Background
Globally, breast cancer has been identified as the most common cancer among women. The clinical efficacy of adjuvant oral antiestrogen therapy—including tamoxifen and aromatase inhibitors—has been proven to be clinically efficacious for breast cancer survivors. However, medication adherence for these therapies remains suboptimal among breast cancer survivors.
Objective
The aim of this study was to evaluate the effect of a reminder intervention—a smart pill bottle paired with the Pillsy mobile application—on medication adherence, medication self-efficacy, and depression, among breast cancer survivors who were undergoing oral antiestrogen therapy.
Methods
This study is a randomized controlled trial. Sixty-one women were allocated to an experimental group (n = 31) and the control group (n = 30). The experimental group received the reminder intervention of a smart pill bottle for 4 weeks. Study outcomes were identified as medication adherence, medication self-efficacy, and depression.
Results
Fifty-seven women completed the follow-up measurement. Significant differences in favor of the experimental group were noted for medication adherence (P = .004) and medication self-efficacy (P = .004). There was no statistically significant difference between the 2 groups with regard to depression (P = .057).
Conclusions
Reminder intervention using smart pill bottles was effective in improving medication adherence and medication self-efficacy among breast cancer survivors undergoing oral antiestrogen therapy.
Implications for Practice
A smart pill bottle method of intervention can be a useful reminder strategy to improve medication adherence among breast cancer survivors.
KEY WORDS: Breast neoplasms, Depression, Intervention study, Medication adherence, Self-efficacy, Survivors
Globally, breast cancer has been identified as the most common cancer among women, especially in developed countries.1–3 In South Korea, the prevalence of breast cancer is steadily increasing, with hormone receptor–positive being the most common subtype.4 Adjuvant oral antiestrogen therapy for women with estrogen receptor–positive breast cancer significantly reduces cancer recurrence and prolongs survival/mortality.5,6 Medication adherence is critical for receiving the benefits of adjuvant oral antiestrogen therapy; however, studies have consistently found nonadherence to adjuvant hormonal therapy among a considerable proportion of breast cancer survivors.7–9
Factors associated with nonadherence and nonpersistence to adjuvant oral antiestrogen therapy among breast cancer survivors are multidimensional, including debilitating adverse effects, inadequate information, and a lack of support.10,11 In addition, perceived self-efficacy12–16 and depression have been reported to influence medication adherence in this population.13,14,17 Furthermore, forgetfulness was seen as a common factor influencing medication adherence.9,18,19
Existing intervention studies concerning medication adherence in breast cancer survivors receiving adjuvant oral antiestrogen therapy have focused on patient navigation,20 provision of educational materials21 or information,22 and text message reminder programs.23,24 However, a systematic review reported that the provision of educational materials, which is the most common type of intervention, did not result in a significant improvement in adherence to adjuvant oral antiestrogen therapy.25 In contrast, a meta-analysis demonstrated a significant improvement in adherence among groups receiving a reminder intervention compared with control group participants.26 Although these studies have assessed medication adherence reminder messages on a weekly23,27 or daily24 schedule, the researchers did not account for the variation among individual patients’ medication schedules. Consequently, it is important to examine this research gap to obtain accurate outcomes accounting for patients’ diversity in terms of individual schedules for medication adherence.
Oral medication adherence has most commonly been measured by self-report21,23,24,28 or pill count at the clinic.29,30 To ensure that there is a consistent amount of medication in the body throughout the day, it is essential to follow the prescription. In light of this evidence, electronic pillbox monitoring could be a better alternative to pill counts and patient self-reports in medication adherence assessment. In particular, smart pill bottles include a function to automatically track the time of taking the medication, which then enables an objective assessment of the pills taken at the scheduled time. The smart pill bottles have several other functions that could promote medication adherence, such as providing a reminder at the scheduled dose time and sending messages to designated helpers if a person is more than 1 hour late for the scheduled dose.31–33
Depressive symptoms have been reported as a barrier to oral endocrine therapy adherence among breast cancer survivors.14 In a previous study on women with breast cancer, nonadherence to adjuvant oral hormone therapy was 2.3 times higher in patients experiencing depressive symptoms.34 Furthermore, studies have shown that social support is helpful in improving depressive symptoms and medication adherence in breast cancer survivors.35,36 Because the smart pill bottle reminder has support functions to help users take medication at the scheduled time, through beeping, blinking, and caregiver support, it is worthwhile to explore whether this intervention could also improve depressive symptoms in breast cancer survivors.
So far, studies using smart pill bottles have been restricted to people living with multiple myeloma,37 heart failure,33 and HIV.32 To the best of our knowledge, no previous studies have explored promoting medication adherence among breast cancer survivors. The purpose of this study was to explore the effects of a smart pill bottle reminder intervention on medication adherence and medication self-efficacy and whether this intervention also alleviates the levels of depressive symptoms among breast cancer survivors undergoing adjuvant oral antiestrogen therapy.
Methods
Design
This study was a single center, 2-armed randomized controlled trial.
Participants
The participants are breast cancer survivors who were recruited from the outpatient breast surgical clinic of a single urban tertiary hospital in South Korea. The initial candidate list was created by a researcher based on electronic medical records of women who needed adjuvant oral antiestrogen therapy. Eligible women were approached by a nurse in an oncology breast clinic during their routine clinic visit. They were invited to participate in this study after being informed of the study. Inclusion criteria were (1) being at least 18 years or older, (2) having survived breast cancer stage I to III, (3) receiving adjuvant oral antiestrogen therapy after breast surgery (mastectomy or partial mastectomy), (4) possessing a smartphone, and (5) being willing to utilize a smart pill bottle for adjuvant oral antiestrogen therapy during the study period. Women were excluded from this study if they (1) had a stage IV metastatic breast cancer diagnosis, (2) had breast cancer recurrence, (3) were younger than 18 years, or (4) had cognitive impairments.
Procedure
After participants eligible for the study were invited to participate, written informed consent was obtained before completion of the baseline test via a self-reported questionnaire. After enrollment, participants were randomly assigned (allocation ratio 1:1) to receive either the smart pill bottle reminder intervention (n = 31) or usual care (n = 30). Randomization was performed by drawing lots. Each participant received a sealed envelope with information indicating the group to which they were assigned. Among the participants, 1 woman from the intervention group dropped out because she disliked the alarm, and 3 women from the control group dropped out because of international travel plans (n = 1) and difficulties in recording information on medication taken (n = 2). At the 4-week follow-up, 57 women provided postintervention data. Figure 1 outlines the Consolidated Standards of Reporting Trials diagram37 detailing study enrollment and retention.
Figure 1.
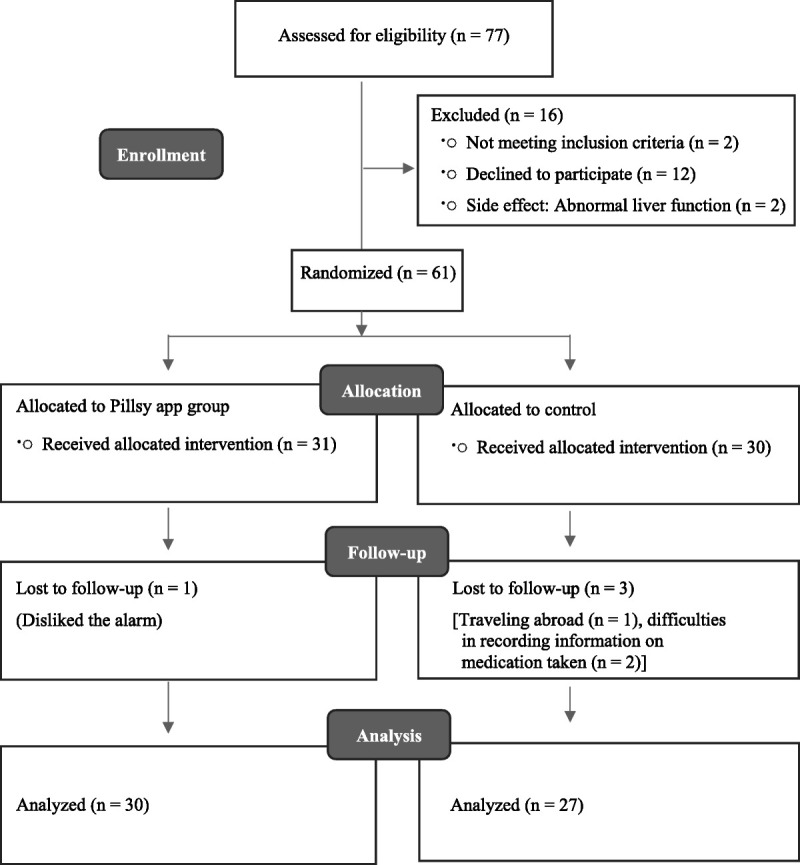
Enrollment flowchart.
A total of 77 eligible participants were screened and invited to participate (Figure 1). Of those screened, 61 women agreed to participate, and 16 were excluded because of lack of interest in participation (n = 12), not meeting inclusion criteria (n = 2), and temporary medication withdrawal owing to increase in liver function test (n = 2).
Data Collection
Data were collected before and after the intervention using a structured questionnaire survey in the outpatient clinic and from the records on the opening of the bottle cap. For the pretest, data regarding participants’ demographic and clinical characteristics, medication self-efficacy, and depression were collected. For the posttest, participants were asked to provide data on medication adherence, medication self-efficacy, and depression. Data were collected from April 2019 to June 2020, with both the pretest and posttest taking 10 to 15 minutes to complete.
Intervention
Participants in the control group received usual care and were asked to complete the medication logs daily. The usual standard of care consisted of routine clinical follow-ups, without smart pill bottle reminders, for adjuvant oral antiestrogen therapy.
After enrollment, participants in the intervention group received a smart pill bottle (Pillsy, Inc, Seattle, WA). The smart pill bottle beeps and blinks to remind a user when it is time for a dose. It syncs with the free Pillsy mobile application (app) via Bluetooth. The mobile app has several functions, including recording the users’ dose when the bottle cap is opened, tracking the remaining medications (automated tracking), beeping to remind users when it is time for a dose, sending alarm reminders when users forget a dose (alarm reminders), and sending notifications to designated helpers when users miss a dose (sharing with designated helpers). For medication adherence, it was assumed that the opening of the bottle cap signified the consumption of medication by the participants. All participants were taking their entire dose at one time once a day.
Participants in the intervention group received the smart pill bottle reminder intervention for 4 weeks (28 days) with the time of medication intake being recorded automatically. After participants were informed about the intervention, the Pillsy mobile app was downloaded, and their accounts were created. Then, the smart pill bottle was paired with the mobile app, and information including name of the oral antiestrogen, dosage, frequency per day, and preferred scheduled dosage time was individually entered on each participant’s smartphone. In addition, participants were informed that they could designate anyone of their choosing—such as family members, friends, or medical personnel—as the designated helper. All participants chose a researcher as the helper.
Participants were reminded to take their medication as per the beeping and blinking function of the smart pill bottle cap, which indicated the scheduled time. They also received smartphone notifications that the time of taking the medication was recorded automatically. Further, participants received text message reminders 20 minutes after the scheduled dose time if they had not already taken the dose. If a participant missed a dose, the designated helper received automated phone call reminders 1 hour after the scheduled dose time. The helper provided phone counseling on any issue related to missing the dose or if there were any physical or psychosocial difficulties. Figure 2 presents the functions of the smart pill bottle paired with the Pillsy mobile app.
Figure 2.
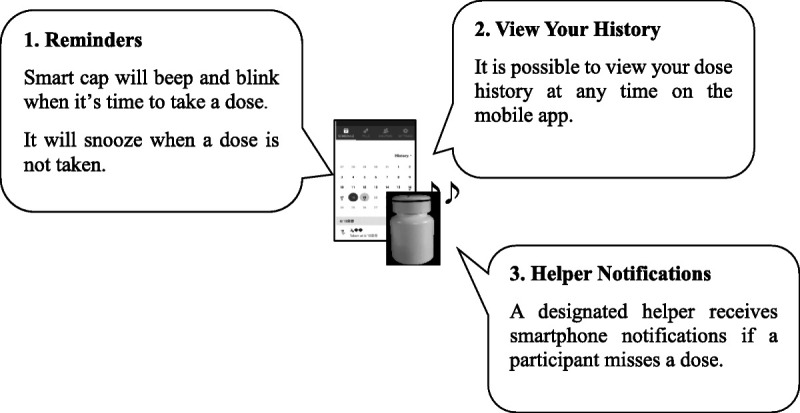
Smart pill bottle paired with the Pillsy mobile app.
Outcome Measures
The questionnaires used in this study surveyed for demographic and clinical information, medication adherence, medication self-efficacy, and depression.
MEDICATION ADHERENCE
Medication adherence for 28 days was calculated based on the monthly medication logs in the control group and automatic smartphone records in the experimental group. Medication adherence was defined as taking a dose within 1 hour before or after the scheduled administration time. This was established based on a previous study utilizing a smart pill bottle with patients diagnosed with multiple myeloma who were receiving oral anticancer therapy.38 Medication adherence was calculated as an adherence percentage by dividing the number of days on which at least 1 bottle opening was registered by the total number of monitored days, multiplied by 100.
MEDICATION SELF-EFFICACY
Self-efficacy was measured using a medication self-efficacy scale.39 This tool consists of 8 items rated on a 5-point Likert scale ranging from 1 (not at all) to 5 (always). Scores ranged from 8 to 40, with higher scores indicating higher medication self-efficacy. The Cronbach’s α values were .81 in the original study and .71 in this study.
DEPRESSION
Depression was measured using the Center for Epidemiologic Studies Depression scale developed by Radloff40 and validated for the South Korean population.41 It consists of 20 items rated on a 4-point Likert scale (from 0: rarely or none of the time, to 3: most or all of the time). Scores ranged from 0 to 60, with higher scores indicating a higher frequency of depressive symptoms during the last week. For the original tool, a total score of ≥16 is considered indicative of subthreshold depression. The Cronbach’s α values were .91 in the original study40 and .90 in this study.
Ethical Considerations
This study was approved by the institutional review board of H Medical Center (IRB no. 2019-01-036-009). Participants were fully informed about the study’s aims, as well as their rights to anonymity and confidentiality. In addition, they were informed that they could withdraw at will, and their data would not be used in this study. Participants provided signed written informed consent before initiating the study. The participants received a small gift ($10) after completing the posttest. This study was conducted in accordance with the Declaration of Helsinki and the General Data Protection Regulation. The study did not involve any changes to participants’ regular care routines.
Data Analysis
Data were assessed using SPSS for Windows, version 26.0 (IBM Corp, Armonk, New York). Descriptive statistics were used, and we calculated frequency and percentage for categorical variables and mean and SD values for continuous variables. Homogeneity between the 2 groups was analyzed with the χ2 test and t test. Medication adherence was calculated as an adherence percentage with mean adherence for both groups being reported. An independent-samples t test was used to compare the difference between medication adherence of groups. The analysis of covariance, with the posttest score as the outcome and pretest score as a covariate, was used to conduct adjusted between-group analysis of medication self-efficacy and depression. The significance level was set at .05.
Results
Sample Characteristics
Demographic and clinical characteristics of the 57 participants who completed the follow-ups are provided in Table 1. Their mean age was 53.33 ± 8.71 years, and 78.9% of them were of midlevel socioeconomic status. The average time since diagnosis was 16.81 ± 9.98 months, and the average period of undergoing adjuvant oral antiestrogen therapy was 12.16 ± 8.58 months. The groups did not differ with regard to baseline demographic and clinical characteristics (Table 1).
Table 1.
Participants’ Characteristics and Comparisons between Groups (N = 57)
| Characteristics | Categories | Total (N = 57) | Exp. (n = 30) | Cont. (n = 27) | t or χ2 | P |
|---|---|---|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | Mean ± SD/n (%) | ||||
| Age, y | 53.33 (8.71) | 52.07 (9.34) | 54.74 (7.87) | −1.161 | .744 | |
| Education | ≤ Middle school | 19 (33.3) | 7 (23.3) | 12 (44.4) | 5.80 | .056 |
| High school | 30 (52.7) | 16 (53.4) | 14 (51.9) | |||
| ≥ College | 8 (14.0) | 7 (23.3) | 1 (3.7) | |||
| Religion | None | 31 (54.4) | 17 (56.7) | 14 (51.9) | 2.88 | .436 |
| Protestant | 10 (17.5) | 3 (10.0) | 7 (25.9) | |||
| Catholic | 7 (12.3) | 4 (13.3) | 3 (11.1) | |||
| Buddhist | 9 (15.8) | 6 (20.0) | 3 (11.1) | |||
| Employment | Self-employed | 6 (10.5) | 5 (16.7) | 1 (3.7) | 3.73 | .150 |
| Unemployed | 40 (70.2) | 18 (60.0) | 22 (81.5) | |||
| Employed | 11 (19.3) | 7 (23.3) | 4 (14.8) | |||
| Economic status | High | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.73 | .519 |
| Middle | 45 (78.9) | 25 (83.3) | 20 (74.1) | |||
| Low | 12 (21.1) | 5 (16.7) | 7 (25.9) | |||
| Time since diagnosis, mo | 16.81 (9.98) | 14.83 (8.61) | 19.00 (11.0) | −1.59 | .076 | |
| Type of surgery | BCS | 45 (78.9) | 22 (73.3) | 23 (85.2) | 1.20 | .340 |
| MRM | 12 (21.1) | 8 (26.7) | 4 (14.8) | |||
| Stage at diagnosis | 0 or I | 35 (61.4) | 18 (60.0) | 17 (63.0) | 1.22 | .631 |
| II | 15 (26.3) | 7 (23.3) | 8 (29.6) | |||
| III | 7 (12.3) | 5 (16.7) | 2 (7.4) | |||
| AET agent | Tamoxifen | 32 (56.2) | 21 (70.0) | 11 (40.8) | 5.51 | .074 |
| Anastrozole | 17 (29.8) | 7 (23.3) | 10 (37.0) | |||
| Letrozole | 8 (14.0) | 2 (6.7) | 6 (22.2) | |||
| Period of AET, mo | 12.16 (8.58) | 11.30 (8.0) | 13.11 (9.24) | −0.79 | .487 |
Abbreviations: AET, antiestrogen therapy; BCS, breast-conserving surgery; Con., control group; Exp., experimental group; MRM, modified radical mastectomy.
Homogeneity of Dependent Variables Between the 2 Groups
In the pretest, the average medication self-efficacy score was 34.10 ± 4.82 in the experimental group and 32.70 ± 4.62 in the control group. The average depression score was 20.90 ± 7.74 in the experimental group and 21.56 ± 8.30 in the control group. There were no significant differences in medication self-efficacy (t = 1.11, P = .270) and depression (t = −0.31, P = .759) between the groups in the pretest.
Intervention Effects
MEDICATION ADHERENCE RATES
At 28 days, a larger proportion of participants in the experimental group displayed medication adherence in comparison with those in the control group (97.30% vs 88.33%). Mean medication adherence rates were higher among the experimental rather than the control group (t = 2.96, P = .004; Table 2).
Table 2.
Comparison of Variables Between Groups (N = 57)
| Variables | Exp. (n = 30) | Cont. (n = 27) | t | P | Adjusted Pa | |
|---|---|---|---|---|---|---|
| Mean % ± SD/ Mean ± SD | Mean % ± SD/Mean ± SD | |||||
| Medication adherence | 97.30 ± 4.91 | 88.33 ± 15.75 | 2.96 | .004 | ||
| Medication self-efficacy | Pretest | 34.10 ± 4.82 | 32.70 ± 4.62 | 1.11 | .270 | |
| Posttest | 37.13 ± 2.92 | 33.93 ± 4.56 | 3.13 | .003 | .004 | |
| Depression | Pretest | 20.90 ± 7.74 | 21.56 ± 8.30 | −.31 | .759 | |
| Posttest | 17.77 ± 6.52 | 20.41 ± 7.75 | −1.40 | .168 | .057 |
Abbreviations: Cont., control group; Exp., experimental group.
aAnalysis of covariance.
SELF-EFFICACY AND DEPRESSION
In the experimental group, the average medication self-efficacy score was 34.10 ± 4.82 at baseline and 37.13 ± 2.92 at posttest; in the control group, these scores were 32.70 ± 4.62 and 33.93 ± 4.56, respectively. Participants in the experimental group were more likely to display medication self-efficacy compared with the control group (F = 9.07, P = .004).
In the experimental group, the average depressive symptoms score was 20.90 ± 7.74 at baseline and 17.77 ± 6.52 at posttest; in the control group, these scores were 21.56 ± 8.30 and 20.41 ± 7.75, respectively. There were no statistically significant differences in depression between the groups (F = 3.79, P = .057; Table 2).
Discussion
This study demonstrated the efficacy of a reminder intervention by utilizing a smart pill bottle for improving medication self-efficacy and adherence among breast cancer survivors taking antihormonal agents. Medication adherence was higher in the group using a smart pill bottle paired with the mobile app rather than in participants receiving the usual care. The combined functions of the smart pill bottle, such as alarms at medication time (including snoozing if medication is not taken), and the requirement of a designated helper could have contributed to medication adherence among participants. Previous studies found no positive impact of providing standardized information or educational materials on persistence with and compliance to adjuvant endocrine therapy among women with breast cancer.21,42,43 In contrast, reminder interventions have been effective for ensuring adherence to adjuvant hormone therapy.23,24 Our study thus builds on previous results by demonstrating the efficacy of a reminder intervention for improving medication adherence in breast cancer survivors. However, in our study, the time period of reminders and reminder methods were somewhat different from those previously used, such as weekly reminders using an app23 and daily reminders using a smart pill bottle.38 The present results are in line with those of previous studies reporting a positive impact of smart pill bottle interventions on medication adherence in patients with multiple myeloma38 and people with HIV.32
Considering that antihormonal aromatase must be taken daily and that forgetfulness is one of the barriers to medication adherence among breast cancer survivors,18–20 interventions involving daily reminders would be more beneficial than those based on weekly reminders. In this study, the duration of reminder intervention was 28 days. Given that adjuvant oral antiestrogen therapies are taken for multiple years,5 the 4-week period was too short to assess whether they improve the participants’ overall medication adherence and self-efficacy in the long run. Therefore, to obtain better results regarding adherence using a smart pill bottle reminder intervention, future research on the long-term benefits of this intervention is warranted. Furthermore, smart pill bottles are easy to use44 and have the advantage of not only an alarm function but also messages to helpers (caregivers) when patients miss a dose. In our study, all participants designated non–family members as helpers. There are limitations in explaining the reasons for this because these data were not fully explored. However, participants reported their preference in designating healthcare providers as “helpers” (support persons) because they thought healthcare providers could understand and support them better. Furthermore, they disliked being a burden to their family members who might worry and be concerned. Studies reported that breast cancer survivors might consider taking adjuvant oral antiestrogen therapy to be a private issue45 and be reluctant to talk about problems for fear that their family members would worry.16 Nevertheless, social support by healthcare providers and family members is seen to play an important role in medication adherence in breast cancer survivors.11 Thus, future research exploring the benefits of designating family members as helpers in the use of smart pill bottles is warranted.
A significant improvement was observed in the experimental group regarding medication self-efficacy. The difference in medication adherence between the 2 groups is probably due partly to their self-efficacy. This is confirmed by a previous study reporting that self-efficacy in medication management had a positive impact in the context of oral anticancer medications among breast cancer survivors.13 Thus, assessing and monitoring the self-efficacy of women with breast cancer, both before and during adjuvant oral antiestrogen therapy, should be incorporated into comprehensive care plans.
In a systematic review, depression was identified as a predictor of the success of adjuvant endocrine therapy.17 Social support also plays a positive role in improving depressive symptoms.35,36 Such results indicate that evaluating and supporting breast cancer survivors with depressive symptoms would positively impact medication adherence. In this study, the smart pill bottle reminder intervention was not effective in improving depressive symptoms. Therefore, we recommend providing psychological support as a supplement to reminder interventions in breast cancer survivors undergoing adjuvant oral antiestrogen therapy.
Limitations
Despite its strengths, this study has certain limitations. First, as the setting was a single university medical center, the results have limited generalizability. Second, we did not consider the time since diagnosis, which may influence adherence rates as an inclusion criterion or stratification variable. Thus, caution is required when interpreting these data for other clinical centers. Third, as the intervention was provided for a short period, its long-term effects remain unknown, necessitating longitudinal studies. Fourth, participants chose a member of the research staff as their helper rather than a family member, which could have limited the findings’ generalizability. Fifth, while we based our medication adherence rates on recorded data, there is a possibility that these were overestimated because smart pill bottles can be opened without the medication being taken. Thus, it is recommended that the questionnaires administered to participants include whether at times they did not take their medication immediately after opening the bottle. On the contrary, there is a possibility of medication adherence having been underestimated, because we only counted the medication taken within 1 hour before or after the scheduled time. When participants are not at home during the time when medication intake is scheduled, timely intake could be difficult unless they carry the smart pill bottle with them. This suggests the need for a smaller bottle that can be used for travel or for daily on-the-go activities.
Implications for Practice
In this study, we included participants regardless of their history of medication adherence. The rates of medication adherence among breast cancer survivors have varied in the literature, ranging from about 60%22 to 80%.21 The rate of medication adherence in this study (97.3% vs 88.3%, P = .004) is better than a study that assessed the impact of educational intervention (82% vs 81%, P = .45)21 and in line with other studies that assessed the impact of a weekly short message service for 6 months (72.4% vs 59.5%; P = .034)27 and an App+Reminder (weekly) for 8 weeks (100% vs 72%; P < .05).23 The results of this study support that smart pill bottle reminder interventions can be useful for breast cancer survivors undergoing adjuvant oral antiestrogen therapy. In particular, this intervention may be recommended for women who have difficulties with regular medication intake because of forgetfulness. Among women with depressive symptoms, this study also indicates the need for supportive interventions in addition to smart pill bottle reminders. Furthermore, designating a helper for reminders with a smart pill bottle could be beneficial, especially for exploring the reasons for not taking the medication on time. Thus, daily monitoring and provision of early intervention for women who need support with medication adherence would lead to positive outcomes. Knowledge of the exact time of intake is an important aspect of evaluating medication adherence, which is not always possible just by counting pills. In relation to this, smart pill bottles may be helpful for determining the formation of habits related to adjuvant oral antiestrogen therapy, especially as this therapy can continue for several years.46 It would also be helpful for determining the exact time of medication intake because it allows the assessment of “when,” and not just “if,” the medication was taken and for maintaining the proper medication level in the body by taking it as scheduled.
With regard to the sustainability of smart pill bottle reminders, breast cancer survivors can use the smart pill bottle continuously once they purchase it (approximately $56), although the battery (approximately $7) has to be changed annually. Accordingly, the cost of purchasing it could be burdensome for some women.47 Thus, considering women’s need for financial support would be helpful, particularly for women with low medication compliance. In terms of information sharing, healthcare providers could check medication adherence data that are sent in real time to a dedicated platform if a user’s weekly adherence drops to less than 80%.38 Furthermore, they could be informed by a support person when breast cancer survivors miss their medications repeatedly. One study indicated that healthcare providers’ challenge in assessing adherence was the patient not disclosing discontinuation immediately or overreporting adherence.48 Real-time information on adherence using the smart pill bottle would therefore aid practitioners in monitoring medication adherence.
Conclusions
This study demonstrated that reminder interventions utilizing a smart pill bottle paired with the mobile app would be helpful for improving short-term medication adherence and medication self-efficacy in breast cancer survivors undergoing adjuvant oral antiestrogen therapy. Such reminder interventions may improve the timing of dosage in addition to the number of doses taken as scheduled. Furthermore, incorporation of psychological support may be needed to improve depressive symptoms in this population. Adapting this smartphone technology to improve medication adherence may benefit breast cancer survivors undergoing adjuvant oral antiestrogen therapy, leading to overall positive outcomes.
ACKNOWLEDGMENT
The research team thanks all the participants, who generously invested their time for the purpose of this study.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Contributor Information
Hyang Rang Park, Email: angela58300@naver.com.
Soo Hyun Kim, Email: soohyun@inha.ac.kr.
Savitri Singh-Carlson, Email: ssinghcarlson@sdsu.edu.
References
- 1.Ahmad A. Breast cancer statistics: recent trends. Adv Exp Med Biol. 2019;1152:1–7. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol. 2019;1152:9–29. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE Ma J Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451. [DOI] [PubMed] [Google Scholar]
- 4.Kang SY Kim YS Kim Z, et al. Breast cancer statistics in Korea in 2017: data from a breast cancer registry. J Breast Cancer. 2020;23(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burstein HJ Lacchetti C Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37(5):423–438. [DOI] [PubMed] [Google Scholar]
- 6.Cuzick J Sestak I Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camacho FT, Tan X, Alcalá HE, Shah S, Anderson RT, Balkrishnan R. Impact of patient race and geographical factors on initiation and adherence to adjuvant endocrine therapy in Medicare breast cancer survivors. Medicine (Baltimore). 2017;96(24):e7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hershman DL Kushi LH Hillyer GC, et al. Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res Treat. 2016;157(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawesi S, Carpenter JS, Jones J. Reasons for nonadherence to tamoxifen and aromatase inhibitors for the treatment of breast cancer: a literature review. Clin J Oncol Nurs. 2014;18(3):E50–E57. [DOI] [PubMed] [Google Scholar]
- 10.Clancy C, Lynch J, OConnor P, Dowling M. Breast cancer patients’ experiences of adherence and persistence to oral endocrine therapy: a qualitative evidence synthesis. Eur J Oncol Nurs. 2020;44:101706. [DOI] [PubMed] [Google Scholar]
- 11.Toledo G, Ochoa CY, Farias AJ. Exploring the role of social support and adjuvant endocrine therapy use among breast cancer survivors. Support Care Cancer. 2020;28(1):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert LK, Balneaves LG, Howard AF, Gotay CC. Patient-reported factors associated with adherence to adjuvant endocrine therapy after breast cancer: an integrative review. Breast Cancer Res Treat. 2018;167(3):615–633. [DOI] [PubMed] [Google Scholar]
- 13.Lin C, Clark R, Tu P, Bosworth HB, Zullig LL. Breast cancer oral anti-cancer medication adherence: a systematic review of psychosocial motivators and barriers. Breast Cancer Res Treat. 2017;165(2):247–260. [DOI] [PubMed] [Google Scholar]
- 14.Paranjpe R, John G, Trivedi M, Abughosh S. Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res Treat. 2019;174(2):297–305. [DOI] [PubMed] [Google Scholar]
- 15.Wouters H Stiggelbout AM Bouvy ML, et al. Endocrine therapy for breast cancer: assessing an array of women’s treatment experiences and perceptions, their perceived self-efficacy and nonadherence. Clin Breast Cancer. 2014;14(6):460–467.e2. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Zhang XJ, Wang DQ, Xu L, Wang AP. Factors influencing medication-taking behaviour with adjuvant endocrine therapy in women with breast cancer: a qualitative systematic review. J Adv Nurs. 2020;76(2):445–458. [DOI] [PubMed] [Google Scholar]
- 17.Mausbach BT, Schwab RB, Irwin SA. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015;152(2):239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali EE, Cheung KL, Lee CP, Leow JL, Yap KY, Chew L. Prevalence and determinants of adherence to oral adjuvant endocrine therapy among breast cancer patients in Singapore. Asia Pac J Oncol Nurs. 2017;4(4):283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philipovskiy A Campbell A Heydarian R, et al. Adherence to adjuvant aromatase inhibitor therapy among postmenopausal Hispanic/Latino women with breast cancer. Anticancer Res. 2020;40(2):857–864. [DOI] [PubMed] [Google Scholar]
- 20.Ell K Vourlekis B Xie B, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115(19):4606–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neven P Markopoulos C Tanner M, et al. The impact of educational materials on compliance and persistence rates with adjuvant aromatase inhibitor treatment: first-year results from the Compliance of Aromatase Inhibitors Assessment in Daily Practice Through Educational Approach (CARIATIDE) study. Breast. 2014;23(4):393–399. [DOI] [PubMed] [Google Scholar]
- 22.Ziller V, Kyvernitakis I, Knöll D, Storch A, Hars O, Hadji P. Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment—the COMPAS study. BMC Cancer. 2013;13:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graetz I, McKillop CN, Stepanski E, Vidal GA, Anderson JN, Schwartzberg LS. Use of a web-based app to improve breast cancer symptom management and adherence for aromatase inhibitors: a randomized controlled feasibility trial. J Cancer Surviv. 2018;12(4):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krok-Schoen JL Naughton MJ Young GS, et al. Increasing adherence to adjuvant hormone therapy among patients with breast cancer: a smart phone app-based pilot study. Cancer Control. 2019;26(1):1073274819883287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ekinci E Nathoo S Korattyil T, et al. Interventions to improve endocrine therapy adherence in breast cancer survivors: what is the evidence? J Cancer Surviv. 2018;12(3):348–356. [DOI] [PubMed] [Google Scholar]
- 26.Fenerty SD, West C, Davis SA, Kaplan SG, Feldman SR. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan EH Wong ALA Tan CC, et al. Improving medication adherence with adjuvant aromatase inhibitor in women with breast cancer: a randomised controlled trial to evaluate the effect of short message service (SMS) reminder. Breast. 2020;53:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmes EA, Hughes DA, Morrison VL. Predicting adherence to medications using health psychology theories: a systematic review of 20 years of empirical research. Value Health. 2014;17(8):863–876. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Garg S, Sharma N, Singh MM. Improving the assessment of medication adherence: challenges and considerations with a focus on low-resource settings. Ci Ji Yi Xue Za Zhi. 2019;31(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf MS Davis TC Curtis LM, et al. A patient-centered prescription drug label to promote appropriate medication use and adherence. J Gen Intern Med. 2016;31(12):1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi EPH. A pilot study to evaluate the acceptability of using a smart pillbox to enhance medication adherence among primary care patients. Int J Environ Res Public Health. 2019;16(20):3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellsworth GB Burke LA Wells MT, et al. Randomized pilot study of an advanced smart-pill bottle as an adherence intervention in patients with HIV on antiretroviral treatment. J Acquir Immune Defic Syndr. 2021;86(1):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohn A Speier W Lan E, et al. Integrating remote monitoring into heart failure patients’ care regimen: a pilot study. PLoS One. 2020;15(11):e0242210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang HY, Sayers SL, Weissinger G, Riegel B. The role of depression in medication adherence among heart failure patients. Clin Nurs Res. 2014;23(3):231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markovitz LC, Drysdale NJ, Bettencourt BA. The relationship between risk factors and medication adherence among breast cancer survivors: what explanatory role might depression play? Psychooncology. 2017;26(12):2294–2299. [DOI] [PubMed] [Google Scholar]
- 36.Bright EE, Stanton AL. Prospective investigation of social support, coping, and depressive symptoms: a model of adherence to endocrine therapy among women with breast cancer. J Consult Clin Psychol. 2018;86(3):242–253. [DOI] [PubMed] [Google Scholar]
- 37.Schulz KF Altman DG Moher D, CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauro J, Mathews KB, Sredzinski ES. Effect of a smart pill bottle and pharmacist intervention on medication adherence in patients with multiple myeloma new to lenalidomide therapy. J Manag Care Spec Pharm. 2019;25(11):1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee HJ. Factors Affecting Medication Compliance of Hypertensive Patients [master’s thesis]. Cheonan, South Korea: Dankook University; 2002. [Google Scholar]
- 40.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur. 1977;1(3):385–401. [Google Scholar]
- 41.Chon KK, Choi SC, Yang BC. Integrated adaptation of CES-D in Korea. Korean J Health Psychol. 2001;6(1):59–76. [Google Scholar]
- 42.Hadji P Blettner M Harbeck N, et al. The Patient’s Anastrozole Compliance to Therapy (PACT) program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013;24(6):1505–1512. [DOI] [PubMed] [Google Scholar]
- 43.Heiney SP, Parker PD, Felder TM, Adams SA, Omofuma OO, Hulett JM. A systematic review of interventions to improve adherence to endocrine therapy. Breast Cancer Res Treat. 2019;173(3):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zijp TR, Touw DJ, van Boven JFM. User acceptability and technical robustness evaluation of a novel smart pill bottle prototype designed to support medication adherence. Patient Prefer Adherence. 2020;14:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert LK Balneaves LG Howard AF, et al. Understanding adjuvant endocrine therapy persistence in breast cancer survivors. BMC Cancer. 2018;18(1):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayala de la Peña F Andrés R Garcia-Sáenz JA, et al. SEOM clinical guidelines in early stage breast cancer (2018). Clin Transl Oncol. 2019;21(1):18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farias AJ, Du XL. Association between out-of-pocket costs, race/ethnicity, and adjuvant endocrine therapy adherence among Medicare patients with breast cancer. J Clin Oncol. 2017;35(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Mendoza AH Cabling M Dilawari A, et al. Providers’ perspectives on adherence to hormonal therapy in breast cancer survivors. Is there a role for the digital health feedback system? Health Technol (Berl). 2019;9(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]


