ABSTRACT
We report the case of a 61-year-old woman with painful chronic pancreatitis related to proximal pancreatic duct pancreatolithiasis who underwent successful fragmentation with pancreatic extracorporeal shock wave lithotripsy (ESWL). Two weeks later, she developed abdominal pain, nausea, and vomiting and was found to have a new 4.6 × 2.3 cm hepatic abscess. She was treated with antibiotics but did not require additional intervention. Reported etiologies of post-ESWL abdominal pain include local irritation and bruising at the interface and pancreatitis, which has been reported in 4.2% of cases. We suggest that hepatic abscess ought to be considered in the differential diagnosis of post-ESWL abdominal pain.
INTRODUCTION
Pancreatolithiasis of the main pancreatic duct may contribute to attacks of acute pancreatitis (AP) or chronic ductal hypertension causing chronic pain among patients with chronic pancreatitis (CP). Strategies for treatment include pancreatic surgery, endoscopic retrograde cholangiopancreatography (ERCP) and peroral pancreatoscopy with intraductal lithotripsy, and extracorporeal shock wave lithotripsy (ESWL). The use of pancreatic ESWL has been increasing after reports of its efficacy and safety.1,2
Urologic and pancreatic ESWLs have been in use for over 40 years and are generally considered safe.3–5 In a series including over 5,000 patients who underwent pancreatic ESWL, 22.5% reported adverse events, which included bruising (19%), mild AP (3.1%), and severe AP (0.5%).6 Cases of bacteremia have been reported after urologic ESWL and biliary ESWL, but not after pancreatic ESWL.7,8 Unlike the urinary and biliary tracts, manipulation of the pancreatic duct is generally considered to be unlikely to lead to local infection or bacteremia, perhaps in part because of antibacterial properties of the pancreatic fluid.9 In this case report, we describe the development of a hepatic abscess after pancreatic ESWL.
CASE REPORT
A 61-year-old woman with painful CP was referred to our pancreas clinic after an AP hospitalization. Other medical conditions include hypertension, hyperlipidemia, gastroesophageal reflux, and diet-controlled type 2 diabetes mellitus. Her index AP occurred in her late 30s and was initially attributed to alcohol use. During her second hospitalization for AP, she underwent cholecystectomy. She was symptom-free for several years until she recently experienced a third AP and was diagnosed with CP. She had not consumed alcohol in many months and has never smoked. The most recent AP episode was attributed to pancreatic duct obstruction from a pancreatolith measuring 11 mm × 10 mm within the proximal main pancreatic duct, leading to dilation of the main pancreatic duct (Figure 1). Furthermore, there was no evidence of biliary obstruction or pancreatic duct stricture. She was referred for pancreatic ESWL to fragment and remove the main duct pancreatolith.
Figure 1.
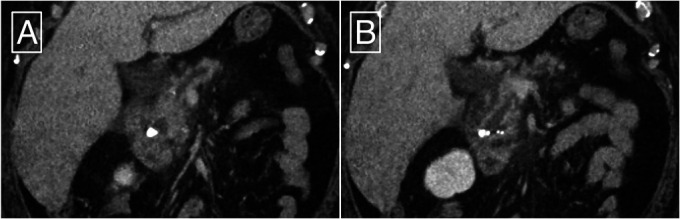
Computed tomography showing (A) pancreatolithiasis at the head of the pancreas, mild peripancreatic fat stranding, and (B) main pancreatic duct dilation.
Pancreatic ESWL (Dornier Delta III; Dornier MedTech, Weßling, Germany) was performed with the administration of 1,800 total shocks delivering 81,320 mJ of energy. Successful stone fragmentation to fragments < 3 mm in size was achieved (Figure 2). Endoscopic retrograde pancreatography was not performed.
Figure 2.
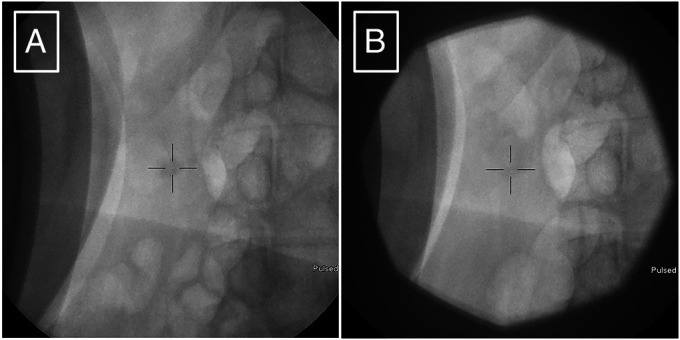
Fluoroscopy obtained during ESWL showing (A) pre-ESWL pancreatolithiasis, and (B) post-ESWL pancreatolithiasis is fractured into smaller fragments. ESWL, extracorporeal shock wave lithotripsy.
Two weeks after pancreatic ESWL, she presented with new abdominal pain, fatigue, nausea, and vomiting. Her pain localized to the right upper quadrant and radiated toward the epigastrium, whereas her pancreatitis pain was previously felt in the epigastrium with radiation toward the back. Initially, she was afebrile with stable vital signs. Her liver function panel showed a total bilirubin of 2.5 mg/dL (reference <1.5 mg/dL) and an alkaline phosphatase of 316 U/L (reference 32–126 U/L), but normal transaminases. The white blood cell count was 7.05 × 103/µL, and her other chemistries were normal, including a normal lipase. Imaging demonstrated a 4.6 × 2.3 cm loculated liver abscess near the hilum, which was new compared with her imaging 3 months before pancreatic ESWL (Figure 3). Interventional radiology was consulted for drainage, but determined that the size and location of the abscess was not amenable to percutaneous drainage. Infectious disease consultants recommended that intravenous antibiotics should be commenced, and percutaneous drainage could be considered in the event of clinical deterioration. No other inciting event, such as recent travel or invasive procedures, could be identified. Her hospital course was otherwise significant for the development of bacteremia and persistent fevers. Echocardiography and spine imaging did not reveal any other organ involvement. She was treated with a 4-week course of ertapenem.
Figure 3.
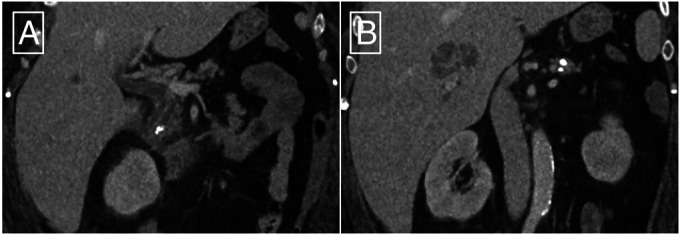
Computed tomography on postprocedure day 14 showing (A) a reduction in the size of the pancreatolith at the head of the pancreas and (B) a multiloculated abscess in the right lobe of the liver.
She was seen for follow-up in the pancreas clinic and denied any abdominal pain and steatorrhea. Bilirubin normalized, and alkaline phosphatase improved (down to 210 U/L). Repeated computed tomography showed near-resolution of the hepatic abscess.
DISCUSSION
Pancreatic ESWL is now considered the first-line intervention for the management of large (≥5 mm), symptomatic pancreatolithiasis in the proximal main pancreatic duct.10 Contraindications to pancreatic ESWL include pregnancy, ongoing AP, and coagulopathy, and pancreatic ESWL ought not be performed when there is consideration for an underlying malignancy, aortic aneurysm, or large right-sided renal cysts. Logistically, pancreatic ESWL can be performed as the first intervention for radiopaque pancreatoliths because these can be visualized on fluoroscopy to target the shock waves. By contrast, radiolucent pancreatoliths may require pancreatic duct stent placement to assist with targeting the beam. Finally, pancreatic ESWL does not require endoscopic cannulation of the pancreatic duct, so it may be considered when pancreatic duct cannulation is challenging, such as after pancreatic surgery.11
Recently, there are increasing reports of technical success (e.g., clearance of pancreatolithiasis) and clinical success (e.g., pain or other patient-reported outcome measurement) after pancreatic ESWL. A systematic review conducted in 2015 concluded that ductal clearance was achieved in 70.7% of patients (95% confidence interval 68.97%–72.38%), including 3,189 patients from 27 studies.1 Regarding clinical success, pain relief was achieved in most patients, and an improvement in quality of life was reported by nearly 90% of patients.1 However, this systematic review did not distinguish between studies that applied ESWL or ESWL plus ERCP. In a separate randomized controlled trial, pancreatic ESWL with or without ERCP had similar rates of technical success and clinical success.12 An additional trial to assess the clinical outcomes of ESWL plus ERCP compared with sham is ongoing.13
Only a minority of patients who undergo pancreatic ESWL develop adverse events, and most of these are mild. The most common adverse outcomes include bruising (19%) and pain at the site of the interface (13.5%), which are self-limited.6 Single reports of gastrointestinal bleeding, hematuria, and a colonic hematoma have been described after pancreatic ESWL.14–16 Mild AP has been reported in 4.2% of cases and severe AP requiring hospitalization in less than 1% of cases.1,6 Rates of post-ESWL pancreatitis are similar in adult and pediatric populations and may be marginally reduced with the use of rectal indomethacin.17,18 Bacteremia and hepatic abscess has not been reported as an adverse event of pancreatic ESWL until now. Abscesses after urologic ESWL have been rarely described and have been attributed to the combination of transient bacteremia and local tissue inflammation related to shock wave trauma.19,20 In our case, we speculate that the hepatic abscess developed because of transient bacteremia and local inflammation of the right hepatic lobe due to the positioning of the transducer.
In conclusion, pancreatic ESWL is an efficacious intervention for symptomatic pancreatolithiasis of the main pancreatic duct but carries a number of potential adverse outcomes. In addition to post-ESWL pancreatitis and local irritation, clinicians should consider infectious etiologies when patients present with abdominal pain after ESWL.
DISCLOSURES
Author contributions: ML Ramsey: initial draft of the manuscript, substantial contribution to acquisition of data, approval of the final manuscript, and accountable for accuracy of the work. M. Bender, LF Lara, and S. Han: critical review of the manuscript, substantial contribution to acquisition of data, approval of the final manuscript, and accountable for accuracy of the work. S. Han is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Matthew Bender, Email: matthew.bender@osumc.edu.
Luis F. Lara, Email: luis.lara@osumc.edu.
Samuel Han, Email: samuel.han@osumc.edu.
REFERENCES
- 1.Moole H, Jaeger A, Bechtold ML, Forcione D, Taneja D, Puli SR. Success of extracorporeal shock wave lithotripsy in chronic calcific pancreatitis management: A meta-analysis and systematic review. Pancreas. 2016;45(5):651–8. [DOI] [PubMed] [Google Scholar]
- 2.Jaben IL, Coté GA, Forster E, et al. Comparison of urologist- vs gastroenterologist-directed extracorporeal shock wave lithotripsy for pancreaticolithiasis. Clin Gastroenterol Hepatol. 2021;19(6):1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaussy C, Brendel W, Schmiedt E. Extracorporeally induced destruction of kidney stones by shock waves. Lancet. 1980;2(8207):1265–8. [DOI] [PubMed] [Google Scholar]
- 4.Greiner L, Jakobeit C. ESWL in pancreatic duct stones [in German]. Dtsch Med Wochenschr. 1989;114(49):1940. [PubMed] [Google Scholar]
- 5.Sauerbruch T. Extracorporeal shockwave lithotripsy dof pancreatic calculi [in German]. Leber Magen Darm. 1990;20(3):146–8. [PubMed] [Google Scholar]
- 6.Tandan M, Nageshwar Reddy D, Talukdar R, et al. ESWL for large pancreatic calculi: Report of over 5000 patients. Pancreatology. 2019;19(7):916–21. [DOI] [PubMed] [Google Scholar]
- 7.Zimhony O, Goland S, Malnick SD, Singer D, Geltner D. Enterococcal endocarditis after extracorporeal shock wave lithotripsy for nephrolithiasis. Postgrad Med J. 1996;72(843):51–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kullman E, Jönsson KA, Lindström E, Dahlin LG, Ansehn S, Borch K. Bacteremia associated with extracorporeal shockwave lithotripsy of gallbladder stones. Hepatogastroenterology. 1995;42(6):816–20. [PubMed] [Google Scholar]
- 9.Rubinstein E, Mark Z, Haspel J, et al. Antibacterial activity of the pancreatic fluid. Gastroenterology. 1985;88(4):927–32. [DOI] [PubMed] [Google Scholar]
- 10.Kitano M, Gress TM, Garg PK, et al. International consensus guidelines on interventional endoscopy in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020;20(6):1045–55. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Ji JT, Xin L, et al. Extracorporeal shock wave lithotripsy for chronic pancreatitis patients with stones after pancreatic surgery. Pancreas. 2018;47(5):609–16. [DOI] [PubMed] [Google Scholar]
- 12.Dumonceau JM, Costamagna G, Tringali A, et al. Treatment for painful calcified chronic pancreatitis: Extracorporeal shock wave lithotripsy versus endoscopic treatment: A randomised controlled trial. Gut. 2007;56(4):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olesen SS, Drewes AM, Gaud R, et al. Combined extracorporeal shock wave lithotripsy and endoscopic treatment for pain in chronic pancreatitis (SCHOKE trial): Study protocol for a randomized, sham-controlled trial. Trials. 2020;21(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozarek RA, Brandabur JJ, Ball TJ, et al. Clinical outcomes in patients who undergo extracorporeal shock wave lithotripsy for chronic calcific pancreatitis. Gastrointest Endosc. 2002;56(4):496–500. [DOI] [PubMed] [Google Scholar]
- 15.Vaysse T, Boytchev I, Antoni G, et al. Efficacy and safety of extracorporeal shock wave lithotripsy for chronic pancreatitis. Scand J Gastroenterol. 2016;51(11):1380–5. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Hao L, Wang T, Li ZS, Xu ZL, Hu LH. Colonic hematoma after extracorporeal shock wave lithotripsy for pancreatic stones: A case report. BMC Gastroenterol. 2019;19(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Bi YW, Ji JT, et al. Extracorporeal shock wave lithotripsy is safe and effective for pediatric patients with chronic pancreatitis. Endoscopy. 2017;49(5):447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian YY, Ru N, Chen H, et al. Rectal indometacin to prevent pancreatitis after extracorporeal shock wave lithotripsy (RIPEP): A single-centre, double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2022;7(3):238–44. [DOI] [PubMed] [Google Scholar]
- 19.Unal B, Kara S, Bilgili Y, Basar H, Yilmaz E, Batislam E. Giant abdominal wall abscess dissecting into thorax as a complication of ESWL. Urology. 2005;65(2):389. [DOI] [PubMed] [Google Scholar]
- 20.Gasser TC, Frei R. Risk of bacteraemia during extracorporeal shock wave lithotripsy. Br J Urol. 1993;71(1):17–20. [DOI] [PubMed] [Google Scholar]


