Abstract
Currently, there are no approved pharmacological treatments for the management of patients with idiopathic pulmonary fibrosis (IPF) in the USA or Europe. Pirfenidone is an orally bio-available small molecule that exhibits antifibrotic and anti-inflammatory properties in a variety of in vitro and animal models.
Pirfenidone has been evaluated in four randomised, double-blind, placebo-controlled clinical trials conducted in Japan, North America and Europe. The totality of the data from these trials indicates that pirfenidone is able to reduce the rate of decline in lung function, measured as change in per cent predicted forced vital capacity (FVC) or vital capacity. There was also an effect on secondary end-points of progression free survival, categorical change in per cent predicted FVC, and the 6-min walk test.
A recent meta-analysis of the three phase III studies in IPF demonstrated that pirfenidone significantly reduced the risk of disease progression by 30%. The efficacy of pirfenidone is associated with an acceptable tolerability and safety profile.
Keywords: Idiopathic pulmonary fibrosis, meta-analysis, N-acetylcysteine, pirfenidone, progression-free survival, vital capacity
Idiopathic pulmonary fibrosis (IPF) is a progressive, debilitating and currently incurable disease [1], affecting 80,000–85,000 people in Europe [2, 3]. IPF follows an insidious clinical course with progressive decline in pulmonary function that increasingly restricts routine physical activity [4, 5]. Median survival time from diagnosis is usually between 2 to 5 yrs, with a 5-yr survival rate of ∼20% [5, 6]. Biologically, IPF behaves similarly to cancer in terms of patient decline [7]. Patients’ short survival time, high mortality and rapid decline highlight the necessity of early diagnosis and more optimal treatment [1].
PHARMACOLOGICAL THERAPIES FOR IPF: CURRENT STATUS
There are currently no approved pharmacological therapies for IPF in Europe (refer to footnote) or the USA and treatment traditionally consists of corticosteroids and immunosuppressants, as proposed by the joint American Thoracic Society (ATS)/European Respiratory Society (ERS) consensus statement on IPF in 2000 [1]. However, recent guidelines, including an evidence-based update from the ATS/ERS joint task force, make a strong recommendation against the use of corticosteroids plus immunomodulator therapy in patients with IPF because of drug-related adverse effects and lack of clinically proven efficacy [8, 9].
Partly based on the poor outcomes associated with corticosteroids and immunosuppressant therapy, it is now thought that the underlying process in IPF may be associated with repeated epithelial cell injury and a resulting fibroproliferative process [10–12]. Recent clinical trials have evaluated a number of new therapeutic approaches for IPF including the dual endothelin receptor antagonist bosentan [13], imatinib [14], sildenafil [15], etanercept [16] and interferon-γ-1b [17]. These studies utilised a number of different outcome measures but none met their primary end-point (table 1).
Table 1. Selected recent clinical trials in idiopathic pulmonary fibrosis.
| Trial | Drug (putative MoA) | Patients n | End-point | Outcome |
| IFIGENIA | NAC (anti-oxidant; plus prednisone and AZT) | 155 | ΔVC, ΔDL,CO | Positive |
| NCT0063869 | Etanercept (TNF antagonist) | 88 | ΔFVC, ΔDL,CO, PA-a,O2 | Negative |
| INSPIRE | IFN-γ-1b (antifibrotic) | 826 | Survival | Negative |
| BUILD-1 | Bosentan (endothelin inhibitor) | 158 | 6MWT | Negative |
| BUILD-3 | Bosentan (endothelin inhibitor) | 600 | Progression-free survival | Negative |
| NCT00131274 | Imatinib (tyrosin kinase inhibitor) | 119 | Disease progression or death | Negative |
| STEP-IPF | Sildenafil (PD5 inhibitor) | 180 | 6MWT | Negative |
| Shionogi phase III | Pirfenidone (antifibrotic) | 267 | ΔVC | Positive |
| PIPF 004 (CAPACITY 2) | Pirfenidone (antifibrotic) | 435 | ΔFVC % pred | Positive |
| PIPF 006 (CAPACITY 1) | Pirfenidone (antifibrotic) | 344 | ΔFVC % pred | Negative |
MoA: mechanism of action; NAC: N-acetylcysteine; AZT: azathioprine; TNF: tumour necrosis factor; IFN: interferon; PD5: phosphodiesterase-5; VC: vital capacity; DL,CO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity; PA–a,O2: alveolar–arterial oxygen tension difference; 6MWT: 6-min walk test; % pred: % predicted.
TRIPLE THERAPY WITH PREDNISONE, AZATHIOPRINE AND N-ACETYLCYSTEINE
One of the first studies to report a positive outcome in patients with IPF was the European IFIGENIA trial [18]. IFIGENIA was a double-blind, randomised, placebo-controlled, multicentre study evaluating the effects of additional N-acetylcysteine (NAC) high-dose in patients under treatment with prednisone plus azathioprine. Patients were randomly assigned to recieve NAC or placebo. In this study, patients receiving NAC for 12 months demonstrated slower deterioration of vital capacity (VC) and diffusing capacity of the lung for carbon monoxide (DL,CO) than patients receiving prednisone and azathioprine therapy alone [18]. These results suggest that the addition of NAC to low-dose prednisone and azathioprine may help to preserve pulmonary function in patients with IPF [18]. However, in view of a drop-out rate of ∼30% (including deaths) questions have been raised regarding the clinical relevance and robustness of the treatment effect.
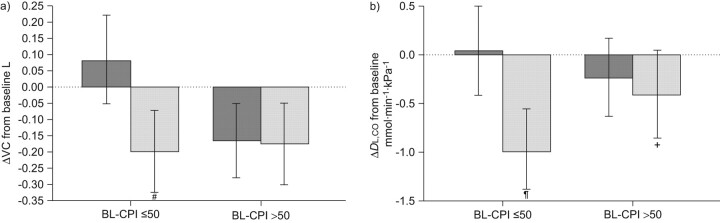
A further exploratory analysis from the IFIGENIA trial using a composite physiological index (CPI) suggests that NAC may be more effective in less advanced disease [19]. The CPI was calculated according to Wells et al. [20] (CPI = 91.0−0.65 × DL,CO (% pred) − 0.53 VC (% pred) + 0.34 forced expiratory volume in 1 s (% pred)). CPI is between 20 and 80 points with higher points indicating more fibrosis and poorer prognosis [20]. In this analysis baseline CPI appears to influence the outcome of therapy. Patients with less advanced disease (baseline CPI ≤50 points) showed effects favouring NAC on changes in forced vital capacity (FVC) and DL,CO. In contrast, only small, nonsignificant trends favouring NAC were observed in patients with a baseline CPI >50 points (fig. 1). The authors highlighted how these results suggest an influence of disease stage on treatment efficacy with NAC [19]. An ongoing trial evaluating the effectiveness of prednisone, azathioprine and NAC in IPF (PANTHER-IPF) is currently recruiting patients [21].
Figure 1.
Effects of N-acetylcysteine (▒) and placebo (░) on vital capacity (VC) and diffusing capacity of the lung for carbon monoxide (DL,CO) depending on baseline composite physiological index (BL-CPI) being lower or higher than 50 points. #: p = 0.0031; ¶: p = 0.0015; +: p = 0.067. Reproduced from [19] with permission from BioMed Central.
PIRFENIDONE
Pirfenidone, an orally active small molecule, is the first substance for which more than one positive phase III randomised, placebo-controlled trial has been reported with a statistically significant effect on a primary end-point in patients with IPF.
Pirfenidone exhibits antifibrotic and anti-inflammatory properties in a variety of in vitro and animal models [22–25], although the molecular target is not known. Pirfenidone has been shown to ameliorate drug-induced lung fibrosis in animal models [23]. In vitro studies have demonstrated that pirfenidone inhibits transforming growth factor (TGF)-β-stimulated collagen synthesis, decreases extracellular matrix deposition [22, 24] and blocks the mitogenic effects of platelet-derived growth factor in lung fibroblasts derived from patients with IPF [23]. Pirfenidone has demonstrated broad antifibrotic activity in several animal models of fibrosis in lung and other organs. In lung, pirfenidone reduced fibrosis in response to bleomycin, lung transplant and repeated allergen exposure. In these studies, treatment-related reductions in fibrosis were associated with modulation of several fibrogenic mediators including TGF-β [25].
Pirfenidone has been evaluated in a number of clinical trials which have reported on the efficacy and safety of pirfenidone in patients with IPF.
JAPANESE PHASE II AND III TRIALS
Following an open-label phase II pilot study [26] and an open-label 1-yr study [27], a double-blind, placebo-controlled clinical trial of pirfenidone was conducted in 107 Japanese patients with IPF [28]. This study was terminated early due to a higher number of acute exacerbations in the placebo group than in the pirfenidone group [28]. Based on the positive results from this trial, which demonstrated a reduced decline in VC at 9 months (p = 0.036) in patients receiving pirfenidone [28], a phase III trial was conducted in Japanese patients with well-defined IPF and mild-to-moderate impairment in lung function.
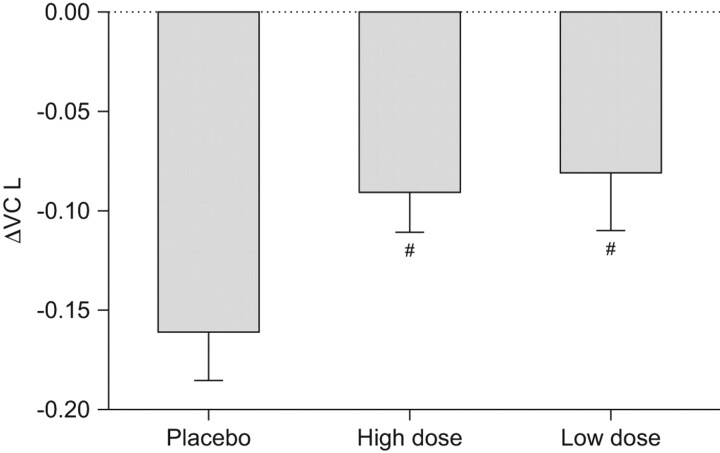
This three-armed phase III, multicentre, double-blind, placebo-controlled study randomised patients to either high-dose pirfenidone (600 mg three times per day; n = 108), low-dose pirfenidone (400 mg three times per day; n = 55) or placebo (n = 104) in the ratio 2:1:2. The pirfenidone dose was increased in a stepwise manner up to the full dose over 4 weeks. A total of 267 patients were evaluated for efficacy of pirfenidone [29]. The primary end-point was decline in VC from baseline at 52 weeks, which was changed from lowest arterial oxygen saturation measured by pulse oximetry (Sp,O2) during the 6-min, steady-state exercise test (6MET) prior to unblinding. Secondary end-points included progression-free survival (PFS; decline in VC ≥10% and/or death) and change in the lowest Sp,O2 during the 6MET [29].
Significant differences were observed in the primary end-point of VC decline between the placebo group (-0.16 L) and the high-dose pirfenidone group (-0.09 L), representing a 44% reduction in VC decline compared to placebo favouring pirfenidone (p = 0.042) (fig. 2). A significant difference was also seen between the low-dose pirfenidone and placebo groups (p = 0.0394). Pirfenidone was also associated with a significant increase in the secondary end-point of PFS, defined as the period until a first progressive event (either categorical decrease of >10% in FVC or death; p = 0.028). Pirfenidone was relatively well-tolerated, with photosensitivity being the major adverse event (observed in 51% and 53% of patients in the high-dose and low-dose group, respectively), although this was rated mild in the majority of patients (∼70% and 80% in the high-dose and low-dose groups, respectively). Discontinuation rates were 37% in the high-dose pirfenidone group and 29.8% in the placebo group [27]. Based on these studies pirfenidone was approved for IPF patients in Japan in 2008.
Figure 2.
Effects of pirfenidone on vital capacity (VC) at week 52 in a Japanese phase III trial. The last observation carried forward method was used for drop-outs in each group. Data are presented as mean±se. Placebo group: n = 103; high-dose group: n = 104; low-dose group: n = 54. #: p<0.1, comparison of adjusted means based on ANCOVA (negative and positive of the changes represent deterioration and improvement from baseline, respectively). Reproduced from [31].
STUDIES 004 AND 006: EUROPEAN AND NORTH AMERICAN PHASE III TRIALS
Two concurrent, multinational, randomised, double-blind controlled trials with pirfenidone in IPF patients have been conducted in Europe and North America. Results from these two phase III studies were first presented at the 2009 International Conference of the ATS [30], the 2009 and 2010 ERS Annual Congress [31–33] and during an FDA Pulmonary-Allergy Drugs Advisory Committee in 2010 [34]. Final results were published in early 2011 [35].
Given the generally limited understanding of end-points in IPF trials, a relevant spectrum of clinical outcomes were assessed to investigate different aspects of the disease process. Patient eligibility required a confident diagnosis of IPF, baseline FVC % pred ≥50% and DL,CO % pred ≥35%. Exclusion criteria included significant obstructive lung disease and patients on medications for IPF. The primary end-point in both studies was absolute change in FVC % pred from baseline to week 72 (intention to treat analysis, rank ANCOVA). Secondary end-points included categorical change in FVC % pred, PFS, 6-min walk test (6MWT) distance, lowest Sp,O2 during 6MWT, DL,CO % pred and time to worsening. Pre-specified exploratory analyses across both studies were also conducted to estimate the magnitude of the treatment effect in the combined population [30].
In study 004, 435 patients were randomised 2:2:1 to receive a total daily dose of 2,403 mg pirfenidone, placebo or a total daily dose of 1,197 mg pirfenidone, respectively, for 72 weeks. The lower dose of pirfenidone in study 004 was included to explore dose–effect relationships. In study 006, 344 patients were randomised to treatment with either 2,403 mg pirfenidone orally or placebo for 72 weeks with a 1:1 randomisation [35]. Patient demographics and baseline characteristics were well balanced across treatment groups in the two trials, although there were more US patients and more patients on supplemental oxygen in study 006 than in study 004 [35].
Results from study 004 showed that pirfenidone was statistically significantly superior compared to placebo in the primary end-point (ΔFVC % pred) at week 72 (p = 0.001). Secondary end-points of PFS (defined as death or decline ≥10% of FVC or ≥15% in DL,CO; p = 0.023) and categorical change in FVC (decline in FVC ≥10%; p = 0.001) were also statistically significant [35].
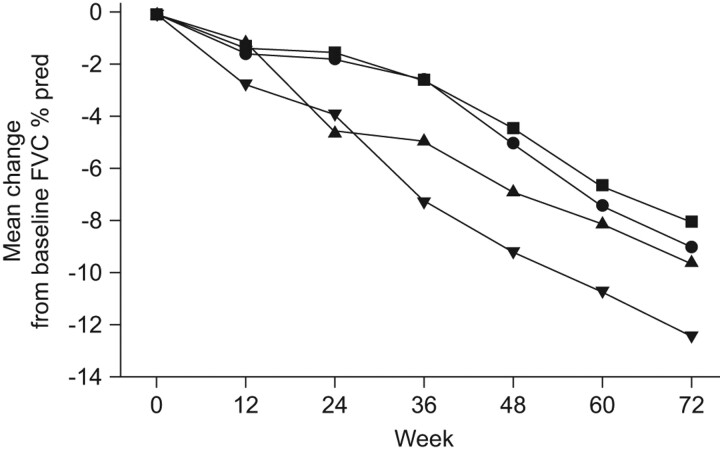
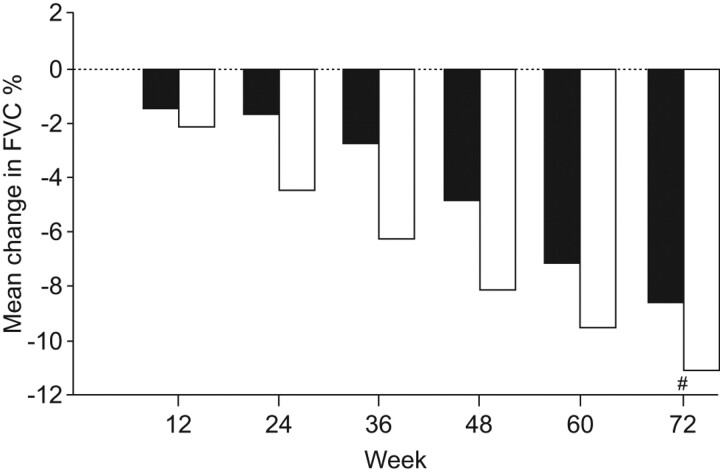
However, in study 006 pirfenidone was not statistically significantly superior compared to placebo for the primary end-point (p = 0.501), although the results were generally consistent with and supportive of the results from study 004 [30]. Interestingly, there was not the clinically expected decline in FVC % pred in the placebo arm of study 006 that was seen in study 004 (fig. 3) [35]. In study 006, pirfenidone treatment was associated with a significant beneficial effect on the secondary end-point of the 6MWT distance when compared to placebo (p = 0.001) [35]. A temporal analysis of FVC % pred decline over time during the 72 weeks showed that the reduction in FVC decline with pirfenidone was also statistically significant through week 48 in study 006 (fig. 4). Overall, the treatment effect observed in these two studies was generally consistent with that observed in the phase III study of pirfenidone in IPF patients conducted in Japan [29, 35].
Figure 3.
Change in forced vital capacity (FVC) % predicted (% pred) in study 004 and study 006 comparing pirfenidone or placebo in patients with idiopathic pulmonary fibrosis [29, 35]. ▪: study 004 pirfenidone 2,403 mg·day−1; •: study 006 pirfenidone 2,403 mg·day−1; ▴: study 006 placebo; ▾: study 004 placebo.
Figure 4.
Temporal analysis of change in forced vital capacity per cent predicted with pirfenidone in patients with idiopathic pulmonary fibrosis [34].
As stated previously, a low-dose pirfenidone group (1,197 mg·day−1; n = 87) was included in study 004 for exploratory descriptive purposes. Exploratory analyses of clinical efficacy parameters in the three treatment arms in study 004 revealed a clear dose–response relationship, with low-dose pirfenidone having a more modest treatment effect compared with high-dose pirfenidone. The observed dose–response relationship provides additional support for the overall clinical efficacy observations in studies 004 and 006 [32].
EXPLORATORY POOLED ANALYSIS OF STUDIES 004 AND 006
An exploratory analysis of pooled primary end-point data from both studies was also conducted to provide more precise estimates of treatment effect [30, 33]. This was considered valid since study 004 and study 006 were designed as nearly identical studies to facilitate pooling of data and was supported by the fact that the overall results were directionally similar with no treatment affected by study interaction [34, 35].
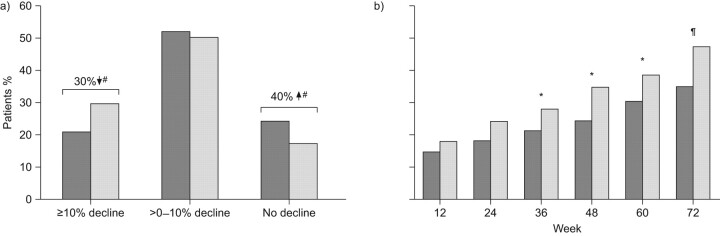
The results of this exploratory pooled analysis, assessing treatment effect over the full duration of the study, showed that pirfenidone (n = 345) significantly (p = 0.005) reduced decline in FVC % pred compared to placebo (n = 347) (fig. 5) [30, 33]. Exploratory analyses of pooled data for the secondary end-points also demonstrated a statistically significant treatment benefit of pirfenidone on PFS (p = 0.029), categorical FVC change (p = 0.003) and 6MWT distance (p = 0.001) (fig. 6) [30, 33]. The exploratory pooled analysis of categorical FVC change showed that 30% fewer patients experienced >10% decrease in FVC at week 72 in the pirfenidone group than in the placebo group [30]. This magnitude of decline is considered clinically meaningful as a 10% decline in FVC % pred has been shown to be an independent predictor of mortality in patients with IPF in multiple studies [33].
Figure 5.
Exploratory pooled analysis (studies 004 and 006 combined) of change in forced vital capacity (FVC) with pirfenidone 2,403 mg·day−1 (□: n = 345) or placebo (▪: n = 347) in patients with idiopathic pulmonary fibrosis [29, 35]. #: p = 0.005, rank ANCOVA (pirfenidone 2,403 mg·day−1 versus placebo at week 72).
Figure 6.
Exploratory pooled analysis (studies 004 and 006 combined) of a) categorical forced vital capacity change at week 72 and b) 6-min walk test distance decrement >50 m (p = 0.03 Cochran–-Haenszel row mean score test based on five categories) [28, 31]. ▒: pirfenidone 2,403 mg·day−1 (n = 345); ░: placebo (n = 347). *: p<0.05; #: p = 0.003; ¶: p≤0.001.
SAFETY AND TOLERABILITY OF PIRFENIDONE IN STUDIES 004 AND 006
Results from studies 004 and 006 showed that pirfenidone 2,403 mg·day−1 was safe and generally well tolerated [35]. There were fewer treatment-emergent deaths observed among patients treated with pirfenidone 2,403 mg than with placebo during the treatment periods. In study 004, 5.7% of patients in the pirfenidone group died during the treatment period, compared with 8.0% in the placebo group. In study 006, 5.3% of patients in the pirfenidone group died during the treatment period, compared to 8.7% in the placebo group [30]. This difference appeared to be driven by a significant reduction in IPF-related deaths among pirfenidone-treated patients. Specifically, the incidence of IPF-related deaths was 3.5% in patients treated with pirfenidone compared with 7.2% in those treated with placebo (51% relative reduction, p = 0.031) [30].
There was no difference between pirfenidone and placebo in the percentage of patients who experienced a treatment emergent serious adverse event [35]. As previously reported, gastrointestinal discomfort and photosensitivity were the commonest side-effects [35]. Nausea and diarrhoea were the most commonly reported gastrointestinal adverse events in all three treatment groups. Grade 3 or 4 nausea or diarrhoea was uncommon, as was treatment discontinuation due to either nausea or diarrhoea. Gastrointestinal adverse events were generally mild to moderate and rarely resulted in treatment discontinuation [35]. Rash and photosensitivity occurred with a greater frequency in patients treated with pirfenidone 2,403 mg·day−1. These events were generally mild to moderate in severity and rarely resulted in treatment discontinuation. A dose–response relationship was observed on a number of adverse events although relatively few adverse events resulted in treatment discontinuations [35].
META-ANALYSIS OF PHASE III STUDIES
The Cochrane Collaboration recently published the results of a meta-analysis to assess the efficacy of nonsteroid agents in adults with IPF, including pirfenidone [36]. Four trials involving 1,155 patients were reviewed comparing pirfenidone with placebo, including the three phase III pirfenidone trials that reported PFS as an outcome. The result of the meta-analysis suggested that pirfenidone significantly reduced the risk of disease progression by 30% (fig. 7) [36]. In this Cochrane review the effect of pirfenidone on pulmonary function could be assessed based on two studies [28–30] analysing 314 patients. Results showed that pirfenidone significantly reduced the decline in VC from baseline (p = 0.0006) [36].
Figure 7.
Forest plot of pirfenidone versus placebo in improving progression-free survival in patients with idiopathic pulmonary fibrosis. Reproduced with modification from [36].
PROGNOSTIC FACTORS FOR IPF AND ASSESSMENT OF NEW TREATMENT OPTIONS
The variable clinical course of patients with IPF underscores the heterogeneous nature of the disease and the inherent challenges associated with the design of clinical trials in IPF. One outcome shown to be a reliable predictor of disease progression and mortality is change in FVC. FVC is widely used and accepted as a clinically meaningful measure of IPF disease status [1]. In a large study by du Bois et al. [37] utilising prospectively collected data, notable predictors of mortality were age, FVC change, baseline DL,CO and respiratory hospitalisation. In terms of FVC change, patients experiencing a >10% decline in FVC during the preceding 24 weeks were at a greater than four-fold increased risk of death over the next 24 weeks compared to patients with an FVC decline of <5% [37].
These data further emphasise that agents which affect the rate of change in FVC, such as pirfenidone, may be expected to play an important role in the management of patients with IPF through delaying disease progression and the risk of death.
SUMMARY
IPF is an inevitably progressive fatal lung disease and effective treatment options are urgently needed. The current treatment paradigm utilising corticosteroids and immunosuppressants is associated with adverse effects and the efficacy is not clinically proven. To date there is some evidence that the combination of corticosteroid, azathioprine and NAC may be beneficial in patients with mild to moderate disease but this is based on a single study with a number of limitations.
The only agent that has proven to be clinically effective in several randomised, double-blind, placebo-controlled studies is pirfenidone. Pirfenidone has demonstrated a statistically significant and clinically meaningful effect on change in FVC % pred or VC, as well as on PFS and categorical change in FVC % pred. Although one of these studies did not achieve statistical significance on the primary end-point, because there was a less than expected decline in FVC in the placebo group, results are generally consistent and supportive of the other trials with pirfenidone. The efficacy of pirfenidone is also associated with an acceptable tolerability and safety profile.
Acknowledgments
This article is based on the proceedings of a satellite symposium held at the 2010 ERS Annual Congress (Barcelona, Spain), which was sponsored by InterMune Inc. The author was assisted in the preparation of the text by professional medical writers at IntraMed International (Milan, Italy). The medical writing support was funded by InterMune Inc.
Footnotes
Following the presentation sponsored by InterMune Inc. at the 2010 ERS Annual Congress (Barcelona, Spain), pirfenidone has received marketing authorisation in the European Union in 2011.
Provenance
Publication of this peer-reviewed article was supported by InterMune Inc., Brisbane, CA, USA (article sponsor, European Respiratory Review issue 121).
Statement of Interest
U. Costabel has received consultancy fees from Actelion, Boehringer Ingelheim, Centocor, Gilead and InterMune, and has received lecture fees from InterMune.
REFERENCES
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000; 161: 646–664. [DOI] [PubMed] [Google Scholar]
- 2.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and tamilial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax 2002; 57: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orphanet. Orphanet Report Series Rare Diseases Collection. Prevalence of rare diseases: bibliographic data. May 2011 Number 1. . www.orphanet.net
- 4.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med 2002; 165: 277–304. [DOI] [PubMed] [Google Scholar]
- 5.Vancheri C, Failla M, Crimi N, et al. Idiopathic pulmonary fibrosis: a disease with similarities and links to cancer biology. Eur Respir J 2010; 35: 496–504. [DOI] [PubMed] [Google Scholar]
- 6.Olson AL, Swigris JJ, Lezotte DC, et al. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007; 176: 277–284. [DOI] [PubMed] [Google Scholar]
- 7.Harari S, Caminati A. Update on diffuse parenchymal lung disease. Eur Respir Rev 2010; 19: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63: Suppl. 5, v1–v58. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Collard HR, Egan J, et al. An Official ATS/ERS/JRS/ALAY Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.du Bois RMStrategies for treating idiopathic pulmonary fibrosis Nat Rev Drug Discov 2010; 9: 129–140. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 2008; 3: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001; 134: 136–151. [DOI] [PubMed] [Google Scholar]
- 13.King TE, Behr J, Brown KK, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008; 177: 75–81. [DOI] [PubMed] [Google Scholar]
- 14.Daniels CE, Lasky JA, Limper AH. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am J Respir Crit Care Med 2010; 181: 604–610. [DOI] [PubMed] [Google Scholar]
- 15.Zisman DA, Schwarz M, Anstrom KJ, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med 2010; 363: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghu G, Brown KK, Costabel U, et al. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med 2008; 178: 948–955. [DOI] [PubMed] [Google Scholar]
- 17.King TE, Albera C, Bradford WZ, et al. Effect of interferon gamma-1b on survival in patients with idiopathic pulmonary fibrosis (INSPIRE): a multicentre, randomised, placebo-controlled trial. Lancet 2009; 374: 222–228. [DOI] [PubMed] [Google Scholar]
- 18.Demedts M, Behr J Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2005; 353: 2229–2242. [DOI] [PubMed] [Google Scholar]
- 19.Behr J, Demedts M, Buhl R, et al. Lung function in idiopathic pulmonary fibrosis – extended analyses of the IFIGENIA trial. Respir Res 2009; 10: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003; 167: 962–969. [DOI] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov. Evaluating the effectiveness of prednisone, azathioprine, and N-acetylcysteine in people with idiopathic pulmonary fibrosis (PANTHER-IPF). Identifier NCT00650091. http://clinicaltrials.gov/ct2/home.
- 22.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999; 291: 367–373. [PubMed] [Google Scholar]
- 23.Gurujeyalakshmi G, Hollinger MA, Giri SN. Pirfenidone inhibits PDGF isoforms in bleomycin hamster model of lung fibrosis at the translational level. Am J Physiol 1999; 276: L311–L318. [DOI] [PubMed] [Google Scholar]
- 24.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 1999; 289: 211–218. [PubMed] [Google Scholar]
- 25.Oku H, Shimizu T, Kawabata T, et al. Antifibrotic action of pirfenidone and prednisolone: different effects on pulmonary cytokines and growth factors in bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol 2008; 590: 400–408. [DOI] [PubMed] [Google Scholar]
- 26.Raghu G, Johnson WC, Lockhart D, et al. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label phase II study. Am J Respir Crit Care Med 1999; 159: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 27.Nagai S, Hamada K, Shigematsu M, et al. Open-label compassionate use one year-treatment with pirfenidone to patients with chronic pulmonary fibrosis. Intern Med 2002; 41: 1118–1123. [DOI] [PubMed] [Google Scholar]
- 28.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 29.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 821–829. [DOI] [PubMed] [Google Scholar]
- 30.Noble PW, Albera C, Bradford W, et al. The CAPACITY (CAP) trials: randomized, double−blind, placebo−controlled, phase III trials of pirfenidone (PFD) in patients with idiopathic pulmonary fibrosis (IPF). 2009 American Thoracic Society International Conference (San Diego, CA, USA). Am J Respir Crit Care Med 2009; 179: A1129. [Google Scholar]
- 31.du Bois R, Albera C, Bradford W, et al. Pirfenidone treatment for idiopathic pulmonary fibrosis (IPF): a comprehensive analysis of safety. Eur Respir J 2009; 34: Suppl. 53, 494s. [Google Scholar]
- 32.Costabel U, Albera C, Bradford W, et al. Pirfenidone dose-response in patients with idiopathic pulmonary fibrosis (IPF). A comprehensive analysis of outcomes in CAPACITY 2. Eur Respir J 2010; 36: Suppl. 54, 41s. [Google Scholar]
- 33.Albera C, Bradford W, Costabel U, et al. The magnitude of pirfenidone treatment effect in patients with idiopathic pulmonary fibrosis (IPF): a pooled analysis of outcomes in the CAPACITY (CAP) studies. Eur Respir J 2010; 36: Suppl. 54, 41s. [Google Scholar]
- 34.Karimi-Shah BA. Pulmonary-Allergy Drugs Advisory Committee (PADAC): Pirfenidone Capsules NDA 22-535, S-000. U.S. Food and Drug Administration. www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM206398.pdf. [Google Scholar]
- 35.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY: two randomised trials). Lancet 2011; 377: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 36.Spagnolo P, Del Giovane C, Luppi F, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev 2010; 9: CD003134. [DOI] [PubMed] [Google Scholar]
- 37.du Bois R, Albera C, Bradford W, et al. Predictors of mortality in patients with idiopathic pulmonary fibrosis (IPF). 2009 American Thoracic Society International Conference (San Diego, CA, USA). Am J Respir Crit Care Med 2009; 179: A1114. [Google Scholar]