42
3.3.2. LOX Inhibition
The results were measured by the common colorimetric method43 and as previously reported.22 The % inhibition was calculated as the absorbance change for the minute of enzyme activity compared to absorbance change for a minute of the tested oils and compounds. Nordihydroguaiaretic acid was also used as a positive control. The analyses were performed in duplicate, and results are given as mean and standard deviation (SD).
3.4. Statistical Analysis
The statistical analysis was carried out using the GraphPad Prism, version 7.02 (La Jolla, California, USA). In vitro data was expressed as mean ± standard deviation (mean ± SD). The p < 0.05 was accepted as statistically significant.
Acknowledgments
This study was supported by a research project of Anadolu University Scientific Research Projects Commission (BAP 2005S058).
Author Contributions
Conceptualization was done by A.E.K and F.D. Data curation was done by S.N.B., A.E.K., and B.D. Formal analysis was done by S.N.B., A.E.K, and B.D. Funding acquisition was done by F.D. Investigation was done by A.E.K., S.N.B., and F.D. Methodology was contributed by S.N.B. Project administration was done by F.D. Resources were contributed by F.D. Supervision was done by F.D. and B.D. Validation was done by S.N.B. Visualization was done by A.E.K. Roles/writing (original draft) was done by S.N.B. and A.E.K. Writing (review and editing) was done by F.D.
The authors declare no competing financial interest.
References
- Woronuk G.; Demissie Z.; Rheault M.; Mahmoud S. Biosynthesis and Therapeutic Properties of Lavandula Essential Oil Constituents. Planta Med. 2010, 77, 7–15. 10.1055/s-0030-1250136. [DOI] [PubMed] [Google Scholar]
- Gilani A. H.; Aziz N.; Khan M. A.; Shaheen F.; Jabeen Q.; Siddiqui B. S.; Herzig J. W. Ethnopharmacological evaluation of the anticonvulsant, sedative and antispasmodic activities of Lavandula stoechas L. J. Ethnopharmacol. 2000, 71, 161–167. 10.1016/S0378-8741(99)00198-1. [DOI] [PubMed] [Google Scholar]
- Blazekovic B.; Stanic G.; Pepeljnjak S.; Vladimir-Knezevic S. In Vitro Antibacterial and Antifungal Activity of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’. Molecules 2011, 16, 4241–4253. 10.3390/molecules16054241. [DOI] [Google Scholar]
- Bajalan I.; Rouzbahani R.; Pirbalouti A. G.; Maggi F. Chemical Composition and Antibacterial Activity of Iranian Lavandula × hybrida. Chem. Biodiversity 2017, 14, e1700064 10.1002/cbdv.201700064. [DOI] [PubMed] [Google Scholar]
- Wells R.; Truong F.; Adal A. M.; Sarker L. S.; Mahmoud S. S. Lavandula Essential Oils: A Current Review of Applications in Medicinal, Food, and Cosmetic Industries of Lavender. Nat. Prod. Com. 2018, 13, 1403–1417. 10.1177/1934578X180130103. [DOI] [Google Scholar]
- Nikšić H.; Kovač-Bešović E.; Makarević E.; Durić K.; Kusturica J.; Muratovic S. Antiproliferative, Antimicrobial, and Antioxidant Activity of Lavandula angustifolia Mill. Essential Oil. J. Health Sci. 2017, 7, 35–43. 10.17532/jhsci.2017.412. [DOI] [Google Scholar]
- Adam K.; Sivropoulou A.; Kokkini S.; Lanaras T.; Arsenakis M. Antifungal Activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa Essential Oils against Human Pathogenic Fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. 10.1021/jf9708296. [DOI] [Google Scholar]
- Abou Baker D. H.; Amarowicz R.; Kandeil A.; Ali M. A.; Ibrahim E. A. Antiviral Activity of Lavandula angustifolia L. and Salvia officinalis L. Essential Oils Against Avian Influenza H5N1 Virus. J. Agric. Food. Res. 2021, 4, 100135. 10.1016/j.jafr.2021.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gören A. C.; Topçu G.; Bilsel G.; Bilsel M.; Aydoǧmusç Z.; Pezzuto J. M. The Chemical Constituents and Biological Activity of Essential Oil of Lavandula stoechas ssp.stoechas. Z. Naturforsch., C. 2002, 57, 797–800. 10.1515/znc-2002-9-1007. [DOI] [PubMed] [Google Scholar]
- Alberto A.; Andrea B.; Valentina C.; Sandro D.; Cabras P. Chemical Composition, Seasonal Variability, and Antifungal Activity of Lavandula stoechas L.ssp. stoechas Essential Oils from Stem/Leaves and Flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. 10.1021/jf0603329. [DOI] [PubMed] [Google Scholar]
- Rai V. K.; Sinha P.; Yadav K. S.; Shukla A.; Saxena A.; Bawankule D. U.; Tandon S.; Khan F.; Chanotiya C. S.; Yadav N. P. Anti-psoriatic Effect of Lavandula angustifolia Essential Oil and its Major Components Linalool and Linalyl acetate. J. Ethnopharmacol. 2020, 261, 113127. 10.1016/j.jep.2020.113127. [DOI] [PubMed] [Google Scholar]
- Kim M. G.; Kim S. M.; Min J. H.; Kwon O. K.; Park M. H.; Park J. W.; Ahn H. I.; Hwang J. Y.; Oh S. R.; Lee J. W.; Ahn K. S. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. 10.1016/j.intimp.2019.105706. [DOI] [PubMed] [Google Scholar]
- Kasper S. An orally administered lavandula oil preparation (Silexan) for anxiety disorder and related conditions: an evidence based review. Int. J. Psych. Clin. Pract. 2013, 17, 15–22. 10.3109/13651501.2013.813555. [DOI] [PubMed] [Google Scholar]
- Al-Ansari M. M.; Andeejani A. M. I.; Alnahmi E. Insecticidal, antimicrobial and antioxidant activities of essential oil from Lavandula latifolia L. and its deterrent effects on Euphoria leucographa. Ind. Crops Prod. 2021, 170, 113740. 10.1016/j.indcrop.2021.113740. [DOI] [Google Scholar]
- De Rapper S.; Viljoen A.; Van Vuuren S. The in vitro antimicrobial effects of lavandula angustifolia essential oil in combination with conventional antimicrobial agents. Evid.-based Complement. Altern. Med. 2016, 2016, 1–9. 10.1155/2016/2752739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh H. M. A.; Wilkinson J. M. Biological activities of Lavender essential oil. Phyther. Res. 2002, 16, 301–308. 10.1002/ptr.1103. [DOI] [PubMed] [Google Scholar]
- Yang S. K.; Yusoff K.; Ajat M.; Wee C. Y.; Yap P. S. X.; Lim S. H. E.; Lai K. S. Combinatorial Antimicrobial Efficacy and Mechanism of Linalool Against Clinically Relevant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 382. 10.3389/fmicb.2021.635016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F.; Chen Q.; Liang Q.; Zhang M.; Cheng W.; Chen H.; Yun Y.; Zhong Q.; Chen W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.; Yao L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. 10.3390/molecules25112627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S. A.; Nagarajan S. K.; Ramesh V.; Palaniyandi V.; Selvam S. P.; Madhavan T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. 10.1016/j.molstruc.2020.128823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. J.; Biswas Roy S.; Mehta H. J.; Joo M.; Sadikot R. T. Alternative and Natural Therapies for Acute Lung Injury and Acute Respiratory Distress Syndrome. Biomed. Res. Int. 2018, 2018, 1–9. 10.1155/2018/2476824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci F.; Terali K.; Karadaǧ A. E.; Biltekin S. N.; Sakallı E. A.; Koşar M.; Demirci B.; Baser K. H. C. In Vitro and In Silico Evaluation of ACE2 and LOX Inhibitory Activity of Origanum Essential Oils and Carvacrol. Planta Med. 2022, 10.1055/a-1828-2479. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Penninger J. M.; Li Y.; Zhong N.; Slutsky A. S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. J.; Hiscox J. A.; Hooper N. M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004, 25, 291–294. 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Bodiga S.; Das S. K.; LO J.; Patel V.; Oudit G. Y. Role of ACE2 in diastolic and systolic heart failure. Heart Fail. Rev. 2012, 17, 683–691. 10.1007/s10741-011-9259-x. [DOI] [PubMed] [Google Scholar]
- Tipnis S. R.; Hooper N. M.; Hyde R.; Karran E.; Christie G.; Turner A. J. A Human Homolog of Angiotensin-converting Enzyme. J. Biol. Chem. 2000, 275, 33238–33243. 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- Robison K.; Malik K. A Novel Angiotensin-Converting Enzyme-Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9 Mechanism of High Glucose Induced Angiotensin II Production in Rat Vascular Smooth Muscle Cells. Circ. Res. 2000, 87, 1–9. 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- Hamming I.; Timens W.; Bulthuis M.; Lely A.; Navis G.; van Goor H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer D.; Gilbert M.; Borman R.; Clark K. L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 532, 107–110. 10.1016/S0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- Amirfakhryan H.; Safari F. Outbreak of SARS-CoV2: Pathogenesis of infection and cardiovascular involvement. Hell. J. Cardiol. 2021, 62, 13–23. 10.1016/j.hjc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K.; Imai Y.; Rao S.; Gao H.; Guo F.; Guan B.; Huan Y.; Yang P.; Zhang Y.; Deng W.; Bao L.; Zhang B.; Liu G.; Wang Z.; Chappell M.; Liu Y.; Zheng D.; Leibbrandt A.; Wada T.; Slutsky A. S.; Liu D.; Qin C.; Jiang C.; Penninger J. M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Moore M. J.; Vasilieva N.; Sui J.; Wong S. K.; Berne M. A.; Somasundaran M.; Sullivan J. L.; Luzuriaga K.; Greenough T. C.; Choe H.; Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E.; Ntanasis-Stathopoulos I.; Elalamy I.; Kastritis E.; Sergentanis T. N.; Politou M.; Psaltopoulou T.; Gerotziafas G.; Dimopoulos M. A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayola-Serrano N. C.; Roy N.; Fathah Z.; Anwar M. M.; Singh B.; Ammar N.; Sah R.; Elba A.; Utt R. S.; Pecho-Silva S.; Rodriguez-Morales A. J.; Dhama K.; Quraishi S. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflammation Res. 2021, 70, 877–889. 10.1007/s00011-021-01473-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco A.; Martinez-Gutierrez R.; Tomas V.; Tudela J. Lavandula angustifolia and Lavandula latifolia Essential Oils from Spain: Aromatic Profile and Bioactivities. Planta Med. 2016, 82, 163–170. 10.1055/s-0035-1558095. [DOI] [PubMed] [Google Scholar]
- Hammer K. A.; Carson C. F.; Dunstan J. A.; Hale J.; Lehmann H.; Robinson C. J.; Prescott S. L.; Riley T. V. Antimicrobial and anti-inflammatory activity of five Taxandria fragrans oils in vitro. Microbiol. Immunol. 2008, 52, 522–530. 10.1111/j.1348-0421.2008.00070.x. [DOI] [PubMed] [Google Scholar]
- Juhás Š.; Bukovská A.; Čikoš Š.; Czikková S.; Fabian D.; Koppel J. Anti-Inflammatory Effects of Rosmarinus officinalis Essential Oil in Mice. Acta Vet. Brno. 2009, 78, 121–127. 10.2754/avb200978010121. [DOI] [Google Scholar]
- Azkur A. K.; Akdis M.; Azkur D.; Sokolowska M.; Veen W.; Brüggen M. C.; O’Mahony L.; Gao Y.; Nadeau K.; Akdis C. A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B.; Chu H.; Yang D.; Sze K. H.; Lai P. M.; Yuan S.; Shuai H.; Wang Y.; Kao R. Y. T.; Chan J. F. W.; Yuen K. Y. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses 2019, 11, 73. 10.3390/v11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Euch S. K.; Hassine D. B.; Cazaux S.; Bouzouita N.; Bouajila J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. 10.1016/j.sajb.2018.07.010. [DOI] [Google Scholar]
- Demirci B.; Karadaǧ A. E.; Biltekin S. N.; Demirci B. In vitro ACE2 and 5-LOX enzyme inhibition by menthol and three different mint essential oils. Nat. Prod. Commun. 2021, 16, 1934578X2110550. 10.1177/1934578X211055014. [DOI] [Google Scholar]
- Demirci F.; Karadaǧ A. E.; Biltekin S. N.; Demirci B. In vitro ACE2 and 5-LOX Inhibition of Rosmarinus officinalis L. Essential Oil and its Major Component 1,8-Cineole. Rec. Nat. Prod. 2021, 16, 1307–6167. 10.25135/rnp.265.21.05.2080. [DOI] [Google Scholar]
- Baylac S.; Racine P. Inhibition of 5-Lipoxygenase by essential oils and other natural fragment extracts. Int. J. Aromather. 2003, 13, 138–142. 10.1016/S0962-4562(03)00083-3. [DOI] [Google Scholar]