Abstract
Frequencies of vaccine-responsive T-lymphocyte precursors in peripheral blood mononuclear cells (PBMC) prior to and after administration of peptide-based vaccines in patients with cancer can be measured by limiting-dilution assays (LDA) or by ELISPOT assays. We have used a modified version of the ELISPOT assay to monitor changes in the frequency of gamma interferon (IFN-γ)-producing T cells in a population of lymphocytes responding to a relevant peptide or a nonspecific stimulator, such as phorbol myristate acetate-ionomycin. Prior to its use for monitoring of patient samples, the assay was validated and found to be comparable to the LDA performed in parallel, using tumor-reactive cytolytic T-lymphocyte (CTL) lines. The sensitivity of the ELISPOT assay was found to be 1/100,000 cells, with an interassay coefficient of variation of 15%, indicating that it could be reliably used for monitoring of changes in the frequency of IFN-γ-secreting responder cells in noncultured or cultured lymphocyte populations. To establish that the assay is able to detect the T-cell precursor cells responsive to the vaccine, we used CD8+ T-cell populations positively selected from PBMC of HLA-A2+ patients with metastatic melanoma, who were treated with dendritic cell-based vaccines containing gp100, MELAN-A/MART-1, tyrosinase, and influenza virus matrix peptides. The frequency of peptide-specific responder T cells ranged from 0 to 1/2,600 before vaccination and increased by at least 1 log unit after vaccination in two patients, one of whom had a clinical response to the vaccine. However, no increases in the frequency of peptide-responsive T cells were observed in noncultured PBMC or PBMC cultured in the presence of the relevant peptides after the melanoma patients enrolled in another trial were treated with the intramuscular peptide vaccine plus MF59 adjuvant. Thus, while the ELISPOT assay was found to be readily applicable to assessments of frequencies of CTL precursors of established CTL lines and ex vivo-amplified PBMC, its usefulness for monitoring of fresh PBMC in patients with cancer was limited. In many of these patients antitumor effector T cells are present at frequencies of lower than 1/100,000 in the peripheral circulation. Serial monitoring of such patients may require prior ex vivo amplification of specific precursor cells.
The ELISPOT assay has been described as a method which can measure the frequency in a clonal population of T cells capable of responding to the antigen by secretion of cytokines (5, 9, 10, 28, 29). While the assay has been extensively evaluated for its ability to estimate the frequencies of antiviral effector cells, only a few studies used ELISPOT for the assessment of antitumor responses (22, 29). With the recent introduction of antitumor vaccines, a great deal of interest has developed in ELISPOT and its utilization for monitoring of antigen- or peptide-specific responses to tumor vaccines in patients with cancer. A number of vaccine trials have been in progress, mainly with patients with metastatic melanoma, as a result of recent successes in the identification of a rapidly increasing number of unique HLA-restricted melanoma peptides (2, 33, 34, 40). In contrast to the case for viral infections, however, it has been difficult to demonstrate the presence of tumor-specific cytotoxic T lymphocytes (CTL) (4, 11) or their generation as a result of vaccine administration to patients with advanced cancer (12, 23). Even in patients with metastatic melanoma who had complete or partial clinical responses following vaccination with MAGE-3, the presence of MAGE-3-specific CTL circulating in the peripheral blood could not be demonstrated (16). In other vaccination trials, CTL responses were detectable only after several cycles of in vitro stimulation of peripheral blood mononuclear cells (PBMC) with the immunizing peptides (26). This is in contrast to vaccinations with viral peptides, e.g., influenza virus peptides, where the ELISPOT assay is able to detect peptide-specific memory CD8+ T cells in freshly isolated PBMC (6, 15). It is reasonable to anticipate that, unlike T cells mediating antiviral immune responses (1, 19), T cells with specificity for self or differentiation epitopes (which are potentially tolerogenic) might be infrequent or absent. Therefore, a sensitive and reliable assay that allows for accurate detection of frequencies, and particularly for demonstration of increased postvaccination frequencies, of T cells responsive to the peptides or proteins used in the vaccine is essential for monitoring patient responses or for confirming their absence.
The only assay known to reliably measure frequencies of single-antigen-responding T cells is the limiting-dilution assay (LDA), which has been extensively utilized in human tumor antigen studies to estimate the numbers of proliferating T-lymphocyte precursors or CTL precursors (CTL-p) in various effector cell populations (32). However, with immune cells obtained from cancer patients, LDA has generally detected low CTL-p frequencies (17, 37). Furthermore, LDA does not lend itself to routine clinical monitoring, largely because of its technical complexity, and efforts to replace it with a more practical but equally sensitive method have been undertaken in a number of different laboratories (7, 9, 10, 13, 14, 20, 28, 29).
We describe here the development, preclinical assessment, and application to cancer patient monitoring of a modified ELISPOT assay for individual T cells which secrete gamma interferon (IFN-γ) in response to specific, major histocompatibility complex (MHC)-restricted stimulating antigens or antigenic peptides. The assay is applicable to frequency measurements with ex vivo-activated lymphocyte populations or established CTL lines. However, its utility may be limited for a routine evaluation of patient samples such as fresh PBMC obtained from patients with cancer.
MATERIALS AND METHODS
Tumor and lymphocyte cell lines.
The HLA-A2+ human tumor cell lines PCI-13, a squamous cell carcinoma of the head and neck (SCCHN), and HR, a gastric carcinoma, were established from tumor biopsies and maintained in culture as previously described (8, 30). A human melanoma cell line, Mel 526, was obtained from Steven A. Rosenberg, Surgery Branch, National Cancer Institute, Bethesda, Md. Human CTL lines specific for the PCI-13 SCCHN were generated by in vitro sensitization of HLA-A2+ normal PBMC with irradiated PCI-13 cells, as described below. Melanoma-specific CTL lines (no. 1520 and 1088) established by outgrowth of tumor-infiltrating lymphocytes (TIL) were obtained from Steven A. Rosenberg. CTL line 1520 is specific for the gp100209-217 peptides, and CTL line 1088 is specific for the MELAN-A/MART-127-35 peptide. Both lines are restricted by HLA-A2. The CTL lines were cultured in the presence of 100 IU of interleukin-2 (IL-2) per ml and stimulated with irradiated Mel 526 cells (10,000 rads; 1 tumor/10 T cells), which express both gp100 and MELAN-A/MART-1 antigens, at weekly intervals.
PBMC.
Venous blood was obtained from normal volunteers and collected into heparinized tubes. PBMC were isolated by Ficoll-Hypaque gradient centrifugation. PBMC recovered from the interface were washed, counted in trypan blue, and either immediately used for ELISPOT assays and intracytoplasmic IFN-γ assays by flow cytometry or cryopreserved in liquid N2 for additional studies. Cryopreserved samples were thawed, and the recovered cells were washed, counted, and used for ELISPOT assays. In some cases, fresh or cryopreserved PBMC were separated into CD8+ and CD4+ fractions, using positive selection with immunobeads (MACS MicroBeads; Miltenyi Biotech, Auburn, Calif.).
Generation and culture of PCI-13-specific CTL lines.
The CTL lines were induced from leukopaks obtained from HLA-A2+ platelet donors through the Central Blood Bank of Pittsburgh, Pittsburgh, Pa. Mononuclear cells were separated on Ficoll-Hypaque gradients, washed and used for induction of CTL lines as follows.
(i) In the case of CTL lines 1 to 3, PBMC (106) were incubated with 105 irradiated (100 Gy) PCI-13 cells (a ratio of 10 responder cells to 1 stimulator cell) in wells of a 24-well tissue culture plate containing 2 ml of AIM-V medium (Gibco) supplemented with 5% human AB serum (NABI, Miami, Fla.), 100 IU of IL-2 (Chiron, Emeryville, Calif.) per ml, 10 U of IL-1β (Genzyme Corp., Cambridge, Mass.) per ml, 50 IU of IL-4 (Schering Plough, Kennilworth, N.J.) per ml, and 125 U of IL-6 (Sandoz, Vienna, Austria) per ml. Prior to its use as a stimulator, the PCI-13 cell line was treated with 1000 IU of IFN-γ (Roussel UCLAF, Romainville, France) per ml for 48 h to increase expression of MHC class I molecules. Responder T lymphocytes were cultured at 37°C in an atmosphere of 5% CO2 in air and were restimulated every 7 to 10 days with irradiated, IFN-γ-treated PCI-13 cells at a responder/stimulator cell ratio of 10:1. A total of six in vitro stimulations were performed. Between days 30 and 55 of culture, the CTL lines were tested in ELISPOT assays, and in selected cases, an LDA was done as well.
(ii) In the case of CTL lines 4 and 5, first and second ex vivo stimulations were performed with autologous dendritic cells (DC) pulsed with a peptide preparation obtained from PCI-13 cells. After the third stimulation, irradiated PCI-13 cells were used as stimulators, exactly as described above.
The CTL lines were tested for specificity in 4-h 51Cr release assays against PCI-13 cells and a panel of HLA-A2+ and HLA-A2− tumor cell lines and normal cell targets. Blocking with anti-MHC class I and anti-HLA-A2 antibodies (Abs) was used to confirm that the CTL lines were HLA-A2 restricted.
PCI-13-derived peptide preparation.
PCI-13 cells were grown in a cell factory (Nunc, Fisher Scientific) until they were 80% confluent in culture medium consisting of Dulbecco modified Eagle medium supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 100 IU of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, and 50 μg of gentamicin per ml (all from GIBCO). Prior to trypsinization, the monolayers were washed twice with Hanks' balanced salt solution (HBSS). Trypsin-EDTA solution was added, and a single-cell suspension of tumor cells was harvested and pelleted by centrifugation. Trifluoroacetic acid (TFA) lysates were then generated as previously described by Rotzschke et al. (27). Briefly, PCI-13 cells were resuspended in 0.1% TFA in double-distilled water, Dounce homogenized, sonicated, and incubated for 30 min at 4°C. Lysates were then centrifuged at 12,000 × g for 30 min at 4°C, and the peptide-containing supernatants were recovered. Tumor-associated peptides were then isolated as the flowthrough fraction obtained by centrifugal filtration on Centricon-3 ultrafiltration devices (peptides with Mrs of <3,000; Amicon, Bedford, Mass.). The peptide solutions were then lyophilized to near-complete dryness to remove TFA and were then reconstituted in 100 μl of HBSS containing 10% dimethyl sulfoxide (Sigma) prior to storage at −80°C.
DC generation.
To generate autologous DC, PBMC obtained from a leukapak were suspended in AIM-V medium at a cell density of 107/ml in T162 flasks. After 1 h of incubation in 5% CO2 in air at 37°C, nonadherent cells were decanted. Residual nonadherent cells and platelets were removed by five vigorous washes with 40 ml of HBSS prior to addition of AIM-V medium, containing 1,000 IU of IL-4 per ml and 1,000 IU of granulocyte-macrophage colony-stimulating factor per ml, to the adherent PBMC. The cultures were incubated in 5% CO2 in air at 37°C for 7 days. On day 7, DC were harvested, irradiated (30 Gy), and pulsed with a peptide preparation previously coincubated with Dynabeads at room temperature for 2 h. The presence of peptide-coated Dynabeads facilitated uptake and processing of tumor-derived antigens by the DC. Responder T lymphocytes (nonadherent PBMC) were incubated in AIM-V medium containing 5% heat-inactivated human AB serum without any cytokines and stimulated with the peptide-pulsed DC at a responder/stimulator cell ratio of 50:1. After a period of 1 week, aliquots of IL-2 (10 IU/ml), IL-1β (0.2 ng/ml), and IL-7 (0.2 ng/ml) were added to the cultures. The peptide-pulsed DC were used for first and second stimulations, but for third and fourth stimulations, irradiated PCI-13 cells were used for all subsequent weekly restimulations. After the third stimulation, negative selection was performed with each CTL line to enrich for CD8+ T cells by using magnetic beads coated with anti-CD4 Abs. ELISPOT assays and LDA were performed on day 31 of culture with CTL line 4 and on day 39 with CTL line 5.
LDA with PCI-13-specific CTL lines.
The LDA was performed as previously described (37). Lymphocytes were seeded in wells of 96-well plates at 10, 3, and 1 cell/well with 5 × 103 irradiated (100 Gy) PCI-13 cells used as stimulators and 2 × 104 to 5 × 104 irradiated (30 Gy) allogeneic PBMC obtained from a healthy donor used as feeder cells. The culture medium was AIM-V containing 5% (vol/vol) heat-inactivated human AB serum, IL-2 (10 IU/ml), IL-1β (0.2 ng/ml), and IL-7 (0.2 ng/ml). At 2 to 3 weeks later, wells containing proliferating lymphocytes were quantitated in order to determine the frequency of proliferating T-lymphocyte precursors.
Clonal analysis.
Wells seeded with 1 cell/well with visible lymphocyte growth were selected for cytotoxicity assays. With the CTL line 3, 105 proliferating microcultures were obtained from a total of 480 wells (21.9%); with CTL line 4, 18 microcultures were obtained from 480 wells (3.85%); and with CTL line 5, 20 microcultures were obtained from 464 wells (4.31%). Since the proliferating wells represented <30% of the total wells by Poisson distribution analysis, these microcultures were assumed to be clonal expansions. The microcultures were tested in 4-h 51Cr release assays at an effector/target cell ratio of 10:1, and the levels of specific lysis were determined, using PCI-13 cells as targets. Lysis of >10% was considered significant, with these lytic microcultures designated PCI-13-reactive CTL-p. CTL-p frequencies were determined as described previously by us (37) and compared to the frequency of IFN-γ secretors determined in the ELISPOT assay.
ELISPOT assay.
For determinations of the frequency of T cells capable of responding to a specific stimulus by secretion of IFN-γ, an ELISPOT assay has been established. A single-cell, plaque-like assay, it is a modification of that described earlier by Tanguay and Killion (31) and by Ronnelid and Klareskog (25). The capture and detection monoclonal Abs were selected on the basis of their performance in ELISPOT assays and the ability of capture Abs to bind to plastic. The assay was performed in wells of 96-well flat-bottom microtiter polystyrene enzyme-linked immunosorbent assay (ELISA) plates (Immulon; Fisher, Pittsburgh, Pa.) coated with 5 μg of capture anti-IFN-γ Ab (Pharmingen catalog no. 18891D) per ml in 50 μl of the diluent (phosphate-buffered saline [PBS], pH 7.2) per well. The plates were incubated overnight in a moist chamber, washed extensively in 0.05% Tween 20–PBS, and blocked with 1% (vol/vol) bovine serum albumin in PBS for 2 h at 37°C. PBMC, purified T cells, or cultured T cells resuspended in AIM-V medium supplemented with 10% (vol/vol) AB human serum were then added at various numbers, e.g., from 105 to 500 cells per well in triplicate wells, and the plates were spun at 200 × g for <1 min. The stimulatory peptides pulsed onto irradiated presenting cells (e.g., T2 or C1R.A2) or irradiated tumor cells were then added to each well, and the plates were incubated for 24 h (for CTL lines or T cells cultured in the presence of IL-2) or 48 h (for noncultured PBMC plus stimulators) at 37°C. Next, the plates were vigorously washed six times with the solution of 0.05% Tween 20–PBS, and the biotinylated detection anti-IFN-γ Ab (Pharmingen catalog no. 18902D) was added at 5 μg/ml. The plates were again incubated for 2 h at 37°C, washed, and developed with the avidin-horseradish peroxidase conjugate for 1 h. Following addition of the substrate 3,3′,5,5′-tetramethylbenzidine (TBM) and an additional 20 to 30 min of incubation, the plates were prepared for counting of blue spots, microscopically or using image analysis. The number of blue spots per well was determined microscopically, using an inverted phase-contrast microscope (Olympus model SZH10), or with a computer-assisted image analysis system (KS ELISPOT; Carl Zeiss, Hallbergmoos, Germany). The frequency of positive (IFN-γ-producing) cells per the total number of plated cells was calculated after the number of spots in control wells had been subtracted from that in experimental wells. These control wells contained T2 cells and responding lymphocytes but no peptides. Additional control wells for the assay included reagents alone (blank), nonstimulated effector cells (spontaneous IFN-γ production), or normal PBMC stimulated with phorbol myristate acetate (PMA) at 1 ng/ml and ionomycin at 1 μM as positive assay controls. Statistical analysis of the number of spots in nonstimulated versus stimulated wells was performed, and significant differences were noted. The sensitivity of the assay was determined by titrations of melanoma-specific CTL, which are known to produce IFN-γ in response to the gp100 peptide, into ELISPOT wells containing a constant number of peripheral blood lymphocytes (PBL) per well. Interassay reproducibility was determined in 19 consecutive assays, using cryopreserved, thawed, and PMA-ionomycin-stimulated lymphocytes obtained from the same normal volunteer.
Synthetic peptides.
Peptides were synthesized using 9-fluorenylmethoxy carbonyl chemistry by the Peptide Synthesis Facility at the University of Pittsburgh Cancer Institute (UPCI). Each peptide was purified to >95% homogeneity by reverse-phase high-pressure liquid chromatography, and the identity of each peptide was confirmed by mass spectrometry. The following peptides were synthesized and used in the present study: MART-127-35 (AAGIGILTV) (recognized by TIL 1088), gp100209-217 (ITDQVPFSV) (recognized by TIL 1520), tyrosinase (YMDGTMSQV), and an influenza virus matrix peptide, flu MI58-66 (GILGFVFTL).
Cytotoxicity assays.
Four-hour 51Cr release assays were performed in triplicate, as previously described (35), using tumor cells (PCI-13 or Mel 562) as targets. The percent specific lysis was calculated as [(experimental cpm − spontaneous-release cpm)/(maximal cpm − spontaneous-release cpm)] × 100. The data were expressed as lytic units20 (LU20)/107 effector cells, using a computer program, as previously described (35). Unlabeled K562 cells were used as cold targets in all cytotoxicity experiments (35).
Flow cytometry.
PBMC or cultured lymphocytes were stained with fluorescein- or phycoerythrin-labeled monoclonal Abs to surface antigens CD3, CD8, CD4, and HLA-A2 (Becton Dickinson, San Jose, Calif.) and examined in a flow cytometer as described earlier (39). Isotype control Abs were included in all experiments.
Patient specimens.
Blood specimens from patients with metastatic melanoma who participated in two phase I vaccination trials at the UPCI were obtained pre- and postvaccination. In one trial, 24 patients with metastatic melanoma were randomized to receive a vaccine of either MELAN-A/MART-1, gp100, or tyrosinase peptide given intramuscularly with MF59 as an adjuvant. Patients received between 1 and 5 weekly courses of a vaccine, except for one patient who received 11 courses. In the second, phase I-II clinical trial, 25 patients with high-risk stage III or IV metastatic melanoma received one to four courses of intravenous vaccine with autologous DC pulsed with five different peptides: MELAN-A/MART-1 (ILTVILGVL), MELAN-A/MART-1 (AAGIGILTV), gp100 (YLEPGPVTA), tyrosinase (YMDGTMSQV), or flu matrix (GILGFVFTL).
Patients' PBMC were recovered on Ficoll-Hypaque gradients and cryopreserved to allow for testing of pre- and postvaccination samples in the same ELISPOT assay. Two approaches were used to enrich PBMC in T cells responsive to the immunizing peptides. One used PBMC which were thawed, washed, and placed in culture with the peptide(s) (10 μM) used for vaccinations for 14 days. Conditions for expansion of bulk T-cell cultures were the same as those described for PCI-13-specific CTL lines above. The second approach utilized PBMC which were thawed on the day of the assay and, following enrichment in CD8+T cells, were tested in ELISPOT assays without ex vivo expansion.
Statistical analysis.
Differences between paired groups of values were tested using the Wilcoxon test. Whenever applicable, Student's t test was also used. Differences with a P value of <0.05 were considered significant.
RESULTS
Selection and titration of anti-IFN-γ Abs.
The ELISPOT assay used two monoclonal Abs directed against different determinants of human IFN-γ. To determine the optimal concentrations of these capture and detection Abs, either a gp100-specific CTL line or normal human PBMC activated with PMA-ionomycin were plated in triplicate wells of 96-well plates coated with various dilutions of the capture Ab. After 24 or 48 h of incubation, supernatants were removed, and after extensive washing, the detection Ab was added at various dilutions. These checkerboard titrations were performed in at least three independent experiments. As shown in Table 1, the optimal Ab dilutions were determined to be 5 μg/ml for capture Abs and 2.5 μg/ml for detection Abs. The lots of Abs titrated as described above were reserved and purchased in bulk to ensure the reproducibility of the assay.
TABLE 1.
Titration of the capture and detection Abs for use in ELISPOTa
| Detection Ab concn (μg/ml) | Spots counted/105 CTL plated with capture antibody concn (μg/ml) of:
|
|||
|---|---|---|---|---|
| 1 | 2.5 | 5 | 10 | |
| 1 | NSb | 26,000 | 35,000 | 49,000 |
| 2.5 | NS | 38,000 | 60,000c | 46,000 |
| 5 | NS | 25,000 | 50,000 | 45,000 |
| 10 | NS | 44,000 | 46,000 | 52,000 |
Checkerboard titrations were performed to determine the optimal number of spots per well in an ELISPOT assay for IFN-γ production. Melanoma gp100-reactive CTL (line 1520), used as responders, were titrated into wells and stimulated with gp100 (1 μg/ml)-pulsed T2 cells for 24 h. The capture and detection Abs described in Materials and Methods were used at various concentrations, as indicated. The numbers shown are extrapolated. Results from a representative experiment of three performed is shown.
NS, no spots detected.
Largest number of spots detected.
Counting of spots.
Spots associated with IFN-γ secretion by single cells were evaluated microscopically using an inverted phase-contrast microscope or a computer-assisted image analysis system. To avoid bias, microscopic counts were independently determined by two technologists, and the results were averaged. Comparisons between the two scoring methods indicated that the numbers of spots counted were comparable for wells containing the same number of plated cells. In 20 individual comparisons of the two scoring methods at various cell concentrations per well, the mean values ± standard deviations (SD) were 164 ± 146 spots counted microscopically and 144 ± 129 spots counted by image analysis (P > 0.05). Spot counts could be performed faster and with fewer dilutions of the plated cells in the image analysis system than by eye. The advantage of the image analysis system is that wells containing large numbers of spots can be accurately scored. Also, for monitoring of large numbers of wells, computer-assisted analysis is more practical.
Frequency of IFN-γ-producing cells in populations of PMA-ionomycin-stimulated normal PBMC.
Using the ELISPOT assay, we first determined the frequency of T lymphocytes able to secrete IFN-γ after stimulation with PMA-ionomycin in PBMC obtained from normal donors. As indicated in Table 2, this frequency was found to range from 1/2 to 1/500 in PBMC obtained from 20 normal donors, with 47 distinct assays performed. The determined mean frequency (± SD) of 10% ± 11.8% of IFN-γ-producing cells in response to the nonspecific T-cell activators PMA and ionomycin (Table 2) was used as a reference normal control value. In all ELISPOT assays, PBMC of at least one normal donor were routinely used as a positive assay control.
TABLE 2.
ELISPOT assays for IFN-γ production by PBMC obtained from normal donorsa
| Assay conditions | Frequency | IFN-γ-producing cells (%) |
|---|---|---|
| Medium (spontaneous release) | <1/100,000b | <0.001b |
| Medium + PMA (1 ng/ml) + ionomycin (1 μM) | 1/10 (1/500–1/2)c | 10 ± 11.8 (0.2–50)d |
PBMC were obtained from 20 normal volunteers and were tested in 47 distinct assays over a period of 6 months. PBMC were titrated into wells of ELISPOT plates (105 to 102 cells/well) in triplicate and incubated in medium alone or with the stimulating agents for 48 h at 37°C. Spots were counted to determine frequencies of responding cells in each population.
Theoretical sensitivity of the ELISPOT assay with 100,000 cells plated per well.
Mean (range).
Mean ± SD (range).
Optimal conditions for ELISPOT assays with different types of effector cells.
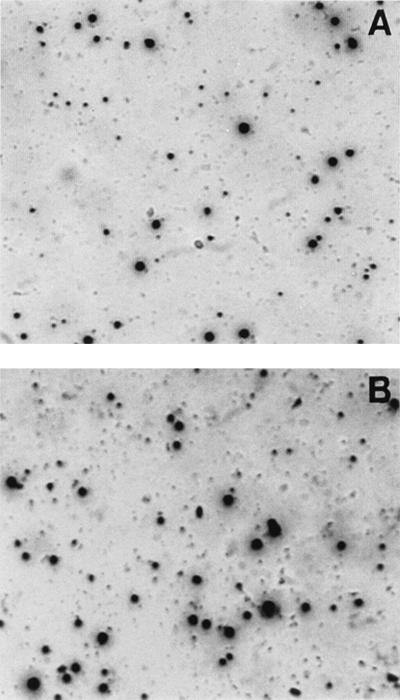
In initial experiments, attempts were made to optimize the assay for different types of effector cells, e.g., fresh PBMC or antigen-specific CTL lines. We observed that the quality of the spots was markedly different depending on the effector cells used. The quality of spots obtained in an ELISPOT assay performed with a PCI-13-specific CTL line is shown in Fig. 1. Large, diffuse spots signify the secretion of considerable levels of IFN-γ. In contrast to spots seen with CTL lines cultured in the presence of IL-2, the quality of spots seen with fresh PBMC stimulated with PMA-ionomycin was notably different (Fig. 2). Intense well-defined small or large spots were seen in this system (Fig. 2), with the sizes of spots reflecting the variable quantities of IFN-γ secreted by individual T-cell clones.
FIG. 1.
Representative well from an ELISPOT assay performed with CTL cultured in the presence of IL-2. Note the presence of large, diffuse spots. Magnification, ×45.
FIG. 2.
Two different representative wells from an ELISPOT plate. The well shown in panel A contains twofold-fewer T cells than that shown in panel B. The sizes of spots vary, but their number can be precisely determined microscopically or by image analysis. Magnification, ×45.
An advantage of ELISPOT assays performed in conventional plastic plates without nitrocellulose inserts is that supernatants can be easily recovered from each well, without disturbing the spots, and tested for levels of IFN-γ by ELISA. We were therefore able to confirm that ELISPOT supernatants from wells with diffuse, large spots formed by CTL (Fig. 1) contained 610 ± 192 pg of IFN-γ per ml (mean ± SD from four determinations), while wells with the compact, small spots seen with PMA-ionomycin-activated PBMC (Fig. 2) contained only 34 ± 20 pg of IFN-γ per ml. This observation suggested that the conditions of the assay may have to be very carefully adjusted in order to achieve optimal spot formation. To this end, we performed ELISPOT assays with PCI-13-specific CTL lines and fresh or frozen and thawed PBMC activated with PMA-ionomycin under various experimental conditions (data not shown). Comparisons of fresh with cryopreserved PBMC (n = 10) showed no significant differences (P > 0.5) in the frequencies of IFN-γ-producing cells stimulated with PMA-ionomycin. These experiments allowed for the following conclusions to be made. (i) With fresh or frozen and thawed PBMC, the ELISPOT assay should be performed in the presence of 10% (vol/vol) AB serum and 20 IU of IL-2 per ml and the effector cells need to be incubated with a stimulator (antigen) for 24 to 48 h, with small, intense, pin-like spots to be expected. (ii) With CTL lines or PBMC sensitized in vitro in the presence of antigens and IL-2, the assay should be performed in the absence of serum or exogenous IL-2 for 20 to 24 h, with large, diffuse spots to be expected. In either case, it is necessary to select the optimal antigen concentration in preliminary experiments or to perform the ELISPOT assay at several different antigen concentrations.
Reproducibility and sensitivity of ELISPOT assays.
In order to assess the performance characteristics of the ELISPOT assay for IFN-γ, we first determined its intra- and interassay variabilities, using PMA-ionomycin as a nonspecific T-cell stimulator. As shown in Table 3, both the interassay reproducibility and that of assays performed on the same day but plated in different plates were excellent. In general, the frequency of lymphocytes responding to PMA-ionomycin by secretion of IFN-γ remained remarkably constant in individual normal donors tested months apart.
TABLE 3.
Reproducibility of ELISPOT assaysa
| Comparison | Assay date (mo/day/yr) | Frequency (%) of IFN-γ-producing cells |
|---|---|---|
| Assays performed on different days | 10/14/97 | 1/20 (5) |
| 3/10/98 | 1/15 (7) | |
| 10/27/97 | 1/25 (4) | |
| 12/2/97 | 1/25 (4) | |
| 11/27/97 | 1/30 (3) | |
| 1/6/98 | 1/40 (2.5) | |
| 10/27/97 | 1/25 (4) | |
| 3/24/98 | 1/25 (4) | |
| 4/21/98 | 1/16 (4) | |
| 4/8/98 | 1/6 (17) | |
| 5/12/98 | 1/7 (15) | |
| Assays performed on the same day | 1/21/98 | 1/6 (17) |
| 1/21/98 | 1/6 (17) | |
| 12/29/97 | 1/21 (5) | |
| 12/29/97 | 1/36 (3) | |
| 4/15/98 | 1/10 (10) | |
| 4/15/98 | 1/9 (11) | |
| 1/28/98 | 1/20 (5) | |
| 1/28/98 | 1/18 (6) | |
| 4/1/98 | 1/10 (10) | |
| 4/1/98 | 1/12 (8) |
ELISPOT assays were performed with PBMC obtained from normal donors. Cells were stimulated with PMA (1 ng/ml) plus ionomycin (1 μM) for 48 h. Assays were performed on the same or different days with PBMC of the same donors. Assays performed on the same day were set up on different plates in parallel. Spot counts in triplicate wells containing the same numbers of cells were not significantly different (P > 0.05). The mean of the difference ± SD for all paired values was 1% ± 1%.
Our results indicated that the ELISPOT assay yields comparable frequencies of IFN-γ-secreting cells whether freshly harvested or frozen and thawed PBMC are used as a source of responders. Based on these data, cryopreserved PBMC obtained from a normal donor were tested in 19 consecutive ELISPOT assays performed on different days over a period of 6 months. The interassay coefficient of variation was found to be 15%.
In the next series of experiments, we compared the frequencies of IFN-γ-secreting cells in PBMC, obtained from the same normal individual, which were cryopreserved, thawed, stimulated with PMA-ionomycin, and tested in ELISPOT assays performed on different days. Limiting dilution of the samples was performed (Table 4), and the samples plated in triplicate were scored for the number of spots at each cell concentration. The mean numbers of spots in assays run on different days were compared and were found to differ minimally (<1%). The representative limiting-dilution plots for cryopreserved PBMC obtained from another normal individual and tested in five independent ELISPOT assays are shown in Fig. 3. They confirm the excellent reproducibility of the assay.
TABLE 4.
Limiting dilution of samples and interassay reproducibility in ELISPOTa
| Day | Cells/well | Spot count
|
Spots/106 cells | Frequency (1/x) | ||||
|---|---|---|---|---|---|---|---|---|
| Well 1 | Well 2 | Well 3 | Mean | SD | ||||
| A | 10,000 | 400 | 400 | 400 | 400 | 0 | 40,000 | 25 |
| 2,000 | 73 | 82 | 78 | 78 | 3.7 | 39,000 | 26 | |
| 400 | 16 | 18 | 17 | 17 | 0.8 | 42,500 | 24 | |
| 80 | 4 | 4 | 2 | 3.3 | 1.1 | 41,250 | 25 | |
| B | 10,000 | 381 | 352 | 368 | 367 | 11.9 | 36,700 | 27 |
| 2,000 | 99 | 95 | 97 | 97 | 1.6 | 48,500 | 21 | |
| 400 | 15 | 21 | 18 | 18 | 2.4 | 45,000 | 22 | |
| 80 | 4 | 3 | 5 | 4 | 1.0 | 50,000 | 20 | |
| C | 10,000 | 500 | 425 | 410 | 445 | 48.2 | 48,200 | 21 |
| 2,000 | 108 | 80 | 94 | 94 | 11.4 | 47,000 | 21 | |
| 400 | 20 | 21 | 15 | 19 | 3.2 | 47,500 | 21 | |
| 80 | 5 | 4 | 5 | 4.6 | 0.6 | 57,500 | 17 | |
Representative ELISPOT results selected from over 50 distinct assays performed with cryopreserved PBMC of the same normal individual are shown. The PBMC were thawed, titrated into wells of ELISPOT plates (105 to 80 cells/well) in triplicate, and activated with PMA-ionomycin (see Materials and Methods). Triplicate control wells containing cells incubated in medium alone were also plated. Following 48 h of incubation at 37°C, all control wells were negative (no spots), while the wells plated with 105 cells stimulated with PMA plus ionomycin contained too many spots to be counted. The presented data are spot counts obtained with titrated PBMC of the same individual tested by ELISPOT on three different days (A, B, and C). The mean difference (± SD) in the frequency of IFN-γ-secreting cells was not significant between the assays performed on different days (<1% ± 0.2%).
FIG. 3.
Limiting-dilution plots for five PBMC samples obtained from the same normal individual and tested in ELISPOT assays performed on different days. The cells were stimulated with PMA-ionomycin as described in Materials and Methods. The data points are mean numbers of spots in the triplicate wells ± SD.
To determine the sensitivity of the assay, a melanoma-specific CTL line, with a predetermined frequency of IFN-γ-producing cells, was titrated into wells containing nonactivated HLA-A2+ PBL (105 per well). As shown in Table 5, the frequency of IFN-γ-producing cells was 1/3 in the CTL line, and the ELISPOT assay detected even 1 IFN-γ-producing CTL in 100,000 PBL. Thus, the theoretical and practical sensitivities of the ELISPOT assay were found to be the same at 1 positive cell per 100,000 cells plated.
TABLE 5.
Sensitivity of the ELISPOT assaya
| Plated cells/well | No. of expected spots/well | No. of counted spots/well | Frequency of IFN-γ-producing cells |
|---|---|---|---|
| CTL alone (100) | 33 | 33 | 1/3 |
| PBL alone (105) | 0 | 0 | <1/100,000 |
| PBL (105) + CTL (100) | 33 | 32 | 1/3,128 |
| PBL (105) + CTL (50) | 16 | 16 | 1/6,253 |
| PBL (105) + CTL (25) | 8 | 9 | 1/11,113 |
| PBL (105) + CTL (5) | 1 | 1 | 1/100,000 |
| PBL (105) + CTL (3) | 1 | 1 | 1/100,000 |
CTL line 1520 (gp100 peptide specific) was used in titration experiments. This line was pretested in ELISPOT for the frequency of IFN-γ-producing T cells in response to gp100 peptide on the day preceding the titration experiment. The frequency was 1/3. Decreasing numbers of CTL were mixed with normal nonactivated PBL (105/well), and ELISPOT assays were performed, using the gp100 peptide (1 μg/ml) for T-cell stimulation in triplicate wells. Results are from one of two titration experiments performed.
Frequencies of melanoma peptide-specific T cells in cultured CTL lines.
We next applied the ELISPOT assay to determine the frequencies of peptide-specific T cells among CTL lines (no. 1520 and 1088) with known specificity for melanoma targets, such as Mel 526, which express gp100 and MELAN-A/MART-1. The CTL lines were maintained in culture in the presence of 100 IU of IL-2 per ml. Line 1520 recognizes an HLA-A2-binding gp100209-217 epitope, while line 1088 recognizes the MELAN-A/MART-127-35 peptide (personal communication). The frequency of T cells able to respond to the gp100 peptide (1 μg/ml) presented on T2 cells was 1/2 in CTL line 1520. T-cell line 1088 had a frequency of 1/260 for T cells specific for the MELAN-A/MART-1 peptide (Table 6). When irradiated Mel 526 targets were used as stimulators, the frequency was 1/33 for the gp100-specific and 1/434 for the MELAN-A/MART-1-specific T-cell lines. These results suggest that the assay is able to effectively detect and quantitate tumor antigen-specific T cells in bulk cultured T-cell lines.
TABLE 6.
ELISPOT assays for IFN-γ production by individual T cells responding to melanoma-associated antigensa
| Assay conditions | Frequency (%) of IFN-γ-producing T cells |
|---|---|
| CTL.1 (spontaneous release) | 1/351 (0.3) |
| CTL.1 + T2 cells | 1/330 (0.3) |
| CTL.1 + T2 cells + gp100 | 1/2 (50) |
| CTL.1 + Ir Mel 526 | 1/33 (3) |
| CTL.1 + T2 cells + MELAN/MART-1 | 1/350 (0.4) |
| CTL.2 (spontaneous release) | 1/2,000 (0.05) |
| CTL.2 + T2 cells | 1/1,666 (0.06) |
| CTL.2 + MELAN-A/MART-1 | 1/1,538 (0.07) |
| CTL.2 + T2 cells + MELAN-A/MART-1 | 1/260 (0.4) |
| CTL.2 + Ir Mel 526 | 1/434 (0.2) |
| CTL.2 + T2 cells + gp100 | 1/1500 (0.06) |
The CTL.1 line (no. 1520) was specific for gp100, and CTL.2 line (no. 1088) was specific for MELAN-A/MART-1. The irradiated (Ir) Mel 526 tumor cell line was used as a stimulator to maintain the CTL, as discussed in Materials and Methods. For the 24-h ELISPOT assay, either the gp100 peptide (1 μg/ml) or MELAN-A/MART-1 peptide (1 μg/ml) alone or pulsed on T2 cells was used as a stimulator. The effector/target cell ratios were 10:1.
To further show that the production of IFN-γ by human tumor-specific CTL upon stimulation with the autologous tumor depends on MHC class I-restricted antigen presentation, we have evaluated a series of CTL lines which recognize a shared antigen expressed on HLA-A2+ SCCHNs but not on other HLA-A2+ human carcinomas (our unpublished data). These lines were established and characterized in our laboratory (21, 36). As shown in Table 7, preincubation of PCI-13 tumor cells with anti-HLA class I Ab (0.4 μg of w6/32) completely eliminated tumor-specific IFN-γ production by the CTL, reducing the frequency of detectable spots from 1/32 to 1/1,000, which is equal to the frequency observed for T cells alone. These data show that the modified ELISPOT assay could be readily used to quantitate the frequency of tumor-specific T cells in cultures of lymphocytes obtained from patients with melanoma, head and neck cancer, or other malignancies. It should be noted, however, that this frequency varied widely even in the same CTL line tested at different time points of ex vivo culture.
TABLE 7.
ELISPOT assay for IFN-γ production by PCI-13-reactive HLA-A2-restricted CTLa
| Assay conditions | Frequency (%) of IFN-γ-producing cells |
|---|---|
| CTL.1 (spontaneous release) | 1/1,000 (0.1) |
| CTL.1 + Ir PCI-13 | 1/8 (12.5) |
| CTL.2 (spontaneous release)b | 1/1,000 (0.1) |
| CTL.2 + Ir PCI-13 | 1/32 (3.1) |
| CTL.2 + Ir PCI-13 | 1/50 (2.0) |
| CTL.2 + Ir PCI-13 + W6/32 Abc | 1/1,000 (0.1) |
| CTL.2 + Ir HRd | 1/1,000 (0.1) |
Two different PCI-13 (SCCHN)-reactive CTL lines were tested. T cells were titrated into wells of ELISPOT plates (6 × 104 to 2 × 103 cells) in triplicate and coincubated with irradiated (Ir) PCI-13 cells (10 lymphocytes/1 tumor cell) for 48 h at 37°C. Spots were counted to determine the frequency of IFN-γ-producing T cells in each coculture.
The CTL.2 line was tested on different days 2 weeks apart.
PCI-13 cells were preincubated with anti-MHC class I Ab (w6/32) for 30 min.
CTL.2 cells were coincubated with the HLA-A2+ gastric carcinoma cell line used as a control.
Comparison of ELISPOT assay with LDA.
To further confirm that ELISPOT assay can be reliably performed in lieu of LDA, we directly compared the two assays, using CTL lines generated from normal PBMC sensitized with PCI-13 cells or PCI-13-derived peptides in vitro. The bulk CTL lines generated as described in Materials and Methods were first tested in cytotoxicity assays against PCI-13 targets, in the presence or absence of anti-class I (w6/32) or anti-HLA-A2 (BB7.2) blocking Abs. At the time when anti-PC13 reactivity was detectable in bulk T-cell cultures, LDA and ELISPOT assay were simultaneously performed. As the results presented in Table 8 indicate, the frequencies of single cells capable of recognizing PCI-13, as measured either by IFN-γ secretion (ELISPOT) or by cytotoxicity against PCI-13 targets (LDA-clonal analysis), were similar. This comparison verifies that ELISPOT assay serves as an appropriate substitute for the more laborious LDA for effective estimation of CTL-p frequencies in polyclonal populations of ex vivo-generated human effector T lymphocytes.
TABLE 8.
Comparison of ELISPOT with LDA for the ability to measure the frequency of T cells in CTL lines responding to PCI-13 by IFN-γ productiona
| CTL line | ELISPOT assay [frequency (%) of cells secreting IFN-γ] | LDA [no. of clones with specific lysis of >10%/no. of wells] (%) |
|---|---|---|
| CTL.3 | 1/71 (1.41) | 5/480 (1.04) |
| CTL.4 | 1/179 (0.56) | 3/480 (0.63) |
| CTL.5 | 1/91 (1.10) | 4/464 (0.86) |
PCI-13-specific bulk CTL lines were established from PBMC of normal donors by multiple rounds of in vitro sensitization with irradiated PCI-13 (CTL.3) or with the peptide preparations derived from PCI-13 and presented on DC (CTL.4 and CTL.5). The cells were plated in LDA at 1 cell/well. All proliferating microcultures were studied for the ability to kill PCI-13 targets in 4-h 51Cr release assays. The ELISPOT assays are described in Materials and Methods.
Utilization of ELISPOT assays for monitoring of patients with melanoma.
Specimens obtained from patients with metastatic melanoma who participated in two melanoma peptide-based vaccination protocols at the UPCI were also studied in ELISPOT assays. These patients were also immunized with the flu MI58-66 peptide. The PBMC samples were obtained from the patients prior to and after peptide vaccinations and tested either without culture (as total mononuclear cells or after selection of CD8+ cells) or after culture for 7 to 14 days, using two cycles of stimulation with the relevant peptides (i.e., peptides contained in the vaccine) pulsed onto irradiated autologous PBMC. When fresh, unstimulated PBMC obtained either before or after vaccination were tested in ELISPOT assays, no responses were detectable, indicating that the frequency of T cells responsive to the melanoma peptides or to the influenza virus peptide was very low (i.e., <105) in these patients' peripheral blood. However, following in vitro expansion of PBMC obtained from the patients randomized to receive the MELAN-A/MART-1 vaccine, MELAN-A/MART-1-specific T cells were detectable in the prevaccination as well as postvaccination samples, as shown in Table 9 for three of the patients. However, after two ex vivo stimulations, no significant postvaccination increase in the frequency of MART-1-responding T cells was observed in the patients immunized intramuscularly with the peptide plus MF59 adjuvant (Table 9). There were no clinical responses observed among the participants in this vaccination protocol.
TABLE 9.
ELISPOT assay for melanoma peptide-specific T lymphocytes in the peripheral blood of patients with melanoma vaccinated with peptide-based tumor vaccinea
| Patient | Time | Frequency of IFN-γ-secreting T cells |
|---|---|---|
| 1 | Prevaccination | 1/3,300 |
| Postvaccination | 1/1,500 | |
| 2 | Prevaccination | 1/727 |
| Postvaccination | 1/800 | |
| 3 | Prevaccination | 1/4,000 |
| Postvaccination | 1/3,333 |
The patients with metastatic melanoma were participants in a phase I vaccination trial performed at the UPCI (see Materials and Methods). The data shown are for three patients randomized to receive the MELAN-A/MART 1 peptide vaccine plus MF59 as adjuvant. Their PBMC were obtained prior to and after intramuscular vaccine administration, expanded in culture during two cycles of stimulation with a mixture of melanoma peptides (including the MELAN-A/MART-1 peptide [AAGIGILTV] used for vaccination), and on day 17 tested in ELISPOT assays for IFN-γ production in response to the same MELAN-A/MART-1 peptide (1 μg/ml) pulsed onto C1R.A2 cells. No MELAN-A/MART 1-responsive lymphocytes were detected in fresh, noncultured PBMC of the three patients. These patients' data were randomly selected to illustrate ELISPOT responses obtained pre- and postvaccination in this clinical trial.
To evaluate responses of patients who received multiepitope melanoma peptide and DC-based vaccines, we enriched PBMC in CD8+ T cells by positive selection on immunobeads prior to ELISPOT assays. Such enriched preparations generally contained 80 to 90% of CD8+ T cells, as determined by flow cytometry. In some but not all patients tested, CD8+-cell-enriched fractions gave positive results in ELISPOT assays, which had been negative with unseparated PBMC (data not shown). Again, postvaccination specimens often did not show higher frequencies of peptide-specific T cells than prevaccination specimens. However, in two melanoma patients, one who was a clinical responder and the other who was not a responder to the DC-based multipeptide vaccine, increased frequencies of T cells specific for the melanoma peptides or for the influenza virus peptide were detected by ELISPOT assay (Table 10). Thus, the assay was able to detect changes from low or undetectable prevaccine to higher postvaccine T-cell frequencies in some of the vaccinated melanoma patients.
TABLE 10.
ELISPOT responses of positively selected CD8+ T cells to melanoma peptides in peripheral blood of patients with metastatic melanoma vaccinated with peptide-based tumor vaccinea
| Patient | Stimulating peptide | Frequency of IFN-γ-secreting T cells:
|
|
|---|---|---|---|
| Prevaccination | Postvaccination | ||
| 1 | gp100 | 0 | 0 |
| Tyrosinase | 0 | 0 | |
| MELAN-A/MART-127-35 | 1/10,000 | 1/1,000 | |
| Flu matrix58-66 | 1/7,000 | 1/600 | |
| 2 | gp100 | 0 | 1/10,000 |
| Tyrosinase | 0 | 1/2,500 | |
| MELAN-A/MART-127-35 | 1/2,600 | 1/200 | |
| Flu matrix58-66 | 1/14,000 | 1/1,500 | |
| 3 | gp100 | 0 | 0 |
| Tyrosinase | 0 | 0 | |
| MELAN-A/MART-127-35 | 0 | 0 | |
| Flu matrix58-66 | 1/6,250 | 1/16,600 | |
The patients with metastatic melanoma were participating in a DC-based multiepitope melanoma peptide vaccine at the UPCI. Their PBMC were obtained prior to and after vaccine administration and cryopreserved. Prior to ELISPOT assay, PBMC were thawed, and positively selected CD8+ T cells were tested in ELISPOT assay for IFN-γ secretion in response to individual peptides pulsed on T2 cells at a concentration of 1 μg/ml.
While the ELISPOT assay was capable of discriminating between various frequencies of T cells responsive to influenza virus or melanoma peptides in CD8+-cell-enriched or ex vivo-amplified PBMC, it has failed to detect significant increments in the frequency of the peptide-responsive T cells among freshly harvested PBMC in most patients with metastatic melanoma vaccinated in our two phase I clinical trials.
DISCUSSION
Cytokine release determined by ELISA or 51Cr release cytotoxicity assays, which are MHC restricted, has been frequently used to measure T-cell responses to antigenic epitopes (3, 19, 33). However, these assays estimate bulk effector responses, without providing an estimate of the number of cells which are functionally responsive to a given stimulus. In situations when a comparison of the frequencies of responding T cells in two populations, e.g., prior to and after vaccination, is necessary, these assays are particularly uninformative. On the other hand, the only assay available for the analysis of frequencies of specific T cells, the LDA, is not applicable to serial monitoring of patient responses, largely owing to its complexity and a labor-intense format. The ELISPOT assay for secretion of cytokines (TNF-α, IFN-γ, or granulocyte-macrophage colony-stimulating factor) by single responders is widely considered to be the best replacement for LDA (10, 24, 28, 29), although few direct comparisons between the two types of assays have been reported so far (15, 18). Studies comparing the two assays have utilized viral antigens, which induce robust memory responses (see, e.g., reference 15). Human tumor-specific responses, on the other hand, are always difficult to quantitate, especially by LDA, perhaps because of the self nature of epitopes involved or tumor-induced immunosuppression which impairs lymphocyte proliferation (17, 37). A great need exists for a clinically applicable assay, such as ELISPOT, to measure frequencies of antigen-responsive T cells, preferably without ex vivo amplification, which could introduce in vitro artifacts.
To be able to utilize the ELISPOT assay for monitoring of those patients with cancer who receive cancer vaccines, it was first necessary to establish its feasibility, sensitivity, and reliability. We performed these studies with PBMC obtained from normal donors stimulated with nonspecific activators (such as PMA and ionomycin), with tumor antigen-specific CTL lines, or with bulk T-cell lines generated by in vitro sensitization of normal or patient-derived lymphocytes with tumor peptides or irradiated tumor cells. Our results allowed us to determine performance characteristics and make recommendations for the optimal utilization of this assay, depending on the type of effector cell tested. As the assay is antibody based, the quality and titer of the capture and detection antibodies are of particular importance. Also, effector cells are plated in a single cell layer in this assay, and, thus background effects, levels of the cytokine produced by individual cells, interactions between the plated cells, and the presence or absence of exogenous cytokines are likely to influence the assay results. Similarly, conditions used for in vitro sensitization, including the concentration and presentation of peptides, the effector/target cell ratio, the presence of AB serum, and time of incubation of effector cells with the stimulating agents were found to be critically important for the number and appearance (intensity and size) of spots. For these various reasons, ELISPOT assays have to be performed under carefully defined and strictly quality-controlled conditions. Nevertheless, the assay, when established and routinely executed by an experienced laboratory, was found to be highly reproducible, with an interassay coefficient of variation of 15%. Its sensitivity was found to be 1/100,000 cells.
The question arises as to the rationale for selection of IFN-γ as the cytokine of choice for the ELISPOT assay. While activated T cells produce a variety of cytokines, both TNF-α and IFN-γ have been reported to correlate with specific antitumor cytotoxicity in clonal as well as nonclonal ex vivo assays (9, 10, 13, 28, 33). In our hands, the IFN-γ ELISPOT assay gave lower nonspecific background and discriminated better between low and high cytokine secretion (as judged by the quality of small, dense spots versus large, diffuse spots) than the TNF-α ELISPOT assay. In addition, IFN-γ secretion was shown to be MHC class I restricted, using CTL lines with specificity for SCCHN. However, the most convincing result was the positive correlation observed between the IFN-γ secretion by a tumor antigen-specific CTL line and the LDA (Table 8). It is necessary to realize, however, that this comparison was performed with an established CTL line which proliferated well and generated numerous clones. Nevertheless, we were reassured that the ELISPOT assay measured the frequency of individual antigen-specific effector T cells in bulk lymphocyte populations responding to the tumor as accurately as the LDA.
Having established the optimal conditions for the ELISPOT assay and having confirmed its reproducibility, we next applied this assay to the assessment of patients' specimens obtained as a part of two different peptide-based vaccination protocols open to patients with metastatic melanoma at our institution. While the final results of these studies will be reported separately, it is interesting that we were unable to detect melanoma peptide-responsive T cells in the blood of several of these patients (Table 9) prior to or after vaccinations, using nonenriched PBMC for ELISPOT assays. These results are in agreement with reports by others (22, 26). On the other hand, following positive selection of CD8+ T lymphocytes, a procedure designed to enrich CTL-p in this fraction, ELISPOT assays were positive in some but not all patients with metastatic melanoma tested as a part of the DC-based vaccine trial (Table 10). For example, two of three patients immunized with the DC-based multiepitope vaccine showed increased postvaccination frequencies of T cells specific to MELAN-A/MART-1 and influenza virus peptides or to all vaccinating peptides. Patient 1 in Table 10 was a clinical responder to the vaccine.
Because the ELISPOT assay was negative in nonenriched PBMC of the majority of patients with metastatic melanoma initially tested, we next used rounds of in vitro stimulation with the relevant peptides presented on autologous PBMC to expand and amplify T-cell responses. With this ex vivo amplification, high frequencies of CTL were detected by ELISPOT assay both prior to and after vaccination in PBMC of patients with melanoma. In examples presented in Table 9, the prevaccination frequency of MELAN-A/MART-1-specific T cells as assessed in ELISPOT assays in three HLA-A2+ patients with melanoma ranged between 1/727 and 1/3,333. The frequencies of T cells responsive to the flu MI55-56 peptide ranged between 1/500 and 1/10,000 (not shown). However, these frequencies did not change appreciably following vaccinations.
Overall, our results indicate that the ELISPOT assay is able to detect relatively low frequencies (i.e., 1/105) of antitumor-specific T cells and thus is about 2 log units more sensitive than cytotoxicity assays estimated to be able to detect 1/1,000 specific effector cells (24). However, even this sensitive assay cannot detect antitumor reactive T cells if their frequency is lower than 1/105 in the peripheral blood of patients with cancer. Two strategies are presently available to determine the frequency of such rare CTL-p: (i) enrichment in CD8+ T cells prior to ELISPOT assay or (ii) culture of PBMC with at least two cycles of in vitro sensitization with relevant peptides to expand CTL-p. Both of these strategies increase the assay complexity, but in the presence of appropriate controls they can reliably detect differences, if any, between pre- and postvaccination specimens.
At present it is not clear why antitumor CTL-p are rare in the circulation of most patients with cancer, including those who receive antitumor vaccines (22, 26). Many factors might contribute to the lack of increase in the frequency of circulating CTL following vaccination with peptide-based vaccines in patients with cancer, as observed by us and others. Methodologic differences, the use of various vaccination regimens, or the existence of tolerance to self epitopes and of tumor-induced immunosuppression (38) in cancer patients could, in part, explain these results. The availability of a sensitive and reliable ELISPOT assay which can be used in the clinical setting to prospectively monitor patients' antigen-specific responses to tumor vaccines should facilitate investigation of these factors. Additional studies employing ELISPOT as well as highly specific tetramer assays (24) for frequency analysis of antigen-specific T cells are now in progress in many laboratories. It is expected that application of these technologies to patient monitoring will allow for meaningful correlations to be made between clinical responses and the frequencies of tumor antigen- or peptide-reactive T cells in the circulation of patients receiving tumor vaccines.
ACKNOWLEDGMENTS
This work was supported in part by grant P0-1DE 12321 to Theresa L. Whiteside from the National Institutes of Health.
We acknowledge the expert technical assistance of Lena Lowelander and Linda Guzik.
REFERENCES
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Boon T, Gajewski T F, Coulie P G. From defined human tumor antigens to effective immunization. Immunol Today. 1995;16:334–336. doi: 10.1016/0167-5699(95)80149-9. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the HIV-1-specific CTL response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulie P, Somville M, Lehmann F, Hainaut P, Brasseur F, Devos R, Boon T. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int J Cancer. 1992;50:289–297. doi: 10.1002/ijc.2910500220. [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Anderson G, Ekre H-P, Nilsson L-A, Klareskog L, Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 6.Doherty P C. The numbers game for virus-specific CD8+ T cells. Science. 1998;280:227. doi: 10.1126/science.280.5361.227. [DOI] [PubMed] [Google Scholar]
- 7.Fujihashi K, McGhee J R, Beagley K W, Mcpherson D T, Mcpherson S A, Huang C M, Kiyono H. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J Immunol Methods. 1993;160:181–189. doi: 10.1016/0022-1759(93)90176-8. [DOI] [PubMed] [Google Scholar]
- 8.Heo D S, Snyderman C H, Gollin S M, Pan S, Walker E, Deka R, Barnes E L, Johnson J T, Herberman R B, Whiteside T L. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;49:5167–5175. [PubMed] [Google Scholar]
- 9.Herr W, Linn B, Leister N, Wandel E, Meyer Zum Buschenfelde K-H, Wolfel T. The use of computer-assisted video image analysis for the quantification of CD8+ T lymphocytes producing tumor necrosis factor α spots in response to peptide antigens. J Immunol Methods. 1997;203:141–151. doi: 10.1016/s0022-1759(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 10.Herr W, Schneider J, Ansgar L W, Meyer Zum Buschenfelde K-H, Wolfel T. Detection and quantification of blood-derived CD8+ T lymphocytes secreting tumor necrosis factor α in response to HLA-A2.1-binding melanoma and viral peptide antigens. J Immunol Methods. 1996;191:131–142. doi: 10.1016/0022-1759(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 11.Herr W, Wolfel T, Heike M, Meyer Zum Buschenfelde K-H, Knuth A. Frequency analysis of tumor-reactive cytotoxic T lymphocytes in peripheral blood of a melanoma patient vaccinated with autologous tumor cells. Cancer Immunol Immunother. 1994;39:93–99. doi: 10.1007/BF01525314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jager E, Bernhard H, Romero P, Ringhoffer M, Arand M, Karbach J, Ilsemann C, Hagedorn M, Knuth A. Generation of cytotoxic T-cell responses with synthetic melanoma-associated peptides in vivo: implications for tumor vaccines with melanoma-associated antigens. Int J Cancer. 1996;66:162–169. doi: 10.1002/(SICI)1097-0215(19960410)66:2<162::AID-IJC4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Kabilan L, Andersson G, Lolli F, Ekre H-P, Olsson T, Troye-Blomberg M. Detection of intra-cellular expression and secretion of interferon-γ at the single-cell level after activation of human T cells with tetanus toxoid in vitro. Eur J Immunol. 1990;20:1085–1089. doi: 10.1002/eji.1830200521. [DOI] [PubMed] [Google Scholar]
- 14.Klinman D M. ELISPOT assay to detect cytokine-secreting murine and human cells. Curr Protocols Immunol. 1994;19:1–8. doi: 10.1002/0471142735.im0619s83. [DOI] [PubMed] [Google Scholar]
- 15.Lalvani A, Brookes R, Hambleton S, Briton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–864. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G, Cascinelli N, Bourlond A, Vanwijck R, Humblet Y, Canon J-L, Laurent C, Naeyaert J-M, Plagne R, Deraemaeker R, Knuth A, Jager E, Brasseur F, Herman J, Coulie P G, Boon T. Tumor regression responses in melanoma patients treated with a peptide encoded by gene MAGE-3. Int J Cancer. 1995;63:883–885. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 17.Miescher S, Whiteside T L, Moretta L, Von Fliedner V. Clonal and frequency analyses of tumor-infiltrating T lymphocytes from human solid tumors. J Immunol. 1987;138:4004–4011. [PubMed] [Google Scholar]
- 18.Miyahira Y, Murata K, Rodriguez D, Rodriguez J R, Esteban M, Rodriguez M M, Zavala F. Quantification of antigen-specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-s. [DOI] [PubMed] [Google Scholar]
- 19.Morris A G, Lin Y L, Askonas B A. Immune interferon release when a cloned cytotoxic T cell line meets its correct influenza-infected target cell. Nature. 1982;295:150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- 20.Nordstrom L, Ferrua B. Reverse elispot assay for clonal analysis of cytokine production. II. Enumeration of interleukin-1-secreting cells by amplified (avidin-biotin antiperoxidase) assay. J Immunol Methods. 1992;150:199–206. doi: 10.1016/0022-1759(92)90079-9. [DOI] [PubMed] [Google Scholar]
- 21.Okada K, Yasumura S, Muller-Fleckenstein I, Fleckenstein B, Talib S, Koldovsky U, Whiteside T L. Interactions between autologous CD4+ and CD8+ T lymphocytes and human squamous cell carcinoma of the head and neck. Cell Immunol. 1997;177:35–48. doi: 10.1006/cimm.1997.1079. [DOI] [PubMed] [Google Scholar]
- 22.Pass H A, Schwarz S L, Wunderlich J E, Rosenberg S A. Immunization of patients with melanoma peptide vaccines: immunologic assessment using ELISPOT assay. Cancer J Sci Am. 1998;4:316–323. [PubMed] [Google Scholar]
- 23.Rivoltini L, Loftus D J, Squarcina P, Castelli C, Rini F, Arienti F, Belli F, Marincola F M, Geisler C, Borsatti A, Appella E, Parmiani G. Recognition of melanoma-derived antigens by CTL: possible mechanisms involved in downregulating anti-tumor T-cell reactivity. Crit Rev Immunol. 1998;18:55–63. doi: 10.1615/critrevimmunol.v18.i1-2.70. [DOI] [PubMed] [Google Scholar]
- 24.Romero P, Cerottini J-C, Waanders G. Novel methods to monitor antigen-specific cytotoxic T-cell responses in cancer immunotherapy. Mol Med Today. 1998;4:305–312. doi: 10.1016/s1357-4310(98)01280-5. [DOI] [PubMed] [Google Scholar]
- 25.Ronnelid J, Klareskog L. A comparison between ELISPOT methods for the detection of cytokine producing cells: grated sensitivity and specificity using ELISA plates as compared to nitrocellulose membranes. J Immunol Methods. 1997;20:17–26. doi: 10.1016/s0022-1759(96)00170-6. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S M, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, Wonderlich J R, Parkhurst M R, Kawakami Y, Seipp C A, Einhorn J H, White D E. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee H G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990;348:252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- 28.Scheibenbogen C, Lee K-H, Mayer S, Stevanovic S, Moebius U, Herr W, Rammensee H-G, Keilholz U. A sensitive ELISPOT assay for detection of CD8+ T lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin Cancer Res. 1997;3:221–226. [PubMed] [Google Scholar]
- 29.Scheibenbogen C, Lee K-H, Stevanovic S, Witzens M, Willhauck M, Waldmann V, Naeher H, Rammensee H-G, Keilholz U. Analysis of the T-cell response to tumor and viral peptide antigens by an IFN-γ-ELISPOT assay. Int J Cancer. 1997;71:1–5. doi: 10.1002/(sici)1097-0215(19970611)71:6<932::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu Y, Iwatsuki S, Herberman R B, Whiteside T L. Clonal analysis of tumor-infiltrating lymphocytes from human primary and metastatic liver tumors. Int J Cancer. 1990;46:878–993. doi: 10.1002/ijc.2910460521. [DOI] [PubMed] [Google Scholar]
- 31.Tanguay S, Killion J. Direct comparison of ELISPOT and ELISA-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994;13:259–263. [PubMed] [Google Scholar]
- 32.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;126:614–619. [PubMed] [Google Scholar]
- 33.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, de Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 34.Van der Eynde B, van der Bruggen P. T-cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. doi: 10.1016/s0952-7915(97)80050-7. [DOI] [PubMed] [Google Scholar]
- 35.Whiteside, T. L., J. Bryant, R. Day, and R. B. Herberman. Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J. Clin. Lab. Anal. 4:102–114. [DOI] [PubMed]
- 36.Whiteside T L, Chikamatsu K, Nagashima S, Okada K. Antitumor effects of cytolytic T lymphocytes (CTL) and natural killer (NK) cells in head and neck cancer. Anticancer Res. 1996;16:2357–2364. [PubMed] [Google Scholar]
- 37.Whiteside T L, Miescher S, Hurlimann J, von Fliedner V. Separation, phenotyping and limiting-dilution analysis of T-lymphocytes infiltrating human solid tumors. Int J Cancer. 1986;37:803–811. doi: 10.1002/ijc.2910370602. [DOI] [PubMed] [Google Scholar]
- 38.Whiteside T L, Rabinowich H. The role of Fas/FasL in immunosuppression induced. by human tumors. Cancer Immunol Immunother. 1998;46:175–184. doi: 10.1007/s002620050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whiteside T L, Sung M-W, Nagashima S, Chikamatsu K, Okada K, Vujanovic N L. Human tumor antigen-specific T lymphocytes and IL2-activated natural killer (A-NK) cells: comparisons of antitumor effects in vitro and in vivo. Clin Cancer Res. 1998;4:1135–1145. [PubMed] [Google Scholar]
- 40.Wolfel T, van Pel A, Brichard V, Schneider J, Selinger B, Meyer zum Buschenfelde K H, Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]