Abstract
Background
The randomized phase 3 CeTeG/NOA-09 trial assessed whether CCNU plus temozolomide was superior to temozolomide alone in newly diagnosed MGMT promoter methylated glioblastoma patients. Survival was significantly improved from 31.4 months (temozolomide) to 48.1 months (CCNU plus temozolomide). In view of this encouraging data, we assessed safety and efficacy of this regimen under real-life conditions.
Methods
We retrospectively collected clinical and radiographic data from adult newly diagnosed MGMT promoter methylated IDH wildtype glioblastoma patients from five neuro-oncology centers in Germany. For inclusion in our analysis, treatment with CCNU and temozolomide had to be performed for at least six weeks (one course).
Results
Seventy patients were included. Median progression-free survival was 14.4 months and median overall survival 33.8 months. Patients with TTFields treatment for at least 8 weeks and CCNU plus temozolomide (n = 22, 31%) had a prolonged progression-free survival compared to those with TTFields treatment for less than eight weeks (n = 48, 69%) (21.5 versus 11.2 months; P = .0105). In a multivariable Cox regression analysis, TTFields treatment for eight weeks or longer together with CCNU plus temozolomide and a Karnofsky performance score ≥ 90% were independent prognostic factors for progression-free and overall survival. Pseudoprogression occurred in n = 16 (33%) of investigated n = 49 (70%) patients. In n = 31 (44%) patients high-grade hematotoxicity was observed.
Conclusions
The results from this multicentric trial indicate that—under real-life conditions—toxicity and survival estimates are comparable to the CeTeG/NOA-09 trial. TTFields therapy for at least eight weeks in combination with this regimen was independently associated with prolonged survival.
Keywords: CCNU and temozolomide, CeTeG, glioblastoma, NOA-09, TTFields
Key Points.
Treatment according to the CeTeG protocol was feasible under real-life conditions.
TTFields therapy for at least eight weeks was associated with improved survival.
Karnofsky performance score ≥ 90% was linked to improved survival.
Importance of the Study.
This study builds on the findings of two phase 3 trials in newly diagnosed glioblastoma. The CeTeG/NOA-09 trial suggested that the combination of CCNU plus temozolomide could have a significant impact on the survival of newly diagnosed MGMT promoter methylated glioblastoma patients. The EF-14 trial reported a significant improvement in overall survival through the addition of TTFields to maintenance temozolomide treatment of newly diagnosed glioblastoma patients. This study provides real-life evidence for the toxicity and efficacy of CCNU plus temozolomide and suggests that the triple combination of TTFields with temozolomide plus CCNU could offer an additional survival benefit in newly diagnosed MGMT promoter methylated glioblastoma patients.
Glioblastoma treatment remains challenging despite steady improvement in the understanding of glioma biology. Median overall survival (mOS) of molecularly unselected newly diagnosed glioblastoma patients in clinical trials is limited to a time span of less than 2 years.1 The CeTeG/NOA-09 trial, along with the EF-14 trial, was one of the rare positive randomized phase 3 chemotherapy trials in the past 17 years to evaluate a novel tumor therapy in patients ≤ 70 years of age with newly diagnosed glioblastoma. In the CeTeG/NOA-09 trial it was demonstrated that mOS could be extended to 4 years compared to standard temozolomide by combining temozolomide with CCNU in newly diagnosed glioblastoma patients harboring the prognostically favorable methylation of the O(6)-methylguanine-DNA methyltransferase (MGMT) promoter.2 The number of adverse events of grade 3 or higher (according to the Common Terminology Criteria for Adverse Events—CTCAE—Version 5—https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm) in the CCNU plus temozolomide group was only slightly higher compared to standard temozolomide, indicating reasonable feasibility. The CeTeG/NOA-09 trial was also among the first biomarker-driven glioblastoma trials by showing that combined CCNU plus temozolomide treatment was superior to standard temozolomide in MGMT promoter methylated newly diagnosed glioblastoma only, indicating a predictive property of MGMT promoter methylation status.3 Nevertheless, the trial has been challenged for the small sample size and for including patients from a single country only. In addition, there was no significant difference in terms of progression-free survival (PFS) between the temozolomide-CCNU and standard temozolomide arm and few patients with isocitrate dehydrogenase (IDH) mutant glioblastoma were considered eligible—as per the valid World Health Organization (WHO) Classification of Tumors of the Central Nervous System from 2016.4 TTFields were not used in the CeTeG/NOA-09 study. However, a previously published analysis with limited sample size indicates that the triple therapy including temozolomide, CCNU and TTFields was feasible in newly diagnosed MGMT promoter methylated glioblastoma.5
In this multicenter analysis conducted at five neuro-oncology centers in Germany, we aimed to investigate real-life data on tolerability and efficacy of treatment with CCNU and temozolomide in a homogeneous cohort of newly diagnosed MGMT promoter methylated IDH wildtype glioblastoma patients in tandem with the update of the World Health Organization (WHO) Classification of Tumors of the Central Nervous System of 2021.6
Materials and Methods
Study Design
We gathered clinical and radiographic data from patients newly diagnosed with glioblastoma between May 2012 and August 2020 at five neuro-oncology centers in Germany (University Hospital Essen, University Hospital Leipzig, University Hospital Münster, University Hospital Regensburg, University Hospital Würzburg). The following selection criteria had to be met:
(1) Adult patients, newly diagnosed with glioblastoma.
(2) Patients with any Karnofsky performance score (KPS) and any age equal to or greater than 18 were included.
(3) Methylation of MGMT promoter and IDH wildtype status. MGMT promoter methylation status was determined locally through quantitative methylation-specific polymerase chain reaction (PCR) or deoxyribonucleic acid (DNA) pyrosequencing.
(4) First-line treatment with the combination of temozolomide and CCNU for at least 6 weeks (one course). Concomitant and adjuvant CCNU plus temozolomide were allowed as well as combined treatment with TTFields.
All data were obtained within the framework of routine clinical assessments. Radiographic data were shared as Digital Imaging and Communications in Medicine (DICOM) files. All clinical and radiographic data were collected in an anonymized format. The available radiographic data were independently reviewed to determine the extent of initial resection and for validation of tumor recurrence or putative pseudoprogression by an experienced board-certified neuro-radiologist (E.H.). Treatment response was evaluated as per the updated response assessment criteria for high-grade gliomas7 using the archived magnetic resonance imaging (MRI) scans performed at 8–12 weeks intervals. Toxicity was determined corresponding to CTCAE (Version 5).
This study was approved by the local ethics committee of the University Duisburg-Essen (application number: 20-9431-BO).
Diagnosis of Tumor Progression and Pseudoprogression
Putative pseudoprogression was detected either by subsequent MRI and/or positron emission tomography (PET) imaging according to the updated response assessment criteria for high-grade gliomas.7 The interpretation of PET findings was conducted pursuant to established guidelines.8–10 In brief, the diagnosis of tumor progression was made when progressive contrast-enhancing lesions according to response assessment in neuro-oncology (RANO) criteria were noted in MRI under treatment and when further progression of contrast-enhancement was noted in a follow-up MRI at least 4 weeks later. By contrast, the diagnosis of pseudoprogression was applied when the follow-up MRI showed stabilization or regression of the contrast-enhancing lesions. In a few cases (n = 9, 18%) repeat brain surgery was performed so that the appropriate histopathologic findings could be used to distinguish between true tumor recurrence and pseudoprogression.
Statistics
Canonical clinical features were presented descriptively in a tabular format. To facilitate comparison, we selected the cutoff levels for age groups, KPS, and extent of resection reported in the EF-14 trial.11 Moreover, we determined the median number of applied CeTeG courses to allow comparison to the CeTeG/NOA-09 trial.2 For comparison of feature distribution in the established subgroups we used the Mann–Whitney U test (continuous variables) and the Fisher’s exact test (categorical variables). To assess the survival function from lifetime data we used the Kaplan–Meier estimator. If progression or death respectively had not occurred at the time of data analysis (December 1, 2021), the corresponding patients were considered censored for further survival analysis. Multivariable Cox regression models were used to determine independently significant predictors for PFS and overall survival (OS). For data visualization, GraphPad Prism version 9 (GraphPad Software Inc., San Diego, USA) and Affinity Designer version 1.9.0 (Serif Europe, West Bridgford, UK) were used.
Results
Patients’ Characteristics
A total of n = 70 newly diagnosed MGMT promoter methylated IDH wildtype glioblastoma patients treated with the combination of CCNU plus temozolomide at five neuro-oncology centers in Germany were included in this retrospective trial. Most patients (n = 54, 77 %) were younger than 65 years of age and had a KPS of at least 90% (n = 45, 64%) at the time of diagnosis. There was an imbalance in the sex distribution towards slightly more male patients (n = 39, 56% men versus n = 31, 44% women). About 39% of patients (n = 27) underwent complete resection at initial diagnosis (before treatment with the combination of CCNU/temozolomide). The median number of CeTeG courses was five and identical to the number observed in the CeTeG trial. Thirty-nine percent of patients (n = 27) started the chemotherapy with CCNU and temozolomide after completion of radiotherapy (adjuvant group) whereas 61% (n = 43) started, adhering to the CeTeG trial protocol, with radiotherapy onset (concomitant group). Information on chemotherapy dose escalation was available in a fraction of patients (n = 32, 46%), among whom dose escalation was performed according to the CeTeG trial protocol in 3% (n = 1) of the patients. Additionally, n = 29 patients (41%) were treated with adjuvant TTFields in keeping with the EF-14 trial. The follow-up interval ranged from four to 102 months with a median follow-up time of 25 months. Detailed patients’ characteristics of the full multicentric cohort are listed in Table 1.
Table 1.
Patient’s Characteristics of the Full Multicentric Cohort
| Full Cohort (n = 70) | |
|---|---|
| Age at diagnosis in years, median (range) | 55 (21–76) |
| Age at diagnosis, n | |
| ≥ 65 years | 16 (23%) |
| < 65 years | 54 (77%) |
| Karnofsky performance score in %, median (range) | 90 (60–100) |
| Karnofsky performance score, n | |
| ≥ 90% | 45 (64%) |
| < 90% | 25 (36%) |
| Sex, n | |
| Men | 39 (56%) |
| Women | 31 (44%) |
| Extent of resection, n | |
| Biopsy | 8 (11%) |
| Partial resection | 35 (50%) |
| Complete resection | 27 (39%) |
| No. of CeTeG courses, n | |
| ≥ 5 courses | 43 (61%) |
| < 5 courses | 27 (39%) |
| CeTeG onset, n | |
| Adjuvant | 27 (39%) |
| Concomitant | 43 (61%) |
| Treatment with CeTeG plus TTFields, n | 29 (41%) |
| Follow-up in months, median (range) | 25 (4–102) |
No., number; TTFields, tumor treating fields.
Toxicity
In this analysis of combined CCNU and temozolomide treatment no treatment-related death occurred. We observed adverse events of CTCAE grade 3 or higher in n = 47 (67%) patients. Forty-four percent (n = 31) of the 70 patients experienced hematotoxicities of CTCAE grade 3 or higher, 23% (n = 16) had nonhematological adverse events of CTCAE grade 3 or higher. From the patients with CTCAE grade 3 or higher hematotoxicities, n = 11 patients (35%) had isolated lymphopenia. We observed CTCAE grade 4 hematological adverse events in n = 4 (5%) of the patients (n = 2 with pancytopenia, n = 2 with thrombocytopenia or neutropenia). None of the patients in our multicentric cohort developed grade 4 nonhematological toxicities. Table 2 synoptically displays the toxicity data of all patients in detail.
Table 2.
Adverse Events According to the Common Terminology Criteria for Adverse Events (CTCAE—Version 5)
| Full cohort (n = 70) | |
|---|---|
| Treatment-related deaths, n | 0 |
| Patients with adverse events CTCAE ≥ grade 3, n | 47 (67%) |
| Patients with hematotoxicity CTCAE ≥ grade 3, n | 31 (44%) |
| Neutropenia | 8 (26%) |
| Lymphopenia | 11 (35%) |
| Pancytopenia | 7 (23%) |
| Thrombopenia | 5 (16%) |
| Patients with nonhematological adverse events CTCAE ≥ grade 3, n | 16 (23%) |
| Dysgeusia | 1 (6%) |
| Epidural empyema | 1 (6%) |
| GGT elevation | 3 (19%) |
| Hypernatremia | 1 (6%) |
| Lipase elevation | 1 (6%) |
| Nausea | 1 (6%) |
| Seizure | 2 (13%) |
| Transaminase elevation | 6 (38%) |
GGT, gamma-glutamyltransferase.
Overall Treatment Efficacy and Subgroup Analysis
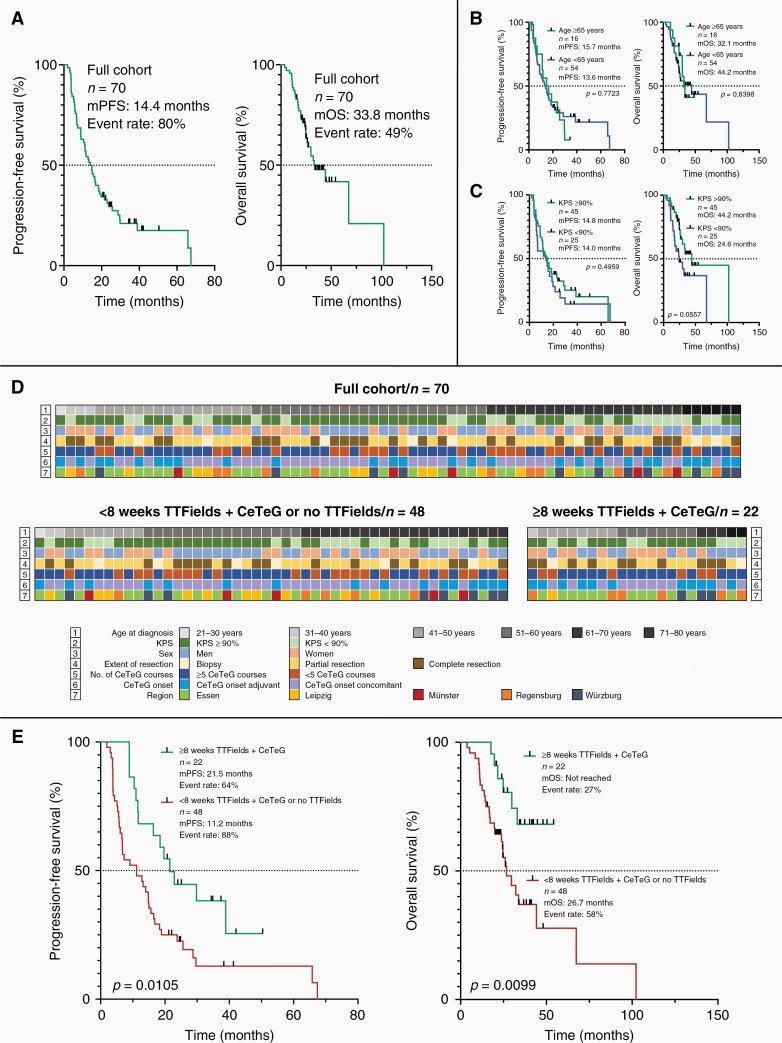
In the overall cohort, median progression-free survival (mPFS) was 14.4 months at an event rate of 80% and mOS was 33.8 months at an event rate of 49% (Figure 1a). The PFS rate at 6 months was 77%, the OS rate at 12 months was 89%. Patients of at least 65 years of age did not significantly perform worse than those younger in terms of OS and PFS (mOS: 32.1 months versus 44.2 months, P = .8398; mPFS: 15.7 months versus 13.6 months, P = .7723; Figure 1b). Regarding KPS, OS appeared slightly longer, however statistically not significant, in patients with a KPS ≥ 90% as opposed to those with a KPS < 90% (mOS: 44.2 months versus 24.6 months, P = .0557; mPFS: 14.8 months versus 14.0 months, P = .4959; Figure 1c). PFS and OS were not markedly different in patients who received concomitant CCNU plus temozolomide (concomitant group, n = 43) as opposed to patients who received CCNU plus temozolomide post radiotherapy (adjuvant group, n = 27) (mPFS: concomitant group 14.8 months versus adjuvant group 13.2 months, P = .7393; mOS: concomitant group 67.4 months versus adjuvant group 32.1 months, P = .5353) (Supplementary Figure S1a + b).
Figure 1.
Kaplan–Meier curves and individual patient information. For the full multicentric cohort mPFS was 14.4 months at an event rate of 80% and mOS was 33.8 months at an event rate of 49% (a). The subgroup of patients ≥ 65 years of age did not perform significantly worse in respect to OS and PFS than the patients < 65 years of age (b). For KPS, overall survival appeared slightly longer in patients with a KPS ≥ 90% compared to those with a KPS < 70% (c). In (d) individual patient’s characteristics for all patients as well as comparatively for the subgroup of patients with < 8 weeks TTFields + CeTeG or no TTFields treatment and for the subgroup of patients with ≥ 8 weeks TTFields + CeTeG treatment are illustrated. Treatment in the latter subgroup resulted in a longer median progression-free and overall survival than in the subgroup of patients with < 8 weeks TTFields + CeTeG or no TTFields treatment (e). KPS, Karnofsky performance score; mOS, median overall survival; mPFS, median progression-free survival; No., number; OS, overall survival; PFS, progression-free survival; TTFields, tumor treating fields.
Combination of CeTeG and TTFields
In patients who received CeTeG and TTFields therapy for at least 8 weeks, mPFS was extended compared to those patients who underwent CeTeG and TTFields therapy for less than 8 weeks or had no TTFields treatment (21.5 months versus 11.2 months, HR: 2.118, 95% CI: 1.25–3.60, P = .0105). A similar observation was found for OS, where mOS in patients who received CeTeG and TTFields therapy for at least eight weeks was not reached in comparison to 26.7 months (HR: 2.551, 95% CI: 1.25–5.20, P = .0099) in the patient subgroup of patients who received CeTeG and TTFields therapy for less than 8 weeks or without TTFields treatment (Figure 1e).
To estimate the robustness of the survival benefit in CeTeG and TTFields treated patients, we investigated the clinical profiles with respect to TTFields treatment. In total, a subgroup of n = 48 patients had less than 8 weeks combined TTFields and CeTeG treatment or no TTFields treatment, whereas n = 22 patients received combined TTFields and CeTeG treatment for at least 8 weeks. Between the two groups, the distribution of clinical features (age at diagnosis, KPS, sex, extent of resection, number of applied CeTeG courses, and onset of CeTeG therapy) was statistically balanced. The respective patient characteristics are shown in Table 3. In Figure 1d individual clinical features are comparatively illustrated for every individual patient.
Table 3.
Patients’ Characteristics of the Subgroup with < 8 weeks TTFields + CeTeG or no TTFields Treatment and of the Subgroup with ≥ 8 weeks TTFields + CeTeG Treatment
| < 8 weeks TTFields + CeTeG or no TTFields (n = 48) | ≥ 8 weeks TTFields + CeTeG (n = 22) | P-value | |
|---|---|---|---|
| Age at diagnosis, n | .7601 | ||
| ≥ 65 years | 12 (25%) | 4 (18%) | |
| < 65 years | 36 (75%) | 18 (82%) | |
| Karnofsky performance score, n | .7898 | ||
| ≥ 90% | 30 (63%) | 15 (68%) | |
| < 90% | 18 (37%) | 7 (32%) | |
| Sex, n | .6072 | ||
| Men | 28 (58%) | 11 (50%) | |
| Women | 20 (42%) | 11 (50%) | |
| Extent of resection, n | .7117 | ||
| Biopsy | 6 (13%) | 2 (10%) | |
| Partial resection | 25 (52%) | 10 (45%) | |
| Complete resection | 17 (35%) | 10 (45%) | |
| No. of CeTeG courses, n | .1911 | ||
| ≥ 5 courses | 27 (56%) | 16 (73%) | |
| < 5 courses | 21 (44%) | 6 (27%) | |
| CeTeG onset, n | .1987 | ||
| Adjuvant | 16(33%) | 11 (50%) | |
| Concomitant | 32 (67%) | 11 (50%) | |
| Follow-up in months, median (range) | 23 (4–102) | 34 (17–54) |
No., number; TTFields, tumor treating fields.
Multivariable Analysis
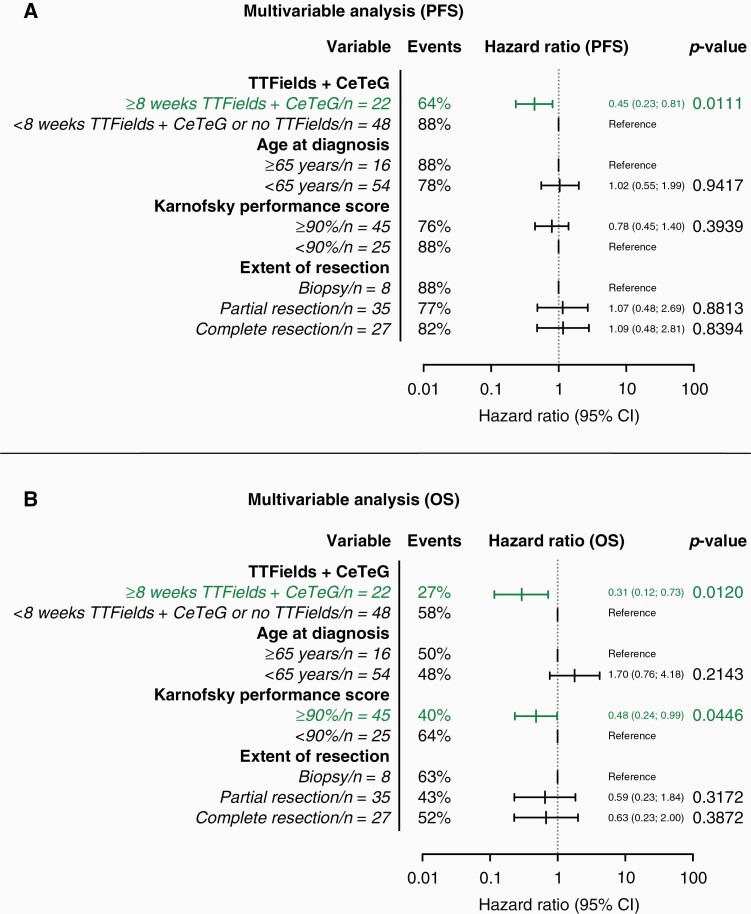
In a multivariable Cox regression analysis featuring the canonical—prognostically relevant—clinical factors (age at diagnosis, KPS, extent of resection), the combination of CeTeG and TTFields for at least 8 weeks (HR: 0.45, 95% CI: 0.23–0.81, P = .0111) emerged as the only statistically significant and independent prognostic marker regarding PFS (Figure 2a). Concerning OS, besides combination of CeTeG + TTFields for at least 8 weeks (HR: 0.31, 95% CI: 0.12–0.73; P = .0120) also a KPS ≥ 90% emerged as a statistically significant prognostic marker (HR: 0.48, 95% CI: 0.24–0.99; P = 0.0446) (Figure 2b).
Figure 2.
Multivariable analysis for progression-free and overall survival. Combination of CeTeG + TTFields for at least 8 weeks emerged as the only statistically significant prognostic marker regarding PFS (a). Concerning OS, the combination of CeTeG + TTFields for at least 8 weeks and a KPS of at least 90% emerged as statistically significant prognostic markers. CI, confidence interval; KPS, Karnofsky performance score; No., number; PFS, progression-free survival; OS, overall survival; TTFields, tumor treating fields.
MRI-Based Evaluation of Pseudoprogression
In n = 49 patients (70%) MRI data before and after initial brain surgery, after completion of radiotherapy, and at the timepoint of first putative tumor progression/pseudoprogression were available. In 16/49 patients (33%), we detected pseudoprogression by independent radiological assessment. Fifteen of these 16 patients (94%) had pseudoprogression within the first three months after completion of radiotherapy (early pseudoprogression). Ten out of 16 patients (63%) with pseudoprogression in our cohort started combined temozolomide/CCNU treatment after completion of radiotherapy (adjuvant) and six out of 16 patients (37%) with pseudoprogression in our cohort started combined temozolomide/CCNU treatment concomitant to radiotherapy (concomitant). Validation of tumor progression/pseudoprogression was based mostly on follow-up MRI (in 20/49 cases, 41%). In nine patients (18%), recurrent brain surgery was performed following MRI showing putative tumor progression/pseudoprogression. Four patients (8%) underwent monomodal follow-up PET investigation and n = 16 patients (33%) underwent multimodal PET-MRI investigation for progression/pseudoprogression validation. In all n = 49 patients, tumor treatment was not altered between MRI obtained at the time of suspected tumor progression/pseudoprogression and follow-up MRI, PET, or recurrent brain surgery. The corresponding data is presented in Table 4.
Table 4.
Patient’s Characteristics of the Patient Cohort with Available Radiographic Information
| MRI Cohort (n = 49) | |
|---|---|
| Age at diagnosis, n | |
| ≥ 65 years | 12 (24%) |
| < 65 years | 37 (76%) |
| Karnofsky performance score, n | |
| ≥ 90% | 27 (55%) |
| < 90% | 22 (45%) |
| Sex, n | |
| Men | 28 (57%) |
| Women | 21 (43%) |
| Extent of resection, n | |
| Biopsy | 7 (14%) |
| Partial resection | 23 (47%) |
| Complete resection | 19 (39%) |
| No. of CeTeG courses, n | |
| ≥ 5 courses | 28 (57%) |
| < 5 courses | 21 (43%) |
| CeTeG onset, n | |
| Adjuvant | 20 (41%) |
| Concomitant | 28 (59%) |
| Pseudoprogression, n | 16 (33%) |
| < 12 weeks after radiotherapy | 15 (94%) |
| ≥ 12 weeks after radiotherapy | 1 (6%) |
| Validation of progression/pseudoprogression, n | |
| Follow-up MRI | 20 (41%) |
| Recurrent brain surgery | 9 (18%) |
| Follow-up PET | 4 (8%) |
| Follow-up PET/MRI | 16 (33%) |
| Follow-up in months, median (range) | 25 (9–102) |
MRI, magnetic resonance imaging; No., number; PET, positron emission tomography.
In the cohort of patients who received TTFields treatment (n = 20), the pseudoprogression rate was 40% (n = 8/20). This percentage was higher (but statistically insignificant, P = .5363) compared to the pseudoprogression rate (28%, n = 8/29) of patients who did not receive TTFields treatment (n = 29). The pseudoprogression rates of the concomitant group versus the adjuvant group did not differ markedly (30%, n = 6/20 versus 34%, n = 10/29) (Supplementary Figure S1c).
Discussion
Our analysis provides evidence that the combination of CCNU and temozolomide is feasible and safe in the treatment of newly diagnosed MGMT promoter methylated IDH wildtype glioblastoma patients outside of study conditions in real-life routine patient care. Furthermore, in our real-life cohort high KPS (≥ 90%) and TTFields treatment (≥ 8 weeks) were prognostic factors associated with improved survival. Noteworthy, only 61% of patients started with the combination of CCNU/temozolomide concomitant to radiation therapy and were treated in exact agreement with the CeTeG regimen. Thirty-nine percent of the patients received temozolomide only in the concomitant phase followed by CCNU/temozolomide in the adjuvant phase.
In our multicentric patient cohort the combination of CCNU and temozolomide resulted in a mPFS of 14.4 months and a mOS of 33.8 months. These survival times exceed the survival times for newly diagnosed MGMT promoter methylated glioblastoma patients after standard treatment with temozolomide (mOS: 21.7 months) reported in the EORTC 26981/22981-NCIC CE3 trial and in the control arm of the CENTRIC trial (mOS: 26.3 months).11–13 However, the survival times were substantially shorter than those reported in the experimental CeTeG arm (mOS: 48.1 months) and rather comparable to the times reported in the control arm of the CeTeG trial (mOS: 31.4 months) and the control arm of the CheckMate 548 trial (mOS: 32.1).2,14 Possible explanations for this discrepancy in survival times of our cohort versus the experimental arm of the CeTeG trial could be that our cohort included patients older than 70 years of age (9%), patients with a KPS < 70% (3%), and exclusively included patients with confirmed IDH wildtype status (in the CeTeG trial 23% of patients had either mutant or unknown IDH status). In addition, it must be noted that only 61% of patients in our cohort have been treated according to the CeTeG regimen—with combination chemotherapy starting already during radiotherapy—and 39% received CCNU plus temozolomide only after radiotherapy completion. Reasons for the reduced fraction of patients who received CCNU plus temozolomide already in the concomitant phase are the initial treatment in a non-university center, the then still not established use of CCNU plus temozolomide in clinical routine, the decision of the treating physician—particularly in elderly patients with reduced KPS—to start conservatively with temozolomide, and the patients’ preference.
We have identified factors significantly and independently associated with improved OS. The first factor was KPS, showing that patients with a KPS ≥ 90% had improved OS as opposed to those with a KPS < 90%, indicating that under real-life conditions the combination of CCNU plus temozolomide might be particularly useful for patients with a very good clinical condition. In addition, our investigation revealed that patients with newly diagnosed MGMT promoter methylated IDH wildtype glioblastoma who received a triple therapy with CCNU, temozolomide and TTFields for at least 8 weeks performed much better in terms of OS—compared to those who either had no TTFields treatment or less than eight weeks—independently from known canonical factors. This finding sheds light on the putative additive effect TTFields may have on the CeTeG regimen and should be further evaluated in prospective controlled clinical trials. At least our analysis shows, along with a previous retrospective analysis that the triple therapy (CCNU plus temozolomide plus TTFields) appears feasible.5 Notably, no single patient suffered from high-grade skin toxicity after triple therapy with CCNU, temozolomide, and TTFields. In hindsight, data on putative reasons for early discontinuation or for not receiving TTFields at all, was available for only a fraction of study patients (32/70, 46%). In most of the patients of this group, discontinuing or refusing TTFields was their own preference (15/32, 47%). In the group of patients who received TTFields (17/32, 53%), those who discontinued TTFields before 8 weeks time interval (5/32, 16%) did so because of tumor progression.
The rate of high-grade adverse events (67%) in our study was comparable to that observed in the CeTeG/NOA-09 trial (59%). In detail, the rate of high-grade non-hematological adverse events was identical in our cohort and in the CeTeG/NOA-09 cohort (23%), whereas the rate of high-grade hematological adverse events was slightly higher in our cohort (44%) compared to the CeTeG/NOA-09 cohort (36%). In both cohorts, no treatment-related death occurred. In the cohort of patients above the age of 70 (n = 6), which did not meet the eligibility criteria for the CeTeG/NOA-09 trial, the rate of high-grade adverse events was 50% and quite comparable to the CeTeG/NOA-09 trial. In the patient cohort with a KPS < 70% (n = 2) both patients had a high-grade adverse event, inhibiting a reliable conclusion as to whether treatment in this subcohort according to the CeTeG/NOA-09 regimen is feasible. Taken together, our study shows the treatment of elderly patients (> 70 years of age) with the CeTeG/NOA-09 regimen is feasible.
In our study, we noted a high rate of pseudoprogression events (32.7%) in the patients where MRI evaluation was possible. This number is higher than the pseudoprogression event rate reported in the CeTeG/NOA-09 trial (10.6%). While in the CeTeG/NOA-09 trial 86% of pseudoprogression cases were confirmed histologically, only 10% of patients received histological confirmation in our cohort, possibly explaining the discrepancy. The high pseudoprogression rate observed in our cohort may be explained by the fact that not all MRI scans were available for analysis of pseudoprogression/progression. Only 49 of 70 patients (70%) had MRI data suitable for the evaluation of pseudoprogression. Moreover, the pseudoprogression rate of 33% that superficially creates the impression of being very high has to be put in perspective of published literature of pseudoprogression in temozolomide-treated high-grade glioma, where radiographic pseudoprogression rates within a 95% confidence interval of 33–40% have been reported.15 Nonetheless, it remains questionable whether the pseudoprogression data obtained from our real-life study can be compared to data obtained from a phase 3 trial (Herrlinger et al.2). It has to be noted, however, that the histological diagnosis of pseudoprogression may be challenging as tumor tissue and radionecrosis are regularly observed at the same time.16 Given the paucity of treatment options post CCNU or temozolomide, it is particularly important to have a high degree of suspicion for pseudoprogression to avoid discontinuing a putatively efficacious treatment. However, misclassification of pseudoprogression as progression is unlikely to affect the overall survival rates observed in our study.
Lastly, limitations inherent to this analysis have to be mentioned. The retrospective study design, the low number of samples, the lack of a balanced control cohort, and the different therapy onsets (adjuvant therapy onset versus concomitant therapy onset, although there was no statistically significant impact on survival) limit conclusions on efficacy.
In summary, our study provides real-life data for the first time regarding the treatment of CCNU plus temozolomide in newly diagnosed MGMT promotor methylated IDH wildtype glioblastoma showing that this treatment regimen is feasible and might be effective. The data from our multicentric analysis provide a very reasonable rationale for a follow-up study of a larger cohort of MGMT promoter methylated IDH wildtype glioblastoma patients with a triple treatment of CCNU, temozolomide and TTFields, arguing for the combination of the regimens used in the CeTeG/NOA-09 and EF-14 trials. A more resilient foundation of data can be expected after the analysis of the TTFields In Germany in Routine Clinical Care (TIGER; NCT03258021) trial and the Phase 3 Trial of Gleostine® (Lomustine)-Temozolomide Combination Therapy Versus Standard Temozolomide in Patients With Methylated MGMT Promoter Glioblastoma (NCT05095376).
Supplementary Material
Contributor Information
Lazaros Lazaridis, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK).
Elisabeth Bumes, Department of Neurology and Wilhelm Sander-NeuroOncology Unit, University Hospital Regensburg, Regensburg, Germany.
Dorothee Cäcilia Spille, Department of Neurosurgery, University Hospital Münster, Münster, Germany.
Tim Schulz, Department of Neurosurgery, University Hospital of Würzburg, Würzburg, Germany.
Sina Heider, Department of Radiotherapy and Radiation Oncology, University Hospital Leipzig, Leipzig, Germany.
Sarina Agkatsev, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Teresa Schmidt, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK).
Tobias Blau, DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); Institute of Neuropathology, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Christoph Oster, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK).
Jonas Feldheim, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany.
Walter Stummer, Department of Neurosurgery, University Hospital Münster, Münster, Germany.
Almuth Friederike Kessler, Department of Neurosurgery, University Hospital of Würzburg, Würzburg, Germany.
Clemens Seidel, Department of Radiotherapy and Radiation Oncology, University Hospital Leipzig, Leipzig, Germany.
Oliver Grauer, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Münster, Germany.
Peter Hau, Department of Neurology and Wilhelm Sander-NeuroOncology Unit, University Hospital Regensburg, Regensburg, Germany.
Ulrich Sure, German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; Department of Neurosurgery and Spine Surgery, University Medicine Essen, University Duisburg-Essen, Essen, Germany; Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Essen, Germany.
Kathy Keyvani, Institute of Neuropathology, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Ulrich Herrlinger, Division of Clinical Neurooncology, Department of Neurology and Center for Integrated Oncology, University Hospital Bonn, Bonn, Germany.
Christoph Kleinschnitz, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Martin Stuschke, Department of Radiotherapy, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Ken Herrmann, German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; Department of Nuclear Medicine, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Cornelius Deuschl, German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Medicine Essen, University Duisburg-Essen, Essen, Germany.
Stella Breuer, Institute of Neuroradiology, University Hospital Frankfurt, Frankfurt, Germany.
Elke Hattingen, Institute of Neuroradiology, University Hospital Frankfurt, Frankfurt, Germany.
Björn Scheffler, DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK); German Cancer Research Center (DKFZ), Heidelberg, Germany.
Sied Kebir, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK).
Martin Glas, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences (C-TNBS), Division of Clinical Neurooncology, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK), Partner Site University Medicine Essen, Essen, Germany; DKFZ-Division Translational Neurooncology at the West German Cancer Center (WTZ), DKTK Partner Site, University Medicine Essen, University Duisburg-Essen, Essen, Germany; German Cancer Consortium (DKTK).
Funding
None declared.
Conflict of interest statement. Lazaros Lazaridis received honoraria and travel support from Novocure. Teresa Schmidt received honoraria and travel support from Novocure. Almuth F. Kessler received travel support and research grants from Novocure. Clemens Seidel received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: AbbVie, Bristol-Myers Squibb, HRA Pharma, Medac, Novocure, Roche, Seagen. Peter Hau received honoraria from Roche, Novartis, Novocure, Bayer, Medac and travel support from Novocure and Medac. Ulrich Herrlinger received lecture and/or advisory board honoraria from Medac, Noxxon, AbbVie, Bayer, Janssen, and Karyopharm. Björn Scheffler is supported by the German Cancer Consortium (DKTK). Sied Kebir received honoraria and travel support from Novocure. Martin Glas reports honoraria from Roche, Novartis, UCB, AbbVie, Daiichi Sankyo, Novocure, Bayer, Janssen-Cilag, Medac, Merck, Kyowa Kirin, travel support from Novocure and Medac, research grant from Novocure. All remaining authors have declared no conflicts of interest.
Authorship statement
Writing and reviewing of the manuscript: L.L., E.B., D.C.S., TI.S., S.H., S.A., TE.S., T.B., C.O., J.F., W.S., A.F.K., C.S., O.G., P.H., U.S., K.K., U.H., C.K., M.S., K.H., C.D., S.B., E.H., B.S., S.K., M.G. Statistical analysis: L.L., S.K., M.G. Medical data assessment: L.L., E.B., D.C.S., TI.S., S.H., S.K. Conceptualization: L.L., S.K., M.G.
References
- 1. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herrlinger U, Tzaridis T, Mack F, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678–688. [DOI] [PubMed] [Google Scholar]
- 3. Herrlinger U, Rieger J, Koch D, et al. Phase II trial of lomustine plus temozolomide chemotherapy in addition to radiotherapy in newly diagnosed glioblastoma: UKT-03. J Clin Oncol. 2006;24(27):4412–4417. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 5. Lazaridis L, Schafer N, Teuber-Hanselmann S, et al. Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J Cancer Res Clin Oncol. 2020;146(3):787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [(18)F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kebir S, Fimmers R, Galldiks N, et al. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-l-tyrosine PET. Clin Cancer Res. 2016;22(9):2190–2196. [DOI] [PubMed] [Google Scholar]
- 10. Galldiks N, Langen KJ, Holy R, et al. Assessment of treatment response in patients with glioblastoma using O-(2-18F-fluoroethyl)-l-tyrosine PET in comparison to MRI. J Nucl Med. 2012;53(7):1048–1057. [DOI] [PubMed] [Google Scholar]
- 11. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 12. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 14. Weller M, Lim M, Idbaih A, et al. A randomized phase 3 study of nivolumab or placebo combined with radiotherapy plus temozolomide in patients with newly diagnosed glioblastoma with methylated MGMT promoter: CheckMate 548 [abstract]. Neuro Oncol. 2021;23:vi55–vi56. [Google Scholar]
- 15. Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A. Incidence of tumour progression and pseudoprogression in high-grade gliomas: a systematic review and meta-analysis. Clin Neuroradiol. 2018;28(3):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Fevre C, Lhermitte B, Ahle G, et al. Pseudoprogression versus true progression in glioblastoma patients: a multiapproach literature review: part 1—molecular, morphological and clinical features. Crit Rev Oncol Hematol. 2021;157:103188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.