Abstract
Nitrate increases the tolerance of plants to hypoxia, although the mechanisms related to this beneficial effect are still unclear. Recently, we observed that cultivation of soybean plants with nitrate reduced hypoxic accumulation of fermentation end products by isolated root segments compared with the ammonium treatment. Interestingly, the same decrease in the intensity of fermentation was detected when ammonium-grown root segments were incubated with nitrite, suggesting the involvement of this anion in the nitrate-mediated modulation of fermentative metabolism. Here we extended these experiments to intact plants subjected to root hypoxia and observed similar effects of nitrate and nitrite in reducing root ethanol production, which indicates the physiological relevance of the in vitro results. In both experimental systems, nitrite stimulated nitric oxide emission by ammonium-grown roots to levels similar to that of nitrate-cultivated ones. The involvement of mitochondrial reduction of nitrite to nitric oxide in the root response to hypoxia is suggested.
Keywords: ammonium, ethanol, fermentation, hypoxia, nitric oxide, nitrate, nitrite
Root hypoxia is a highly stressful condition which may occur as a consequence of soil flooding resulting from excessive rainfall or irrigation usually associated with poor soil drainage.1 The deficiency of oxygen, the terminal acceptor of the mitochondrial respiratory chain, exerts negative effects on the growth and productivity of economically important species, as well as affecting the abundance and distribution of native species in natural environments.2 At the biochemical level, oxygen deficiency leads to inhibition of oxidative phosphorylation followed by the induction of anaerobic processes of ATP production and NAD(P)+ regeneration, such as fermentation pathways.2 Despite the transient induction of lactic acid fermentation which occurs in some plant species at the initial phase of the hypoxic response, ethanol is considered to be the main fermentative end product in nearly all plant roots subjected to hypoxia.3
There is strong experimental evidence, also supported by observations in the field, that the addition of nitrogen in the form of nitrate (NO3-), but not ammonium (NH4+), reduces the deleterious effects of oxygen deficiency in diverse plant species.4-8 A role for NO3- reduction by the nitrate reductase (NR) enzyme in proton consumption and NAD(P)+ regeneration during hypoxia has been suggested,9,10 although there is some controversy about this hypothesis.11,12 Thus, the mechanisms by which NO3- improves plant tolerance to hypoxia are still under debate.
In a recent study,13 we showed that segments of soybean roots isolated from NO3--cultivated plants produced lower amounts of fermentation end products under hypoxia in comparison with those of NH4+-cultivated plants. Interestingly, the same decrease in the intensity of fermentation was detected when root segments from ammonium-grown plants were incubated with nitrite (NO2-), the product of NO3- reduction by NR. The effect of NO2- on fermentation occurred irrespective of the presence of NO3- and of the extremely low NR activity of ammonium-grown root segments. Therefore, the involvement of NO2- in the NO3--mediated modulation of fermentative metabolism was suggested.13
It is noteworthy that our previous study,13 as well as most studies regarding the effect of NO3- and/or NO2- on hypoxic metabolism,11,12,14,15 was performed in vitro, using isolated root segments or coleoptiles as a model system. In order to verify whether our hypothesis of the involvement of NO2- in the NO3--mediated response during hypoxia could have physiological relevance in a whole-plant context, here soybean plants were subjected to root hypoxia and the effect of distinct nitrogen regimes on ethanol production by intact roots was evaluated (Fig. 1A). In agreement with our previous in vitro results, roots from NO3--cultivated plants released significantly lower amounts of ethanol in the nutrient solution under hypoxia when compared with NH4+-cultivated roots. Moreover, the addition of NO2- significantly reduced the intense hypoxic ethanol production by NH4+-cultivated roots. Thus, the role of NO3- in decreasing the alcoholic fermentative metabolism of intact roots and the involvement of NO2- in this mechanism can be suggested. It remains to be established what impact different nitrogen treatments might have on the hypoxic levels of other carbon metabolites, such as sugars and organic acids, not only in roots but also in leaves.
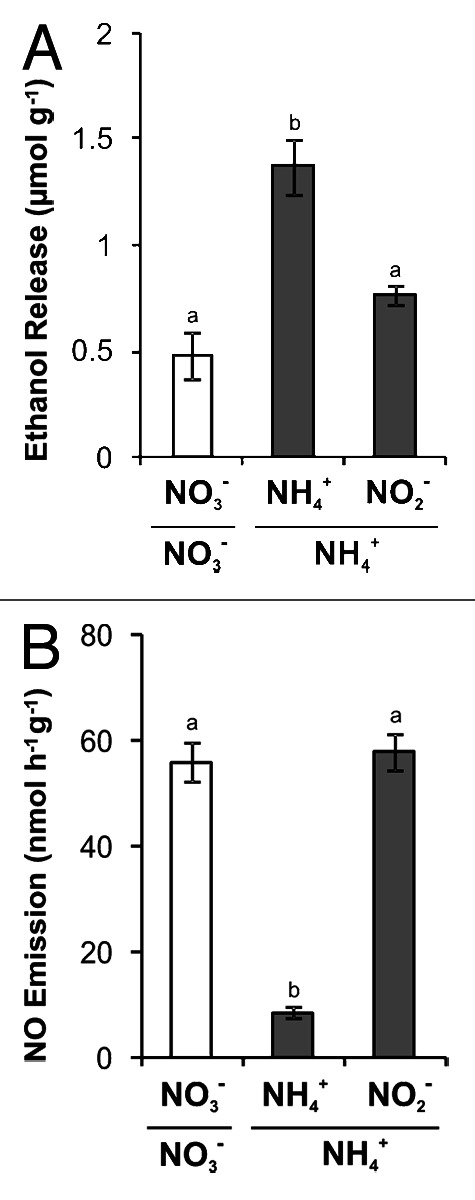
Figure 1. Effect of distinct nitrogen treatments on ethanol production and nitric oxide emission by hypoxic soybean roots. Non-nodulated soybean plants [Glycine max (L.) Merril cv IAC-23] were hydroponically cultivated in a greenhouse under natural conditions of light and temperature (mean min/max: 19°C/36°C). Hoagland and Arnon’s N-free nutrient solution at 1/3 strength was supplemented with 5 mM NO3- or 5 mM NH4+ as the nitrogen source.13 Plants at the V4 stage were transferred to a N2-purged nutrient solution which was then covered with mineral oil in order to maintain root hypoxia. Measurements using an O2 electrode demonstrated that O2 levels in the solution were maintained at less than 5% of saturation. In the case of NO3--cultivated plants (white bars), the hypoxic nutrient solution always contained 5 mM NO3-, whereas NH4+-cultivated plants (gray bars) were treated with 5 mM NH4+ or 1 mM NO2-. (A) Ethanol release in nutrient solution was analyzed enzimatically after 24 h of root hypoxia treatment. (B) Nitric oxide emission by roots submitted to hypoxia for 3 h was determined using the fluorescent probe DAF-2, as described before.13 Data represent mean ± SE (n = 6). Different letters indicate significantly different values according to one-way ANOVA followed by the Tukey test (p < 0.05).
During both in vitro13 and in vivo (Fig. 1B) experimental approaches, it was also determined that hypoxic roots from NO3--cultivated plants emitted much higher levels of nitric oxide (NO) than those from NH4+-cultivated ones, which is consistent with recent data showing that a considerable amount of root NO3- is directed to NO synthesis during oxygen deficiency.16 Furthermore, a clear effect of NO2- in stimulating NO emission by NH4+-grown roots was observed in vitro13 and in vivo (Fig. 1B). Interestingly, NO2--induced NO emission by root segments was prevented in the presence of potassium cyanide, suggesting the involvement of mitochondrial respiratory chain in this mechanism.13 These results are in accordance with the growing evidence concerning the importance of mitochondrial reduction of NO2- in NO synthesis under oxygen deficiency.17
Overall, results shown in Figure 1 indicate a negative correlation between NO emission and ethanol production in intact roots, similar to that observed in our in vitro study.13 Indeed, NO2- reduction to NO by the mitochondrial respiratory chain has been associated with anaerobic ATP production and NAD(P)+ regeneration,18 which may constitute an alternative to fermentation, thus reducing the intensity of this process.13 The relevance of the mechanism of NO synthesis from NO2- in maintaining mitochondrial functionality during this stress condition has been suggested.17,18 Therefore, a striking link between NO3- nutrition, mitochondrial reduction of NO2- and plant response to hypoxia arises from these observations.
Another interesting possibility is a signaling function of the NO produced by roots during hypoxia. Although we showed that NO per se did not directly modulate the fermentative metabolism of soybean root segments,13 it cannot be excluded that NO may act as a mediator of other aspects of plant response to hypoxia, such oxygen sensing19 and hormone signaling.16,20 Given the broad effect of NO on gene regulation,21 the involvement of this radical in the hypoxia-induced transcriptional reprogramming in plant tissues is promising. A NO-mediated control at the post-translational level requires further investigation, since hypoxia has been recently shown to increase the level of S-nitrosylated compounds in Arabidopsis thaliana roots.16
Acknowledgments
This work was supported by grant 303931/2009-4 from the Conselho Nacional de Desenvolvimento Científico e Tecnológico. H.C.O. was supported by a post-doc fellowship (2009/17583-3) from the Fundação de Amparo à Pesquisa do Estado de São Paulo.
Glossary
Abbreviations:
- NH4+
ammonium
- NO
nitric oxide
- NO2-
nitrite
- NO3-
nitrate
- NR
nitrate reductase
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Irfan M, Hayat S, Hayat Q, Afroz S, Ahmad A. . Physiological and biochemical changes in plants under waterlogging. Protoplasma 2010; 241:3 - 17; http://dx.doi.org/ 10.1007/s00709-009-0098-8; PMID: 20066446 [DOI] [PubMed] [Google Scholar]
- 2.Bailey-Serres J, Voesenek LACJ. . Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 2008; 59:313 - 39; http://dx.doi.org/ 10.1146/annurev.arplant.59.032607.092752; PMID: 18444902 [DOI] [PubMed] [Google Scholar]
- 3.Gibbs J, Greenway H. . Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Funct Plant Biol 2003; 30:1 - 47; http://dx.doi.org/ 10.1071/PP98095 [DOI] [PubMed] [Google Scholar]
- 4.Arnon DI. . Ammonium and nitrate nitrogen nutrition of barley at different seasons in relation to hydrogen-ion concentration, manganese, copper, and oxygen supply. Soil Sci 1937; 44:91 - 122; http://dx.doi.org/ 10.1097/00010694-193708000-00001 [DOI] [Google Scholar]
- 5.Malavolta E. . Studies on the nitrogenous nutrition of rice. Plant Physiol 1954; 29:98 - 9; http://dx.doi.org/ 10.1104/pp.29.1.98; PMID: 16654622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trought MCT, Drew MC. . Alleviation of injury to young wheat plants in anaerobic solution cultures in relation to the supply of nitrate and other inorganic nutrients. J Exp Bot 1981; 32:509 - 22; http://dx.doi.org/ 10.1093/jxb/32.3.509 [DOI] [Google Scholar]
- 7.Allègre A, Silvestre J, Morard P, Kallerhoff J, Pinelli E. . Nitrate reductase regulation in tomato roots by exogenous nitrate: a possible role in tolerance to long-term root anoxia. J Exp Bot 2004; 55:2625 - 34; http://dx.doi.org/ 10.1093/jxb/erh258; PMID: 15475378 [DOI] [PubMed] [Google Scholar]
- 8.Thomas AL, Sodek L. . Development of nodulated soybean plant after flooding of the root system with different sources of nitrogen. Braz J Plant Physiol 2005; 17:291 - 7; http://dx.doi.org/ 10.1590/S1677-04202005000300003 [DOI] [Google Scholar]
- 9.Garcia-Novo F, Crawford RMM. . Soil aeration, nitrate reduction and flooding tolerance in higher plants. New Phytol 1973; 72:1031 - 9; http://dx.doi.org/ 10.1111/j.1469-8137.1973.tb02079.x [DOI] [Google Scholar]
- 10.Roberts JKM, Andrade FH, Anderson IC. . Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiol 1985; 77:492 - 4; http://dx.doi.org/ 10.1104/pp.77.2.492; PMID: 16664083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoimenova M, Libourel IGL, Ratcliffe RG, Kaiser WM. . The role of nitrate reduction in the anoxic metabolism of roots I. Characterization of root morphology and normoxic metabolism of wild type tobacco and a transformant lacking root nitrate reductase. Plant Soil 2003; 253:155 - 67; http://dx.doi.org/ 10.1023/A:1024591116697 [DOI] [Google Scholar]
- 12.Libourel IG, van Bodegom PM, Fricker MD, Ratcliffe RG. . Nitrite reduces cytoplasmic acidosis under anoxia. Plant Physiol 2006; 142:1710 - 7; http://dx.doi.org/ 10.1104/pp.106.088898; PMID: 17071644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira HC, Salgado I, Sodek L. . Involvement of nitrite in the nitrate-mediated modulation of fermentative metabolism and nitric oxide production of soybean roots during hypoxia. Planta 2013; 237:255 - 64; http://dx.doi.org/ 10.1007/s00425-012-1773-0; PMID: 23011570 [DOI] [PubMed] [Google Scholar]
- 14.Fan TW, Higashi RM, Lane AN. . An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Arch Biochem Biophys 1988; 266:592 - 606; http://dx.doi.org/ 10.1016/0003-9861(88)90292-5; PMID: 3190244 [DOI] [PubMed] [Google Scholar]
- 15.Fan TWM, Higashi RM, Frenkiel TA, Lane AN. . Anaerobic nitrate and ammonium metabolism in flood-tolerant rice coleoptiles. J Exp Bot 1997; 48:1655 - 66 [Google Scholar]
- 16.Hebelstrup KH, van Zanten M, Mandon J, Voesenek LA, Harren FJ, Cristescu SM, et al. . Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana.. J Exp Bot 2012; 63:5581 - 91; http://dx.doi.org/ 10.1093/jxb/ers210; PMID: 22915746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta KJ, Igamberdiev AU, Manjunatha G, Segu S, Moran JF, Neelawarne B, et al. . The emerging roles of nitric oxide (NO) in plant mitochondria. Plant Sci 2011; 181:520 - 6; http://dx.doi.org/ 10.1016/j.plantsci.2011.03.018; PMID: 21893247 [DOI] [PubMed] [Google Scholar]
- 18.Stoimenova M, Igamberdiev AU, Gupta KJ, Hill RD. . Nitrite-driven anaerobic ATP synthesis in barley and rice root mitochondria. Planta 2007; 226:465 - 74; http://dx.doi.org/ 10.1007/s00425-007-0496-0; PMID: 17333252 [DOI] [PubMed] [Google Scholar]
- 19.Borisjuk L, Rolletschek H. . Nitric oxide is a versatile sensor of low oxygen stress in plants. Plant Signal Behav 2008; 3:391 - 3; http://dx.doi.org/ 10.4161/psb.3.6.5403; PMID: 19704575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igamberdiev AU, Baron K, Manac’h-Little N, Stoimenova M, Hill RD. . The haemoglobin/nitric oxide cycle: involvement in flooding stress and effects on hormone signalling. Ann Bot 2005; 96:557 - 64; http://dx.doi.org/ 10.1093/aob/mci210; PMID: 16027133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grün S, Lindermayr C, Sell S, Durner J. . Nitric oxide and gene regulation in plants. J Exp Bot 2006; 57:507 - 16; http://dx.doi.org/ 10.1093/jxb/erj053; PMID: 16396997 [DOI] [PubMed] [Google Scholar]


