ABSTRACT
Purpose
High-intensity interval training (HIIT) has been shown to improve cardiorespiratory fitness (CRF) and health-related outcomes in various chronic diseases, including cancer. However, data on feasibility and efficacy of HIIT in advanced cancer patients are still sparse, presumably because of safety concerns, like suspected immunosuppression after vigorous exercise. This randomized, sham-intervention controlled study aimed to investigate feasibility, safety, and preliminary efficacy of very low-volume HIIT (LOW-HIIT) in advanced cancer patients.
Methods
Twenty-seven patients (55.4 ± 13.2 yr) with different advanced cancers (Union for International Cancer Control [UICC] III/IV) were randomly allocated to LOW-HIIT (n = 13), consisting of 5 × 1 min cycle ergometer intervals (14 min per session total duration) at 80% to 95% HRpeak (two sessions per week for 12 wk), or a sham intervention (n = 14) performing light physical mobilization exercises (SHAM). Primary outcomes were attrition and attendance rates, with values of ≤25% and ≥80%, respectively, considered acceptable. Secondary outcomes were safety, protocol fidelity, physiological (including CRF measures) and patient-reported outcomes (including fatigue and quality of life).
Results
One of 13 patients (8%) receiving LOW-HIIT dropped out. Mean attendance rate was ~93%. The prescribed minimum exercise intensity was consistently reached by all patients. Low-volume HIIT was well tolerated and not associated with any serious adverse event nor with increased infection susceptibility. There were no biochemical signs of acute immunosuppression after LOW-HIIT. Contrarily, differentiation and degranulation of natural killer cells was acutely increased postexercise. Low-volume HIIT improved CRF measures including peak oxygen uptake, self-reported fatigue, physical, and social functioning. No significant changes occurred in the SHAM group.
Conclusions
Low-volume HIIT can be regarded as feasible and safe in advanced cancer patients. Our preliminary data indicate favorable acute effects on NK-cells and beneficial chronic adaptations in CRF, fatigue, and aspects of quality of life.
Key Words: HIIT, ONCOLOGY, SUPPORTIVE CARE, AEROBIC EXERCISE, CARDIOPULMONARY FITNESS, NK CELLS
It is well established that regular physical activity is associated with decreased risk for the occurrence of several cancer types, including some of the most common cancers (1). Notably, these associations appear to be independent from body mass index (BMI), smoking history and other modifiable lifestyle factors (1). Beyond the preventive effects of physical activity, targeted exercise programs are also recognized as an important adjuvant cornerstone of multimodal cancer treatment. Although until the late 1990s, cancer patients were commonly advised to rest and to avoid physical activity, there is now increasingly strong evidence that exercise during cancer treatment is generally safe and can improve patients’ fitness, physical functioning, fatigue and health-related quality of life (QoL) (2).
According to the current American College of Sports Medicine guidelines, cancer survivors should be encouraged to follow the physical activity recommendations for the general population, namely to achieve at least 150 min of moderate-intensity aerobic exercise per week or alternatively 75 min of vigorous-intensity aerobic exercise per week, as far as their cancer status, age, physical abilities, and comorbidities allow (2).
Compared with cancer survivors, available data on feasibility, safety and effects of exercise in advanced cancer patients are still relatively sparse (2). To date, two meta-analyses have provided promising evidence that traditional exercise modalities, in particular aerobic and resistance training at low-to-moderate intensity appear to be feasible and safe in patients with advanced cancer (3) and bone metastases (4). However, both studies have acknowledged that more research is required to enhance the knowledge about feasibility and safety aspects and physiological efficacy of exercise interventions in this specific population, particularly with regard to exercise volume, intensity and frequency.
Patients diagnosed with advanced cancer typically experience multiple symptoms and physical limitations, such as pronounced fatigue, pain, nausea and dyspnea, making participation in exercise more challenging (5). These physical complaints may lead to a significant reduction in physical activity and, in turn, further worsen physical capacity and disease prognosis (6). Furthermore, advanced cancer patients are at increased risk of suffering from a number of adverse cancer-specific treatment side effects, including cardiotoxic effects of chemotherapy, which can additionally contribute to muscle wasting, weakness (7) and a decrease in cardiorespiratory fitness (CRF) (8). Thus, the recommended amounts of physical activity for cancer survivors may not or only hardly be achievable by patients with advanced cancer. Apart from physical constraints, it has been reported that “lack of time” is the most common reason for declining participation in exercise programs among advanced cancer patients (5), which is in line with other chronic disease cohorts (9).
Consequently, there is an eminent need for the development and evaluation of exercise modalities that are feasible but still physiologically effective to evoke health-related benefits in advanced cancer patients. In this regard, high-intensity interval training (HIIT) has emerged as a more time-efficient exercise option to traditional higher-volume moderate-intensity continuous training. High-intensity interval training is a type of cardiovascular training that typically involves brief intense exercise bouts at intensities of ≥80% of peak heart rate (HRpeak), interspersed by recovery periods of low-intensity activity or rest (10). In recent years, a number of meta-analyses have demonstrated that HIIT can also induce beneficial effects on a broad range of health outcomes in various clinical cohorts, including cancer patients (11–15). More specifically, it has been reported that HIIT was found be feasible and safe for breast cancer survivors (14) as well as for patients with several other cancer types undergoing prehabilitation (13) and other stages of oncologic therapy (12,15). Collectively, meta-analyses revealed significant improvements in CRF (quantified as peak oxygen uptake, V̇O2peak) in cancer patients when HIIT was compared with usual care, with pooled mean differences ranging between 2.1 and 3.7 mL·kg−1·min−1 (12,13,15). Moreover, a recent meta-analysis has demonstrated that the regular practice of interval training, including low-volume HIIT, can contribute to improvements in immune functions, such as increases in peripheral lymphocyte T helper cells, enhanced lymphocyte function and beneficial adaptations in neutrophil function in healthy populations (16). In addition, a very recent study has reported a remarkable improvement in natural killer (NK) cell cytotoxicity (i.e., “NK cell killing capacity”) in previously untrained, healthy individuals after 4 wk of HIIT (17).
However, to date, there is still a lack of knowledge on the effects of HIIT in advanced cancer patients, which may be largely attributed to safety concerns regarding vigorous physical activity in this vulnerable population. Mainly based on research from the 1980s and 1990s, it has been suggested that acute bouts of strenuous (in particular prolonged) exercise can temporarily suppress immune function, a theory referred to as the “open window hypothesis.” Although the open window theory has been challenged in recent years (18), it has indeed to be considered that advanced cancer is typically associated with an impaired immune system resulting in an increased susceptibility to virus infections (19). Consequently, it is paramount to shed light on the effects of vigorous exercise on immune function in patients with advanced cancer when it comes to evaluate the safety and feasibility of HIIT in this particular vulnerable group.
Therefore, the aim of the present pilot study was twofold: First, we aimed to investigate the feasibility and safety of an extremely time-efficient, very low-volume HIIT protocol (LOW-HIIT), previously proven effective in improving CRF, cardiometabolic health markers and well-being in sedentary healthy individuals (20) and obese metabolic syndrome patients (21,22), in a cohort of advanced cancer patients, including the investigation of its acute effects on immune parameters. Second, we proposed to explore the preliminary efficacy of LOW-HIIT on patients’ CRF, selected biochemical markers and self-reported outcomes including fatigue, health-related QoL and physical performance status compared with a group of advanced cancer patients performing a light physical mobilization program that served as sham-intervention group (SHAM). Based on data obtained from previous research in other chronic disease patients (21,22), we hypothesized that (i) LOW-HIIT would be feasible and safe for advanced cancer patients and would not induce adverse effects on immune function due to its extremely low exercise volume, and (ii) LOW-HIIT would provide greater benefits on patients’ CRF, selected blood biomarkers and self-reported outcomes compared with the sham intervention.
METHODS
Study design
The present investigation was a 12-wk randomized, sham-intervention controlled pre–post feasibility study, which was conducted as a preliminary project to assist in the design of a larger scale randomized clinical trial (ClinicalTrials.gov: NCT04065815). Patients were randomly assigned to the LOW-HIIT and SHAM group, respectively. Randomization was conducted employing a computerized random number generator (MinimPy, GNU GPL v3), independently of the researchers who were involved in data collection. Before randomization, patients were stratified according to their sex, age (<60 yr and ≥60 yr), UICC grade (III and IV) and V̇O2peak (<20 mL·kg−1·min−1 and ≥20 mL·kg−1·min−1) to achieve a homogenous distribution of patients’ main characteristics among both groups. Both groups received standard care nutritional counseling to support patients’ energy, macronutrient and micronutrient intakes. The study’s primary feasibility outcomes were the attrition and session attendance rates. Secondary measures were safety, protocol fidelity and patient-reported exercise enjoyment. Key secondary outcomes to estimate preliminary efficacy of LOW-HIIT were V̇O2peak and self-reported fatigue. Further secondary outcomes to obtain first estimates on physiological and self-reported effects included acute responses of immune parameters to a single session of LOW-HIIT and chronic changes in blood variables, body composition, CRF measures, and self-reported QoL and physical functioning.
All patients were fully informed about the aims and procedures of the study, which conformed to the Helsinki Declaration. Before study enrolment, all patients provided written consent for participation and the use of data for research purposes. The study protocol was approved by the Medical Ethical Committee of the Friedrich-Alexander University Erlangen-Nürnberg (approval number: 79_19B). A schematic illustration of the study design is shown in Figure 1.
FIGURE 1.
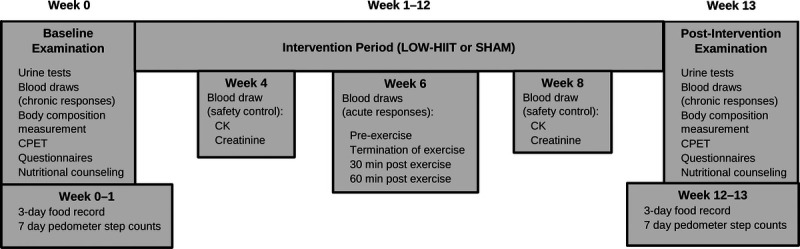
Schematic illustration of study design. Blood draws in week 4 and week 8 before exercise session.
Patients
Patients were recruited through flyers and physician and nurse referral from different departments of the University Hospital Erlangen treating oncological patients, including the Department of Medicine 1—Gastroenterology, Pneumology and Endocrinology, Department of Medicine 5—Hematology and Oncology, Department of Obstetrics and Gynecology, Department of Dermatology, Department of Radiation Oncology and Department of Urology. Adult patients (≥18 yr) diagnosed with advanced cancer (UICC stages III and IV) and an ongoing anticancer therapy were considered as eligible for study inclusion. Exclusion criteria were the following: clinical diagnosis of severe cardiac, orthopedic, or neurological diseases; acute vein thrombosis; acute infectious diseases; oncological surgery in the last 3 months and pregnancy. As this was a pilot study, formal sample size calculations were not conducted. However, it has been suggested that 12 participants per group is a good rule of thumb for estimating feasibility with sufficient precision in pilot studies (23).
Interval training
Training was performed on electronically braked cycle ergometers (Corival cpet; Lode, Groningen, Netherlands) and supervised by certified physiotherapists/sports therapists. Exercise sessions were scheduled twice a week (with at least 1 d rest in between) for a total period of 12 wk. The LOW-HIIT protocol was in accordance with the protocol developed by Reljic et al. (20) and as previously described in detail. In brief, the protocol consisted of a 2-min warm-up period followed by five interval bouts of 1 min at 80% to 95% HRpeak interspersed with 1 min of low intensity recovery and a concluding 3 min cooldown phase (total time per session, 14 min). The minimum exercise intensity to be achieved during each interval was >80% HRpeak. However, to achieve a progressive training stimulus, patients were instructed to increase the load intensity (if possible) during the course of the exercise intervention according to the following pattern: weeks 1 to 4, 80% to 85% HRpeak; weeks 5 to 8, 85% to 90% HRpeak; and weeks 9 to 12, 90% to 95% HRpeak. To reach their individual target HR for each 1-min interval bout, patients were instructed to adjust the pedal cadence and/or increase/decrease load resistance. Patients were provided with a chest strap HR monitor (acentas, Hörgertshausen, Germany) to track their HR in real-time during exercise. Patients’ HR was recorded continuously each exercise session and subsequently, HR responses during each interval were analyzed using a specific HR monitoring system (HR monitoring team system, acentas, Hörgertshausen, Germany). Patients were able to schedule their exercise sessions individually during the opening hours of our Training Center. Patients were continuously monitored throughout all exercise sessions and regularly questioned about their condition.
Sham Intervention
Patients in the SHAM group performed 2 weekly sessions of a light physical mobilization program. The program consisted of two sets of light dynamic movements, each repeated for 10 times, including trunk flexion and extension, partial squats (i.e., knee bends to ~80°–100° of flexion), butterfly movements (i.e., forward and backward movements with extended arms), and pull-down movements (i.e., overhead arm extension and pulldown) (total session time: 20 min). During these exercises, patients wore a specific vest, a hip belt and upper arm and thigh cuffs with integrated electrodes (miha bodytec, Gersthofen, Germany), which are usually used to induce electrical muscle stimulation (“whole-body electromyostimulation training”). For this sham intervention, however, electric current intensity was below the threshold that triggers a muscle contraction.
Feasibility and safety assessment
Based on previous research, reporting attrition and attendance rates in the range of 6% to 58% and 44% to 100%, respectively, in exercise interventions with advanced cancer patients (3–5), we defined feasibility as ≥75% of patients completing the LOW-HIIT intervention and ≥80% attendance in scheduled exercise sessions. Protocol fidelity was assessed through analyzing the degree to which the intended exercise intensity (i.e., the prescribed exercise heart rate) and number of intervals were achieved by the patients. For this purpose, heart rate was continuously recorded during all exercise sessions and subsequently evaluated as described in detail above. Patient-reported exercise enjoyment was assessed at the end of the intervention using a seven-point rating scale ranging from 1 (“not enjoyable at all”) to 7 (“extremely enjoyable”). In addition, patients were asked whether the LOW-HIIT protocol was helpful to overcome previous barriers to participate in exercise, as well as they intended to further engage in LOW-HIIT or in exercise in general after termination of the intervention using a four-item measure. Given that similar data on LOW-HIIT protocol fidelity and enjoyment are not available for advanced cancer patients, we did not predefine specific assumptions for these two outcomes. Safety was evaluated through recording and analyzing of adverse events (AE). According to the National Cancer Institute (24), an AE was defined as “an unexpected medical problem that happens during treatment (with a drug or other therapy).” Adverse events were considered to be related to exercise if they occurred during the exercise session or within 1 h after cessation of exercise (25) or if a physician detected a clear relationship between exercise participation and the occurrence of an AE. All AE were immediately recorded in a study-specific protocol for each patient when they occurred or when they were reported to the therapists or research staff by the patient by phone or at the exercise sessions. Specific log sheets were handed out to assist patients in the recording of AE that occurred outside of the exercise sessions. Adverse event severity rating was based on the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 5.0, ranging from grade 1 (“asymptomatic or mild symptoms”) to grade 5 (“death related to AE”) (26). The safety of the study was defined as the absence of exercise-related AE classified as grade 3 (i.e., “significant symptoms requiring hospitalization and/or limitations in self-care activities of daily living”) or above (26).
Health examinations
The baseline examination was conducted 1 wk before the start of the intervention and outcome reassessment was performed within the first week after completion of the intervention, at least 3 d apart from the last exercise session and at a similar time of day to ensure sufficient recovery and to avoid potential circadian effects. Patients were instructed to present in an overnight-fasted state at our Research Laboratory and to abstain from alcohol consumption as well as from vigorous physical activities for at least 24 h preceding the examination. During the examination, patients were carefully screened to ensure safe participation in the exercise intervention, including resting and exercise electrocardiography, blood pressure measurements in rest and during exercise testing and evaluation of routine blood and urine parameters. The assessments were conducted under laboratory conditions and were strictly standardized as further outlined below. All examinations were made in a single-blinded fashion, meaning that the researchers who were involved in data collection were unaware of the patients’ group allocation.
Urine tests
Upon arrival to the laboratory, patients were first asked to provide a urine sample to routinely screen for conditions like urinary tract infections, kidney disorders or diabetes, and to determine urine-specific gravity (USG). Urine samples were analyzed within 30 min of collection using Multistix® 10 SG dipsticks (Siemens HealthCare, Erlangen, Germany).
Blood collection for determination of chronic responses
Preintervention and postintervention blood samples were drawn by puncture of an antecubital arm vein into collection tubes using a disposable cannula (S-Monovette, Sarstedt, Nürmbrecht, Germany). All blood samples were analyzed at the diagnostic laboratories of the University Hospital Erlangen. Analyses included blood count using a hematology analyzer (ADVIA 120/2120, Siemens HealthCare, Erlangen, Germany), serum concentrations of glucose, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) using a photometrical method (Clinical Chemistry Analyzer Beckman Coulter DxC AU700 or AU5800; Beckman Coulter, Brea, CA) and C-reactive protein (CRP) (Turbidimetric method, Clinical Chemistry Analyzer Beckman Coulter DxC AU700 or AU5800; Beckman). In addition, creatine kinase (CK) levels were analyzed at the baseline examination, week 4, week 8, and postintervention examination to monitor muscular stress using a photometrical method (Clinical Chemistry Analyzer Beckman Coulter DxC AU700 or AU5800, Beckman Coulter).
Blood collection for determination of acute responses
During week 6 of the intervention, the acute responses to a LOW-HIIT exercise session on blood immune parameters were investigated. Week 6 (midintervention) was chosen as the time point for blood sampling to allow sufficient time for patients to adapt to the training and thus, to better reflect the acute responses of immune parameters under steady state conditions.
For this purpose, an indwelling cannula was placed in an antecubital vein and blood samples were drawn before exercise, directly after termination of exercise and after 30 and 60 min of exercise. Subsequently, the tubes with the blood samples were immediately processed for the isolation of peripheral blood mononuclear cells (PBMC) and frozen at −80°C until they were transferred to the research laboratory of the Department of Medicine 5, University Hospital Erlangen for further processing. The acute response analyses included the determination of standard differential blood count parameters using a hematology analyzer (ADVIA 120/2120, Siemens HealthCare, Erlangen, Germany). All blood count parameters were corrected according to the following formula to address acute plasma volume shifts after exercise (27):
where BM and Hb are the concentrations for the blood biomarker and hemoglobin before (pre) and after (post) exercise.
Moreover, the acute response analysis included a comprehensive characterization of peripheral blood natural killer cells (NK cells). For this purpose, cell lines and culture media were established as follows: The tumor cell line K562 was persuaded from the American Type Culture Collection (ATCC, Manassas, VA) and cells were maintained at 37°C in complete medium (CM) consisting of RPMI1640 media (GIBCO) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Biochrom, Berlin, Germany) and antibiotics (penicillin/streptomycin; GIBCO). Next, fluorescent cell barcoding of live cells was conducted. To increase the accuracy and reproducibility of the functional and phenotypical NK cell analyses, the isolated PBMC from all four time points of a single cancer patient were each labeled with an individual concentration of a fluorescent live-cell dye (VPD) (Thermo Fisher Scientific, Erlangen, Germany) and later pooled together for the subsequent analysis steps. Briefly, PBMC were washed once with PBS (Gibco) and then labeled with different concentrations of the live-cell dye VPD for 5 min at 37°C. The labeling was stopped by adding 100 μL FCS and incubating them for another 2 min at 37°C. Peripheral blood mononuclear cells were washed twice with CM and subsequently pooled together. Therefore, cells were labeled with the dead-cell maker Zombie® aqua (Biolegend, San Diego, CA), FITC-labeled anti-CD16 (Biolegend), PE-labeled anti-CD56 (Biolegend), PerCP-Cy5.5-labeled anti-CD14 (Biolegend), APC-labeled anti-CD45 (BD Bioscience, Heidelberg, Germany) and APC-Cy7-labeled anti-CD3 (Biolegend) for 20 min at 4°C in staining buffer (PBS/1% FCS). Afterward the cells were washed twice with staining buffer and fixed in 4% paraformaldehyde for 10 min at 4°C. Finally, the cells were analyzed at a FACS canto II machine (BD Bioscience). FlowJo software (FlowJo LLC, Ashland, OR) was used to perform the subsequent FACS data analysis.
Subsequently, functional analysis of NK cells was performed. After thawing, PBMC were incubated overnight at 37°C in CM supplemented with 100 U IL-2 mL−1 (Miltenyi, Bergisch Gladbach, Germany). Next day, cells were harvested and fluorescent cell barcoding was performed as specified above. Pooled PBMC were then incubated for an additional 4 h in CM with AF647-labeled CD107a antibodies (1:200; Biolegend) in the presence or absence of K562 cells (ratio 1:1). After 1 h the protein transport inhibitors brefeldin A (GolgiPlug™; BD Bioscience) and monensin (GolgiStop™; BD Bioscience) were added to the culture for the remaining 3 h. Afterward, cells were harvested and stained with FITC-labeled anti-CD16 (Biolegend), PE-labeled anti-CD56 (Biolegend), PE-Cy7–labeled anti-CD3 (Biolegend). Subsequently, intracellular labeling of IFNγ expression (APC-Cy7 anti-IFNγ; Biolegend) was done using a fixation/permeabilization solution kit (BD Bioscience) according to the manufacturer’s instructions. Finally, the cells were analyzed at a FACS canto II machine (BD Bioscience).
Body composition measurement
For body composition measurements, patients were still in a fasted state and instructed again to empty their bladder if necessary. Before measurements, patients were visually screened for peripheral edema to ensure a euhydrated status and to exclude the presence of significant body fluid disturbances, respectively. A segmental multifrequency bioelectrical impedance analysis device (seca mBCA 515, Seca, Hamburg, Germany), which has previously been validated against magnetic resonance imaging (28), was used to determine body mass, skeletal muscle mass, the percentage of body fat, total body water, and extracellular water.
Cardiopulmonary exercise test
Patients performed the standardized cardiopulmonary exercise test (CPET) on an electronically braked cycle ergometer (Corival cpet; Lode) to determine V̇O2peak, peak power output (Wpeak) and peak heart rate (HRpeak). Briefly, after a 1-min familiarization period, the CPET started at 50 W and then workrate gradually increased in ramp fashion corresponding to 12.5 W·min−1 (1 W every 5 s) for female patients and 15 W·min−1 (1 W every 4 s) for male patients, respectively, until volitional exhaustion. Criteria to assume that maximal effort was reached were at least two of the following: a leveling-off of oxygen uptake, peak RER (RERpeak) ≥1.1, age predicted HRpeak (APHRpeak) ≥90% (using the equation: 220 − age) and maximal RPE (RPEpeak) ≥19 on the Borg scale (29). HR was recorded continuously using a 12-lead ECG system (custo cardio 110, custo med, Ottobrunn, Germany). Oxygen uptake (V̇O2) and carbon dioxide output (V̇CO2) were measured with an open-circuit breath-by-breath spiroergometric system (Metalyzer 3B-R3; Cortex Biophysik, Leipzig, Germany). All measurements were averaged over every 10 s. Furthermore, ventilatory threshold (VT) was determined according to the V-slope method by plotting V̇CO2 against V̇O2 to assess submaximal exercise capacity (30).
Assessment of self-reported outcomes
Self-reported outcomes were determined using standardized questionnaires, which were all previously validated and/or carefully translated in the German language. Fatigue was determined by the use of the Functional Assessment of Chronic Illness Therapy—Fatigue Scale (13-item FACIT Fatigue Scale) where a higher score indicates less fatigue and better function (31). Health-related QoL was examined using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire—C30 (EORTC QLQ-C30), consisting of different health-related subdimensions. Here, higher scores in global health and functional scales represent a better health-related QoL whereas high scores in symptom scales indicate a higher symptomatic burden (32). Physical functioning status was assessed by the Karnofsky Index (33). The questionnaires were completed by the patients at the baseline and postintervention examinations in a separate waiting area of our Research Center. Potential queries about the questionnaires could be clarified with the investigators at any time. At the final examination, the patients additionally provided an evaluation sheet, consisting of a seven-point scale to rate their enjoyment of the exercise program (1, not enjoyable at all; 7, extremely enjoyable) and of questions regarding their main personal barriers to regular exercise and whether the applied LOW-HIIT protocol was helpful in overcoming these more easily.
Physical activity assessment
Patients’ daily physical activity was assessed using pedometers (Walking Style One 2.1; Omron, Mannheim, Germany), which were worn for seven consecutive days before the baseline and postintervention examinations. For the analysis, the averaged over 7 d step counts were used.
Dietary support
In both groups, nutritional intakes were monitored using 24-h dietary records (Freiburger Ernährungsprotokoll; Nutri-Science, Freiburg, Germany) assessed on three consecutive days at study entry and within the last week of the study intervention. Analysis of mean caloric and nutrient intake was done using the software PRODI 6 expert (Nutri-Science). Based on these data, patients received individual nutritional counseling by a registered dietician by face-to-face conversation. Nutritional advices followed current dietary guidelines for patients with malignant disease undergoing anticancer treatment (34). In brief, energy intake was calculated according to the estimated resting energy expenditure using the Harris-Benedict equation (35), physical activity level and nutritional status. It was targeted that patients ingest at least 25 to 30 kcal·kg−1·d−1. Given increased protein requirements in cancer diseases, patients were instructed to achieve a daily protein intake of >1.0 g·kg−1 (34). Patients with renal failure were advised not to exceed a daily protein intake of 1.0 g·kg−1 in acute or 1.2 g·kg−1 in chronic disease, respectively. In overweight patients (BMI ≥25 kg·m−2), nutritional intake was adjusted to their normal body mass according to the Broca Index (i.e., height [cm] − 100) to prevent excessive energy consumption.
Statistical analysis
All statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL), with the exception of the specific NK cell analyses, which were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). First, the Shapiro–Wilk test was applied to check the distribution of data. A 2 × 2 repeated-measures ANOVA was conducted to determine main effects of group, time, and interaction between both factors. Homogeneity of variance was assured using the Levene’s test. In case of significant main or interaction effects, post hoc paired t tests were performed to determine differences between preintervention and postintervention values in each group and independent t tests were carried out to analyze between group differences, respectively. In case of nonnormally distributed data, log or square root transformation was applied, and subsequently, the same analyses were applied to the transformed values. If data transformation did not improve the data distribution to meet assumptions of parametric tests, the nonparametric Friedman two-way analysis of variance by ranks was conducted, followed by Wilcoxon’s and Mann–Whitney tests for post hoc comparisons. Furthermore, effect sizes were calculated according to the partial eta squared (ɳp2) for ANOVA and Kendall’s coefficient of concordance (W) for the Friedman test. Effect sizes were classified as follows: small ≤0.01, medium ≥0.06, and large ≥0.14 for ɳp2, and small ≥0.10, medium ≥0.30, and large ≥0.50 for W (36). Pearson (r) correlation analyses were calculated to investigate the relationship between selected parameters. For all analyses, the significance level was defined to be P < 0.05. Data are reported as means ± SD and preintervention/postintervention changes are shown with 95% confidence intervals (95% CI).
RESULTS
Study flow, attrition, and attendance rates
A total of 30 patients were screened for eligibility. Twenty-seven patients who met the eligibility criteria were enrolled in the study and randomly assigned to either the LOW-HIIT or SHAM group. Baseline characteristics of all included patients are shown in Table 1. Three patients dropped out during the intervention period (LOW-HIIT: n = 1, 8% and SHAM: n = 2, 14%). The reasons for dropout are displayed in Figure 2 (study flowchart). Thus, a total of 24 patients (LOW-HIIT: n = 12, SHAM: n = 12) completed the study and were included in the final data analysis. Patients’ anticancer treatments and comorbidities are reported in Supplemental Table 1 (see Supplemental Digital Content 1, Patients’ anticancer treatments and comorbidities, http://links.lww.com/MSS/C659). The attendance rates (the percentage of the scheduled training sessions that the patients completed) were 92.5% ± 9.7% in the LOW-HIIT group, and 97.3% ± 6.0% in the SHAM group, respectively.
TABLE 1.
Baseline characteristics of all included patients.
| HIIT (n = 13) | SHAM (n = 14) | |
|---|---|---|
| Men/women, n (%) | 7 (54)/6 (46) | 6 (43)/8 (57) |
| Age (yr) | 52.5 (45.0–60.1) | 58.0 (50.1–65.9) |
| BMI (kg·m−2) | 24.3 (21.6–26.9) | 24.0 (22.0–26.1) |
| V̇O2peak (mL·kg−1·min−1) | 26.7 (22.3–31.2) | 26.3 (22.4–30.3) |
| Cancer sites | ||
| Colon/rectum, n (%) | 4 (30.8) | 2 (14.3) |
| Stomach, n (%) | 3 (23.1) | 2 (14.3) |
| Melanoma, n (%) | 2 (15.4) | 1 (7.1) |
| Liver, n (%) | 1 (7.7) | 1 (7.1) |
| Pancreas, n (%) | 1 (7.7) | 0 (0) |
| Esophagus, n (%) | 1 (7.7) | 1 (7.1) |
| Ovary, n (%) | 1 (7.7) | 3 (21.4) |
| Myeloma, n (%) | 0 (0) | 2 (14.3) |
| Breast, n (%) | 0 (0) | 2 (14.3) |
| Lung, n (%) | 0 (0) | 1 (7.1) |
| UICC stagea | ||
| III, n (%) | 1 (7.7) | 1 (7.1) |
| IV, n (%) | 12 (92.3) | 11 (78.6) |
Numbers are given as mean and 95% confidence intervals and absolute numbers and percentages, respectively.
aTwo myeloma-patients of the SHAM not included in UICC data.
FIGURE 2.
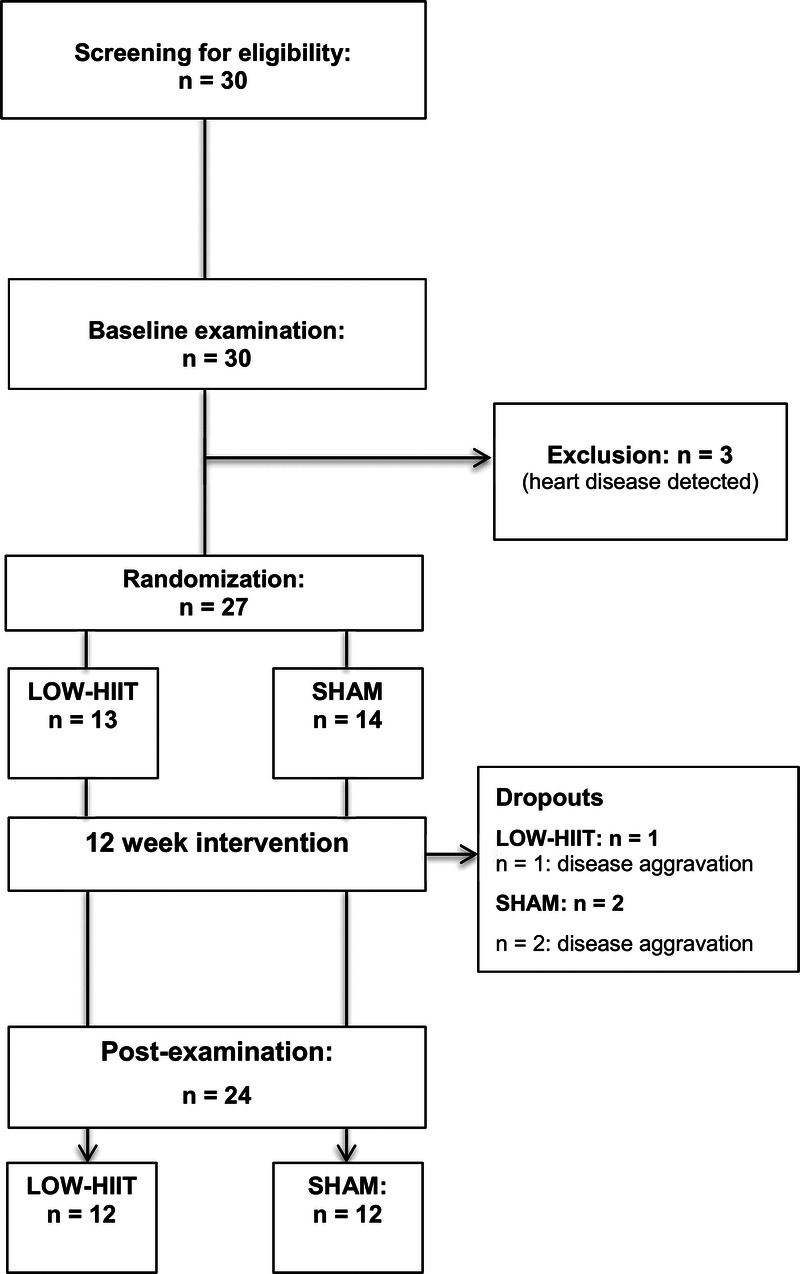
Study flowchart.
Safety data and acceptability
In both groups, no serious AE occurred at any point during the training sessions. Minor AE that were recorded during or after a LOW-HIIT or SHAM exercise session and other incidents that occurred throughout the study period are reported in Supplemental Table 2 (see Supplemental Digital Content 2, Adverse events and patient-reported physical complaints during study, http://links.lww.com/MSS/C660). The majority of physical complaints that were experienced by the patients during the study period occurred shortly after chemotherapy treatment and were thus considered unrelated to the exercise interventions. None of the patients reported severe muscle soreness nor displayed clinically relevant disturbances in serum CK levels. In both groups, serum CK values did not change significantly throughout the four time points (baseline, week 4, week 8, and postintervention) and averaged 97 U·L−1 (95% CI, 66–129 U·L−1) in the LOW-HIIT group, and 100 U·L−1 (95% CI, 81–120 U·L−1) in the SHAM group, respectively.
The major barriers to regular exercise reported by patients were “too busy” (67%), followed by “feeling too weak/tired” (63%), “physical complaints/pain” (50%), “unsure how to exercise” and “lack of support” (each 46%). The majority of patients (92%) in the LOW-HIIT group stated that the exercise protocol was helpful to overcome at least one of the perceived major barriers to regular exercise, in particular “lack of time” (83%) and “lack of knowledge and support” (92%). Eighty-three percent of patients stated that they would like to continue participating in LOW-HIIT after termination of the study and 92% stated that they intended to engage in more exercise in general because they felt physically better again.
Protocol fidelity
The average heart rate reached during the interval bouts was equivalent to 93% ± 7% of HRpeak (weeks 1–4: 93% ± 6%, week 5–8: 93% ± 7%, week 9–12: 94% ± 7%), verifying that the prescribed minimum level of exercise intensity (i.e., ≥80% HRpeak) was achieved in the LOW-HIIT group. On average, the intended exercise intensity for weeks 9 to 12 (i.e., 90%–95% HRpeak during intervals) was already achieved from the beginning of the intervention. The individual cumulative HR responses during the interval bouts are shown in Figure 5A. The average HR during the exercise sessions (including warm-up, intervals, recovery periods between intervals and cooldown) was equal to 79 ± 4% HRpeak (weeks 1–4, 79% ± 3%; weeks 5–8, 79% ± 4%; weeks 9–12, 78% ± 4%).
FIGURE 5.
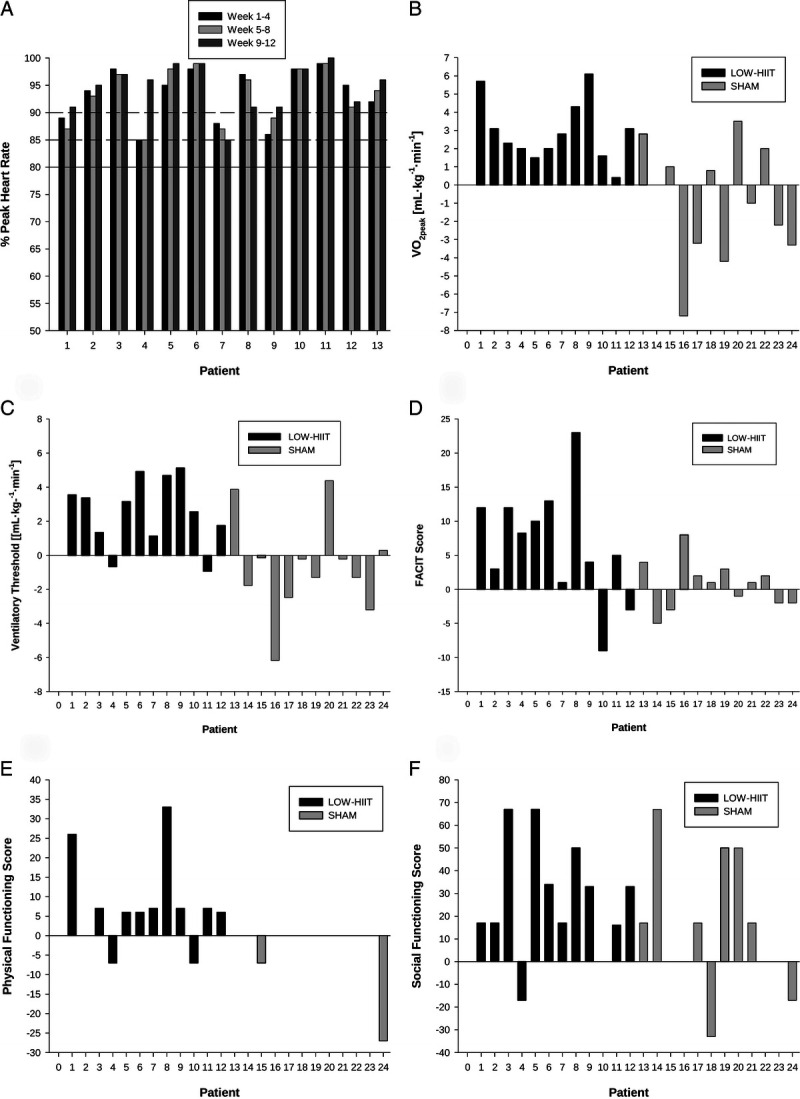
Individual heart rate responses and chronic adaptations to LOW-HIIT. Individual mean heart rate responses during exercise intervals in weeks 1 to 4, weeks 5 to 8, and weeks 9 to 12 (full line indicates minimum required heart rate [≥80% HRpeak), dashed lines indicate lower bounds of the intended heart rates for weeks 5 to 8 [≥85% HRpeak] and week 9 to 12 [≥90% HRpeak] (A), individual changes in maximal oxygen uptake (B), individual changes in VT (C), individual changes in FACIT score (D), individual changes in physical functioning (E), and individual changes in social functioning (F).
Acute effects of LOW-HIIT on immune outcomes
An acute bout of LOW-HIIT led to a sharp increase and subsequent fall in concentrations of leukocytes, lymphocytes, neutrophils and monocytes within 60 min after termination of exercise. However, no statistically significant differences were found in the concentrations of these blood markers between the four time points. Moreover, none of the concentrations dropped significantly below the baseline value during the postexercise period (Fig. 3). NK cells showed an enhanced degranulation 60 min postexercise after contact with K562 and BL-41 cells (Fig. 4A, 4B) compared with preexercise. Moreover, we observed that directly after exercise the composition of the NK cell population was significantly skewed toward the more differentiated CD56dim subset, but returned to baseline levels 60 min postexercise (Fig. 4C, 4D).
FIGURE 3.
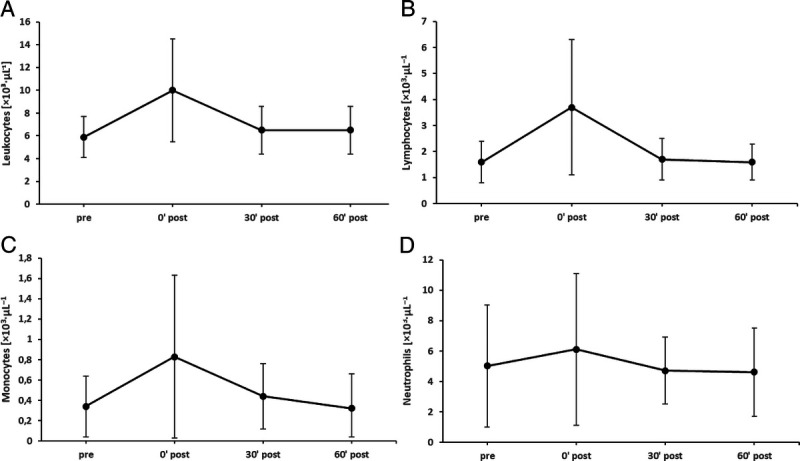
Acute responses of leukocytes (A), lymphocytes (B), monocytes (C), and neutrophils (D) to LOW-HIIT.
FIGURE 4.
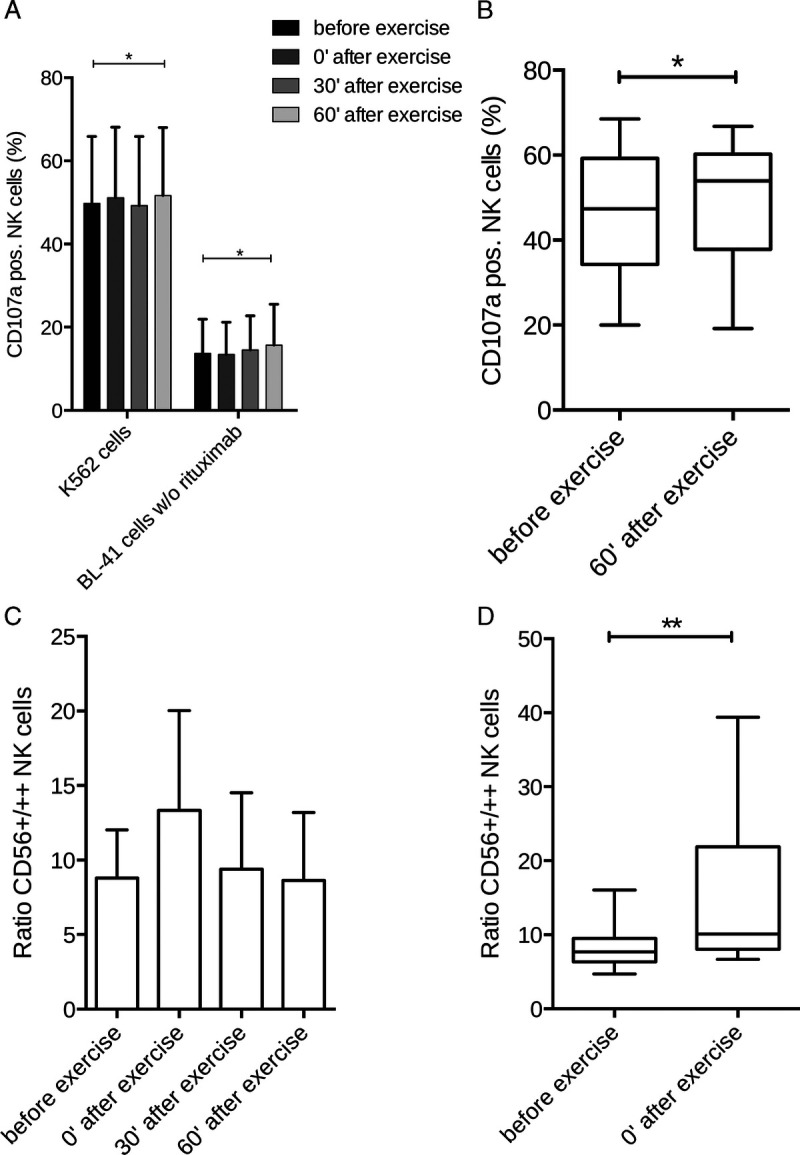
Acute responses of NK cells to LOW-HIIT. NK cell degranulation after contact with K562 and BL-41 cells within 60-min postexercise (A), NK cell degranulation preexercise vs 60-min postexercise (B), NK cell differentiation within 60-min postexercise (C), and NK cell differentiation preexercise vs directly postexercise (D). *Significant difference (P < 0.05) preexercise vs. 60-min postexercise; **Significant difference (P < 0.01) preexercise vs directly postexercise.
Body composition variables and hydration status
No significant within- or between-group effects were found in body weight and any of body composition outcomes. Group-specific preintervention and postintervention body composition values of all data are shown in Table 2. There were no significant within- or between-group effects in USG. In all patients, USG values were within the normal ranges at both measurement times (preintervention, 1022 ± 10 and postintervention, 1020 ± 11).
TABLE 2.
Preintervention and postintervention values of all physiological outcomes.
| HIIT (n = 12) | SHAM (n = 12) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Body weight (kg) | 73.7 (62.6–84.8) | 74.4 (64.9–83.9) | 73.1 (63.9–82.3) | 74.1 (64.0–84.2) |
| Skeletal muscle mass (kg) | 23.6 (19.7–27.5) | 23.8 (20.4–27.2) | 23.4 (19.7–27.1) | 23.3 (19.6–27.1) |
| Fat mass (%) | 28.0 (20.6–35.3) | 28.6 (21.2–35.9) | 29.2 (21.3–37.1) | 30.4 (23.9–37.0) |
| Total body water (L) | 38.6 (33.7–43.4) | 38.6 (34.6–42.7) | 37.8 (32.6–42.9) | 37.8 (32.8–42.8) |
| Extracellular water (L) | 17.5 (15.7–19.3) | 17.5 (16.1–18.9) | 17.0 (14.9–19.2) | 17.1 (15.0–19.2) |
| Absolute V̇O2peak (L·min−1) | 1.86 (1.54–2.18) | 2.09 (1.74–2.44)a | 1.95 (1.62–2.56) | 1.92 (1.68–2.41) |
| Relative V̇O2peak (mL·kg−1·min−1) | 25.8 (21.5–30.0) | 28.8 (24.2–33.1)a | 27.1 (23.2–33.5) | 26.2 (23.0–32.4) |
| Absolute peak power output (W) | 157 (132–183) | 174 (148–200)a | 154 (125–202) | 157 (133–202) |
| Relative peak power output (W kg−1) | 2.2 (1.8–2.5) | 2.4 (2.0–2.8)a | 2.1 (1.8–2.7) | 2.1 (1.8–2.7) |
| VT (mL·kg−1·min−1) | 13.3 (11.3–15.4) | 15.8 (13.5–18.1)a | 15.2 (12.6–17.9) | 14.6 (12.2–17.0) |
| Erythrocytes (×106·μL−1) | 4.22 (3.67–4.78) | 4.35 (4.05–4.66) | 4.03 (3.63–4.44) | 3.95 (3.50–4.40) |
| Hemoglobin (g·dL−1) | 12.1 (10.4–13.8) | 12.9 (12.1–13.7) | 12.6 (11.4–13.7) | 12.6 (11.2–13.9) |
| Hematocrit (%) | 36.4 (31.3–41.5) | 38.6 (36.1–41.1) | 37.1 (33.7–40.5) | 37.4 (33.6–41.1) |
| Leucocytes (×103·μL−1) | 5.57 (4.06–7.07) | 5.01 (4.29–5.73) | 4.12 (3.27–5.00) | 4.53 (3.38–5.68) |
| Thrombocytes (×103·μL−1) | 248 (191–305) | 250 (215–286) | 234 (194–282) | 226 (169–282) |
| Glucose (mmol·L−1) | 5.8 (5.3–6.3) | 5.7 (5.1–6.3) | 5.6 (5.2–6.1) | 5.7 (5.3–6.1) |
| Triglycerides (mmol·L−1) | 1.2 (1.0–1.3) | 1.2 (1.0–1.5) | 1.4 (0.7–2.0) | 1.5 (1.0–1.9) |
| Total cholesterol (mmol·L−1) | 5.3 (4.6–6.1) | 5.8 (4.9–6.7) | 5.6 (4.6–6.6) | 5.5 (4.5–6.4) |
| HDL cholesterol (mmol·L−1) | 1.5 (1.2–1.8) | 1.7 (1.5–2.0) | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) |
| LDL cholesterol (mmol·L−1) | 3.3 (2.7–3.8) | 3.6 (2.9–4.3) | 3.5 (2.8–4.3) | 3.5 (2.7–4.2) |
| CRP (mg·dL−1) | 8.6 (1.7–15.5) | 5.6 (0–11.2) | 4.9 (0.7–9.1) | 7.1 (0–14.2) |
Numbers are given as mean and 95% CIs.
aSignificant difference (P < 0.001) vs preintervention.
CRF data
The mean baseline V̇O2peak (26.5 ± 6.9 mL·kg−1·min−1) and VT (14.3 ± 3.3 mL·kg−1·min−1) indicated that the CRF level was generally poor in the studied collective. All patients reached at least two maximal effort criteria during the preintervention and postintervention CPET. Plateau in V̇O2 and RPEpeak ≥ 19 were reached by 100% of patients at both CPETs. RERpeak ≥ 1.10 was reached by 89% of patients at both time points. HRpeak ≥ 90% of APHRpeak was reached by 75% of patients preintervention and by 79% postintervention, respectively.
A significant group–time interaction and main effect of time was observed for absolute V̇O2peak (P = 0.031, ή2 = 0.20 and P = 0.008, ή2 = 0.28, respectively), absolute Wpeak (P < 0.026, ή2 = 0.21 and P < 0.003, ή2 = 0.34, respectively), and relative Wpeak (P < 0.029, ή2 = 0.20 and P < 0.012, ή2 = 0.26, respectively). A significant group–time interaction was found for relative V̇O2peak (P = 0.001, ή2 = 0.38) and VT (P < 0.010, ή2 = 0.29). In the LOW-HIIT group, post hoc tests revealed significant increases in absolute V̇O2peak (+0.23 L·min−1; 95% CI, 0.11–0.35 L·min−1; P < 0.001), relative V̇O2peak (3.0 mL·kg−1·min−1; 95% CI, 1.8–4.0 mL·kg−1·min−1; P < 0.001), absolute Wpeak (16 W, 95% CI, 8–25 W, P < 0.001), relative Wpeak (0.2 W·kg−1, 95% CI: 0.1–0.3 W·kg−1, P < 0.001), and VT (2.5 mL·kg−1·min−1, 95% CI: 1.2–3.8 mL·kg−1·min−1, P < 0.001). In the SHAM group, there were no significant changes in any of the CRF outcomes (Fig. 5). Preintervention and postintervention CRF values for each group are shown in Table 2.
Chronic response of blood markers
There were no significant effects for any blood markers that were used to assess the chronic biochemical responses to both interventions. Group-specific preintervention and postintervention values of all blood markers are shown in Table 2.
Self-reported outcomes
A significant group–time interaction was detected for the FACIT score (P = 0.034, ή2 = 0.19) and physical functioning (P = 0.017, ή2 = 0.23). Furthermore, a significant main effect of time was found for the FACIT score (P = 0.011, ή2 = 0.26) and social functioning (P = 0.001, ή2 = 0.39). Group-specific post hoc analyses revealed significant improvements of the FACIT score (+7 points; 95% CI, 1–13 points; P = 0.010), physical functioning (+8 points; 95% CI, 1–15 points; P = 0.022), and social functioning (+28 points; 95% CI, 12–44 points; P = 0.001) in the LOW-HIIT group. Improvements in physical functioning were significantly correlated with increases in absolute V̇O2peak (r = 0.52, P = 0.004), relative V̇O2peak (r = 0.51, P = 0.006), and WVT (r = 0.37, P = 0.037). No significant changes in self-reported outcomes were observed for the SHAM group (Fig. 5). Group-specific preintervention and postintervention values of all data are displayed in Table 3.
TABLE 3.
Preintervention and postintervention values of all self-reported outcomes.
| HIIT (n = 12) | SHAM (n = 12) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| ECOG performance status | 1.18 (0.78–1.59) | 1.00 (1.00–1.00) | 1.08 (0.76–1.41) | 1.08 (0.76–1.41) |
| Karnofsky Index | 73 (69–77) | 75 (70–80) | 73 (67–78) | 71 (66–76) |
| FACIT score | 32 (26–39) | 39 (35–44)a | 38 (34–42) | 39 (35–44) |
| EORTC QLQ-C30 | ||||
| Physical functioning | 74 (62–86) | 82 (73–90)a | 79 (69–89) | 76 (66–86) |
| Role functioning | 47 (25–70) | 65 (53–78) | 63 (47–78) | 60 (48–71) |
| Emotional functioning | 62 (50–73) | 74 (64–85) | 69 (57–80) | 68 (53–84) |
| Cognitive functioning | 69 (49–89) | 74 (60–87) | 76 (63–90) | 81 (69–92) |
| Social functioning | 40 (25–55) | 68 (57–80)b | 57 (37–77) | 70 (54–87) |
| Pain | 37 (14–60) | 26 (10–42) | 22 (9–35) | 18 (7–29) |
| Dyspnea | 30 (16–45) | 25 (15–34) | 36 (13–59) | 36 (13–59) |
| Insomnia | 39 (11–67) | 27 (8–47) | 30 (13–47) | 28 (8–48) |
| Appetite loss | 28 (6–50) | 22 (5–38) | 30 (7–53) | 19 (3–36) |
| Constipation | 0 (0–0) | 11 (0–22) | 11 (0–22) | 14 (0–28) |
| Diarrhea | 22 (6–39) | 14 (0–28) | 14 (0–28) | 19 (3–36) |
| Financial difficulties | 19 (3–36) | 14 (0–28) | 8 (0–16) | 8 (0–16) |
| Nausea | 18 (5–31) | 18 (9–27) | 10 (2–18) | 7 (2–12) |
| Fatigue | 55 (36–73) | 41 (29–54) | 40 (27–53) | 40 (32–49) |
| Global health | 53 (42–64) | 60 (48–72) | 63 (52–74) | 68 (58–78) |
Numbers are given as mean and 95% CI.
*Significant difference (P < 0.05).
**Significant difference (P < 0.01) vs. preintervention.
Nutritional analysis and pedometer data
We found a significant group–time interaction for energy intake per kg body weight (P < 0.018, ή2 = 0.23) and a main effect of time for fat intake (P = 0.018, ή2 = 0.23), respectively. Subsequent post hoc tests, however, did not detect any between- and within-group differences for energy and macronutrient intakes. Group-specific preintervention and postintervention nutritional intakes are shown in Supplemental Table 3 (see Supplemental Digital Content 3, Patients’ daily nutritional intakes preintervention and during the last week of intervention, http://links.lww.com/MSS/C661). ANOVA revealed a strong trend toward a group–time interaction for daily steps (P = 0.053, ή2 = 0.20), with post hoc tests indicating a significant increase in physical activity in the LOW-HIIT group from 4937 ± 811 to 6171 ± 895 steps·per day (+1233 steps per day, 95% CI, 153–2620 steps·per day; P = 0.037), and (nonsignificant) reduction in step count in the SHAM group (8417 ± 991 to 7336 ± 812 steps per day, P = 0.263).
DISCUSSION
Although there is ample of evidence to indicate that exercise is a crucial factor in cancer prevention and rehabilitation, there is still a lack of data regarding the effects of more vigorous exercise such as HIIT in advanced cancer patients (2). To our knowledge, this study was the first to comprehensively investigate the feasibility, as well as the acute and chronic responses to a very low-volume HIIT protocol in a cohort of advanced cancer patients. The key findings were that (i) LOW-HIIT was well accepted and tolerated by advanced cancer patients as became evident by high attendance and completion rates and low adverse effects profiles; (ii) acute LOW-HIIT may increase NK cell differentiation and degranulation postexercise; and (iii) 12 wk of LOW-HIIT appears to improve CRF and various aspects of QoL, including self-reported fatigue, physical functioning and social functioning.
Thanks to the advances in medical science and treatment, the life expectancy of patients with advanced cancers has considerably increased in the last decades (3). Consequently, there is a growing need for effective supportive care measures to maintain patients’ physical condition and QoL while reducing or eliminating disease-related symptoms and adverse side effects of long-term anticancer treatment. Given that along with physical limitations, time constraints are among the most frequently reported barriers to exercise participation in advanced cancer patients (3), it is an important finding of this study that as little as 28 min of LOW-HIIT per week were well tolerated and manageable by our patients. Simultaneously, our preliminary data provide first evidence that LOW-HIIT may be effective enough to induce clinically meaningful improvements in physiological and psychological outcomes.
Notably, the attendance (~93%) and completion (~92%) rates in our study were higher compared with the average rates reported in previous exercise interventions with advanced cancer patients (attrition: 24% and attendance rates: ~78%, respectively) (3). In this respect, it is to consider that the weekly time commitment for our LOW-HIIT protocol (28 min) was substantially lower compared with previous studies with advanced cancer patients applying exercise programs of durations ranging between 75 and 240 min·wk−1 (average time commitment per week: ~128 min) (3). In conjunction with the results obtained from the patients’ evaluation sheets regarding their perceived exercise barriers, it is therefore plausible to assume that LOW-HIIT may circumvent time-related exercise obstacles and may be (perceived as) more manageable by advanced cancer patients when compared with exercise programs with higher volumes. In line with this assumption, a recent meta-analysis from our group has indicated that exercise volume (including session duration and time effort per week) appears to be a significant predictor of dropout from exercise interventions among previously untrained individuals (37). Moreover, it has been suggested that the common recommendation of 150 min·wk−1 for the minimum amount of moderate physical activity required for health benefits may have negative impact on motivation because it is not a realistic goal for most adults (38). Exercise interventions applying higher intensities in advanced cancer patients are very rare to date. With the exception of two pilot studies, also applying cycle ergometer-based HIIT protocols with intensities of 80% to 95% HRpeak that were incorporated into combined aerobic/resistance training programs (39,40), exercise intensity did typically not exceed 80% to 85% HRpeak in previous interventions (3).
One of the main reasons for the reluctant application of HIIT in advanced cancer patients so far might be safety concerns, particularly with regard to potential adverse impact on patients’ immune system. In this context, it is an important finding that our LOW-HIIT protocol (with average HR values during intervals bouts equal to 93% HRpeak) was well manageable and apparently, not associated with clinical or biochemical manifestation of immunosuppression in our patients. A temporarily impaired immune function (“open window”) has occasionally been reported in athletes after the completion of long and intense exercise and/or insufficient recovery between workout sessions or competitions, respectively (16,18). However, in accordance with recent research (16–18), our results suggest that brief vigorous exercise should not generally be regarded as harmful for the immune system, even in immune-compromised advanced cancer patients. Interestingly, we observed that directly after termination of an acute bout of LOW-HIIT, the composition of the NK cell population was significantly skewed toward the more differentiated CD56dim subset. Importantly, this subset is known to exhibit higher natural cytotoxicity against tumor cells compared with their CD56bright counterpart and is able to perform antibody-dependent cellular cytotoxicity because of their expression of the Fc gamma III receptor (CD16). Therefore, higher numbers of CD56dim NK cells might be beneficial for patients receiving therapeutic antibody as part of their antitumor treatment (41). Furthermore, 60 min postexercise, we found a significant increase in NK cell degranulation. Together, these findings point to an activation and increased cytotoxic activity of NK cells after LOW-HIIT. The exercise-dependent regulation of NK cells and its potential anticancer effects have been well established in the excellent pioneering work of Hojman and colleagues (42). In this context, it has been suggested that higher-intensity exercise stimulates NK cell mobilization and cytotoxicity to a greater extent than exercise with moderate intensity (43). The precise physiological mechanisms by which exercise might increase NK cell number and/or function is still not completely understood and under active research. Preclinical research has indicated, for example, that exercise may “prepare” the tumor microenvironment for a stronger infiltration with NK-cells. Furthermore, it has been suggested, that increased secretions of epinephrine and the myokine interleukine-6 that occur during and within hours after exercise (which apparently is more pronounced after more intense exercise) trigger NK cell mobilization (16). Accordingly, Barra et al. (44) have demonstrated that HIIT increases NK cell number and function in obese mice and in overweight/obese women. Similar results have been recently reported by Llavero et al. (17), who observed significant improvements in NK cell function with respect to cytotoxicity after 4 wk of HIIT in previously untrained but otherwise healthy individuals. Our study provides novel preliminary evidence to the field by showing for the first time that LOW-HIIT appears to be a potent stimulus to induce a mobilization and activation of NK cells in immune-compromised advanced cancer patients as well. Further research will be needed to investigate the long-term effects of LOW-HIIT on NK cells in cancer patients and to explore its potential beneficial impact on tumor control.
Advanced cancer is typically associated with a significant reduction in CRF, which may be related to the disease itself, decrease in physical activity, loss of muscle mass and negative side effects of anticancer treatment (6). Given that CRF is a significant predictor for survival after cancer diagnosis (45) it is a crucial preliminary finding that LOW-HIIT increased V̇O2peak by 3.0 mL·kg−1·min−1 in our patients. This increase in V̇O2peak could provide a clinical benefit as each 1 mL·kg−1·min−1 improvement has been associated with a 16% reduction in cancer mortality (46). In addition, we found an average 2.5 mL·kg−1·min−1 increase in VT, a submaximal measure of CRF, which is more specific to estimate the ability to perform physical activities of daily living. The achieved increase in VT after LOW-HIIT is an encouraging preliminary result, as a change by 2 mL·kg−1·min−1 has been deemed clinically relevant in disease cohorts including patients undergoing chemotherapy (47). In this context, it is particularly important to mention that VT is often used as an indicator to assess a patient’s preoperative risk and to decide on further procedures. The observed improvements in CRF are consistent with two previous studies investigating the effects of HIIT in advanced cancer patients. Quist et al. (39) reported significant improvements in the estimated aerobic capacity and the 6-min walking test performance in advanced lung cancer patients after a 6-wk exercise intervention involving HIIT on cycle ergometers, strength training and relaxation. Van Dungen et al. (40) found that the 6-min walking test distance improved significantly in patients with different advanced cancers after a 6-wk exercise program consisting of HIIT and resistance training. The current study expands the knowledge in the field in that we directly measured V̇O2peak (the gold standard for CRF evaluation) to assess the effects of our HIIT protocol on patients’ physical performance. Taken together with the results of these previous studies, our findings provide encouraging preliminary evidence that targeted HIIT programs can produce physiological adaptions that are associated with enhanced CRF—even in advanced cancer disease stages.
Although not statistically significant (most likely due to the small sample size), we would additionally like to point on the striking reduction in serum CRP levels in the LOW-HIIT group. Advanced cancer patients typically present elevated CRP levels due to cancer-related cachexia. It has been shown that patients with markedly increased serum concentrations have a poorer overall survival prognosis (48). Thus, a reduction in CRP levels by 3.0 mg·dL−1, as observed in our patients, points to an important improvement of inflammation status that could subsequently translate into better prognosis of patients.
Apart from physiological benefits related to higher levels of CRF (e.g., increased cardiac output, greater oxygen transport and utilization) improvements in physical capacity have also been associated with favorable changes in QoL and well-being in various populations (49). In line with the literature, we found a significant relationship between increases in measures of CRF and improvements in patients’ self-reported physical functioning. Moreover, patients reported significantly improved fatigue levels after 12 wk of LOW-HIIT. This is a very important finding since fatigue is the most prevalent symptom associated with advanced cancer, affecting 60% to 90% of patients (4). Given that a FACIT score improvement of 3.1 has been considered clinically important (50), the average 7.0 score increase observed in our patients may be associated with meaningful health benefits. Subsequently, improved physical functioning and fatigue resulting from LOW-HIIT might have had a positive impact on daily physical activity as became evident by an increase daily steps and other important aspects of QoL, such as social-functioning.
There are some limitations of this study that should be considered. First, the present investigation is an initial feasibility study with a relatively small sample size. Thus, the effects of LOW-HIIT on physiological and psychological outcomes in advanced cancer patients need to be confirmed in further research. In particular, we note that the analysis of acute immune marker responses after a HIIT session was performed only once after 6 wk of exercise (specifically, a period in which a certain steady state was most likely reached). Thus, the corresponding results are rather to be considered a first “snapshot.” Given that the acute responses blood collection required the insertion of an indwelling cannula and demanded a nonnegligible investment of time, this procedure, however, posed an additional burden on the patients. Consequently, the majority of patients refused multiple blood collections. Future research, thus, may wish to investigate the longitudinal pattern of acute blood responses to LOW-HIIT sessions.
Second, we acknowledge that the present intervention was conducted in a very well controlled clinical setting with a careful supervision of all exercise sessions. The safe application of LOW-HIIT in advanced cancer patients in other settings (e.g., rehabilitation and health centers) will therefore need to be critically evaluated. However, based on our encouraging results, including high completion and attendance rates and low adverse event profiles, we expect that the good tolerability and acceptance of LOW-HIIT will also be confirmed in other (“real-world”) settings—provided that proper medical clarification is carried out beforehand. Third, because of the multiple information sources for patients on how to register for participation in a program at our Research and Treatment center (e.g., referrals by physicians and nurses in several departments within our University Hospital, referrals by other patients and posted flyers), a meaningful evaluation of the study-specific recruitment process as feasibility outcome was not possible. We concede, however, that in addition to the outcomes we assessed in our study, precise data on the recruitment rate (i.e., how many of the patients who were made aware of our LOW-HIIT program through the different information sources finally decided to participate) would be useful for a more comprehensive evaluation of feasibility. In this context, we also note that the excellent accessibility of our Research and Treatment Center for patients and the close connection to other Medical Departments treating oncological patients are unlikely to be representative conditions and therefore may limit generalizability to some extent. Fourth, given that the present intervention lasted 12 wk, the longer-term effects of LOW-HIIT, in particular, the potential impact on disease prognosis and survival, remain unclear. Thus, larger-scale (ideally multicenter) trials involving long-term intervention periods will be needed to answer such questions. In addition, we point out the relatively wide range of diagnoses and tumor types in our patient collective as a limitation of the study. Future studies would therefore probably also benefit from examining a more homogeneous collective. Finally, apart from the pioneering character of our study, we would like to point out that the inclusion of a SHAM group represents a particular strength of this investigation, as a sham intervention is typically associated with less confounders on study outcomes compared with an inactive control group. We note that the type of SHAM intervention (“low-threshold” whole-body electromyostimulation training) was chosen because this type of training is already performed for several years at our Training Center as part of the oncologic exercise therapy and well known among patients, respectively. Therefore, it was considered a better blinding modality compared with light cycle ergometer training. However, we acknowledge that for comparing purposes, a time- and mode-matched SHAM-intervention (e.g., a light cycle ergometer training for 14 min) would potentially have been a better measure for comparative purposes.
CONCLUSIONS
This is the first study to investigate the impact of very low-volume HIIT on acute immune response and chronic adaptations of several physiological and psychological outcomes in a cohort of advanced cancer patients. We provide novel evidence that LOW-HIIT appears to be feasible and safe in this vulnerable group of patients. Our findings underpin previous research indicating that brief vigorous exercise appears not to pose a risk for an increased susceptibility to virus infections—even in immune-compromised advanced cancer patients. Instead, our acute response analysis points to an improved mobilization and activation of NK cells after termination of a LOW-HIIT session. Moreover, our data indicate that less than 30 min of LOW-HIIT—corresponding to only a fifth of the physical activity recommendations for the general population and cancer survivors—may induce clinically relevant positive chronic effects on CRF, fatigue and aspects of QoL, including physical functioning and social functioning. Based on our preliminary data, health professionals working with advanced cancer patients can be encouraged to incorporate LOW-HIIT into exercise programs and/or to implement it as an initial preparatory training modality before higher-volume exercise regimes. Further research is needed to clarify the longer-term effects of LOW-HIIT in advanced cancer patients, in particular its potential impact on tumor biology, disease prognosis and survival.
Supplementary Material
Acknowledgments
This study has been supported by the H.W. & J. Hector Foundation, the Manfred Roth Foundation, and Research Foundation for Medicine at the University Hospital Erlangen. The authors would like to thank Alisia Gerl, Melanie Klaußner, Maike Tobschall, and Kerstin Weidlich for supervising and instructing the exercise sessions. The authors would also like to thank Maria Ehnis for her assistance in data collection. The authors are especially grateful to all study participants for their willingness to participate in this study.
Conflicts of Interest: The authors declare no conflicts of interest. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
HANS J. HERRMANN, Email: Hans.Herrmann@uk-erlangen.de.
BENEDIKT JAKOBS, Email: benedikt.jacobs@uk-erlangen.de.
WALBURGA DIETERICH, Email: Walburga.Dieterich@uk-erlangen.de.
DIMITRIOS MOUGIAKAKOS, Email: dimitrios.mougiakakos@med.ovgu.de.
MARKUS F. NEURATH, Email: markus.neurath@uk-erlangen.de.
YURDAGÜL ZOPF, Email: yurdaguel.zopf@uk-erlangen.de.
REFERENCES
- 1.Moore SC Lee IM Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell KL Winters-Stone KM Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heywood R, McCarthy AL, Skinner TL. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support Care Cancer. 2017;25(10):3031–50. [DOI] [PubMed] [Google Scholar]
- 4.Weller S Hart NH Bolam KA, et al. Exercise for individuals with bone metastases: a systematic review. Crit Rev Oncol Hematol. 2021;166:103433. [DOI] [PubMed] [Google Scholar]
- 5.Sheill G, Guinan E, Brady L, Hevey D, Hussey J. Exercise interventions for patients with advanced cancer: a systematic review of recruitment, attrition, and exercise adherence rates. Palliat Support Care. 2019;17(6):686–96. [DOI] [PubMed] [Google Scholar]
- 6.Albrecht TA, Taylor AG. Physical activity in patients with advanced-stage cancer: a systematic review of the literature. Clin J Oncol Nurs. 2012;16(3):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilliam LAA, St Clair DK. Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress. Antioxid Redox Signal. 2011;15(9):2543–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulkes SJ Howden EJ Bigaran A, et al. Persistent impairment in cardiopulmonary fitness after breast cancer chemotherapy. Med Sci Sports Exerc. 2019;51(8):1573–81. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CL, Sheane BJ, Cunnane G. Attitudes towards exercise in patients with chronic disease: the influence of comorbid factors on motivation and ability to exercise. Postgrad Med J. 2011;87(1024):96–100. [DOI] [PubMed] [Google Scholar]
- 10.Gibala MJ, Gillen JB, Percival ME. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med. 2014;44(2 Suppl):127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattioni Maturana F, Martus P, Zipfel S, Nieß AM. Effectiveness of HIIE versus MICT in improving cardiometabolic risk factors in health and disease: a meta-analysis. Med Sci Sports Exerc. 2021;53(3):559–73. [DOI] [PubMed] [Google Scholar]
- 12.Mugele H Freitag N Wilhelmi J, et al. High-intensity interval training in the therapy and aftercare of cancer patients: a systematic review with meta-analysis. J Cancer Surviv. 2019;13(2):205–23. [DOI] [PubMed] [Google Scholar]
- 13.Palma S, Hasenoehrl T, Jordakieva G, Ramazanova D, Crevenna R. High-intensity interval training in the prehabilitation of cancer patients-a systematic review and meta-analysis. Support Care Cancer. 2021;29(4):1781–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji K, Matsuoka YJ, Ochi E. High-intensity interval training in breast cancer survivors: a systematic review. BMC Cancer. 2021;21(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallen MP Hennessy D Brown S, et al. High-intensity interval training improves cardiorespiratory fitness in cancer patients and survivors: a meta-analysis. Eur J Cancer Care. 2020;29(4):e13267. [DOI] [PubMed] [Google Scholar]
- 16.Souza D Vale AF Silva A, et al. Acute and chronic effects of interval training on the immune system: a systematic review with meta-analysis. Biology (Basel). 2021;10(9):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llavero F Alejo LB Fiuza-Luces C, et al. Exercise training effects on natural killer cells: a preliminary proteomics and systems biology approach. Exerc Immunol Rev. 2021;27:125–41. [PubMed] [Google Scholar]
- 18.Campbell JP, Turner JE. There is limited existing evidence to support the common assumption that strenuous endurance exercise bouts impair immune competency. Expert Rev Clin Immunol. 2019;15(2):105–9. [DOI] [PubMed] [Google Scholar]
- 19.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reljic D, Wittmann F, Fischer JE. Effects of low-volume high-intensity interval training in a community setting: a pilot study. Eur J Appl Physiol. 2018;118(6):1153–67. [DOI] [PubMed] [Google Scholar]
- 21.Reljic D, Frenk F, Herrmann HJ, Neurath MF, Zopf Y. Effects of very low volume high intensity versus moderate intensity interval training in obese metabolic syndrome patients: a randomized controlled study. Sci Rep. 2021;11(1):2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reljic D, Frenk F, Herrmann HJ, Neurath MF, Zopf Y. Low-volume high-intensity interval training improves cardiometabolic health, work ability and well-being in severely obese individuals: a randomized-controlled trial sub-study. J Transl Med. 2020;18(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–91. [Google Scholar]
- 24.National Cancer Institute Web site [Internet] . Rockville (MD): National Cancer Institute; [cited 2022 April 6]. Available from: https://www.cancer.gov. [Google Scholar]
- 25.Vicent L Ariza-Solé A González-Juanatey JR, et al. Exercise-related severe cardiac events. Scand J Med Sci Sports. 2018;28(4):1404–11. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute Web site [Internet] . Rockville (MD): National Cancer Institute; [cited 2022 April 6]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf. [Google Scholar]
- 27.Matomäki P, Kainulainen H, Kyröläinen H. Corrected whole blood biomarkers - the equation of dill and Costill revisited. Phys Rep. 2018;6(12):e13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosy-Westphal A, Jensen B, Braun W, Pourhassan M, Gallagher D, Müller MJ. Quantification of whole-body and segmental skeletal muscle mass using phase-sensitive 8-electrode medical bioelectrical impedance devices. Eur J Clin Nutr. 2017;71(9):1061–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howley ET, Bassett DR, Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc. 1995;27(9):1292–301. [PubMed] [Google Scholar]
- 30.Meyer T, Lucía A, Earnest CP, Kindermann W. A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters—theory and application. Int J Sports Med. 2005;26(1 Suppl):S38–48. [DOI] [PubMed] [Google Scholar]
- 31.Montan I, Löwe B, Cella D, Mehnert A, Hinz A. General population norms for the functional assessment of chronic illness therapy (FACIT)—fatigue scale. Value Health. 2018;21(11):1313–21. [DOI] [PubMed] [Google Scholar]
- 32.Singer S Wollbrück D Wulke C, et al. Validation of the EORTC QLQ-C30 and EORTC QLQ-H&N35 in patients with laryngeal cancer after surgery. Head Neck. 2009;31(1):64–76. [DOI] [PubMed] [Google Scholar]
- 33.Karnofsky DA, Burchenal JH. “The Clinical Evaluation of Chemotherapeutic Agents in Cancer,” in Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- 34.Arends J Bachmann P Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48. [DOI] [PubMed] [Google Scholar]
- 35.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(1 Suppl):5–41. [PubMed] [Google Scholar]
- 36.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Routledge, UK: Taylor and Francis; 1988. [Google Scholar]
- 37.Reljic D, Lampe D, Wolf F, Zopf Y, Herrmann HJ, Fischer J. Prevalence and predictors of dropout from high-intensity interval training in sedentary individuals: a meta-analysis. Scand J Med Sci Sports. 2019;29(9):1288–304. [DOI] [PubMed] [Google Scholar]
- 38.Knox ECL, Webb OJ, Esliger DW, Biddle SJH, Sherar LB. Using threshold messages to promote physical activity: implications for public perceptions of health effects. Eur J Pub Health. 2014;24(2):195–9. [DOI] [PubMed] [Google Scholar]
- 39.Quist M Rørth M Langer S, et al. Safety and feasibility of a combined exercise intervention for inoperable lung cancer patients undergoing chemotherapy: a pilot study. Lung Cancer. 2012;75(2):203–8. [DOI] [PubMed] [Google Scholar]
- 40.Van den Dungen IA, Verhagen CA, van der Graaf WT, van den Berg JP, Vissers KC, Engels Y. Feasibility and impact of a physical exercise program in patients with advanced cancer: a pilot study. J Palliat Med. 2014;17(10):1091–8. [DOI] [PubMed] [Google Scholar]
- 41.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. [DOI] [PubMed] [Google Scholar]
- 42.Idorn M, Hojman P. Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med. 2016;22(7):565–77. [DOI] [PubMed] [Google Scholar]
- 43.Bigley AB Rezvani K Chew C, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun. 2014;39:160–71. [DOI] [PubMed] [Google Scholar]
- 44.Barra NG Fan IY Gillen JB, et al. High intensity interval training increases natural killer cell number and function in obese breast cancer-challenged mice and obese women. J Cancer Prev. 2017;22(4):260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fardman A Banschick GD Rabia R, et al. Cardiorespiratory fitness and survival following cancer diagnosis. Eur J Prev Cardiol. 2021;28(11):1242–9. [DOI] [PubMed] [Google Scholar]
- 46.Imboden MT Harber MP Whaley MH, et al. The association between the change in directly measured cardiorespiratory fitness across time and mortality risk. Prog Cardiovasc Dis. 2019;62(2):157–62. [DOI] [PubMed] [Google Scholar]
- 47.Sinclair RCF, Sumpter K, Griffin SM. Fitness after chemotherapy. Br J Anaesth. 2016;116(1):140. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. Clinical significance of pretreatment serum C-reactive protein level in soft tissue sarcoma. Cancer. 2012;118(4):1055–61. [DOI] [PubMed] [Google Scholar]
- 49.Gillison FB, Skevington SM, Sato A, Standage M, Evangelidou S. The effects of exercise interventions on quality of life in clinical and healthy populations; a meta-analysis. Soc Sci Med. 2009;68(9):1700–10. [DOI] [PubMed] [Google Scholar]
- 50.Cella D Wilson H Shalhoub H, et al. Content validity and psychometric evaluation of functional assessment of chronic illness therapy—fatigue in patients with psoriatic arthritis. J Patient Rep Outcomes. 2019;3(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


