Abstract
The purpose of the present study was to investigate the effects of drinking water alkaline mineral complex (AMC) supplementation on growth performance, intestinal morphology, inflammatory response, immunity, antioxidant defense system, and barrier functions in weaned piglets. In a 15-d trial, 240 weaned piglets (9.35 ± 0.86 kg) at 28 d of age (large white × landrace × Duroc) were randomly divided into two groups: the control (Con) group and the AMC group. Drinking water AMC supplementation improved (P < 0.01) final body weight (BW) and average daily gain (ADG) in weaned piglets compared to the Con group. Importantly, AMC reduced (P < 0.01) the feed-to-gain (F:G) ratio. AMC water improved the physical health conditions of piglets under weaning stress, as reflected by the decreased (P < 0.05) hair score and conjunctival score. Moreover, there was no significant (P > 0.05) difference in relatively small intestinal length, organ (liver, spleen, and kidney) indices, or gastrointestinal pH value in weaned piglets between the two groups. Of note, AMC significantly promoted the microvilli numbers in the small intestine and effectively ameliorated the gut morphology damage induced by weaning stress, as evidenced by the increased (P < 0.05) villous height (VH) and ratio of VH to crypt depth. Additionally, AMC lessened the levels of lipopolysaccharide (LPS, P < 0.01) and the contents of IL1β (P<0.05), and TNF-α (P<0.05) in the weaned piglet small intestine. Conversely, the gut immune barrier marker, secretory immunoglobulin A (sIgA) levels in serum and small intestine mucosa were elevated after AMC water treatment (P < 0.01). Furthermore, AMC elevated the antioxidant mRNA levels of (P < 0.05) SOD 1-2, (P < 0.01) CAT, and (P < 0.01) GPX 1-2 in the small intestine. Likewise, the mRNA levels of the small intestine tight junction factors Occludin (P < 0.01), ZO-1 (P < 0.05), Claudin 2 (P < 0.01), and Claudin 5 (P<0.01) in the AMC treatment group were notably higher than those in the Con group. In conclusion, drinking water AMC supplementation has an accelerative effect on growth performance by elevating gut health by improving intestinal morphology, the inflammatory response, the antioxidant defense system, and barrier function in weaned piglets.
Keywords: alkaline mineral complex water, growth performance, intestinal barrier function, intestinal inflammation, weaned piglets
The results of this study provide a new strategy for alleviating weaning stress and elevating the growth rate of weaned piglets and highlight the application potential of drinking water alkaline mineral complex supplementation in swine production.
Introduction
Weaning is one of the most threatening but inevitable stressors for piglets (Upadhaya and Kim, 2021). The stress induced by weaning, especially within the first two weeks, can cause intestinal injury, immune system disorder, and digestive system dysfunction (Campbell et al., 2013), which then inhibit growth and decrease the feed intake of piglets. After weaning, piglets experience many stresses, such as transport stress (Johnson and Lay, 2017), handling stress, feed change (from liquid to solid) stress (Campbell et al., 2013), and social stress (Corbett et al., 2021), together with a gradual reduction in maternal antibodies (Hao et al., 2021). Meanwhile, for piglets, changes in the physical environment (space, building, farm, water supply, etc.) increase exposure to pathogens as well as dietary and environmental antigens. Therefore, the weaning stage is also known as the “immune blank period,” in which piglets have decreased disease resistance. They must adapt quickly to all these stressors to increase productivity and efficiency; however, when the stress exceeds a threshold that the piglet cannot overcome, it contributes to decreased performance and increased mortality (Xiong et al., 2019). It is well known that since the discovery of antibiotics, they have been extensively used in the pig industry as a promoter of growth at subtherapeutic doses, effectively alleviating the damage to piglets caused by weaning stress (Chen et al., 2020). However, a series of potential hazards caused by antibiotic application have also caused great concern in modern society, including bacterial resistance and residues (Chen et al., 2019). Therefore, finding an antibiotic alternative to alleviate the performance decline in weaned piglets is crucial for pork production and food security.
Water is an essential part of the diet and exerts a key role in nutrition (Dore et al., 2021). Alkaline minerals, such as Na, K, and Zn, are not only ample inorganic constituents in biota but are also the most studied elements because of their pivotal roles in biophysiological metabolism and catalytic processes (Nan et al., 2020). A previous study demonstrated that greater BW gain and lower morbidity were observed in animals treated with ionized alkaline mineral complex (AMC) water compared to untreated animals (Shin et al., 2014). The biologically beneficial and therapeutic effects of AMC water composed of various electrolytes have been demonstrated, such as controlling the proliferation of cancer cells (Pavelic et al., 2001), eliminating reactive oxygen species (ROS) in vivo (Zhu et al., 2021), and treating functional bowel disease and irritable bowel syndrome (Shin et al., 2018). The AMC water used in this study was an alkaline solution (pH 9.1) containing silicon, sodium, potassium, zinc, and germanium, and its properties are based on its mineral composition. Among these minerals, silicon has been linked to skin, hair, and nail health, bone mineralization, collagen production, Alzheimer’s disease, atherosclerosis, immune system augmentation, and a variety of other diseases and pharmacological effects (Jurkic et al., 2013). Silicon is an essential trace element related to longevity, and the content of metasilicic acid is one of the indicators used to identify natural mineral water. Unfortunately, the biologically beneficial effects of silicon appear to be overlooked. On the other hand, minerals are involved in digestion, biosynthesis, and many physiological processes, including maintaining the normal function of bones, muscles, the heart, and the brain (Domingo and Marques, 2021). Meanwhile, they are also key components in the generation of enzymes and hormones (Wang et al., 2020a), thus exerting a vital role in sustaining metabolism and homeostasis. Moreover, as a nonspecific immunostimulator, drinking water AMC supplementation has been widely used to promote the growth and development of pigs as early as 2001 (Choi et al., 2001). However, the biologically beneficial effects of AMC water on weaned piglets have not been evaluated.
The gut is one of the major target organs of weaning stress (Hu et al., 2018; Lauridsen, 2020). Given its multiple functions (Campbell et al., 2013), including digestion and absorption of electrolytes and nutrients, regulation of fluid transport, and secretion of mucins, immunoglobulins, and digestive enzymes, it serves as a host barrier against harmful pathogens and antigens. Hence, our study aimed to investigate the effects of drinking water AMC supplementation on growth performance, intestinal morphology, intestinal inflammatory response, intestinal immune and antioxidant function, and intestinal barrier integrity in weaned piglets.
Materials and Methods
Animal and experimental design
All experimental methods and humane end points for decreasing pain in animals were performed after ratification from the Guide for the Care and Use of Laboratory Animals at Northeast Agricultural University, Harbin, China. Animal research was performed in conformity with the procedures and rules of the laboratory of animals defined by the state council (Decree No. 676).
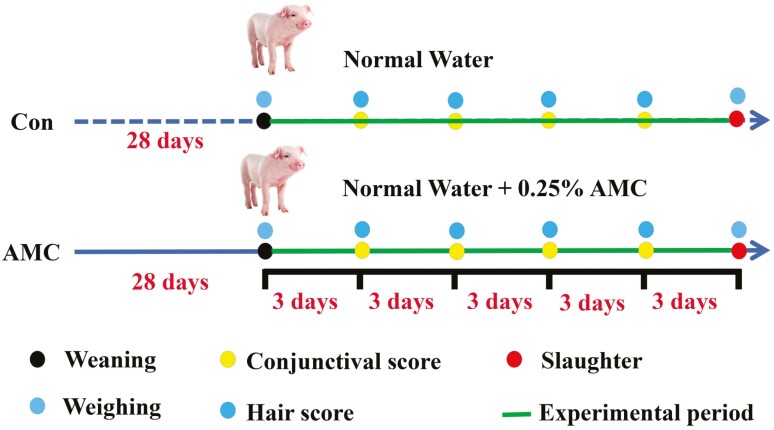
The experimental design and basic data collection details are shown in Figure 1. In total, 240 weaned piglets at 28 d of age (large white × landrace × Duroc) were randomly allocated into two groups (six replicate pens per group and 20 piglets per pen) based on body weight (BW; 9.35 ± 0.86 kg) and sex. All piglets were fed the same basal diet (NRC, 2012, Table 1). For ease of administration, and based on the ratio of the animal’s demand for the element, we employed an AMC concentrate, which contains sodium metasilicate pentahydrate (5H2O·Na2SiO3, 200 g/L), potassium bicarbonate (KHCO3, 100 g/L), zinc oxide (ZnO, 10 mg/L), and bis-(carboxyethylgermanium) sesquioxide (Ge-132, 1 mg/L). Piglets obtained water ad libitum from two different tanks that were fitted with a water meter, agitator, and a reactor system: one tank contained basal water plus 0.25% AMC concentrate (AMC, pH 9.1), and the second contained basal water without AMC supplementation (Con, pH 7.0). The calculated content of ions in AMC water and the analyzed content in basal water are shown in Table 2. The mineral content of AMC water used in this study was assessed based on our preliminary test, which showed that piglets had the best growth performance and lowest diarrhea rates (data not presented here) at this dose. All piglets were weaned at 28 d and had free access to food and water during this 15-d trial. All raw materials for AMC concentrate were purchased from Nail Biotechnology Co., Ltd, Beijing, China.
Figure 1.
The time flow chart for treatment and data collection.
Table 1.
Composition of the basal diet (as-fed basis)
| Item | Ingredient, % | Nutrient composition2 | Content, g/kg |
|---|---|---|---|
| Corn | 43.3 | DE(Mcal/kg) | 3.35 |
| Soybean meal | 25.4 | Crude protein (%) | 21.51 |
| Soy protein isolate | 3.00 | Lysine (%) | 1.41 |
| Whey powder | 7.50 | Methionine + Cystine (%) | 0.81 |
| Soybean oil | 1.50 | Threonine (%) | 0.94 |
| Lactose | 10.0 | Calcium (%) | 0.86 |
| Stone powder | 0.75 | Total phosphorus (%) | 0.70 |
| Calcium hydrogen phosphate | 1.05 | ||
| 50% choline chloride | 0.10 | ||
| Lysine | 0.22 | ||
| l-Methionine | 0.10 | ||
| l-Threonine | 0.08 | ||
| Vitamin and mineral premix1 | 1.00 | ||
| Total | 100 |
Vitamin and mineral premix supplied per kilogram diet: vitamin A, 18,000 IU; vitamin D, 4,000 IU; vitamin E, 50 mg; vitamin K3, 4 mg; vitamin B1, 4 mg; vitamin B2, 10 mg; vitamin B6, 4 mg; vitamin B12, 30 μg; pantothenic acid, 30 mg; folic acid, 2 mg; biotin, 0.16 mg; Fe, 150 mg; Cu, 18 mg; Mn, 48 mg; Zn, 150 mg; I, 1.5 mg; and Se, 0.3 mg.
Values were calculated according to NRC (2012).
Table 2.
The calculated content of ions in AMC water and analyzed content in basal water
| Ion ingredients | AMC water | Basal water |
|---|---|---|
| calculated contents, mg/L | analyzed contents, mg/L | |
| SiO32- | 179.25 | ND1 |
| Na+ | 108.49 | 2.79 |
| K+ | 97.50 | 0.95 |
| Zn2+ | 0.02 | ND |
| Ge4+ | 0.0005 | ND |
| HCO3- | 152.50 | 13.20 |
ND, not detected
Growth performance determination
The initial BW of all experimental piglets was determined on day 1 (weaning point), and the end of the trial (Day 15); four piglets were randomly selected from each pen to determine the final BW. Meanwhile, daily feed intakes were recorded every day. On the basis of these data, the average daily feed intake (ADFI), average daily gain (ADG), and the feed-to-gain (F:G) ratio were obtained.
Conjunctival score and hair score
The dorsal hair and conjunctiva were scored on two piglets randomly selected in each replicate (n = 12) every 3 d. The hair scores are defined as follows: 1 point, shiny and smooth hair; 2 points, slightly shiny and coarse hair; and 3 points, dull, rough, and dirty hair. The conjunctiva scoring of weaned piglets is as follows: 1 point, normal conjunctiva, and no tear stain; 2 points, normal conjunctiva with tear stain; 3 points, conjunctival flushing with tear stain.
Sample collection and gastrointestinal pH detection
On day 15, one piglet from each pen (n = 6) was randomly selected and slaughtered for sample collection. By jugular venipuncture, blood samples were obtained, and serum was obtained as described in a previous report (Chen et al., 2021). The piglets were euthanized, and the small intestine length was measured with a tape measure. The liver, spleen, and kidney were weighed with an electronic scale. Based on these data, the organ indices of the liver, spleen, and kidney and the relative length of the small intestine in piglets were calculated. The abdominal cavity was opened to directly collect the midsection of the duodenum, jejunum, and ileum. Meanwhile, the pH values of the stomach, midduodenum, midjejunum, midileum, midcecum, and midcolon contents were detected using a mobile pH detector (testo 205, Chunan Electronic Co., Ltd, Shanghai, China). Correspondingly sized sections were taken from the midduodenum, midjejunum, and midileum and fixed in 4% paraformaldehyde or 2.5% glutaraldehyde for paraffin section and scanning electron microscopy (SEM). A 10-cm section was snap-frozen in liquid nitrogen and then stored at –80 °C for various analyses. Mucosal samples from small intestines were collected by scraping with a glass slide.
Small intestine morphology analysis
Hematoxylin and eosin (H&E) staining and SEM were performed to analyze intestinal morphology as described previously (Yi et al., 2016; Dai et al., 2021). The SEM visualized using a Philips Model SU8010 FASEM (HITACHI, Japan). The villous height (VH) and crypt depth (CD) of each intestinal segment were measured with ImageJ software, and VH:CD ratio was calculated. A minimum of 10 villi from each sample were measured for each group.
Inflammatory marker detection
The levels of inflammatory markers (LPS/IL1β/IL6/TNF-α) in the small intestinal mucosa were measured using LPS (Beijing Chenglin Biological Technology Co. Ltd, China, AD11746Po), IL1β (Beijing Chenglin Biological Technology Co. Ltd, China, AD0125Po), IL-6 (Beijing Chenglin Biological Technology Co. Ltd, China, AD0120Po), and TNF-α (Beijing Chenglin Biological Technology Co. Ltd, China, AD0070Po) ELISA kits according to the manufacturer’s protocol.
Intestinal immune function evaluation
The levels of the gut immune marker sIgA in the small intestinal mucosa and serum were detected by a commercial kit (Beijing Chenglin Biological Technology Co. Ltd, China, AD12416Po).
Quantitative real-time PCR
Total RNA was extracted from midduodenum, midjejunum, and midileum tissues (100 mg) using RNAout reagent (Beijing Tiandi, Inc., Beijing, P.R. China) following the manufacturer’s description. The quality and concentration of the total RNA were determined spectrophotometrically at 260/280 nm. First-strand complementary DNA (cDNA) was produced from 4 μg total RNA using a commercial reagent kit (product category: AU311-02, TransGen Biotech, Beijing, China) following the manufacturer’s description. The cDNA was stored at −80 °C before the quantitative real-time PCR (qRT-PCR). The primers used in this study for qRT-PCR (Table 3) were designed by Primer Premier software 6.
Table 3.
Primers used in this study for qRT-PCR analysis
| Gene name1 | Accession number | Primer and probe sequences (5ʹ to 3ʹ)2 |
|---|---|---|
| GAPDH1 | NM_001206359.1 | F: TCGGAGTGAACGGATTTGGC R: TGACAAGCTTCCCGTTCTCC |
| GAPDH2 | NM_001206359.1 | F: CGGAGTGAACGGATTTGGC R: CACCCCATTTGATGTTGGCG |
| SOD-1 | NM_001190422.1 | F: AAGGCCGTGTGTGTGCTGAA R: AGTGGCCACACCATCTTTGC |
| SOD-2 | NM_214127.2 | F: GGCCTACGTGAACAACCTGA R: TGATTGATGTGGCCTCCACC |
| SOD-3 | NM_001078688.1 | F: TGACGCTGCTCTGTGCTTAC |
| R: AACTCCTGCCAGATCTCCGT | ||
| CAT | NM_214301.2 | F: CCTGCAACGTTCTGTAAGGC |
| R: GCTTCATCTGGTCACTGGCT | ||
| GPX-1 | NM_214201.1 | F: CCTAGCAGTGCCTAGAGTGC |
| R: CGCCCATCTCAGGGGATTTT | ||
| GPX-2 | NM_001115136.1 | F: CTGGACGGGGAGAAGGTAGA |
| R: CGGACGTACTTGAGGCTGTT | ||
| ZO-1 | XM_021098856.1 | F: TCAAGGTCTGCCGAGACAAC |
| R: ATCACAGTGTGGTAAGCGCA | ||
| Claudin-2 | NM_001161638.1 | F: AACGAGTTCTTACGTCGGGG |
| R: CGAGGAGATGGCGCTAGATG | ||
| Claudin-5 | NM_001161636.1 | F: CCTGTCAAGTATTCGGCCCC |
| R: CGACACCCTCAGACGTAGTT | ||
| Occludin | NM_001163647.2 | F: CAGGTGCACCCTCCAGATTG |
| R: ATGTCGTTGCTGGGTGCATA |
GAPDH, Housekeeping gene; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; ZO-1, zonula occludens-1.
F, forward; R, reverse.
Statistical analysis
All statistical data were analyzed with GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA) software. Statistical analysis was performed using Student’s t-tests to compare differences between the two groups. A significant difference and extremely significant difference were considered if P < 0.05 and P < 0.01, respectively, and a trend was considered if the P-value was between 0.05 and 0.10.
Results
Growth performance
As shown in Table 4, under the precondition of no difference (P > 0.05) in initial BW, drinking water supplementation with AMC significantly improved the final BW (P < 0.01) and ADG (P < 0.01) in weaned piglets compared to the Con group. However, no changes in ADFI were observed (P > 0.05) between the Con and AMC groups. In the field of economic benefits, AMC treatment significantly reduced the F:G (P < 0.01) ratio. These results indicated that AMC water promoted growth performance in piglets under weaning stress. Importantly, although there was no significant change in the ADFI, the economic benefit was significantly improved by decreasing the F:G ratio.
Table 4.
Effect of AMC water on the growth performance of weaned piglets1
| Item2 | Con | AMC | SD | P-value |
|---|---|---|---|---|
| BW, kg | ||||
| Initial BW | 9.22 | 9.49 | 0.86 | 0.274 |
| Final BW | 12.29A | 14.37B | 1.00 | <0.0001 |
| ADG, kg/d | 0.20A | 0.33B | 0.05 | <0.0001 |
| ADFI, g/d | 335.7 | 379.7 | 200.65 | 0.554 |
| F:G ratio | 1.70A | 1.20B | 0.05 | <0.0001 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups (n = 24); Data are presented as the mean and SD.
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; F:G, feed to gain ratio.
Means in a row with different superscripts differ significantly (P < 0.01).
Health status
To evaluate the health status of weaned piglets, we regularly scored dorsal hair and conjunctiva. As presented in Table 5, compared to the Con group, the hair score of the AMC treatment group was notably decreased on Days 6 (P < 0.01) and 12 (P < 0.05). Meanwhile, a decreasing trend of hair score was observed in the AMC group on Day 15 (P = 0.080). Likewise, the conjunctival score was apparently reduced (P < 0.01) on Day 6 after AMC water treatment, but no difference was recorded on the other experimental days (P > 0.05). These results suggested that treating piglets under weaning stress with AMC water has a positive effect on health.
Table 5.
Effect of AMC water on the hair score and conjunctival score of weaned piglets1
| Item | Con | AMC | SD | P-value |
|---|---|---|---|---|
| Hair score | ||||
| Day 3 | 1.80 | 1.70 | 0.56 | 0.696 |
| Day 6 | 1.90A | 1.20B | 0.49 | 0.006 |
| Day 9 | 1.90 | 1.50 | 0.64 | 0.180 |
| Day12 | 1.70a | 1.20b | 0.45 | 0.024 |
| Day15 | 1.70 | 1.30 | 0.48 | 0.080 |
| Conjunctival score | ||||
| Day 3 | 1.20 | 1.20 | 0.42 | >0.999 |
| Day 6 | 1.70A | 1.10B | 0.40 | 0.004 |
| Day 9 | 1.60 | 1.30 | 0.50 | 0.196 |
| Day12 | 1.60 | 1.40 | 0.51 | 0.3979 |
| Day15 | 1.50 | 1.20 | 0.47 | 0.177 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups (n = 12); Data are presented as the mean and SD.
Means in a row with different superscripts differ significantly (P < 0.05).
Means in a row with different superscripts differ significantly (P < 0.01).
Relative length of the small intestine and organ indices
As shown in Table 6, compared to the Con group, water supplementation with AMC did not significantly affect the relative length of the small intestine (P > 0.05) or the organ indices of the liver, spleen, or kidney in weaned piglets (P > 0.05).
Table 6.
Effect of AMC water on the relative length of the small intestine and organ indices of weaned piglets1
| Item | Con | AMC | SD | P-value |
|---|---|---|---|---|
| Relative length | ||||
| Small intestine | 0.97 | 0.98 | 0.04 | 0.796 |
| Organs indexes | ||||
| Liver | 1.00 | 0.94 | 0.10 | 0.311 |
| Spleen | 1.00 | 0.85 | 0.18 | 0.176 |
| Kidney | 1.00 | 0.99 | 0.09 | 0.856 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups (N = 6); Data are presented as the mean and SD.
Means in a row with different superscripts differ significantly (P < 0.05).
Means in a row with different superscripts differ significantly (P < 0.01).
Gastrointestinal pH value
The gastrointestinal pH value is an important parameter for intestinal homeostasis (Table 7). As an alkaline liquid, there was no obvious difference in the pH value of the stomach and small intestinal contents (including the duodenum, jejunum, and ileum) or the contents of the cecum and colon in piglets of the AMC treatment group compared to the Con group (P>0.05).
Table 7.
Effect of AMC water on the gastrointestinal pH value of weaned piglets1
| Item | Con | AMC | SD | P-value |
|---|---|---|---|---|
| Digestive tract pH | ||||
| Stomach | 2.62 | 2.74 | 0.56 | 0.796 |
| Duodenum | 5.41 | 6.10 | 0.72 | 0.259 |
| Jejunum | 6.20 | 6.56 | 0.25 | 0.067 |
| Ileum | 6.86 | 6.92 | 0.20 | 0.625 |
| Cecum | 6.41 | 6.25 | 0.31 | 0.407 |
| Colon | 6.28 | 6.30 | 0.27 | 0.921 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups (N = 6); Data are presented as the mean and SD.
Means in a row with different superscripts differ significantly (P < 0.05).
Means in a row with different superscripts differ significantly (P < 0.01).
Intestinal morphology
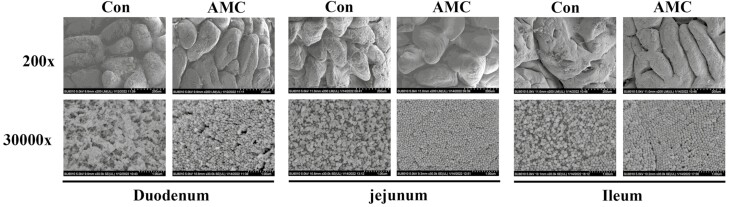
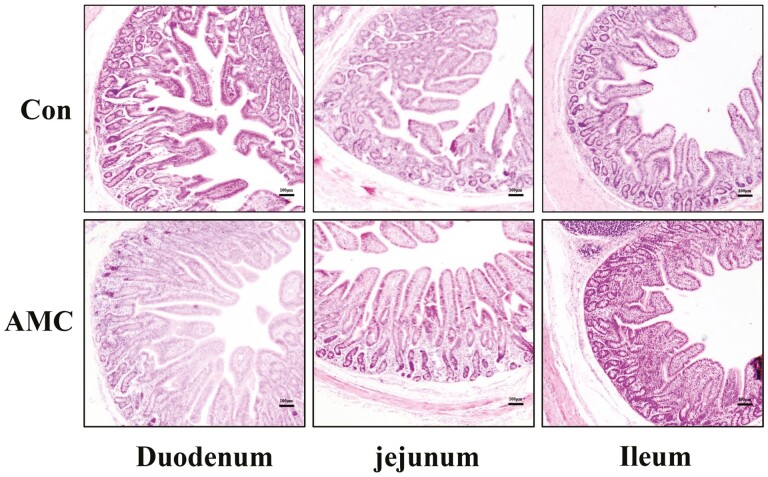
SEM and H&E staining were used to evaluate the effect of AMC treatment on morphological injury of the small intestinal epithelium in weaned piglets. SEM at 200x magnification revealed that surface injury to villi in the duodenum, jejunum, and ileum was alleviated by AMC water (Figure 2). Interestingly, AMC also promoted the number of microvilli in the small intestine, manifesting as a more tidy and dense microvilli morphology at 30,000 times magnification. This result was also supported by H&E staining (Figure 3). As shown in Table 8, AMC treatment markedly increased VH in the duodenum (P < 0.01), jejunum (P < 0.05), and ileum (P < 0.05) compared to the Con group. However, there was no significant difference in CD in the small intestine between the Con and AMC groups (P > 0.05). Accordingly, the VH:CD ratio was obviously increased in the duodenum (P < 0.01), jejunum (P < 0.05), and ileum (P < 0.01) of weaned piglets after AMC treatment. These results demonstrated that AMC ameliorated the surface injury to villi in the small intestine and repaired the intestinal damage of piglets induced by weaning stress.
Figure 2.
The effect of AMC water on small intestinal morphology shown by SEM. Upper, SEM at 200 times magnification; Nether, SEM at 30,000 times magnification.
Figure 3.
The effect of AMC water on small intestinal morphology shown by H&E staining.
Table 8.
Effect of AMC water on the intestinal morphology of weaned piglets1
| Item2 | Con | AMC | SD | P-value |
|---|---|---|---|---|
| Duodenum | ||||
| VH, μm | 321.10A | 464.60B | 62.17 | <0.0001 |
| CD, μm | 161.30 | 141.70 | 47.28 | 0.369 |
| VH:CD | 1.99A | 3.28B | 0.42 | <0.0001 |
| Jejunum | ||||
| VH, μm | 352.90a | 416.10b | 56.05 | 0.023 |
| CD, μm | 151.50 | 149.60 | 31.35 | 0.900 |
| VH:CD | 2.33a | 2.78b | 0.37 | 0.016 |
| Ileum | ||||
| VH, μm | 272.30a | 327.80b | 49.65 | 0.022 |
| CD, μm | 120.60 | 105.60 | 35.72 | 0.248 |
| VH:CD | 2.29A | 3.02B | 0.44 | 0.001 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups N =10); Data are presented as the mean and SD.
VH, villus height; CD, crypt depth; VH:CD, villus height to crypt depth ratio.
Means in a row with different superscripts differ significantly (P < 0.05).
Means in a row with different superscripts differ significantly (P < 0.01).
Intestinal inflammatory marker levels
Activation of the inflammatory response and LPS activity is a hallmark of weaning stress-induced intestinal damage in piglets. As shown in Table 9, AMC water significantly reduced the mucosal level of LPS in the duodenum, jejunum, and ileum (P < 0.01). Meanwhile, the levels of IL1β (P<0.05) and TNF-α (P<0.05) in the small intestinal mucosa were markedly decreased after AMC water treatment. Similarly, a significant decrease in IL6 levels (P < 0.01) was noticed in the duodenal and jejunal mucosa of piglets treated with AMC water, whereas only a decreasing trend was observed in the ileal mucosa (P = 0.067). These results showed that AMC water effectively reduced intestinal inflammation induced by weaning stress in piglets.
Table 9.
Effect of AMC water on small intestinal inflammatory markers of weaned piglets1
| Item2 | Con | AMC | SD | P-value |
|---|---|---|---|---|
| Duodenum | ||||
| LPS, ng/L | 262.10A | 188.80B | 27.30 | 0.001 |
| IL1β, ng/L | 141.30a | 104.40b | 24.21 | 0.025 |
| IL6, ng/L | 57.83A | 39.28B | 5.94 | 0.001 |
| TNF-α, ng/L | 97.83a | 84.82b | 7.79 | 0.021 |
| Jejunum | ||||
| LPS, ng/L | 291.00A | 166.80B | 33.91 | <0.0001 |
| IL1β, ng/L | 153.50A | 106.80B | 18.54 | 0.002 |
| IL6, ng/L | 56.00A | 39.45B | 7.46 | 0.007 |
| TNF-α, ng/L | 102.30a | 84.82b | 11.13 | 0.024 |
| Ileum | ||||
| LPS, ng/L | 326.90A | 250.30B | 26.86 | 0.0006 |
| IL1β, ng/L | 138.50A | 98.43B | 8.47 | <0.0001 |
| IL6, ng/L | 49.33 | 41.11 | 6.88 | 0.067 |
| TNF-α, ng/L | 103.80a | 88.16b | 11.75 | 0.045 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups (N = 6); data are presented as the mean and SD.
LPS, lipopolysaccharide; IL1β, interleukin 1β; IL6, interleukin 6; TNF-α, tumor necrosis factor-alpha.
Means in a row with different superscripts differ significantly (P < 0.05).
Means in a row with different superscripts differ significantly (P < 0.01).
Intestinal immune function
sIgA is considered an immune barrier on the mucosal surface, and can effectively inhibit pathogen adhesion, colonization, and invasion. Surprisingly, the mucosa level of sIgA in the duodenum, jejunum, and ileum was prominently elevated after treatment with AMC water (Table 10). The results of sIgA levels obtained in this study indicated that AMC treatment could improve the mucosal immune barrier by promoting the secretion of sIgA in the small intestine of weaned piglets.
Table 10.
Effect of AMC water on intestinal immune function markers of weaned piglets
| Item2 | Con | AMC | SD | P-value |
|---|---|---|---|---|
| sIgA, µg/mL | ||||
| Serum | 9.17A | 13.89B | 0.74 | <0.0001 |
| Duodenum | 7.43A | 9.80B | 0.79 | 0.0005 |
| Jejunum | 7.95A | 9.29B | 0.45 | 0.0004 |
| Ileum | 8.64A | 10.34B | 0.85 | 0.007 |
Statistical significance was determined using Student’s t-tests to compare differences between two groups (N = 6); Data are presented as the mean and SD.
sIgA secretory immunoglobulin A.
Means in a row with different superscripts differ significantly (P < 0.01).
Intestinal expression of genes related to antioxidant enzymes
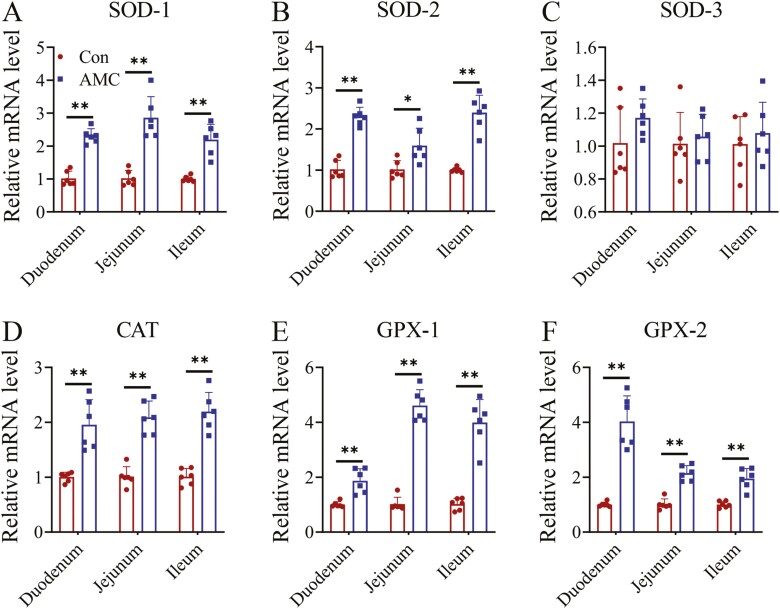
In this study, the effect of AMC on the antioxidant defense system was determined by analyzing the mRNA levels of key antioxidants, including SOD 1-3, CAT, and GPX 1-2 (Figure 4). Compared to the Con group, the relative expression levels of SOD-1 and SOD-2 in the duodenum (P < 0.01), jejunum (P < 0.05), and ileum (P < 0.01) were markedly increased by AMC treatment, while no change was noticed in the SOD-3 mRNA level (P > 0.05). Likewise, water supplementation with AMC significantly boosted the mRNA expression of peroxidases, including CAT, GPX-1, and GPX-2, in the small intestine (P < 0.01). These results suggested that the repair of weaning stress-induced piglet intestinal damage by AMC may be related to the enhancement of the antioxidant defense system.
Figure 4.
The relative mRNA expression levels of antioxidant-related genes in the small intestine of weaned piglets. (A) SOD-1, (B) SOD-2, (C) SOD-3, (D) CAT, (E) GPX-1, and (F) GPX-2. Data are presented as the means ± SD (n = 6 per group). Statistical significance was determined using Student’s t-tests to compare differences between two groups. *P < 0.05, **P < 0.01.
Intestinal expression of genes related to barrier function
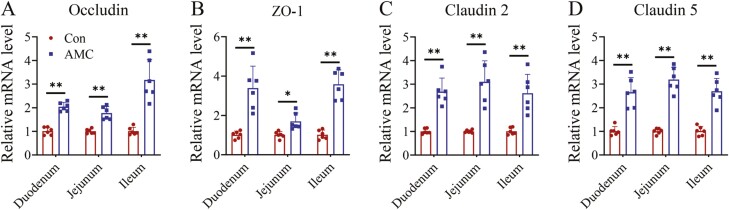
Small intestinal tight junction function was determined by measuring the Occludin, ZO-1, Claudin-2, and Claudin-5 expression in the duodenum, jejunum, and ileum. As shown in Figure 5, the mRNA levels of Occludin, Claudin-2, and Claudin-5 were extremely significantly elevated in the small intestine of weaned piglets after AMC treatment (P < 0.01). Additionally, the relative mRNA expression of ZO-1 was remarkably promoted in the duodenum (P < 0.01), jejunum (P < 0.05), and ileum (P < 0.01) when the piglets were treated with AMC water. These results revealed that AMC improved gut barrier function by increasing the expression of tight junction-related genes.
Figure 5.
The relative mRNA expression levels of intestinal epithelium integrity-related genes in the small intestine of weaned piglets. (A) Occludin, (B) ZO-1, (C) Claudin 2, and (D) Claudin 5. Data are presented as the means ± SD (n = 6 per group). Statistical significance was determined using Student’s t-tests to compare differences between two groups. *P < 0.05, **P < 0.01.
Discussion
ADG and ADFI are important parameters for evaluating the growth performance and health status of piglets, which are closely related to the economic benefits of the farm (Bai et al., 2021). Weaning stress induces reduced appetite, decreased feed intake, and inhibited growth in piglets and, in more severe cases, leads to secondary infection and even death of piglets (Sun et al., 2020). Since antibiotics have been banned from feed additives, veterinarians, and livestock practitioners have been looking for effective alternatives. AMC is considered one of the most promising alternatives to antibiotics (Koo et al., 2006; Shin et al., 2014). Previous studies have shown that drinking water supplemented with AMC improves growth performance in a variety of animal models, including piglets (Park et al., 2002), cattle (Kim et al., 2018), ostriches (Seyfori et al., 2018), and olive flounder (Shin et al., 2014). In agreement with these studies, our results also indicated that drinking water supplementation with AMC increased piglet growth performance, as evidenced by the promoted ADG and decreased F:G ratio. Of note, the improved performance of weaned piglets may be associated with improved overall piglet health.
After weaning, most piglets are in a subhealthy state, showing messy and rough hair, tear stains in the conjunctiva of the eye, and even color changes due to disease factors (Campbell et al., 2013). To determine whether AMC water treatment could improve piglet health, hair scoring and conjunctival scoring were performed in this study. Surprisingly, we observed that AMC water significantly decreased the hair score and conjunctival score of weaned piglets on day 6 after weaning, suggesting that AMC has the potential to ameliorate the subhealthy state of weaned piglets. On the other hand, the relative intestinal length (Wang et al., 2020b) and organ index (Yu et al., 2021) can partly reflect the growth and development of piglets. In this study, there was no significant difference in the relative length of the small intestine and indices of the liver, spleen, and kidney in weaned piglets. The period of this trial was 15 d, which may explain the abovementioned changes in the length of the small intestine and organ indices. We speculated that the beneficial effects of AMC in promoting intestinal and organ development may be amplified with prolonged treatment. It is worth emphasizing again that the main purpose of this study was to evaluate the effect of AMC water on the growth performance and intestinal health of weaning-stressed piglets. Thus, we may extend the experimental period in future studies to explore the effect of AMC water on the gut or organ development of piglets.
It has been recognized that an acidic gut environment may be more conducive to gut health (Williams, 2010), leading to the subconscious belief that alkaline substances may damage the gut. A previous study on AMC showed that AMC water has a protective effect on ethanol-induced hemorrhagic gastric injury in mice (Nassini et al., 2010). The mucus-bicarbonate-phospholipid “barrier,” which is made up of mucus gel, bicarbonate, and surfactant phospholipids, is the first line of defense against gastrointestinal tissue injury (Kao and Lichtenberger, 1991). Thus, the statement that a more acidic internal environment is better for gastrointestinal health is not absolute. Our results indicated that there was no difference in the pH values of the stomach and gut lumen, which may be attributed to the strong humoral buffering system in mammals.
Due to the immature digestive system and low nutrient digestibility, changes in structure and physiological function (enzymatic activity and absorption or secretion) in the gut occur in weaned piglets (Gu et al., 2017). The majority of studies showed that long-lasting structural and functional changes in the small intestine were induced after weaning, including villous atrophy and increased CD (Pluske et al., 1997; Boudry et al., 2004). The SEM result showed that weaning caused marked injury to the epithelium and a decrease in microvilli density in all small intestine segments of piglets, whereas these changes were reversed by AMC treatment. Meanwhile, intestinal VH and the VH/CD ratio are the gold standard for assessing intestinal morphology (Xie et al., 2021). It plays an essential role in nutrient absorption and provides a protective barrier. Moreover, the reduction in VH/CD caused by weaning can cause malabsorption, which in turn hinders intestinal functional repair (Song et al., 2018; Wang et al., 2019). The intestinal H&E staining results in this study proved that oral administration of AMC to weaned piglets had a better effect on VH and the VH:CD ratio in the duodenum, jejunum, and ileum, which are beneficial to intestinal function. Emerging evidence indicates that intestinal epithelial injury is usually accompanied by bacterial translocation, leading to a dramatic inflammatory response, oxidative stress, and metabolic endotoxemia (Ma et al., 2021). Our results indicated that the levels of LPS and proinflammatory factors, such as IL1β, IL6, and TNF-α, were significantly reduced in the weaned piglet small intestine after AMC treatment, which may be related to AMC promoting the secretion of sIgA in the small intestine (Noval Rivas et al., 2019). Moreover, AMC water alleviated the oxidative stress induced by weaning or inflammation by boosting the transcriptional levels of antioxidant enzymes, including SOD 1-2, CAT, and GPX 1-2, accordingly enhancing the gut antioxidant defense system of weaned piglets. The alleviation of the inflammatory response and oxidative stress may be linked to the increase in intestinal goblet cell number by AMC treatment (Shin et al., 2014), whose main function is to synthesize and secrete mucins to form a mucosal barrier to protect epithelial cells.
On the other hand, the alteration of LPS levels and mRNA expression of proinflammatory factors and antioxidant enzymes also indirectly supported the improvement of the intestinal epithelial barrier. The promotion of small gut epithelial barrier function may be a consequence of elevated gut morphology, immunological function, and lower inflammation levels in weaned piglets (Barbara et al., 2021). The breakdown of the intestinal barrier provides favorable conditions for the proliferation and invasion of pathogens and exacerbates more severe injury (Xie et al., 2021). In this study, we found that the mRNA expression levels of tight junction proteins, including Occludin, ZO-1, Claudin2, and Claudin5, in the duodenum, jejunum and ileum were significantly increased by AMC water treatment. Tight junctions are structures that connect epithelial cells, giving them the function of sealing paracellular spaces between cells, accordingly limiting bacterial toxins and pathogens. A variety of transmembrane and cytoplasmic proteins, including Occludins, Claudins, and ZOs, are important components of tight junctions, which interact and form complex closed structures with the cytoskeleton (Chelakkot et al., 2018). A study found that the increase in intestinal permeability in weaned piglets was negatively correlated with the mRNA expression of Occludin, Claudins, and ZO-1 in the intestinal epithelium (Hu et al., 2013). Therefore, our results demonstrated that AMC water plays a crucial role in maintaining intestinal permeability in weaned piglets, and the mechanism may involve promotion of the expression of tight junction proteins.
In conclusion, our study found that drinking water AMC supplementation could effectively enhance growth performance by alleviating the inflammatory response, promoting the immune barrier and antioxidant defense system, improving small intestinal morphology, and maintaining small intestinal barrier function in weaned piglets. Further studies should be performed to determine the optimal dose of AMC supplementation.
Acknowledgments
This study has received assistance from National Natural Science Foundation of China (No. 32172932), Key Program of Natural Science Foundation of Heilongjiang Province of China (No. ZD2021C003), China Agriculture Research System of MOF and MARA (No. CARS-35), Distinguished Professor of Longjiang Scholars Support Project (No. T201908) and Heilongjiang Touyan Innovation Team Program. We acknowledge Qing-Lin Li, Dr. Song Qi, and all members of Nail Biotechnology Co. Ltd (Beijing, China) for their assistance in this study.
Glossary
Abbreviations
- AMC
alkaline mineral complex
- ADG
average daily gain
- ADFI
average daily feed intake
- BW
body weight
- CAT
catalase
- Con
Control
- CD
crypt depth
- cDNA
complementary DNA
- F: G
feed to gain ratio
- GPX
glutathione peroxidase
- H&E staining
Hematoxylin and Eosin staining
- IL1β
interleukin1β
- IL6
interleukin6
- LPS
lipopolysaccharide
- qRT-PCR
quantitative Real-Time polymerase chain reaction
- ROS
reactive oxygen species
- sIgA
secretory immunoglobulin A
- SOD
superoxide dismutase
- SEM
scanning electron microscopy
- TNF-α
tumor necrosis factor-alpha
- VH
villous height
- ZO-1
zonula occludens-1
Contributor Information
Jian Chen, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Ya-Ru Xu, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Jian-Xun Kang, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Bi-Chen Zhao, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Xue-Yan Dai, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Bai-Hao Qiu, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Jin-Long Li, College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, P.R. China; Key Laboratory of the Provincial Education Department of Heilongjiang for Common Animal Disease Prevention and Treatment, Northeast Agricultural University, Harbin 150030, P.R. China; Heilongjiang Key Laboratory for Laboratory Animals and Comparative Medicine, Northeast Agricultural University, Harbin 150030, P.R. China.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Bai, K., Jiang L., Li Q., Zhang J., Zhang L., and Wang T.. . 2021. Dietary dimethylglycine sodium salt supplementation improves growth performance, redox status, and skeletal muscle function of intrauterine growth-restricted weaned piglets. J. Anim. Sci. 99(7):skab186. doi: 10.1093/jas/skab186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara, G., Barbaro M. R., Fuschi D., Palombo M., Falangone F., Cremon C., Marasco G., and Stanghellini V.. . 2021. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front. Nutr 8:718356. doi: 10.3389/fnut.2021.718356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry, G., Peron V., Le Huerou-Luron I., Lalles J. P., and Seve B.. . 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 134:2256–2262. doi: 10.1093/jn/134.9.2256 [DOI] [PubMed] [Google Scholar]
- Campbell, J. M., Crenshaw J. D., and Polo J.. . 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechno. 4:19. doi: 10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelakkot, C., Ghim J., and Ryu S. H.. . 2018. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 50:1–9. doi: 10.1038/s12276-018-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Ying G. G., and Deng W. J.. . 2019. Antibiotic residues in food: extraction, analysis, and human health concerns. J. Agric. Food Chem. 67:7569–7586. doi: 10.1021/acs.jafc.9b01334 [DOI] [PubMed] [Google Scholar]
- Chen, J., Mao Y., Xing C., Hu R., Xu Z., Cao H., and Luo J.. . 2020. Traditional chinese medicine prescriptions decrease diarrhea rate by relieving colonic inflammation and ameliorating caecum microbiota in piglets. Evid. Based. Complement. Alternat. Med. 2020:3647525. doi: 10.1155/2020/3647525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Xie Y., Zhong R., Han H., Liu L., Chen L., Zhang H., Beckers Y., and Everaert N.. . 2021. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J. Anim. Sci. 99. doi: 10.1093/jas/skab183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, I. S., Shin S. J., and Yoo H. S.. . 2001. Modulatory effects of ionized alkali mineral complex (IAMC) on mRNA expression of porcine cytokines. J. Vet. Med. Sci. 63:1179–1182. doi: 10.1292/jvms.63.1179 [DOI] [PubMed] [Google Scholar]
- Corbett, R. J., Luttman A. M., Wurtz K. E., Siegford J. M., Raney N. E., Ford L. M., and Ernst C. W.. . 2021. Weaning induces stress-dependent DNA methylation and transcriptional changes in piglet PBMCs. Front. Genet. 12:633564. doi: 10.3389/fgene.2021.633564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, X. Y., Li X. W., Zhu S. Y., Li M. Z., Zhao Y., Talukder M., Li Y. H., and Li J. L.. . 2021. Lycopene ameliorates Di(2-ethylhexyl) phthalate-induced pyroptosis in spleen via suppression of classic caspase-1/NLRP3 pathway. J. Agric. Food Chem. 69:1291–1299. doi: 10.1021/acs.jafc.0c06534 [DOI] [PubMed] [Google Scholar]
- Domingo, J. L., and Marques M.. . 2021. The effects of some essential and toxic metals/metalloids in COVID-19: A review. Food Chem. Toxicol. 152:112161. doi: 10.1016/j.fct.2021.112161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore, M. P., Pes G. M., and Realdi G.. . 2021. Health properties of the Italian San Martino® mineral-rich water: a self-controlled pilot study. Biomed. Pharmacother. 138:111509. doi: 10.1016/j.biopha.2021.111509 [DOI] [PubMed] [Google Scholar]
- Gu, Y., Song Y., Yin H., Lin S., Zhang X., Che L., Lin Y., Xu S., Feng B., Wu D., . et al. 2017. Dietary supplementation with tributyrin prevented weaned pigs from growth retardation and lethal infection via modulation of inflammatory cytokines production, ileal expression, and intestinal acetate fermentation. J. Anim. Sci. 95:226–238. doi: 10.2527/jas.2016.0911 [DOI] [PubMed] [Google Scholar]
- Hao, Y., Wang J., Teng D., Wang X., Mao R., Yang N., and Ma X.. . 2021. A prospective on multiple biological activities of lactoferrin contributing to piglet welfare. Biochem. Cell Biol. 99:66–72. doi: 10.1139/bcb-2020-0078 [DOI] [PubMed] [Google Scholar]
- Hu, C. H., Xiao K., Luan Z. S., and Song J.. . 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi: 10.2527/jas.2012-5796 [DOI] [PubMed] [Google Scholar]
- Hu, J., Ma L., Nie Y., Chen J., Zheng W., Wang X., Xie C., Zheng Z., Wang Z., Yang T., . et al. 2018. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe. 24:817–832.e8 e818. doi: 10.1016/j.chom.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Johnson, J. S., and Lay D. C.. . 2017. Evaluating the behavior, growth performance, immune parameters, and intestinal morphology of weaned piglets after simulated transport and heat stress when antibiotics are eliminated from the diet or replaced with l-glutamine. J. Anim. Sci. 95:91–102. doi: 10.2527/jas.2016.1070. [DOI] [PubMed] [Google Scholar]
- Jurkic, L. M., Cepanec I., Pavelic S. K., and Pavelic K.. . 2013. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: new perspectives for therapy. Nutr. Metab. (Lond) 10:2. doi: 10.1186/1743-7075-10-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, Y. C., and Lichtenberger L. M.. . 1991. Phospholipid- and neutral lipid-containing organelles of rat gastroduodenal mucous cells. Possible origin of the hydrophobic mucosal lining. Gastroenterology 101:7–21. doi: 10.1016/0016-5085(91)90454-s. [DOI] [PubMed] [Google Scholar]
- Kim, T. I., Lim D. H., Jang S. S., Kim S. B., Park S. M., Park J. H., Ki K. S., and Mayakrishnan V.. . 2018. Effects of supplementing Barodon, Bacillus subtilis, and Ampbio on growth performance, biochemical metabolites, and hormone levels in Korean native heifers. Trop. Anim. Health Prod. 50:1637–1643. doi: 10.1007/s11250-018-1606-7. [DOI] [PubMed] [Google Scholar]
- Koo, H., Ryu S. H., Ahn H. J., Jung W. K., Park Y. K., Kwon N. H., Kim S. H., Kim J. M., Yoo B. W., Choi S. I., . et al. 2006. Immunostimulatory effects of the anionic alkali mineral complex Barodon on equine lymphocytes. Clin. Vaccine Immunol. 13:1255–1266. doi: 10.1128/CVI.00150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen, C. 2020. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 98(4):skaa086. doi: 10.1093/jas/skaa086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X., Zhang Y., Xu T., Qian M., Yang Z., Zhan X., and Han X.. . 2021. Early-life intervention using exogenous fecal microbiota alleviates gut injury and reduce inflammation caused by weaning stress in piglets. Front. Microbiol. 12:671683. doi: 10.3389/fmicb.2021.671683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan, H., Zhao L., Yang F., Liu Y., Xiao Z., Cao X., and Qiu H.. . 2020. Different alkaline minerals interacted with biomass carbon during pyrolysis: which one improved biochar carbon sequestration? J. Clean Prod. 255:120162. doi: 10.1016/j.jclepro.2020.120162 [DOI] [Google Scholar]
- Nassini, R., Andre E., Gazzieri D., De Siena G., Zanasi A., Geppetti P., and Materazzi S.. . 2010. A bicarbonate-alkaline mineral water protects from ethanol-induced hemorrhagic gastric lesions in mice. Biol. Pharm. Bull. 33:1319–1323. doi: 10.1248/bpb.33.1319 [DOI] [PubMed] [Google Scholar]
- Noval Rivas, M., Wakita D., Franklin M. K., Carvalho T. T., Abolhesn A., Gomez A. C., Fishbein M. C., Chen S., Lehman T. J., Sato K., . et al. 2019. Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation. Immunity 51:508–521 e506. doi: 10.1016/j.immuni.2019.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington, DC: National Academy Press. [Google Scholar]
- Park, B. K., Lyoo K. S., Park Y. H., Koh J. H., and Seo K.. . 2002. Host immune responses against hog cholera virus in pigs treated with an ionized alkali mineral complex. J. Vet. Sci. 3:315–319. doi: 10.4142/jvs.2002.3.4.315 [DOI] [PubMed] [Google Scholar]
- Pavelic, K., Hadzija M., Bedrica L., Pavelic J., Dikic I., Katic M., Kralj M., Bosnar M. H., Kapitanovic S., Poljak-Blazi M., . et al. 2001. Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J. Mol. Med. (Berl.) 78:708–720. doi: 10.1007/s001090000176 [DOI] [PubMed] [Google Scholar]
- Pluske, J. R., Hampson D. J., and Williams I. H.. . 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- Seyfori, H., Ghasemi H. A., Hajkhodadadi I., Nazaran M. H., and Hafizi M.. . 2018. Growth performance, mineral digestibility, and blood characteristics of ostriches receiving drinking water supplemented with varying levels of chelated trace mineral complex. Biol. Trace Elem. Res. 183:147–155. doi: 10.1007/s12011-017-1117-9 [DOI] [PubMed] [Google Scholar]
- Shin, C. H., Cha J. H., Rahimnejad S., Jeong J. B., Yoo B. W., Lee B. K., Ahn H. J., Choi S. I., Choi Y. J., Park Y. H., . et al. 2014. Effects of dietary supplementation of Barodon, an anionic alkali mineral complex, on growth performance, feed utilization, innate immunity, goblet cell and digestibility in Olive Flounder (Paralichthys olivaceus). Asian-Australas. J. Anim. Sci. 27:383–390. doi: 10.5713/ajas.2013.13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, D. W., Yoon H., Kim H. S., Choi Y. J., Shin C. M., Park Y. S., Kim N., and Lee D. H.. . 2018. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: A randomized double-blind, placebo-controlled pilot study. Evid. Based. Complement. Alternat. Med 2018:9147914. doi: 10.1155/2018/9147914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y. M., Kim M. H., Kim H. N., Jang I., Han J. H., Fontamillas G. A., Lee C. Y., and Park B. C.. . 2018. Effects of dietary supplementation of lipid-coated zinc oxide on intestinal mucosal morphology and expression of the genes associated with growth and immune function in weanling pigs. Asian Austral. J. Anim. 31(3):403–409. doi: 10.5713/ajas.17.0718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Z., Li H., Li Y., and Qiao J.. . 2020. Lactobacillus salivarius, a potential probiotic to improve the health of LPS-challenged piglet intestine by alleviating inflammation as well as oxidative stress in a dose-dependent manner during weaning transition. Front. Vet. Sci 7:547425. doi: 10.3389/fvets.2020.547425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhaya, S. D., and Kim I. H.. . 2021. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets-A review. Animals (Basel) 11(8):2418. doi: 10.3390/ani11082418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. W., Liu J., Zhao W. P., Zhang Z. H., Li S. Q., Li S. H., Zhu S. Q., and Zhou B. H.. . 2019. Effect of fluoride on small intestine morphology and serum cytokine contents in rats. Biol. Trace Elem. Res. 189(2):511–518. doi: 10.1007/s12011-018-1503-y [DOI] [PubMed] [Google Scholar]
- Wang, C., Zhang R., Wei X., Lv M., and Jiang Z.. . 2020a. Metalloimmunology: The metal ion-controlled immunity. Adv. Immunol. 145:187–241. doi: 10.1016/bs.ai.2019.11.007 [DOI] [PubMed] [Google Scholar]
- Wang, M., Yang C., Wang Q. Y., Li J. Z., Li Y. L., Ding X. Q., Yin J., Yang H. S., and Yin Y. L.. . 2020b. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal 14:1196–1203. doi: 10.1017/S175173111900288X [DOI] [PubMed] [Google Scholar]
- Williams, N. T. 2010. Probiotics. Am. J. Health Syst. Pharm. 67:449–458. doi: 10.2146/ajhp090168 [DOI] [PubMed] [Google Scholar]
- Xie, W., Song L., Wang X., Xu Y., Liu Z., Zhao D., Wang S., Fan X., Wang Z., Gao C., . et al. 2021. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 13:1956281. doi: 10.1080/19490976.2021.1956281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, X., Tan B., Song M., Ji P., Kim K., Yin Y., and Liu Y.. . 2019. Nutritional intervention for the intestinal development and health of weaned pigs. Front. Vet. Sci. 6:46. doi: 10.3389/fvets.2019.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H., Zhang L., Gan Z., Xiong H., Yu C., Du H., and Wang Y.. . 2016. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci. Rep. 6:25679. doi: 10.1038/srep25679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L., Li H., Peng Z., Ge Y., Liu J., Wang T., Wang H., and Dong L.. . 2021. Early weaning affects liver antioxidant function in piglets. Animals (Basel) 11(9):2679. doi: 10.3390/ani11092679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S. Y., Liu L. L., Huang Y. Q., Li X. W., Talukder M., Dai X. Y., Li Y. H., and Li J. L.. . 2021. In silico analysis of selenoprotein N (Gallus gallus): absence of EF-hand motif and the role of CUGS-helix domain in antioxidant protection. Metallomics 13:mfab004. doi: 10.1093/mtomcs/mfab004 [DOI] [PubMed] [Google Scholar]