Abstract
Arsenic exposure produces significant hematotoxicity in vitro and in vivo. Our previous work shows that arsenic (in the form of arsenite, AsIII) interacts with the zinc finger domains of GATA-1, inhibiting the function of this critical transcription factor, and resulting in the suppression of erythropoiesis. In addition to GATA-1, GATA-2 also plays a key role in the regulation of hematopoiesis. GATA-1 and GATA-2 have similar zinc finger domains (C4-type) that are structurally favorable for AsIII interactions. Taking this into consideration, we hypothesized that early stages of hematopoietic differentiation that are dependent on the function of GATA-2 may also be disrupted by AsIII exposure. We found that in vitro AsIII exposures disrupt the erythromegakaryocytic lineage commitment and differentiation of erythropoietin-stimulated primary mouse bone marrow hematopoietic progenitor cells (HPCs), producing an aberrant accumulation of cells in early stages of hematopoiesis and subsequent reduction of committed erythro-megakaryocyte progenitor cells. Arsenic significantly accumulated in the GATA-2 protein, causing the loss of zinc, and disruption of GATA-2 function, as measured by chromatin immunoprecipitation and the expression of GATA-2 responsive genes. Our results show that the attenuation of GATA-2 function is an important mechanism contributing to the aberrant lineage commitment and differentiation of early HPCs. Collectively, findings from the present study suggest that the AsIII-induced disruption of erythro-megakaryopoiesis may contribute to the onset and/or exacerbation of hematological disorders, such as anemia.
Keywords: Arsenic, Arsenite (AsIII), Hematopoietic progenitor cells, Erythromegakaryocytic progenitor cells, GATA-2, Zinc finger proteins
1. Introduction
Anemia is a blood disorder that affects over a billion people worldwide (Kassebaum et al., 2014; Koury, 2014; Organization, 2015). Many deleterious health effects are associated with anemia, including fatigue, cognitive and motor impairments, increased susceptibility to infection, heart failure, low birth weights, pre-term births, and risk of maternal and/or neonatal mortality (Balarajan et al., 2011; Kassebaum et al., 2014; Organization, 2015). Although the etiology is complex, there is clear epidemiological evidence supporting the association between arsenic exposure and anemia (Hopenhayn et al., 2006; Heck et al., 2008; Surdu et al., 2015; Kile et al., 2016; Parvez et al., 2017). Despite this understanding, the underlying molecular mechanisms remain to be fully elucidated.
Arsenic is a widespread environmental toxicant commonly found in food and drinking water across the world (Registry, A.F.T.S.A.D, 2007; Organization, 2011; Agency, United States Enivironmental Protection Agency, 2012; Registry, A.F.T.S.A.D, 2016). As a result, many people are exposed to arsenic in drinking water at levels near or exceeding the maximum contaminant limit of 10 μg/L (ppb) (Registry, A.F.T.S.A.D, 2007; Organization, 2011; Agency, United States Enivironmental Protection Agency, 2012; Naujokas et al., 2013; Registry, A.F.T.S.A.D, 2016). Previous work from our lab has shown that arsenic exposures (in the form of arsenite, AsIII), at environmentally relevant doses, can cause anemia in mice via the inhibition of red blood cell (RBC) production in the bone marrow (Medina et al., 2017; Zhou et al., 2020). We identified that very early erythropoietin (EPO)-dependent stages of RBC development (i.e., BFU-E, CFU-E, and proerythroblasts) are highly sensitive to AsIII exposures (Medina et al., 2017; Zhou et al., 2020; Medina et al., 2021a; Wan et al., 2021). AsIII was found to directly interact with GATA-1, a zinc finger transcription factor and master regulator of erythropoiesis, resulting in zinc loss and impaired DNA binding activity (Zhou et al., 2020; Medina et al., 2021b). This loss of GATA-1 regulation was found to substantially contribute to the observed suppression of erythropoiesis (Medina et al., 2017; Zhou et al., 2020; Medina et al., 2021a; Wan et al., 2021).
Hematopoiesis is the process by which multipotent hematopoietic stem cells (HSCs) give rise to all major cell lineages of the blood, including lymphocytes, myeloid cells, monocytes, megakaryocytes, and RBCs (Metcalf, 2007; Rieger and Schroeder, 2012). One path of HSC lineage commitment is to the multipotent common myeloid progenitor (CMP) stage, which subsequently gives rise to myeloid cells, monocytes, megakaryocytes, and RBCs (Metcalf, 2007; Seita and Weissman, 2010; Rieger and Schroeder, 2012). The lineage commitment fate of CMPs is a topic of debate; however, a current hypothesis is that the fate of these cells is intrinsically regulated through the expression and subsequent functional antagonism of major transcriptional regulators of erythro-megakaryopoiesis and myelopoiesis; i.e., GATA-2, GATA-1, and PU.1, respectively (Rekhtman et al., 1999; Nerlov et al., 2000; Zhang et al., 2000).
GATA-2 is a zinc finger transcription factor that has a critical role in regulating the proliferation and expansion of HSCs and during the lineage commitment of hematopoietic progenitor cells (HPCs) (Tsai and Orkin, 1997; Ferreira et al., 2005; Vicente et al., 2012). In particular, GATA-2 orchestrates the expression of many essential transcription factors, including GATA-1, PBX1, RHAG1, and MLLT3, whose collective functions govern the activation or suppression of many target genes that support the generation, self-renewal, or survival of HPCs (Igarashi et al., 2002; Orkin and Zon, 2008; Gao et al., 2015; Calvanese et al., 2019). The activity of GATA-2 further facilitates the erythromegakaryocytic lineage commitment of HPCs through the induction of many essential genes including, Gata-1, promotion of GATA factor switching, and by repressing the function of PU.1, the myeloid specific transcription factor (Fujiwara et al., 2009; Bresnick et al., 2010; Vicente et al., 2012; Suzuki et al., 2013).
Taking into account that GATA-2 and GATA-1 have similar zinc finger domains, which contain C4-type zinc finger motifs known to be structurally favorable for interactions with AsIII (Ferreira et al., 2005; Zhou et al., 2011; Zhou et al., 2014; Zhou et al., 2020), we hypothesized that the inhibition of GATA-2 activity in early HPCs may be an important mechanism of AsIII-induced hematotoxicity. Results from the present study highlight demonstrate that AsIII disrupts erythro-megakaryopoiesis via the attenuation of GATA-2 DNA binding activity, which may contribute to the onset and/or exacerbation of blood disorders, such as anemia.
2. Methods
2.1. Chemicals and reagents
Sodium meta-AsIII (NaAsO2, ≥95% purity, CAS 774–46-5, Cat. No. S7400) was purchased from Sigma Aldrich (St. Louis, MO). Refer to Supplementary Materials and Methods for a complete list of reagents used in this study.
Stock solutions of AsIII were prepared immediately prior to use in experiments using cell culture grade water and culture medium. The high dose of AsIII used in this study, 500 nM (37.5 ppb), is above the United States Environmental Protection Agency and World Health Organization drinking water standard of 10 ppb, but is within the range of arsenic exposures that many human populations around the world experience in their drinking water (Hopenhayn et al., 2006; Heck et al., 2008; Naujokas et al., 2013; Surdu et al., 2015; Kile et al., 2016; Parvez et al., 2017).
2.2. Primary mouse bone marrow cell isolation
All experiments were performed in accordance with protocols approved by the Institutional Animal Use and Care Committee at the University of New Mexico Health Sciences Center (UNM HSC). Male C57BL/6 J mice (11 weeks of age) were purchased from Jackson Laboratory (Bar Harbor, ME) and acclimated in the UNM HSC animal facility for one week prior to the onset of experiments.
Bone marrow cells were isolated from the femurs and tibias of each mouse as described by (Ezeh et al., 2016). Cells were resuspended in Isocove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% heat-inactivated fetal bovine serum (HI FBS), 2 mM l-glutamine, 100 mg/ml streptomycin, and 100 units/mL penicillin then pooled together and used for the isolation of HPCs. The concentration and viability of isolated cells was measured using acridine orange/propidium iodide (AO/PI) staining and a Nexcelom Cellometer Auto 2000.
2.3. Hematopoietic progenitor cell isolation and in vitro erythropoiesis model
HPCs were purified from whole bone marrow using the EasySep™ Mouse HPC Isolation Kit according to the manufacturer’s instructions and as previously described (Zhou et al., 2020; Medina et al., 2021a). In brief, 1 × 108 bone marrow cells/mL were stained in EasySep™ Buffer (DPBS without calcium and magnesium (DPBS−), 2% HI FBS, and 1 mM EDTA) with a combination of biotinylated lineage specific antibodies (i. e., CD5, CD11b, CD19, CD45R/B220, Ly6G/C (Gr-1), and TER119) for 15 min at 4 °C. These lineage positive cells were then removed from the bone marrow cell mixture using streptavidin-coated magnetic particles and a BigEasy Easy Sep™ magnet (STEMCELL Technologies, Cambridge, MA).
Isolated HPCs (~ > 75% Lin−, >45% cKit+) were used in an in vitro model of erythropoiesis as described previously (Shuga et al., 2007; Zhou et al., 2020; Medina et al., 2021a). In brief, HPCs were cultured in Serum Free StemSpan Hematopoietic Progenitor Expansion medium (STEMCELL Technologies, Cambridge, MA) supplemented with 5 IU/mL human recombinant erythropoietin (EPO) (31.25 ng/mL) and 100 ng/mL murine stem cell factor (SCF) to promote erythroid lineage commitment and differentiation (Shuga et al., 2007; Zhou et al., 2020; Medina et al., 2021a).
2.4. Flow cytometry
Erythromegakaryocytic and myeloid progenitor subsets were assessed based on cell surface marker phenotype as detailed by (Pronk and Bryder, 2011; Grover et al., 2014; Zhou et al., 2020; Medina et al., 2021a) and as depicted in Supplementary Fig. S1: CMP (Lin−, cKit+, SCA-1−, CD16/32−, CD34+); megakaryocyte-erythroid progenitor (MEP), Lin−, cKit+, SCA-1−, CD16/32−, CD34−), pre-granulocyte macrophage progenitor (Pre-GM), Lin−, cKit+, SCA-1−, CD16/32−, CD150−, CD105−); granulocyte macrophage progenitor (GMP), Lin−, cKit+, SCA-1−, CD16/32+, CD150−). Analysis of cell surface marker phenotype was conducted as described by (Zhou et al., 2020; Medina et al., 2021a). At least 1 × 106 cells were stained in 100 μL BD Horizon Brilliant Stain Buffer with 0.5 μg of the following monoclonal antibodies (all from BD Biosciences): cKit-APC-R700, SCA-1-BV605, CD34-PE-Cy7, CD16/32-BV510, CD150-BV421, and CD105-BB515. Samples were analyzed using a BD LSRFortessa flow cytometer. Fluorescence compensation was performed using AbC Total Antibody Compensation Beads (ThermoFisher Scientific, Waltham, MA), and gating was performed with the aid of fluorescence-minus-one controls.
2.5. RNA isolation and quantitative real-time PCR
RNA was isolated using the QIAshredder and RNeasy kit according to manufacturer’s instructions (Qiagen, Germantown, MD). The concentration and purity of RNA was measured using an Agilent Nanodrop spectrophotometer (Santa Clara, California). Total RNA (1.5 μg) was utilized for cDNA synthesis using the High-Capacity Reverse Transcription Kit (ThermoFisher Scientific, Waltham, MA). cDNA was diluted 1:10 (v/v) in RNase/DNase free water and stored at −80 °C prior to use in quantitative real-time PCR (qPCR). qPCR reactions were performed in technical replicates of three per sample using TaqMan Gene Expression Master Mix with TaqMan gene expression probes for Gapdh (Mm99999915_g1), Gata-2 (Mm00492300_m1), Pbx1 (Mm01701536_m1), Rhag1 (Mm00488027_m1), Runx1 (Mm01213404_m1), Cd34 (Mm00519283_m1), Bmp4 (Mm00432087_m1), Klf1 (Mm00516096_m1), and Tal1 (Mm01187033_m1). Samples were analyzed using a BioRad CFX384 Touch Real-Time PCR Detection System (BioRad, Hercules, CA). Relative expression was calculated using the delta-delta CT method using Gapdh as an endogenous control.
2.6. Immunoprecipitation
Following AsIII exposures, GATA-2 protein was purified from whole cell lysates using the Pierce™ Crosslink Magnetic Immunoprecipitation (IP)/Co-Immunoprecipitation Kit (ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions or as described previously (Zhou et al., 2011; Zhou et al., 2020). GATA-2 was purified from 150 μg of total protein using 3 μg of anti-GATA2 antibody (ab109241) or a concentration and isotype matched control antibody (ab172730). Following IP, GATA-2 or IgG were eluted from the beads using a nondenaturing method and stored at −80 °C prior to preparation for inductively coupled plasma-mass spectrometry (ICP-MS).
2.7. Chromatin Immunoprecipitation-qPCR
The DNA binding activity of GATA-2 was measured using the Chromatin Immunoprecipitation (ChIP)-IT High Sensitivity®, ChIP-IT Control, and ChIP-IT® Control qPCR Kits (Active Motif, Carlsbad, CA) following the manufacturer’s instructions or with slight modifications as described by (Zhou et al., 2020). Chromatin was fragmented using a micro ultrasonic cell disrupter (Kontes, Vineland, NJ) to approximately 200–1000 bp. Size of fragmented chromatin was verified using a 1.5% (w/v) agarose gel. Immunoprecipitation was performed using 7 μg of fragmented chromatin and 3 μg of recombinant anti-GATA2 antibody (ab109241), normal mouse IgG (2 μg; Active Motif, Carlsbad, CA), or RNA Polymerase II + bridging antibody (2 μg each; Active Motif, Carlsbad, CA). ChIP assays were verified to meet or exceed the quality control standards established by Active Motif for the ChIP-IT High Sensitivity® Kit prior to performing qPCR.
Following ChIP, purified DNA was used to assess the enrichment of GATA-2 binding at four well established GATA-2 binding sites (Grass et al., 2003; Yu et al., 2009; Suzuki et al., 2013) using the ChIP-IT® qPCR Analysis Kit (Active Motif, Carlsbad, CA). Primer sequences used for ChIP-qPCR were obtained from Grass et al., 2003 and Yu et al., 2009 and are listed in Supplementary Table S1. To validate that these genomic regions were suitable for the evaluation of GATA-2 DNA binding activity, sequences were cross referenced to available ChIP-sequencing data from the University of California Santa Cruz Genomics Institute Encyclopedia of DNA Elements (https://genome.ucsc.edu/ENCODE/index.html).
The amplification efficiency of all primer sets (i.e., enhancer region −2.8 kb upstream of the Gata-2 promoter (G2P −2.8 kb), Pbx1, Rhag1, and Mllt3) were assessed and verified to be ~100%. In accordance with the ChIP-IT® qPCR Analysis Kit (Active Motif, Carlsbad, CA), universal negative control primers (Gapdh2) were also used for qPCR and subsequent data analysis. qPCR was performed using SsoAdvanced Universal SYBR Green Supermix (BioRad. Hercules, CA) and a BioRad CFX384 Touch Real-Time PCR Detection System (BioRad, Hercules, CA). Data are expressed as binding events per 1000 cells, which can be converted to percent input by dividing the values by 1000.
2.8. Inductively coupled plasma-mass spectrometry
Arsenic and zinc content in immunoprecipitated GATA-2 was measured as described by (Zhou et al., 2011; Zhou et al., 2020). Briefly, immunoprecipitated GATA-2 protein was digested in 70% trace metal grade nitric acid prior to analysis of total arsenic and zinc content using a PerkinElmer NexION 300D Inductively coupled plasma-mass spectrometry (ICP-MS) (Perkin Elmer, Waltham, MA). Experimental samples were analyzed in conjunction with numerous quality control measures, including internal standards, blank samples, and standard curves for data quantification, validation, and verification.
2.9. Statistics
Data were analyzed with GraphPad Prism 9.4.0. Flow cytometry data were analyzed using FlowJo version 10 (FlowJo LLC). Statistically relevant changes between untreated control and AsIII exposed groups were determined using a one-way ANOVA followed by Tukey’s post hoc test or a two-tailed Student’s t-test at a significance level of p < 0.05. All experiments were performed by preparing samples in at least triplicate for each assay with two to three independent experiments performed and consistent results attained. ANOVA and two-tailed Student’s t-test results are summarized in Supplementary Table S2.
3. Results
3.1. Erythro-megakaryocytic lineage commitment of HPCs is disrupted by AsIII exposure
To determine the effects of AsIII on the erythromegakaryocytic lineage commitment and differentiation of HPCs, we utilized primary mouse bone marrow HPCs stimulated with EPO and SCF to promote erythroid lineage commitment and differentiation (Shuga et al., 2007). Erythromegakaryocytic differentiation of HPCs was assessed following 48 h exposure to 0, 100, or 500 nM AsIII based on cell surface marker phenotype using multi-parameter flow cytometry (Pronk and Bryder, 2011; Grover et al., 2014; Zhou et al., 2020).
A significant, dose dependent accumulation of CMPs was observed following 48 h exposure to 100 nM (~14% increase; p = 0.0484) or 500 nM (~41% increase; p < 0.0001) AsIII (Fig. 1A and Supplementary Fig. S1). The accumulation of CMPs was accompanied by a significant reduction of MEPs following exposure to 100 or 500 nM AsIII (p = 0.0397 or p < 0.0001, respectively), suggesting that AsIII suppressed the erythromegakaryocytic differentiation of EPO-stimulated HPCs (Fig. 1B and Supplementary Fig. S1). Intriguingly, a significant increase in early pre-GM cells (p = 0.0031) as well as GMPs (p = 0.0157) was also identified following exposure to 500 nM AsIII (Fig. 1C and Supplementary Fig. S1). Collectively, these results suggest that AsIII compromises the lineage commitment fate of CMPs, causing a shift from erythromegakaryocytic to myeloid cell lineages (Fig. 1A–C and Supplementary Fig. S1).
Fig. 1.
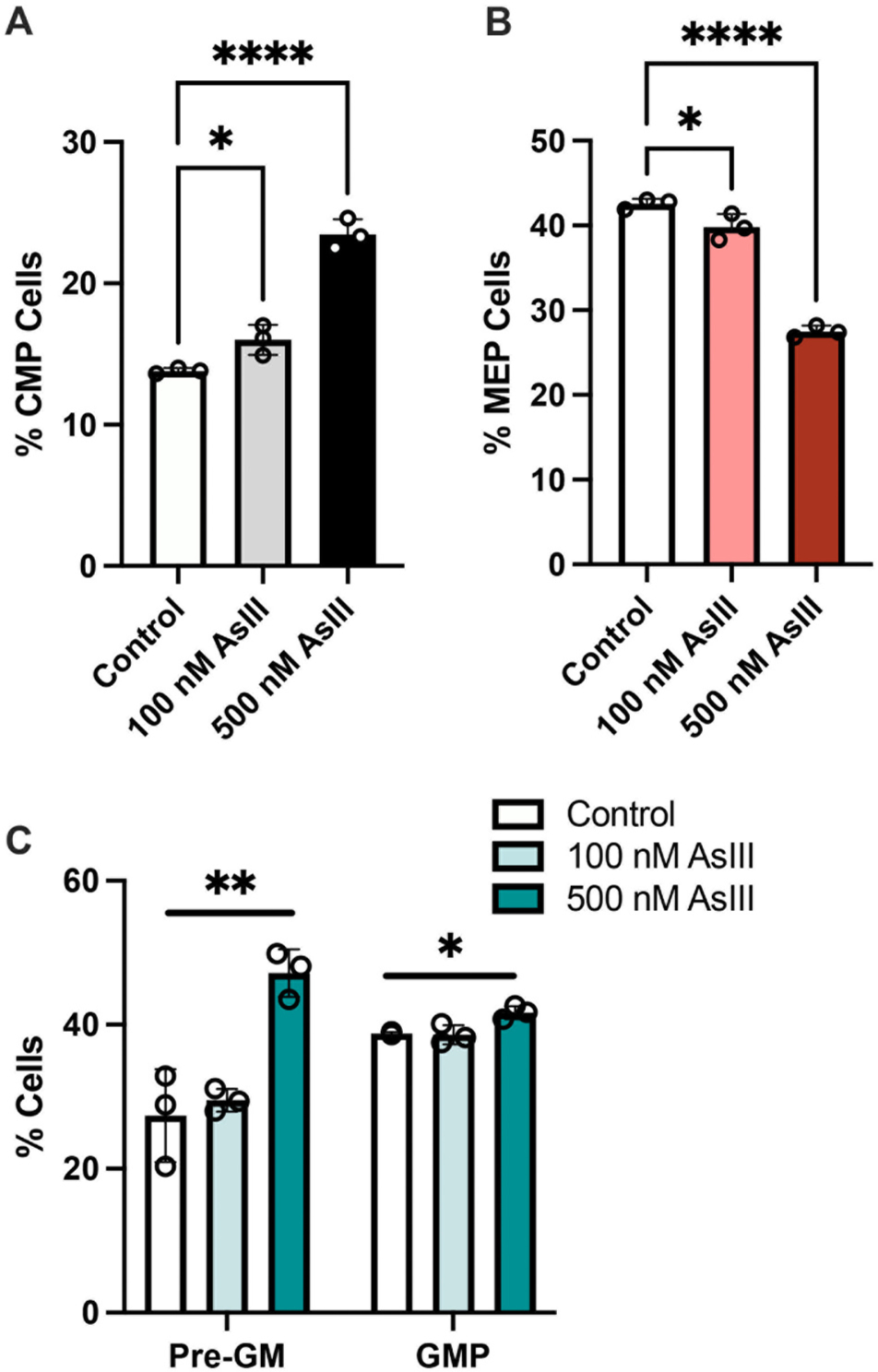
AsIII impairs the erythro-megakaryocytic lineage commitment of early HPCs. (A) Percentages of surface marker defined common myeloid progenitors (CMP) and (B) erythro-megakaryocytic progenitors (MEP), and (C) myeloid progenitors (Pre-GM and GMP) after 48 h exposure of HPCs to 0, 100, or 500 nM AsIII. Data are expressed as mean ± SD. Statistically significant differences compared to untreated control in one-way ANOVA followed by Tukey’s post hoc test (n = 3/group, *p < 0.05, **p < 0.01, ****p < 0.0001).
3.2. AsIII binds to the GATA-2 protein causing the displacement of zinc
Our previous work shows that AsIII interacts with the zinc finger domains of GATA-1, inhibiting the function of this critical transcription factor and resulting in the suppression of erythropoiesis (Zhou et al., 2020).Taking into account that GATA-2 and GATA-1 have similar zinc finger domains, which contain zinc finger motifs (C4-type) that are structurally favorable for AsIII interactions (Zhou et al., 2011; Zhou et al., 2014), we hypothesized that AsIII-induced zinc finger disruption may also be an important contributing molecular mechanism to the impairment of GATA-2 function and loss of erythromegakaryocytic differentiation of EPO-stimulated HPCs (Fig. 1).
To determine whether the disruption of erythromegakaryocytic differentiation was mediated by AsIII interactions with the zinc finger motifs of GATA-2, we immunoprecipitated GATA-2 from cell lysates and measured the arsenic and zinc content in the purified protein (i.e., GATA-2 or non-specific IgG) by ICP-MS. A significant increase in relative arsenic content (p = 0.0012; Fig. 2A), along with a corresponding decrease in relative zinc content (p = 0.0308; Fig. 2B) was found in the GATA-2 protein following exposure to 500 nM AsIII. These results suggest that AsIII binds to the GATA-2 protein causing the displacement of zinc, likely via interactions with the C4 zinc finger motifs.
Fig. 2.
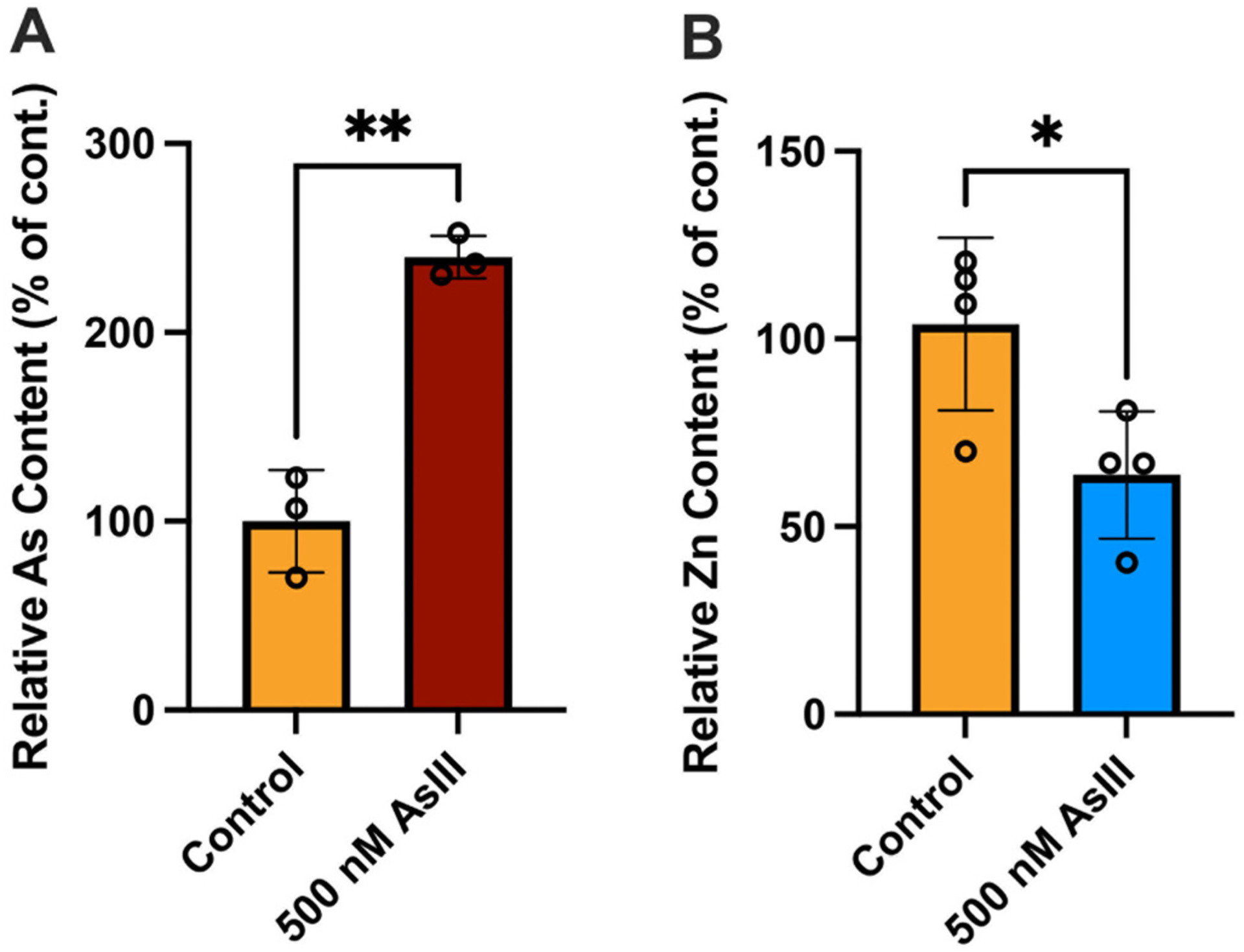
AsIII binds to and displaces zinc from the GATA-2 protein. Primary mouse bone marrow HPCs were treated with 0 or 500 nM AsIII for 48 h. GATA-2 was purified from whole cell lysates by IP with a specific GATA-2 antibody. Following IP, and arsenic and zinc content in purified GATA-2 was measured by ICP-MS. (A) Arsenic (As) binds to the GATA-2 protein. (B) Loss of zinc (Zn) from the GATA-2 protein following AsIII exposure. Data were normalized relative to the control group and expressed as mean ± SD, n = 3–4/group, *p < 0.05, **p < 0.01 in Student’s t-test compared to untreated control.
3.3. GATA-2 DNA binding activity and the expression of GATA-2-regulated genes is attenuated by AsIII exposure
Taking into consideration that AsIII significantly binds to, and displaces zinc from the GATA-2 transcription factor, along with the importance of GATA-2 in regulating genes essential for the normal lineage commitment and subsequent erythromegakaryocytic differentiation of HPCs (Tsai et al., 1994; Ferreira et al., 2005; Fujiwara et al., 2009; Vicente et al., 2012; Suzuki et al., 2013), we evaluated whether AsIII disrupts the DNA binding activity of GATA-2 using ChIP-qPCR. GATA-2 DNA binding was significantly suppressed following AsIII exposure at several well-established genomic loci (Grass et al., 2003; Yu et al., 2009; Suzuki et al., 2013), including an enhancer region −2.8 kb upstream of the G2P (p < 0.0001) and regions in the Pbx1 (p = 0.0045), Rhag1 (p = 0.0402), and Mllt3 (p = 0.0051) promoters (Fig. 3A–D). To verify the reduction of GATA-2 DNA binding activity was resultant of functional and not expression level changes, we evaluated GATA-2 protein levels in EPO-stimulated HPCs following AsIII exposure. Exposure to 500 nM AsIII did not significantly modify GATA-2 protein levels (Supplementary Fig. S2).
Fig. 3.
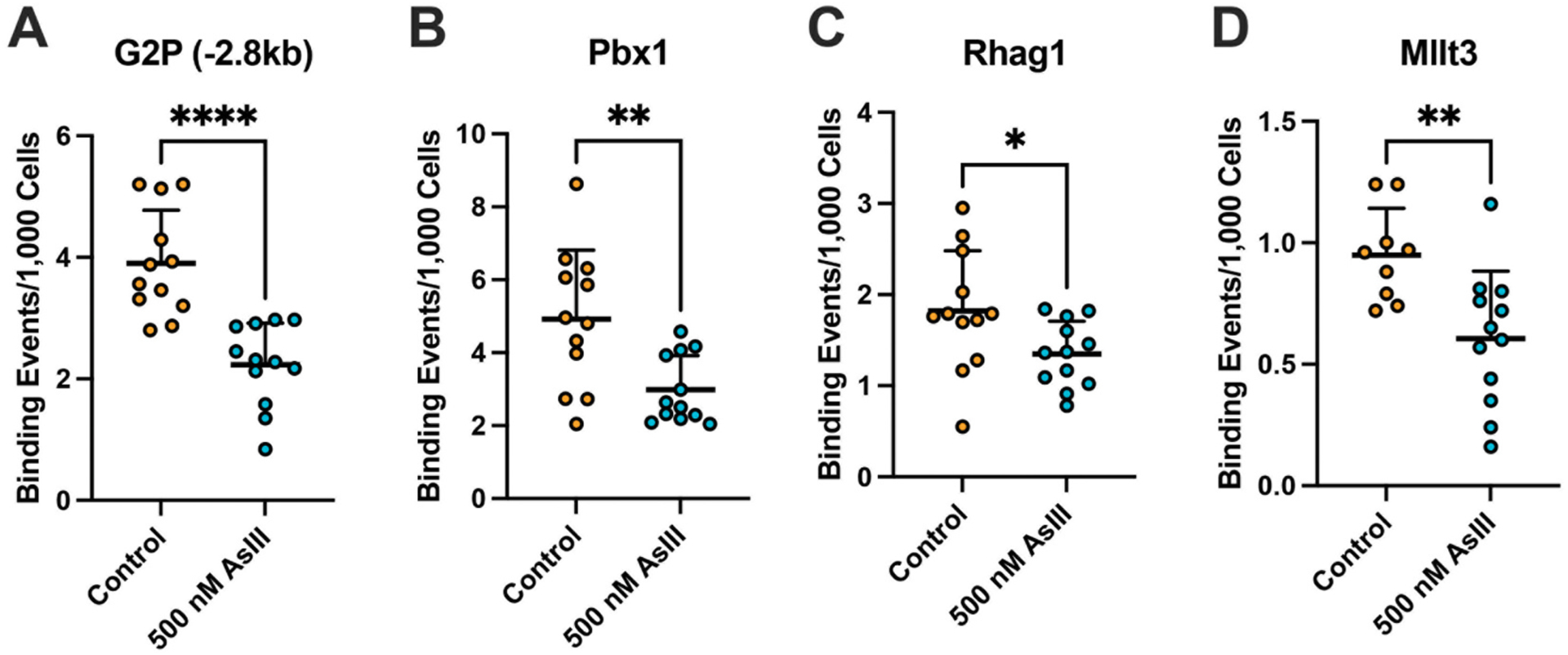
AsIII impairs the DNA binding activity of GATA-2. Primary mouse bone marrow HPCs stimulated with EPO and SCF to undergo erythroid lineage commitment and differentiation were treated with 0 or 500 nM AsIII for 48 h. Following AsIII exposure, GATA-2 DNA binding activity was measured by ChIP-qPCR at four known GATA-2 regulatory sites: (A) −2 kb upstream of GATA-2 promoter (−2.8 kb), (B) Pbx1, (C) Rhag1, and (D) Mllt3. Data are expressed as mean ± SD, n = 12/group, *p < 0.05, **p < 0.01 in Student’s t-test compared to untreated control.
To investigate the downstream ramifications of the loss of GATA-2 DNA binding activity, we evaluated the expression of several GATA-2-regulated genes critical for the erythromegakaryocytic lineage commitment and subsequent differentiation of EPO-stimulated HPCs after 500 nM AsIII exposure, including Gata-2 (p = 0.0018), Gata-1 (p = 0.0185), Pbx1 (p = 0.0396), Rhag1 (p = 0.0061), Runx1 (p = 0.0310), Cd34 (p = 0.0209), Bmp4 (p = 0.0123), Klf1 (p = 0.0059), and Tal1 (p = 0.0439) (Fig. 4A–I).
Fig. 4.
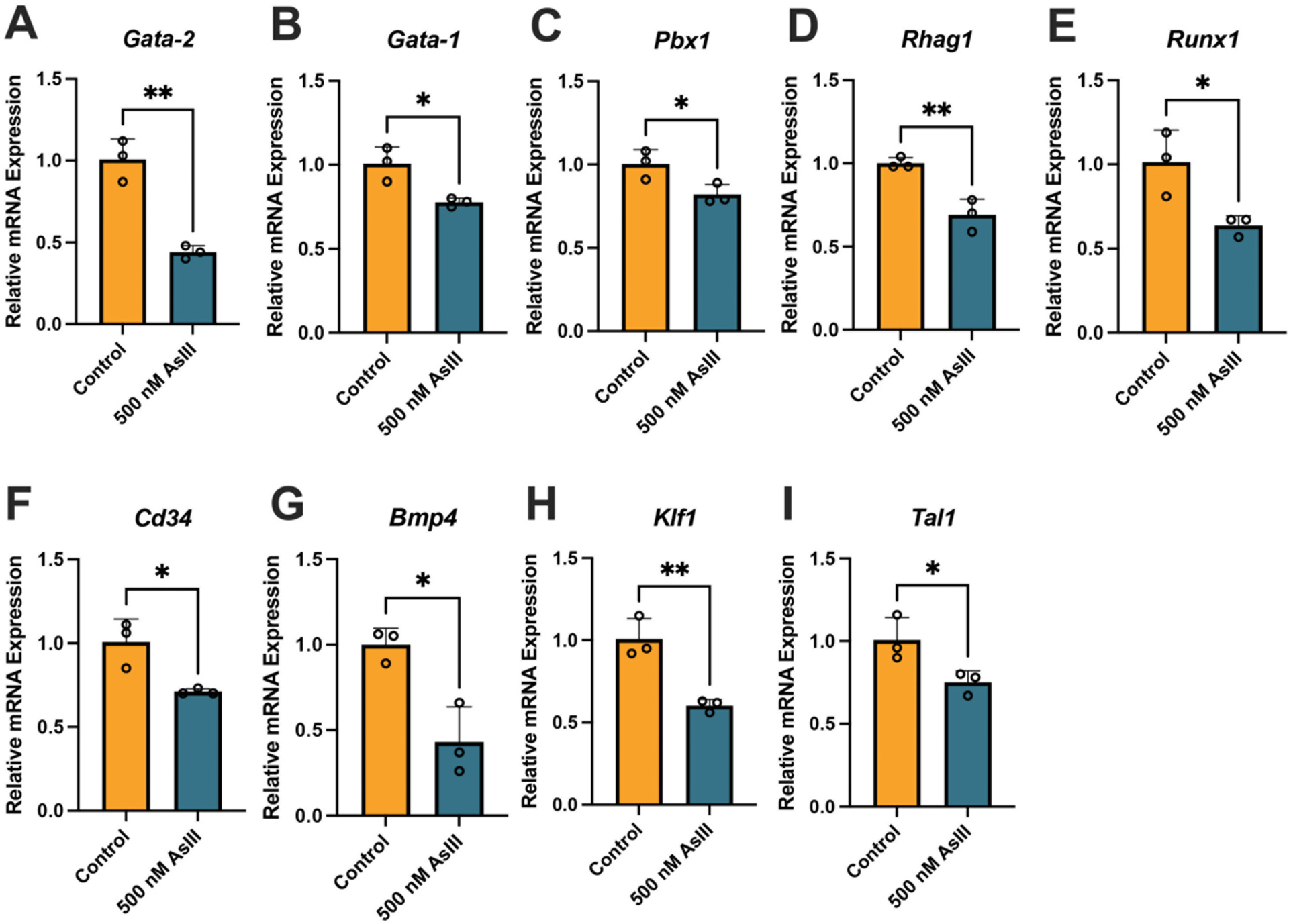
AsIII suppresses the expression of GATA-2-regulated genes. Primary mouse bone marrow HPCs stimulated with EPO and SCF to undergo erythroid lineage commitment and differentiation were treated in vitro with 0 or 500 nM AsIII for 48 h and the expression of GATA-2-responsive genes was measured using qPCR. Relative expression of GATA-2-regulated genes (normalized to Gapdh) (A) Gata-2, (B) Gata-1, (C) Pbx1, (D) Rhag1, (E) Runx1, (F) Cd34, (G) Bmp4, (H) Klf1, (I) Tal1. Data are expressed as mean ± SD. Statistically significant differences compared to untreated control in in Student’s t-test compared to untreated control (n = 3/group; *p < 0.05, **p < 0.01).
Taken together, these results suggest that AsIII disrupts the DNA binding activity of GATA-2, resulting in the reduced expression of multiple GATA-2-responsive genes important for the erythromegakaryocytic differentiation of CMPs.
4. Discussion
The regulation of hematopoiesis is complex and relies on both extrinsic and intrinsic regulation to ensure the successful production of millions of blood cells everyday including, immune and RBCs (Orkin and Zon, 2008; Rieger and Schroeder, 2012). GATA-2 has a critical role in the intrinsic regulation of erythromegakaryocytic lineage commitment and subsequent differentiation of HPCs (Tsai et al., 1994; Tsai and Orkin, 1997; Ferreira et al., 2005; Fujiwara et al., 2009; Vicente et al., 2012; Suzuki et al., 2013). Of central importance in the maintenance of normal hematopoiesis, is the GATA-2-regulated induction of GATA-1 expression, which is essential in promoting the transition from CMP to subsequent stages of erythromegakaryocytic differentiation (Fujiwara et al., 2009; Vicente et al., 2012; Suzuki et al., 2013).
The interaction between AsIII with zinc binding proteins is well documented and has been demonstrated across a variety of cell types and zinc finger proteins (Hartwig et al., 2002; Zhang et al., 2010; Zhou et al., 2011; Sun et al., 2014; Zhou et al., 2014; Zhou et al., 2015; Banerjee et al., 2020; Zhou et al., 2020; Vergara-Geronimo et al., 2021). Zinc finger proteins containing ≥3 cysteine residues are well established targets of AsIII toxicity (Zhou et al., 2011; Zhou et al., 2014; Zhou et al., 2020). We previously reported in committed erythroid progenitor cells that GATA-1 is also a target to AsIII-induced zinc finger disruption (Zhou et al., 2020; Medina et al., 2021a). GATA-2 and GATA-1 have similar zinc finger domains, containing C4-type zinc finger motifs known to be structurally favorable for molecular interactions with AsIII (Zhou et al., 2011; Zhou et al., 2014; Zhou et al., 2020). Similar to GATA-1, AsIII was also found to bind with the GATA-2 protein, causing the displacement of zinc, and attenuation of GATA-2 DNA binding activity.
We found that AsIII exposure resulted in the aberrant accumulation of CMPs, which failed to properly transition to later stages of erythromegakaryocytic differentiation, even in the presence of direct extrinsic stimulation by EPO. Loss of GATA-2-regulated signals, including the induction of GATA-1, and other genes whose actions are essential for commitment to erythromegakaryocytic differentiation, is likely responsible for the accumulation of cells at the CMP stage. Interestingly, a study by Menendez-Gonzalez et al., showed that loss of GATA-2 enhances myeloid differentiation, while simultaneously impairing the differentiation of erythro-megakaryocytic cell lineages (Menendez-Gonzalez et al., 2019). Although this was not a focus of the aforementioned study, it is possible that the enhanced differentiation of myeloid progenitors observed in the present study was resultant from the lack of GATA-2 regulation in the repression of PU.1 during the lineage commitment of early HPCs. The impacts of AsIII exposure on the balance between GATA-2/1 and PU.1 in CMPs and associated implications for HPC differentiation will be the focus of future experiments.
During the normal erythromegakaryocytic lineage commitment and differentiation of HPCs, GATA-2 induces the expression of GATA-1 and subsequently works in cooperation with GATA-1, to regulate the expression of many genes important for erythro-megakaryopoiesis (Ferreira et al., 2005; Hattangadi et al., 2011; Dzierzak and Philipsen, 2013). This regulation involves the selective recognition and replacement of GATA-2 by GATA-1 at multiple genomic loci, termed GATA-switching sites (Bresnick et al., 2010; Suzuki et al., 2013). Several of the GATA-2 binding sites investigated in the present study occur at GATA-switching sites (Bresnick et al., 2010; Suzuki et al., 2013), including G2P −2.8 kb, Rhag1, and Mllt3. Switching from GATA-2- to GATA-1-mediated regulation of gene expression is a critical event in the lineage commitment and differentiation of erythromegakaryocytic progenitor cells (Bresnick et al., 2010; Suzuki et al., 2013). Based on our previous findings that GATA-1 function is suppressed by AsIII exposure, combined with the current results showing similar disruptions to GATA-2, suggests that impairment of GATA-switching may be an important link between the aberrant erythromegakaryocytic lineage commitment of HPCs and the inhibition of erythropoiesis observed in our previous studies (Medina et al., 2017; Zhou et al., 2020; Medina et al., 2021a; Wan et al., 2021).
The present study provides novel support for the sensitivity of zinc finger proteins to AsIII-induced toxicity, and further underscores the diversity of critical regulatory proteins impacted and the diverse biological ramifications of such molecular interactions. This study provides evidence that AsIII can influence the erythromegakaryocytic lineage commitment of early HPCs by disrupting the DNA binding activity of GATA-2. Our findings highlight a mechanism by which AsIII disrupts GATA-2, providing useful information for the design of future intervention strategies. Collectively, findings from the present study demonstrate that the AsIII-induced disruption of erythro-megakaryopoiesis may have a role in the development and/or exacerbation of hematopoietic disorders, such as anemia.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Environmental Health Sciences (NIEHS) Grant Numbers RO1 ES029369, R01 ES029369-03S1; NIEHS and UNM METALS Superfund Research Program Grant Number P42 ES025589; UNM Center for Metals in Biology and Medicine (CMBM) through National Institutes of Health (NIH), National Institute of General Medical Sciences (NIGMS) Grant Number P20 GM130422; Institutional Development Award (IDeA) through the NIGMS of the NIH Grant Number P20 GM103451; and the National Science Foundation Louis Stokes Alliance for Minority Participation, Undergraduate Research Scholars Program Grant Number 23680-23680-160.
The authors would also like to extend thanks and appreciation to Dr. Rui Lui from the UNM CMBM, Integrated Molecular Analysis Core and Dr. Abdul Mehdi S. Ali from the Department of Earth and Planetary Sciences, UNM Analytical Chemistry Laboratory for their assistance and expertise with ICP-MS sample preparation and analyses.
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2022.116193.
Data availability
Data will be made available on request.
References
- Agency, United States Enivironmental Protection Agency, 2012. 2012. Edition of the Drinking Water Standards and Health Advisories Table
- Balarajan Y, Ramakrishnan U, Ozaltin E, Shankar AH, Subramanian SV, 2011. Anaemia in low-income and middle-income countries. Lancet 378, 2123–2135. [DOI] [PubMed] [Google Scholar]
- Banerjee M, Ferragut Cardoso AP, Lykoudi A, Wilkey DW, Pan J, Watson WH, Garbett NC, Rai SN, Merchant ML, States JC, 2020. Arsenite exposure displaces zinc from ZRANB2 leading to altered splicing. Chem. Res. Toxicol 33, 1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S, 2010. GATA switches as developmental drivers. J. Biol. Chem 285, 31087–31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvanese V, Nguyen AT, Bolan TJ, Vavilina A, Su T, Lee LK, Wang Y, Lay FD, Magnusson M, Crooks GM, Kurdistani SK, Mikkola HKA, 2019. MLLT3 governs human haematopoietic stem-cell self-renewal and engraftment. Nature 576, 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E, Philipsen S, 2013. Erythropoiesis: development and differentiation. Cold Spring Harb. Perspect Med 3, a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeh PC, Xu H, Wang SC, Medina S, Burchiel SW, 2016. Evaluation of toxicity in mouse bone marrow progenitor cells. Curr. Protoc. Toxicol 67, 18–19, 11–18 19 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R, Ohneda K, Yamamoto M, Philipsen S, 2005. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol. Cell. Biol 25, 1215–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, O’Geen H, Keles S, Blahnik K, Linnemann AK, Kang YA, Choi K, Farnham PJ, Bresnick EH, 2009. Discovering hematopoietic mechanisms through genome-wide analysis of GATA Factor chromatin occupancy. Mol. Cell 36, 667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Chen YH, Peterson LC, 2015. GATA family transcriptional factors: emerging suspects in hematologic disorders. Exp. Hematol. Oncol 4 (28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH, 2003. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. U. S. A 100, 8811–8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A, Mancini E, Moore S, Mead AJ, Atkinson D, Rasmussen KD, O’Carroll D, Jacobsen SE, Nerlov C, 2014. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J. Exp. Med 211, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig A, Asmuss M, Ehleben I, Herzer U, Kostelac D, Pelzer A, Schwerdtle T, Burkle A, 2002. Interference by toxic metal ions with DNA repair processes and cell cycle control: molecular mechanisms. Environ. Health Perspect 110 (Suppl. 5), 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattangadi SM, Wong P, Zhang L, Flygare J, Lodish HF, 2011. From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood 118, 6258–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Chen Y, Grann VR, Slavkovich V, Parvez F, Ahsan H, 2008. Arsenic exposure and anemia in Bangladesh: a population-based study. J. Occup. Environ. Med 50, 80–87. [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Bush HM, Bingcang A, Hertz-Picciotto I, 2006. Association between arsenic exposure from drinking water and anemia during pregnancy. J. Occup. Environ. Med 48, 635–643. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW, 2002. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 17, 117–130. [DOI] [PubMed] [Google Scholar]
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ, Murray CJ, 2014. A systematic analysis of global anemia burden from 1990 to 2010. Blood 123, 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Faraj JM, Ronnenberg AG, Quamruzzaman Q, Rahman M, Mostofa G, Afroz S, Christiani DC, 2016. A cross sectional study of anemia and iron deficiency as risk factors for arsenic-induced skin lesions in Bangladeshi women. BMC Public Health 16, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury MJ, 2014. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev 28, 49–66. [DOI] [PubMed] [Google Scholar]
- Medina S, Xu H, Wang SC, Lauer FT, Liu KJ, Burchiel SW, 2017. Low level arsenite exposures suppress the development of bone marrow erythroid progenitors and result in anemia in adult male mice. Toxicol. Lett 273, 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S, Bolt AM, Zhou X, Wan G, Xu H, Lauer FT, Liu KJ, Burchiel SW, 2021a. Arsenite and monomethylarsonous acid disrupt erythropoiesis through combined effects on differentiation and survival pathways in early erythroid progenitors. Toxicol. Lett 350, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina S, Zhou X, Lauer FT, Zhang H, Liu KJ, Lewis J, Burchiel SW, 2021b. Modulation of PARP activity by Monomethylarsonous (MMA(+3)) acid and uranium in mouse thymus. Toxicol. Appl. Pharmacol 411, 115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Gonzalez JB, Vukovic M, Abdelfattah A, Saleh L, Almotiri A, Thomas LA, Agirre-Lizaso A, Azevedo A, Menezes AC, Tornillo G, Edkins S, Kong K, Giles P, Anjos-Afonso F, Tonks A, Boyd AS, Kranc KR, Rodrigues NP, 2019. Gata2 as a crucial regulator of stem cells in adult hematopoiesis and acute myeloid leukemia. Stem Cell Rep 13, 291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D, 2007. On hematopoietic stem cell fate. Immunity 26, 669–673. [DOI] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA, 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ. Health Perspect 121, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C, Querfurth E, Kulessa H, Graf T, 2000. GATA-1 interacts with the myeloid PU.1 transcription factor and represses PU.1-dependent transcription. Blood 95, 2543–2551. [PubMed] [Google Scholar]
- Organization, W.H, 2011. Arsenic in Drinking-Water WHO Press, Geneva. [Google Scholar]
- Organization, W.H, 2015. The Global Prevalence of Anaemia in 2011
- Orkin SH, Zon LI, 2008. Hematopoiesis: an evolving paradigm for stem cell biology. Cell 132, 631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Medina S, Santella RM, Islam T, Lauer FT, Alam N, Eunus M, Rahman M, Factor-Litvak P, Ahsan H, Graziano JH, Liu KJ, Burchiel SW, 2017. Arsenic exposures alter clinical indicators of anemia in a male population of smokers and non-smokers in Bangladesh. Toxicol. Appl. Pharmacol 331, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk CJ, Bryder D, 2011. Flow cytometry-based identification of immature myeloerythroid development. Methods Mol. Biol 699, 275–293. [DOI] [PubMed] [Google Scholar]
- Registry, A.F.T.S.A.D, 2007. Toxicological Profile for Arsenic U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Registry, A.F.T.S.A.D, 2016. Addendum to the Toxicological Profile for Arsenic U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. [Google Scholar]
- Rekhtman N, Radparvar F, Evans T, Skoultchi AI, 1999. Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: functional antagonism in erythroid cells. Genes Dev 13, 1398–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger MA, Schroeder T, 2012. Hematopoiesis. Cold Spring Harb. Perspect. Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Weissman IL, 2010. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med 2, 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuga J, Zhang J, Samson LD, Lodish HF, Griffith LG, 2007. In vitro erythropoiesis from bone marrow-derived progenitors provides a physiological assay for toxic and mutagenic compounds. Proc. Natl. Acad. Sci. U. S. A 104, 8737–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhou X, Du L, Liu W, Liu Y, Hudson LG, Liu KJ, 2014. Arsenite binding-induced zinc loss from PARP-1 is equivalent to zinc deficiency in reducing PARP-1 activity, leading to inhibition of DNA repair. Toxicol. Appl. Pharmacol 274, 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surdu S, Bloom MS, Neamtiu IA, Pop C, Anastasiu D, Fitzgerald EF, Gurzau ES, 2015. Consumption of arsenic-contaminated drinking water and anemia among pregnant and non-pregnant women in northwestern Romania. Environ. Res 140, 657–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kobayashi-Osaki M, Tsutsumi S, Pan X, Ohmori S, Takai J, Moriguchi T, Ohneda O, Ohneda K, Shimizu R, Kanki Y, Kodama T, Aburatani H, Yamamoto M, 2013. GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation. Genes Cells 18, 921–933. [DOI] [PubMed] [Google Scholar]
- Tsai FY, Orkin SH, 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89, 3636–3643. [PubMed] [Google Scholar]
- Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH, 1994. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371, 221–226. [DOI] [PubMed] [Google Scholar]
- Vergara-Geronimo CA, Leon Del Rio A, Rodriguez-Dorantes M, Ostrosky-Wegman P, Salazar AM, 2021. Arsenic-protein interactions as a mechanism of arsenic toxicity. Toxicol. Appl. Pharmacol 431, 115738. [DOI] [PubMed] [Google Scholar]
- Vicente C, Conchillo A, Garcia-Sanchez MA, Odero MD, 2012. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit. Rev. Oncol. Hematol 82, 1–17. [DOI] [PubMed] [Google Scholar]
- Wan G, Medina S, Zhang H, Pan R, Zhou X, Bolt AM, Luo L, Burchiel SW, Liu KJ, 2021. Arsenite exposure inhibits the erythroid differentiation of human hematopoietic progenitor CD34(+) cells and causes decreased levels of hemoglobin. Sci. Rep 11, 22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Riva L, Xie H, Schindler Y, Moran TB, Cheng Y, Yu D, Hardison R, Weiss MJ, Orkin SH, Bernstein BE, Fraenkel E, Cantor AB, 2009. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol. Cell 36, 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhang X, Iwama A, Yu C, Smith KA, Mueller BU, Narravula S, Torbett BE, Orkin SH, Tenen DG, 2000. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96, 2641–2648. [PubMed] [Google Scholar]
- Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de The H, Chen SJ, Chen Z, 2010. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science 328, 240–243. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG, 2011. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J. Biol. Chem 286, 22855–22863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun X, Mobarak C, Gandolfi AJ, Burchiel SW, Hudson LG, Liu KJ, 2014. Differential binding of monomethylarsonous acid compared to arsenite and arsenic trioxide with zinc finger peptides and proteins. Chem. Res. Toxicol 27, 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Cooper KL, Sun X, Liu KJ, Hudson LG, 2015. Selective sensitization of zinc finger protein oxidation by reactive oxygen species through arsenic binding. J. Biol. Chem 290, 18361–18369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Medina S, Bolt AM, Zhang H, Wan G, Xu H, Lauer FT, Wang SC, Burchiel SW, Liu KJ, 2020. Inhibition of red blood cell development by arsenic-induced disruption of GATA-1. Sci. Rep 10, 19055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


