Abstract
Purpose of review
The prevalence and burden of obesity has reached alarming levels. The assessment of human energy expenditure enables the identification of obesity-prone and obesity-resistant individuals and helps to explain the short and long-term success of weight loss treatments. In this review, we describe the state-of-the-art methods used in the assessment of human energy expenditure and the impact of dietary intake on the interpretation of the data.
Recent findings
The reference techniques to assess energy expenditure in humans have not significantly changed during the last century. Today, indirect calorimetry, either using a metabolic chamber or a metabolic cart, is the favored method to assess human energy expenditure and is the only method enabling the assessment of macronutrient oxidation. The doubly labeled water method however provides accurate assessment of human energy expenditure under free living conditions.
Summary
Although energy expenditure and macronutrient oxidation can be assessed by simple calculations from oxygen consumption and carbon dioxide production, these calculations can provide erroneous results or require corrections and/or more complex interpretation when several biochemical pathways are simultaneously engaged. Such physiological mechanisms are often elicited by dietary interventions including, among other, gluconeogenesis, lipogenesis, ketogenesis, alcohol oxidation and under or overfeeding.
Keywords: doubly labeled water, energy balance, indirect calorimetry, nutrient oxidation, substrate balance
INTRODUCTION
The prevalence of obesity has increased worldwide during the past few decades [1,2], making obesity one of the largest public health burdens [3,4]. Obesity is the result of complex interactions between individuals’ genetic background, environmental, behavioral and socioeconomic factors (Fig. 1) [4]. However, regardless of its cause, obesity is produced by a sustained positive energy balance, that is energy intake exceeding energy expenditure. In normal circumstances, energy balance is tightly regulated and many biological processes maintain weight stability over time by matching energy intake to energy expenditure [4]. However, when exposed to the modern obesogenic environment, a large part of the human population presents a biological predisposition to excessive weight gain [5].
FIGURE 1.
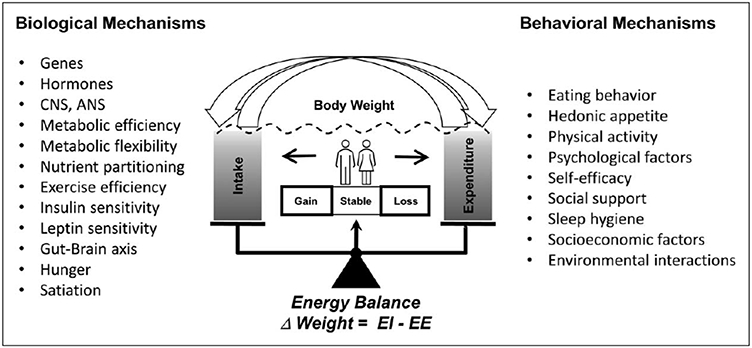
Determinants of human body weight regulation. Despite a strong homeostatic regulation of energy balance, biological and behavioral factors can perturb its regulation. Therefore, the variations on body weight are explained by complex interactions between the individual’s genetic background, environmental, behavioral and socioeconomic factors.
Reproduced by courtesy of Redman, Martin and Krakoff
Over the past few decades, the assessment of energy expenditure has enabled the identification of several factors underlying the biological predisposition to excessive weight gain occurring in many individuals [4,6]. For instance, we were the first to describe that a low relative energy expenditure (adjusted for fat-free mass, fat mass, age and sex) is a risk factor for body weight gain. More recently, changes in energy expenditure in response to acute perturbations in energy intake (e.g. 24-h fasting or 24-h of 200% overfeeding) have been shown to be predictive of weight gain [6-8]. Nowadays, the presence of biologically determined thrifty (prone to weight gain) or spendthrift (resistant to weight gain) phenotypes is recognized [8]. Such thrifty and spendthrift phenotypes also predict the individual response to weight loss treatment [9]. Even more importantly, a reduction in energy expenditure larger than expected by changes in body weight and composition (metabolic adaption) is triggered in response to weight loss and is partially responsible for weight regain [10]. In summary, alterations of energy expenditure determine the propensity of many individuals to become obese and seem to cause a resistance to weight loss and a propensity to regain the weight after a weight loss intervention.
The maintenance of energy balance requires the maintenance of the balance of the three major macronutrients, that is fat, protein and carbohydrate (Fig. 2). In contrast to dietary carbohydrates, protein and alcohol, increased dietary fat intake does not induce rapid increases in fat oxidation thus causing body fat accretion. Moreover, the interindividual variability in the capacity to adjust substrate oxidation to substrate availability (i.e. metabolic flexibility) is hypothesized to be a determinant of ectopic fat accumulation and insulin resistance [11,12]. Together, the above observation highlights the importance of assessing not only total energy expenditure, but the contribution of different macronutrient oxidation rates to energy expenditure.
FIGURE 2.
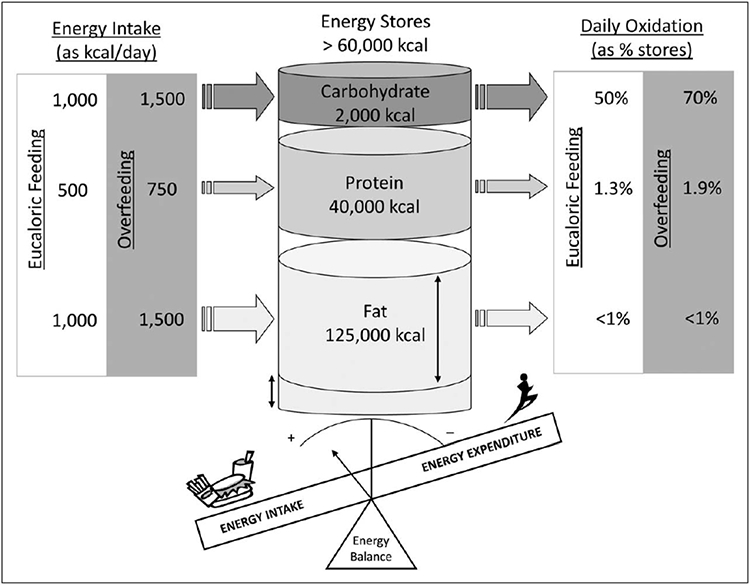
Energy balance. In humans, energy balance depends upon the separate maintenance of macronutrient balance. The daily turnover of carbohydrates is around 50% of the body reserves in energy balance conditions and can be increased up to 100% under overfeeding circumstances. Daily protein turnover typically represents a bit more than 1% of the body reserves and is directly determined by protein ingestion resulting in a robust maintenance of body protein stores. Daily fat turnover represents less than 1% of the fat body reserves. Importantly, while changes in carbohydrate and protein intakes are rapidly mirrored by proportional changes in their oxidation, changes in fat intake are not rapidly compensated by changes in fat oxidation. Therefore, both carbohydrate and protein balances are well regulated and do not impact much the overall energy balance. In contrast, fat balance is the main determinant of energy balance in humans. Adapted from [13].
Below we describe the state-of-the-art methods to assess human energy expenditure and substrate oxidation, and the impact of dietary intake on the interpretation of the data.
METHODS TO ASSESS HUMAN ENERGY EXPENDITURE
The reference techniques to assess energy expenditure in humans have not much changed during the last century. The most accurate methods are direct calorimetry and indirect calorimetry, which both require the individual to be confined in small rooms (Table 1). Therefore, many methods for assessing energy expenditure in free-living conditions have been developed including heart rate (HR) monitors, accelerometry and the doubly labeled water (DLW) method.
Table 1.
Comparison of methods to assess human energy expenditure
| Method | Duration of use | Accuracy | Cost | Advantages | Limitations |
|---|---|---|---|---|---|
| Direct calorimetry | One to several days | High except for thermal inbalance conditions | High (set up and maintenance) | Direct measure of heat production, complete control of environmental factors | Technically demanding, unable to detect acute changes, restricted to confined space |
| Indirect calorimetry, metabolic chamber | Hours to several days | High | High (set up and maintenance) | Real-time minute-by-minute data, allow measurement of components of energy expenditure and macronutrient utilization | Technically demanding, restricted to confined space |
| Indirect calorimetry, metabolic cart | Hours | High for RMR, moderate for other components of 24 h EE | Moderate (set up and maintenance) | Quick response time, easy to operate, feasible in clinical setting, allow measurement of macronutrient utilization | Restricted mobility |
| DLW | 4-21 days | Pretty high | High (isotope cost and analysis) | Gold standard in free-living conditions, applicable to wide range of protocols | No time-course data, unable to differentiate components of EE unless combined with chamber |
| Physical activity log | Flexible | Low | Low | Easy to administer | Participant burden may compromise data quality and some physical activities (e.g. cycling) are difficult to detect |
| Kinematic measurements | Flexible | Low | Low | Easy to administer, objective and unbiased | Pedometers provide no data on patterns and intensity of physical activity |
| HR monitoring | Flexible | Low | Low | Easy to administer, objective and unbiased | Requires individualized calibration, significant loss of data points |
| Ventilation monitoring | Hours | Low | Low | Easy to administer, objective and unbiased | Low applicability in free-living conditions |
DLW, doubly labeled water; EE, energy expenditure; HR, heart rate. Adapted from [14].
Direct calorimetry
As mandated by the first law of thermodynamics, all energy used by the human body will eventually be dissipated as heat, except for the energy used to perform mechanical work [15]. Thus, in conditions of thermal balance, energy expenditure can be accurately assessed by measuring heat production. Direct calorimetry has been used to assess human energy expenditure since the 18th century. Direct calorimeters are small, insulated chambers in which heat released by the person is measured. Due to the high cost of building and maintaining the required equipment as well as some limitations, such as the inability to adequately assess energy expenditure in periods shorter than 24 h due to circadian variations of body temperature [16], direct calorimetry is rarely used nowadays. Nonetheless, it can be argued that it is still the gold-standard technique for the assessment of energy expenditure [15].
Indirect calorimetry
Indirect calorimetry estimates heat production from measurements of oxygen consumption (VO2) and carbon dioxide production (VCO2) [17]. It is based on the known amounts of heat released per liter of VO2 and VCO2 during the combustion of different macronutrients [17]. Importantly, the oxidation of different organic compounds involves different pro- portions of VO2 and VCO2, and thus, indirect calorimetry does allow to determine the individual contribution of different macronutrients oxidation to total energy expenditure. Today, two types of indirect calorimeters are widely used: metabolic chambers and metabolic carts [15,18]. Metabolic chambers are airtight rooms, large enough for an individual to live comfortably during periods that usually extend from 12 h to several days. Ambient air is continuously introduced, mixed and with- drawn from the chamber at a constant rate. Gas concentrations of O2 and CO2 are determined in the incoming and outgoing air flows after drying it. VO2 and VCO2 can be calculated knowing the flow of fresh air and the volume of the chamber [15,18]. The individual’s spontaneous physical activity is commonly assessed by motion detectors while in the chamber to determine the different components of energy expenditure. In contrast to metabolic chambers, which are somewhat rare (approximately 30 worldwide [15]) because of their cost and required maintenance, metabolic carts are much more widely used. Metabolic carts are designed to assess respiratory gas exchanges using a ventilated hood or canopy, a facemask or a mouthpiece [18]. Metabolic carts determine VO2 and VCO2 capturing expired gases either breath-by-breath or by an open circuit flow (three to five times the volunteer’s ventilation) similar to the one used in metabolic chambers [15]. Metabolic carts are easy to use and inexpensive, but limits movement and locomotion, only allowing short-term (up to some hours) assessments. The Deltatrac metabolic cart (DTC; Datex Instrumentarium Corp., Helsinki, Finland), often considered as the gold-standard, is unfortunately no longer manufactured. There are many commercially available metabolic carts on the market [19], although the validity and reliability of some of these instruments are often debatable [19-22].
Doubly labeled water
Ideally, energy expenditure must be calculated from both VO2 and VCO2 since the energy equivalent of VO2 and VCO2 varies if proteins, carbohydrates or lipids are oxidized. However, energy expenditure can also be estimated by just VCO2 with the caveat that speculations have to be made on the source of the macronutrient ratio oxidized [17]. Since the early 1980s, the DLW method has been used to estimate human energy expenditure from VCO2 [23]. The DLW method relies on the enrichment of the body water with heavy hydrogen (2H) and heavy oxygen (18O) after drinking an appropriate dose of DLW (2H218O). After reaching enrichment peaks inversely proportional to total body water, the rate of daily disappearance of both isotopes from the body is determined from collected body fluid samples [23]. VCO2 is then calculated from the differ-that the respiratory quotient (RQ) is equal to the food quotient, estimated from the diet macronutrient composition. Despite other noncalorimetric methods have been used for assessing VCO2, such as the labeled bicarbonate [17], the DLW method unequivocally is the reference method for assessing energy expenditure in free living humans [23].
Other methods
Other methods have been used to estimate human energy expenditure, including observed or self- reported physical activity, kinematic recordings (e.g. pedometers, accelerometers) and physiological measurements (e.g. HR, body temperature, galvanic skin response) [14,16]. Despite the rapid growth in commercial activity trackers during the recent years and ongoing efforts for improving energy expenditure estimations [24], the validity of these devices to estimate energy expenditure remains limited [25].
CALCULATION AND INTERPRETATION OF INDIRECT CALORIMETRY
Indirect calorimetry has been used to assess energy expenditure since the time of Lavoisier, but it is in the second half of the 20th century that more precise systems have been developed taking advantage of the tremendously improved sensitivity of flowmeters and gas analyzers [16]. Moreover, indirect calorimetry, ideally in combination with urinary nitrogen, is the only technique that allows precise estimations of the contribution of different energy substrates to energy expenditure. Consequently, indirect calorimetry has become the widely preferred method for assessing human energy expenditure. However, even if indirect calorimetry allows a precise assessment of VO2 and VCO2, many sources of error can contaminate and bias the read- outs. A detailed description of how VO2 and VCO2 are obtained is beyond the scope of this review and has been recently reviewed [15].
Several equations for assessing energy expenditure from VO2, VCO2 and urinary nitrogen were proposed during the 20th century (Table 2). More- over, equations to estimate carbohydrate and fat oxidation have also been derived from the known stoichiometric energy equivalents of O2 and CO2 of different macronutrients (Table 2). Since most of urinary nitrogen (>80%) is in the form of urea, the amount of protein oxidation, can be estimated from urinary nitrogen, assuming that 6.25 g of protein are oxidized for every gram of urinary nitrogen.
Table 2.
Commonly used equations for estimating energy expenditure, carbohydrates oxidation and fat oxidation
| Author | Equation |
|---|---|
| Energy expenditure | |
| Weir | EE = 3.941_x VO2+1.106_x VCO2 −_2.17 x N |
| Consolazio | EE = 3.78 x VO2 + 1.16 x VCO2 − 2.98 x N |
| Brouwer | EE = 3.866 x VO2 + 1.20 x VCO2 − 1.43 x N |
| Jequier | EE = [4.686 + 1.096_(NPRQ x 0.707)] x NPVO2 + 4.60 x PVO2 |
| Ferrannini | EE = 3.91 x VO2 + 1.10 VCO2 − 3.34 x N |
| Carbohydrates oxidation | |
| Jequier | CHOox = 4.113 x VCO2 − 2.907 x VO2 − 0.375 x PROox |
| Frayn | CHOox = 4.55 x VCO2 − 3.21 x VO2 − 2.87 x N |
| Fat oxidation | |
| Jequier | FATox = 1.689 x VO2 − 1.689 x VCO2 − 0.324 x PROox |
| Frayn | FATox = 1.67 x VO2 − 1.67 x VCO2 − 1.92 x N |
CHOox, carbohydrates oxidation (g/min); EE, energy expenditure (kcal/min); FATox, fat oxidation (g/min); N, urinary nitrogen (g/min); NPRQ, nonprotein respiratory quotient; NPVO2, nonprotein oxygen consumption (l/min); PROox, protein oxidation (g/min); PVO2, protein oxygen consumption (l/min); VCO2, carbon dioxide production (l/min); VO2, oxygen consumption (l/min).
It should be noted that the stoichiometry of a biomolecule oxidation does not depend on the intermediate metabolic process (e.g. the RQ remains the same if a glucose molecule is completely oxidized within a tissue or if it is converted to lactate in one tissue to be oxidized in another one [26]). Consequently, the equations to estimate energy expenditure and macronutrient oxidation remain valid regardless of the intermediate metabolic process by which nutrient are oxidized. However, these equations rely on some assumptions and therefore, provide erroneous results when these assumptions are not met. Caution is needed for interpreting these calculations in conditions other than normal physiological circumstances, which are commonly caused by dietary interventions, pathological states and others [16,17].
Macronutrient interconversion
The equations to estimate nutrient oxidation require a more complex interpretation when bio- synthetic process involves biochemical pathways such as lipogenesis or gluconeogenesis [16]. In this case, the result of the calculation does not represent the rate of nutrient oxidation, but the net rate of nutrient utilization regardless of the biochemical pathway [26]. Therefore, whenever lipid synthesis is taking place, the calculated carbohydrate ‘oxidation’ should be interpreted as the net rate of carbo- hydrate utilization, that is oxidation and conversion to fat. Similarly, when lipogenesis takes place, the fat oxidation equation calculates the net balance between fat oxidation and fat synthesis. In rare cases, calculated fat oxidation may provide negative values when the nonprotein RQ is above 1.0, an indication of net de-novo lipogenesis (i.e. the rate of lipogenesis exceeds the rate of fat oxidation). Conversely, when gluconeogenesis from amino acids takes place (lactate and pyruvate conversion to glucose does not affect any of the calculated substrate oxidation), the calculated value for carbohydrate oxidation reflect the net rate of carbohydrate disappearance (i.e. oxidation – synthesis). In this circum- stance, the rate of fat oxidation rate will be slightly underestimated by approximately 10% of the rate of glucose synthesis [26]. Protein oxidation rate will similarly be affected, since urinary nitrogen will be the consequence of not only protein oxidation, but also alanine deamination. Importantly, although de-novo lipogenesis does not affect the calculation of total energy expenditure, it is slightly affected when gluconeogenesis takes place.
Generation of intermediate metabolites
An important assumption of the macronutrient oxidation equations is that there is no accumulation or excretion of any intermediate metabolite, and there- fore, oxidation leads only to the production of end products such as CO2, water and urinary nitrogen [26]. Therefore, whenever changes occur in the body pool or the excretion rate of intermediate metabolites, the interpretation of indirect calorimetry should be made with caution [17]. The CO2 body pool is physiologically well maintained but can be impacted soon after intense exercise, during hyper or hypoventilation or as a consequence of a shift in acid–base balance [16,26]. Under these circumstances, indirect calorimetry calculations are likely invalid and misleading, and should be discussed accordingly. In case of a decreased body urea pool, protein oxidation will be proportionally overestimated [16]. Similarly, erroneous results are generated in the presence of metabolic processes that produce significant amounts of nitrogenous end products other than urea, such as uric acid (RQ = 0.707) or ammonia (RQ = 0.950) [17].
Another cause of erroneous estimations can be the accumulation of intermediate metabolites such as lactate or ketone bodies [17,27]. Corrections can be made in the nutrient oxidation equations to account for changes in circulating, urine and breath levels of intermediate metabolites [17,27]. However, it should be noted that even if it can be considered in the calculation, a change in lactate or ketone bodies concentration will be coupled with an increase pro- duction of hydrogen ions, which is likely to produce CO2 retention.
Alcohol intake
The nutrient oxidation equations assume that the oxidation mixture is composed of carbohydrates, fat and proteins. However, if alcohol is ingested, it will be preferentially oxidized since it cannot be stored within the body. Since alcohol is rapidly oxidized, alcohol intake provides a good estimation of alcohol oxidation and can be used to correct the calculation of the other nutrient oxidation rates [17].
Type of macronutrient ingested
One should realize that the equations to calculate macronutrient oxidation rates were built assuming a single representative value for the energy equivalents of O2 and CO2. However, the real energy equivalents of O2 or CO2 depend on the type of biomolecules being oxidized, so the composition of uncommon nutrient intake does introduce minor errors in the estimation of energy expenditure. For instance, while the RQ for fat oxidation is normally considered at 0.71, the oxidation of medium chain triglycerides provides an RQ of 0.74 [17]. Similarly, the oxygen consumed per gram of nutrient can be underestimated depending on the dietary source of protein and carbohydrates. For instance, zein protein oxidation consume 9% more oxygen than the one considered by the standard values, while sucrose or lactose oxidation underestimate the carbohydrate oxidation up to 6% [16].
Oxygen consumption estimation from carbon dioxide production
As mentioned above, the food quotient is commonly used for estimating RQ when energy expenditure is calculated only from VCO2 such as with the DLW method. It should be noted however, that the food quotient is equal to RQ only under conditions of energy and macronutrient balance [17,28▪,29▪]. Therefore, corrections are needed for estimating VO2 from VCO2 measurements when the individual is in positive (the RQ will be higher than the food quotient) or negative (the RQ will be lower than the food quotient) energy balance [17,28▪,29▪].
CONCLUSION
The assessment of energy expenditure and macro- nutrient oxidation is of great relevance in the obesity research field. Indirect calorimetry, the reference method, enables the calculations of energy expenditure and macronutrient oxidation in confined conditions, while the DLW is the preferred method to assess energy expenditure in free living conditions. Both methods imply simple calculations using VO2 and/or VCO2. However, these calculations can provide erroneous results and/or require more complex interpretation when several physiological processes, often elicited by dietary interventions, take place.
KEY POINTS.
Indirect calorimetry, either using a metabolic chamber or a metabolic cart, is the preferred method to assess human energy expenditure and is the only method enabling the assessment of macronutrient oxidation.
Unlike indirect calorimetry, the doubly labeled water method allows an accurate calculation of human energy expenditure under free living conditions.
Indirect calorimetry and the doubly labeled water method imply simple calculations using oxygen consumption and/or carbon dioxide production. However, these calculations can provide erroneous results and therefore require corrections or more complex interpretation in presence of unusual biochemical pathways. This is elicited during prolonged fasting or overfeeding as well as in response to extreme dietary composition.
Dietary interventions and nutritional status can affect the calculations of energy expenditure and macronutrient oxidation when eliciting processes such as gluconeogenesis, lipogenesis, ketogenesis, alcohol oxidation, under and overfeeding.
Acknowledgements
We thank Caitlin Hebert for the assistance with the article revision.
Financial support and sponsorship
G.S-D. is supported by a grant Becas de investigacio´n en universidades o centros en el extranjero from the Alfonso Martin Escudero Foundation. E.R. acknowledge the sup- port from a NORC Center Grant no. P30DK072476 entitled ‘Nutrition and Metabolic Health through the Lifespan’ sponsored by NIDDK.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪ ▪ of outstanding interest
- 1.Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism 2018; 92:6–10. [DOI] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128-9 million children, adolescents, and adults. Lancet 2017; 390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshin A, Forouzanfar MH, Reitsma MB, et al. , GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev 2017; 38:267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speakman JR. The evolution of body fatness: trading off disease and predation risk. J Exp Biol 2018; 221:jeb167254. [DOI] [PubMed] [Google Scholar]
- 6.Piaggi P. Metabolic determinants of weight gain in humans. Obesity (Silver Spring) 2019; 27:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlogl M, Piaggi P, Pannacciuli N, et al. Energy expenditure responses to fasting and overfeeding identify phenotypes associated with weight change. Diabetes 2015; 64:3680–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollstein T, Ando T, Basolo A, et al. Metabolic response to fasting predicts weight gain during low-protein overfeeding in lean men: further evidence for spendthrift and thrifty metabolic phenotypes. Am J Clin Nutr 2019; 110:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinhardt M, Thearle MS, Ibrahim M, et al. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes 2015; 64:2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after ‘The Biggest Loser’ competition. Obesity (Silver Spring) 2016; 24:1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granger A, Mott R, Helenius T, et al. Exercise metabolism, set 2. Cell Metab 2017; 25:977. [DOI] [PubMed] [Google Scholar]
- 12.Smith RL, Soeters MR, Wu€st RCI, Houtkooper RH. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev 2018; 39:489–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flatt JP. Issues and misconceptions about obesity. Obesity (Silver Spring) 2011; 19:676–686. [DOI] [PubMed] [Google Scholar]
- 14.Lam YY, Ravussin E. Analysis of energy metabolism in humans: a review of methodologies. Mol Metab 2016; 5:1057–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoffelen PFM, Plasqui G. Classical experiments in whole-body metabolism: open-circuit respirometry-diluted flow chamber, hood, or facemask systems. Eur J Appl Physiol 2017; 118:33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jéquier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr 1987; 7:187–208. [DOI] [PubMed] [Google Scholar]
- 17.Elia M, Livesey G. Energy expenditure and fuel selection in biological systems: the theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev Nutr Diet 1992; 70:68–131. [DOI] [PubMed] [Google Scholar]
- 18.Lam YY, Ravussin E. Indirect calorimetry: an indispensable tool to understand and predict obesity. Eur J Clin Nutr 2016; 71:318–322. [DOI] [PubMed] [Google Scholar]
- 19.Kaviani S, Schoeller DA, Ravussin E, et al. Determining the accuracy and reliability of indirect calorimeters utilizing the methanol combustion technique. Nutr Clin Pract 2018; 33:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcantara JMA, Sanchez-Delgado G, Martinez-Tellez B, et al. Congruent validity and inter-day reliability of two breath by breath metabolic carts to measure resting metabolic rate in young adults. Nutr Metab Cardiovasc Dis 2018; 28:929–936. [DOI] [PubMed] [Google Scholar]
- 21.Delsoglio M, Dupertuis YM, Oshima T, et al. Evaluation of the accuracy and precision of a new generation indirect calorimeter in canopy dilution mode. Clin Nutr 2019; 39:1927–1934. [DOI] [PubMed] [Google Scholar]
- 22.Oshima T, Delsoglio M, Dupertuis YM, et al. The clinical evaluation of the new indirect calorimeter developed by the ICALIC project. Clin Nutr 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Westerterp KR. Doubly labelled water assessment of energy expenditure: principle, practice, and promise. Eur J Appl Physiol 2017; 117:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Driscoll R, Turicchi J, Hopkins M, et al. Improving energy expenditure estimates from wearable devices: a machine learning approach. J Sports Sci 2020. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.O’Driscoll R, Turicchi J, Beaulieu K, et al. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br J Sports Med 2018; 54:332–340. [DOI] [PubMed] [Google Scholar]
- 26.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 1983; 55:628–634. [DOI] [PubMed] [Google Scholar]
- 27.Hall KD, Chen KY, Guo J, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 2016; 104:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall KD, Guo J, Speakman JR. Do low-carbohydrate diets increase energy & expenditure? Int J Obes (Lond) 2019; 43:2350–2354. ▪In this commentary, Hall et al. reexamined previous data in regard to the effect of low-carbohydrate diets on energy expenditure. They illustrate that failing to capture the real respiratory quotient might change the overall conclusion of a study using doubly labeled water to assess energy expenditure.
- 29. Hall KD, Guo J, Chen KY, et al. Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. Am J Clin Nutr 2019; 109:1328–1334. ▪The study analyzed changes in energy expenditure in response to a ketogenic diet. Although no effect was detected by indirect calorimetry, the doubly labeled water method showed a marked increase in energy expenditure, which was attenuated when it was corrected for the diet induced respiratory quotient. This constitutes a good example of the need of conducting proper corrections when the basic assumptions of the methods for assessing energy expenditure are not met.


