Abstract
Cryptococcus neoformans is an opportunistic fungal pathogen that causes meningitis in ∼10% of patients with AIDS. New selectable markers which confer resistance to G418 or phleomycin when transformed into C. neoformans were made. A hygromycin-selectable marker was modified to allow selection with a single copy of the marker.
Cryptococcus neoformans is a haploid heterothallic basidiomycetous yeast that causes cryptococcal meningitis in immunocompromised patients, particularly those with AIDS (for a review, see reference 1). There are several different serotypes of C. neoformans, with serotype A being the most prevalent clinical isolate in the United States, followed by serotype D. C. neoformans is amenable to molecular genetics, with transformation systems and reverse genetics available (3–5). Although there are a few selectable markers available, most require the generation of a particular mutation in the recipient strain, including ade2 (5), ura5 (3), and nmt487D (4). A marker encoding resistance to hygromycin (2), which has been shown to be useful for transforming both wild-type strains and clinical isolates, was developed. Some studies, such as generation of insertion mutants and subsequent complementation or transformation with separate expression plasmids, require the use of multiple selectable markers. We have determined that C. neoformans is sensitive to several different antibiotics, including phleomycin and G418, and have constructed genes which encode resistance to phleomycin and G418, which can be used as selectable markers.
Determination of sensitivity of C. neoformans to antibiotics.
Fresh colonies of the commonly used serotype A strain H99 were streaked onto YPD plates (1% yeast extract, 2% peptone, 2% glucose, 2% agar) plates containing the following antibiotics: phleomycin (Cayla) at 100, 10, or 1 μg/ml: G418 (Geneticin; Life Technologies) at 100, 10, or 1 μg/ml; netropsin (Roche) at 100 or 10 μg/ml; and oligomycin (Calbiochem) at 10, 1, or 0.1 μg/ml. C. neoformans showed good sensitivity in the assay to 100 μg of G418 per ml and 100 μg of netropsin per ml and some sensitivity to phleomycin at 100 μg/ml. No sensitivity to oligomycin was detected at any of the tested concentrations.
Phleomycin and G418 were tested further because there were genes available which confer resistance to these antibiotics in other systems. H99 cells were grown to late log phase, washed in phosphate-buffered saline (PBS), and plated at a concentration of ∼107 cells per plate onto YPD agar plates containing different concentrations of antibiotic. It was determined that no detectable growth occurred on plates containing 200 μg of G418 per ml or on plates containing 250 μg of phleomycin per ml at pH 7.0.
Development of vectors for transformation studies.
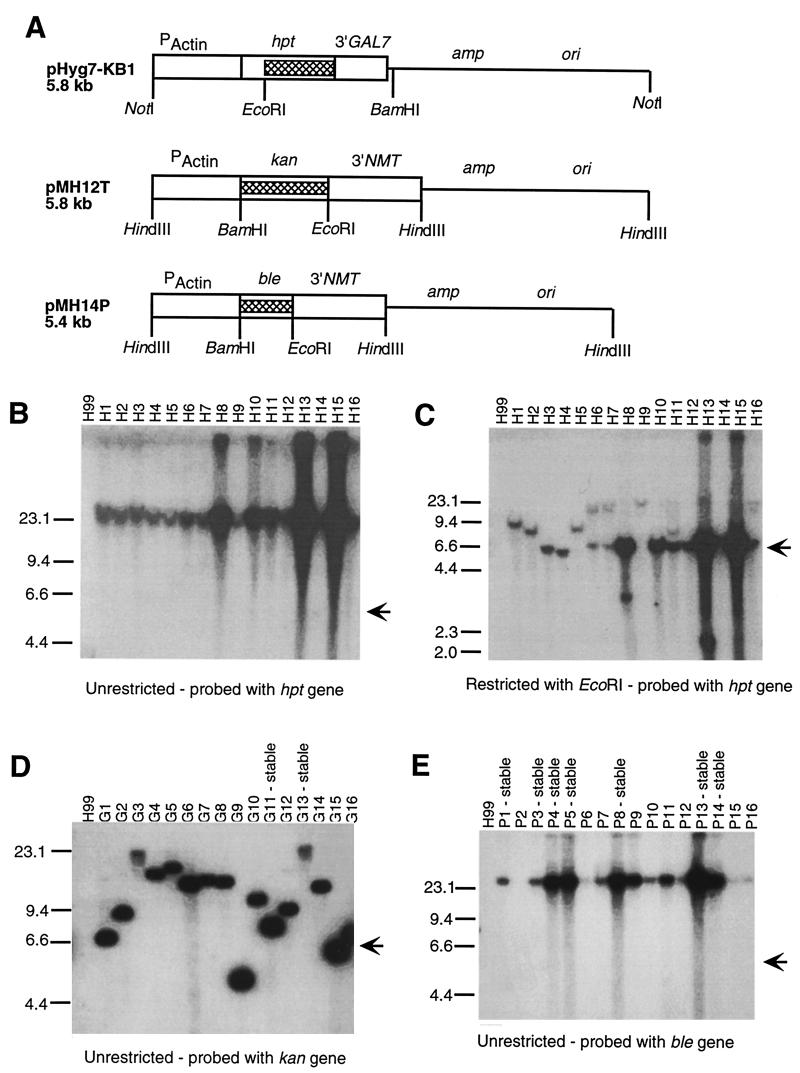
Figure 1A is a schematic of the three transformation vectors. To create the modified hygromycin resistance cassette, the plasmid pTELHYG (2) was digested with NotI and religated to remove the telomeric sequences and create plasmid pHYG. To provide a poly(A) addition site and possibly improve mRNA stability, the 3′ untranslated sequences from the GAL7 gene (7) on plasmid pGUST11 (a generous gift from Brian Wickes) from the NruI-KpnI sites were inserted into the EcoRV-KpnI sites just 3′ to the end of the hygromycin coding sequences (hpt) in the pHYG plasmid. The KpnI site was then blunted, and a BamHI linker was added to create the plasmid pHYG7-KB1.
FIG. 1.
(A) Schematic linear diagrams of the transformation plasmids. Relevant restriction sites are marked below the diagram. The portion of the plasmid used as a probe in the Southern blots are indicated with hatched boxes. (B, C, D, and E) Southern blot analysis of the transformants. H99 is the parental strain. H1 to H16 are stable hygromycin-resistant H99 transformants, G1 to G16 are G418-resistant H99 transformants, and P1 to P16 are phleomycin-resistant H99 transformants. Some of the G418 and phleomycin resistant transformants were stable, and those are marked above the lane. The size markers are indicated along the left side of the blots. Whether the genomic DNA is uncut or restricted with EcoRI is indicated below each blot. Uncut genomic DNA migrates with the 23.1-kb size marker. The position at which the linearized parent plasmid migrates is marked on the right of each blot with an arrow.
Coding sequences from the kan and ble genes from the bacterial transposable element Tn5 were isolated by PCR with primers that introduced a BamHI site just 5′ to the initiator ATG and an EcoRI site just 5′ to the terminator codon. To create pMH12-T, the synthesized 800-bp kan gene was cloned between an ∼850-bp fragment containing the promoter from the C. neoformans actin gene (3) and an ∼1,000-bp fragment containing the 3′ untranslated region from the C. neoformans NMT gene (4) in a pGEM13-derived vector. The synthesized ∼450-bp ble gene was cloned into a similar vector to create pMH14-P. The PCR-synthesized kan and ble coding regions were sequenced, and no PCR-induced errors were incorporated into the genes. Similar plasmids containing the zeocin resistance coding sequence from pZERO (Invitrogen) and the kan gene from Tn903 were also made and tested but never produced any transformants.
Transformation of C. neoformans.
C. neoformans serotype A strain H99 and serotype D strain JEC20 were transformed by biolistic techniques (5). Plasmid DNAs used for the transformations were either uncut or cleaved with a restriction enzyme which cleaves once in the plasmid (see Fig. 1 and Table 1). The cells were grown to late log phase in YPD, concentrated, and plated onto YPD agar for transformation. The cells were bombarded with 0.6-μm-diameter gold particles coated with plasmid DNA and allowed to sit for 4 h on nonselective medium following transformation. The cells were resuspended in 0.5 ml of 1× PBS liquid medium and plated onto selective medium (YPD supplemented with 400 U of hygromycin (Calbiochem) per ml, YPD supplemented with 200 μg of G418 or YPD per ml, 10 mM MOPS (morpholine propanesulfonic acid; pH 7.0) supplemented with 250 μg of phleomycin per ml). Phleomycin plates were incubated at 4°C for 12 h before transfer to incubation at 30°C. All other plates were immediately incubated at 30°C. Transformants were observed within 3 to 7 days. The transformation frequencies for each condition, plasmid, and strain are shown in Table 1 and are given in transformants per microgram of plasmid DNA. Transformation with pMH12T was very efficient, with frequencies of well over 100/μg in both H99 and JEC20. Transformation with pHYG7-KB1 and pMH14P produced more modest frequencies, and we were unable to obtain any transformations with pMH14P with JEC20 as the recipient strain. The large standard deviations in the transformation frequencies reflects the variability of the process. We observed that different plates transformed at the same time with the same DNA preparation and cell culture would vary in transformation frequency and that there was even more variability from day to day.
TABLE 1.
Transformation frequencies
| Plasmid | Restricteda | Selection | Recipient strain | Frequency of transformantsb | % Stable transformantsc |
|---|---|---|---|---|---|
| pHYG7-KB1 | Hygromycin | H99 | 20 (±16) | 90 | |
| pHYG7-KB1 | BamHI | Hygromycin | H99 | 5 (±2) | 83 |
| pHYG7-KB1 | Hygromycin | JEC20 | 36 (±34) | 2 | |
| pMH12T | G418 | H99 | 404 (±340) | 6 | |
| pMH12T | NotI | G418 | H99 | 180 (±158) | 7 |
| pMH12T | G418 | JEC20 | 355 (±286) | 8 | |
| pMH14P | Phleomycin | H99 | 125 (±169) | 11 | |
| pMH14P | NotI | Phleomycin | H99 | 70 (±89) | 5 |
| pMH14P | Phleomycin | JEC20 | 0 (±0) | n/a |
The plasmids used for transformation were either uncut or restricted with the indicated restriction enzyme.
The transformation frequencies are shown in transformants per microgram of DNA. They were determined from a minimum of six independent transformation events during at least two separate experiments. The standard deviation is shown in parenthesis.
At least 50 independent transformants were passaged three times on nonselective medium and then tested for resistance on selective medium. n/a, not applicable.
Analysis of transformants.
To determine whether the transformants were stable or unstable, independent transformants were passaged three times on nonselective YPD medium and then tested for resistance to the appropriate antibiotic; the results are shown in Table 1. Only those transformants that grew equally well on the selective medium as on nonselective medium were counted as stable transformants. We tested the stability of the transformants by passage in both liquid and solid medium and found no difference (data not shown). In H99, most of the hygromycin-resistant transformants were stable, whereas very few of the G418- or phleomycin-resistant transformants were stable. The percentage of stable transformants was unaffected by prior cleavage with a restriction enzyme. In JEC20, both the hygromycin- and G418-resistant transformants had a very low percentage of stable transformants.
In C. neoformans, unstable transformants are often the result of the transforming DNA forming extrachromosomal plasmids rather than integrating into the genome (3). These plasmids can often be detected by Southern blot analysis of uncut genomic DNA because they migrate significantly faster than the genomic DNA during agarose gel electrophoresis. Genomic DNA was isolated from cells grown on selective medium before they were passaged on nonselective medium. Unrestricted DNA or DNA cleaved with EcoRI was electrophoresed on 1% agarose gels and transferred to nylon membranes. The probes were fragments of the resistance genes, as indicated in Fig. 1A.
Southern blot analysis of the genomic DNA from 16 hygromycin-resistant H99 transformants using unrestricted genomic DNA (Figure 1B) and probed with a portion of the hpt gene showed that the hygromycin gene migrates with the genomic DNA and is not present as small extrachromosomal plasmids. All of these transformants were stable transformants. Southern blot analysis using the same genomic DNAs cleaved with EcoRI (Fig. 1C) indicated that several of the transformants (e.g., H1, H2, H3, H4, H5, and H9) had the marker present in a single copy integrated into the genome, as indicated by the presence of a single band. This result is in contrast to results with the parent plasmid, pTELHYG (2), where multiple insertion sites and extrachromosomal plasmids were observed in most transformants. The remaining transformants had multiple copies of the plasmid inserted in tandem arrays, as indicated by the presence of a band at 5.6 kb, the same size as pHYG7-KB1, and a second band indicating the flanking sequence. The flanking sequences are of different sizes, suggesting that the plasmid is inserted at different sites in the genome. These data suggest that pHYG7-KB1 would be a useful plasmid for experiments that require random integration into the genome.
Southern blot analysis of uncut genomic DNA from G418-resistant transformants (Fig. 1D) probed with the kan gene suggests that the transforming DNA is present as extrachromosomal plasmids and is not integrated into the genomic DNA. There was not a perfect correlation between the presence of plasmid DNA that migrates with the uncut genomic DNA and the stability of the transformant. For example, G11 was stable after multiple passages on nonselective medium, but the transforming DNA was clearly not integrated into the genome.
Southern blot analysis of the phleomycin-resistant transformants (Fig. 1E) showed that the ble gene probe hybridized to DNA that comigrated with the genomic DNA in most of the samples. Some samples showed little or no hybridization, but no samples showed hybridization to bands that migrated more quickly than the genomic DNA. However, more than half of the transformants tested were unstable. If the transforming DNA is extrachromosomal, then it might be present as very large multimers of the plasmid or as an unstable minichromosome (6). Alternatively, the plasmid may be integrated into the chromosome in a manner that allows it to be lost very rapidly or it may be integrated into the mitochondrial genome. In general, the stable transformants had more signal than the unstable transformants, suggesting that the plasmid DNA was already being lost from the population of cells. Southern blot analysis using EcoRI-digested genomic DNA showed that the stable transformants had very high copy numbers of tandem arrays of the plasmid integrated into a single site (data not shown).
We have developed three (two new and one modified) positive selectable markers for use in transformation of the pathogenic fungus C. neoformans. The markers differ in transformation efficiency and frequency of stable integration into the genome. One possible source of the differences in transformation frequencies and the stability of the transformants is the difference in the 3′ sequences that were used. pHYG7-KB1 has a 3′ poly(A) site from the GAL7 gene and produced a high percentage of stable transformants, whereas both pMH12T and pMH14P plasmids produced low percentages of stable transformants and have the 3′ poly(A) site from the NMT gene. The three plasmids clearly have different fates during transformation and therefore will have different utilities. The pHYG7-KB1 plasmid may be useful for generating stable insertions for insertional mutagenesis experiments. The pMH12T plasmid will be more useful for situations which require nonintegrative transformation. An example would be studies which require the transient expression of a protein, such as expression of an enzyme which modifies the genome like transposase or Cre recombinase. In addition, this plasmid may be useful in complementation studies. The pMH14P plasmid may be most appropriate for developing stable transformants in which a very high copy number is desired, e.g., overexpression studies.
Acknowledgments
This work was supported by Public Health Service grants from the National Institute of Allergy and Infectious Diseases (AI41962 and AI01577) and by a grant from the Burroughs Wellcome Fund.
We thank Michelle Higgins and Amy Brotherton for technical assistance, Brian Wickes for plasmid pGUST-11, Gary Cox for plasmid pTELHYG, and June Kwon-Chung for strain JEC20.
REFERENCES
- 1.Casadevall A, Perfect J R. Cryptococcus neoformans. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 2.Cox G M, Toffaletti D L, Perfect J R. Dominant selection system for use in Cryptococcus neoformans. J Med Vet Mycol. 1996;34:385–391. [PubMed] [Google Scholar]
- 3.Edman J C, Kwon-Chung K J. Isolation of the URA5 gene from Cryptococcus neoformans var. neoformans and its use as a selective marker for transformation. Mol Cell Biol. 1990;10:4538–4544. doi: 10.1128/mcb.10.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodge J K, Jackson-Machelski E, Higgins M, Devadas B, McWherter C A, Gordon J I. Genetic and biochemical studies establish that the fungicidal effect of a fully depeptidized inhibitor of Cryptococcus neoformans myristoylCoA:protein N-myristoyltransferase is Nmt dependent. J Biol Chem. 1998;273:12482–12491. doi: 10.1074/jbc.273.20.12482. [DOI] [PubMed] [Google Scholar]
- 5.Toffaletti D L, Rude T H, Johnston S A, Durack D T, Perfect J R. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175:1405–1411. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varma A, Kwon-Chung K J. Formation of a minichromosome in Cryptococcus neoformans as a result of electroporative transformation. Curr Genet. 1994;26:54–61. doi: 10.1007/BF00326305. [DOI] [PubMed] [Google Scholar]
- 7.Wickes B L, Edman J C. The Cryptococcus neoformans GAL7 gene and its use as an inducible promoter. Mol Microbiol. 1995;16:1099–1109. doi: 10.1111/j.1365-2958.1995.tb02335.x. [DOI] [PubMed] [Google Scholar]