Abstract
Purpose:
A better understanding of the relationship between the spread of head and neck squamous cell carcinoma (HNSCC) to regional lymph nodes (LNs) and the frequency and manner of treatment failure should help design better treatment intensification strategies. In this study, we evaluated the relationship between recurrence patterns, mortality, and number of pathologically positive (+) LNs in HNSCC in 3 prospective randomized controlled trials.
Methods and Materials:
We performed a secondary analysis of 947 patients with HNSCC enrolled in RTOG 9501 (n = 410), RTOG 0234 (n = 203), and EORTC 22931 (n = 334) undergoing surgery and postoperative radiation ± systemic therapy. Multivariable models were constructed for overall survival (OS), disease-free survival (DFS), locoregional relapse (LRR), and distant metastases (DM). Restricted cubic splines were used to model the nonlinear relationship between +LN number and outcomes.
Results:
In multivariable analysis, OS and DFS decreased with each +LN without plateau, most pronounced up to 5 +LNs (OS: hazard ratio [HR], 1.21 per +LN; 95% confidence interval [CI], 1.10–1.34; P < .001; DFS: HR per +LN, 1.19; 95% CI, 1.08–1.30; P < .001) and more gradually beyond this (OS: HR per +LN, 1.02; 95% CI, 1.01–1.06; P < .001; DFS: HR per +LN, 1.04; 95% CI, 1.02–1.06; P < .001). In contrast to LRR risk, which increased sharply up to 5 +LNs (HR per +LN, 1.28; 95% CI, 1.10–1.50; P < .001) but plateaued beyond this (HR per +LN, 1.00; 95% CI, 0.96–1.04; P = .98), DM risk increased continuously with increasing +LNs (≤5 +LNs: HR per +LN, 1.10; 95% CI, 1.01–1.20; P = .04; >5 +LNs: HR per +LN, 1.05; 95% CI, 1.02–1.08; P = .003).
Conclusions:
In high-risk resected HNSCC, increased mortality was associated with increased +LN count. LRR and DM risk both increased in parallel up to 5 +LNs, but only DM continued to increase for further +LN increases. These differing recurrence patterns can help inform design of future treatments.
Introduction
Regional lymph node (LN) metastases are associated with increased mortality in head and neck squamous cell carcinoma (HNSCC).1 However, there is substantial heterogeneity in outcomes based on specific nodal features. For example, the eighth edition of the American Joint Committee on Cancer staging manual (AJCC 8E) pathologic nodal classification for HNSCC not related to human papillomavirus (HPV) uses a variety of nodal factors, including size, laterality, and extracapsular extension, to stratify patient prognosis.2,3
There is mounting evidence that the absolute number of pathologically positive (+) LNs may be more important for prognosis than other factors.4–8 Several studies have suggested that quantitative nodal burden can be used to create a simple, objective, and more accurate nodal classification system than the current AJCC system for oral cavity,5 larynx/hypopharynx,4 oropharynx,8 and salivary cancers.6 However, these studies were retrospective, using national cancer registry data. Moreover, these studies do not provide information regarding patterns of recurrence. Therefore, they cannot tell us if the increased mortality observed with increasing +LN number is related to increased locoregional recurrence (LRR), increased distant metastasis (DM), both, or another unidentified factor. Understanding these patterns of recurrence is consequential for designing clinical trials testing novel treatment strategies, given differing strategies likely are needed to reduce LRR and DM.
In this study, we analyzed data from 3 prospective, randomized clinical trials investigating postoperative radiation with or without systemic therapy in surgically resected high-risk HNSCC, Radiation Therapy Oncology Group (RTOG)-9501, RTOG-0234, and European Organization for Research and Treatment of Cancer(EORTC)-22931,9–12 to better evaluate the effect of numerical LN burden on survival and patterns of failure in HNSCC.
Methods and Materials
Patient selection and study design
This study was a secondary analysis of NRG Oncology’s RTOG 9501 and RTOG 0234, and EORTC 22931. Clinical trial data were obtained through approved data sharing agreements and we received access to individual patient-level clinical trial data directly from the EORTC and NRG Oncology. These 3 trials were selected because they are the only randomized trials that involve upfront surgery and postoperative radiation therapy for head and neck cancer available from EORTC and NRG Oncology that have been published to date. Information about the data sharing process is available at https://www.nrgoncology.org/Resources/Ancillary-ProjectsData-Sharing-Application and https://www.eortc.org/data-sharing. Although data were prepared by EORTC and NRG Oncology, and data and results were reviewed by an NRG Oncology statistician (P.A.T-S), all analysis was performed at Cedars-Sinai Medical Center. Full details of patients enrolled in these trials and inclusion and exclusion criteria have been published previously.9,11,12 All 3 studies were randomized trials of patients with HNSCC (oropharynx, oral cavity, larynx, and hypopharynx subsites) with “high-risk” features undergoing postoperative radiation after surgical resection and neck dissection. RTOG 9501 and EORTC 22931 randomized patients to adjuvant radiation therapy with or without concomitant cisplatin, whereas RTOG 0234 randomized patients to adjuvant cetuximab together with either cisplatin or docetaxel-based chemoradiation. All patients signed informed consent to enroll in the trials. Given this was a study using deidentified patient data, our institutional review board deemed it exempt from review.
The major differences between the trials were differing definitions of “high-risk” disease, primary endpoints, and countries where the trials were conducted. RTOG 9501 included patients with 2 or more histologically +LNs, extracapsular extension of nodal disease, and/or positive margins and had a primary endpoint of locoregional control. RTOG 0234 was a phase II trial enrolling patients with extranodal extension, 2 or more +LNs, or positive margins, and the primary endpoint was disease-free survival (DSF). EORTC 22931 enrolled patients with pT3–4 (except T3N0 larynx), positive margins, extranodal extension, perineural involvement, or vascular tumor embolism, and the primary endpoint was progression-free survival.
Statistical analysis
Baseline patient characteristics among patients across the 3 randomized trials RTOG 9501 and RTOG 0234, and EORTC 22931 were compared using the Kruskal-Wallis test and the Pearson’s χ2 test for continuous and categorical variables, respectively. Trials were used as a covariate in the multivariable models. Survival functions were estimated using Kaplan-Meier method, and comparisons of survival curves were evaluated with the log-rank test.13 Rates of LRR and DM were estimated with the cumulative incidence function using competing risks methodology and compared using the K-sample test as proposed by Gray.14
Missing data were imputed using predictive mean matching from the multivariate imputation by chained equations (MICE) algorithm as developed by Buuren.15 Overall survival (OS) was defined as the time from randomization to death or last follow-up. For disease-free survival (DFS), failure was defined as local, regional, or distant progression, second primary tumor, or death. LRR was defined as the time from randomization to local or regional relapse, death, or last follow-up. DM was defined as the time from randomization to DM, death, or last follow-up. For LRR and DM, any death was considered a competing risk. The univariate and multivariable analysis for OS and DFS was performed using a Cox proportional hazards model, and LRR and DM were analyzed using the Fine and Gray competing risks model. The number of histologically involved LN metastases was modeled in multivariable analysis as a restricted cubic spline function with 3 knots corresponding to the 10th, 50th, and 90th quantiles, as proposed by Harrell.16 The choice of modeling LN metastases as a restricted cubic spline function allowed us to avoid the assumption of linearity in the effect of increasing LN metastases with the outcome of interest. The choice of the number of knots was determined based on optimizing for Akaike information criterion, where the locations of the knots were prespecified in fixed quantiles of the variable’s marginal distribution. The location of the knots in these fixed quantiles ensures enough data are available in each interval and prevents outliers from overly influencing knot placement. Estimates from the multivariable model as presented are the averaged estimates after fitting the models on 5 imputed complete data sets with variance equal to the imputation-corrected variance-covariance matrix.17 The identification of the change points in the log relative hazard of increasing number of +LNs was determined using a piecewise linear regression model.18,19 Interaction between nodal count and systemic therapy was fit using a 2 degrees-of-freedom continuous nonlinear spline of the number of +LNs (given the nonlinear relationship between +LN number and outcomes) with 3 knots and the categorical radiation versus chemoradiation variable.20 The test of interaction was performed using a multivariable model with a joint Wald’s test. Test of interaction between +LN number and radiation dose was also performed.
All statistical analyses were performed using R statistical software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria) with 2-sided tests and a significance level of 0.05. The statistics were also reviewed by the statistician who prepared the data for RTOG.
Results
Patient cohort
Overall, 947 patients were included (RTOG 9501: n = 410; RTOG 0234: n = 203; EORTC 22931: n = 334). The median follow-up was 7.4 years (95% confidence interval [CI], 7.0–7.6). Comparisons of baseline characteristics of patients across the 3 trials are shown in Table 1. The mean number of examined LNs was 33 (standard deviation, ± 20), and the mean number of +LNs was 4.2 (standard deviation, ± 4.4). Overall, there were 552 deaths, 238 LRR events, and 259 DM events.
Table 1.
Baseline characteristics of patients across XEORTC 22931, RTOG 9501, and RTOG 0234
| Characteristic | Overall, N = 947 | EORTC 22931, n = 334 | RTOG 9501, n = 410 | RTOG 0234, n = 203 | P value* |
|---|---|---|---|---|---|
|
| |||||
| Age, y | .015 | ||||
| Median (IQR) | 55 (49, 62) | 54 (48, 61) | 55 (50, 62) | 56 (49, 62) | |
| Mean (SD) | 55 (9) | 54 (9) | 56 (9) | 55 (10) | |
| Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Sex | <.001 | ||||
| Male | 816 (86%) | 308 (92%) | 357 (87%) | 151 (74%) | |
| Female | 130 (14%) | 25 (8%) | 53 (13%) | 52 (26%) | |
| Missing | 1 (0.11%) | 1 (0.30%) | 0 (0.0%) | 0 (0.0%) | |
| Anatomic site | <.001 | ||||
| Oral cavity | 293 (31%) | 87 (26%) | 111 (27%) | 95 (47%) | |
| Larynx | 183 (19%) | 75 (22%) | 86 (21%) | 22 (11%) | |
| Hypopharynx | 120 (13%) | 68 (21%) | 40 (10%) | 12 (6%) | |
| Oropharynx | 347 (37%) | 101 (31%) | 172 (42%) | 74 (36%) | |
| Missing | 4 (0.42%) | 3 (0.90%) | 1 (0.24%) | 0 (0.0%) | |
| Tumor classification† | <.001 | ||||
| T1 | 122 (13%) | 27 (8%) | 51 (12%) | 44 (22%) | |
| T2 | 257 (27%) | 83 (25%) | 109 (27%) | 65 (32%) | |
| T3 | 235 (25%) | 93 (28%) | 106 (26%) | 36 (18%) | |
| T4 | 331 (35%) | 129 (39%) | 144 (35%) | 58 (28%) | |
| Missing | 2 (0.21%) | 2 (0.60%) | 0 (0.0%) | 0 (0.0%) | |
| Node classification† | <.001 | ||||
| N0 | 68 (8%) | 54 (18%) | 7 (2%) | 7 (4%) | |
| N1 | 25 (3%) | 17 (6%) | 4 (1%) | 4 (3%) | |
| N2 | 289 (33%) | 53 (18%) | 194 (47%) | 42 (25%) | |
| N3 | 490 (56%) | 173 (58%) | 205 (50%) | 112 (68%) | |
| Missing | 75 (7.9%) | 37 (11.1%) | 0 (0.0%) | 38 (18.7%) | |
| Extranodal extension | <.001 | ||||
| No | 380 (42%) | 143 (43%) | 192 (47%) | 45 (27%) | |
| Yes | 529 (58%) | 191 (57%) | 218 (53%) | 120 (73%) | |
| Missing | 38 (4.01%) | 0 (0.0%) | 0 (0.0%) | 38 (18.7%) | |
| Number of nodes positive | <.001 | ||||
| Median (IQR) | 3.0 (2.0, 5.0) | 2.0 (1.0, 4.0) | 4.0 (2.0, 6.0) | 3.0 (2.0, 5.0) | |
| Mean (SD) | 4.2 (4.4) | 3.0 (3.8) | 5.2 (4.8) | 4.2 (4.2) | |
| Missing | 35 (3.7%) | 7 (2.1%) | 13 (3.2%) | 15 (7.4%) | |
| Number of nodes examined | <.001 | ||||
| Median (IQR) | 31 (19, 43) | 25 (16, 40) | 34 (22, 46) | 30 (19, 44) | |
| Mean (SD) | 33 (20) | 29 (19) | 37 (21) | 33 (18) | |
| Missing | 38 (4.0%) | 10 (3.0%) | 13 (3.2%) | 15 (7.4%) | |
| Surgical margins | <.001 | ||||
| Negative | 722 (77%) | 237 (71%) | 370 (90%) | 115 (58%) | |
| Positive | 218 (23%) | 95 (29%) | 40 (10%) | 83 (42%) | |
| Missing | 7 (0.7%) | 2 (0.6%) | 0 (0.0%) | 5 (2.5%) | |
| Adjuvant therapy | <.001 | ||||
| Radiation therapy | 375 (40%) | 167 (50%) | 208 (51%) | 0 (0%) | |
| Chemoradiation | 572 (60%) | 167 (50%) | 202 (49%) | 203 (100%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Number of events | |||||
| Overall survival | 552 (58.3%) | 174 (52.1%) | 289 (70.5%) | 89 (443.8%) | <.001 |
| Disease-free survival | 620 (65.5%) | 183 (554.8%) | 323 (798.8%) | 114 (56.2%) | <.001 |
| Locoregional recurrence | 238 (25.1%) | 83 (254.9%) | 103 (25.1%) | 52 (265.6%) | .981 |
| Distant metastasis | 259 (27.3%) | 77 (23.1%) | 132 (32.2%) | 50 (254.6%) | .013 |
Abbreviations: AJCC = American Joint Committee on Cancer; IQR = interquartile range; SD = standard deviation.
Statistical tests performed: Kruskal-Wallis test and χ2 test of independence.
AJCC seventh edition TNM (tumor, nodes, metastases) staging.
Data are presented as n (%) unless otherwise indicated.
Multivariable survival analyses
In multivariable analysis, increasing number of +LNs was strongly associated with worse OS (P < .001) (Table 2). Because +LN number and mortality have been reported to have a nonlinear relationship,4–6,8 we modelled this with a restricted cubic spline function. Five +LNs was determined mathematically to be the optimal cut-point across all outcomes using a piecewise linear regression based on use of a restricted cubic spline function adjusted for other variables of interest in multivariable analysis. For OS (Fig. 1A, Table 3), the association of increasing number of +LNs with increased mortality was strongest up to the change point in the slope of the spline at 5 +LNs (hazard ratio [HR] per +LN, 1.21; 95% CI, 1.10–1.34; P < .001). Beyond this, overall mortality continued to increase with each +LN without plateau but at a more gradual rate (HR per +LN, 1.02; 95% CI, 1.01–1.06; P < .001). DFS followed an almost identical pattern (Fig. 1B), with continuous increase in risk with increasing LN number that was more pronounced for the first 5 +LNs (HR per +LN, 1.19; 95% CI, 1.08–1.30; P < .001) in comparison with +LNs beyond 5 (HR per +LN, 1.04; 95% CI, 1.02–1.06; P < .001).
Table 2.
Multivariable Cox regression analysis of OS, DFS, LRR, and DM in patients across XRTOG 9501, RTOG 0234, and EORTC 22931
| OS |
DFS |
LRR |
DM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P value | HR | 95% CI | P value | sHR | 95% CI | P value | sHR | 95% CI | P value |
|
| ||||||||||||
| Age, y | 1 | 0.99, 1.01 | .903 | 1 | 0.99, 1.01 | .859 | 0.98 | 0.96, 0.99 | .005 | 0.99 | 0.97, 1.00 | .15 |
| Sex | ||||||||||||
| Male | - | - | - | - | - | - | - | - | ||||
| Female | 0.82 | 0.63, 1.08 | .163 | 0.89 | 0.69, 1.14 | .344 | 0.96 | 0.65, 1.43 | .857 | 0.84 | 0.57, 1.26 | .406 |
| Anatomic site | ||||||||||||
| Oral cavity | - | - | - | - | - | - | - | - | ||||
| Larynx | 0.63 | 0.49, 0.81 | <.001 | 0.71 | 0.56, 0.89 | .004 | 0.61 | 0.41, 0.89 | .011 | 1.16 | 0.79, 1.71 | .437 |
| Hypopharynx | 0.75 | 0.56, 0.99 | .04 | 0.8 | 0.61, 1.05 | .108 | 0.55 | 0.35, 0.86 | .009 | 1.88 | 1.26, 2.80 | .002 |
| Oropharynx | 0.5 | 0.40, 0.63 | <.001 | 0.56 | 0.46, 0.70 | <.001 | 0.66 | 0.47, 0.91 | .011 | 0.84 | 0.59, 1.20 | .344 |
| Tumor classification* | ||||||||||||
| T1 | - | - | ||||||||||
| T2 | 1.89 | 1.33, 2.69 | <.001 | 1.72 | 1.26, 2.36 | <.001 | 2.62 | 1.40, 4.91 | .003 | 1.73 | 0.99, 3.04 | .055 |
| T3 | 2.58 | 1.80, 3.68 | <.001 | 2.38 | 1.73, 3.28 | <.001 | 2.94 | 1.54, 5.61 | .001 | 2.08 | 1.18, 3.65 | .011 |
| T4 | 2.75 | 1.94, 3.91 | <.001 | 2.39 | 1.74, 3.26 | <.001 | 3.36 | 1.78, 6.33 | <.001 | 2.16 | 1.23, 3.77 | .007 |
| Number of nodes positive (spline)† | - | - | <.001 | - | - | <.001 | - | - | <.001 | - | - | <.001 |
| Number of nodes examined (spline) | - | - | .325 | - | - | .202 | - | - | .884 | - | - | .41 |
| Extranodal extension | ||||||||||||
| No | - | - | - | - | - | - | - | - | ||||
| Yes | 1.59 | 1.31, 1.93 | <.001 | 1.48 | 1.24, 1.77 | <.001 | 1.29 | 0.97, 1.73 | .085 | 1.59 | 1.20, 2.11 | .001 |
| Surgical margins | ||||||||||||
| Negative | - | - | - | - | - | - | - | - | ||||
| Positive | 1.23 | 0.99, 1.53 | .063 | 1.22 | 0.99, 1.50 | .059 | 1.38 | 1.02, 1.88 | .039 | 1.17 | 0.85, 1.63 | .332 |
| Adjuvant therapy | ||||||||||||
| Radiation therapy | - | - | - | - | ||||||||
| Chemoradiation | 0.8 | 0.66, 0.97 | .022 | 0.82 | 0.69, 0.99 | .034 | 0.62 | 0.46, 0.84 | .002 | 0.9 | 0.68, 1.19 | .459 |
| Trial | ||||||||||||
| EORTC 22931 | - | - | - | - | - | - | - | - | ||||
| RTOG 9501 | 1.17 | 0.94, 1.46 | .151 | 1.36 | 1.10, 1.68 | .004 | 0.94 | 0.67, 1.31 | .701 | 1.51 | 1.10, 2.07 | .01 |
| RTOG 0234 | 0.66 | 0.49, 0.88 | .005 | 0.9 | 0.68, 1.17 | .426 | 1.01 | 0.65, 1.56 | .981 | 1.21 | 0.79, 1.84 | .384 |
Abbreviations: AJCC = American Joint Committee on Cancer; CI = confidence interval; DFS = disease-free survival; DM= distant metastasis; HR = hazard ratio; LRR = locoregional recurrence; OS = overall survival; sHR = subdistributional HR.
AJCC seventh edition TNM (tumor, nodes, metastases) staging.
Left blank, as these variables were included in the multivariable model as a restricted cubic spline function due to nonlinear effect. Refer to Table 3 for results of piecewise linear regression for the HR for the spline segments.
Fig. 1.
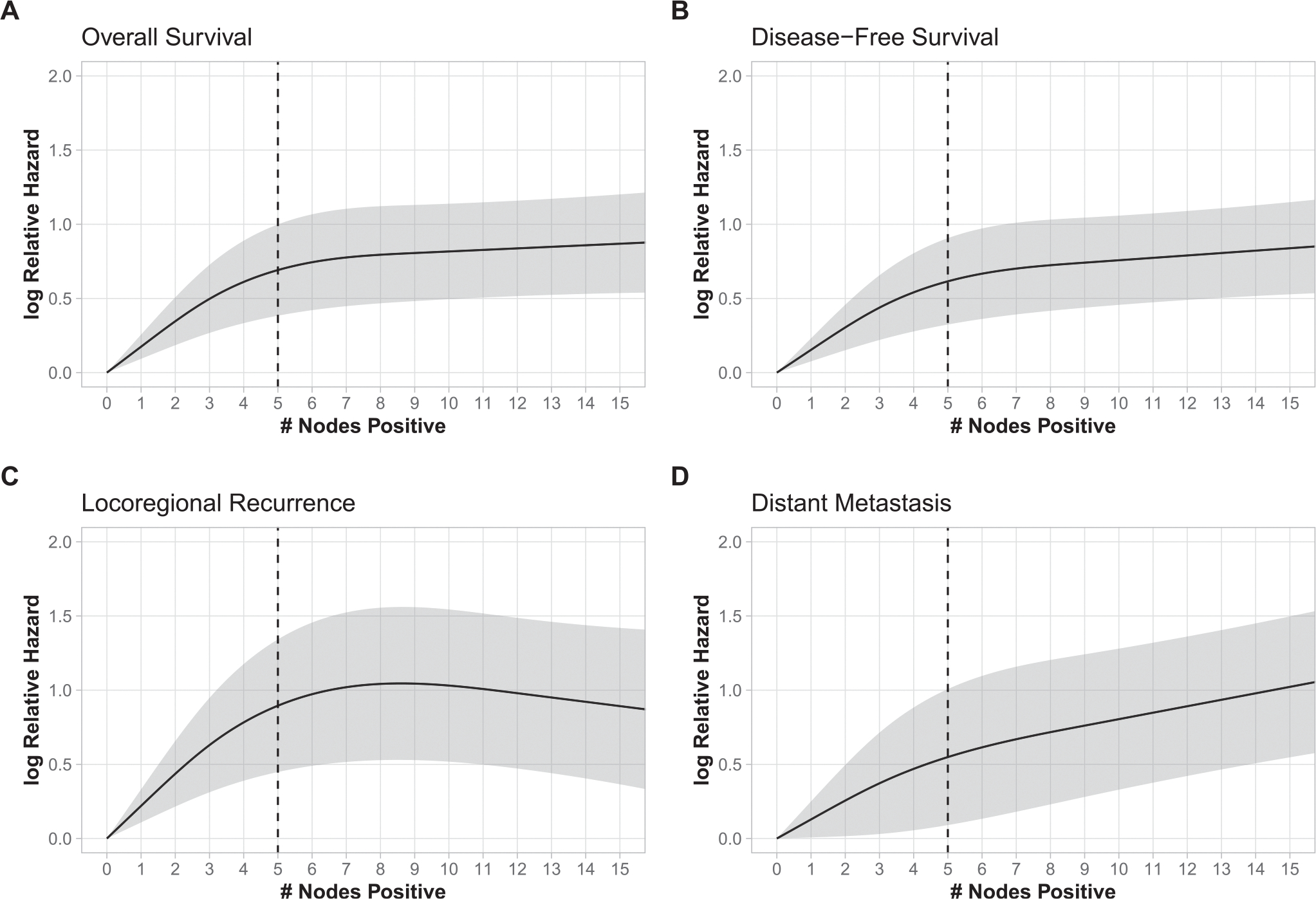
The natural logarithm of the adjusted hazard ratio of risk/event as a nonlinear function of the number of positive lymph nodes, with 0 positive lymph nodes as a reference for (A) overall survival, (B) disease-free survival, (C) locoregional recurrence, and (D) distant metastasis. The gray area represents the 95% pointwise confidence interval of the natural logarithm of the predicted hazard ratios. The black curve represents the smoothed restricted cubic spline plot of the natural logarithm of the predicted adjusted hazard ratio versus the number of positive lymph nodes. The black vertical dotted line represents the calculated change point.
Table 3.
Summary of HRs for number of +LNs stratified by change point of 5 +LNs for overall survival and disease-free survival and subdistributional HRs for locoregional recurrence and distant metastases
| HR/sHR | 95% CI | P value | |
|---|---|---|---|
|
| |||
| Overall survival | |||
| 0–5 +LNs | 1.21 | 1.1, 1.34 | <.001 |
| ≥6 +LNs | 1.02 | 1.01, 1.06 | <.001 |
| Disease-free survival | |||
| 0–5 +LNs | 1.19 | 1.08, 1.30 | <.001 |
| ≥6 +LNs | 1.04 | 1.02, 1.06 | <.001 |
| Locoregional recurrence | |||
| 0–5 +LNs | 1.28 | 1.10, 1.50 | <.001 |
| ≥6 +LNs | 1.00 | 0.96, 1.04 | .98 |
| Distant metastasis | |||
| 0–5 +LNs | 1.10 | 1.01, 1.20 | .028 |
| ≥6 +LNs | 1.05 | 1.02, 1.08 | .003 |
Abbreviations: CI = confidence interval; HR = hazard ratio (expressed as 1 unit increment); LN = lymph node; sHR = subdistributional HR.
Given the limited information regarding HPV status, which is a strong prognostic factor for oropharyngeal cancer, we performed a sensitivity analysis excluding oropharynx patients. As in the main cohort, +LN number was associated with worse OS, DFS, LRR, and DM, with a cut-point determined to be 5 +LNs (Tables E1 and E2).
Patterns of recurrence
When examining patterns of recurrence, LRR and DM demonstrated somewhat differing relationships with number of +LNs. Parallel to mortality, both LRR risk and DM risk increased with each +LN up to 5 (Fig. 1C, 1D). Notably, the relationship between +LN number and LRR risk (subdistributional HR [sHR] per +LN, 1.28; 95% CI, 1.10–1.50; P < .001) was somewhat stronger than for DM risk (sHR per +LN, 1.10; 95% CI, 1.01–1.20; P = .028) in this +LN range. By contrast, for each LN beyond 5 +LNs, there was no additional risk of LRR (sHR per +LN, 1.00; 95% CI, 0.96–1.04; P = .98), but DM risk continued to increase significantly without plateau (sHR per +LN, 1.05; 95% CI, 1.02–1.08; P = .003). (Table 3).
We analyzed the influence of systemic therapy on these results There was no statistically significant interaction between +LN number and delivery of systemic therapy for OS (P = .161), DFS (P = .45), DM (P = .802), or LRR (P = .07). Thus, there was no detectable difference in the association of +LN and outcomes in patients receiving chemoradiation versus those receiving radiation alone. There was also no significant interaction between +LN and radiation dose on LRR (P = .11).
Composite nodal burden and oncologic outcomes
Given the change point identified at 5 +LNs, we compared outcomes for patients with ≤5 LNs versus >5 +LNs to describe the absolute differences in outcomes between these groups. There were marked differences in 5-year outcomes for OS (54% vs 32%, P < .001), DFS (47% vs 27%, P < .001), LRR (21% vs 32%, P = .003), and DM (22% vs 38%, P < .001) when comparing patients with ≤5 and >5 +LNs, respectively (Fig. 2A–D).
Fig. 2.
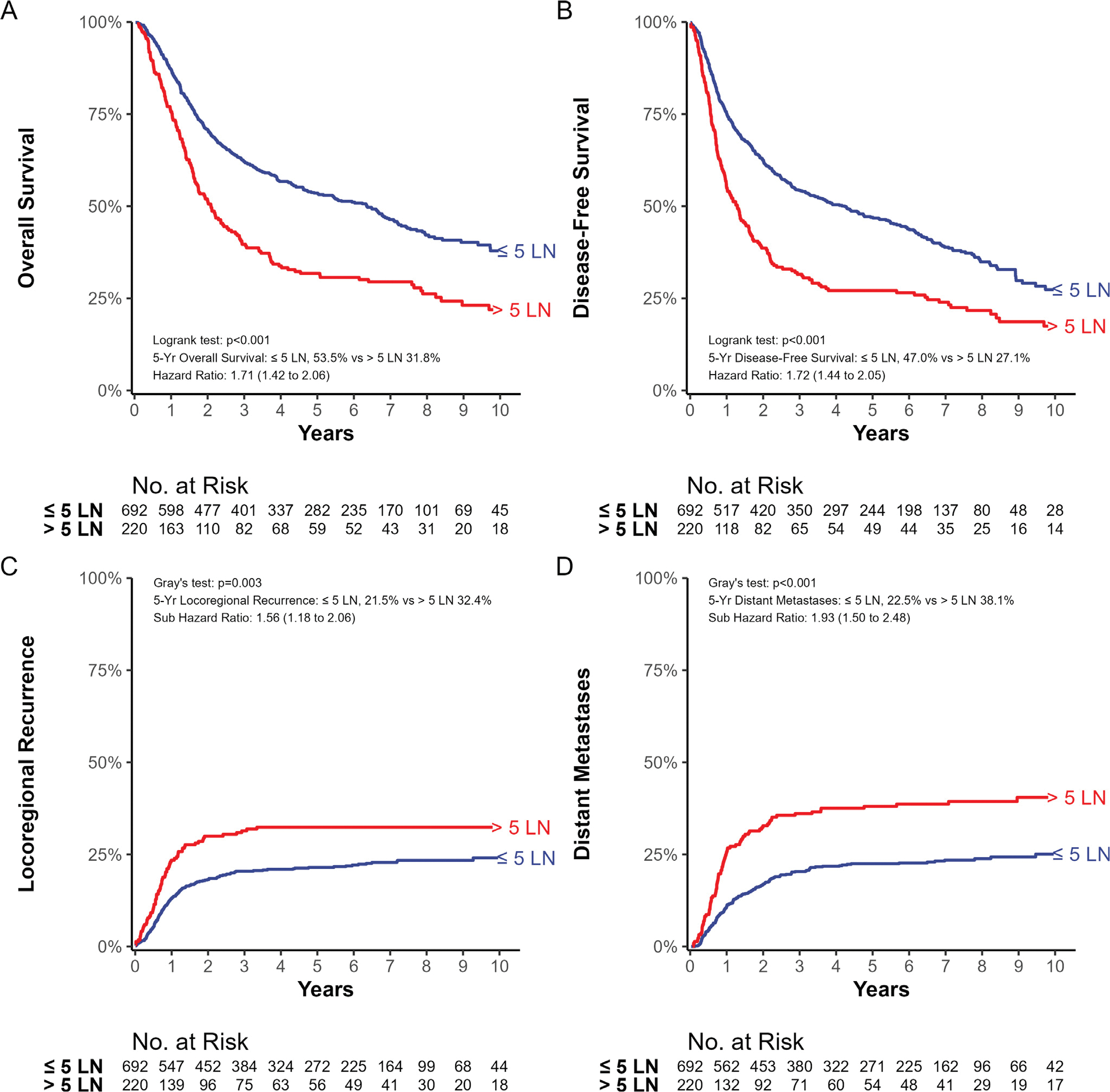
(A) Kaplan-Meier estimate of overall survival. (B) Kaplan-Meier estimate of disease-free survival. (C) Cumulative incidence of locoregional recurrence. (D) Cumulative incidence of distant metastases. All outcomes are stratified by the change point of ≤5 versus >5 lymph nodes.
Discussion
Using data from 3 prospective, randomized controlled clinical trials, we confirmed the importance of the number of +LNs on outcomes in an international cohort of patients with high-risk HNSCC uniformly treated with surgery, neck dissection, and postoperative radiation. Although this has been shown in prior National Cancer Database (NCDB)/Surveillance, Epidemiology, and End Results (SEER) analyses, this is the first study using a prospective collection of data and multi-institutional centers to validate these findings. The association of +LN on outcomes was strongest up to 5 +LNs, with each metastatic LN being associated with an independent additional 19% increased risk of death. Each subsequent +LN beyond 5 continued to be associated with an increased risk of death, but at a more gradual rate of 2% per +LN. This pattern was seen both in patients who received postoperative radiation therapy alone and in those who received radiation therapy and systemic therapy. Notably, low-risk patients not needing postoperative radiation were excluded from these studies. Because of this, there is underrepresentation of low-nodal burden patients in our analysis of these trials, and our results may not apply to low-risk situations.
Our results from multiple prospective trials validate previous retrospective studies in HNSCC that have consistently demonstrated an association between number of +LNs and survival outcomes. Higher +LN burden has been associated with worse survival in these studies in various head and neck cancers,21 including oral cavity,5,22 larynx and hypopharynx,4 salivary gland cancers,6 and oropharyngeal cancer.8,23 Moreover, all of these studies observed similar mortality risk curves as a function of +LN count as this study, with each +LN conveying a 19% to 34% increase in mortality up to a change point of 4 to 5 +LN, followed subsequently by a more modest increase in mortality for each +LN beyond this in all studies. The notable consistency of these findings across various data sets, treatment paradigms, and head and neck cancer subsites, suggests these findings may represent a fundamental relationship between +LN count and outcomes in head and neck cancer, although the underlying biologic mechanisms remain unclear. Nevertheless, the number of +LNs should be considered for a key component of future staging systems. AJCC 8E pathologic nodal staging does use +LN count as the backbone for p16-positive oropharyngeal cancer, which may represent a model for other pathologic nodal staging systems for other head and neck cancer subsites.
Our results show that outcomes are extremely poor for patients with high quantitative metastatic nodal burden. Patients who had more than 5 +LNs had a 5-year DFS of 27%. Our findings demonstrate both LRR and DM play a role in these outcomes, although their relative contributions vary with number of +LNs. For patients with lower nodal burden (≤5 +LNs), increased risk of both LRR and DM play major roles, with LRR risk (28% per +LN) increasing more sharply with each additional involved node than DM risk (10% per +LN). By contrast, for patients with higher nodal burden (>5 +LNs), only DM risk continued to increase without plateau for each additional +LN (5% per +LN), paralleling overall mortality, whereas LRR risk was essentially constant. A higher number of +LNs may be a surrogate for overall volume of cancer, or it may be possible that a tumor capable of seeding 5 or more nodal metastases has inherently higher biologic metastatic potential than one that seeds fewer nodes. It is unclear why LRR risk plateaus beyond 5 +LNs whereas DM does not. One possible factor could be that adjuvant radiation, which was delivered to the locoregional sites of disease in all patients in this study, flattened the risk curve for LRR. There is a natural reduction in the slope of the risk curves for OS, DFS, and DM beyond 5 +LNs (2%–5% per LN), and it is possible that radiation further alters this slope from what would naturally be seen for LRR without adjuvant treatment to a flat line. In any case, given the poor outcomes and high absolute risk of recurrence in the high nodal burden group, in future studies, +LN number should be explored as a factor that may predict greater absolute benefit from concomitant chemotherapy or other treatment intensifications such as immunotherapy and DNA damage repair pathway inhibitors. Notably, novel therapies will need reduce DM in order to strongly influence outcomes in those with high metastatic LN counts.
This study has several limitations. It was an unplanned secondary analysis with data derived from 3 randomized trials enriched for patients at high risk of recurrence, and patients with low-risk disease are not represented. Thus, our data may actually underestimate the effect of increasing nodal burden, given that the patients with 0 and 1 +LN in these studies, the reference cohort for comparison, all had other high-risk features and had higher risk of adverse outcomes than the vast majority of 0 to 1 +LN patients with head and neck cancer. By contrast, all patients with 2 or more +LNs were eligible for RTOG 9501 and RTOG 0234, and thus this cohort much more accurately represents the entire spectrum of disease for these patients. Another limitation is that these databases have limited information regarding laterality of LNs, nodal level, or size of LNs. Some studies suggest these factors have limited independent prognostic effect when accounting for number of +LNs,4,5 but this could not be validated with the data available for our study. Future studies with detailed anatomic nodal information are needed to better understand how nodal factors interact. Information regarding lymphovascular invasion and perineural invasion was also not available for 2 of the 3 trials. Similarly, we were not able to report on local and regional recurrence separately because only 1 of the 3 trial databases reported these separately. Thus, although we think it is probable that most, if not all, of the increase in locoregional recurrence with increasing +LN number is due to regional, rather than local, recurrence risk, this remains speculative. In addition, there were limited HPV data available for patients with oropharyngeal cancer, and this could act as an unmeasured confounder in our analysis given the strong prognostic importance of HPV. Moreover, it has been hypothesized that the number of +LNs has less prognostic effect in HPV-positive versus HPV-negative oropharyngeal cancer, and the AJCC 8E clinical and pathologic nodal staging systems for these diseases use this factor very differently. However, a recently published study refuted this hypothesis, demonstrating that HPV-positive and HPV-negative tumors have remarkably similar relationships between metastatic LN count and outcome.8 Additionally, we note that only about one-third of patients in this study had oropharyngeal cancers, and the prevalence of HPV-related cancers was relatively low at the time the majority of these patients were enrolled. We conducted a sensitivity analysis excluding oropharyngeal cancer patients, which demonstrated very similar to the results in the overall cohort, including the multivariable analysis, cut point, and HRs. Taken together, although it is uncertain how applicable our data are to HPV-positive patients, we also believe that HPV is unlikely to represent a major confounding factor undermining our results. In addition, our results should not be extrapolated to clinical staging for patients treated nonsurgically, given the potential confounding of false negative LNs on imaging and greater difficulty identifying extranodal extension with imaging. Lastly, our study could be considered a “mini–meta-analysis,” and biases of this study design should be considered. One such potential source of bias is if there is a lack of transparency regarding how researchers decide when to stop conducting further replications.24 As an example, researchers could theoretically combine studies up until the point that they achieved their desired outcome and then choose not to add data from additional studies. This is not relevant to our project, however, as our decision to include these 3 trials was decided upfront based on data availability without any analysis of the data. We sought to include only randomized controlled trials for patients with HNSCC undergoing surgery included in international cooperative groups with robust data sharing policies given the importance of individual patient level data on cancer-specific outcomes for this type of analysis. We acknowledge that there may be other relevant studies performed in other settings that were not included in our analysis.
Conclusions
Using data sets from cooperative group prospective randomized controlled trials enrolling an international cohort of patients with high-risk HNSCC, we validated increasing number of +LNs as an independent factor associated with increasing overall mortality. These data strongly demonstrate the influential role of +LN number and support its use to refine future staging for HNSCC and provide better risk stratification. Additionally, we found both LRR and DM contribute to increasing mortality at modest +LN counts, but DM is the predominant factor that increases without plateau for very high nodal burden patients. These unique patterns of recurrence may help us better understand the mechanisms of metastasis and their influence on current outcomes and suggest risk-directed strategies for future clinical trials.
Supplementary Material
Acknowledgments—
The authors thank the European Organisation for Research and Treatment of Cancer (EORTC) for permission to use the data from EORTC 22931, and NRG Oncology for permission to use data from Radiation Therapy Oncology Group (RTOG) 9501 and RTOG 0234 for this research. The contents of this publication and the methods used are solely the responsibility of the authors and do not necessarily represent the official views of the EORTC or RTOG.
Footnotes
Disclosures: Q.T.L. has been a consultant for Nanobiotix (honoraria) and Merck (travel expenses). The spouse of Z.S.Z. does legal work for Johnson & Johnson and Allergan through her law firm. All other authors have no disclosures to declare.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijrobp.2022.03.033.
Data sharing statement:
Clinical trial data was obtained through approved data sharing agreements, and we received access to raw clinical trial data directly from the European Organisation for Research and Treatment of Cancer and NRG Oncology. Information about the data sharing process is available at https://www.nrgoncology.org/Resources/Ancillary-Projects-Data-Sharing-Application and https://www.eortc.org/data-sharing.
References
- 1.Cerezo L, Millan I, Torre A, et al. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer. A multivariate study of 492 cases. Cancer 1992;69:1224–1234. [DOI] [PubMed] [Google Scholar]
- 2.Amin MB. American Joint Committee on Cancer. American Cancer Society: AJCC Cancer Staging Manual. 8th ed. Chicago, IL: Springer; 2017. [Google Scholar]
- 3.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005;27:843–850. [DOI] [PubMed] [Google Scholar]
- 4.Ho AS, Kim S, Tighiouart M, et al. Association of quantitative metastatic lymph node burden with survival in hypopharyngeal and laryngeal cancer. JAMA Oncol 2018;4:985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho AS, Kim S, Tighiouart M, et al. Metastatic lymph node burden and survival in oral cavity cancer. J Clin Oncol 2017;35:3601–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aro K, Ho AS, Luu M, et al. Development of a novel salivary gland cancer lymph node staging system. Cancer 2018;124:3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts TJ, Colevas AD, Hara W, et al. Number of positive nodes is superior to the lymph node ratio and American Joint Committee on Cancer N staging for the prognosis of surgically treated head and neck squamous cell carcinomas. Cancer 2016;122:1388–1397. [DOI] [PubMed] [Google Scholar]
- 8.Ho AS, Luu M, Kim S, et al. Nodal staging convergence for HPV− and HPV+ oropharyngeal carcinoma. Cancer 2021;127:1590–1597. [DOI] [PubMed] [Google Scholar]
- 9.Harari PM, Harris J, Kies MS, et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J Clin Oncol 2014;32:2486–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper JS, Zhang Q, Pajak TF, et al. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys 2012;84:1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004;350:1937–1944. [DOI] [PubMed] [Google Scholar]
- 12.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 14.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Annal Stat 1988;16:1141–1154. [Google Scholar]
- 15.Buuren SV. Flexible Imputation of Missing Data (Chapman and Hall/CRC Interdisciplinary Statistics). 2nd ed. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2018. 1 (online resource). [Google Scholar]
- 16.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Location: Springer International Publishing; 2015. [Google Scholar]
- 17.Little RJA, Rubin DB. Statistical Analysis With Missing Data. Location: Wiley; 1987. [Google Scholar]
- 18.Muggeo V Segmented: An R package to fit regression models with broken-line relationships. R News; 2008;8:20–25. [Google Scholar]
- 19.Muggeo VMR. Estimating regression models with unknown breakpoints. Stat Med 2003;22:3055–3071. [DOI] [PubMed] [Google Scholar]
- 20.Agresti A Categorical Data Analysis. 3rd ed. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 21.Reinisch S, Kruse A, Bredell M, et al. Is lymph-node ratio a superior predictor than lymph node status for recurrence-free and overall survival in patients with head and neck squamous cell carcinoma? Ann Surg Oncol 2014;21:1912–1918. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimi A, Gil Z, Amit M, et al. The prognosis of N2b and N2c lymph node disease in oral squamous cell carcinoma is determined by the number of metastatic lymph nodes rather than laterality: Evidence to support a revision of the American Joint Committee on Cancer staging system. Cancer 2014;120:1968–1974. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Kallogjeri D, Gay H, et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol 2015;51:514–520. [DOI] [PubMed] [Google Scholar]
- 24.Ueno T, Fastrich GM, Murayama K. Meta-analysis to integrate effect sizes within an article: Possible misuse and Type I error inflation. J Exp Psychol Gen 2016;145:643–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical trial data was obtained through approved data sharing agreements, and we received access to raw clinical trial data directly from the European Organisation for Research and Treatment of Cancer and NRG Oncology. Information about the data sharing process is available at https://www.nrgoncology.org/Resources/Ancillary-Projects-Data-Sharing-Application and https://www.eortc.org/data-sharing.


