Abstract
A bioresorbable, mono-crystalline magnesium (Mg) ring device and suture implantation technique were designed to connect the ends of a transected anterior cruciate ligament (ACL) to restabilize the knee and load the ACL to prevent disuse atrophy of its insertion sites and facilitate its healing. To test its application, cadaveric goat stifle joints were evaluated using a robotic/universal force-moment sensor testing system in three states: Intact, ACL-deficient, and after Mg ring repair, at 30°, 60°, and 90° of joint flexion. Under a 67-N anterior tibial load simulating that used in clinical examinations, the corresponding anterior tibial translation (ATT) and in-situ forces in the ACL and medial meniscus for 0 and 100 N of axial compression were obtained and compared with a control group treated with suture repair. In all cases, Mg ring repair reduced the ATT by over 50% compared to the ACL-deficient joint, and in-situ forces in the ACL and medial meniscus were restored to near normal levels, showing significant improvement over suture repair. These findings suggest that Mg ring repair could successfully stabilize the joint and load the ACL immediately after surgery, laying the framework for future in vivo studies to assess its utility for ACL healing.
Keywords: anterior cruciate ligament (ACL), ACL repair, magnesium ring device, robotic testing system
The anterior cruciate ligament (ACL) is the most frequently injured ligament in the knee. It has been estimated that there are approximately 200,000 cases of ACL injuries per year in the United States.1 Since midsubstance ACL ruptures have a limited capacity for healing, surgical reconstruction of the ACL using a soft tissue replacement autograft or cadaveric allograft has been the standard of treatment.2,3 In the short-term, these reconstruction procedures can restore knee stability and relieve pain, allowing for return to sports after months of rehabilitation. However, longer term clinical follow-up studies have found that up to 25% of patients have unsatisfactory results that include complications attributed to graft donor site morbidity, residual knee pain, and prevalence of osteoarthritis.4–7
With the recent advances in functional tissue engineering, scientists, and surgeons have begun to explore novel ways to heal an injured ACL.8–11 Healing the ACL would be an attractive alternative treatment option for many patients, as it would preserve its complex anatomy and proprioceptive function while avoiding some of the complications following reconstruction. To this end, large animal studies12–14 as well as clinical studies15,16 using cells, growth factors, and scaffolds have shown that these augmentation procedures could promote and accelerate the healing response of an injured ACL.
In our research center, biological scaffolds derived from porcine extracellular matrix (ECM) have been used to improve the healing of a patellar tendon defect17 and a 6 mm wide MCL gap injury.18,19 These positive findings led to its application together with the same ECM in its hydrogel form to successfully heal a fully transected ACL in a goat model.8,9 At 12 weeks post-surgery, the healing ACL had an organized collagen matrix with spindle-shaped cells, but without tissue hypertrophy. Uniaxial tensile testing revealed that the stiffness of the healing femur-ACL-tibia complex (FATC) was 2.5 times greater than suture repair alone, and the ultimate load was 1.9-fold higher. However, at a subsequent longer time point; that is, 26 weeks, the mode of failure was found to have moved from the midsubstance (at 12 weeks) to its femoral insertion. This was a result of continuing improvement of the healing ACL while its insertion sites became weakened due to the lack of adequate stress in the FATC.20 These results suggested that mechanical loading of the FATC would be needed to limit the negative effects of disuse atrophy of its insertion sites.
Published in vitro21,22 and in vivo23 studies have shown that mechanical augmentation of an injured ACL could help to stabilize the knee, leading to improvement in its healing. For example, when sutures were applied to provide bone-to-bone fixation between the femur and tibia, joint stability as well as the function of the ACL and medial meniscus could be restored when the knee was subjected to externally applied loads.22 The same technique used in an in vivo study also led to an improved healing response of a transected ACL when compared with a suture repair technique alone.23 In this study, a new repair technique was developed using a novel, bioresorbable, magnesium (Mg) ring-shaped device secured with sutures to provide joint stability and to simultaneously load the ligament as well as its insertion sites. Mg was chosen as a material for the ring because it is biodegradable and bioresorbable; as such, this ring-suture complex was intended to gradually transfer loading to the ACL throughout the healing process, eventually leaving behind only a functional ACL.
To evaluate the potential application of the Mg ring device, an experiment was performed to address the following research question: Could the use of the Mg ring and corresponding suture repair technique (“Mg ring repair”) restore joint function and the in-situ forces in the ACL and medial meniscus to normal levels immediately after implantation using our established goat model?24,25 To address this question, a robotic/UFS testing system26 was employed to quantitatively evaluate the function of cadaveric goat stifle joints following Mg ring repair when external loads were applied mimicking those used in clinical examinations.
METHODS
The Mg ring device was designed based on the size and geometry of the goat stifle joint. Notable features of the ring included its conical frustum shape with a larger diameter on the distal (tibial) end to match the anatomy of the goat ACL, one set of notches on the anterior side, as well as four staggered holes around the medial and lateral sides (Fig. 1). It was manufactured from pure Mg with a mono-crystalline atomic structure, which was selected due to its high ductility and plasticity to allow it to deform in the joint space without fracture.
Figure 1.
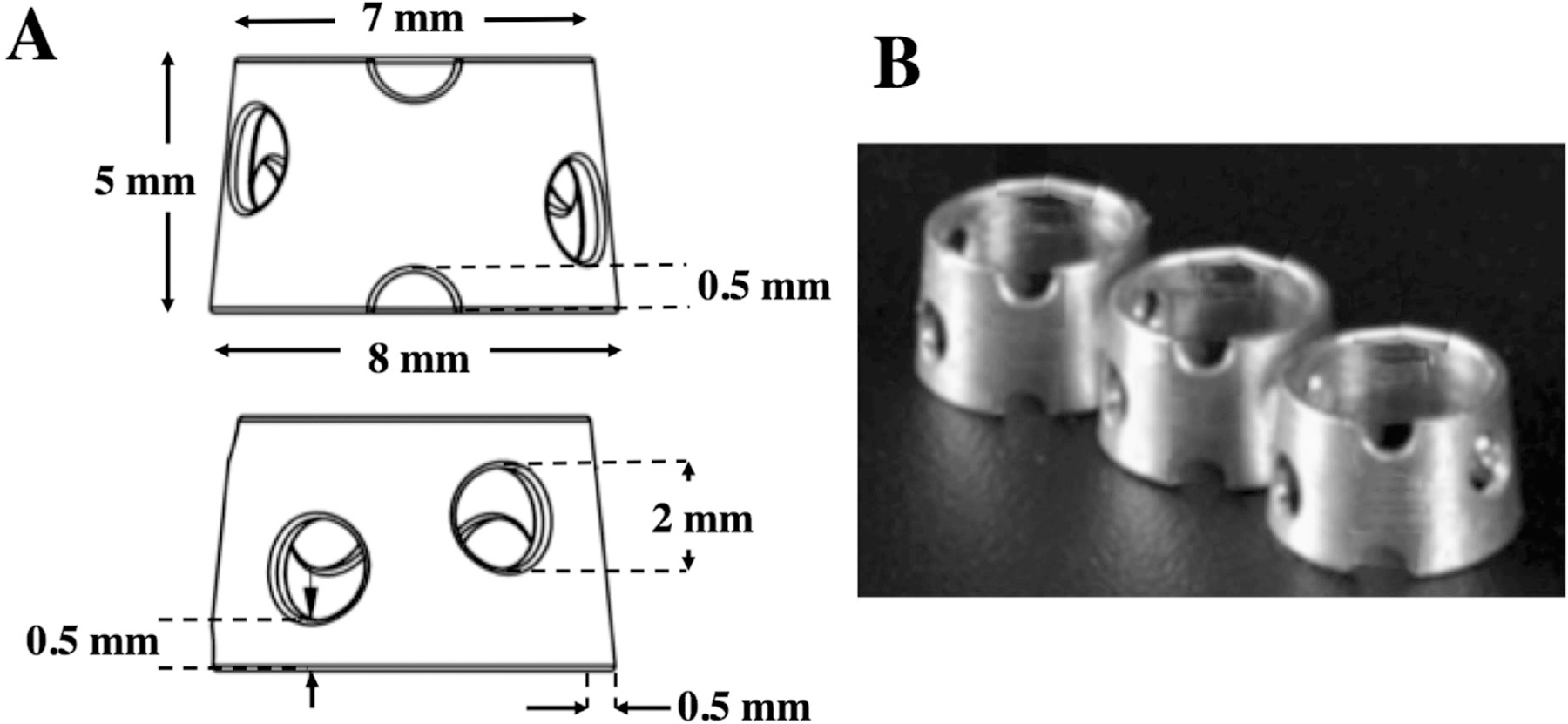
(A) Schematic diagram, and (B) a photograph of the Mg ring device.
For the in vitro evaluation, fresh-frozen stifle joints from Boer goats were used. A total of eight joints was determined to be the required sample size by an a priori power analysis using previous data to determine differences of 2 mm in stifle joint kinematics and 10 N in in-situ forces in the ACL. The function of the Mg ring-repaired ACL was tested using the robotic/universal force-moment sensor (UFS) testing system developed in our research center, which is capable of measuring 6-DOF joint kinematics and the in-situ forces in knee structures.22,25–28 The data obtained from this method of testing represents an improvement over the linkage systems that had been used historically.29,30
Intact stifle joints were thawed 24 h prior to testing and prepared as previously described.25,31 The potted femur and tibia were bolted to the robotic/UFS testing system and secured within custom clamps, with the femur fixed in a rigid pedestal base and the tibia attached to the end effector of the robot (Puma Model 762, Unimate, Pittsburgh, PA). An anatomical coordinate system describing the position of the joint relative to the UFS (Model 4015, JR3, Inc., Woodland, CA) was defined.32 The subsequent testing procedure is summarized in Table 1. First, the passive path of flexion-extension was found from full extension to 90° of flexion, to be used as a reference position throughout testing to move between joint flexion angles. Next, the robotic/UFS testing system was operated in force-control mode to determine the stifle joint kinematics in response to externally applied loads simulating those used clinically to test for ACL function: (i) a 67-N anterior tibial load, and (ii) a 67-N anterior tibial load with 100 N axial compression.22,33,34 Each loading condition was applied at three preselected angles of joint flexion (30°, 60°, and 90°), while the robot recorded the resulting 5-DOF kinematics.
Table 1.
Experimental Protocol and Data Obtained Using the Robotic/UFS Testing System Under a 67-N Anterior Tibial Load Without (I–III.A) and With (I–III.B) 100 N Axial Compression
| Protocol | Data Obtained |
|---|---|
| I. Intact joint | |
| Path of passive flexion-extension | |
| External loading conditions | |
| A. 67-N anterior tibial load | Intact kinematics (I.A) |
| B. 67-N anterior tibial load + 100 N axial compression Transect ACL | Intact kinematics (I.B) |
| II. ACL-deficient joint | |
| Repeat kinematics I.A, I.B | In-situ forces in the ACL |
| Apply loads I.A, I.B | ACL-deficient kinematics (II.A, II.B) |
| III. Mg ring repair of the ACL | |
| Perform Mg ring repair | |
| Apply loads I.A, I.B | Mg ring repair kinematics (III.A, III.B) |
| Release Mg ring | |
| Repeat kinematics III.A, III.B | In-situ forces in Mg ring-repaired ACL |
| IV. Medial meniscus-deficient joint | |
| Repeat kinematics I, II, III | In-situ forces in the medial meniscus |
Next, the ACL was transected through its midsubstance using a medial parapatellar incision. The previously recorded kinematics of the joint with an intact ACL were then repeated, while the UFS recorded new forces and moments. The in-situ forces in the intact ACL could then be determined in response to each of the loading conditions using the principle of superposition. The ACL-deficient kinematics were also recorded using loading conditions (1) and (2).
Then, Mg ring repair of the ACL was performed. First, four 1.5 mm bone tunnels were drilled, with two passing anterior to the ACL’s femoral insertion site and two passing medial and lateral to its tibial insertion site. Then, two sets of “repair sutures” (PDS #0, Ethicon, Inc., Bridgewater, NJ) and “fixation sutures” (Ethibond #2, Ethicon, Inc., Bridgewater, NJ) were attached to each end of the transected ACL (Fig. 2A). The ring was then attached to the ACL, with the repair sutures passing directly through the ring and tied around it. The fixation sutures were passed through the suture holes and bone tunnels, and fixed on the outside of the bones using an Endobutton on the femoral side (Smith & Nephew, Andover, MA) and double-spiked plate and fixation post on the tibial side (Smith & Nephew) under 40 N applied tension (Fig. 2B). Once Mg ring repair was complete, the kinematics of the repaired joint were obtained using loading conditions (1) and (2). Finally, the ring was then removed and the previously recorded kinematics were repeated to obtain the in-situ forces in the repaired ACL.
Figure 2.
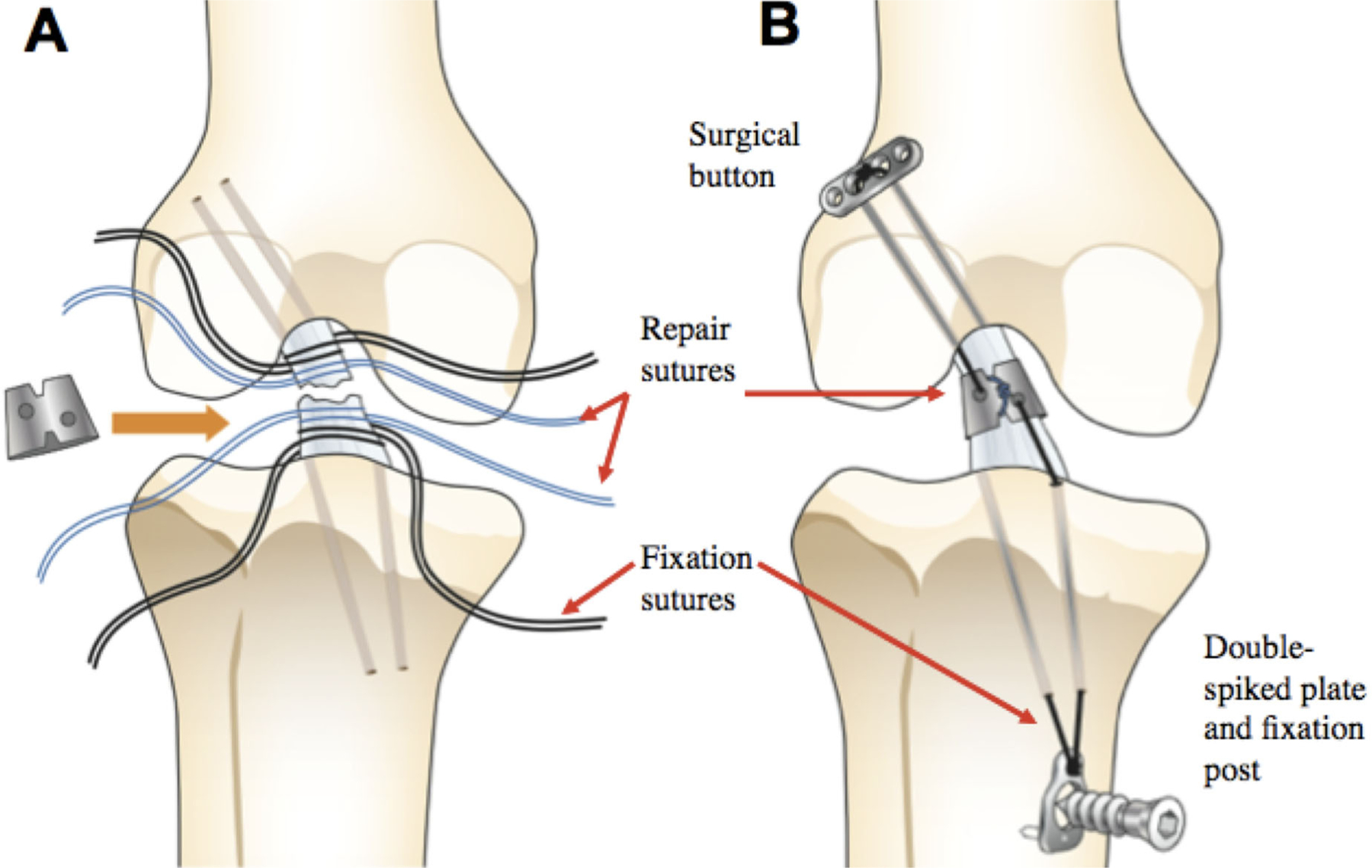
Procedural details for Mg-based ring repair, depicting attachment of repair and fixation sutures to the ACL (A), followed by attachment and fixation of the ring (B).
To measure the in-situ forces in the medial meniscus under each condition, it was carefully removed from the joint. Then, the kinematics of the joint with an intact ACL, ACL-deficient joint, and repaired joint were repeated to determine its in-situ forces in each state using the principle of superposition.
SPSS Statistical Software (Version 21, IBM, Armonk, NY) was used for statistical analyses. Because the same specimen were used for all testing conditions, a repeated measures analysis of variance (ANOVA) was performed for statistical comparisons between joint states. Bonferroni post-hoc tests were done for pairwise comparisons, with statistical significance set at p ≤ 0.05.
RESULTS
Mg ring repair could successfully connect the two ends of the transected ACL and provide mechanical augmentation to stabilize the goat stifle joint. Data on anterior tibial translation (ATT) in response to an applied 67-N anterior tibial load are summarized in Table 2. For the joint with an intact ACL, the ATT was between 1.8 and 2.7 mm. These values were similar at 30° and 60°, but became lower at 90°. Following ACL transection, it increased to more than 12 mm (p < 0.05). However, following Mg ring repair, it was reduced to 4.3–5.8 mm, which was 8–12 mm lower than the ACL-deficient state (p < 0.05) and 2–3 mm higher than that of the intact joint (p < 0.05).
Table 2.
Anterior Tibial Translation in mm (Mean ± SD) of the Goat Stifle Joints Tested at 30°, 60°, and 90° of Flexion in Response to: (A) a 67-N Anterior Tibial Load and (B) a 67-N Anterior Tibial Load + 100 N Axial Compression
| Flexion Angle |
|||
|---|---|---|---|
| 30° | 60° | 90° | |
| A. 67-N anterior tibial load | |||
| Intact | 2.5 ± 0.6 | 2.7 ± 0.9 | 1.8 ± 1.0 |
| ACL-deficient | 15.2 ± 2.3*,** | 15.8 ± 1.7*,** | 12.4 ± 1.6*,** |
| Mg ring repair | 5.0 ± 1.0* | 5.8 ± 1.0* | 4.3 ± 1.3* |
| B. 67-N anterior tibial load + 100 N axial compression | |||
| Intact | 3.8 ± 0.8 | 4.6 ± 0.7 | 3.3 ± 1.0 |
| ACL-deficient | 17.4 ± 2.7*,** | 17.8 ± 2.7*,** | 13.6 ± 2.9*,** |
| Mg ring repair | 8.0 ± 1.4* | 9.5 ± 1.6* | 7.0 ± 1.6* |
Significantly different compared to the intact knee (p < 0.05).
Significantly different from Mg ring repair (p < 0.05).
The in-situ force data are detailed in Table 3. Under the 67-N anterior tibial load, the in-situ force in the intact ACL was between 51 and 61 N, which was 73–90% of the applied load. Following Mg ring repair of the ACL, it was 55–63 N and was within ±5 N of that in the intact ACL for all three joint flexion angles tested (p > 0.05). The corresponding in-situ force in the medial meniscus was found to be negligibly small when the ACL was intact, but it increased significantly to 29–40 N (p < 0.05) following ACL transection. With Mg ring repair, it was reduced to levels that were not statistically different from those in the joint with an intact ACL (p > 0.05).35
Table 3.
In-Situ Forces in N (mean ± SD) in the: (A) ACL and (B) Medial Meniscus of the Goat Stifle Joints at 30°, 60°, and 90° of Flexion in Response to (I) a 67-N Anterior Tibial Load and (II) a 67-N ATL With 100 N Axial Compression
| A. ACL |
B. Medial Meniscus |
|||||
|---|---|---|---|---|---|---|
| Flexion Angle | 30° | 60° | 90° | 30° | 60° | 90° |
| I. 67-N anterior tibial load | ||||||
| Intact joint | 61 ± 8 | 59 ± 4 | 51 ± 9 | 5 ± 3 | 4 ± 2 | 6 ± 4 |
| ACL-deficient joint | — | — | — | 37 ± 16*,** | 46 ± 16*,** | 35 ± 15*,** |
| Mg ring repaired joint | 62 ± 7 | 63 ± 7 | 55 ± 7 | 7 ± 4 | 8 ± 8 | 16 ± 9 |
| II. 67-N anterior tibial load + 100 N axial compression | ||||||
| Intact joint | 82 ± 13 | 91 ± 19 | 78 ± 33 | 9 ± 6 | 8 ± 6 | 22 ± 12 |
| ACL-deficient joint | — | — | — | 57 ± 29*,** | 88 ± 40*,** | 85 ± 39*,** |
| Mg ring repaired joint | 74 ± 15 | 92 ± 22 | 79 ± 33 | 14 ± 9 | 26 ± 13* | 50 ± 21* |
Significantly different from the intact knee (p < 0.05).
Significantly different from Mg ring repair (p < 0.05).
When a 100 N axial compressive load was added to the 67-N anterior tibial load, the ATT of the joint with an intact ACL was consistently 1–3 mm higher than those under the 67-N anterior tibial load alone (Table 2). Following ACL transection, the ATT increased four to sixfold (p < 0.05). After Mg ring repair, it was reduced by about 50% of those in the ACL-deficient state (p < 0.05), but remained 4–6 mm higher than that of the intact joint (p < 0.05).
Due to the additional compressive load, the in-situ force in the intact ACL was 18–31 N higher than under the anterior tibial load alone (Table 3). Following Mg ring repair, it was restored to within ±8N of the intact ACL for all joint flexion angles tested. The in-situ force in the medial meniscus with an intact ACL was unchanged with the additional compressive load at 30° and 60°, but it increased by about threefold at 90° of knee flexion. After transection of the ACL, it increased significantly to 57–88 N (p < 0.05). Following Mg ring repair, it was again reduced by 74–85% compared to the ACL-deficient joint and was not statistically different from that in the joint with an intact ACL at 30° (p > 0.05).
To further determine the efficacy of Mg ring repair in restoring stifle joint stability and ACL function, the data obtained were compared to those collected from an earlier study from our research center whereby only sutures were applied to repair a transected ACL (“suture repair”).22 For this comparison, the data on the knee kinematics and in-situ forces in the repaired ACL and medial meniscus were normalized by values in the intact joint in order to reduce interspecimen variation, such that a value of 1.0 would indicate restoration to normal levels.
Under the 67-N anterior tibial load, the normalized ATT after Mg ring repair ranged from 2.1 to 2.9 at the tested flexion angles, while for suture repair it was more than twice that and ranged from 5.3 to 6.2 (p < 0.05; Fig. 3A). The corresponding normalized in-situ force in the ACL following Mg ring repair was similar to that in that intact joint and was between 1.0 and 1.1, which was significantly higher than 0.6–0.8 following suture repair (p < 0.05; Fig. 4A). This lower in-situ force in the ACL was concomitant with a significantly higher in-situ force in the medial meniscus following suture repair compared to Mg ring repair (4.7–13.3 and 1.8–4.4, respectively; p < 0.05; Fig. 4A).
Figure 3.
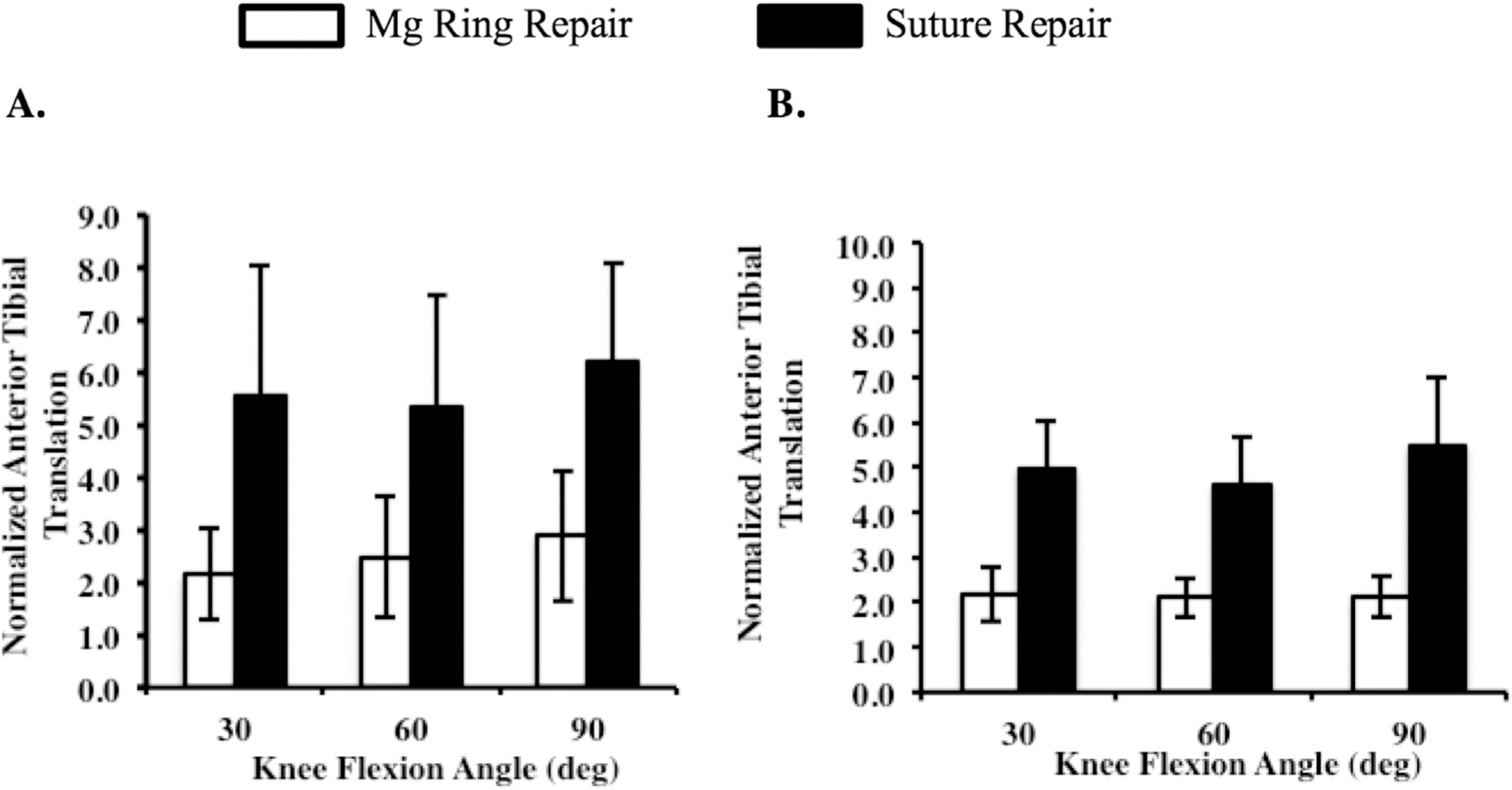
Normalized anterior tibial translation of the Mg ring-repaired and suture-repaired goat stifle joints at 30°, 60°, and 90° of flexion under (A) a 67-N anterior tibial load and (B) a 67-N anterior tibial load with 100 N axial compression.
Figure 4.
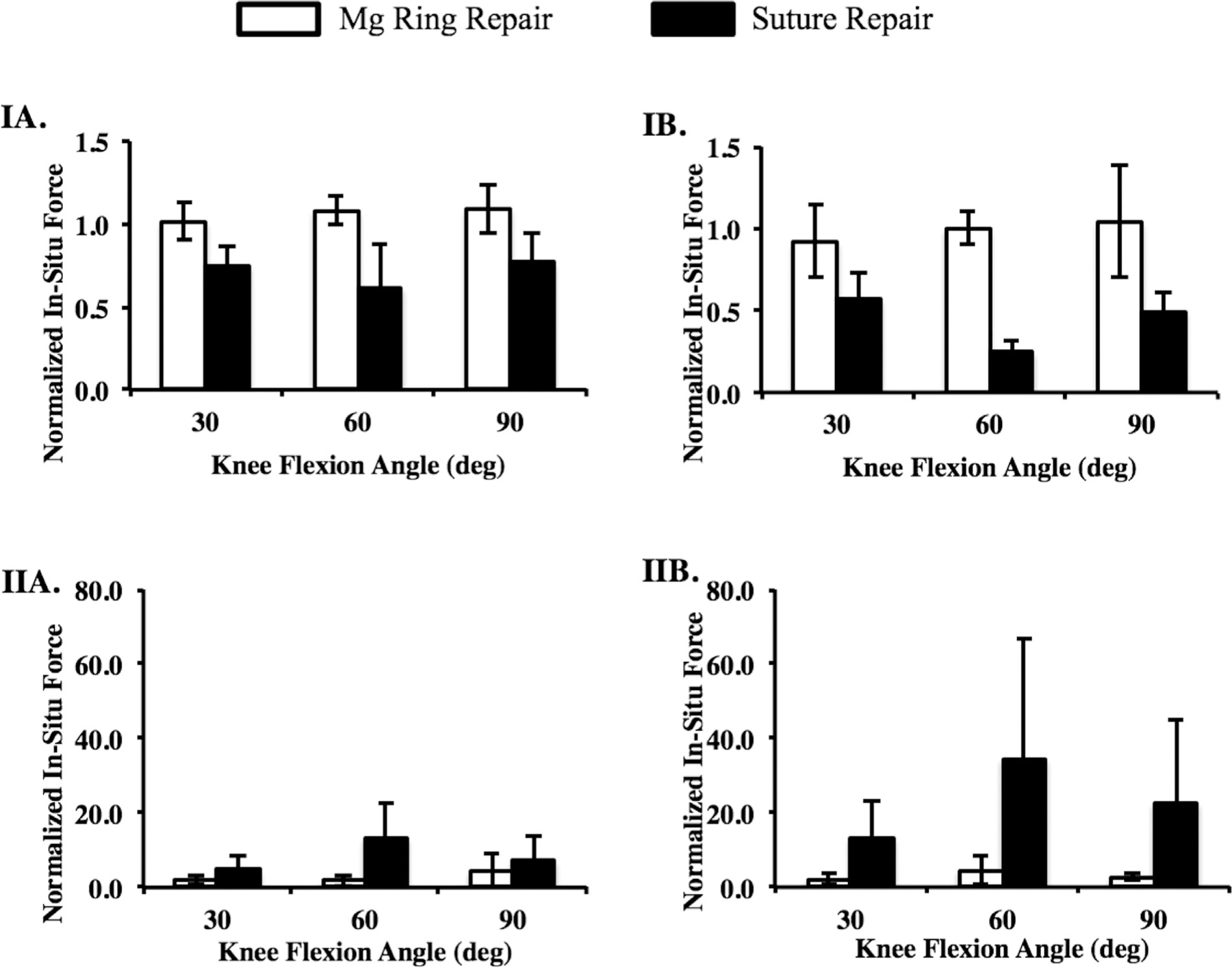
Normalized in-situ forces in (I) the ACL and (II) medial meniscus in the Mg ring- and suture-repaired goat stifle joints at 30°, 60°, and 90° of flexion under (A) a 67-N anterior tibial load and (B) a 67-N anterior tibial load with 100 N axial compression.
With the addition of 100 N axial compression, the differences between Mg ring repair and suture repair were more pronounced. Again, the normalized ATT after suture repair was more than two times higher than after Mg ring repair at the tested flexion angles (4.6–5.5 and 2.1–2.2, respectively; p < 0.05; Fig. 3B). However, the in-situ force in the ACL following suture repair was reduced by more than 50% to just 0.1–0.3. This resulted in increases in in-situ force in the medial meniscus of up to 35 times that in the joint with the intact ACL. In contrast, after Mg ring repair, the in-situ force in the ACL was maintained at 0.9–1.0 (p < 0.05; Fig. 4B), and the normalized in-situ force in the medial meniscus was 2.0–4.5 (p < 0.05; Fig. 4B).
DISCUSSION
In this study, a Mg ring device and suture implantation technique were designed and used to successfully repair a transected ACL in goat stifle joints. Mg ring repair could stabilize the cadaveric stifle joint by restoring its ATT to within 2–6 mm of the stifle joint with an intact ACL following an applied 67-N anterior tibial load with or without 100 N axial compression. The corresponding in-situ force in the repaired ACL was also returned to those in the joint with the intact ACL. Thus, these findings give positive answers to the research question by demonstrating that Mg ring repair could successfully restore the stifle joint stability and the function of the ACL. In addition, Mg ring repair was also able to reduce excessive loading of the medial meniscus, which could reduce the potential of secondary meniscal injury following ACL injury.35–37 The ATT and in-situ forces in the ACL and medial meniscus were also significantly improved compared to suture repair, and were close to those obtained in previous studies using sutures for bone-to-bone fixation as well as those following ACL reconstruction.21,22,31 As restoration of joint function would be crucial for successful ACL healing, an improvement of these parameters at time zero may result in enhancement of ACL healing in vivo.
There are a few limitations to the present study. The in vitro loading conditions were chosen to simulate those used in clinical examinations to test for ACL function,33 but they do not represent complex loading conditions in a live animal. In future studies, kinematics data recorded using biplanar fluoroscopy38,39 could be repeated on the robotic/UFS testing system to determine the in-situ forces in the Mg ring-repaired ACL and the surrounding knee structures during activities of daily living. Secondly, the advantages of Mg ring repair in a goat model may not be directly analogous to the human knee due to differences in its size, shape, range of motion, loading conditions, and so on. However, the goat model has been successfully used in a number of preclinical studies involving the ACL,8,24,25,31,40 and is considered to be an acceptable animal model. Finally, the ACL was transected through its midsubstance prior to Mg ring repair, which is not representative of ACL rupture clinically. However, our goal was to first demonstrate the feasibility of using the Mg ring for ACL repair, and transection is the most consistent model of injury. Of course, with successful results, future studies with a more realistic injury model can proceed.
This study has yielded important data on joint stability and function using Mg ring repair of a transected ACL in a goat model. These results lay the groundwork for in vivo studies at multiple healing time points to determine if this technique would improve ACL healing when used alongside an ECM bioscaffold over our previous data using ECM alone, as well as whether it could prevent disuse atrophy at its insertion sites by loading the healing ACL throughout the healing process. In these multidisciplinary studies, testing of joint stability and stifle joint function using the robotic/UFS testing system, structural and viscoelastic properties using uniaxial tensile testing of the healing femur-ACL-tibia complex, and the biological response as well as quality and composition of healing tissue using histological assessment will be important outcome measures to assess ACL healing. All of this data will be needed to assess whether Mg ring repair could eventually be suitable for clinical applications to regenerate an injured ACL.
ACKNOWLEDGMENTS
Financial support from the Commonwealth of Pennsylvania, an NSF ERC grant (#08012348), and an NIH NIBIB training grant (T32 EB0003392; Biomechanics in Regenerative Medicine) is gratefully acknowledged.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Beaty JH. 1999. Knee and leg: soft tissue trauma. In: EA A, editor. OKU orthopaedic knowledge update, 1st ed. Rosemont, IL: American Academy of Orthopaedic Surgeons. p xix, 442. [Google Scholar]
- 2.Beynnon BD, Johnson RJ, Abate JA, et al. 2005. Treatment of anterior cruciate ligament injuries, part I. Am J Sport Med 33:1579–1602. [DOI] [PubMed] [Google Scholar]
- 3.Jones KG. 1963. Reconstruction of the anterior cruciate ligament—a technique using the central 1/3 of the patellar ligament. J Bone Joint Surg Am 45:925–932. [PubMed] [Google Scholar]
- 4.von Porat A, Roos EM, Roos H. 2004. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis 63:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salmon LJ, Russell VJ, Refshauge K, et al. 2006. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft—minimum 13-year review. Am J Sport Med 34:721–732. [DOI] [PubMed] [Google Scholar]
- 6.Pinczewski LA, Lyman J, Salmon LJ, et al. 2007. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft—a controlled, prospective trial. Am J Sport Med 35:564–574. [DOI] [PubMed] [Google Scholar]
- 7.Barenius B, Ponzer S, Shalabi A, et al. 2014. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med 42:1049–1057. [DOI] [PubMed] [Google Scholar]
- 8.Fisher MB, Liang R, Jung HJ, et al. 2012. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc 20:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen DT, Geel J, Schulze M, et al. 2013. Healing of the goat anterior cruciate ligament after a new suture repair technique and bioscaffold treatment. Tissue Eng Pt A 19:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vavken P, Fleming BC, Mastrangelo AN, et al. 2012. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy 28:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu HH, Cooper JA Jr., Manuel S, et al. 2005. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: in vitro optimization studies. Biomaterials 26:4805–4816. [DOI] [PubMed] [Google Scholar]
- 12.Kanaya A, Deie M, Adachi N, et al. 2007. Intra-articular injection of mesenchymal stromal cells in partially tom anterior cruciate ligaments in a rat model. Arthroscopy 23:610–617. [DOI] [PubMed] [Google Scholar]
- 13.Joshi SM, Mastrangelo AN, Magarian EM, et al. 2009. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sport Med 37:2401–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiig ME, Amiel D, VandeBerg J, et al. 1990. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res 8:425–434. [DOI] [PubMed] [Google Scholar]
- 15.Steadman JR, Cameron-Donaldson ML, Briggs KK, et al. 2006. A minimally invasive technique (“healing response”) to treat proximal ACL injuries in skeletally immature athletes. J Knee Surg 19:8–13. [DOI] [PubMed] [Google Scholar]
- 16.Gobbi A, Bathan L, Boldrini L. 2009. Primary repair combined with bone marrow stimulation in acute anterior cruciate ligament lesions: results in a group of athletes. Am J Sports Med 37:571–578. [DOI] [PubMed] [Google Scholar]
- 17.Karaoglu S M BF, Woo SL-Y, et al. 2008. Use of a bioscaffold to improve healing of a patellar tendon defect after graft harvest for ACL reconstruction: a study in rabbits. J Orthop Res 26:255–263. [DOI] [PubMed] [Google Scholar]
- 18.Musahl V, Abramowitch SD, Gilbert TW, et al. 2004. The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament—a functional tissue engineering study in rabbits. J Orthop Res 22:214–220. [DOI] [PubMed] [Google Scholar]
- 19.Liang R, Woo SL-Y, Takakura Y, et al. 2006. Long-term effects of porcine small intestine submucosa on the healing of medial collateral ligament: a functional tissue engineering study. J Orthop Res 24:811–819. [DOI] [PubMed] [Google Scholar]
- 20.Woo SLY, et al. 1990. The response of ligaments to injury: healing of the collateral ligaments. In: Daniel D, Akeson W, O’Connor J, editors. Knee ligaments: structure, function, injury, and repair New York: Raven Press. p 351–364. [Google Scholar]
- 21.Fleming BC, Carey JL, Spindler KP, et al. 2008. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res 26:1500–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher MB, Jung HJ, McMahon PJ, et al. 2011. Suture augmentation following ACL injury to restore the function of the ACL, MCL, and medial meniscus in the goat stifle joint. J Biomech 44:1530–1535. [DOI] [PubMed] [Google Scholar]
- 23.Murray MM, Magarian E, Zurakowski D, et al. 2010. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy 26:S49–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papageorgiou CD, Ma CB, Abramowitch SD, et al. 2001. A multidisciplinary study of the healing of an intraarticular anterior cruciate ligament graft in a goat model. Am J Sport Med 29:620–626. [DOI] [PubMed] [Google Scholar]
- 25.Abramowitch SD, Papageorgiou CD, Withrow JD, et al. 2003. The effect of initial graft tension on the biomechanical properties of a healing ACL replacement graft: a study in goats. J Orthop Res 21:708–715. [DOI] [PubMed] [Google Scholar]
- 26.Livesay GA, Fujie H, Kashiwaguchi S, et al. 1995. Determination of the in-situ forces and force distribution within the human anterior cruciate ligament. Ann Biomed Eng 23:467–474. [DOI] [PubMed] [Google Scholar]
- 27.Rudy TW, Livesay GA, SL-Y Woo, et al. 1996. A combined robotic/universal force sensor approach to determine in situ forces of knee ligaments. J Biomech 29:1357–1360. [DOI] [PubMed] [Google Scholar]
- 28.Woo SL-Y, Kanamori A, Zeminski J, et al. 2002. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon—a cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg Am 84A:907–914. [DOI] [PubMed] [Google Scholar]
- 29.Inoue M, McGurk-Burleson E, Hollis JM, et al. 1987. Treatment of the medial collateral ligament injury .1: the importance of anterior cruciate ligament on the varus-valgus knee laxity. Am J Sport Med 15:15–21. [DOI] [PubMed] [Google Scholar]
- 30.Hollis JM, Takai S, Adams DJ, et al. 1991. The effects of knee motion and external loading on the length of the Anterior Cruciate Ligament (Acl)—a kinematic study. J Biomech Eng 113:208–214. [DOI] [PubMed] [Google Scholar]
- 31.Ma CB, Papageogiou CD, Debski RE, et al. 2000. Interaction between the ACL graft and MCL in a combined ACL plus MCL knee injury using a goat model. Acta Orthop Scand 71:387–393. [DOI] [PubMed] [Google Scholar]
- 32.Livesay GA, Rudy TW, SL-Y Woo, et al. 1997. Evaluation of the effect of joint constraints on the in situ force distribution in the anterior cruciate ligament. J Orthop Res 15:278–284. [DOI] [PubMed] [Google Scholar]
- 33.Holden JP, Grood ES, Korvick DL, et al. 1994. In-vivo forces in the anterior cruciate ligament—direct measurements during walking and trotting in a quadruped. J Biomech 27:517–526. [DOI] [PubMed] [Google Scholar]
- 34.Li GA, Rudy TW, Allen C, et al. 1998. Effect of combined axial compressive and anterior tibial loads on in situ forces in the anterior cruciate ligament: a porcine study. J Orthop Res 16:122–127. [DOI] [PubMed] [Google Scholar]
- 35.Daniel DM, Stone ML, Dobson BE, et al. 1994. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med 22:632–644. [DOI] [PubMed] [Google Scholar]
- 36.Papageorgiou CD, Gil JE, Kanamori A, et al. 2001. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sport Med 29:226–231. [DOI] [PubMed] [Google Scholar]
- 37.Allen CR, Wong EK, Livesay GA, et al. 2000. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res 18:109–115. [DOI] [PubMed] [Google Scholar]
- 38.Li G, Kozanek M, Hosseini A, et al. 2009. New fluoroscopic imaging technique for investigation of 6DOF knee kinematics during treadmill gait. J Orthop Surg Res 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers CA, Torry MR, Peterson DS, et al. 2011. Measurements of tibiofemoral kinematics during soft and stiff drop landings using biplane fluoroscopy. Am J Sport Med 39:1714–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuda E, Fukuda Y, Loh JC, et al. 2002. The effect of soft-tissue graft fixation in anterior cruciate ligament reconstruction on graft-tunnel motion under anterior tibial loading. Arthroscopy 18:960–967. [DOI] [PubMed] [Google Scholar]


