Abstract
Tests based on tuberculin purified protein derivative (PPD) cannot distinguish between tuberculosis infection, Mycobacterium bovis BCG vaccination, or exposure to environmental mycobacteria. The present study investigated the diagnostic potential of two Mycobacterium tuberculosis-specific antigens (ESAT-6 and CFP10) in experimental animals as well as during natural infection in humans and cattle. Both antigens were frequently recognized in vivo and in vitro based on the induction of delayed-type hypersensitivity responses and the ability to induce gamma interferon production by lymphocytes, respectively. The combination of ESAT-6 and CFP10 was found to be highly sensitive and specific for both in vivo and in vitro diagnosis. In humans, the combination had a high sensitivity (73%) and a much higher specificity (93%) than PPD (7%).
Tuberculosis (TB) remains a major global health problem. Human TB is the most frequent cause of death from a single infectious agent, being responsible for eight million new cases and approximately two million deaths annually (39). The AIDS epidemic and the appearance of multidrug-resistant strains of Mycobacterium tuberculosis have contributed to the resurgence of TB in humans. TB is also a significant problem in cattle and bovine TB represents a significant zoonotic infection, particularly in human immunodeficiency virus-infected humans in developing countries (8). Early diagnosis of infection is crucial to prevent the spread of both of these diseases, and improved methods are urgently required.
Purified protein derivative (PPD), a crude, poorly defined mixture of mycobacterial antigens containing both secreted and somatic proteins, has been used for TB diagnosis and epidemiological studies for more than half a century. It has been used for many years as an in vivo skin test reagent in both humans and cattle. Alternatives to skin testing have been investigated. In 1990, an in vitro diagnostic test for Mycobacterium bovis infection in cattle was developed, based on the detection of gamma interferon (IFN-γ) liberated in whole blood cultures incubated in vitro with PPD (38). In 1994, an adaptation of this test was developed for the diagnosis of M. tuberculosis and Mycobacterium avium infection in humans, again using PPD-type antigens (33, 34). PPD contains many mycobacterial antigens, some of which are shared among pathogenic mycobacteria belonging to the M. tuberculosis complex (M. tuberculosis, M. bovis, and Mycobacterium africanum), environmental nontuberculous mycobacteria (NTM), and the vaccine substrain M. bovis bacille Calmette-Guerin (BCG) (13). Thus, although responsiveness to PPD is an important aid in the diagnosis of TB and can give an indication of exposure to mycobacteria, it is often impossible to distinguish BCG vaccination and exposure to NTM from M. tuberculosis infection (12). It has been apparent, therefore, that a new diagnostic reagent with specificity for M. tuberculosis and M. bovis is needed to overcome the limitations of PPD.
The recent identification of regions of the M. tuberculosis genome that are missing from BCG and most NTM provides a new opportunity for the development of novel diagnostic tools (6, 9). One such region is the RD1 region, which is deleted from all BCG strains but present in the M. tuberculosis complex (14, 32). This region encodes the T-cell antigen ESAT-6, which was originally isolated from a highly stimulatory low-molecular-mass fraction of M. tuberculosis culture filtrate (1, 32). With both humans and cattle, in vitro studies measuring either soluble IFN-γ or IFN-γ-secreting T cells have indicated that ESAT-6 is a potential diagnostic reagent which is highly specific for active tuberculosis and is frequently recognized in disease (7, 16, 23, 24, 28, 35). However, as might be expected in a genetically diverse population, the sensitivity of a diagnosis based on a single antigen was lower than that with a complex antigen mixture like PPD (16, 28, 35).
Recently, another antigen (CFP10) was identified in the low-molecular-mass fraction of culture filtrate, and interestingly, the gene which encodes this antigen is located in the same operon as ESAT-6 (4). Southern blotting of genomic DNA has shown the presence of both the esat-6 and cfp10 genes in M. tuberculosis, M. africanum, and virulent M. bovis, whereas these two genes could not be demonstrated in any BCG vaccine strains and in NTM, with a few exceptions (Mycobacterium kansasii, Mycobacterium szulgai, and Mycobacterium marinum) (14, 30). In agreement with this distribution, it was recently demonstrated that ESAT-6 was able to discriminate TB patients from both BCG-vaccinated individuals and M. avium patients (16).
This study has compared the diagnostic potentials of these two novel M. tuberculosis-specific antigens for in vivo and in vitro TB diagnosis. The results show that the combined use of ESAT-6 and CFP10 improves the diagnostic specificity without loss of sensitivity and offers a realistic alternative to PPD.
MATERIALS AND METHODS
Antigens.
The PPDs used in the skin tests and IFN-γ assays were prepared from M. tuberculosis (PPD; tuberculin RT 23 [for in vivo studies] and RT 49 [for in vitro studies]; Statens Serum Institute, Copenhagen, Denmark) and M. bovis (PPDB). PPDB was obtained from the Veterinary Laboratories Agency (Addlestone, United Kingdom). Short-term culture filtrate (ST-CF) was produced as previously described (3). Briefly, M. tuberculosis (8 × 106 CFU/ml) was grown in modified Sauton medium without Tween 80 on an orbital shaker at 37°C for 4 to 7 days. The culture supernatants were sterile filtered and concentrated with a PM 10 membrane (Amicon, Danvers, Mass.). Recombinant ESAT-6 and CFP10 were produced as previously described (4, 15).
Guinea pigs.
All animal experiments were approved by the institutes' animal welfare committees in the United Kingdom and Denmark. Female outbred guinea pigs of strain Dunkin Hartley (Møllegaard Breeding and Research Center A/S, Lille Skensved, Denmark) were used in this study. TB infection was carried out by the aerosol route in an exposure chamber of a Glas-Col Inhalation Exposure System, which was calibrated to deliver approximately 20 to 25 M. tuberculosis Erdman bacilli into the lungs of each animal. A group of guinea pigs was injected intradermally with 2 × 106 CFU of BCG (BCG Danish 1331; Statens Serum Institut); these animals are referred to as BCG vaccinated. Another group was intradermally given 2 × 106 CFU of a clinical isolate of M. avium (Atyp.1443; Statens Serum Institut); they are referred as being M. avium sensitized. One group was left untreated as a control naive group. Skin tests were performed 28 days after infection or sensitization with 10 tuberculin units of PPD (1 tuberculin unit = 0.02 μg) as a positive control or with 1 μg of ESAT-6 or CFP10 in 0.1 ml of 0.005% Tween 80–phosphate-buffered saline. These protein concentrations were previously determined to be optimal (data not shown). Skin test responses (diameter of erythema) were independently read 24 h later by two experienced examiners, and the results were expressed as the mean of the two readings. The variation between the two readings was less than 10%. Skin test responses larger than 5 mm were regarded as positive.
Cattle.
Blood samples were obtained from skin test-positive cattle on farms where M. bovis infection had been confirmed by conventional culture methods. In the field cases, M. bovis infection was confirmed at postmortem examination by conventional culture methods and histopathology. For experimentally infected cattle, groups of Friesian-cross castrated males 3 to 6 months of age were obtained from herds known to be free of M. bovis infection for at least the previous 5 years. The calves were placed in strict isolation and infected by intranasal instillation with approximately 106 CFU of a field strain of M. bovis (T/91/1378; Veterinary Science Division, Belfast, United Kingdom) as previously described (21). Heparinized whole blood was dispensed into flat-bottom 96-well microtiter plates, and duplicate cultures were stimulated with PPDB (4 μg/ml), pokeweed mitogen (Sigma Chemical Company, Poole, United Kingdom) (4 μg/ml) as a positive control (only positive cultures were included), ESAT-6 (2 μg/ml), CFP10 (2 μg/ml), or ESAT-6–CFP10 (1 μg of each antigen). An equal volume of phosphate-buffered saline was added to control, unstimulated cultures. The plates were then incubated at 37°C in 6% CO2 for 24 h, after which the duplicate supernatants were pooled and assayed for IFN-γ by enzyme-linked immunosorbent assay (ELISA) (Commonwealth Serum Laboratories, Parkville, Australia). Color development was measured at 450 nm, and results were expressed as optical density (OD) indices (ODI) (ODI = OD for stimulated cultures/OD for control cultures) as previously described (25). An ODI of ≥2 was considered positive. The coefficient of variation between duplicate wells was less than 5%, and the OD for control wells was usually less than 0.1.
Human donors.
The human study was approved by the Local Ethical Committee for Copenhagen and Frederiksberg, Denmark (RH 01-282/96). Danish TB patients diagnosed and treated at the Department of Pulmonary Medicine, University Hospital of Copenhagen, Copenhagen, Denmark, were asked to participate in the study. Only patients with minimal TB (n = 24), as defined by the International Union against Lung Disease, were included (11), since most TB patients with advanced TB have cellular immune responses that are significantly suppressed (31). Blood samples were drawn after diagnosis but before the start of antimycobacterial drug treatment. In Denmark, half of the TB patients are foreign-born immigrants from Africa, the Middle East, and the Far East (26). BCG-vaccinated (n = 8) and nonvaccinated (n = 6) healthy individuals were recruited as controls. Healthy donors with a history of person-to-person or laboratory exposure to M. tuberculosis were excluded.
Blood samples were drawn into tubes with Li-heparin and processed within 6 h of sampling. Peripheral blood mononuclear cells (PBMC) were separated from whole blood by density centrifugation as previously described (29) and frozen in liquid nitrogen (27). PBMC were thawed and resuspended in RPMI 1640 supplemented with 40 μg of streptomycin per ml, 40 U of penicillin per ml, 0.04 mM glutamine (Life Technologies Laboratory, Paisley, Scotland), and 10% normal human AB serum (local blood bank, Rigshospitalet, Copenhagen, Denmark). IFN-γ determinations were performed as previously described (28). Briefly, triplicates of 2.0 × 105 PBMC in a total volume of 0.2 ml were stimulated in vitro in round-bottom microtiter wells (Nunc, Roskilde, Denmark) with PPD (2.5 μg/ml), ST-CF (5 μg/ml), and ESAT-6–CFP10 (2.5 μg/ml each) at 37°C in 5% CO2 for 5 days. Phytohemagglutinin (2 μg/ml) was included as a positive control. The IFN-γ releases in response to ST-CF and PPD were not significantly different (P = 0.8) and for some patients for whom PPD values were not available, IFN-γ release from ST-CF-stimulated wells are shown instead (see Fig. 2).
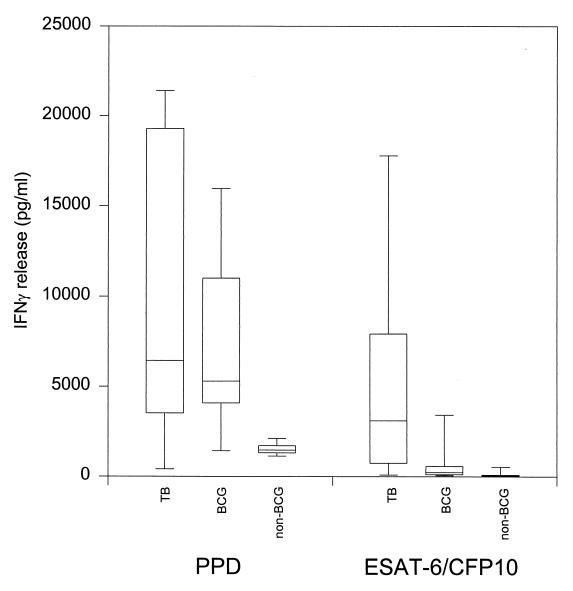
FIG. 2.
Human IFN-γ responses to specific antigens. PBMC from 11 patients with minimal TB, 8 BCG-vaccinated donors, and 6 nonvaccinated donors were stimulated with PPD or ESAT-6–CFP10. IFN-γ in the supernatants was determined after 5 days of culture by ELISA and expressed as the mean picograms per milliliter for triplicate wells. Results are shown as box plots with median values in each group, 25 and 75% quartiles, and 5 and 95% percentile points.
After 5 days of antigen stimulation, supernatants were harvested and IFN-γ was determined by ELISA using commercially available reagents (Pharmingen, San Diego, Calif.). Each IFN-γ ELISA included a standard, recombinant human IFN-γ (Endogen, Woburn, Mass.), and the absorbance as a function of concentration (picograms per milliliter) was fitted to a lin-log sigmoid curve (r > 0.95). The detection limit of the assay was 25 pg/ml, and individual antigen responses are shown as delta values (IFN-γ release in the stimulated wells minus IFN-γ release in the control wells). The coefficient of variation between triplicate wells and the reproducibility between assays with the same patients were less than 10%.
RESULTS
DTH responses specific for M. tuberculosis.
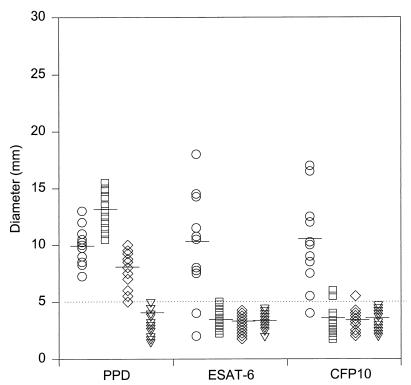
ESAT-6 has previously been investigated in the guinea pig model, and delayed-type hypersensitivity (DTH) responses were found to be highly specific for infection with M. tuberculosis (10). We therefore started by comparing the specificities of DTH responses to CFP10 and ESAT-6 in the guinea pig model. Individual DTH responses in M. tuberculosis-infected, BCG-vaccinated, M. avium-sensitized, and naive guinea pigs to PPD, ESAT-6, and CFP10 are shown in Fig. 1. As expected, PPD did not discriminate between the exposure to the different mycobacteria and induced positive DTH responses in either TB-infected, M. avium-sensitized, or BCG-vaccinated guinea pigs. The DTH responses were 10.0 ± 1.7 (mean response ± standard deviation), 7.7 ± 3.1, and 13.0 ± 1.7, respectively. The CFP10 and ESAT-6 antigens elicited almost identical DTH reactions in TB-infected guinea pigs, and these were comparable to the responses induced by PPD. Both ESAT-6 and CFP10 were strongly recognized in infected guinea pigs, with 9 to 10 responders out of 11 guinea pigs and with responses of 11.4 ± 3.6 and 10.9 ± 3.7. In contrast to PPD, both antigens were highly specific for M. tuberculosis, but compared to ESAT-6, a few animals gave marginally positive nonspecific DTH responses to CFP10 in M. avium-sensitized and BCG-vaccinated guinea pigs.
FIG. 1.
Guinea pig DTH responses to specific antigens. Individual DTH responses and mean responses (—) in M. tuberculosis-infected (○), BCG-vaccinated (□), M. avium-sensitized (◊), and naive (▿) guinea pigs to PPD, ESAT-6, or CFP10 are shown. DTH responses were read after 24 h, and the dotted line indicates the limit for positive responses (5 mm).
Combination of single specific antigens for diagnosis of TB in humans and cattle.
The in vitro release of IFN-γ in response to PPD, ESAT-6, and CFP10 was investigated with 20 TB patients and 20 M. bovis-infected cattle. As shown in Table 1, all of the TB patients except one were PPD responsive (>300 pg of IFN-γ/ml). Both ESAT-6 and CFP10 stimulated the release of large quantities of IFN-γ and were recognized by 60% of the patients tested (12 out of 20). Fifty percent of the donors (10 out of 20) recognized both antigens, although often with different intensities. Of importance is that four donors recognized only one of the two antigens. Taken together, the two antigens were recognized in 14 out of the 20 donors (70%).
TABLE 1.
IFN-γ responses of patients with minimal TB and M. bovis-infected cattle to PPD or PPDB, ESAT-6, and CFP10
| Subject | IFN-γ responsea
|
|||||
|---|---|---|---|---|---|---|
| Patients
|
Cattle
|
|||||
| PPD | ESAT-6 | CFP10 | PPDB | ESAT-6 | CFP10 | |
| 1 | 4,321 | 2,542 | 3,815 | 4.2 | 4.7 | 3.9 |
| 2 | 3,565 | 108 | 48 | 5.3 | 5.0 | 1.3 |
| 3 | 4,700 | 4,484 | 2,055 | 7.4 | 6.0 | 5.8 |
| 4 | 5,376 | 700 | 514 | 0.9 | 0.8 | 0.9 |
| 5 | 208 | 134 | 155 | 2.2 | 0.7 | 1.0 |
| 6 | 10,675 | 3,051 | 8,003 | 0.9 | 1.1 | 1.8 |
| 7 | 7,658 | 22 | 3,895 | 1.4 | 1.0 | 0.9 |
| 8 | 4,500 | 1,752 | 875 | 18.5 | 12.8 | 14.6 |
| 9 | 3,916 | 651 | 41 | 40.4 | 23.8 | 17.8 |
| 10 | 2,143 | 80 | 18 | 4.7 | 3.6 | 2.7 |
| 11 | 5,969 | 75 | 1,276 | 18.5 | 2.8 | 1.4 |
| 12 | 20,827 | 2,269 | 215 | 28.8 | 20.6 | 11.6 |
| 13 | 5,892 | 2,766 | 1,995 | 19.9 | 8.2 | 1.4 |
| 14 | 5,804 | 3,327 | 1,287 | 21.7 | 12.7 | 1.7 |
| 15 | 5,797 | 1,318 | 3,467 | 6.4 | 2.9 | 1.4 |
| 16 | 2,856 | 27 | 295 | 2.0 | 0.9 | 1.0 |
| 17 | 16,418 | 4,372 | 6,248 | 18.7 | 17.3 | 11.4 |
| 18 | 543 | 54 | 67 | 11.0 | 6.4 | 0.7 |
| 19 | 15,543 | 5,742 | 2,021 | 1.9 | 1.2 | 2.2 |
| 20 | 7,283 | 101 | 234 | 30.1 | 27 | 1.1 |
| No. of positive respondersb | 19 | 12 | 12 | 15 | 14 | 8 |
IFN-γ measured by ELISA. Results for humans are expressed as mean picograms per milliliter from triplicate wells and are shown as delta values (see Materials and Methods). Results for cattle are expressed as mean ODI values (OD of antigen-stimulated well/OD of control well) from duplicate cultures.
For the TB patient group the cutoff point was an IFN-γ release of >300 pg/ml; for M. bovis-infected cattle the cutoff point was an ODI of >2.
In infected cattle, ESAT-6 was recognized in 14 out of 20 cases (70%), while 8 cattle (40%) recognized CFP10. PPDB was recognized in 15 cases (75%). As seen in TB patients, seven cattle responded to both antigens, but in eight cases only one of the two antigens was recognized. In total, the antigens were recognized in 75% of the cattle, which is similar to the percentage of PPD responders in this investigation.
To investigate the potential effect of a combination of ESAT-6 and CFP10, four additional TB patients and four experimentally infected cattle were tested with the antigens individually or combined. As shown in Table 2, the four TB patients and four infected cattle responded strongly to the combination of ESAT-6 and CFP10. As would be expected from Table 2, nonresponders to one of the antigens had responses to the combination which reflected the antigen recognized. Furthermore, all (n = 11) TB-infected guinea pigs had positive DTH responses to the combination (0.5 μg/antigen), while some nonresponders were observed with the single antigens. No positive responders to the combination were observed among the BCG-vaccinated, M. avium-sensitized, and naive guinea pigs (data not shown).
TABLE 2.
Comparison of IFN-γ release in four patients with minimal TB and four infected cattle in response to the combination of ESAT-6 and CFP10 or the single components
| Group | Subject | IFN-γ releasea in response to:
|
|||
|---|---|---|---|---|---|
| PPD or PPDB | ESAT-6 | CFP10 | ESAT-6/CFP10 | ||
| Patients | A | 20,927 | 2,169 | 225 | 2,910 |
| B | 17,164 | 6,363 | 2,642 | 9,173 | |
| C | 3,004 | 50 | 3,257 | 4,181 | |
| D | 21,032 | 6,870 | 6,150 | 13,620 | |
| Cattle | A | 31.4 | 5.9 | 1.8 | 6.4 |
| B | 23.9 | 6.2 | 9.6 | 8.7 | |
| C | 55.5 | 12.0 | 22.1 | 19.8 | |
| D | 19.1 | 2.1 | 10.8 | 7.2 | |
IFN-γ was measured by ELISA. Results for humans are expressed as mean picograms per milliliter from triplicate wells and are shown as delta values (see Materials and Methods). Results for cattle are expressed as mean ODI values (OD of antigen-stimulated well/OD of control well) from duplicate cultures.
TB diagnosis by a specific antigen combination or PPD.
In Denmark and many European countries, BCG vaccination has been in use until recently, and a large proportion of the population at risk of contracting TB will have been vaccinated with BCG. We therefore decided to continue the comparison of the diagnostic potential of the ESAT-6–CFP10 combination and PPD in patients with TB and a control group consisting of both BCG-vaccinated and nonvaccinated healthy individuals. The IFN-γ release by PBMC from TB patients (n = 11; randomly picked from the 24 TB patients) and healthy individuals (n = 14; 8 BCG vaccinated and 6 nonvaccinated) was monitored after in vitro stimulation with PPD or ESAT-6–CFP10 (Fig. 2). The TB patients responded to PPD with a median IFN-γ release of 6,430 pg/ml (25th and 75th percentiles, 3,591 and 19,291 pg/ml, respectively). IFN-γ was also released by PPD-stimulated PBMC from the healthy controls with median responses from the BCG-vaccinated individuals of 5,277 pg/ml (4,077 and 11,000 pg/ml) and from the nonvaccinated individuals of 1,455 pg/ml (1,306 and 1,693 pg/ml). The IFN-γ responses to PPD by PBMC from TB patients were not significantly different from those of the healthy BCG-vaccinated controls, but the nonvaccinated controls had significantly (P < 0.01) lower responses than the TB patients and BCG-vaccinated individuals (by the Mann-Whitney rank sum test). The median IFN-γ release in response to the combination of ESAT-6 and CFP10 in the TB patients was 3,098 pg/ml (25th and 75th percentiles, 699 and 7,905 pg/ml, respectively), whereas the BCG-vaccinated controls and nonvaccinated controls gave responses with medians of 234 pg/ml (80 and 565 pg/ml) and 35 pg/ml (25 and 70 pg/ml), respectively. This difference between the TB patients and the healthy controls taken together was highly significant (P < 0.01). In TB patients the IFN-γ responses to PPD and the combination of ESAT-6 and CFP10 were not significantly different (by the Kruskal-Wallis test), but more importantly, in healthy controls this difference was highly significant (P < 0.0001).
The numbers of positive responders to PPD and the specific antigen combination among 11 TB patients and 14 healthy controls were used to calculate the sensitivities and specificities of the diagnostic preparations (Table 3). The sensitivity of PPD was 91% with 300 pg/ml as the cutoff point and decreased slightly to 82% when 1,000 pg/ml was used as the cutoff point. The ESAT-6–CFP10 combination, on the other hand, was recognized by 73% (8 of 11) of the TB patients irrespective of the cutoff point. This was comparable to the recognition of the single antigens by 14 out of 20 TB patients. The sensitivities of PPD (82%) and the combination (73%) were not significantly different (by Fisher's exact test on 95% confidence intervals calculated from the standard binomial). In this study population, the specificity of PPD increased from 0 to 7% when the cutoff point was increased from 300 to 1,000 pg/ml, while the specificity of the combination increased from 71 to 93%. The specificity of the combination was significantly higher (P < 0.0001 by Fisher's exact test) than that of PPD.
TABLE 3.
Sensitivity and specificity of PPD and the combination of ESAT-6 and CFP-10 in TB patients and healthy controlsa
| Cutoff value for IFN-γ release pg/ml | Antigen | No. of responders/ total no.
|
Sensitivity (%)a | Specificity (%)b | |
|---|---|---|---|---|---|
| TB patients | Healthy controlsc | ||||
| >300 | PPD | 10/11 | 14/14 | 91 | 0 |
| ESAT-6/CFP10 | 8/11 | 4/14 | 73 | 71 | |
| >1,000 | PPD | 9/11 | 13/14 | 82 | 7 |
| ESAT-6/CFP10 | 8/11 | 1/14 | 73 | 93 | |
Percentage of positive responders in the TB-infected group.
Percentage of nonresponders to the antigen in the noninfected group.
BCG-vaccinated and nonvaccinated individuals.
DISCUSSION
A new diagnostic reagent which can differentiate between BCG-vaccinated, NTM-sensitized, and TB-infected humans and cattle is urgently needed. As confirmed in the present study, when diagnosis is based on PPD, a large number of false-positive cases are seen after BCG vaccination and exposure to NTM as PPD contains a large number of antigens shared among different mycobacterial strains. However, the combined use of ESAT-6 and CFP10 shows a selective discrimination of TB and potential for TB diagnosis.
Previous attempts to increase the specificity of diagnosis with PPD in humans (36) and cattle (18) have utilized comparative testing in which responses to PPD prepared from mycobacteria within the M. tuberculosis complex are compared with responses to PPD from NTM. The recent identification of regions of the M. tuberculosis genome that are missing from BCG and most NTM provides a new opportunity for the development of novel diagnostic tools (6, 9). In 1996, Stover et al. (17a) used subtractive hybridization to identify regions on the genome of M. bovis that were deleted in BCG. These regions were called RD-1, RD-2, and RD-3, and the first two regions contained the genes for ESAT-6 and the 24-kDa antigen MPT64, which previously had attracted a great deal of interest as an antigen with diagnostic potential. MPT64 has consistently been shown to induce strong and TB-specific DTH responses in the guinea pig model of TB (10, 22). However, contrasting results have been obtained so far regarding the potential of this molecule for human TB diagnosis. In a small-scale clinical evaluation of mycobacterial antigens as skin test reagents, 50% of PPD-reactive TB patients had a positive intradermal reaction to Ag85B (MPT59), but only 6% had a positive reaction to MPT64 (37). In contrast to this, a recent study applied MPT64 as a patch test in humans and found both high sensitivity (98.1%) and high specificity (100%) (20). The background for this discrepancy is unclear at present, but on the other hand, it has been a consistent finding that in vitro this antigen is weakly recognized and induces only low levels of IFN-γ release by PBMC from TB patients (35, 37). Similar low in vitro responses have been seen in M. bovis-infected cattle (J. Pollock et al., unpublished observation). MPT64 is therefore not an obvious candidate molecule for use in an in vitro diagnostic assay based on the release of IFN-γ as described in the present study.
ESAT-6, for comparison, is broadly recognized early during disease in different species infected with M. tuberculosis or M. bovis (2, 5, 24), and this antigen is generally reported to trigger the release of high levels of IFN-γ by sensitized PBMC from TB patients (19, 28, 35). This antigen discriminates TB patients from both BCG-vaccinated and M. avium patients and has therefore been suggested as a candidate for in vitro TB diagnosis (16, 28, 35). However, as the sensitivity of ESAT-6 was reported to be significantly lower than that of PPD (16, 28, 35), further progress in this field has awaited the identification of additional specific antigens to be incorporated into a combination useful for specific TB diagnosis with acceptable sensitivity.
The present study represents this next step, by testing of ESAT-6 together with CFP10, another recently identified highly specific mycobacterial antigen (4). This antigen shares several characteristics with ESAT-6; both antigens are small molecules encoded within the same operon in the RD-1 region, and in the present study both molecules were demonstrated to be very strong T-cell antigens recognized by similar percentages (60%) of TB patients. The fact that both of these antigens are very prominent T-cell targets during early TB infection is intriguing and may reflect an important role of the gene products from this operon in certain stages of mycobacterial growth and intracellular survival. The practical consequence of this immunodominance is that the two molecules are ideal partners for a diagnostic composition either given together, as done in the present study, or fused in the form of a recombinant hybrid molecule.
Combining M. tuberculosis complex-specific antigens into cocktails has recently become feasible due to the increasing number of mycobacterial antigens being cloned and characterized. The potential of diagnostic cocktails has been demonstrated in two recent animal model studies. In one study, all M. tuberculosis-infected guinea pigs responded to MPT64 combined with ESAT-6, while nonresponders to the individual antigens were found (10). Moreover, strong DTH responses were found in BCG-vaccinated guinea pigs to a cocktail of M. tuberculosis complex-specific antigens containing MPT63, MPT70, MPT64, and MTC28, whereas no responses were seen in M. avium-sensitized animals (17).
The combination of ESAT-6 and CFP10 used in the present study resulted in an increased diagnostic performance reflected by higher sensitivity than with the single antigens (60%) without jeopardizing the specificity of the assay. The two antigens combined had approximately the sensitivity found with PPD (73 versus 82%), and large-scale studies are needed to define the cutoff point for optimal sensitivity and specificity of this new diagnostic test. The difference in the specificities of the antigen combination and PPD is clear, and this high specificity could be of crucial importance for any future diagnostic test for screening, contact tracing, and rapid diagnosis and onset of correct treatment of TB patients in the pulmonary clinic. Unfortunately, the sensitivity of our specific antigen combination is not high enough for a negative result to be used on its own as a criterion for exclusion of active TB.
In humans, PPD is a highly sensitive reagent both as an in vivo skin test reagent and as an antigen for in vitro studies. The major drawback, as mentioned, is the poor specificity in populations sensitized to mycobacterial antigens as a consequence of either BCG vaccination or exposure to NTM. Since BCG vaccination has been in use in Europe until the last decade and BCG is the most widely used vaccine in the world, any alternative to PPD must be specific for infections with M. tuberculosis. We have shown that the combination of ESAT-6 and CFP10 is highly specific for M. tuberculosis, with a low reactivity in BCG-vaccinated individuals responsive to PPD. In addition, a previous study has provided an indication that such a specific diagnostic reagent may be used for detection of early cases of TB even before the development of active disease (28). A long-term follow-up study is currently under way to study the breakdown with active TB in ESAT-6-positive and -negative contacts, and this may give an indication of the potential of such antigens for the diagnosis of preclinical or latent TB.
In conclusion, our study clearly suggests that a combination of specific antigens containing antigens such as ESAT-6 and CFP10 can form the basis of an improved next-generation diagnostic reagent which can play a role in the diagnosis of active TB in humans and cattle as well as in epidemiological studies and disease control programs. Such a specific reagent would have the potential to play a much more important role in the prevention and control of TB than PPD has done in many years.
ACKNOWLEDGMENTS
We thank Axel Kok Jensen from the Department of Pulmonary Medicine, Rigshospitalet, Copenhagen, Denmark, for the recruitment of TB patients. We thank Inger Brock, Thomas Thomassen, Vita Skov, and Martyn Girvin for excellent technical assistance and Mark Doherty for a critical review of the manuscript.
Financial support was from the Third Research and Development Program “Life Sciences and Technologies for Developing Countries,” The European Commission DRXII contract TS3*CT 940313, the Danish National Research Center for Medical Biotechnology, the Danish National Association Against Lung Diseases, and the Danish International Development Assistance (DANIDA).
REFERENCES
- 1.Andersen P. The T cell response to secreted antigens of Mycobacterium tuberculosis. Immunobiology. 1994;191:537–547. doi: 10.1016/S0171-2985(11)80460-2. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Andersen A B, Sorensen A L, Nagai S. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J Immunol. 1995;154:3359–3372. [PubMed] [Google Scholar]
- 3.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthet F X, Rasmussen P B, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–3203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 5.Brandt L, Oettinger T, Holm A, Andersen P. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J Immunol. 1996;157:3527–3533. [PubMed] [Google Scholar]
- 6.Brosch R, Gordon S V, Billault A, Garnier T, Eiglmeier K, Soravito C, Barrel B G, Cole S T. Use of Mycobacterium tuberculosis H37Rv bacterial artificial chromosome library for genome mapping, sequencing, and comparative genomics. Infect Immun. 1998;66:2221–2229. doi: 10.1128/iai.66.5.2221-2229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buddle B M, Parlane N A, Keen D L, Aldwell F E, Pollock J M, Lightbody K, Andersen P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by recombinant mycobacterial antigens. Clin Diagn Lab Immunol. 1999;6:1–5. doi: 10.1128/cdli.6.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daborn C J, Grange J M. HIV/AIDS and its implications for the control of animal tuberculosis. Br Vet J. 1993;149:405–417. doi: 10.1016/S0007-1935(05)80107-1. [DOI] [PubMed] [Google Scholar]
- 9.Deuskar N J, Deo M G. Mycobacterial cell surface proteins revealed by labeling with 125I. Int J Lepr Other Mycobact Dis. 1991;59:482–486. [PubMed] [Google Scholar]
- 10.Elhay M J, Oettinger T, Andersen P. Delayed-type hypersensitivity responses to ESAT-6 and MPT64 from Mycobacterium tuberculosis in the guinea pig. Infect Immun. 1998;66:3454–3456. doi: 10.1128/iai.66.7.3454-3456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enarson D A, Murray F. Global epidemiology of tuberculosis. In: Rom W M, Garay S, editors. Tuberculosis. Boston, Mass: Brown and Co.; 1996. pp. 57–75. [Google Scholar]
- 12.Fogan L. PPD antigens and the diagnosis of mycobacterial diseases. Arch Intern Med. 1969;124:49–54. [PubMed] [Google Scholar]
- 13.Harboe M. Antigens of PPD, old tuberculin, and autoclaved Mycobacterium bovis BCG studied by crossed immunoelectrophoresis. Am Rev Respir Dis. 1981;124:80–87. doi: 10.1164/arrd.1981.124.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Harboe M, Oettinger T, Wiker H G, Rosenkrands I, Andersen P. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun. 1996;64:16–22. doi: 10.1128/iai.64.1.16-22.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe M, Wiker H G, Ulvund G, Malin A S, Dockrell H, Holm A, Jørgensen M C, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lein A D, von-Reyn C F, Ravn P, Horsburgh C R, Alexander L N, Andersen P. Cellular immune responses to ESAT-6 discriminate between patients with pulmonary disease due to Mycobacterium tuberculosis. Clin Diagn Lab Immunol. 1999;6:606–609. doi: 10.1128/cdli.6.4.606-609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyashchenko K, Manca C, Colangeli R, Heijbel A, Williams A, Gennaro M L. Use of Mycobacterium tuberculosis complex-specific antigen cocktails for a skin test specific for tuberculosis. Infect Immun. 1998;66:3606–3610. doi: 10.1128/iai.66.8.3606-3610.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Mahairas G G, Sabo P J, Hickey M J, Singh D C, Stover C K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178:1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monaghan M L, Doherty M L, Collins J D, Kazda J F, Quinn P J. The tuberculin test. Vet Microbiol. 1994;40:111–124. doi: 10.1016/0378-1135(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa A S, Amoudy H A, Wiker H G, Abal A T, Ravn P, Oftung F, Andersen P. Comparison of antigen specific T cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand J Immunol. 1998;48:535–543. doi: 10.1046/j.1365-3083.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura R M, Velmonte M A, Kawajiri K, Ang C F, Frias R A, Mendoza M T, Montoya J C, Honda I, Haga S, Toida I. MPB64 mycobacterial antigen: a new skin-test reagent through patch method for rapid diagnosis of active tuberculosis. Int J Tuberc Lung Dis. 1998;2:541–546. [PubMed] [Google Scholar]
- 21.Neill S D, Hanna J, O'Brien J J, McCracken R M. Excretion of Mycobacterium bovis by experimentally infected cattle. Vet Rec. 1988;123:340–343. doi: 10.1136/vr.123.13.340. [DOI] [PubMed] [Google Scholar]
- 22.Oettinger T, Holm A, Mtoni I M, Andersen A B, Haslov K. Mapping of the delayed-type hypersensitivity-inducing epitope of secreted protein MPT64 from Mycobacterium tuberculosis. Infect Immun. 1995;63:4613–4618. doi: 10.1128/iai.63.12.4613-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pathan A, Brookes R, Pritchard H, Wilkinson R, Pasvol G, Hill A, Lalvani A. Human T cell responses to the antigen ESAT-6 characterize a vaccine candidate and potential diagnostic test for tuberculosis. Immunology. 1998;95(Suppl. 1):90. [Google Scholar]
- 24.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock J M, Andersen P. The potential of the ESAT-6 antigen secreted by virulent mycobacteria for specific diagnosis of tuberculosis. J Infect Dis. 1997;175:1251–1254. doi: 10.1086/593686. [DOI] [PubMed] [Google Scholar]
- 26.Poulsen S, Miörner H. Tuberculosis 1995. EPI-NEWS. Copenhagen, Denmark: Statens Serum Institut; 1996. [Google Scholar]
- 27.Ravn P, Boesen H, Wilcke J T, Andersen P. In: Secreted antigens and immune responses to Mycobacterium tuberculosis. Karger S, editor. London, United Kingdom: Medical principles and practice. Medical and Scientific; 1997. pp. 74–83. [Google Scholar]
- 28.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy H, Mustafa A S, Jensen A K, Holm A, Rosenkrands I, Oftung F, Olobo J, von-Reyn C F, Andersen P. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–645. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 29.Ravn P, Pedersen B K. Mycobacterium avium and purified protein derivative-specific cytotoxicity mediated by CD4+ lymphocytes from healthy HIV-seropositive and -seronegative individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:433–441. doi: 10.1097/00042560-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 30.Skjøt R L V, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. Comparative evaluation of low-molecular-mass T-cell antigens from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant. Infect Immun. 2000;68:214–220. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sodhi A, Gong J, Silva C, Qian D, Barnes P F. Clinical correlates of interferon gamma production with tuberculosis. Clin Infect Dis. 1997;25:617–620. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen A L, Nagai S, Houen G, Andersen P, Andersen A B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–1717. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Streeton J A. A blood test reliable for spotting TB infection. TB Weekly. 1995;2:3–4. [Google Scholar]
- 34.Streeton J A, Desem N, Jones S L. Sensitivity and specificity of a gamma interferon blood test for tuberculosis infection. Int J Tuberc Lung Dis. 1998;2:443–450. [PubMed] [Google Scholar]
- 35.Ulrichs T, Munk M E, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro M L, Kaufmann S H. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–3958. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.von-Reyn C F, Green P A, McCormick D, Huitt G A, Marsh B J, Magnusson M, Barber T W. Dual skin testing with Mycobacterium avium sensitin and purified protein derivative: an open study of patients with M. avium complex infection or tuberculosis. Clin Infect Dis. 1994;19:15–20. doi: 10.1093/clinids/19.1.15. [DOI] [PubMed] [Google Scholar]
- 37.Wilcke J T, Jensen B N, Ravn P, Andersen A B, Haslov K. Clinical evaluation of MPT-64 and MPT-59, two proteins secreted from Mycobacterium tuberculosis, for skin test reagents. Tuber Lung Dis. 1996;77:250–256. doi: 10.1016/s0962-8479(96)90009-x. [DOI] [PubMed] [Google Scholar]
- 38.Wood P R, Corner L A, Plackett P. Development of a simple, rapid in vitro cellular assay for bovine tuberculosis based on the production of gamma interferon. Res Vet Sci. 1990;49:46–49. [PubMed] [Google Scholar]
- 39.World Health Organization. 23 March 1999, revision date. Global tuberculosis control—WHO report 1999. [Online.] http://www.who.int. [22 December 1999, last date accessed.]