Abstract
Tendons are collagen-rich musculoskeletal tissues that possess the mechanical strength needed to transfer forces between muscles and bones. The mechanical development and function of tendons are impacted by collagen crosslinks. However, there is a limited understanding of how collagen crosslinking is regulated in tendon during development and aging. Therefore, the objective of the present review was to highlight potential regulators of enzymatic and non-enzymatic collagen crosslinking and how they impact tendon function. The main collagen crosslinking enzymes include lysyl oxidase (LOX) and the lysyl oxidase-like isoforms (LOXL), whereas non-enzymatic crosslinking is mainly mediated by the formation of advanced glycation end products (AGEs). Regulators of the LOX and LOXL enzymes may include mechanical stimuli, mechanotransducive cell signaling pathways, sex hormones, transforming growth factor (TGF)β family, hypoxia, and interactions with intracellular or extracellular proteins. AGE accumulation in tendon is due to diabetic conditions and aging, and can be mediated by diet and mechanical stimuli. The formation of these enzymatic and non-enzymatic collagen crosslinks plays a major role in tendon biomechanics and in the mechanisms of force transfer. A more complete understanding of how enzymatic and non-enzymatic collagen crosslinking is regulated in tendon will better inform tissue engineering and regenerative therapies aimed at restoring the mechanical function of damaged tendons.
Keywords: Tendon, development, collagen, crosslinking, advanced glycation end products (AGEs), lysyl oxidase (LOX)
Introduction
Tendon is predominantly composed of collagen, which is hierarchically arranged in fibrils, fibers, and fascicles (Provenzano and Vanderby, 2006). The stretching and sliding of these collagen structures play a role in the ability of tendons to transfer forces between muscles and bones, and ultimately contribute to the strength and deformation mechanisms of tendons (Hijazi et al., 2019; Peterson and Szczesny, 2020; Provenzano and Vanderby, 2006; Screen et al., 2004; Svensson et al., 2013; Szczesny and Elliott, 2014). Collagen crosslinking may be a critical regulator of tendon mechanics and these crosslinks are essential for tendons to withstand physiological forces over an entire lifetime (Heinemeier et al., 2013; Thorpe et al., 2010b). The mechanisms that regulate the formation of collagen crosslinking, as well as the types and extent of crosslinking, are beginning to be elucidated. As collagen crosslinking impacts both tendon development and aging, an improved understanding of the regulators of crosslinking has the potential to significantly impact strategies focused on tendon tissue engineering, maintenance of tendon function, and healing.
The interfibrillar and intrafibrillar crosslinks between collagen molecules are formed by both enzymatic and non-enzymatic processes, which contribute in a unique way to tendon functions and changes over time. Collagen crosslinking that occurs during embryonic tendon development appears to be mainly regulated by enzymatic processes, such as by LOX activity (Marturano et al., 2013; 2014). In collagen, LOX enzymatically catalyzes the reaction of Hyl and lysine residues into reactive aldehyde species, which subsequently spontaneously link to aldehyde species on adjacent molecules (Lucero and Kagan, 2006; Pinnel and Martin, 1958). These LOX-mediated crosslinks lead to the formation of trivalent and divalent crosslinks between collagen molecules.
Non-enzymatic crosslinks, such as AGEs (with glucosepane being a predominant AGE), are spontaneously formed through glycation processes and yield divalent crosslinks between neighboring fibril residues (Sajithlal et al., 1998). AGE accumulation is associated with aging and diseases, such as diabetes, and may contribute to increased brittleness as well as limited collagen remodeling. Together, enzymatic and non-enzymatic collagen crosslinks play an important role in elaboration of tendon mechanical properties during development and aging by reinforcing the collagen matrix and possibly facilitating the transfer of mechanical forces throughout the tissue (Gerriets et al., 1993).
The effects of enzymatic and non-enzymatic collagen crosslinks on tendon mechanical properties have been explored in several recent reviews (Eekhoff et al., 2018; Gjaltema and Bank, 2017; Snedeker and Gautieri, 2014; van der Slot et al., 2003). Briefly, both enzymatic and non-enzymatic crosslinks affect the collagen structure, altering both tissue biomechanics (e.g., altered tissue elastic modulus, ultimate tensile strength, stress relaxation, etc.) and failure mechanisms (e.g., crosslink breakage, load transfer, etc.). However, future work will need to directly compare how enzymatic vs. non-enzymatic crosslinks impact tendon mechanical properties. Despite the important and complex role collagen crosslinking plays in tendon formation and pathology, it is less clear how these crosslinks are regulated. A thorough understanding of the regulation of enzymatic and non-enzymatic crosslinks is needed to better determine how tendon mechanical properties are regulated, to guide the development of mechanically functional engineered tendon tissues, and to better characterize tendon pathogenesis. Further, manipulation of crosslinks may be applied to a variety of tissue engineering techniques that use collagen-based biomaterials to modulate mechanical properties and failure mechanisms. Therefore, the present review aimed to highlight recent findings on the potential regulators of enzymatic and non-enzymatic collagen crosslinks in tendon, as well as to discuss the limited knowledge on collagen crosslinking in tendon development, disease, and aging. To further develop novel effective treatments for damaged tendons, an improved understanding of collagen crosslinks and the mechanisms governing their formation is needed.
Collagen fibrillogenesis and role of enzymatic crosslinking
Intracellular fibrillogenesis
Embryonic tendon is characterized by a transition from a dense cellular network, with relatively minimal collagen content, to a structure defined by an organized collagen matrix (Richardson et al., 2007; Schiele et al., 2013; Schiele et al., 2015; Theodossiou et al., 2020). This process of collagen fibrillogenesis has been previously described (Banos et al., 2008; Bayer et al., 2010; Canty et al., 2006; Canty and Kadler, 2005; Kadler et al., 2008) but will be briefly reviewed along with the formation of the two primary forms of mature enzymatic crosslinks, HP and LP, in tendon.
Collagen fibrillogenesis begins with the translation of collagen pre-pro-α-polypeptide chains, which subsequently undergo hydroxylation of proline and lysine residues. Lysine hydroxylation is mediated by the enzyme LH. Consisting of three distinct isoforms, LH1 primarily hydroxylates lysine along the helical domain of the α-chains to form Hyl (Gjaltema and Bank, 2017). Together, glycosylation of Hyl residues, hydroxylation of proline via the complex P4HA/PDI and P3H isoforms, and formation of disulfide bonds initiate aggregation of α-chains into a triple helix to form a procollagen molecule that is secreted by a collagen-producing cell into the ECM through a vesicle. This secreted molecule is cleaved at the N- and C-terminal by procollagen peptidases to produce mature tropocollagen (Gjaltema and Bank, 2017) (Fig. 1). Remaining telopeptide lysine residues can be hydroxylated through LH isoforms, possibly LH2 (Gjaltema and Bank, 2017; van der Slot et al., 2003). Notably, LH1 and LH2 (consisting of the splicing isoform LH2a and LH2b) are primarily associated with the major collagen types (I, II, and III), whereas LH3 is primarily associated with the minor collagen types (e.g., IV and V) (Gjaltema and Bank, 2017; Risteli et al., 2004; van der Slot et al., 2003). LH1 primarily hydroxylates lysines in the helical domain of procollagen, whereas LH2 functions within the telopeptidyl domain (Myllylä et al., 2007; Takaluoma et al., 2007; van der Slot et al., 2003). In a mouse development model, all LH isoforms were found expressed at various levels during embryogenesis and in a tissue-dependent manner (Salo et al., 2006). Specifically, all LH isoforms were expressed in the murine mesoderm at embryonic day (E)7.5 and had continued expression in later mesoderm-derived tissues. LH1 is highly expressed throughout embryonic development of mice within collagen type I, II, and III. LH2a is expressed in whole mouse embryos until E11.5 and, then, localizes to the brain, kidney, and testis (Salo et al., 2006; Walker et al., 2005). LH2b (long splice form) expression begins at E11.5 and is the primary LH isoform found throughout adulthood in muscle, lung, and connective tissue. LH3 appears to have a more housekeeping function (Salo et al., 2006).
Fig. 1. Summary of collagen fibrillogenesis.
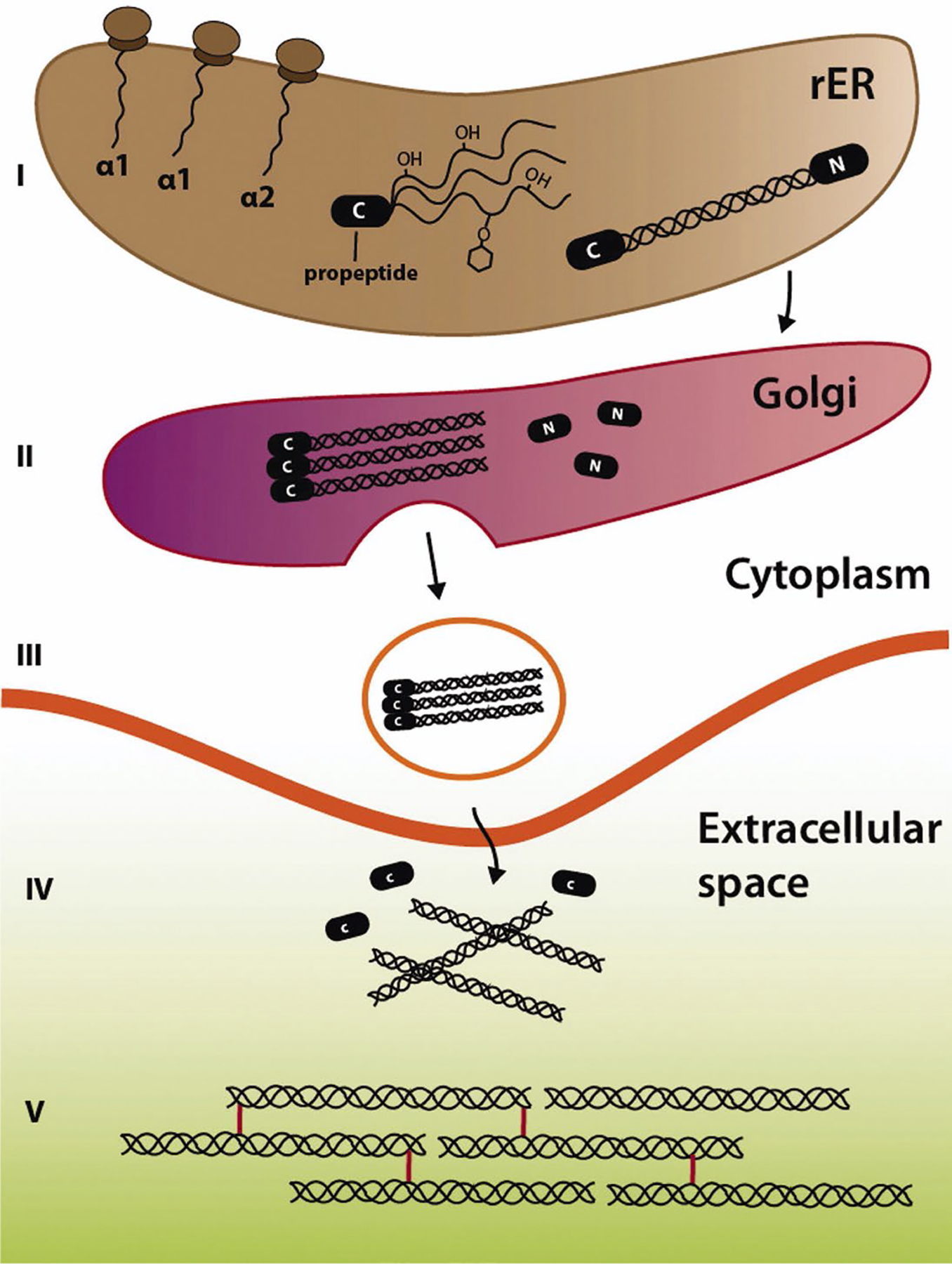
(I) Fibrillogenesis begins with production of procollagen-α-chains (α1–3) and their aggregation into collagen pro-peptides in the rough ER (rER). (II) Pro-peptides are processed and packaged in the Golgi apparatus before being secreted into the (III) extracellular space for maturation and crosslinking, (IV) including cleavage of remaining N-terminal (N) and C-terminal (C) domains to produce (V) mature tropocollagen. Reprinted with permission from Gjaltema and Bank (2017).
Extracellular fibrillogenesis
Following secretion and hydroxylation of the triple-helical procollagen, LOX catalyzes the formation of Hyl and lysine residues into reactive aldehyde species. In turn, these species spontaneously link to aldehydes on adjacent collagen molecules by covalent bonds to form immature divalent crosslinks (Fig. 2a). Immature divalent crosslinks may subsequently link with further residues on another telopeptidyl residue to create a single mature trivalent crosslink (Eyre et al., 1984; 2008). The most abundant types of enzyme-mediated crosslinks within adult tendon are mature trivalent HP (HP or HylPyr, or pyridinoline, 428 Da) crosslinks – composed of two telopeptide Hyl and one helix Hyl – and, to a lesser extent, trivalent LP (LP or LysPyr, or deoxypyridinoline, 412 Da) crosslinks – composed of two telopeptide Hyl and one helix lysine (Fig. 3) (Eyre et al., 1984; Eyre et al., 2008).
Fig. 2. Enzymatic versus non-enzymatic processes.
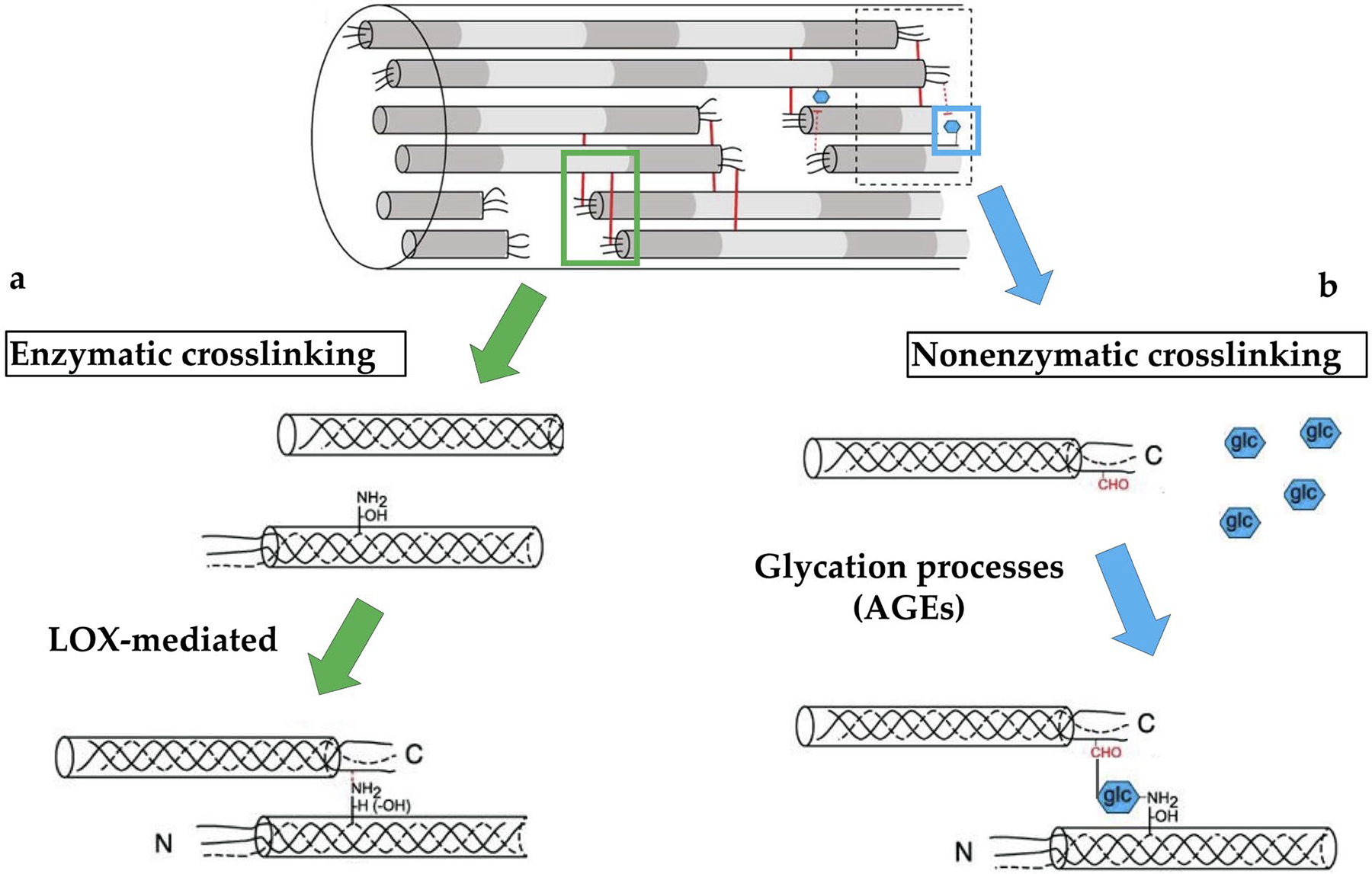
The two primary forms of crosslinks in mature tendon collagen can be identified as being driven by enzyme activity (i.e., enzymatic crosslinks) or other processes (i.e., non-enzymatic). (a) Enzymatic crosslinks are primarily mediated by LOX, which catalyzes the conversion of lysine residues (NH2) into reactive aldehyde species (-H) and yield immature or mature divalent and trivalent crosslinks. (b) The dominant non-enzymatic crosslinks are AGEs, which are formed through glycation of protein residues, such as lysine (NH2), by reducing sugars, such as glucose (glc). Modified with permission from Hudson et al. (2018).
Fig. 3. LOX-mediated collagen crosslinking.
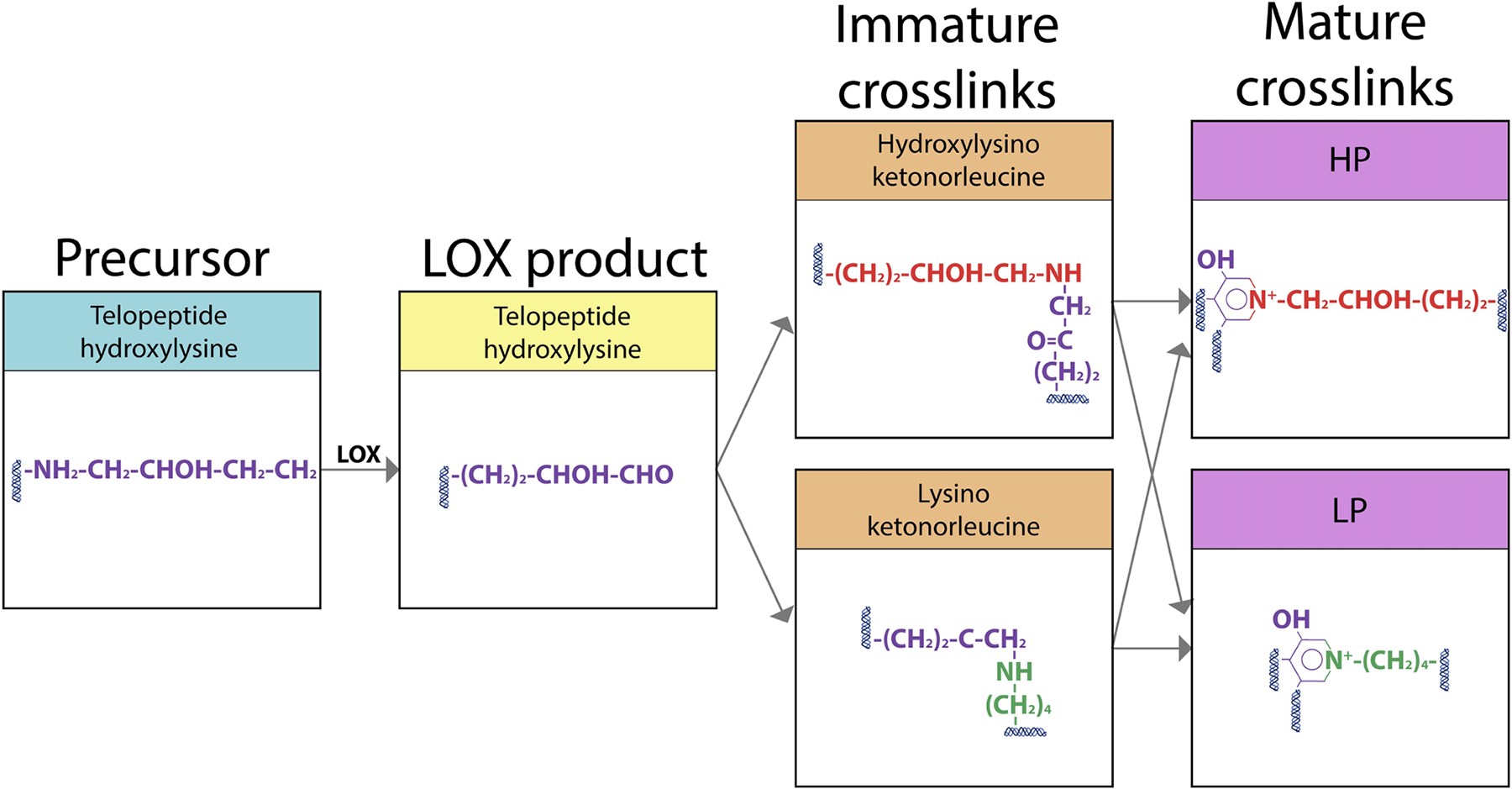
LOX-mediated formation of divalent and trivalent crosslinks in collagen fibrils in tendons. Modified with permission from Makris et al. (2013).
A recent study has suggested that these LOX-mediated crosslinks are formed at the fibril surface during fibrillogenesis because the LOX molecule (3.7 nm radius) may be too large to diffuse within the collagen fibril (Leighton et al., 2021). While unknown in tendon, previous work in bone has identified the gap spacing (i.e., porosity) between the tropocollagen molecules within a collagen fibril to be around 2 nm (Xu et al., 2020), thereby inhibiting LOX diffusion. Furthermore, the molecular weight of LOX (32 kDa) approaches the upper limits of collagen type I pore size, estimated to be between 5.7 kDa and 48 kDa, which may limit LOX ability to diffuse into the fibril (Toroian et al., 2007). Differences in HP and LP forms (e.g., Hyl vs. lysine) are driven by LH1 and LH2 during fibrillogenesis (Hudson et al., 2017; Terajima et al., 2016). However, the specific mechanisms governing LH-mediated processes are not well understood in tendons or ligaments. Other types of enzymatic crosslinking are more or less prevalent among different types of tendons, such as Hyl pyrrole and lysyl pyrrole (Eekhoff et al., 2018; Thorpe et al., 2010a).
While LOX appears to be the primary mediator of enzymatic collagen crosslinking in tendon, other enzymes, such as TGs, may also impact ECM crosslinking (Taye et al., 2020) as well as cell behavior and tissue pathologies (Eckert et al., 2014; Lorand and Graham, 2003). Recently, TG2 was found in the myotendinous junction of 5-month old mice (Jacobson et al., 2020). TGs have been identified in human articular cartilage (Summey et al., 2002) and may play a role in supraspinatus tendon pathogenesis (Oliva et al., 2009). The specific effects of TGs and similar enzymes on tendon formation remain largely unknown and need further study. Therefore, the present review will focused on LOX-mediated crosslinks in tendon development and aging.
Crosslinks identified in mature tendon
Concentrations of HP and LP crosslinks appear to increase over time throughout maturation in mouse tail tendon (Stammers et al., 2020a) (Fig. 4a). Functionally distinct tendons contain different amounts of mature trivalent crosslinks (Carroll et al., 2012; Svensson et al., 2013; Svensson et al., 2018). For example, in 16-week-old rats, HP crosslink content was 139-fold higher in Achilles tendons, an energy-storing tendon, compared to tail tendons, a positional tendon, whereas LP crosslink content was 1.6-fold higher in tail tendons (Svensson et al., 2018). Furthermore, flexor and extensor tendons have different crosslink types and densities, with flexor tendons containing more thermally stable crosslinks (i.e., more mature crosslinks) and a higher crosslink density (Herod et al., 2016). Despite this, extensor tendons are significantly stronger and tougher than flexor tendons (Herod et al., 2016). The role of crosslinking and its contribution to these mechanical properties requires further investigation, but it is proposed that a trade-off between strength and fatigue resistance and crosslinking may play a role in the mechanical differences between tendon types.
Fig. 4. Collagen crosslinking changes over time in mouse tail tendon.
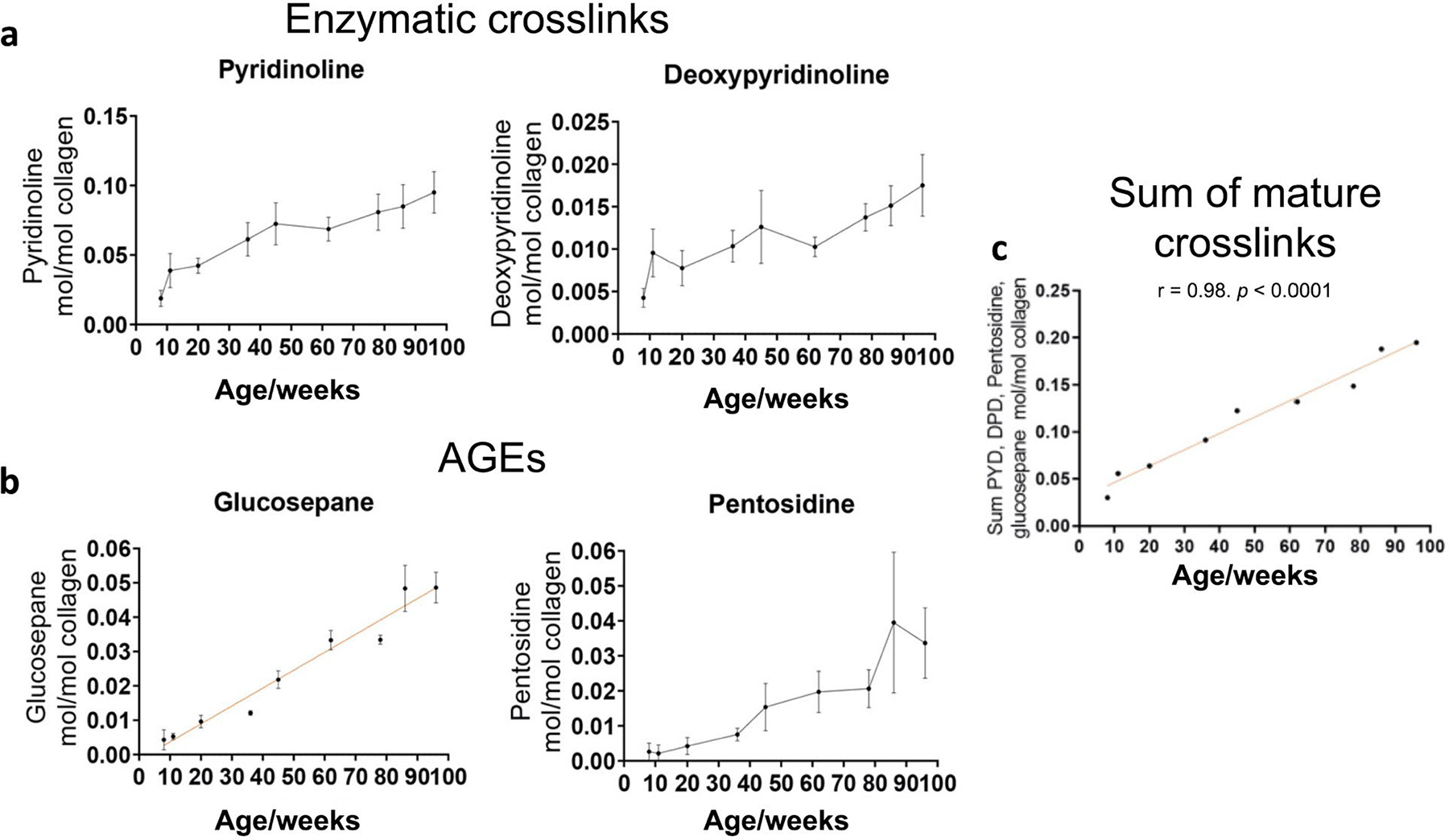
(a) Mature enzymatic crosslinks, HP and LP, increase with aging. (b) AGEs (glucosepane and pentosidine) and (c) total mature crosslinks increase with aging. Modified with permission from Stammers et al. (2020a).
Crosslinking may also differ between mature tendons and ligaments. Comparing human cadaveric ACL, patellar tendon, and semitendinosus tendon, ACLs were found to have a larger total number of enzymatic crosslinks than either tendons and no differences were observed between patellar tendon and semitendinosus tendon (Suzuki et al., 2008). Similarly, cells derived from rabbit ACL, MCL, and patellar tendon and cultured for 4 weeks showed distinct biochemical profiles (Kato et al., 2015). ACL-derived fibroblasts had significantly larger total enzymatic crosslinking content (total divalent crosslinks plus pyridinoline crosslinks) and increased PLOD1/2, LOX, Col1a1, and Col3a1 expression, compared to MCL-and patellar-tendon-derived fibroblasts, with no differences between MCL and patellar tendon cells (Kato et al., 2015). These studies suggest that functionally distinct ligaments and tendons have unique crosslink maturation processes (Kato et al., 2015; Suzuki et al., 2008). However, the mechanisms governing these maturation processes are not well characterized.
Equine SDFTs are commonly used as a functional equivalent to human Achilles tendon (Patterson-Kane and Rich, 2014) and they are the most commonly injured tendon in horses (Thorpe et al., 2010a). Using HPLC, a positive correlation between pyrrole crosslinking densities and mechanical properties, including elastic modulus, ultimate stress, and yield stress, was identified in SDFTs. However, the predominant crosslink, HP, did not correlate with mechanical function (Thorpe et al., 2010a). Similar work in human patellar tendon corroborated the finding that HP and LP crosslinks are likely to only play a minor role in tissue mechanics after initial tissue formation (Svensson et al., 2013).
The different crosslinks and concentrations within various tendon types, particularly tendons of different functions (i.e., positional vs. energy-storing tendons), raise intriguing questions regarding the specific role of enzymatic crosslinks in these tissues and how they are differentially regulated.
Crosslinks at the tendon-bone interface
The mechanical properties and structure of tendon change spatially as the tissue transitions from the tendon itself (mainly collagen) to its insertion site into the bone (mainly mineralized collagen). The tendon-bone interface (enthesis) is particularly pertinent because partial- and full-thickness tendon tears at the enthesis are a primary cause of rotator cuff injury (Spargoli, 2016; Yamamoto et al., 2010) and typically require a surgical repair. The enthesis demonstrates a lower modulus than other regions of the tendon, possibly leading to increased deformation and energy absorption prior to failure, creating an overall tougher tissue (Deymier et al., 2017). Mineral content, collagen orientation, and protein gradients all vary throughout the length of the enthesis (Deymier-Black et al., 2015; Thomopoulos et al., 2003). Specifically, closer to the tendon side, more aligned collagen fibers along with higher concentrations of decorin and biglycan are observed (Deymier-Black et al., 2015). As the collagen fibers in tendon becomes ossified (transition to bone), collagen becomes less aligned and localized aggrecan (a proteoglycan localized in other regions of the tendon that experience high compressive forces) content becomes prominent (Thomopoulos et al., 2003; Waggett et al., 1998). A significant knowledge gap exists in the heterogeneity of collagen crosslinking and associated mechanisms (including types of crosslinks, LOX, LH, etc.), particularly within critical transition regions of the enthesis. A better understanding of these phenomena may aid in the development of tissue-engineered constructs and regenerative therapies targeted at treating evulsion injuries.
LOX production and activation
The copper-dependent amine oxidase LOX, coded by LOX, is first transcribed as a pre-pro-protein. Pre-proLOX undergoes post-translational modifications within the ER and, then, experiences N-glycosylation of the N-terminal pro-peptide chain (147 aa residues) and folding of the C-terminal (containing the mature protein, 249 aa residues) to form three or more disulfide bonds (Trackman et al., 1992; Williams and Kagan, 1985). Then, proLOX is secreted into the ECM where the glycosylated N-terminal is proteolytically cleaved by BMP-1, also known as PCP, to release the active mature LOX protein and generate a LOX-PP (Cronshaw et al., 1995; Maruhashi et al., 2010; Uzel et al., 2001). The mechanisms regulating BMP-1 in tendon are not currently well known but, considering the role it plays in LOX activation, it would be worth investigating them. LOX-PP may help govern LOX activity by mediating secretion of proLOX into the ECM, and glycosylation of LOX-PP aids in efficient protein folding (Grimsby et al., 2010). To the authors’ knowledge, the specific role of LOX-PP in tendon has not been characterized and further investigation could improve the understanding of LOX regulation. Notably, LOX incorporates a copper cofactor and an organic peptidyl cofactor (LTQ) that are needed for proper function (Gacheru et al., 1990; Wang et al., 1996). As a copper-dependent enzyme, the addition of copper sulfate to cell culture increases the total HP crosslinks and tensile properties (which can be further enhanced by treatment with exogenous Hyl) in engineered neocartilage constructs through enhanced LOX activity (Makris et al., 2013).
LOXL isoforms
The LOX superfamily consists also of 4 additional isoforms, LOXL1-4. A copper-binding domain, LTQ, and a CRL domain on the C-terminal are conserved through all 5 paralogues (Lee and Kim, 2006). LOXL1, while the most morphologically similar to LOX, appears to primarily target elastin and tissues undergoing elastogenic events, making it less relevant to tendon-collagen crosslinking (Liu et al., 2004; Zhao and Zhou, 2012). Furthermore, elastin deficiency has minor impacts on murine Achilles tendon mechanical properties (Eekhoff et al., 2017), suggesting that LOXL1 may have a minimal role in tendon formation. However, this needs further study. Whereas LOX and LOXL1 both require proteolytic cleavage for activation, LOXL2-4 are active in both processed and non-processed forms (Rodriguez et al., 2010; Trackman et al., 1992). LOXL2 and LOXL3 isoforms exhibit amine oxidase activity towards collagen and elastin (Kim et al., 2011; Lee and Kim, 2006). Although not well explored in tendon formation, knockdown of LOXL2 in chondrogenic cell lines blocks further chondrogenesis through increases in SNAIL, a transcriptional repressor of cadherins, and decreases in SOX9, a transcription factor and key regulator of chondrogenesis (Iftikhar et al., 2011). Application of exogenous LOXL2 to cultured cartilage explants (derived from bovine articular cartilage) and engineered chondrogenic constructs (primary chondrocytes cultured to create a self-assembled structure), which were later implanted into athymic mice, promoted tissue maturation (increased mature crosslinks) and enhanced tensile mechanical properties (Makris et al., 2014). LOXL3 appears to have a critical role in facilitating development of the myotendinous junction and proper formation of the fibronectin matrix within mouse embryos (Kraft-Sheleg et al., 2016). Increases in LOXL2-4 correlate with fibrotic conditions in skin and lung, often identified by abnormal stiffness through excessive ECM and collagen deposition and crosslinking. However, this needs further investigation in tendon (Huang et al., 2019; Huang et al., 2020; Jones et al., 2018). Together, the impact of LOX-like protein isoforms on tissue development justifies additional investigation for applications in tendon tissue engineering.
Impacts of LOX inhibition on tendon development
Complete suppression of LOX results in perinatal murine death due to compromised collagen networks in the cardiovascular system and in abnormal collagen fiber morphology and organization within the dermis (Hornstra et al., 2003; Mäki et al., 2002; Mäki et al., 2005). No known animal models have been developed that allow for selective activation or inactivation of LOX during tendon development or in adulthood. Such a model would be beneficial to studying how LOX regulates tissue formation, homeostasis, and healing. However, to investigate LOX, current techniques rely mainly on the use of various ligands to inhibit its activity. These inhibitors act through two mechanisms: i) interaction with the copper-dependent cofactor (e.g., thiram, disulfiram, MCP); ii) interaction with the LTQ prosthetic group at the LOX active site (e.g., BAPN, TCP) (Hajdú et al., 2018).
BAPN is the most widespread irreversible inhibitor of LOX used in tendon studies. Although BAPN is broadly recognized as a standard in the tendon field, evidence suggests that BAPN treatment is not effective against LOXL2 (Kim et al., 2011); while LOXL3 is sensitive to inhibition by BAPN treatment (Kim et al., 2011; Lee and Kim, 2006). Others point to BAPN as an inhibitor of the entire LOX superfamily due to its affinity for amine oxidases (Rodríguez et al., 2008; Tang et al., 1983). Few studies have focused upon elucidating the specific effect of BAPN on individual LOX isoforms and more work should be conducted on how these inhibitory molecules specifically interact within the tendon. As such, interpretations of current studies may be limited by a lack of understanding on the specificity of BAPN inhibition. Specifically, conclusions regarding the effects of LOX using BAPN may not directly consider the potential activity of LOXL2-4 within the tendon. However, BAPN has been a powerful tool in determining how enzymatic collagen crosslinking impacts embryonic tendon formation.
Several studies using BAPN in a chick model of embryonic tendon formation have shown that LOX is essential for increased elastic modulus during development (Marturano et al., 2013; Marturano et al., 2014; Pan et al., 2018). Specifically, treatment of embryonic chick with varying concentrations of BAPN (5 mg/g or 15 mg/g) results in a decreased nanoscale elastic modulus of the calcaneal tendon at HH 40 and HH 43 without identified changes to GAG/dry mass, hydroxyproline/dry mass, cell viability, or cell density (Marturano et al., 2013). A higher concentration of BAPN (15 mg/g) results in a greater decrease in elastic modulus than a lower concentration (5 mg/g). BAPN also decreases the ratio of HP/collagen and LP/collagen, compared to controls (Marturano et al., 2014). The HP/LP ratio remains constant, suggesting BAPN acts on enzymatic collagen crosslinking, but without substrate preference towards HP or LP crosslinks (Marturano et al., 2014). In a different study of chick calcaneal tendon, LOX expression changed between HH 38 and HH 45, with peak expression at HH 42. LOX activity steadily increased over these same time points (Pan et al., 2018). Interestingly, proLOX activity peaked at HH 43, which is when chick embryos exhibit peak motility. Each LOX isoform exhibited a distinct gene expression profile. LOXL2 and LOXL4 decreased at HH 41, 42, 45 and HH 39–45, respectively, relative to HH 38, but LOXL1 and LOXL3 remained relatively constant, emphasizing each isoform may have a distinct role in embryonic tendon development (Pan et al., 2018).
Less work on LOX has been performed in human or mammalian tendon models. However, one study used an in vitro tendon-construct model that incorporated adult human tendon-derived fibroblasts in a fibrin gel (Herchenhan et al., 2015). Constructs immediately treated with BAPN (day 0) were untestable and resulted in a ruptured construct. Constructs first cultured for 14 d, and then treated with BAPN, showed increased collagen type I monomers and dimers compared to controls at 21 d of culture (i.e., less crosslinked). BAPN treatment resulted in a mechanically weaker construct (decreased failure stress, failure strain, and tensile modulus) and abnormal collagen fibrils that resembled Ehlers-Danlos collagen phenotypes (Herchenhan et al., 2015). BAPN treatment did not appear to affect Col1a1 expression or collagen type V, decorin, fibromodulin, or tenascin-X.
Based on these studies, LOX appears to be an important regulator in the formation of tendon mechanical properties. However, separating the role of collagen crosslinking from larger structural changes to collagen over time remains a challenge. Specifically, collagen organization (i.e., fibril overlap and elongation, interweaving, bifurcation/fusion, etc.) changes throughout tendon development, demonstrating greater structural order (i.e., fewer bifurcations and less interweaving) in mature compared to fetal tendons (Provenzano and Vanderby, 2006). Further, some of these interweaving collagen fibrils may be retained in mature tendon and their contributions to mechanical function are being evaluated, with evidence that friction between helical fibrils transfers mechanical loads (Safa et al., 2019; Szczesny et al., 2017). Overall, further work is needed in mammalian systems to identify specifically how LOX and LOXL1-4 are regulated during tendon development, as well as better differentiate between changes to collagen crosslinking and the broader changes to ECM structure.
Potential regulators of enzymatic crosslinking
Enzymatic crosslinking mediated by LOX appears to play an important role in the mechanical formation of tendon. Therefore, knowing the mechanisms that regulate LOX production and activity is needed to understand and ultimately regulate functional tendon development. While there is limited information on specific cell signaling pathways or external factors that contribute to LOX regulation at the cellular level in tendon, potential regulators that will be discussed include mechanical stimuli, hormone interactions, growth factors, hypoxia, and various glycoproteins in the ECM (Table 1, Fig. 5).
Table 1.
Recent studies exploring potential regulators of enzymatic collagen crosslinking in tendon, ligament, and related tissues.
| Potential regulator | Species/tissue | Age/time point | Treatment | Evaluation | Major findings | Source |
|---|---|---|---|---|---|---|
| Mechanical stimuli | Chick tendon | HH 28-HH 45, tendon explant cultured for 48 h | Paralysis, hypermotility, BAPN | Mechanical testing, LOX/LOXL expression and activity | Paralysis (unloading) decreases modulus and LOX activity, LOXL isoforms respond uniquely to mechanical loading | Pan et al., 2018 |
| Human-derived periodontal ligament cells | 24 or 48 h culture | 3 % or 10 % mechanical stretch | RT-PCR, fluorescence imaging | 3 % stretch promotes LOX and collagen production whereas 10 % stretch upregulates MMP2 expression with no changes to LOX activity | Chen et al., 2016 | |
| Human-derived periodontal ligament cells, rat periodontal ligament | 12 or 24 h culture, 3 d of treatment | Compressive loading, excessive occlusal loading | LOX/LH2/collagen gene expression, tissue histology | Low loading increases LOX/LH2 expression, excessive occlusal loading increases collagen maturation and LOX/LH2 expression | Kaku et al., 2016 | |
| Rat tail tendon, mouse tail tendon | 14–18 weeks (rat), 10–39 weeks (mouse) | PIEZO1 gain (or loss) of function | Mechanical testing, calcium imaging, crosslink analyses | PIEZO1 affects enzymatic crosslinking density and crosslinking appears to be mechanosensitive | Passini et al., 2021 | |
| Hypoxia | Bovine patellar tendon and articular cartilage | 4–8 weeks old bovine tissue explant cultured for 4 weeks | Hypoxia, exogenous LOXL2 | Mechanical testing, LOX expression, crosslink analyses | Increased number of mature crosslinks, increased LOX expression (evaluated in articular cartilage only), and increased modulus with hypoxia and LOXL2 treatment | Makris et al., 2014 |
| TGFβ signaling | Human trabecular meshwork cells | 24 h treatment in culture | Exogenous TGFβ, inhibitors of TGFβ signaling | RT-PCR, Western blot | TGFβ1-3 isoforms promote LOX and LOXL1-4 expression through canonical and non-canonical TGFβ signaling pathways | Sethi et al., 2011 |
| Human trabecular meshwork cells | 24 h treatment in culture | BMP antagonist, inhibitors of TGFβ signaling | LOX/LOXL1-4 expression | BMP antagonist gremlin induces LOX and LOXL expression through TGFβ signaling | Sethi et al., 2013 | |
| Protein interactions | Mouse dermis, human-derived fibroblasts | 8 weeks old, 24–96 h culture | TSP-1 knockout | Solid-phase binding assays, fluorescence imaging | TSP-1 binds to intracellular and extracellular collagen and interacts with dermal LOX activity | Rosini et al., 2018 |
| Mouse tail tendon | 5 months old | Fibromodulin knockout | Solid phase proximity ligation assay, histology | Fibromodulin interacted with LOX at the surface of collagen fibrils | Kalamajski et al., 2016 | |
| Mouse tail tendon | 2 months old | Cyp B knockout | TEM imaging, mass spectrometry, histology | Cyp B knockout impairs tendon fibrillogenesis; altered lysine hydroxylation | Terajima et al., 2016 | |
| Estrogen | Human ACL-derived fibroblasts | 20–24 year old fibroblasts cultured for 14 d | Exogenous estradiol, BAPN | Mechanical testing, hydroxyproline assay, LOX activity assay | Estrogen reduces tissue engineered construct mechanical properties and LOX activity | Lee et al., 2015 |
Fig. 5. Potential cell-level regulators of enzymatic crosslinking in tendon.
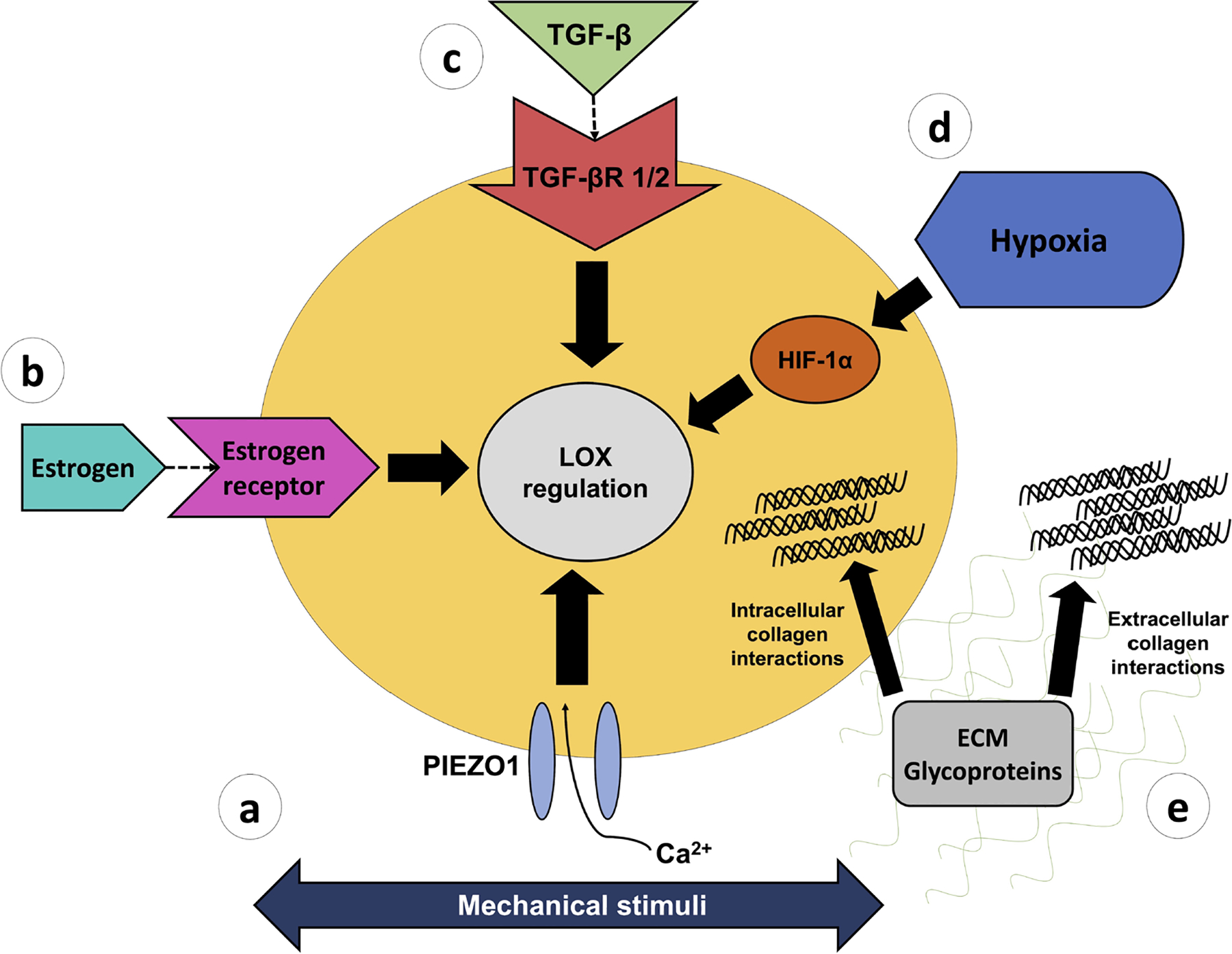
Enzymatic crosslinking, mediated by LOX, may be regulated by (a) mechanoresponsive signaling pathways, such as the calcium ion channel PIEZO1, (b) signaling through the sex hormone estrogen, (c) TGFβ family, (d) hypoxia-induced HIF-1α signaling, and (e) intracellular and extracellular collagen interactions with glycoproteins including fibromodulin and TSP-1.
Mechanical stimuli
Tendons experience mechanical stimulation from muscle contractions. The impact of muscle contractions on tendon and LOX has been explored during embryonic chick development (Pan et al., 2018). Chick embryos exhibiting a hypermotile (i.e., excessive mechanical loading) profile (through treatment with 4-aminopyridine) show an increased calcaneal tendon elastic modulus relative to controls, without changes in LOX expression. However, chick embryos treated with rigid and flaccid paralysis (unloading) phenotypes (through treatment with decamethonium bromide or pancuronium bromide) have a decreased calcaneal tendon elastic modulus that corresponds to decreased LOX activity. Interestingly, a hypermotility plus BAPN treatment does not significantly alter the elastic modulus (Pan et al., 2018). Together, these findings suggest that mechanical stimuli from muscle contractions during embryonic development impact tendon modulus through alterations to LOX production and, therefore, collagen crosslinking.
A recent study using rat tendon explants and genetic manipulations in mice showed that adult tendon cells may detect some modes of mechanical loading through the mechanosensitive ion channel PIEZO1 (Passini et al., 2021). Diminished PIEZO1 activity decreases stiffness, whereas enhanced PIEZO1 activity increases stiffness of rat tail tendons. Furthermore, pharmacologically induced PIEZO1 activation (through Yoda1), combined with mechanical stretch, upregulates Lox expression in tendon fascicle explants. Mice with a PIEZO1 gain-of-function mutation display increased crosslink density, as indicated by thermal calorimetry and fluorescence imaging, and with no apparent changes to fibril structure or organization (Passini et al., 2021). These results may suggest that enzymatic collagen crosslinking is at least partially governed by the ion channel PIEZO1 and by mechanical stimuli. Further research into LOX activation by PIEZO1 and how and which mechanosensing pathways are affected by mechanical stimulation is necessary to help the understanding of LOX regulation and collagen crosslinking by tendon cells.
Mechanoregulation in PDL
hPDL cells embedded within a collagen gel respond uniquely to different magnitudes of mechanical loading. Low tensile strain magnitudes (3 %) upregulate LOX, Col1a1 and Col3a1 expression, LOX activity, and production of secreted collagen (Chen et al., 2013). In contrast, high tensile strain magnitudes (10 %) do not affect LOX but downregulate Col1a1 and upregulate Col3a1, MMP2, and TIMP2 expression (Chen et al., 2013). One potential explanation for these phenomena is that low level mechanical stimulation (3 % strain) facilitates ECM deposition and stabilization via LOX-mediated collagen crosslinking, whereas high level stimulation (10 % strain) favors ECM degradation and tissue remodeling (Chen et al., 2013). A similar study on hPDL cells applying varying magnitudes of compressive mechanical loading found LOX and LH2 expression increased at low but decreased at high loading magnitudes (Kaku et al., 2016). This same study explored the in vivo effects of loading in rats using an excessive occlusal loading model (i.e., a steel wire was added to the left first molar to induce excessive contact between teeth). Excessive loading increased collagen maturation as well as LOX and LH2 positive cells. Excessive loading plus BAPN treatment suppressed the relative increases in LOX and LH2 positive cells, as well as collagen maturation (Kaku et al., 2016). This suggests that PDL collagen maturation in an excessive loading model is at least partially driven by mechanosensitive enzymatic crosslinker activity. Together, these studies on the mechanosensitive behavior of PDL tissue and cells justifies further investigation in tendon tissue, specifically on the impacts of varying magnitudes of mechanical load and the correlation between ECM remodeling (e.g., MMPs) and stabilizing (e.g., LOX) proteins.
Positional tendons versus energy-storing tendons
Further evidence for a mechanosensitive crosslinking-driven mechanism comes from the different types of crosslinks identified in positional versus energy-storing tendons (Carroll et al., 2012; Svensson et al., 2013; Svensson et al., 2018). A possible explanation for the disparate crosslinking quantities are the functional differences between these tendon types and the different loading magnitudes. Energy-storing and weight-bearing tendons, such as the Achilles tendon, are repeatedly exposed to a greater mechanical load than tail tendons, suggesting LOX and the resulting crosslinks may be mechanically regulated. However, it is possible that differences between tendon types are not related to LOX regulation and mechanical loading but to underlying differences during formation due to unknown growth factors, signaling, genetic variation, etc.. These potential regulators of LOX along with mechanical stimuli should be investigated in future studies.
Hypoxia
The oxygen tension of tendon in vivo is not well documented but the minimal vasculature of tendon suggests it is a relatively hypoxic tissue, presumably similar to articular cartilage (1–10 % oxygen) (Grimshaw and Mason, 2000) or bone marrow (1–7 % oxygen) (Antoniou et al., 2004; Chow et al., 2001; D’Ippolito et al., 2006). Low oxygen levels in tendon have the potential to regulate LOX, however literature exploring hypoxia and LOX does not focus primarily on tendons. A hypoxic environment (2 % oxygen) increases LOX expression, Young’s modulus, and the concentration of HP crosslinks in bovine articular cartilage explants after 4-week culture (Makris et al., 2014) (Fig. 6). When BAPN treatment is used in combination with hypoxia, LOX expression increases (although at a level lower than hypoxia treatment alone) but Young’s modulus and HP crosslinks are not impacted, relative to controls. Together, this suggests that hypoxia may mediate LOX expression and activity to impact tissue modulus. Hypoxia and BAPN treatments were also applied to bovine ACL, PCL, patellar tendon, and knee meniscus explants; the same trends in modulus were detected, although LOX expression was not assessed in these tissues (Makris et al., 2014).
Fig. 6. Effect of hypoxia on crosslinking.
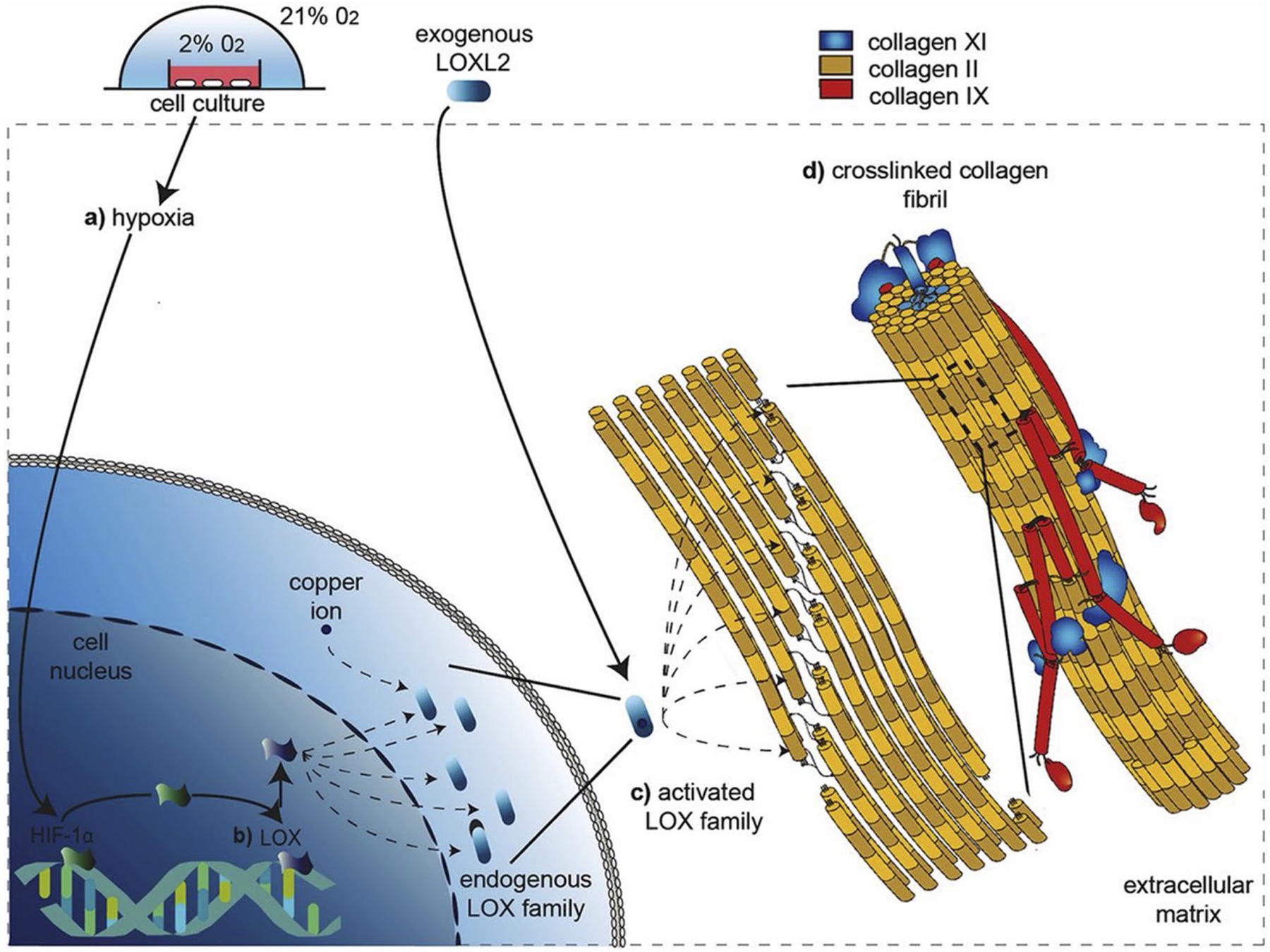
Hypoxic environments may regulate LOX-mediated collagen crosslinking. (a) In vitro cell cultures exposed to hypoxia may upregulate LOX production via the HIF-1 pathway. (b) Promotion of LOX and LOXL increase activity of respective isoforms (LOX, LOXL1-4). (c) LOX and LOXL isoforms drive collagen maturation by catalyzing the formation of intermolecular bonds within and between collagen fibers to form HP and LP crosslinks. (d) Fibrillar collagen crosslinks catalyzed by LOX enhance tissue mechanical properties. Reprinted with permission from Makris et al. (2014).
Treatment of rat lung fibroblasts with cobalt chloride (a chemical inducer of a hypoxia-mimicking cell response) induces activation of LOX via the HIF-1 pathway, specifically, through the hypoxia-response element located in the promoter region of LOX (Gao et al., 2013). Treatments combining cobalt chloride and cadmium chloride (an inducer of reactive oxygen species) inhibit HIF-1 expression and binding with LOX (Gao et al., 2013). A study in dermal fibroblasts found that hypoxia promotes collagen deposition via HIF-1α and TGFβ/Smad signaling and that HIF-1α knockdown inhibits TGF-β/Smad signaling (Mingyuan et al., 2018). Moreover, silencing Smad4 decreases HIF-1α significantly when combined with hypoxia (Mingyuan et al., 2018). Furthermore, multiple studies have identified LH2 expression and LH2 activity to increase under hypoxic conditions (Brinckmann et al., 2005; Hofbauer et al., 2003; Horino et al., 2002; van Vlimmeren et al., 2010). Mouse embryonic fibroblasts cultured in a hypoxic environment show a time-dependent 5- to 12-fold transcription up-regulation of PLOD1 and PLOD2, which code for LHs. However, embryonic fibroblasts lacking the HIF-1α subunit are not affected (Hofbauer et al., 2003).
While LOX appears to be impacted by hypoxia, the specific cellular pathways are poorly understood, particularly in tendon. Further investigation of this topic has potential to significantly impact the understanding of the hypoxia-dependent mechanisms governing enzymatic crosslinking in tendon.
TGFβ signaling
TGFβ signaling is a known regulator of tendon differentiation in both embryo and cell culture (Brown et al., 2015; Chien et al., 2018; Havis et al., 2016; Pryce et al., 2009; Tan et al., 2020; Theodossiou et al., 2019; Theodossiou et al., 2021). However, while the interactions between the TGFβ family and LOX have been previously investigated in various tissues, they are not well understood in tendon and necessitate further study. TGFβ1 is a potent inducer of enzymatic crosslinks during fibrosis and scar formation (Fushida-Takemura et al., 1996; Van Bergen et al., 2013). Three members of the TGFβ family (TGFβ1, 2, 3) promote LOX and LOXL1-4 expression in human trabecular meshwork (sponge-like connective tissue located in the proximal side of the eye) cells via both Smad and non-Smad pathways (MAPK/JNK and AP-1) through TGFβR1 and TGFβR2 (Sethi et al., 2011). Additionally, the specific BMP antagonist gremlin induces LOX and LOXL1-4 expression through constitutively active TGFβ/Smad and inducible MAPK/JNK signaling pathways, suggesting BMPs may at least partially mediate enzymatic collagen crosslinking (Sethi et al., 2013). In tendon, more work is needed to understand how the TGFβ family impacts LOX and LOXL1-4.
Glycoprotein interactions
The KGHR sequence is a highly conserved amino acid binding sequence unique to fibrillar collagen and is involved in collagen crosslinking (Rodriguez-Pascual and Slatter, 2016; Sekiya et al., 2011). Currently, the glycoproteins TSP-1 and fibromodulin are the best understood ECM proteins that specifically interact with the KGHR binding motif in collagen. Thbs1-null mice experience altered crosslinking in the dermis, less abundant levels of proLOX and mature LOX, and an increased ratio of mature LOX to proLOX (Rosini et al., 2018). Moreover, TSP-1 inhibits maturation of proLOX through interactions with BMP-1 and collagen type I-III in vitro and binds both intracellular and extracellular collagen through the KGHR binding motif (Kalamajski et al., 2016; Rosini et al., 2018). TSP-1 is a particularly intriguing indirect regulator of enzymatic collagen crosslinking because it regulates fibrillogenesis at the intracellular procollagen level as well as at the extracellular fibrillar collagen level, and through regulation of LOX maturation. Interestingly, TSP-1 is also a well-documented regulator of the TGFβ superfamily in fibrotic diseases (Murphy-Ullrich and Suto, 2018). Further investigation of the various roles of TSP-1 in tendon development and function is warranted.
Fibromodulin deficient mice (Fmod-null) have altered tendons (mechanically weaker and morphologically altered) that exhibit increased LOX-induced mature crosslinks within collagen type I C-telopeptide domains, but LOX itself does not appear to be affected through post-translational modifications (Kalamajski et al., 2014). Fibromodulin, a SLRP, appears to alter the crosslinking behavior through interactions with LOX at the surface molecules of growing fibrils. This does not directly affect LOX quantity or processing, emphasizing the importance of not only stability and quantity of crosslinks but also location on the collagen fibril (Kalamajski et al., 2014; 2016).
Together, these studies on TSP-1 and fibromodulin highlight the importance of further investigating the interactions between glycoproteins that bind to the surface of collagen fibrils (e.g., the KGHR motif) and collagen crosslinking. The dual binding of these glycoproteins to fibrillar surface domains demonstrates that LOX-mediated crosslinking is modulated through a variety of interactions that are not yet well understood in tendon.
Chaperone proteins
One study on CypB (an ER-resident chaperone protein)-null mice, used as a model for studying recessive osteogenesis imperfecta, showed that tail tendons exhibit poorly organized and abnormal morphology. CypB contributes, but does not appear to be essential, to the P3H complex that regulates LH isoforms and procollagen crosslinking (Terajima et al., 2016). Inhibiting CypB in tendon appears to impair fibrillogenesis and collagen morphology by altering lysine hydroxylation of collagen molecules, potentially through modulation of LH1-3 selectivity and activity, alteration of the P3H complex, or modification of collagen-SLRP interactions (Kalamajski et al., 2014; Robinson et al., 2005). The role of chaperone proteins in the regulation of enzymatic crosslinking needs to be further explored in tendon.
Estrogen
Risk of Achilles tendon rupture may be reduced in females compared to males, suggesting an unknown protective mechanism for females (Sarver et al., 2017). Pardes et al. (2016) identified increased material properties (linear and dynamic moduli), decreased viscoelastic properties (hysteresis, percent relaxation), and decreased failure load in female rat Achilles tendons, suggesting female tendons may have more resistance to deformation under load and more efficient energy transfer. Comparing male and female tendons, no differences in tendon organization, cell shape, cellularity, proteoglycan content, or muscle fiber type were observed, indicating an unknown mechanism driving these disparities (Pardes et al., 2016). Minimal information is available on sex-related crosslinking differences, but a potential mechanism involved in these sex-related mechanical differences may include sex hormones, such as estrogen. An in vitro study found that estrogen can impact LOX levels in ligament cells (Lee et al., 2015). Fibroblasts derived from human ACLs, seeded in fibrin gels, and treated with peak physiologically relevant estrogen levels (i.e., 3–4 d before ovulation) have reduced mechanical properties (ultimate tensile stress, modulus) and LOX activity, but no changes in collagen content. Interestingly, LOX activity is more severely inhibited with estrogen treatment than LOX mRNA, suggesting estrogen may affect LOX primarily post-translationally (Lee et al., 2015).
When comparing the results of these studies, it is important to note that while Achilles tendons in females appear to be more protected from injury than males, females have an increased risk of ACL rupture, especially during menstruation (Arendt and Dick, 1995; Sarver et al., 2017). Unique sex-related differences between ligaments (e.g., ACL) and tendons (e.g., Achilles) and rates of injury may be attributed to the different mechanical roles of the tissues, but also several other confounding factors (Q-angle, age, sport being played, etc.). Together, these results suggest LOX may be partially controlled post-translationally by estrogen and justifies further investigation into the effects of estrogen on collagen crosslinking (e.g., LOX, LH, BMP-1, etc.) within tendon.
Tissue engineering applications of LOX
Manipulation of LOX and associated proteins for tendon tissue engineering applications shows promise and recent interest. A 2020 patent titled “Enhancing Tissue Mechanical Properties” was filed (patent number US20200268935A1; Web ref. 1). This patent proposed to enhance tissue mechanical properties by increasing LOX or LOXL1-4 activity using techniques including mechanical stimulation to treat injuries and related musculoskeletal conditions (Web ref. 1).
Although currently less investigated for tendon tissue engineering applications, scaffold-free approaches have been attempted to regulate LOX-mediated collagen crosslinking for skin and cartilage in order to improve tissue formation. A scaffold-free 3D skin model was constructed from cultured fibroblasts derived from human stromal tissues and, when treated with TGFβ1 and BAPN (to inhibit LOX and LOXL4), appeared to be less fibrogenic compared to TGFβ1-only treated controls (Huang et al., 2019). Thus, inhibition of LOX and/or associated proteins within tissues that experience post-injury fibrosis (e.g., excessive amount of collagen often leading to scar formation), such as tendon, has the potential to facilitate restoration of pre-injury mechanical function. Similarly, injection of BAPN combined with controlled exercise has been used to improve fiber alignment and reduce the risk of reinjury in adult horse tendons (Dyson, 2004), potentially through a similar mechanism relating LOX/LOXL4 and antifibrogenic phenotypes seen in the fibrotic skin models (Huang et al., 2019). For cartilage tissue engineering applications, LOXL2 has been used in combination with TGFβ1 and chondroitinase-ABC to form 3D neocartilage from expanded articular chondrocytes (Kwon et al., 2019). This novel strategy combining manipulation of enzymatic crosslinkers using exogenous LOXL2 supplementation appears to be a promising technique for engineering more physiologically relevant cartilage constructs (Kwon et al., 2019). To the authors’ knowledge, the enhancement of exogenous LOX activity has not been performed towards engineered tendon formation in vitro.
LOX and associated collagen crosslinks have been used in tissue engineering applications but have yet to be fully explored for tendon applications. Further understanding of the effect of LOX on mechanical properties and LOX regulation will prove beneficial to develop novel tendon tissue engineering strategies.
Non-enzymatic crosslinking
Differently from enzymatic crosslinks, non-enzymatic crosslinks typically form after adolescence and accumulate throughout adulthood. It was suggested that slow turnover of collagen in tendon allows for non-enzymatic crosslinking to accumulate (Avery and Bailey, 2005; Mentink et al., 2002), with the most prevalent type of non-enzymatic crosslinks being AGEs (see Table 2 for a summary on recent studies investigating AGE formation and impacts on tendon). AGEs form through Maillard reactions when reducing sugars, such as glucose, react with proteins (Fig. 2b) (Ahmed et al., 2012). The Maillard reaction is the same reaction that causes bread and meat to brown under high heat and tendons that have accumulated a large amount of AGEs appear yellow due to this reaction (Lee and Veres, 2019). Glucosepane is an AGE derived from D-glucose and presents lysine-arginine covalent links. Glucosepane is the most commonly found AGE in tendons, occurring 1,000 times more frequently than other AGEs (Jost et al., 2018). Pentosidine, a similar AGE, commonly used as a quantitative biomarker for AGEs, is formed from ribose and presents lysine-arginine links.
Table 2.
Recent studies on AGE formation and impacts in tendon.
| Treatment | Species/tissue | Age/time point | Analysis | Major findings | Source |
|---|---|---|---|---|---|
| Aging | Human patellar tendon | 67 ± 3 years, 27 ± 2 years | Mechanical testing, collagen crosslink analysis | Aged tendons show increased HP, LP, and pentosidine crosslinks and decreased maximum force | Couppé et al, 2009 |
| Diabetic conditions | Mouse tail tendon | 26 weeks old | HPLC for crosslink analysis, mass spectrometry | AGE-mediated crosslinks interact in the same Hyl domains as enzymatic crosslinks | Hudson et al., 2018 |
| Human Achilles tendon | 58–60 years old | Mechanical testing, TEM | Diabetic patients show increased Young’s modulus in tendon | Couppé et al., 2016 | |
| Rat tail tendon | 9–10 weeks old | Collagen solubility, Ehrlich test, fluorescence imaging | Green tea treatment decreases markers of advanced glycation and increases collagen solubility | Babu et al., 2008 | |
| High AGE diet | Rat Achilles and tail tendons | 40 weeks old | Mass spectrometry, HPLC | High-AGE diet increases AGE content in Achilles and tail tendons, with Achilles having higher AGE levels than tail tendons | Skovgaard et al., 2017 |
| Ribose | Bovine tail tendons | 18–30 months old | Mechanical testing, scanning calorimetry, SEM | Ribose treatment alters nanoscale collagen fibril deformation mechanisms in tendon | Lee and Veres, 2019 |
| Rat tail tendon | 6 months old | Fluorescence imaging, enzyme susceptibility assay, mechanical testing | Ribose-treated and mechanically loaded tendons are more susceptible to collagenase activity | Bourne et al., 2015 | |
| MGO | Rat tail tendon | 3, 12, and 22 months old | Collagen solubility, SDS-PAGE, mass spectrometry | Glucosepane is the predominant AGE; MGO treatment increases tendon stability | Jost et al., 2018 |
| Rat tail tendon | > 17 weeks old | Mechanical testing, multiphoton imaging | MGO treatment diminishes fiber-fiber sliding and increases fiber stretch | Li et al., 2013 | |
| Rat tail tendon | 17–24 weeks old | Mechanical testing, SAXS | MGO treatment alters molecular collagen deformation mechanisms (increased fibril failure resistance and reduced molecular sliding within fibrils) | Fessel et al., 2014 | |
| Glutaraldehyde | Rat tail tendon | Adult | Nanoindentation | Glutaraldehyde treatment results in a more brittle behavior during fibril fracture | Gachon and Mesquida, 2020 |
| Sodium borohydride | Mouse tail tendon | 11 weeks old | Mechanical testing, HPLC mass spectrometry | Reduction with sodium borohydride decreases stress relaxation and plastic deformation and increases failure stress | Stammers et al., 2020 |
Originally, AGEs were thought to result in spontaneous and non-specific crosslinking between helical regions of collagen molecules, which contrasts with mature enzymatic crosslinks that primarily form at the telopeptidyl region of collagen molecules in tendon (Eyre et al., 2008). However, a recent study found that AGE-mediated crosslinks occur on the same helical Hyl sites in the collagen molecule as enzymatic crosslinks, suggesting that AGEs may impair the enzymatic crosslinking profile through steric interactions and have the potential to impact tissue mechanical properties (Hudson et al., 2018). These findings focused on Hyl-derived glycations and only minorly examined lysine-derived glycations (because rat tail tendons have an appreciably lower concentration of mature lysine-derived crosslinks). Therefore, understanding the location of both Hyl- and lysine-glycated crosslinks and how they interact with enzymatic crosslinking in tendon needs further study.
AGE accumulation due to aging
AGEs increase as a function of age and may play a role in the age-related changes of tendon mechanical properties. Biochemical analysis of old (ages 67 ± 3 years) and young (ages 27 ± 2 years) human patellar tendons found aged tendons have decreased total collagen level, increased levels of both HP and LP enzymatic crosslinks, and increased pentosidine level (as a representative AGE), compared to young tendons (Couppé et al., 2009). The increased crosslinking seen in aged patellar tendon was associated with a decreased maximum force; however, other properties (strain, stiffness, stress, modulus, or CSA) were not affected (Couppé et al., 2009). In contrast, a different study using C57BL/6 mice tail tendons found that while immature crosslink levels decreases with age (resulting in a net decrease in total crosslinks), mature crosslinks (pyridinoline, glucosepane, pentosidine) and total lysine glycation levels (Fig. 4b) increase with age, suggesting that changes to both enzymatic and non-enzymatic crosslinks may contribute to tendon stiffening with age (Fig. 4c) (Stammers et al., 2020a). Together, these results justify further investigation of the specific changes of non-enzymatic crosslinks within tendon over time.
Several changes in structural (reduced tendon CSA and fibril diameter), cellular (decreased cell proliferation and stem/progenitor cell quantity), and mechanical properties (reduced modulus and strength), beyond just alterations to AGE-mediated crosslinks, occur in tendon during the aging process and have been recently reviewed in detail (Svensson et al., 2016). Briefly, few studies have specifically examined the relationship between aging and collagen deformation mechanisms (i.e., fiber sliding, helical rotation, etc.). One study explored the age-specific response of equine SDFTs, an energy-storing tendon, and found that aged tendons have a decreased ability to withstand fatigue loading (cyclic loading) due to aged fascicles being less capable of helical rotations (Thorpe et al., 2014). While the specific mechanisms culminating in impaired helical twist were not examined, other age-associated changes, such as age-related AGE accumulation and crosslinking (Stammers et al., 2020a), could account for the diminished mechanical response through interaction with the collagen matrix.
AGE accumulation due to diabetes and diet
The slow turnover rate of collagen molecules within tendon makes it a tissue particularly susceptible to excess free glucose associated with hyperglycemia, as seen in diabetic patients, and it may induce excessive AGE accumulation at more dramatic rates than healthy counterparts (Brennan, 1989; Lee and Veres, 2019). Diabetic conditions are known to increase the elastic modulus in human Achilles tendon (Couppé et al., 2016). However, in diabetic mouse Achilles, supraspinatus, and patellar tendons stiffness decreases but the modulus is not affected (Connizzo et al., 2014). The underlying mechanisms driving these changes in mouse and human are unknown. Diabetic mice have decreased tail tendon collagen solubility compared to non-diabetic mice, suggesting that AGE accumulation may limit collagen remodeling and make the tendon more susceptible to microdamage accumulation (Babu et al., 2008). However, AGE accumulation is partially mitigated by a green tea treatment (Babu et al., 2008). Specifically, a green tea extract decreases Ehlrlich-positive material (i.e., tryptophan positive proteins), a marker of advanced glycation, and increases the tendon solubility of diabetic mice, suggesting hyperglycemic-induced glycation and AGEs can be therapeutically targeted.
AGE accumulation is also affected by the diet. Mice fed a normal diet with a large AGE content show higher levels of AGEs (pentosidine, carboxymethyllysine, methylglyoxal-derived hydroimidazolone) in both Achilles and tail tendons (with the Achilles having higher levels than the tail tendons) compared to mice fed a high-fat low-AGE content diet (Skovgaard et al., 2017). Mechanisms governing these tendon-specific responses to diet are not well understood but could be attributed to functionally distinct loading environments and collagen turnover. More specifically, previous work has identified increased AGE content and reduced collagen turnover in high strain, frequently injured tendons (equine SDFT) compared to low strain, infrequently injured tendons (equine common digital extensor tendon) (Thorpe et al., 2010b), reinforcing a potential relationship between loading environment and diet-induced AGE accumulation. Further study into the mechanisms governing tendon-specific diet-induced AGE accumulation and the potentially protective effects of diet will be needed.
In vitro evaluation of AGE accumulation effects on tendon mechanics
Ribose and MGO are commonly used treatments that induce AGE formation and can be used to study the effects of non-enzymatic crosslinking on tendon. These treatments are effective at accelerating and simulating AGE accumulation starting from glucose in vitro (normally a gradual process in vivo) in tendon.
Ribose-induced AGEs
Ribose-glycated rabbit Achilles tendons show increased mechanical properties (maximum stress, strain, Young’s modulus, stiffness) and pentosidine (as a representative AGE), but decreased total collagen content (Reddy, 2004). In another study, two-month-old mouse tail tendons treated with ribose demonstrated increased hydration levels and decreased transverse stiffness (Andriotis et al., 2019). Accordingly, as tissue hydration can affect mechanical properties (Ntim et al., 2005), AGE accumulation could be impacting tissue mechanics through changes to hydration levels. Further work using ribose treatment has shown that AGE crosslinking negatively affects the ability of collagen fibrils to respond to loading after the yield point and results in a mechanically inferior tissue (Lee and Veres, 2019). An 11 d-glycated rat tail tendon collagen fibril showed that the intermolecular spacing increased by 0.05 nm and the D-period length decreased by 0.04 nm (Gautieri et al., 2017). However, a possible confounding factor is that ribose models tend to use a supraphysiological ribose concentration, which may induce an excessive AGE crosslinking profile. Thus, conclusions from these models will need to be confirmed in vivo (Gautieri et al., 2017; Vicens-Zygmunt et al., 2015). Together, these studies suggest that ribose treatment to stimulate the formation of AGEs is viable and could be further utilized to study the impacts of non-enzymatic crosslinks upon multiscale tendon mechanics.
MGO-induced AGEs
MGO is a dicarbonyl derived as a metabolic by-product of glycolysis and forms AGEs at a quicker rate than in vivo glucose reactions. Although, similarly to ribose, excessive MGO treatments can be less physiologically relevant (Li et al., 2013; Schalkwijk and Stehouwer, 2020). Rat tail tendons with MGO-derived AGEs show diminished fiber sliding and increased fiber-stretch but no obvious fibril-level ultrastructural changes (Li et al., 2013). Furthermore, MGO-treatment affects the tendon biomechanics (reduced stress relaxation, increased yield stress and ultimate failure stress). These results may suggest that the tissue changes associated with AGE accumulation could be driven by alterations to fiber deformation mechanisms, such as fiber-fiber sliding. Further investigation using SAXS found that MGO-treated fibrils exhibited altered molecular deformation (the associated quarter-staggered helical conformation was more resistant to strain) when mechanically loaded compared to untreated controls (Fessel et al., 2014). An increase in fibril failure resistance was also identified by a diminished capacity for collagen molecule-molecule sliding within fibrils, an observation potentially driven by AGEs bonding between fibrils. Interestingly, these changes to fibril deformation mechanics did not correspond to changes in fibril modulus and, thus, lend further support to the previous findings that AGE-derived mechanical changes are likely driven by changes at a larger scale, such as fiber-fiber sliding (Fessel et al., 2014; Li et al., 2013). These findings, while highly informative, are challenged by a lack of understanding regarding the specific number of MGO-mediated crosslinks and their location within the tail tendons. However, MGO models appear to be an effective means for studying AGE accumulation in tendon.
Glutaraldehyde-induced AGEs
Glutaraldehyde is another potent inducer of non-enzymatic crosslinks. Gachon and Mesquida (2020) found that collagen fibrils from adult rat tail tendons treated with glutaraldehyde do not show normal strain-stiffening and strain-softening behavior (i.e., excessive crosslinking causes fibrils to continue stiffening through higher strains, resulting in a more brittle behavior during fracture), differently from untreated control fibrils that exhibit strain-stiffening and strain-softening (i.e., fibrils initially straighten in response to strain before the fibrils themselves undergo softening at higher strains). Notably, these findings contradict a similar work on rat tail tendons using MGO-derived crosslinks that suggests AGE accumulation impacts tissue mechanics through fiber-level mechanisms, not alterations to fibrils (Li et al., 2013). Disparities between these studies may be attributed to different methods to induce non-enzymatic crosslinks (i.e., glutaraldehyde vs. MGO) and mechanical testing platforms. However, it will be important to identify how different crosslinking mechanisms affect fibril and fiber-level mechanics in tendon.
Glucosepane-induced AGEs
The effects of glucosepane, the most abundant lysine-arginine-derived AGE, have been recently investigated using computational modeling. Nash et al. (2019) coupled experimental methods with a computational model. Glucosepane and hydration levels increase in human Achilles and tibialis anterior tendons over time. Using molecular dynamics simulations, intramolecular glucosepane was demonstrated to negatively alter collagen structure and ordered collagen packing, as well as to increase hydration (unbound water molecules) around collagen molecules (Nash et al., 2019). A different simulation found that glucosepane and DOGDIC (another AGE crosslink) accumulation potentially increase Young’s modulus at low-range strains (0–15 %). However, the effects vary depending on where along the collagen molecules the crosslinks occur (Collier et al., 2018). A similar study focusing on arginine-lysine MODIC, GODIC, and DOGDIC crosslinks showed that, differently from glucosepane, DOGDIC seems to not inhibit collagen organization through spatial interactions but rather facilitate ordered collagen packing (Nash et al., 2021). These findings could be attributed to the smaller structure of DOGDIC compared to glucosepane (i.e., glucosepane has a much larger and obstructive structure than DOGDIC) altering its binding characteristics. Experimentally derived observations to validate these models will be an important future direction as well as identifying the specific binding locations. Overall, these findings justify both further investigation of various AGEs in tendon and the development of new methods to study AGEs in vivo.
Regulation of AGEs by mechanical loading
Mechanical loading and AGEs both have protective effects against collagen-remodeling enzymes (Paik et al., 2006; Wyatt et al., 2009; Zareian et al., 2010). However, more recent work investigating AGE crosslinking in tendon found that loading, when combined with AGE accumulation, increases enzymatic degradation rates (Bourne et al., 2014). Specifically, rat tail tendons treated with ribose for 3 (less crosslinked) or 7 d (more crosslinked) to induce AGE crosslinking and, then, exposed to tensile strains were both more susceptible to degradation from bacterial collagenases compared to controls (Bourne et al., 2014). This observation could possibly be explained by the accumulation of AGEs disrupting the regular force-transferring mechanisms of collagen fibrils. The relationship between non-enzymatic crosslinking and loading was explored in mature rat tail tendons treated with sodium borohydride to fix the number of crosslinks and glycated lysine residues within the tissue (Stammers et al., 2020b). Sodium borohydride-fixed tendons stretched to 4 % strain exhibited an impaired mechanical response (reduced stress relaxation, increased failure stress, and reduced plastic deformation, i.e., being more brittle), demonstrating that dynamic crosslinking and glycation (glycations and crosslinks breaking and reforming in response to stimuli to attenuate mechanical load) are necessary for proper tissue function (Stammers et al., 2020b). Another study explored the effects of human life-long endurance running (as an extended loading environment model) and found that both young and aged runners show tendon hypertrophy and a 21 % decrease in AGE density, compared to aged controls (Couppé et al., 2014). The observed decreases in AGE content from endurance running could suggest a mechanoresponsive behavior in tendon AGE accumulation, perhaps relating to a relationship between long-term mechanical loading and dynamic crosslinking. These studies describe a complex relationship between non-enzymatic collagen crosslinks and mechanical loading that deserves further study.
Overall, non-enzymatic crosslinks, specifically AGEs, are physiologically relevant in tendon because of diabetes and aging. AGEs form through the relatively slow process of glycation that, when coupled with the slow turnover rate of collagen in tendon, leads to an accumulation of crosslinks during aging that affects tendon mechanical properties. AGE accumulation appears to impact collagen at the fiber and fibril levels, and conditions that induce excessive AGE formation are linked to negative tendon outcomes. Therefore, further investigation is needed to determine the specific mechanical effects of AGEs, how they interact with collagen molecules, and to identify mechanisms that may regulate AGE accumulation and removal.
Conclusion
Enzymatic and non-enzymatic collagen crosslinking plays a role in regulating the mechanical function of tendon. Multiple collagen crosslinking enzymes (e.g., LOX and LOXL2-4) are needed for proper collagen crosslinking both during and after fibrillogenesis, which mediates the mechanical formation of tendon and may distinguish functionally distinct tendons (energy storing vs. positional). However, the mechanisms governing enzymatic crosslinking are not well understood and require further investigation. Potential regulators of enzyme production at the cellular level may include mechanical stimuli, mechanotransducive cell signaling pathways, sex hormones, TGFβ family, hypoxia, and interactions with intracellular or extracellular proteins.
AGE crosslinks derived from non-enzymatic interactions resulting from free glucose impact tendon function. AGEs, which accumulate during the normal aging process, can be accelerated under diabetic conditions and may also be affected by diet. The specific consequences of AGE accumulation are not entirely understood but may alter both fiber and fibril behavior. An improved understanding of the exact location and process through which these crosslinks form is needed. Overall, enzymatic and non-enzymatic crosslinks affect tendon biomechanics and, thus, a better understanding of their regulators can advance future clinical treatments for tendon disease, injury, and age-related conditions.
Acknowledgments
This work was supported by funding from the Idaho NASA EPSCoR Program, NIH/NIBIB (R03EB024134), INBRE program, P20 GM103408 (National Institutes of General Medical Sciences), and Beckman Scholars Award from the Arnold and Mabel Beckman Foundation (to NMP).
List of Abbreviations
- ACL
anterior cruciate ligament
- AGE
advanced glycation end product
- AP-1
activating protein-1
- BAPN
β-aminopropionitrile
- BMP-1
bone morphogenetic protein-1
- Col1a1
collagen type I alpha 1 chain
- Col3a1
collagen type III alpha 1 chain
- CRL
cytokine receptor-like
- CSA
cross-sectional area
- CypB
cyclophilin B
- DOGDIC
3-deoxyglucosone-derived imidazolium
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- GODIC
glyoxal-derived imidazolium
- HH
Hamburger-Hamilton
- HIF
hypoxia inducible factor
- HP
hydroxylysyl pyridinoline
- hPDL
human periodontal ligament
- HPLC
high-performance liquid chromatography
- Hyl
hydroxylysine
- JNK
c-Jun N-terminal kinases
- LH
lysyl hydroxylase
- LOX
lysyl oxidase
- LOXL
LOX-like protein
- LOX-PP
LOX pro-peptide
- LP
lysyl pyridinoline
- LTQ
lysyltyrosine quinone
- MAPK
mitogen-activated protein kinase
- MCL
medial collateral ligament
- MCP
2-mercaptopyridine-N-oxide
- MGO
metabolite methylglyoxal
- MMP
matrix metalloproteinase
- MODIC
methylglyoxal-derived imidazolium
- P3H
prolyl-3-hydroxylase
- P4HA
prolyl 4-hydroxylase subunit alpha
- PCP
procollagen-C-proteinase
- PDI
protein disulfide isomerase
- PDL
periodontal ligament
- PIEZO1
Piezo type mechanosensitive ion channel component 1
- PLOD
procollagen lysyl hydroxylase
- Pyr
pyridinoline
- RT-PCR
reverse transcription polymerase chain reaction
- SAXS
small-angle X-ray scattering
- SDFT
superficial digital flexor tendon
- SDS-PAGE
sodium dodecyl sulfatepolyacrylamide gel electrophoresis
- SEM
scanning electron microscopy
- SLRP
small leucine-rich proteoglycan
- SNAIL
snail
- SOX9
SRY-box transcription factor 9
- TCP
trans-2-phenylcylopropylamine
- TEM
transmission electron microscopy
- TG
transglutaminase
- TGFβ
transforming growth factor beta
- TGFβR
TGFβ receptor
- Thbs1
thrombospondin-1 gene
- TIMP
tissue inhibitor of MMP
- TSP-1
thrombospondin-1
References
- Ahmed AS, Schizas NL,J, Ahmed M, Ostenson C-G, Salo P, Hewitt C, Hart DA, Ackermann PW (2012) Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol 113: 1784–1791. [DOI] [PubMed] [Google Scholar]
- Andriotis OG, Elsayad K, Smart DE, Nalbach M, Davies DE, Thurner PJ (2019) Hydration and nanomechanical changes in collagen fibrils bearing advanced glycation end-products. Biomed Opt Express 10: 1841. DOI: 10.1364/BOE.10.001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou ES, Sund S, Homsi EN, Challenger LF, Rameshwar P (2004) A theoretical simulation of hematopoietic stem cells during oxygen fluctuations: prediction of bone marrow responses during hemorrhagic shock. Shock 22: 415–422. [DOI] [PubMed] [Google Scholar]
- Arendt E, Dick R (1995) Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med 23: 694–701. [DOI] [PubMed] [Google Scholar]
- Avery NC, Bailey AJ (2005) Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports 15: 231–240. [DOI] [PubMed] [Google Scholar]
- Babu PVA, Sabitha KE, Shyamaladevi CS (2008) Effect of green tea extract on advanced glycation and cross-linking of tail tendon collagen in streptozotocin induced diabetic rats. Food Chem Toxicol 46: 280–285. [DOI] [PubMed] [Google Scholar]
- Banos CC, Thomas AH, Kuo CK (2008) Collagen fibrillogenesis in tendon development: current models and regulation of fibril assembly. Birth Defects Res C Embryo Today 84: 228–244. [DOI] [PubMed] [Google Scholar]
- Bayer ML, Yeung CYC, Kadler KE, Qvortrup K, Baar K, Svensson RB, Magnusson SP, Krogsgaard M, Koch M, Kjaer M (2010) The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials 31: 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JW, Lippell JM, Torzilli PA (2014) Glycation cross-linking induced mechanical-enzymatic cleavage of microscale tendon fibers. Matrix Biol 34: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan M (1989) Changes in solubility, non-enzymatic glycation, and fluorescence of collagen in tail tendons from diabetic rats. J Biol Chem 264: 20947–20952. [PubMed] [Google Scholar]
- Brinckmann J, Kim S, Wu J, Reinhardt DP, Batmunkh C, Metzen E, Notbohm H, Bank RA, Krieg T, Hunzelmann N (2005) Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Biol 24: 459–468. [DOI] [PubMed] [Google Scholar]
- Brown JP, Galassi TV, Stoppato M, Schiele NR, Kuo CK (2015) Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res Ther 6: 89. DOI: 10.1186/s13287-015-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty EG, Kadler KE (2005) Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 118: 1341–1353. [DOI] [PubMed] [Google Scholar]
- Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, Huffman A, O’Toole ET, Kadler KE (2006) Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem 281: 38592–38598. [DOI] [PubMed] [Google Scholar]
- Carroll CC, Whitt JA, Peterson A, Gump BS, Tedeschi J, Broderick TL (2012) Influence of acetaminophen consumption and exercise on Achilles tendon structural properties in male Wistar rats. Am J Regul Integr Comp Physiol 302: R990–R995. [DOI] [PubMed] [Google Scholar]
- Chen Y-J, Jeng J-H, Chang H-H, Huang M-Y, Tsai F-F, Jane Yao C-C (2013) Differential regulation of collagen, lysyl oxidase and MMP-2 in human periodontal ligament cells by low- and high-level mechanical stretching. J Periodontal Res 48: 466–474. [DOI] [PubMed] [Google Scholar]
- Chien C, Pryce B, Tufa SF, Keene DR, Huang AH (2018) Optimizing a 3D model system for molecular manipulation of tenogenesis. Connect Tissue Res 59: 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DC, Wenning LA, Miller WM, Papoutsakis ET (2001) Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J 81: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TA, Nash A, Birch HL, de Leeuw NH (2018) Effect on the mechanical properties of type I collagen of intra-molecular lysine-arginine derived advanced glycation end-product cross-linking. J Biomech 67: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connizzo BK, Bhatt PR, Liechty KW, Soslowsky LJ (2014) Diabetes alters mechanical properties and collagen fiber re-alignment in multiple mouse tendons. Ann Biomed Eng 42: 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjær M, Magnusson SP (2009) Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107: 880–886. [DOI] [PubMed] [Google Scholar]
- Couppé C, Svensson RB, Grosset J-F, Kovanen V, Nielsen RH, Olsen MR, Larsen JO, Praet SFE, Skovgaard D, Hansen M, Aagaard P, Kjaer M, Magnusson SP (2014) Life-long endurance running is associated with reduced glycation and mechanical stress in connective tissue. Age (Dordr) 36: 9665. DOI: 10.1007/s11357-014-9665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppé C, Svensson RB, Kongsgaard M, Kovanen V, Grosset J-F, Snorgaard O, Bencke J, Larsen JO, Bandholm T, Christensen TM, Boesen A, Helmark IC, Aagaard P, Kjaer M, Magnusson SP (2016) Human Achilles tendon glycation and function in diabetes. J Appl Phsiol 120: 130–137. [DOI] [PubMed] [Google Scholar]
- Cronshaw AD, Fothergill-Gilmore LA, Hulmes DJS (1995) The proteolytic processing site of the precursor of lysyl oxidase. Biochem J 306: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deymier AC, An Y, Boyle JJ, Schwartz AG, Birman V, Genin GM, Thomopoulos S, Barber AH (2017) Micro-mechanical properties of the tendon-to-bone attachment. Acta Biomater 56: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deymier-Black AC, Pasteris JD, Genin GM, Thomopoulos S (2015) Allometry of the tendon enthesis: mechanisms of load transfer between tendon and bone. J Biomech Eng 137: 111005. DOI: 10.1115/1.4031571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC (2006) Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 39: 513–522. [DOI] [PubMed] [Google Scholar]
- Dyson SJ (2004) Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992–2000). Equine Vet J 36: 415–419. [DOI] [PubMed] [Google Scholar]
- Eckert RL, Kaartinen MT, Nurminskaya M, Belkin AM, Colak G, Johnson GVW, Mehta K (2014) Transglutaminase regulation of cell function. Physiol Rev 94: 383–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eekhoff JD, Fang F, Kahan LG, Espinosa G, Cocciolone AJ, Wagenseil JE, Mecham RP, Lake SP (2017) Functionally distinct tendons from elastin haploinsufficient mice exhibit mild stiffening and tendon-specific structural alteration. J Biomech Eng 139: 1110031–1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eekhoff JD, Fang F, Lake SP (2018) Multiscale mechanical effects of native collagen cross-linking in tendon. Connect Tissue Res 59: 410–422. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Paz MA, Gallop PM (1984) Cross-linking in collagen and elastin. Annu Rev Biochem 53: 717–748. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Weis MA, Wu J-J (2008) Advances in collagen cross-link analysis. Methods 45: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel G, Li Y, Diederich V, Guizar-Sicairos M, Schneider P, Sell DR, Monnier VM, Snedeker JG (2014) Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS One 9: e110948. DOI: 10.1371/journal.pone.0110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushida-Takemura H, Fukuda M, Maekawa N, Chanoki M, Kobayashi H, Yashiro N, Ishii M, Hamada T, Otani S, Ooshima A (1996) Detection of lysyl oxidase gene expression in rat skin during wound healing. Arch Dermatol Res 288: 7–10. [DOI] [PubMed] [Google Scholar]
- Gacheru SN, Trackman PC, Shah MA, O’Gara CY, Spacciapoli P, Greenaway FT, Kagan HM (1990) Structural and catalytic properties of copper in lysyl oxidase. J Biol Chem 265: 19022–19027. [PubMed] [Google Scholar]
- Gachon E, Mesquida P (2020) Stretching single collagen fibrils reveals nonlinear mechanical behavior. Biophys J 118: 1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Zhou J, Zhao Y, Toselli P, Li W (2013) Hypoxia-Response Element (HRE)-directed transcriptional regulation of the rat lysyl oxidase gene in response to cobalt and cadmium. Toxicol Sci 132: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautieri A, Passini FS, Silván U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaelli A, Berli M, Snedeker JG (2017) Advanced glycation end-products: mechanics of aged collagen from molecule to tissue. Matrix Biol 59: 95–108. [DOI] [PubMed] [Google Scholar]
- Gerriets JE, Curwin SL, Last JA (1993) Tendon hypertrophy is associated with increased hydroxylation of nonhelical lysine residues at two specific cross-linking sites in type I collagen. J Biol Chem 268: 25553–25560. [PubMed] [Google Scholar]
- Gjaltema RAF, Bank RA (2017) Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit Rev Biochem Mol Biol 52: 74–95. [DOI] [PubMed] [Google Scholar]
- Grimsby JL, Lucero HA, Trackman PC, Ravid K, Kagan HM (2010) Role of lysyl oxidase propeptide in secretion and enzyme activity. J Cell Biochem 111: 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw MJ, Mason RM (2000) Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthr Cartil 8: 386–392. [DOI] [PubMed] [Google Scholar]
- Hajdú I, Kardos J, Major B, Fabó G, Lőrincz Z, Cseh S, Dormán G (2018) Inhibition of the LOX enzyme family members with old and new ligands. Selectivity analysis revisited. Bioorg Med Chem Lett 28: 3113–3118. [DOI] [PubMed] [Google Scholar]
- Havis E, Bonnin MA, Esteves de Lima J, Charvet B, Milet C, Duprez D (2016) TGFβ and FGF promote tendon progenitor fate and act downstream of muscle contraction to regulate tendon differentiation during chick limb development. Development 143: 3839–3851. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M (2013) Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J 27: 2074–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herchenhan A, Uhlenbrock F, Eliasson P, Weis M, Eyre D, Kadler KE, Magnusson SP, Kjaer M (2015) Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem 290: 16440–16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod TW, Chambers NC, Veres SP (2016) Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading. Acta Biomater 42: 296–307. [DOI] [PubMed] [Google Scholar]
- Hijazi KM, Singfield KL, Veres SP (2019) Ultrastructural response of tendon to excessive level or duration of tensile load supports that collagen fibrils are mechanically continuous. J Mech Behav Biomed 97: 30–40. [DOI] [PubMed] [Google Scholar]
- Hofbauer K-H, Gess B, Lohaus C, Meyer HE, Katschinski D, Kurtz A (2003) Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur J Biochem 270: 4515–4522. [DOI] [PubMed] [Google Scholar]
- Horino Y, Takahashi S, Miura T, Takahashi Y (2002) Prolonged hypoxia accelerates the posttranscriptional process of collagen synthesis in cultured fibroblasts. Life Sci 71: 3031–3045. [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD (2003) Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem 278: 14387–14393. [DOI] [PubMed] [Google Scholar]
- Huang M, Cai G, Baugh LM, Liu Z, Smith A, Watson M, Popovich D, Zhang T, Stawski LS, Trojanowska M, Georgakoudi I, Black LD, Pioli PA, Whitfield ML, Garlick J (2020) systemic sclerosis dermal fibroblasts induce cutaneous fibrosis through lysyl oxidase-like 4: new evidence from three-dimensional skin-like tissues. Arthritis Rheumatol 72: 791–801. [DOI] [PubMed] [Google Scholar]
- Huang M, Liu Z, Baugh L, DeFuria J, Maione A, Smith A, Kashpur O, Black III LD, Georgakoudi I, Whitfield ML, Garlick J (2019) Lysyl oxidase enzymes mediate TGF-β1-induced fibrotic phenotypes in human skin-like tissues. Lab Invest 99: 514–527. [DOI] [PubMed] [Google Scholar]
- Hudson DM, Archer M, King KB, Eyre DR (2018) Glycation of type I collagen selectively targets the same helical domain lysine sites as lysyl oxidase-mediated cross-linking. J Biol Chem 293: 15620–15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DM, Weis M, Rai J, Joeng KS, Dimori M, Lee BH, Morello R, Eyre DR (2017) P3h3-null and Sc65-null mice phenocopy the collagen lysine under-hydroxylation and cross-linking abnormality of Ehlers-Danlos syndrome type VIA. J Biol Chem 292: 3877–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikhar M, Hurtado P, Bais MV, Wigner N, Stephens DN, Gerstenfeld LC, Trackman PC (2011) Lysyl oxidase-like-2 (loxl2) is a major isoform in chondrocytes and is critically required for differentiation. J Biol Chem 286: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KR, Lipp S, Acuna A, Leng Y, Bu Y, Calve S (2020) Comparative analysis of the extracellular matrix proteome across the myotendinous junction. J Proteome Res 19: 3955–3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MG, Andriotis OG, Roberts JJ, Lunn K, Tear VJ, Cao L, Ask K, Smart DE, Bonfanti A, Johnson P, Alzetani A, Conforti F, Doherty R, Lai CY, Johnson B, Bourdakos KN, Fletcher SV, Marshall BG, Jogai S, Brereton CJ, Chee SJ, Ottensmeier CH, Sime P, Gauldie J, Kolb M, Mahajan S, Fabre A, Bhaskar A, Jarolimek W, Richeldi L, O’Reilly KM, Monk PD, Thurner PJ, Davies DE (2018) Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. Elife 7: e36354. DOI: 10.7554/eLife.36354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost T, Zipprich A, Glomb MA (2018) Analysis of advanced glycation endproducts in rat tail collagen and correlation to tendon stiffening. J Agric Food Chem 66: 3957–3965. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hill A, Canty-Laird EG (2008) Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol 20: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaku M, Rosales Rocabado JM, Kitami M, Ida T, Akiba Y, Yamauchi M, Uoshima K (2016) Mechanical loading stimulates expression of collagen cross-linking associated enzymes in periodontal ligament: mechanical stress stimulates cross-linking in pdl. J Cell Physiol 231: 926–933. [DOI] [PubMed] [Google Scholar]
- Kalamajski S, Bihan D, Bonna A, Rubin K, Farndale RW (2016) Fibromodulin interacts with collagen cross-linking sites and activates lysyl oxidase. J Biol Chem 291: 7951–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamajski S, Liu C, Tillgren V, Rubin K, Oldberg Å, Rai J, Weis M, Eyre DR (2014) Increased C-telopeptide cross-linking of tendon type I collagen in fibromodulin-deficient mice. J Biol Chem 289: 18873–18879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Saito M, Funasaki H, Marumo K (2015) Distinctive collagen maturation process in fibroblasts derived from rabbit anterior cruciate ligament, medial collateral ligament, and patellar tendon in vitro. Knee Surg Sports Traumatol Arthrosc 23: 1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-M, Kim E-C, Kim Y (2011) The human lysyl oxidase-like 2 protein functions as an amine oxidase toward collagen and elastin. Mol Biol Rep 38: 145–149. [DOI] [PubMed] [Google Scholar]
- Kraft-Sheleg O, Zaffryar-Eilot S, Genin O, Yaseen W, Soueid-Baumgarten S, Kessler O, Smolkin T, Akiri G, Neufeld G, Cinnamon Y, Hasson P (2016) Localized LOXL3-dependent fibronectin oxidation regulates myofiber stretch and integrin-mediated adhesion. Dev Cell 36: 550–561. [DOI] [PubMed] [Google Scholar]
- Kwon H, O’Leary SA, Hu JC, Athanasiou KA (2019) Translating the application of transforming growth factor-β1, chondroitinase-ABC, and lysyl oxidase-like 2 for mechanically robust tissue-engineered human neocartilage. J Tissue Eng Regen Med 13: 283–294. [DOI] [PubMed] [Google Scholar]
- Lee CA, Lee-Barthel A, Marquino L, Sandoval N, Marcotte GR, Baar K (2015) Estrogen inhibits lysyl oxidase and decreases mechanical function in engineered ligaments. J Appl Physiol 118: 1250–1257. [DOI] [PubMed] [Google Scholar]
- Lee JM, Veres SP (2019) Advanced glycation end-product cross-linking inhibits biomechanical plasticity and characteristic failure morphology of native tendon. J Appl Physiol 126: 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-E, Kim Y (2006) A tissue-specific variant of the human lysyl oxidase-like protein 3 (loxl3) functions as an amine oxidase with substrate specificity. J Biol Chem 281: 37282–37290. [DOI] [PubMed] [Google Scholar]
- Leighton MP, Kreplak L, Rutenberg AD (2021) Non-equilibrium growth and twist of cross-linked collagen fibrils. Soft Matter 17: 1415–1427. [DOI] [PubMed] [Google Scholar]
- Li Y, Fessel G, Georgiadis M, Snedeker JG (2013) Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol: 9: 169–177. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T (2004) Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet 36: 178–182. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4: 140–156. [DOI] [PubMed] [Google Scholar]
- Lu HH, Thomopoulos S (2013) Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng 15: 201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero HA, Kagan HM (2006) Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci 63: 2304–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, Soininen R (2002) Inactivation of the lysyl oxidase gene lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509. [DOI] [PubMed] [Google Scholar]
- Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J (2005) Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol 167: 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris EA, MacBarb RF, Responte DJ, Hu JC, Athanasiou KA (2013) A copper sulfate and hydroxylysine treatment regimen for enhancing collagen cross-linking and biomechanical properties in engineered neocartilage. FASEB J. 27: 2421–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA (2014) Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci USA 111: E4832–E4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK (2013) Characterization of mechanical and biochemical properties of developing embryonic tendon. P Natl Acad Sci USA 110: 6370–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marturano JE, Xylas JF, Sridharan GV, Georgakoudi I, Kuo CK (2014) Lysyl oxidase-mediated collagen crosslinks may be assessed as markers of functional properties of tendon tissue formation. Acta Biomater 10: 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentink CJAL, Hendriks M, Levels AAG, Wolffenbuttel BHR (2002) Glucose-mediated cross-linking of collagen in rat tendon and skin. Clinica Chimica Acta 321: 69–76. [DOI] [PubMed] [Google Scholar]
- Mingyuan X, Qianqian P, Shengquan X, Chenyi Y, Rui L, Yichen S, Jinghong X (2018) Hypoxia-inducible factor-1α activates transforming growth factor-β1/Smad signaling and increases collagen deposition in dermal fibroblasts. Oncotarget 9: 3188–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Suto MJ (2018) Thrombospondin-1 regulation of latent TGF-β activation: a therapeutic target for fibrotic disease. Matrix Biol 68–69: 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllylä R, Wang C, Heikkinen J, Juffer A, Lampela O, Risteli M, Ruotsalainen H, Salo A, Sipilä L (2007) Expanding the lysyl hydroxylase toolbox: New insights into the localization and activities of lysyl hydroxylase 3 (LH3). J Cell Physiol 212: 323–329. [DOI] [PubMed] [Google Scholar]
- Nash A, Noh SY, Birch HL, Leeuw NH (2021) Lysine-arginine advanced glycation end-product cross-links and the effect on collagen structure: a molecular dynamics study. Proteins 89: 521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A, Notou M, Lopez-Clavijo AF, Bozec L, de Leeuw NH, Birch HL (2019) Glucosepane is associated with changes to structural and physical properties of collagen fibrils. Matrix Biol Plus 4: 100013. DOI: 10.1016/j.mbplus.2019.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntim M, Bembey A, Ferguson V, Bushby A (2005) Hydration effects on the viscoelastic properties of collagen. MRS Proc 898: 0898-L05–02. DOI: 10.1557/PROC-0898-L05-02. [DOI] [Google Scholar]
- Oliva F, Zocchi L, Codispoti A, Candi E, Celi M, Melino G, Maffulli N, Tarantino U (2009) Transglutaminases expression in human supraspinatus tendon ruptures and in mouse tendons. Biochem Biophys Res Commun 379: 887–891. [DOI] [PubMed] [Google Scholar]
- Paik DC, Saito LY, Sugirtharaj DD, Holmes JW (2006) Nitrite-induced cross-linking alters remodeling and mechanical properties of collagenous engineered tissues. Connect Tissue Research 47: 163–176. [DOI] [PubMed] [Google Scholar]
- Pan XS, Li J, Brown EB, Kuo CK (2018) Embryo movements regulate tendon mechanical property development. Philos Trans R Soc Lond B Biol Sci 373: 1759. DOI: 10.1098/rstb.2017.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardes AM, Freedman BR, Fryhofer GW, Salka NS, Bhatt PR, Soslowsky LJ (2016) Males have inferior achilles tendon material properties compared to females in a rodent model. Ann Biomed Eng 44: 2901–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passini FS, Jaeger PK, Saab AS, Hanlon S, Chittim NA, Arlt MJ, Ferrari KD, Haenni D, Caprara S, Bollhalder M, Niederöst B, Horvath AN, Götschi T, Ma S, Passini-Tall B, Fucentese SF, Blache U, Silván U, Weber B, Silbernagel KG, Snedeker JG (2021) Shear-stress sensing by PIEZO1 regulates tendon stiffness in rodents and influences jumping performance in humans. Nat Biomed Eng 5: 1457–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson-Kane JC, Rich T (2014) Achilles tendon injuries in elite athletes: lessons in pathophysiology from their equine counterparts. ILAR Jl 55: 86–99. [DOI] [PubMed] [Google Scholar]
- Peterson BE, Szczesny SE (2020) Dependence of tendon multiscale mechanics on sample gauge length is consistent with discontinuous collagen fibrils. Acta Biomater 117: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnel S, Martin GR (1958) The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoapdipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci USA 61: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Vanderby R (2006) Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol 25: 71–84. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dunker N, Schweitzer R (2009) Recruitment and maintenance of tendon progenitors by TGF signaling are essential for tendon formation. Development 136: 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GK (2004) Cross-linking in collagen by non-enzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diabesity Res 5: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE (2007) Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Mol Cell Biol 27: 6218–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigozzi S, Müller R, Snedeker JG (2009) Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J Biomech 42: 1547–1552. [DOI] [PubMed] [Google Scholar]
- Risteli M, Niemitalo O, Lankinen H, Juffer AH, Myllylä R (2004) Characterization of collagenous peptides bound to lysyl hydroxylase isoforms. J Biol Chem 279: 37535–37543. [DOI] [PubMed] [Google Scholar]
- Robinson PS, Huang T-F, Kazam E, Iozzo RV, Birk DE, Soslowsky LJ (2005) Influence of decorin and biglycan on mechanical properties of multiple tendons in knockout mice. J Biomech Eng 127: 181–185. [DOI] [PubMed] [Google Scholar]
- Rodríguez C, Rodríguez-Sinovasm A, Martínez-González J (2008) Lysyl oxidase as a potential therapeutic target. Drug News Perspect 21: 218–224. [DOI] [PubMed] [Google Scholar]
- Rodriguez HM, Vaysberg M, Mikels A, McCauley S, Velayo AC, Garcia C, Smith V (2010) Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem 285: 20964–20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pascual F, Slatter DA (2016) Collagen cross-linking: insights on the evolution of metazoan extracellular matrix. Sci Rep 6: 37374. DOI: 10.1038/srep37374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosini S, Pugh N, Bonna AM, Hulmes DJS, Farndale RW, Adams JC (2018) Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci Signal 11: eaar2566. DOI: 10.1126/scisignal.aar2566. [DOI] [PubMed] [Google Scholar]
- Safa BN, Peloquin JM, Natriello JR, Caplan JL, Elliott DM (2019) Helical fibrillar microstructure of tendon using serial block-face scanning electron microscopy and a mechanical model for interfibrillar load transfer. J R Soc Interface 16: 20190547. DOI: 10.1098/rsif.2019.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal GB, Chithra P, Chandrakasan G (1998) Advanced glycation end products induce crosslinking of collagen in vitro. Biochim Biophys Acta Mol Basis Dis 1407: 215–224. [DOI] [PubMed] [Google Scholar]
- Salo AM, Sipilä L, Sormunen R, Ruotsalainen H, Vainio S, Myllylä R (2006) The lysyl hydroxylase isoforms are widely expressed during mouse embryogenesis, but obtain tissue- and cell-specific patterns in the adult. Matrix Biol 25: 475–483. [DOI] [PubMed] [Google Scholar]
- Sarver DC, Kharaz YA, Sugg KB, Gumucio JP, Comerford E, Mendias CL (2017) Sex differences in tendon structure and function: sex differences in tendons. J Orthop Res 35: 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalkwijk CG, Stehouwer CDA (2020) Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev 100: 407–461. [DOI] [PubMed] [Google Scholar]
- Schiele NR, von Flotow F, Tochka ZL, Hockaday LA, Marturano JE, Thibodeau JJ, Kuo CK (2015) actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development: actin contributions to tendon development. J Orthop Res 33: 874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele NR, Marturano JE, Kuo CK (2013) Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr Opin Biotechnol 24: 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screen HRC, Lee DA, Bader DL, Shelton JC (2004) An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng H 218: 109–119. [DOI] [PubMed] [Google Scholar]
- Sekiya A, Okano-Kosugi H, Yamazaki CM, Koide T (2011) Pigment epithelium-derived factor (PEDF) shares binding sites in collagen with heparin/heparan sulfate proteoglycans. J Biol Chem 286: 26364–26374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Mao W, Wordinger RJ, Clark AF (2011) Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophth Vis Sci 52: 5240–5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A, Wordinger RJ, Clark AF (2013) Gremlin utilizes canonical and non-canonical TGFβ signaling to induce lysyl oxidase (LOX) genes in human trabecular meshwork cells. Exp Eye Res 113: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgaard D, Svensson RB, Scheijen J, Eliasson P, Mogensen P, Hag AM, Kjaer M, Schalkwijk CG, Schjerling P, Magnusson SP, Couppe C (2017) An advanced glycation endproduct (AGE)-rich diet promotes accumulation of AGEs in Achilles tendon. Physiol Rep 5: e13215. DOI: 10.14814/phy2.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedeker JG, Gautieri A (2014) The role of collagen crosslinks in ageing and diabetes - the good, the bad, and the ugly: 6. Muscles Ligaments Tendons J 4: 303–308. [PMC free article] [PubMed] [Google Scholar]
- Spargoli G (2016) Partial articular supraspinatus tendon avulsion (pasta) lesion. Current concepts in rehabilitation. Int J Sports Phys Ther 11: 462–481. [PMC free article] [PubMed] [Google Scholar]
- Stammers M, Ivanova IM, Niewczas IS, Segonds-Pichon A, Streeter M, Spiegel DA, Clark J (2020a) Age-related changes in the physical properties, cross-linking, and glycation of collagen from mouse tail tendon. J Biol Chem 295: 10562–10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammers M, Niewczas IS, Segonds-Pichon A, Clark J (2020b) Mechanical stretching changes crosslinking and glycation levels in the collagen of mouse tail tendon. J Biol Chem 295: 10572–10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summey BT, Graff RD, Lai T-S, Greenberg CS, Lee GM (2002) Tissue transglutaminase localization and activity regulation in the extracellular matrix of articular cartilage. J Orthop Res 20: 76–82. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Takahashi M, Abe M, Nagano A (2008) Biochemical study of collagen and its crosslinks in the anterior cruciate ligament and the tissues used as a graft for reconstruction of the anterior cruciate ligament. Connect Tissue Res 49: 42–47. [DOI] [PubMed] [Google Scholar]
- Svensson RB, Heinemeier KM, Couppé C, Kjaer M, Magnusson SP (2016) Effect of aging and exercise on the tendon. J Appl Physiol 121: 1353–1362. [DOI] [PubMed] [Google Scholar]
- Svensson RB, Mulder H, Kovanen V, Magnusson SP (2013) Fracture mechanics of collagen fibrils: influence of natural cross-links. Biophys J 104: 2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RB, Smith ST, Moyer PJ, Magnusson SP (2018) Effects of maturation and advanced glycation on tensile mechanics of collagen fibrils from rat tail and Achilles tendons. Acta Biomater 70: 270–280. [DOI] [PubMed] [Google Scholar]
- Szczesny SE, Fetchko KL, Dodge GR, Elliott DM (2017) Evidence that interfibrillar load transfer in tendon is supported by small diameter fibrils and not extrafibrillar tissue components. J Orthop Res 35: 2127–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny SE, Elliott DM (2014) Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater 10: 2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaluoma K, Lantto J, Myllyharju J (2007) Lysyl hydroxylase 2 is a specific telopeptide hydroxylase, while all three isoenzymes hydroxylate collagenous sequences. Matrix Biol 26: 396–403. [DOI] [PubMed] [Google Scholar]
- Tan GK, Pryce BA, Stabio A, Brigande JV, Wang C, Xia Z, Tufa SF, Keene DR, Schweitzer R (2020) TGFβ signaling is critical for maintenance of the tendon cell fate. Elife 9: e52695. DOI: 10.7554/eLife.52695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SS, Trackman PC, Kagan HM (1983) Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem 258: 4331–4338. [PubMed] [Google Scholar]
- Taye N, Karoulias SZ, Hubmacher D (2020) The “other” 15–40%: the role of non-collagenous extracellular matrix proteins and minor collagens in tendon. J Orthop Res 38: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terajima M, Taga Y, Chen Y, Cabral WA, Hou-Fu G, Srisawasdi S, Nagasawa M, Sumida N, Hattori S, Kurie JM, Marini JC, Yamauchi M (2016) Cyclophilin-B Modulates collagen cross-linking by differentially affecting lysine hydroxylation in the helical and telopeptidyl domains of tendon type I collagen. J Biol Chem 291: 9501–9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodossiou SK, Murray JB, Hold LA, Courtright JM, Carper AM, Schiele NR (2021) Akt signaling is activated by TGFβ2 and impacts tenogenic induction of mesenchymal stem cells. Int J Stem Cell Res Ther 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodossiou SK, Murray JB, Schiele NR (2020) Cell-cell junctions in developing and adult tendons. Tissue Barriers 8: 1695491. DOI: 10.1080/21688370.2019.1695491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodossiou SK, Tokle J, Schiele NR (2019) TGFβ2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem Bioph Res Co 508: 889–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ (2003) Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res 21: 413–419. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Stark RJF, Goodship AE, Birch HL (2010a) Mechanical properties of the equine superficial digital flexor tendon relate to specific collagen cross-link levels: tendon mechanical properties and collagen cross-links. Equine Vet J 42: 538–543. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Riley GP, Birch HL, Clegg PD, Screen HRC (2014) Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. J R Soc Interface 11: 20131058. DOI: 10.1098/rsif.2013.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL (2010b) Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem 285: 15674–15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toroian D, Lim JE, Price PA (2007) The size exclusion characteristics of type I collagen. J Biol Chem 282: 22437–22447. [DOI] [PubMed] [Google Scholar]
- Trackman PC, Bedell-Hogan D, Tang J, Kagan HM (1992) Post-translational glycosylation and proteolytic processing of a lysyl oxidase precursor. J Biol Chem 267: 8666–8671. [PubMed] [Google Scholar]
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong H-H, Greenspan DS, Trackman PC (2001) Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem 276: 22537–22543. [DOI] [PubMed] [Google Scholar]
- Van Bergen T, Marshall D, Van de Veire S, Vandewalle E, Moons L, Herman J, Smith V, Stalmans I (2013) The role of LOX and LOXL2 in scar formation after glaucoma surgery. Invest Ophthalmol Vis Sci 54: 5788–5796. [DOI] [PubMed] [Google Scholar]
- van der Slot AJ, Zuurmond A-M, Bardoel AFJ, Wijmenga C, Pruijs HEH, Sillence DO, Brinckmann J, Abraham DJ, Black CM, Verzijl N, DeGroot J, Hanemaaijer R, TeKoppele JM, Huizinga TWJ, Bank RA (2003) Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem 278: 40967–40972. [DOI] [PubMed] [Google Scholar]
- van Vlimmeren MAA, Driessen-Mol A, van den Broek M, Bouten CVC, Baaijens FPT (2010) Controlling matrix formation and cross-linking by hypoxia in cardiovascular tissue engineering. J Appl Physiol 109: 1483–1491. [DOI] [PubMed] [Google Scholar]
- Vicens-Zygmunt V, Estany S, Colom A, Montes-Worboys A, Machahua C, Sanabria AJ, Llatjos R, Escobar I, Manresa F, Dorca J, Navajas D, Alcaraz J, Molina-Molina M (2015) Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Respir Res 16: 82. DOI: 10.1186/s12931-015-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal B de C, dos Anjos EHM, Mello MLS (2015) Optical anisotropy reveals molecular order in a mouse enthesis. Cell Tissue Res 362: 177–185. [DOI] [PubMed] [Google Scholar]
- Waggett AD, Ralphs JR, Kwan APL, Woodnutt D, Benjamin M (1998) Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol 16: 457–470. [DOI] [PubMed] [Google Scholar]
- Walker L, Overstreet M, Yeowell H (2005) Tissue-specific expression and regulation of the alternatively-spliced forms of lysyl hydroxylase 2 (LH2) in human kidney cells and skin fibroblasts. Matrix Biol 23: 515–523. [DOI] [PubMed] [Google Scholar]
- Wang SX, Mure M, Medzihradszky KF, Burlingame AL, Brown DE, Dooley DM, Smith AJ, Kagan HM, Klinman JP (1996) A crosslinked cofactor in lysyl oxidase: redox function for amino acid side chains. Science 273: 1078–1084. [DOI] [PubMed] [Google Scholar]
- Williams MA, Kagan HM (1985) Assessment of lysyl oxidase variants by urea gel electrophoresis: Evidence against disulfide isomers as bases of the enzyme heterogeneity. Anal Biochem 149: 430–437. [DOI] [PubMed] [Google Scholar]
- Wyatt KE-K, Bourne JW, Torzilli PA (2009) Deformation-dependent enzyme mechanokinetic cleavage of type I collagen. J Biochem Eng 131: 051004. DOI: 10.1115/1.3078177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Takagishi K, Osawa T, Yanagawa T, Nakajima D, Shitara H, Kobayashi T (2010) Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg 19: 116–120. [DOI] [PubMed] [Google Scholar]
- Zareian R, Church KP, Saeidi N, Flynn BP, Beale JW, Ruberti JW (2010) Probing collagen/enzyme mechanochemistry in native tissue with dynamic, enzyme-induced creep. Langmuir 26: 9917–9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Zhou J (2012) Decreased expression of elastin, fibulin-5 and lysyl oxidase-like 1 in the uterosacral ligaments of postmenopausal women with pelvic organ prolapse: elastin, fibulin-5 and LOXL1 decreased in POP. J Obstet Gynaecol Res 38: 925–931. [DOI] [PubMed] [Google Scholar]
Web Reference
- 1.https://patents.justia.com/patent/20200268935 [20-04-2121]


