ABSTRACT
There is a growing awareness of the importance of sex and gender in medicine and research. Women typically have stronger immune responses to self and foreign antigens than men, resulting in sex-based differences in autoimmunity and infectious diseases. In both animals and humans, males are generally more susceptible than females to bacterial infections. At the same time, gender differences in health-seeking behavior, quality of health care, and adherence to treatment recommendations have been reported. This review explores our current understanding of differences between males and females in bacterial diseases. We describe how genetic, immunological, hormonal, and anatomical factors interact to influence sex-based differences in pathophysiology, epidemiology, clinical presentation, disease severity, and prognosis, and how gender roles affect the behavior of patients and providers in the health care system.
KEYWORDS: sex differences, gender differences, bacterial infections
INTRODUCTION
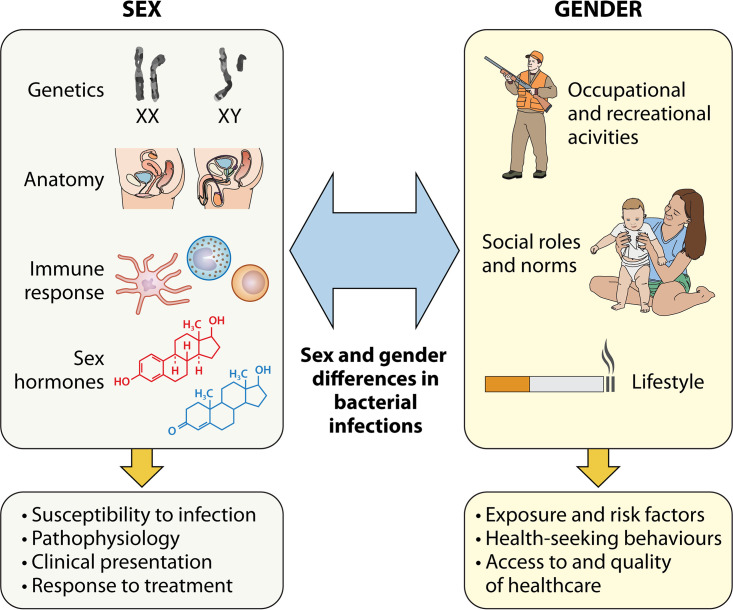
Patient sex is an important determinant in health and disease, and infectious diseases are no exception. Biological sex (defined by sex chromosome complement, sex steroid hormones, and reproductive organs) has been shown to influence susceptibility to infection, pathophysiology, immune responses, clinical presentation, disease severity, and response to treatment and vaccination (1). On the other hand, gender roles (referring to characteristics that are socially constructed) and social norms can influence risk factors and exposure to infection, determine health-seeking behaviors, and impact therapeutic decisions (2). Fig. 1 describes how sex and gender interact to influence differences in bacterial infections.
FIG 1.
Interaction between sex and gender in bacterial diseases.
In this review, we discuss the current knowledge on sex-based differences in bacterial infections, focusing on genetic, anatomical, immunological, hormonal, and behavioral influences and on the epidemiology, pathophysiology, clinical presentation, resolution, and prognosis of selected bacterial diseases.
GENETIC FACTORS
Sex differences begin at conception, with the formation of an embryo carrying XX or XY chromosomes. This establishes a lifelong inequality between male and female cells in the expression of genes encoded in the X and Y chromosomes.
The X chromosome is home to around 1,100 genes and harbors several genes which regulate immune function, such as interleukin-1 (IL-1) receptor-associated kinase 1 (IRAK1), IL-2 receptor-γ chain, IL-3 receptor-α chain, IL-9 receptor, Toll-like receptor 7 (TLR7) and 8, and FOXP3 (3).
Females have two X chromosomes, one of which is randomly silenced in each cell to avoid gene overdosage (4). However, this X chromosome inactivation is only partial, with up to one-third of genes escaping silencing (4). These are often expressed at higher levels in females and can be associated with sex-specific susceptibility to infection and autoimmunity. For example, TLR7 has been shown to escape chromosome X inactivation in immune cells, increasing the risk of autoimmune disease (5).
Furthermore, because the same chromosome is not expressed in each cell, random inactivation leads to female cell mosaicism, which also provides a survival advantage (6). Males, on the contrary, have only a single copy of each of their X chromosome genes, making them vulnerable to X-linked mutations. This is exemplified by X-linked primary immunodeficiencies, which make affected males susceptible to recurrent bacterial, fungal, and viral infections (7).
In addition to evading the harmful effects of these mutations, females benefit from the added diversity when facing new immune challenges, such as invading pathogens (8). The X chromosome is also richer in microRNAs (miRNAs) compared to the Y chromosome, many of which are known to affect immunity (9). For example, miRNA-223, located in the X chromosome, controls susceptibility to tuberculosis (TB) by regulating lung neutrophil recruitment, and its deletion renders mice highly susceptible to infection (10).
The Y chromosome has the lowest number of genes out of all nuclear chromosomes, and it is significantly shorter than the X chromosome. The notion that its function is restricted to sex determination and spermatogenesis has recently been challenged by the discovery of multiple genes with extragonadal expression, with evidence suggesting that the Y chromosome influences immune responses in males (11). For instance, a murine Y chromosome long-arm deletion is associated with deficiencies in B cell and natural killer (NK) cell development, although the precise molecular mechanisms behind this are unclear (12).
IMMUNE RESPONSE
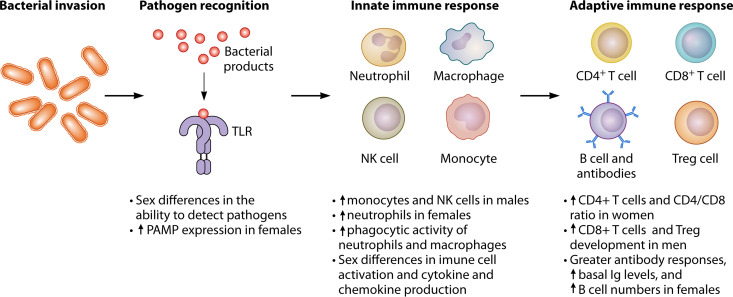
In general, females have stronger innate and adaptive immune responses than males (Fig. 2) (13). These allow better pathogen clearance and response to vaccination, but also make females more prone to inflammatory and autoimmune diseases.
FIG 2.
Sex differences in immune responses associated with bacterial infection. Ig, immunoglobulin; PAMP, pathogen-associated molecular patterns; TLR, Toll-like receptor.
The innate immune system is the first line of immunological defense. There are sex-specific differences in the number and relative distribution of innate immune cells. Males have higher proportions of circulating monocytes (14) and NK cell counts (15), whereas females have higher neutrophil counts in the peripheral blood (16). Antigen-presenting cells (APCs) from females are more efficient in initiating a secondary response from primed lymphocytes compared to APCs from males, and the responsiveness of female cells to alloantigens is superior to that of males (17).
In an ex vivo study, males had a stronger monocyte-derived cytokine response, including IL-1β, tumor necrosis factor alpha (TNF-α), and IL-6 production in response to lipopolysaccharide (LPS), although these differences disappeared after accounting for differences in monocyte concentration (18). Conversely, genes in the type I interferon (IFN) pathway are upregulated in females compared to males, which promotes enhanced responses to TLR agonists (19).
Furthermore, there are sex differences in the ability to detect pathogens, as females have higher expression of pathogen-associated molecular pattern receptors compared to males (20). Compared to male-derived cells, female rodent-derived resident macrophages express higher TLR2 and TLR3 levels and are more efficient at phagocytosis and bacterial killing, while also limiting excessive cytokine production and neutrophil recruitment (21). Bone marrow-derived macrophages from female mice have a significant increase in TLR8 expression compared to male-derived cells. In addition, TLR7 expression is higher in leukocytes from women (5). On the other hand, neutrophils from human males express higher levels of TLR4 and these are increased following activation with LPS, resulting in greater pro-inflammatory cytokine production, which may underlie increased susceptibility to endotoxic shock (22).
Sex also impacts lymphocyte subset distribution. Females have higher absolute and relative CD4+ T cell numbers and higher CD4/CD8 ratios than males, while males have a higher percentage of CD8+ T cells (23). Sex also influences the development of regulatory T (Treg) cells, which are higher in the peripheral blood of males (24). In addition, there are sex differences in humoral immunity. Adult females have greater antibody responses, higher B cell numbers (15), higher IgM and IgG levels, and lower IgA levels compared with males (25, 26). In children, B cell numbers and IgG and IgM levels are comparable between sexes, but females have lower IgA levels (25).
SEX STEROID HORMONES
Sex steroid hormones have immunomodulatory properties, and changes in their levels over the lifespan influence susceptibility and response to infectious diseases. These differences begin in utero with the formation of the testes in male embryos. Once formed, they begin to secrete androgens that cause masculinization and lead to the early development of androgen-dependent sex differences in immunity (27–29). After puberty, concentrations of estrogens and progesterone (P4) in females and androgens in males rise significantly. During this period, there is generally a male bias in infectious diseases, with males being more frequently and more severely affected by bacterial, viral, and parasitic infections, whereas females are more affected by autoimmune disease (30). Differences are also evident during pregnancy, when an increase in the levels of estrogen and, in particular, P4 promote a state of immune tolerance, making pregnant women more susceptible to many infectious diseases (31). During menopause, estrogen and P4 levels drop rapidly in women, while a gradual decline in androgen levels is observed in aging males (1).
Sex steroids can influence immune responses by binding to specific receptors expressed in immune cells, including lymphocytes, macrophages, and dendritic cells (DCs) (13), and can also have a direct effect over bacterial metabolism, growth, and expression of virulence factors (32).
Estrogen.
Estrogens are present in both sexes, but levels are highest in females of reproductive age. The principal endogenous biologically active estrogens are estrone (E1), estradiol (E2), and estriol (E3), the last of these being the main pregnancy estrogen (33). In females, levels vary during the menstrual cycle. They are low before puberty and after menopause and high during pregnancy.
Estrogen receptors (ERs) are ubiquitous in the immune system, and estrogen signals through two different nuclear receptors: ER alpha (ERα) and ER beta (ERβ) (34). Expression of ERs is influenced by age and sex. Monocytes from premenopausal women express ERα at lower levels than monocytes from men and postmenopausal women, whereas no difference was found in ERβ (35). Furthermore, monocytes from men and postmenopausal women contain significantly more ERα than ERβ, suggesting that monocytes from these two groups respond similarly to estrogens (35). On the other hand, ER expression is similar in T or B cells and in plasmacytoid DCs from both sexes (35, 36). In vitro, ERα signaling stimulates differentiation of DCs from monocytes, which produce pro-inflammatory cytokines in response to TLR stimulation. ER signaling also promotes the TLR-driven production of type I interferons (IFNs) in mouse plasmacytoid DCs in vivo (37). In humans, treatment of postmenopausal women with E2 markedly enhances production of IFN-α by plasmacytoid DCs (38).
E2 can augment or dampen immune signaling pathways and enhances both cell-mediated and humoral immunity in a concentration-dependent fashion. Low E2 concentrations promote a Th1-type response, boost cell-mediated immunity, and stimulate type I IFN responses and production of proinflammatory cytokines and chemokines, including IL-1β, IL-6, and TNF-α (39, 40). At high concentrations, E2 promotes Th2-type and humoral responses, inhibits pro-inflammatory pathways, and promotes production of anti-inflammatory cytokines, such as IL-4 and IL-10 (39, 40). Numbers of antibody-secreting cells have been reported to be significantly higher during the peri-ovulatory period in female rhesus macaques (41). Treatment of mice with physiological levels of estrogen results in retention of high-affinity autoreactive B cells, interfering with tolerance induction (42). On the other hand, E2 increases immunoglobulin class-switch recombination and somatic hypermutation in germinal centers. These changes lead to improved responses to vaccination but also increased propensity to autoimmune diseases in women (43).
E2 stimulates the expansion of Treg cells (44), which are higher during the follicular phase of the menstrual cycle (45). Estrogens also reduce the proliferation of immature T lymphocytes and induce thymic involution in mice (46).
Estrogen has been reported to have a protective effect in several infections, such as Vibrio vulnificus, which mostly affects males. In a rat model, ovariectomy was associated with increased mortality and estrogen replacement decreased mortality in both gonadectomized sexes (47). In contrast, in females with cystic fibrosis, estrogen induces conversion of Pseudomonas aeruginosa into the more virulent mucoid form, and the majority of infectious exacerbations occur during high circulating E2 levels (48).
Progesterone.
P4 is produced by the corpus luteum in the ovaries during the menstrual cycle and by the placenta during pregnancy. Progesterone receptors are present in a variety of cell types, including immune cells such as NK cells, macrophages, DCs, and T cells (49). There are sex differences in PR expression, which can explain sex-based disparities in immune responses. For instance, PR expression is higher in female DCs than in male ones, which could justify the differential suppressive effect of progesterone on these cells in female versus male rats (31, 50).
P4 modulates the immune system in order to achieve a successful pregnancy. Increased maternal P4 levels promote a Th2-dominant cytokine phenotype (51, 52) causing an increase in anti-inflammatory cytokines such as IL-4, IL-5, and IL-10 (53–55) and a decrease in proinflammatory cytokines such as TNF-α, IFN-γ, and IL-1β (56, 57). P4 also increases the number of Treg cells and inhibits Th17 cells (58, 59). P4 inhibits DC maturation and DC-mediated proliferation of T cells, favoring immature DCs which have a tolerogenic phenotype (56). This state of immune tolerance, while preventing fetal rejection, increases the susceptibility to and severity of many infections during pregnancy (31). Pregnant women are much more susceptible to Listeria monocytogenes infection than similarly aged healthy adults (60), and P4 increases susceptibility to Chlamydia trachomatis in female rats (61). In contrast, P4 at high doses inhibits the growth of Neisseria gonorrhoeae and N. meningitidis (62) and the germination of Clostridioides difficile spores (63).
Androgens.
Androgens occur in higher concentrations in post-pubertal males than in females (13). Testosterone is the principal androgen, secreted from the testes in males and in small quantities from the ovaries in females. The androgen receptor works as a steroid hormone-activated transcription factor which signals through ligand-dependent and independent signaling pathways (64). Both testosterone and its metabolite, dihydrotestosterone (DHT), generally have suppressive effects on both humoral and cellular immune responses, leading to decreased T and B cell proliferation and decreased immunoglobulin and cytokine production (3).
DHT-treated female mice produce more IL-10 and less IL-12 than untreated female mice, and DHT can act on CD4+ T lymphocytes to increase IL-10 gene expression via androgen receptor signaling (65), thereby promoting an anti-inflammatory response. Treatment of lipopolysaccharide or TNF-α-stimulated human endothelial cells with testosterone controls the inflammatory response mediated by NF-κB (66). In males with symptomatic androgen deficiencies, treatment with testosterone lowers proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6) and increases anti-inflammatory cytokines (such as IL-10) (67). Testosterone deficiency in males is associated with increased CD4+ counts and CD4/CD8 ratios, higher immunoglobulin levels, and increased B cell counts compared with controls, and these changes are reversed by hormonal replacement (68, 69).
In mice, testosterone decreases the expression of TLR4 in macrophages (70). Testosterone suppresses uropathogenic Escherichia coli (UPEC) invasion and colonization by inhibiting the JAK/STAT1 signaling pathway in a prostatitis cell model (71, 72) and also inhibits the expression of pro-inflammatory IL-1β, IL-6, and IL-8 cytokines (72). Male patients with TB show impaired production of gonadal androgens, with lower levels of testosterone compared to healthy controls (73).
GENDER
Gender-related occupational and recreational activities can affect exposure to pathogens. Women are more likely to assume caretaking roles, making them more exposed to childhood diseases (74). On the other hand, men wash their hands less often than women (75). Occupational exposure to animals plays a role in male bias in brucellosis (76) and Q fever (77), while male-predominant mine-related silicosis is a risk factor for TB (78).
Access to care also differs between men and women. In some countries, there is a parental preference for boys over girls. Studies in Bangladesh have shown that parents are more likely to bring their male children to the hospital for pneumonia or diarrhea than their female counterparts, and girls have longer delays to diagnosis, more severe illness on admission, and higher in-hospital mortality (79, 80). In adults, sociocultural and religious norms can also constrain access to health care, and poverty and stigma are important factors in limiting access to care for women in low-income countries. Furthermore, men consistently use more intensive care unit (ICU) resources and are more likely than women to be admitted to an ICU and receive advanced life-supporting measures (81).
SEX AND GENDER DIFFERENCES IN BACTERIAL DISEASES
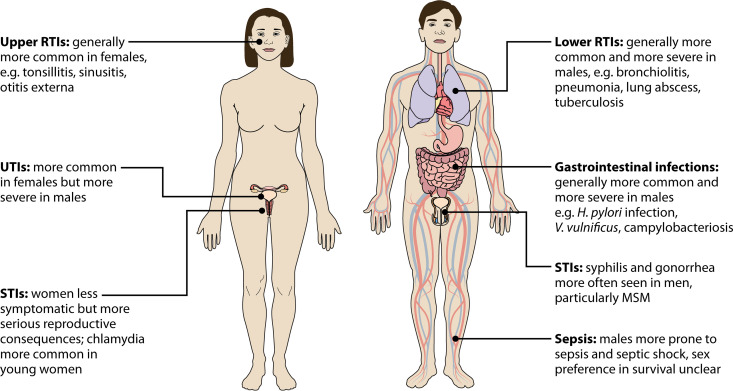
Many bacterial pathogens exhibit a sex preference, and most show a male bias (Table 1). Fig. 3 summarizes differences in the incidence and severity of bacterial diseases across different organ systems.
TABLE 1.
Sex bias by specific bacterial species
| Bacterial species | Bias | Sex- and gender-based risk factors | Reference(s) |
|---|---|---|---|
| Escherichia coli | Female | Food consumption and handling practices, anatomical differences | 245 |
| Streptococcus pneumoniae | Male | Smoking, alcohol use | 127 |
| Legionella pneumophila | Male | Smoking, travel | 131 – 134 |
| Mycobacterium tuberculosis | Male | Occupational (e.g., mining), smoking, travel | 141 |
| Clostridioides difficile | Female | Antibiotic prescription, exposure to infants | 95, 96, 99 |
| Campylobacter spp. | Male | Food-handling practices | 246, 247 |
| Helicobacter pylori | Male | Smoking, low estrogen | 85, 86 |
| Listeria monocytogenes | Young women, elderly men | Pregnancy, waning cellular immunity | 60, 107, 108 |
| Leptospira spp. | Male | Working outdoors or with animals | 248, 249 |
| Francisella tularensis | Male | Outdoor activities, contact with animals | 250 |
| Borrelia burgdorferi | Male predominance in the U.S., female in Europe | Outdoor activities | 220 – 223 |
| Coxiella burnettii | Male | Contact with animals | 77, 232, 233 |
| Brucella spp. | Male | Contact with animals, food consumption habits | 76, 251 |
| Chlamydia trachomatis | Female | Screening bias | 180, 181 |
| Neisseria gonorrhea | Male | High-risk sexual behaviours | 181, 189, 252 |
| Treponema pallidum | Male | High-risk sexual behaviours | 144, 252, 253 |
FIG 3.
Sex and gender bias in bacterial infections. MSM, men who have sex with men; RTIs, respiratory tract infections; STIs, sexual transmitted infections; UTIs, urinary tract infections.
Gastrointestinal tract infections.
Bacterial gastrointestinal infections are a leading cause of illness and death globally and are generally more common and more severe in males (82). This is partly explained by behavioral differences, as men are more likely than women to practice food-handling, preparation, and consumption behaviors that carry a high risk of foodborne diseases (83). Furthermore, differences in the immune response place males at a higher risk of poor outcomes, and sex hormones also play an important role.
(i) Helicobacter pylori. Helicobacter pylori infection is highly prevalent worldwide and is the strongest risk factor for stomach cancer (84). Infection has a slight male bias (85, 86) and males exhibit more severe inflammation, atrophy, and intestinal metaplasia scores compared to females (87). Gastric cancer is twice as common in men as in women (84).
Epidemiological evidence and animal studies suggest a protective effect of female sex hormones, namely, estrogen. A longer fertility window and the use of oral contraceptives or hormone replacement therapy are associated with a lower risk of gastric cancer (88). Transgenic hypergastrinemic mice infected with H. pylori develop gastric carcinoma in a male-predominant fashion (89), and estrogen supplementation, but not castration, attenuates gastric lesions (90). Ovariectomized mice develop significantly more severe H. pylori-induced gastritis and gastric cancer, and E2 supplementation has a protective effect (91).
On the other hand, female sex is associated with clarithromycin and metronidazole resistance (92) and H. pylori eradication failure (93).
(ii) Clostridioides difficile. C. difficile is a major cause of health care-associated infection, although community-acquired cases are increasingly reported (94). C. difficile infection (CDI) is more common in females, who account for 55% to 60% of cases (95, 96). In the United States, females account for 67% of community-acquired CDI (97), potentially due to more frequent antibiotic prescription in women (98). In addition, traditional gender roles result in women generally having more exposure to infants, which is a risk factor for community-acquired CDI (97). Women also account for the majority of hospital-acquired cases and are responsible for 55% of health care-associated CDI in Europe (99). Furthermore, females have an increased risk of recurrent CDI (100) and severe cases have been reported in pregnant and peripartum women (101, 102). On the other hand, male sex is an independent predictor of mortality (103).
(iii) Listeriosis. L. monocytogenes is a foodborne pathogen that can cause septicemia and meningitis as well as fetal infection or abortion in pregnant women (104). Pregnant women are about 20 times more likely to contract this infection compared to the general population (60) due to suppressed cellular immunity and the placental tropism of L. monocytogenes (104). In pregnant women and mice, increased P4 weakens CD8+ T memory cell-mediated IFN-γ responses, which are crucial to host defense against listerial infection (57). Treating female mice with E2 decreased IL-12, TNF-α, and IFN-γ expression and increased IL-4 and IL-10 expression (105). Estrogen also depressed monocyte and lymphocyte accumulation at infective foci and increased mortality in female mice (106).
Incidence rates of invasive listeriosis are higher in females than in males during reproductive years (likely reflecting pregnancy-related listeriosis). In contrast, in older age groups, rates are 2 to 4 times higher in males (107, 108), with similar case-fatality rates (107). In mice, however, infection with L. monocytogenes led to significantly higher lethality rates and bacterial numbers in females than in males (109).
Respiratory tract infections.
Generally, males are more susceptible to respiratory tract infections (RTIs) and have a more severe disease course and higher mortality compared with females. Males are more affected by lower RTIs, such as pneumonia, bronchiolitis, or lung abscess, while females more often develop upper RTIs, such as sinusitis, tonsillitis, and otitis externa (110). However, there are some exceptions. Males are more often affected by otitis media (111) and mastoiditis (112), whereas pertussis has a higher incidence rate in females (113).
Anatomical factors can explain some of these differences. For instance, peripheral airways are narrower during the first year of life in males, which may predispose them to lower rates of RTI (114). On the other hand, after puberty, males have significantly larger central airway luminal areas than females, independently of height (115). This could explain why in cystic fibrosis (which affects prepubescent males and females equally), post-pubescent females have increased rates and severity of exacerbations and more rapid declines in lung function after colonization with P. aeruginosa compared with males (116). It has also been suggested that females have smaller ostia, making them more susceptible to sinus obstruction and infection (117).
(i) Otitis media. Middle ear infections are a leading cause of medical visits and antibiotic prescription in infants and preschool-aged children. Acute otitis media is more common in boys than in girls (111, 118), and children with more severe disease are more often males (119). Studies also show that male sex is a risk factor for recurrent infection (111), as well as a predictor of chronic otitis media (120).
The reasons for these differences are not well understood; however, it has been proposed that abnormal pneumatisation of the mastoid process (with the smaller mastoid cell air system in boys compared with girls), could result in more frequent and severe ear infections in male children (121).
(ii) Pneumonia. Pneumonia is a leading cause of hospitalization and death worldwide, and all types of bacterial pneumonia are more common in males (122). In community-acquired pneumonia, male sex is significantly associated with hospitalization and death, with males 1.3 times more likely to die than females (123, 124). Community-acquired pneumonia is also more common in boys than in girls (125), and male sex is associated with bacteremia in children (126).
Streptococcus pneumoniae is the most common bacterial pathogen in both sexes. Pneumococcal pneumonia and invasive pneumococcal disease are more frequent in males than in females (127), and male sex is associated with mortality in bacteremic pneumococcal pneumonia (128). Although older (over 50 years) females generally have lower antibody responses to pneumococcal vaccines than males (129), the 23-valent pneumococcal vaccine is more effective at preventing hospitalizations caused by S. pneumoniae in women (130). Legionellosis is also more frequently noted in males, with male:female ratios of 1.7 to 5 reported in Europe, the U.S., Australia, and Japan (131–134).
Hospital-acquired pneumonia is also more common in men (122, 135), and male sex is a risk factor for aspiration pneumonia in older patients (136). Furthermore, males are 1.6 times more likely to develop ventilator-associated pneumonia (137), although women have more severe disease and higher mortality (122, 138).
Animal models suggest that sex hormones are involved in pneumonia caused by different pathogens. In some instances, estrogen appears to have a protective role. In a mouse model of pneumococcal pneumonia, E2 promoted control of macrophage inflammatory activity and resolution of lung inflammation (139). In contrast, in a murine model of Acinetobacter baumannii pneumonia, female mice were more susceptible to infection, and treating male mice with E2 increased their susceptibility (140).
(iii) Tuberculosis. TB is the leading cause of death from a bacterial disease among adults worldwide. TB rates are significantly higher in men than in women. According to the World Health Organization, men accounted for 56% of all TB cases in 2020 versus 33% in adult women, with children accounting for the remaining 11% (141). However, the reasons for this bias are not entirely clear. It has been proposed that it could result from systematic underreporting and underdiagnosis of TB in women. Women may be less likely to seek appropriate medical care (142) and present difficulties in diagnostic testing, such as poorer-quality sputum samples (143). In addition, men undergo chest imaging sooner and are more likely to have a sputum smear sample performed (144). However, male bias persists when survey prevalence, rather than notification rates, is analyzed (141), and male predominance is seen even in low-burden countries where differences in access to health care should be negligible (78).
Both gender- and sex-related factors play a role. Men have more social contacts and more often participate in activities that place them at higher risk for TB, such as traveling, smoking, drinking, spending time in settings conducive to transmission (e.g., bars), and engaging in hazardous careers (e.g., mining) (78). However, other risk factors, such as household contacts and HIV infection, are not male-biased.
Both human and animal studies have shown that protective immune responses against M. tuberculosis are largely mediated by CD4+ Th1 cells, which secrete IFN-γ, and this response is mediated by IL-12 (145). However, excessive inflammation can exacerbate lung infection and lead to early death. In a mouse model, elevated M. tuberculosis loads in males were associated with an early exaggerated pulmonary inflammatory response resulting in accelerated disease progression and increased mortality (146). B cells also play a role, and smaller B cell follicles have been reported in male compared with female lungs in mice and are associated with greater disease progression (147).
Male bias does not appear until puberty, suggesting a role for sex steroid hormones. Female and castrated male mice express significantly higher TNF-α, IFN γ, and IL-12 levels than uncastrated males (148), and treatment with testosterone increases their susceptibility to Mycobacterium intracellulare and Mycobacterium marinum infection (149). Conversely, estrogen appears to have a protective role, as ovariectomized mice have a higher susceptibility to Mycobacterium avium, which is lessened by treatment with E2 (150). This is paralleled in humans by postmenopausal women, who are more susceptible to M. avium complex disease (151).
Certain X-linked gene mutations and polymorphisms confer increased risk of TB. Mutations in CYBB result in X-linked chronic granulomatous disease in males and increase susceptibility to mycobacterial disease (152), and TLR8 polymorphisms are linked to tuberculosis susceptibility in males (153).
From a clinical standpoint, women are usually less symptomatic than males. Men are more likely to be smokers, have more comorbidities, and present with hemoptysis, weight loss, and pleural effusion (154). Men also have more advanced radiological findings than women (155) and begin treatment earlier (144), and in a prospective observational study, male sex was associated with worse treatment outcomes (154). Males also have higher treatment dropout rates (156) and are at higher risk of recurrence (157).
For unknown reasons, female sex is a risk factor for developing extrapulmonary TB; studies in the U.S. (158) and Nepal (159) found women to be 1.7 times more likely to develop extrapulmonary TB relative to males. In addition, a prospective cohort study in eight countries showed that significantly more women than men had extensively drug-resistant TB (160).
Urinary tract infections.
Urinary tract infections (UTIs) also exhibit a sex-based preference, with a bias toward women. Etiology is influenced by patient sex, as E. coli, Klebsiella pneumoniae, and Streptococcus agalactiae are more frequently isolated in females than in males, while the opposite is true for Enterococcus faecalis, Proteus mirabilis, and P. aeruginosa (161). The most common causative agent in both sexes is UPEC (162).
Male UTI shows a bimodal distribution at the extremes of age, whereas the burden of infection in women is durable over a lifetime (162). During the first few months of life, the incidence of UTI in boys exceeds that in girls (163), but afterwards, females of all ages are more prone to UTIs than males and around half of all women will experience at least one UTI during their lifetime (164). This gap significantly decreases with age as the incidence of benign prostatic hyperplasia, urinary retention, and incontinence increases in the male population.
The increased female susceptibility to UTI is due to several factors. The female urethra is shorter than its male counterpart, which has been proposed to make it easier for ascending bacteria to reach the bladder (165). Physical proximity of the urethral opening to the rectum and vagina is another important risk factor, as it can lead to colonization of the periurethral mucosa with enteric bacteria (166), and vaginal dysbiosis is associated with an increased risk of UTI (167). A dryer environment at the urethral opening and the anti-bacterial properties of prostate secretions are additional protective features in men (168).
While more common in women, UTIs are more persistent and have higher morbidity and risk of complications in men. Other organs, namely, the prostate, are often involved (168), and male UTIs are usually treated with antibiotics for a longer period compared with female UTIs (169). In UPEC-infected mice, more males than females are unable to clear bacteria and remain chronically infected, and male mice more frequently develop advanced pyelonephritis and kidney abscesses compared with females (170, 171). Furthermore, there is a strong and rapid increase in proinflammatory cytokine expression in female mice which is not observed in males and a larger infiltration by immune cells (170, 171), which may contribute to better bacterial clearance.
Sex hormones are also likely involved. Treatment of UPEC-infected female mice with testosterone leads to persistent bacteriuria and chronic cystitis (170), and castrated male mice have significantly lower bacterial burdens than sham-operated controls (171). After menopause, decreased estrogen levels contribute to physiologic and structural changes which increase the risk of UTI in postmenopausal women, such as reduced urinary flow, increased postvoid residual volume, and incontinence (172) along with a rise in vaginal pH, loss of commensal lactobacilli, and increased vaginal colonization by enteric organisms (165). Furthermore, randomized controlled trials have shown that vaginal estrogen administration reduces UTI recurrence rates in postmenopausal women (173).
Sexually transmitted infections.
Despite being curable, bacterial sexually transmitted infections (STIs) are associated with a significant burden of disease. STI-related morbidity disproportionately affects women, with important implications for women of reproductive age.
In many societies, more restrictive sociocultural norms regarding sexual behavior in women may limit their sexual freedom, restrict their access to information, and reduce their ability to practice safe sexual behaviors (174). Male-to-female transmission of STIs is also thought to be more efficient than female-to-male transmission, possibly due to retention of the infected ejaculate within the vagina and greater tissue injury during intercourse (175).
In addition, STIs are more often asymptomatic in women than in men. Undiagnosed and untreated STIs can result in important long-term reproductive complications, including pelvic inflammatory disease (PID), ectopic pregnancy, and infertility (176, 177). Furthermore, infections in pregnant women are associated with maternal morbidity as well as adverse fetal and perinatal outcomes (178).
(i) Chlamydia. Chlamydia is the most common bacterial STI globally (179). Persons between 15 and 24 years report the highest infection rates, and young women are twice as affected as men (180, 181), although this partly reflects screening programs which primarily target women.
The infection is asymptomatic in a large proportion of cases in both sexes, especially in women (182), but if left untreated can cause severe damage, particularly to the female reproductive tract, and chlamydia is an important cause of PID (176). In men, urethritis can be complicated by epididymitis and male infertility (183). C. trachomatis is the most common genitourinary trigger of reactive arthritis, and Chlamydia-induced arthritis is most often seen in men (184).
The mechanisms by which sex hormones affect C. trachomatis infections are not entirely clear. The likelihood of developing chlamydial or gonococcal salpingitis has been reported to be highest during the estrogen-dominant proliferative phase of the menstrual cycle (185), and a positive correlation was shown between chlamydial load and E2 levels in women (186). In vitro studies have also demonstrated that estrogen enhances chlamydial adherence and intracellular development (187). In contrast, other studies have found increased detection of C. trachomatis during the secretory phase when P4 is higher (188).
(ii) Gonorrhea. Gonorrhea is the second most common bacterial STI (179) and rates of reported infections continue to increase, particularly among men. Rates are highest among adolescents and young adults, and men—especially men who have sex with men (MSM)—are currently more often affected than women in high-income countries (181, 189). In 2018, the male-to-female ratio was 3.2 in Europe and 1.4 in the United States (181, 189).
Urethritis is the most common manifestation of gonococcal infection in men, whereas the endocervical canal is the primary infection site in women (190). Most women show no symptoms of infection (182), while males are often symptomatic (191). Rectal gonorrhea occurs in both sexes and is usually asymptomatic in women, whereas cases in MSM can be associated with complaints of overt proctitis (192). Complications in men include epididymitis, infertility, prostatitis, and seminal vesiculitis (190). Similarly to chlamydial infection, PID is the main complication of gonorrhea in women (176). Disseminated gonococcal infection is the most common systemic complication in both sexes, and probably occurs more frequently in women (190). Estrogen likely plays a role, as E2-treated mice show an enhanced susceptibility to disseminated gonococcal infection (193).
The molecular mechanisms used by the gonococcus to initiate infection, and the resulting inflammatory response, also differ between sexes. In men, interaction with the urethral epithelial cells triggers the release of pro-inflammatory cytokines, promoting an inflammatory response and contributing to the symptomatic nature of gonococcal disease in men (194). Similarly, ascending gonococcal infection of the uterus and fallopian tubes also results in inflammation. In contrast, gonococcal cervicitis is mostly asymptomatic because the gonococcus can evade host immune function by subverting the alternative pathway of complement and does not elicit strong immune responses during uncomplicated genital infections in women (194).
Emergence of gonococcal antimicrobial resistance is a major public health threat, and one study found that men infected with N. gonorrhoeae had 4-fold higher expression of gonococcal antimicrobial resistance genes compared with women (195), which could have implications for sex-specific treatment.
Sepsis.
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (196), and it is a major global health problem (197). If not identified and treated promptly, it can lead to septic shock, multiple organ failure, and death (196). The most common sources are the respiratory tract in males and the urinary tract in females (198, 199). Most studies report higher rates of sepsis and septic shock in males, who account for 55% to 64% of cases (200–202), and male sex has been identified as a predictor of sepsis after trauma (203) and surgery (204). Experimental studies have consistently shown a survival advantage and a protective effect of sex hormones in females. In humans, however, reports on sex and mortality have shown conflicting results. Some have found higher mortality in women (199, 205), others in men (198, 206), whereas others reported no differences (207, 208).
In animal models, estrogen exerts a protective effect by maintaining adequate immune responses and cardiac function. Ovariectomized females show depressed macrophage and splenocyte functions after trauma-hemorrhage, which are associated with significantly increased mortality from subsequent sepsis (209, 210), and addition of E2 normalizes immune functional capacities (210). Sepsis-induced cardiac dysfunction is also less pronounced in female mice than in males (211). In contrast, in humans, circulating E2 levels are increased in non-survivors compared to survivors (212). Testosterone levels are generally low in male patients with sepsis (213), and androgen depletion appears to be protective in animals. Testosterone receptor blockade after trauma-hemorrhage in male mice restores depressed immune functions and improves survival following subsequent sepsis (214). Males also show an inappropriate inflammatory response to sepsis and produce significantly higher levels of pro-inflammatory cytokines (including TNF-α, IL-6, IL-8, IL-1β, and procalcitonin) following endotoxemia induction or sepsis than females (22, 215, 216), which could render them more susceptible to septic shock.
Sex-based differences in health care have also been reported. Women experience significantly longer delays to initial antibiotic administration than men (217, 218), and in a nationwide cohort study, a complete 1-h emergency department sepsis care bundle was fulfilled 38% more often in men (218). In the UCI, women are less likely to receive deep venous thrombosis prophylaxis, hemodialysis catheters, invasive mechanical ventilation (199, 205), or vasopressor support (207), and have a shorter length of stay (206) compared with men.
Other infections.
(i) Lyme borreliosis. Lyme borreliosis is the most common human vector-borne infection in Europe and the U.S. (219). It has a slight male predominance in the U.S., with around 57% of cases being male (220), presumably due to higher occupational risks and outdoor recreational activities. However, in Europe, 55% to 60% of affected patients are female (221–223). Furthermore, females have been reported to attract 33% more tick bites than males, despite spending less time outdoors (224).
One study in Sweden found that erythema migrans in women was less likely to have the classic “bull’s eye” appearance, and the duration from treatment until disappearance of the lesion was significantly longer in women than in men (224).
In the U.S., 70% of patients with Lyme carditis and 60% of cases of Lyme arthritis are male (220). A retrospective study in Slovenia reported that 75% of patients with Lyme arthritis were men, and men accounted for 60% of cases of neuroborreliosis (225). Acrodermatitis chronica atrophicans, a late cutaneous manifestation of Lyme disease, was more common in females, who represented nearly 70% of cases (225). Women may also be at higher risk of developing post-treatment Lyme disease syndrome (226). Reinfection rates are higher in women, particularly postmenopausal women (227), which could be due to falling estrogen levels and differences in the immune response (228).
Sex also impacts diagnosis, because the recommended two-tier testing is male biased. The magnitude of enzyme-linked immunosorbent assay (ELISA) and IgG serologic responses is greater in men (229) and men have on average six reactive bands on the IgG immunoblot, whereas women have only four (230). Current Centers for Disease Control and Prevention criteria require five bands for a positive test, likely underestimating the true number of female cases (230).
(ii) Q fever. Q fever is a zoonosis caused by Coxiella burnetii (231). Seroprevalence is higher in men, a study in Australia reporting a male-to-female ratio of around 1.6 (77). An even greater proportion of men are diagnosed with the disease (sex ratio of 2 to 5) (232, 233), suggesting that men develop symptomatic Q fever more often than women (234). In contrast, boys and girls are almost equally represented (231), suggesting that sex hormones could be involved.
In C. burnetii-infected mice, bacterial loads and granuloma numbers were lower in intact females than in males and ovariectomized females, and treatment with E2 reduced bacterial loads and granuloma numbers in ovariectomized mice (235). P4 inhibits C. burnetii replication in infected placenta-derived cells (236) and bacterial loads increase toward parturition (237) when P4 levels decrease. However, both animals and humans exhibit an increased risk of persistent infection and unfavorable outcomes during pregnancy (231), likely due to impaired cellular immunity.
(iii) Meningitis. Bacterial meningitis is an infection of the membranes that cover the brain and spinal cord caused by a bacterial pathogen. S. pneumoniae, N. meningitidis, and Haemophilus influenzae are the most frequently isolated bacteria. Some studies have reported similar rates of bacterial meningitis in men and women, while others have found a slight male bias (238–241). Male sex has been identified as a predictor of poor outcomes in children (242, 243) and adults (238, 241), despite females having a higher disease severity and higher inflammation markers on admission (238). This may be in part related to a better female response to anti-inflammatory treatment with corticosteroids (244). Sex steroid hormones may also play a role.
CONCLUSIONS
Many bacterial infections exhibit sex and gender differences in pathophysiology, incidence, clinical presentation, disease course, response to treatment, and outcome. Both biological and gender factors come into play and their recognition is essential to improving patient care. Behavioral differences play an important role in the exposure to pathogens, whereas sex differences in the immune response are directly influenced by sex chromosome complement and concentrations of sex steroid hormones.
Nevertheless, these observations have not been systematically integrated into research practices or resulted in changes to medical guidelines, which are mostly not sex-specific. This needs to change, and funding agencies and medical journals should promote scientific research that is sex-conscious and provides sex-disaggregated data. Incorporating implementation science methods to translate existing evidence into sex-specific guidelines is essential to promote improved and more personalized patient care.
ACKNOWLEDGMENTS
S.P.D. has received grants from the European Society of Clinical Microbiology and Infectious Diseases and the European Academy of Neurology. M.C.B. is supported by the Netherlands Organisation for Health Research and Development (ZonMw, NWO-Vidi-Grant [917.17.308]) and the European Research Council (ERC Consolidator Grant [101001237]). D.V.D.B. is funded by the Netherlands Organisation for Health Research and Development (ZonMW, NWO-Vici-Grant [91819627]). No specific funding was provided for this work.
We declare no competing interests.
Contributor Information
Diederik van de Beek, Email: d.vandebeek@amsterdamumc.nl.
Anthony R. Richardson, University of Pittsburgh
REFERENCES
- 1.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. 2015. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14:309–321. 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shannon G, Jansen M, Williams K, Cáceres C, Motta A, Odhiambo A, Eleveld A, Mannell J. 2019. Gender equality in science, medicine, and global health: where are we at and why does it matter? Lancet 393:560–569. 10.1016/S0140-6736(18)33135-0. [DOI] [PubMed] [Google Scholar]
- 3.Fish EN. 2008. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol 8:737–744. 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tukiainen T, Villani A-C, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, GTEx Consortium, Lappalainen T, Regev A, Ardlie KG, Hacohen N, MacArthur DG. 2017. Landscape of X chromosome inactivation across human tissues. Nature 550:244–248. 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souyris M, Cenac C, Azar P, Daviaud D, Canivet A, Grunenwald S, Pienkowski C, Chaumeil J, Mejía JE, Guéry J-C. 2018. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol 3:eaap8855. 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 6.Migeon BR. 2007. Why females are mosaics, X-chromosome inactivation, and sex differences in disease. Gend Med 4:97–105. 10.1016/S1550-8579(07)80024-6. [DOI] [PubMed] [Google Scholar]
- 7.Pessach IM, Notarangelo LD. 2009. X-linked primary immunodeficiencies as a bridge to better understanding X-chromosome related autoimmunity. J Autoimmun 33:17–24. 10.1016/j.jaut.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Libert C, Dejager L, Pinheiro I. 2010. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 10:594–604. 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 9.Pinheiro I, Dejager L, Libert C. 2011. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays 33:791–802. 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- 10.Dorhoi A, Iannaccone M, Farinacci M, Faé KC, Schreiber J, Moura-Alves P, Nouailles G, Mollenkopf H-J, Oberbeck-Müller D, Jörg S, Heinemann E, Hahnke K, Löwe D, Del Nonno F, Goletti D, Capparelli R, Kaufmann SHE. 2013. MicroRNA-223 controls susceptibility to tuberculosis by regulating lung neutrophil recruitment. J Clin Invest 123:4836–4848. 10.1172/JCI67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maan AA, Eales J, Akbarov A, Rowland J, Xu X, Jobling MA, Charchar FJ, Tomaszewski M. 2017. The Y chromosome: a blueprint for men’s health? Eur J Hum Genet 25:1181–1188. 10.1038/ejhg.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun S-l, Horino S, Itoh-Nakadai A, Kawabe T, Asao A, Takahashi T, So T, Funayama R, Kondo M, Saitsu H, Matsumoto N, Nakayama K, Ishii N. 2013. Y chromosome-linked B and NK cell deficiency in mice. J Immunol 190:6209–6220. 10.4049/jimmunol.1300303. [DOI] [PubMed] [Google Scholar]
- 13.Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16:626–638. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 14.Piasecka B, Duffy D, Urrutia A, Quach H, Patin E, Posseme C, Bergstedt J, Charbit B, Rouilly V, MacPherson CR, Hasan M, Albaud B, Gentien D, Fellay J, Albert ML, Quintana-Murci L, Milieu Intérieur Consortium . 2018. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc Natl Acad Sci USA 115:E488–E497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdullah M, Chai P-S, Chong M-Y, Tohit ERM, Ramasamy R, Pei CP, Vidyadaran S. 2012. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell Immunol 272:214–219. 10.1016/j.cellimm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Bain BJ, England JM. 1975. Normal haematological values: sex difference in neutrophil count. Br Med J 1:306–309. 10.1136/bmj.1.5953.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein Y, Ran S, Segal S. 1984. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol 132:656–661. [PubMed] [Google Scholar]
- 18.Beenakker KGM, Westendorp RGJ, de Craen AJM, Chen S, Raz Y, Ballieux BEPB, Nelissen RGHH, Later AFL, Huizinga TW, Slagboom PE, Boomsma DI, Maier AB. 2020. Men have a stronger monocyte-derived cytokine production response upon stimulation with the Gram-negative stimulus Lipopolysaccharide than women: a pooled analysis including 15 study populations. J Innate Immun 12:142–153. 10.1159/000499840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Nakabo S, Blanco LP, O’Neil LJ, Wigerblad G, Goel RR, Mistry P, Jiang K, Carmona-Rivera C, Chan DW, Wang X, Pedersen HL, Gadkari M, Howe KN, Naz F, Dell’Orso S, Hasni SA, Dempsey C, Buscetta A, Frischmeyer-Guerrerio PA, Kruszka P, Muenke M, Franco LM, Sun H-W, Kaplan MJ. 2020. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci USA 117:16481–16491. 10.1073/pnas.2003603117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galligan CL, Fish EN. 2015. Sex differences in the immune response. In Klein SL, Roberts CW (ed). Sex and gender differences in infection and treatments for infectious diseases. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- 21.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. 2011. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118:5918–5927. 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aomatsu M, Kato T, Kasahara E, Kitagawa S. 2013. Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem Biophys Res Commun 441:220–225. 10.1016/j.bbrc.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 23.Uppal SS, Verma S, Dhot PS. 2003. Normal values of CD4 and CD8 lymphocyte subsets in healthy indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom 52:32–36. 10.1002/cyto.b.10011. [DOI] [PubMed] [Google Scholar]
- 24.Afshan G, Afzal N, Qureshi S. 2012. CD4+CD25(hi) regulatory T cells in healthy males and females mediate gender difference in the prevalence of autoimmune diseases. Clin Lab 58:567–571. [PubMed] [Google Scholar]
- 25.Obiandu C, Okerengwo AA, Dapper DV. 2013. Levels of serum immunoglobulins in apparently healthy children and adults in Port Harcourt. Niger J Physiol Sci 28:23–27. [PubMed] [Google Scholar]
- 26.Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, Kumar V, Diavatopoulos DA, Jansen AFM, Lemmers H, Toenhake-Dijkstra H, van Herwaarden AE, Janssen M, van der Molen RG, Joosten I, Sweep FCGJ, Smit JW, Netea-Maier RT, Koenders MMJF, Xavier RJ, van der Meer JWM, Dinarello CA, Pavelka N, Wijmenga C, Notebaart RA, Joosten LAB, Netea MG. 2016. Host and environmental factors influencing individual human cytokine responses. Cell 167:1111–1124.e13. 10.1016/j.cell.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. 2005. A Yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology 146:3280–3285. 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 28.Mitsui T, Araki A, Miyashita C, Ito S, Ikeno T, Sasaki S, Kitta T, Moriya K, Cho K, Morioka K, Kishi R, Shinohara N, Takeda M, Nonomura K. 2019. Effects of prenatal sex hormones on behavioral sexual dimorphism. Pediatr Int 61:140–146. 10.1111/ped.13756. [DOI] [PubMed] [Google Scholar]
- 29.MacLaughlin DT, Donahoe PK. 2004. Sex determination and differentiation. N Engl J Med 350:367–378. 10.1056/NEJMra022784. [DOI] [PubMed] [Google Scholar]
- 30.Pennell LM, Galligan CL, Fish EN. 2012. Sex affects immunity. J Autoimmun 38:J282–J291. 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Robinson DP, Klein SL. 2012. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62:263–271. 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Gómez E, González-Pedrajo B, Camacho-Arroyo I. 2013. Role of sex steroid hormones in bacterial-host interactions. Biomed Res Int 2013:928290. 10.1155/2013/928290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton KJ, Hewitt SC, Arao Y, Korach KS. 2017. Estrogen hormone biology. Curr Top Dev Biol 125:109–146. 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson J-A. 2007. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87:905–931. 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 35.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. 2005. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett 97:107–113. 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Laffont S, Rouquié N, Azar P, Seillet C, Plumas J, Aspord C, Guéry J-C. 2014. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-α production of plasmacytoid dendritic cells from women. J Immunol 193:5444–5452. 10.4049/jimmunol.1303400. [DOI] [PubMed] [Google Scholar]
- 37.Laffont S, Seillet C, Guery JC. 2017. Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol 8:108. 10.3389/fimmu.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seillet C, Laffont S, Trémollières F, Rouquié N, Ribot C, Arnal J-F, Douin-Echinard V, Gourdy P, Guéry J-C. 2012. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood 119:454–464. 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 39.Kovats S. 2015. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol 294:63–69. 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Straub RH. 2007. The complex role of estrogens in inflammation. Endocr Rev 28:521–574. 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 41.Lü FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ. 2002. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol 128:10–20. 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bynoe MS, Grimaldi CM, Diamond B. 2000. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci USA 97:2703–2708. 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakiani S, Olsen NJ, Kovacs WJ. 2013. Gonadal steroids and humoral immunity. Nat Rev Endocrinol 9:56–62. 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 44.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. 2008. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol 214:456–464. 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 45.Arruvito L, Sanz M, Banham AH, Fainboim L. 2007. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: implications for human reproduction. J Immunol 178:2572–2578. 10.4049/jimmunol.178.4.2572. [DOI] [PubMed] [Google Scholar]
- 46.Zoller AL, Kersh GJ. 2006. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol 176:7371–7378. 10.4049/jimmunol.176.12.7371. [DOI] [PubMed] [Google Scholar]
- 47.Merkel SM, Alexander S, Zufall E, Oliver JD, Huet-Hudson YM. 2001. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect Immun 69:6119–6122. 10.1128/IAI.69.10.6119-6122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chotirmall SH, Smith SG, Gunaratnam C, Cosgrove S, Dimitrov BD, O’Neill SJ, Harvey BJ, Greene CM, McElvaney NG. 2012. Effect of estrogen on pseudomonas mucoidy and exacerbations in cystic fibrosis. N Engl J Med 366:1978–1986. 10.1056/NEJMoa1106126. [DOI] [PubMed] [Google Scholar]
- 49.Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. 2006. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol 191:525–535. 10.1677/joe.1.06565. [DOI] [PubMed] [Google Scholar]
- 50.Butts CL, Bowers E, Horn JC, Shukair SA, Belyavskaya E, Tonelli L, Sternberg EM. 2008. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend Med 5:434–447. 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabahi F, Rola-Plesczcynski M, O’Connell S, Frenkel LD. 1995. Qualitative and quantitative analysis of T lymphocytes during normal human pregnancy. Am J Reprod Immunol 33:381–393. 10.1111/j.1600-0897.1995.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 52.Szekeres-Bartho J, Wegmann TG. 1996. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol 31:81–95. 10.1016/0165-0378(96)00964-3. [DOI] [PubMed] [Google Scholar]
- 53.Piccinni MP, Scaletti C, Maggi E, Romagnani S. 2000. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol 109:30–33. 10.1016/S0165-5728(00)00299-X. [DOI] [PubMed] [Google Scholar]
- 54.Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. 2001. Progesterone as an immunomodulatory molecule. Int Immunopharmacol 1:1037–1048. 10.1016/S1567-5769(01)00035-2. [DOI] [PubMed] [Google Scholar]
- 55.Kyurkchiev D, Ivanova-Todorova E, Hayrabedyan S, Altankova I, Kyurkchiev S. 2007. Female sex steroid hormones modify some regulatory properties of monocyte-derived dendritic cells. Am J Reprod Immunol 58:425–433. 10.1111/j.1600-0897.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 56.Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, Tonelli L, Sternberg EM. 2007. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol 19:287–296. 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- 57.Yao Y, Li H, Ding J, Xia Y, Wang L. 2017. Progesterone impairs antigen-non-specific immune protection by CD8 T memory cells via interferon-γ gene hypermethylation. PLoS Pathog 13:e1006736. 10.1371/journal.ppat.1006736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JH, Ulrich B, Cho J, Park J, Kim CH. 2011. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol 187:1778–1787. 10.4049/jimmunol.1003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccinni MP, Giudizi MG, Biagiotti R. 1995. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol 155:128–133. [PubMed] [Google Scholar]
- 60.Janakiraman V. 2008. Listeriosis in pregnancy: diagnosis, treatment, and prevention. Rev Obstet Gynecol 1:179–185. [PMC free article] [PubMed] [Google Scholar]
- 61.Kaushic C, Zhou F, Murdin AD, Wira CR. 2000. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun 68:4207–4216. 10.1128/IAI.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morse SA, Fitzgerald TJ. 1974. Effect of progesterone on Neisseria gonorrhoeae. Infect Immun 10:1370–1377. 10.1128/iai.10.6.1370-1377.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liggins M, Ramirez N, Magnuson N, Abel-Santos E. 2011. Progesterone analogs influence germination of Clostridium sordellii and Clostridium difficile spores in vitro. J Bacteriol 193:2776–2783. 10.1128/JB.00058-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahman F, Christian HC. 2007. Non-classical actions of testosterone: an update. Trends Endocrinol Metab 18:371–378. 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Liva SM, Voskuhl RR. 2001. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 167:2060–2067. 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 66.Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. 2006. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab 91:546–554. 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- 67.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. 2004. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89:3313–3318. 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 68.Koçar IH, Yesilova Z, Ozata M, Turan M, Sengül A, Ozdemir I. 2000. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter’s syndrome. Clin Exp Immunol 121:448–452. 10.1046/j.1365-2249.2000.01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yesilova Z, Ozata M, Kocar IH, Turan M, Pekel A, Sengul A, Ozdemír IC. 2000. The effects of gonadotropin treatment on the immunological features of male patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 85:66–70. 10.1210/jcem.85.1.6226. [DOI] [PubMed] [Google Scholar]
- 70.Rettew JA, Huet-Hudson YM, Marriott I. 2008. Testosterone reduces macrophage expression in the mouse of Toll-like receptor 4, a Trigger for inflammation and innate immunity. Biol Reprod 78:432–437. 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 71.Ho C-H, Lu Y-C, Fan C-K, Yu H-J, Liu H-T, Wu C-C, Chen K-C, Liu S-P, Cheng P-C. 2020. Testosterone regulates the intracellular bacterial community formation of uropathogenic Escherichia coli in prostate cells via STAT3. Int J Med Microbiol 310:151450. 10.1016/j.ijmm.2020.151450. [DOI] [PubMed] [Google Scholar]
- 72.Ho C-H, Fan C-K, Yu H-J, Wu C-C, Chen K-C, Liu S-P, Cheng P-C. 2017. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. PLoS One 12:e0180244. 10.1371/journal.pone.0180244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bini EI, D’Attilio L, Marquina-Castillo B, Mata-Espinosa D, Díaz A, Marquez-Velasco R, Ramos-Espinosa O, Gamboa-Domínguez A, Bay ML, Hernández-Pando R, Bottasso O. 2015. The implication of pro-inflammatory cytokines in the impaired production of gonadal androgens by patients with pulmonary tuberculosis. Tuberculosis (Edinb) 95:701–706. 10.1016/j.tube.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Reves RR, Pickering LK. 1992. Impact of child day care on infectious diseases in adults. Infect Dis Clin North Am 6:239–250. 10.1016/S0891-5520(20)30435-9. [DOI] [PubMed] [Google Scholar]
- 75.Mariwah S, Hampshire K, Kasim A. 2012. The impact of gender and physical environment on the handwashing behaviour of university students in Ghana. Trop Med Int Health 17:447–454. 10.1111/j.1365-3156.2011.02950.x. [DOI] [PubMed] [Google Scholar]
- 76.Fouskis I, Sandalakis V, Christidou A, Tsatsaris A, Tzanakis N, Tselentis Y, Psaroulaki A. 2018. The epidemiology of brucellosis in Greece, 2007–2012: a ‘One Health’ approach. Trans R Soc Trop Med Hyg 112:124–135. 10.1093/trstmh/try031. [DOI] [PubMed] [Google Scholar]
- 77.Gidding HF, Peng CQ, Graves S, Massey PD, Nguyen C, Stenos J, Quinn HE, McIntyre PB, Durrheim DN, Wood N. 2020. Q fever seroprevalence in Australia suggests one in twenty people have been exposed. Epidemiol Infect 148:e18. 10.1017/S0950268820000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nhamoyebonde S, Leslie A. 2014. Biological differences between the sexes and susceptibility to tuberculosis. J Infect Dis 209 Suppl 3:S100–S1006. 10.1093/infdis/jiu147. [DOI] [PubMed] [Google Scholar]
- 79.Naheed A, Breiman RF, Islam MS, Saha SK, Tabassum Naved R. 2019. Disparities by sex in care-seeking behaviors and treatment outcomes for pneumonia among children admitted to hospitals in Bangladesh. PLoS One 14:e0213238. 10.1371/journal.pone.0213238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitra AK, Rahman MM, Fuchs GJ. 2000. Risk factors and gender differentials for death among children hospitalized with diarrhoea in Bangladesh. J Health Popul Nutr 18:151–156. [PubMed] [Google Scholar]
- 81.Fowler RA, Sabur N, Li P, Juurlink DN, Pinto R, Hladunewich MA, Adhikari NK, Sibbald WJ, Martin CM. 2007. Sex-and age-based differences in the delivery and outcomes of critical care. Cmaj 177:1513–1519. 10.1503/cmaj.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, Abdollahi M, Abdollahpour I, Abolhassani H, Aboyans V, Abrams EM, Abreu LG, Abrigo MRM, Abu-Raddad LJ, Abushouk AI, Acebedo A, Ackerman IN, Adabi M, Adamu AA, Adebayo OM, Adekanmbi V, Adelson JD, Adetokunboh OO, Adham D, Afshari M, Afshin A, Agardh EE, Agarwal G, Agesa KM, Aghaali M, Aghamir SMK, Agrawal A, Ahmad T, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmadpour E, Akalu TY, Akinyemi RO, Akinyemiju T, Akombi B, Al-Aly Z, Alam K, Alam N, Alam S, Alam T, et al. 2020. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:1204–1222. 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang S, Leff MG, McTague D, Horvath KA, Jackson-Thompson J, Murayi T, Boeselager GK, Melnik TA, Gildemaster MC, Ridings DL, Altekruse SF, Angulo FJ. 1998. Multistate surveillance for food-handling, preparation, and consumption behaviors associated with foodborne diseases: 1995 and 1996 BRFSS food-safety questions. MMWR CDC Surveill Summ 47:33–57. [PubMed] [Google Scholar]
- 84.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 85.Ferro A, Morais S, Pelucchi C, Dierssen-Sotos T, Martín V, López-Carrillo L, Malekzadeh R, Tsugane S, Hamada GS, Hidaka A, Hernández-Ramírez RU, López-Cervantes M, Zaridze D, Maximovitch D, Pourfarzi F, Zhang Z-F, Yu G-P, Pakseresht M, Ye W, Plymoth A, Leja M, Gasenko E, Derakhshan MH, Negri E, La Vecchia C, Peleteiro B, Lunet N. 2019. Sex differences in the prevalence of Helicobacter pylori infection: an individual participant data pooled analysis (StoP Project). Eur J Gastroenterol Hepatol 31:593–598. 10.1097/MEG.0000000000001389. [DOI] [PubMed] [Google Scholar]
- 86.Ibrahim A, Morais S, Ferro A, Lunet N, Peleteiro B. 2017. Sex-differences in the prevalence of Helicobacter pylori infection in pediatric and adult populations: systematic review and meta-analysis of 244 studies. Dig Liver Dis 49:742–749. 10.1016/j.dld.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Kato S, Matsukura N, Togashi A, Masuda G, Matsuda N, Yamada N, Naito Z, Matsuhisa T, Tajiri T. 2004. Sex differences in mucosal response to Helicobacter pylori infection in the stomach and variations in interleukin-8, COX-2 and trefoil factor family 1 gene expression. Aliment Pharmacol Ther 20 Suppl 1:17–24. 10.1111/j.1365-2036.2004.01985.x. [DOI] [PubMed] [Google Scholar]
- 88.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. 2012. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 21:20–38. 10.1158/1055-9965.EPI-11-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res 63:942–950. [PubMed] [Google Scholar]
- 90.Ohtani M, Ge Z, Garcia A, Rogers AB, Muthupalani S, Taylor NS, Xu S, Watanabe K, Feng Y, Marini RP, Whary MT, Wang TC, Fox JG. 2011. 17 β-estradiol suppresses Helicobacter pylori-induced gastric pathology in male hypergastrinemic INS-GAS mice. Carcinogenesis 32:1244–1250. 10.1093/carcin/bgr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohtani M, Garcia A, Rogers AB, Ge Z, Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC, Fox JG. 2007. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis 28:2597–2604. 10.1093/carcin/bgm150. [DOI] [PubMed] [Google Scholar]
- 92.Osato MS, Reddy R, Reddy SG, Penland RL, Malaty HM, Graham DY. 2001. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch Intern Med 161:1217–1220. 10.1001/archinte.161.9.1217. [DOI] [PubMed] [Google Scholar]
- 93.Chang YW, Ko WJ, Oh CH, Park YM, Oh SJ, Moon JR, Cho J-H, Kim J-W, Jang J-Y. 2019. Clarithromycin resistance and female gender affect Helicobacter pylori eradication failure in chronic gastritis. Korean J Intern Med 34:1022–1029. 10.3904/kjim.2018.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leffler DA, Lamont JT. 2015. Clostridium difficile infection. N Engl J Med 372:1539–1548. 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 95.Turner NA, Grambow SC, Woods CW, Fowler VG, Moehring RW, Anderson DJ, Lewis SS. 2019. Epidemiologic trends in Clostridioides difficile infections in a regional community hospital network. JAMA Netw Open 2:e1914149. 10.1001/jamanetworkopen.2019.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Worth LJ, Spelman T, Bull AL, Brett JA, Richards MJ. 2016. Epidemiology of Clostridium difficile infections in Australia: enhanced surveillance to evaluate time trends and severity of illness in Victoria, 2010–2014. J Hosp Infect 93:280–285. 10.1016/j.jhin.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 97.Chitnis AS, Holzbauer SM, Belflower RM, Winston LG, Bamberg WM, Lyons C, Farley MM, Dumyati GK, Wilson LE, Beldavs ZG, Dunn JR, Gould LH, MacCannell DR, Gerding DN, McDonald LC, Lessa FC. 2013. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med 173:1359–1367. 10.1001/jamainternmed.2013.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Younas M, Royer J, Weissman SB, Winders HR, Dash S, Bookstaver PB, Justo JA, Waites KS, Bell L, Al-Hasan MN. 2020. Clostridioides difficile infection and antibiotic prescription rates in the community: explaining the gender gap. Infect Control Hosp Epidemiol 42:622–624. 10.1017/ice.2020.1268. [DOI] [PubMed] [Google Scholar]
- 99.European Centre for Disease Prevention and Control. 2018. Clostridium difficile infections. Annual epidemiological report for 2016. ECDC, Stockholm, Sweden. [Google Scholar]
- 100.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. 1997. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis 24:324–333. 10.1093/clinids/24.3.324. [DOI] [PubMed] [Google Scholar]
- 101.Rouphael NG, O’Donnell JA, Bhatnagar J, Lewis F, Polgreen PM, Beekmann S, Guarner J, Killgore GE, Coffman B, Campbell J, Zaki SR, McDonald LC. 2008. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol 198:635.e1–635.e6. 10.1016/j.ajog.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 102.de Curraize C, Rousseau C, Corvec S, El-Helali N, Fihman V, Barbut F, Collignon A, Le Monnier A. 2018. Variable spectrum of disease and risk factors of peripartum Clostridium difficile infection: report of 14 cases from French hospitals and literature review. Eur J Clin Microbiol Infect Dis 37:2293–2299. 10.1007/s10096-018-3372-x. [DOI] [PubMed] [Google Scholar]
- 103.Legenza L, Barnett S, Rose W, Bianchini M, Safdar N, Coetzee R. 2018. Epidemiology and outcomes of Clostridium difficile infection among hospitalised patients: results of a multicentre retrospective study in South Africa. BMJ Glob Health 3:e000889. 10.1136/bmjgh-2018-000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Radoshevich L, Cossart P. 2018. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol 16:32–46. 10.1038/nrmicro.2017.126. [DOI] [PubMed] [Google Scholar]
- 105.Salem ML, Matsuzaki G, Madkour GA, Nomoto K. 1999. Beta-estradiol-induced decrease in IL-12 and TNF-alpha expression suppresses macrophage functions in the course of Listeria monocytogenes infection in mice. Int J Immunopharmacol 21:481–497. 10.1016/S0192-0561(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 106.Pung OJ, Luster MI, Hayes HT, Rader J. 1984. Influence of steroidal and nonsteroidal sex hormones on host resistance in mice: increased susceptibility to Listeria monocytogenes after exposure to estrogenic hormones. Infect Immun 46:301–307. 10.1128/iai.46.2.301-307.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernández Escámez PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Takkinen J, Wagner M, Arcella D, Da Silva Felicio MT, Georgiadis M, Messens W, Lindqvist R, EFSA Panel on Biological Hazards (BIOHAZ) . 2018. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J 16:e05134. 10.2903/j.efsa.2018.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Public Health England. 2021. Listeriosis in England and Wales: summary for 2019. Public Health England, London, United Kingdom. [Google Scholar]
- 109.Pasche B, Kalaydjiev S, Franz TJ, Kremmer E, Gailus-Durner V, Fuchs H, Hrabé de Angelis M, Lengeling A, Busch DH. 2005. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun 73:5952–5960. 10.1128/IAI.73.9.5952-5960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Falagas ME, Mourtzoukou EG, Vardakas KZ. 2007. Sex differences in the incidence and severity of respiratory tract infections. Respir Med 101:1845–1863. 10.1016/j.rmed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 111.Kaur R, Morris M, Pichichero ME. 2017. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics 140:e20170181. 10.1542/peds.2017-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Spratley J, Silveira H, Alvarez I, Pais-Clemente M. 2000. Acute mastoiditis in children: review of the current status. Int J Pediatr Otorhinolaryngol 56:33–40. 10.1016/S0165-5876(00)00406-7. [DOI] [PubMed] [Google Scholar]
- 113.Peer V, Schwartz N, Green MS. 2020. A multi-country, multi-year, meta-analytic evaluation of the sex differences in age-specific pertussis incidence rates. PLoS One 15:e0231570. 10.1371/journal.pone.0231570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tepper RS, Morgan WJ, Cota K, Wright A, Taussig LM. 1986. Physiologic growth and development of the lung during the first year of life. Am Rev Respir Dis 134:513–519. [DOI] [PubMed] [Google Scholar]
- 115.Ripoll JG, Guo W, Andersen KJ, Baker SE, Wiggins CC, Shepherd JRA, Carter RE, Welch BT, Joyner MJ, Dominelli PB. 2020. Sex differences in paediatric airway anatomy. Exp Physiol 105:721–731. 10.1113/EP088370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R. 2014. Gender differences in outcomes of patients with cystic fibrosis. J Womens Health (Larchmt) 23:1012–1020. 10.1089/jwh.2014.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen Y, Dales R, Lin M. 2003. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope 113:1199–1205. 10.1097/00005537-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 118.Teele DW, Klein JO, Rosner B. 1989. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis 160:83–94. 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 119.Hotomi M, Yamanaka N, Samukawa T, Suzumot M, Sakai A, Shimada J, Ikeda Y, Faden H. 2005. Treatment and outcome of severe and non-severe acute otitis media. Eur J Pediatr 164:3–8. 10.1007/s00431-004-1564-0. [DOI] [PubMed] [Google Scholar]
- 120.Alho OP, Oja H, Koivu M, Sorri M. 1995. Risk factors for chronic otitis media with effusion in infancy. Each acute otitis media episode induces a high but transient risk. Arch Otolaryngol Head Neck Surg 121:839–843. 10.1001/archotol.1995.01890080011002. [DOI] [PubMed] [Google Scholar]
- 121.Tos M, Stangerup SE. 1985. Secretory otitis and pneumatization of the mastoid process: sexual differences in the size of mastoid cell system. Am J Otolaryngol 6:199–205. 10.1016/S0196-0709(85)80085-5. [DOI] [PubMed] [Google Scholar]
- 122.López-de-Andrés A, Albaladejo-Vicente R, de Miguel-Diez J, Hernández-Barrera V, Ji Z, Zamorano-León JJ, Lopez-Herranz M, Carabantes Alarcon D, Jimenez-Garcia R. 2020. Gender differences in incidence and in-hospital outcomes of community-acquired, ventilator-associated and nonventilator hospital-acquired pneumonia in Spain. Int J Clin Pract 75:e13762. 10.1111/ijcp.13762. [DOI] [PubMed] [Google Scholar]
- 123.Fine MJ, Smith MA, Carson CA, Mutha SS, Sankey SS, Weissfeld LA, Kapoor WN. 1996. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 275:134–141. 10.1001/jama.1996.03530260048030. [DOI] [PubMed] [Google Scholar]
- 124.Pessoa E, Bárbara C, Viegas L, Costa A, Rosa M, Nogueira P. 2020. Factors associated with in-hospital mortality from community-acquired pneumonia in Portugal: 2000–2014. BMC Pulm Med 20:18. 10.1186/s12890-019-1045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clark JE, Hammal D, Hampton F, Spencer D, Parker L. 2007. Epidemiology of community-acquired pneumonia in children seen in hospital. Epidemiol Infect 135:262–269. 10.1017/S0950268806006741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fritz CQ, Edwards KM, Self WH, Grijalva CG, Zhu Y, Arnold SR, McCullers JA, Ampofo K, Pavia AT, Wunderink RG, Anderson EJ, Bramley AM, Jain S, Williams DJ. 2019. Prevalence, risk factors, and outcomes of bacteremic pneumonia in children. Pediatrics 144:e20183090. 10.1542/peds.2018-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wagenvoort GHJ, Sanders EAM, Vlaminckx BJ, de Melker HE, van der Ende A, Knol MJ. 2017. Sex differences in invasive pneumococcal disease and the impact of pneumococcal conjugate vaccination in the Netherlands, 2004 to 2015. Euro Surveill 22:30481. 10.2807/1560-7917.ES.2017.22.10.30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Naucler P, Darenberg J, Morfeldt E, Örtqvist Å, Henriques Normark B. 2013. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax 68:571–579. 10.1136/thoraxjnl-2012-203106. [DOI] [PubMed] [Google Scholar]
- 129.Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. 2009. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50–80 years. Clin Infect Dis 49:1318–1325. 10.1086/606046. [DOI] [PubMed] [Google Scholar]
- 130.Wiemken TL, Carrico RM, Klein SL, Jonsson CB, Peyrani P, Kelley RR, Aliberti S, Blasi F, Fernandez-Gonzalez R, Lopardo G, Ramirez JA, CAPO Investigators . 2014. The effectiveness of the polysaccharide pneumococcal vaccine for the prevention of hospitalizations due to Streptococcus pneumoniae community-acquired pneumonia in the elderly differs between the sexes: results from the Community-Acquired Pneumonia Organization (CAPO) international cohort study. Vaccine 32:2198–2203. 10.1016/j.vaccine.2014.02.048. [DOI] [PubMed] [Google Scholar]
- 131.European Centre for Disease Prevention and Control. 2020. Legionnaire’s disease. Annual epidemiological report for 2018. ECDC, Stockholm, Sweden. [Google Scholar]
- 132.NNDSS Annual Report Working Group. 2019. Australia’s notifiable disease status, 2015: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell (2018) 43. 10.33321/cdi.2019.43.6. [DOI] [PubMed] [Google Scholar]
- 133.Fukushima S, Hagiya H, Otsuka Y, Koyama T, Otsuka F. 2021. Trends in the incidence and mortality of legionellosis in Japan: a nationwide observational study, 1999–2017. Sci Rep 11:7246. 10.1038/s41598-021-86431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Centers for Disease Control and Prevention. 2011. Legionellosis: United States, 2000–2009. MMWR Morb Mortal Wkly Rep 60:1083–1086. [PubMed] [Google Scholar]
- 135.Stenlund M, Sjodahl R, Pia Yngman-Uhlin RN. 2017. Incidence and potential risk factors for hospital-acquired pneumonia in an emergency department of surgery. Int J Qual Health Care 29:290–294. 10.1093/intqhc/mzx018. [DOI] [PubMed] [Google Scholar]
- 136.van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. 2011. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc 12:344–354. 10.1016/j.jamda.2010.12.099. [DOI] [PubMed] [Google Scholar]
- 137.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH. 2002. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 122:2115–2121. 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 138.Sharpe JP, Magnotti LJ, Weinberg JA, Brocker JA, Schroeppel TJ, Zarzaur BL, Fabian TC, Croce MA. 2014. Gender disparity in ventilator-associated pneumonia following trauma: identifying risk factors for mortality. J Trauma Acute Care Surg 77:161–165. 10.1097/TA.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 139.Xiong Y, Zhong Q, Palmer T, Benner A, Wang L, Suresh K, Damico R, D’Alessio FR. 2021. Estradiol resolves pneumonia via ERβ in regulatory T cells. JCI Insight 6:e133251. 10.1172/jci.insight.133251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pires S, Peignier A, Seto J, Smyth DS, Parker D. 2020. Biological sex influences susceptibility to Acinetobacter baumannii pneumonia in mice. JCI Insight 5:e132223. 10.1172/jci.insight.132223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.World Health Organization. 2021. Global tuberculosis report 2021. WHO, Geneva, Switzerland. [Google Scholar]
- 142.Johansson E, Long NH, Diwan VK, Winkvist A. 2000. Gender and tuberculosis control: perspectives on health seeking behaviour among men and women in Vietnam. Health Policy 52:33–51. 10.1016/s0168-8510(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 143.Khan MS, Dar O, Sismanidis C, Shah K, Godfrey-Faussett P. 2007. Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet 369:1955–1960. 10.1016/S0140-6736(07)60916-7. [DOI] [PubMed] [Google Scholar]
- 144.Dale K, Tay E, Trauer JM, Trevan P, Denholm J. 2017. Gender differences in tuberculosis diagnosis, treatment and outcomes in Victoria, Australia, 2002–2015. Int j Tuber Lung Dis 21:1264–1271. 10.5588/ijtld.17.0338. [DOI] [PubMed] [Google Scholar]
- 145.O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP. 2013. The immune response in tuberculosis. Annu Rev Immunol 31:475–527. 10.1146/annurev-immunol-032712-095939. [DOI] [PubMed] [Google Scholar]
- 146.Dibbern J, Eggers L, Schneider BE. 2017. Sex differences in the C57BL/6 model of Mycobacterium tuberculosis infection. Sci Rep 7:10957. 10.1038/s41598-017-11438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hertz D, Dibbern J, Eggers L, von Borstel L, Schneider BE. 2020. Increased male susceptibility to Mycobacterium tuberculosis infection is associated with smaller B cell follicles in the lungs. Sci Rep 10:5142. 10.1038/s41598-020-61503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bini EI, Mata Espinosa D, Marquina Castillo B, Barrios Payán J, Colucci D, Cruz AF, Zatarain ZL, Alfonseca E, Pardo MR, Bottasso O, Hernández Pando R. 2014. The influence of sex steroid hormones in the immunopathology of experimental pulmonary tuberculosis. PLoS One 9:e93831. 10.1371/journal.pone.0093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamamoto Y, Saito H, Setogawa T, Tomioka H. 1991. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun 59:4089–4096. 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsuyuguchi K, Suzuki K, Matsumoto H, Tanaka E, Amitani R, Kuze F. 2001. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 123:428–434. 10.1046/j.1365-2249.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uwamino Y, Nishimura T, Sato Y, Tamizu E, Asakura T, Uno S, Mori M, Fujiwara H, Ishii M, Kawabe H, Murata M, Hasegawa N. 2019. Low serum estradiol levels are related to Mycobacterium avium complex lung disease: a cross-sectional study. BMC Infect Dis 19:1055. 10.1186/s12879-019-4668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, de Beaucoudrey L, Puel A, Feinberg J, Valinetz E, Jannière L, Besse C, Boland A, Brisseau J-M, Blanche S, Lortholary O, Fieschi C, Emile J-F, Boisson-Dupuis S, Al-Muhsen S, Woda B, Newburger PE, Condino-Neto A, Dinauer MC, Abel L, Casanova J-L. 2011. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol 12:213–221. 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dalgic N, Tekin D, Kayaalti Z, Cakir E, Soylemezoglu T, Sancar M. 2011. Relationship between toll-like receptor 8 gene polymorphisms and pediatric pulmonary tuberculosis. Dis Markers 31:33–38. 10.1155/2011/545972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feng J-Y, Huang S-F, Ting W-Y, Chen Y-C, Lin Y-Y, Huang R-M, Lin C-H, Hwang J-J, Lee J-J, Yu M-C, Yu K-W, Lee Y-C, Su W-J. 2012. Gender differences in treatment outcomes of tuberculosis patients in Taiwan: a prospective observational study. Clin Microbiol Infect 18:E331–E337. 10.1111/j.1469-0691.2012.03931.x. [DOI] [PubMed] [Google Scholar]
- 155.Thorson A, Long NH, Larsson LO. 2007. Chest X-ray findings in relation to gender and symptoms: a study of patients with smear positive tuberculosis in Vietnam. Scand J Infect Dis 39:33–37. 10.1080/00365540600951176. [DOI] [PubMed] [Google Scholar]
- 156.Balasubramanian R, Garg R, Santha T, Gopi PG, Subramani R, Chandrasekaran V, Thomas A, Rajeswari R, Anandakrishnan S, Perumal M, Niruparani C, Sudha G, Jaggarajamma K, Frieden TR, Narayanan PR. 2004. Gender disparities in tuberculosis: report from a rural DOTS programme in south India. Int J Tuber Lung Dis 8:323–332. [PubMed] [Google Scholar]
- 157.Korhonen V, Soini H, Vasankari T, Ollgren J, Smit PW, Ruutu P. 2017. Recurrent tuberculosis in Finland 1995–2013: a clinical and epidemiological cohort study. BMC Infect Dis 17:721. 10.1186/s12879-017-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. 2009. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 49:1350–1357. 10.1086/605559. [DOI] [PubMed] [Google Scholar]
- 159.Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN. 2008. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal: a hospital-based retrospective study. BMC Infect Dis 8:8. 10.1186/1471-2334-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dalton T, Cegielski P, Akksilp S, Asencios L, Campos Caoili J, Cho S-N, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, Kim HJ, Kliiman K, Kurbatova E, Kvasnovsky C, Leimane V, van der Walt M, Via LE, Volchenkov GV, Yagui MA, Kang H, Akksilp R, Sitti W, Wattanaamornkiet W, Andreevskaya SN, Chernousova LN, Demikhova OV, Larionova EE, Smirnova TG, Vasilieva IA, Vorobyeva AV, Barry CE, Cai Y, Shamputa IC, Bayona J, Contreras C, Bonilla C, Jave O, Brand J, Lancaster J, Odendaal R, Chen MP, Diem L, Metchock B, Tan K, Taylor A, Wolfgang M, Cho E, Eum SY, Kwak HK, Lee J, Global PETTS Investigators., et al. 2012. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 380:1406–1417. 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Magliano E, Grazioli V, Deflorio L, Leuci AI, Mattina R, Romano P, Cocuzza CE. 2012. Gender and age-dependent etiology of community-acquired urinary tract infections. ScientificWorldJournal 2012:349597. 10.1100/2012/349597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Foxman B. 2010. The epidemiology of urinary tract infection. Nat Rev Urol 7:653–660. 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 163.Shaikh N, Morone NE, Bost JE, Farrell MH. 2008. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J 27:302–308. 10.1097/INF.0b013e31815e4122. [DOI] [PubMed] [Google Scholar]
- 164.Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 113 Suppl 1A:5S–13S. 10.1016/S0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 165.Abelson B, Sun D, Que L, Nebel RA, Baker D, Popiel P, Amundsen CL, Chai T, Close C, DiSanto M, Fraser MO, Kielb SJ, Kuchel G, Mueller ER, Palmer MH, Parker-Autry C, Wolfe AJ, Damaser MS. 2018. Sex differences in lower urinary tract biology and physiology. Biol Sex Differ 9:45. 10.1186/s13293-018-0204-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Czaja CA, Stamm WE, Stapleton AE, Roberts PL, Hawn TR, Scholes D, Samadpour M, Hultgren SJ, Hooton TM. 2009. Prospective cohort study of microbial and inflammatory events immediately preceding Escherichia coli recurrent urinary tract infection in women. J Infect Dis 200:528–536. 10.1086/600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harmanli OH, Cheng GY, Nyirjesy P, Chatwani A, Gaughan JP. 2000. Urinary tract infections in women with bacterial vaginosis. Obstet Gynecol 95:710–712. 10.1016/s0029-7844(99)00632-8. [DOI] [PubMed] [Google Scholar]
- 168.Wagenlehner FM, Weidner W, Pilatz A, Naber KG. 2014. Urinary tract infections and bacterial prostatitis in men. Curr Opin Infect Dis 27:97–101. 10.1097/QCO.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 169.van Nieuwkoop C, van der Starre WE, Stalenhoef JE, van Aartrijk AM, van der Reijden TJK, Vollaard AM, Delfos NM, van ‘t Wout JW, Blom JW, Spelt IC, Leyten EMS, Koster T, Ablij HC, van der Beek MT, Knol MJ, van Dissel JT. 2017. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med 15:70. 10.1186/s12916-017-0835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scharff AZ, Rousseau M, Mariano LL, Canton T, Consiglio CR, Albert ML, Fontes M, Duffy D, Ingersoll MA. 2019. Sex differences in IL-17 contribute to chronicity in male versus female urinary tract infection. JCI Insight 4:e122998. 10.1172/jci.insight.122998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Olson PD, Hruska KA, Hunstad DA. 2016. Androgens enhance male urinary tract infection severity in a new model. J Am Soc Nephrol 27:1625–1634. 10.1681/ASN.2015030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Robinson D, Cardozo L. 2011. Estrogens and the lower urinary tract. Neurourol Urodyn 30:754–757. 10.1002/nau.21106. [DOI] [PubMed] [Google Scholar]
- 173.Perrotta C, Aznar M, Mejia R, Albert X, Ng CW. 2008. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev 16:CD005131. 10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 174.Buvé A, Gourbin C, Laga M. 2008. Gender and sexually transmitted diseases, p 151–164. In Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH (ed). Sexually transmitted diseases, 4th ed. McGraw-Hill, New York, NY. [Google Scholar]
- 175.Wong T, Singh A, Mann J, Hansen L, McMahon S. 2004. Gender differences in bacterial STIs in Canada. BMC Womens Health 4 Suppl 1:S26. 10.1186/1472-6874-4-S1-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brunham RC, Gottlieb SL, Paavonen J. 2015. Pelvic inflammatory disease. N Engl J Med 372:2039–2048. 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 177.Reekie J, Donovan B, Guy R, Hocking JS, Kaldor JM, Mak D, Preen D, Ward J, Liu B, Chlamydia and Reproductive Health Outcome Investigators . 2019. Risk of ectopic pregnancy and tubal infertility following gonorrhea and chlamydia infections. Clin Infect Dis 69:1621–1623. 10.1093/cid/ciz145. [DOI] [PubMed] [Google Scholar]
- 178.Mullick S, Watson-Jones D, Beksinska M, Mabey D. 2005. Sexually transmitted infections in pregnancy: prevalence, impact on pregnancy outcomes, and approach to treatment in developing countries. Sex Transm Infect 81:294–302. 10.1136/sti.2002.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, Chico RM, Smolak A, Newman L, Gottlieb S, Thwin SS, Broutet N, Taylor MM. 2019. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 97:548–562P. 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.European Centre for Disease Prevention and Control. 2020. Chlamydia infection. Annual epidemiological report for 2018. ECDC, Stockholm, Sweden. [Google Scholar]
- 181.Centers for Disease Control and Prevention. 2019. Sexually transmitted disease surveillance 2018. U.S. Department of Health and Human Services, CDC, Atlanta, GA. [Google Scholar]
- 182.Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, Serwadda D, Wawer MJ, Habbema JDF. 2002. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? Int J STD AIDS 13:91–101. 10.1258/0956462021924712. [DOI] [PubMed] [Google Scholar]
- 183.Gimenes F, Souza RP, Bento JC, Teixeira JJV, Maria-Engler SS, Bonini MG, Consolaro MEL. 2014. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol 11:672–687. 10.1038/nrurol.2014.285. [DOI] [PubMed] [Google Scholar]
- 184.Bas S, Scieux C, Vischer TL. 2001. Male sex predominance in Chlamydia trachomatis sexually acquired reactive arthritis: are women more protected by anti-chlamydia antibodies? Ann Rheum Dis 60:605–611. 10.1136/ard.60.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sweet RL, Blankfort-Doyle M, Robbie MO, Schacter J. 1986. The occurrence of chlamydial and gonococcal salpingitis during the menstrual cycle. JAMA 255:2062–2064. 10.1001/jama.1986.03370150104037. [DOI] [PubMed] [Google Scholar]
- 186.Agrawal T, Vats V, Salhan S, Mittal A. 2009. Determination of chlamydial load and immune parameters in asymptomatic, symptomatic and infertile women. FEMS Immunol Med Microbiol 55:250–257. 10.1111/j.1574-695X.2008.00530.x. [DOI] [PubMed] [Google Scholar]
- 187.Bose SK, Goswami PC. 1986. Enhancement of adherence and growth of Chlamydia trachomatis by estrogen treatment of HeLa cells. Infect Immun 53:646–650. 10.1128/iai.53.3.646-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Forcey DS, Hocking JS, Tabrizi SN, Bradshaw CS, Chen MY, Fehler G, Nash JL, Fairley CK. 2014. Chlamydia detection during the menstrual cycle: a cross-sectional study of women attending a sexual health service. PLoS One 9:e85263. 10.1371/journal.pone.0085263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.European Centre for Disease Prevention and Control. 2019. Gonorrhea. Annual epidemiological report for 2018. ECDC, Stockholm, Sweden. [Google Scholar]
- 190.Handsfield HH, Hook EW. 2008. Gonococcal infections in the adult, p 627–645. In Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit JN, Corey L, Cohen MS, Watts DH (ed). Sexually transmitted diseases, 4th ed. McGraw-Hill, New York, NY. [Google Scholar]
- 191.Ong JJ, Fethers K, Howden BP, Fairley CK, Chow EPF, Williamson DA, Petalotis I, Aung E, Kanhutu K, De Petra V, Chen MY. 2017. Asymptomatic and symptomatic urethral gonorrhoea in men who have sex with men attending a sexual health service. Clin Microbiol Infect 23:555–559. 10.1016/j.cmi.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 192.Assi R, Hashim PW, Reddy VB, Einarsdottir H, Longo WE. 2014. Sexually transmitted infections of the anus and rectum. World J Gastroenterol 20:15262–15268. 10.3748/wjg.v20.i41.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kita E, Takahashi S, Yasui K, Kashiba S. 1985. Effect of estrogen (17 beta-estradiol) on the susceptibility of mice to disseminated gonococcal infection. Infect Immun 49:238–243. 10.1128/iai.49.1.238-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Edwards JL, Apicella MA. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 17:965–981. Table of contents. 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nudel K, McClure R, Moreau M, Briars E, Abrams AJ, Tjaden B, Su X-H, Trees D, Rice PA, Massari P, Genco CA. 2018. Transcriptome analysis of Neisseria gonorrhoeae during natural infection reveals differential expression of antibiotic resistance determinants between men and women. mSphere 3:e00312-18. 10.1128/mSphereDirect.00312-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, Angus DC. 2016. The Third International Consensus Definitions for sepsis and septic shock (Sepsis-3). JAMA 315:801–810. 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. 2020. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 395:200–211. 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adrie C, Azoulay E, Francais A, Clec’h C, Darques L, Schwebel C, Nakache D, Jamali S, Goldgran-Toledano D, Garrouste-Orgeas M, Timsit JF. 2007. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest 132:1786–1793. 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 199.Pietropaoli AP, Glance LG, Oakes D, Fisher SG. 2010. Gender differences in mortality in patients with severe sepsis or septic shock. Gend Med 7:422–437. 10.1016/j.genm.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, Parsonnet J, Panzer R, Orav EJ, Snydman DR, Black E, Schwartz JS, Moore R, Johnson BL, Platt R, Academic Medical Center Consortium Sepsis Project Working Group . 1997. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA 278:234–240. 10.1001/jama.1997.03550030074038. [DOI] [PubMed] [Google Scholar]
- 201.Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, Fiore G, Filippini C, Ranieri V. 2013. The influence of gender on the epidemiology of and outcome from severe sepsis. Crit Care 17:R50. 10.1186/cc12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sundararajan V, Macisaac CM, Presneill JJ, Cade JF, Visvanathan K. 2005. Epidemiology of sepsis in Victoria, Australia. Crit Care Med 33:71–80. 10.1097/01.CCM.0000150027.98160.80. [DOI] [PubMed] [Google Scholar]
- 203.Kisat M, Villegas CV, Onguti S, Zafar SN, Latif A, Efron DT, Haut ER, Schneider EB, Lipsett PA, Zafar H, Haider AH. 2013. Predictors of sepsis in moderately severely injured patients: an analysis of the National Trauma Data Bank. Surg Infect (Larchmt) 14:62–68. 10.1089/sur.2012.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Offner PJ, Moore EE, Biffl WL. 1999. Male gender is a risk factor for major infections after surgery. Arch Surg 134:935–938. Discussion 938–940. 10.1001/archsurg.134.9.935. [DOI] [PubMed] [Google Scholar]
- 205.Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, Kartachov M, Zebedies D, Trefzer T, Wernecke K-D, Spies C. 2011. Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care 15:R151. 10.1186/cc10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu J, Tong L, Yao J, Guo Z, Lui KY, Hu X, Cao L, Zhu Y, Huang F, Guan X, Cai C. 2019. Association of sex with clinical outcome in critically ill sepsis patients: a retrospective analysis of the large clinical database MIMIC-III. Shock 52:146–151. 10.1097/SHK.0000000000001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Madsen TE, Simmons J, Choo EK, Portelli D, McGregor AJ, Napoli AM. 2014. The DISPARITY Study: do gender differences exist in Surviving Sepsis Campaign resuscitation bundle completion, completion of individual bundle elements, or sepsis mortality? J Crit Care 29:473.e7–473.e11. 10.1016/j.jcrc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 208.van Vught LA, Scicluna BP, Wiewel MA, Hoogendijk AJ, Klein Klouwenberg PMC, Ong DSY, Cremer OL, Horn J, Franitza M, Toliat MR, Nürnberg P, Bonten MMJ, Schultz MJ, van der Poll T; MARS Consortium . 2017. Association of gender with outcome and host response in critically ill sepsis patients. Crit Care Med 45:1854–1862. 10.1097/CCM.0000000000002649. [DOI] [PubMed] [Google Scholar]
- 209.Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. 2002. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg 235:105–112. 10.1097/00000658-200201000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Knöferl MW, Angele MK, Schwacha MG, Bland KI, Chaudry IH. 2002. Preservation of splenic immune functions by female sex hormones after trauma-hemorrhage. Crit Care Med 30:888–893. 10.1097/00003246-200204000-00029. [DOI] [PubMed] [Google Scholar]
- 211.Chen J, Chiazza F, Collino M, Patel NSA, Coldewey SM, Thiemermann C. 2014. Gender dimorphism of the cardiac dysfunction in murine sepsis: signalling mechanisms and age-dependency. PLoS One 9:e100631. 10.1371/journal.pone.0100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tsang G, Insel MB, Weis JM, Morgan MAM, Gough MS, Frasier LM, Mack CM, Doolin KP, Graves BT, Apostolakos MJ, Pietropaoli AP. 2016. Bioavailable estradiol concentrations are elevated and predict mortality in septic patients: a prospective cohort study. Crit Care 20:335. 10.1186/s13054-016-1525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schröder J, Kahlke V, Staubach KH, Zabel P, Stüber F. 1998. Gender differences in human sepsis. Arch Surg 133:1200–1205. 10.1001/archsurg.133.11.1200. [DOI] [PubMed] [Google Scholar]
- 214.Angele MK, Wichmann MW, Ayala A, Cioffi WG, Chaudry IH. 1997. Testosterone receptor blockade after hemorrhage in males. Restoration of the depressed immune functions and improved survival following subsequent sepsis. Arch Surg 132:1207–1214. 10.1001/archsurg.1997.01430350057010. [DOI] [PubMed] [Google Scholar]
- 215.Oberholzer A, Keel M, Zellweger R, Steckholzer U, Trentz O, Ertel W. 2000. Incidence of septic complications and multiple organ failure in severely injured patients is sex specific. J Trauma 48:932–937. 10.1097/00005373-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 216.Frink M, Pape HC, van Griensven M, Krettek C, Chaudry IH, Hildebrand F. 2007. Influence of sex and age on mods and cytokines after multiple injuries. Shock 27:151–156. 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 217.Madsen TE, Napoli AM. 2014. The DISPARITY-II study: delays to antibiotic administration in women with severe sepsis or septic shock. Acad Emerg Med 21:1499–1502. 10.1111/acem.12546. [DOI] [PubMed] [Google Scholar]
- 218.Sunden-Cullberg J, Nilsson A, Inghammar M. 2020. Sex-based differences in ED management of critically ill patients with sepsis: a nationwide cohort study. Intensive Care Med 46:727–736. 10.1007/s00134-019-05910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mead PS. 2015. Epidemiology of Lyme disease. Infect Dis Clin North Am 29:187–210. 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 220.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. 2017. Surveillance for Lyme disease: United States, 2008–2015. MMWR Surveill Summ 66:1–12. 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wilking H, Stark K. 2014. Trends in surveillance data of human Lyme borreliosis from six federal states in eastern Germany, 2009–2012. Ticks Tick Borne Dis 5:219–224. 10.1016/j.ttbdis.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 222.Geebelen L, Van Cauteren D, Devleesschauwer B, Moreels S, Tersago K, Van Oyen H, Speybroeck N, Lernout T. 2019. Combining primary care surveillance and a meta-analysis to estimate the incidence of the clinical manifestations of Lyme borreliosis in Belgium, 2015–2017. Ticks Tick Borne Dis 10:598–605. 10.1016/j.ttbdis.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 223.Petrulionienė A, Radzišauskienė D, Ambrozaitis A, Čaplinskas S, Paulauskas A, Venalis A. 2020. Epidemiology of Lyme disease in a highly endemic European zone. Medicina (Kaunas) 56:115. 10.3390/medicina56030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bennet L, Stjernberg L, Berglund J. 2007. Effect of gender on clinical and epidemiologic features of Lyme borreliosis. Vector Borne Zoonotic Dis 7:34–41. 10.1089/vbz.2006.0533. [DOI] [PubMed] [Google Scholar]
- 225.Strle F, Wormser GP, Mead P, Dhaduvai K, Longo MV, Adenikinju O, Soman S, Tefera Y, Maraspin V, Lotrič-Furlan S, Ogrinc K, Cimperman J, Ružić-Sabljić E, Stupica D. 2013. Gender disparity between cutaneous and non-cutaneous manifestations of Lyme borreliosis. PLoS One 8:e64110. 10.1371/journal.pone.0064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ljøstad U, Mygland A. 2010. Remaining complaints 1 year after treatment for acute Lyme neuroborreliosis; frequency, pattern and risk factors. Eur J Neurol 17:118–123. 10.1111/j.1468-1331.2009.02756.x. [DOI] [PubMed] [Google Scholar]
- 227.Bennet L, Berglund J. 2002. Reinfection with Lyme borreliosis: a retrospective follow-up study in southern Sweden. Scand J Infect Dis 34:183–186. 10.1080/00365540110080070. [DOI] [PubMed] [Google Scholar]
- 228.Jarefors S, Bennet L, You E, Forsberg P, Ekerfelt C, Berglund J, Ernerudh J. 2006. Lyme borreliosis reinfection: might it be explained by a gender difference in immune response? Immunology 118:224–232. 10.1111/j.1365-2567.2006.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schwarzwalder A, Schneider MF, Lydecker A, Aucott JN. 2010. Sex differences in the clinical and serologic presentation of early Lyme disease: results from a retrospective review. Gend Med 7:320–329. 10.1016/j.genm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 230.Stricker RB, Johnson L, author reply 9–20 . 2009. Gender bias in chronic lyme disease. J Womens Health (Larchmt) 18:1717–1718. 10.1089/jwh.2009.1657. [DOI] [PubMed] [Google Scholar]
- 231.Raoult D, Marrie T, Mege J. 2005. Natural history and pathophysiology of Q fever. Lancet Infect Dis 5:219–226. 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 232.Tissot Dupont H, Raoult D, Brouqui P, Janbon F, Peyramond D, Weiller PJ, Chicheportiche C, Nezri M, Poirier R. 1992. Epidemiologic features and clinical presentation of acute Q fever in hospitalized patients: 323 French cases. Am J Med 93:427–434. 10.1016/0002-9343(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 233.Maurin M, Raoult D. 1999. Q fever. Clin Microbiol Rev 12:518–553. 10.1128/CMR.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tissot-Dupont H, Vaillant V, Rey S, Raoult D. 2007. Role of sex, age, previous valve lesion, and pregnancy in the clinical expression and outcome of Q fever after a large outbreak. Clin Infect Dis 44:232–237. 10.1086/510389. [DOI] [PubMed] [Google Scholar]
- 235.Leone M, Honstettre A, Lepidi H, Capo C, Bayard F, Raoult D, Mege J-L. 2004. Effect of sex on Coxiella burnetii infection: protective role of 17β-estradiol. J Infect Dis 189:339–345. 10.1086/380798. [DOI] [PubMed] [Google Scholar]
- 236.Howard ZP, Omsland A. 2020. Selective inhibition of Coxiella burnetii replication by the steroid hormone progesterone. Infect Immun 88:e00894-19. 10.1128/IAI.00894-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Roest H-J, van Gelderen B, Dinkla A, Frangoulidis D, van Zijderveld F, Rebel J, van Keulen L. 2012. Q fever in pregnant goats: pathogenesis and excretion of Coxiella burnetii. PLoS One 7:e48949. 10.1371/journal.pone.0048949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Dias SP, Brouwer MC, Bijlsma MW, van der Ende A, van de Beek D. 2017. Sex-based differences in adults with community-acquired bacterial meningitis: a prospective cohort study. Clin Microbiol Infect 23:121.e9–121.e15. 10.1016/j.cmi.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 239.Bodilsen J, Storgaard M, Larsen L, Wiese L, Helweg-Larsen J, Lebech A-M, Brandt C, Østergaard C, Nielsen H, DASGIB study group . 2018. Infectious meningitis and encephalitis in adults in Denmark: a prospective nationwide observational cohort study (DASGIB). Clin Microbiol Infect 24:1102.e1–1102.e5. 10.1016/j.cmi.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 240.Polkowska A, Toropainen M, Ollgren J, Lyytikäinen O, Nuorti JP. 2017. Bacterial meningitis in Finland, 1995–2014: a population-based observational study. BMJ Open 7:e015080. 10.1136/bmjopen-2016-015080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tubiana S, Varon E, Biron C, Ploy M-C, Mourvillier B, Taha M-K, Revest M, Poyart C, Martin-Blondel G, Lecuit M, Cua E, Pasquet B, Preau M, Hoen B, Duval X, Duval X, Hoen B, Mourvillier B, Ploy M-C, Tubiana S, Varon E, Caron F, Bollaert P-E, Gaillot O, Taha M-K, Poyart C, Bonacorsi S, Vandenesch F, Cambau E, Lecuit M, Gravet A, Frachet B, Broucker TD, Levy Bruhl D, Raffi F, Anguel N, Argaud L, Arista S, Armand-Lefevre L, Balavoine S, Baraduc R, Barnaud G, Beraud G, Bernard L, Bernars G, Bertei D, Bessede E, Billard Pomares T, Biron C, Bland S, et al. 2020. Community-acquired bacterial meningitis in adults: in-hospital prognosis, long-term disability and determinants of outcome in a multicentre prospective cohort. Clin Microbiol Infect 26:1192–1200. 10.1016/j.cmi.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 242.Oostenbrink R, Moons KG, Derksen-Lubsen G, Grobbee DE, Moll HA. 2002. Early prediction of neurological sequelae or death after bacterial meningitis. Acta Paediatr 91:391–398. 10.1080/080352502317371616. [DOI] [PubMed] [Google Scholar]
- 243.Valmari P, Mäkelä M, Kataja M, Peltola H. 1987. Multivariate prognostication in bacterial meningitis of childhood. Scand J Infect Dis 19:29–34. 10.3109/00365548709032374. [DOI] [PubMed] [Google Scholar]
- 244.Dias SP, Brouwer MC, van de Beek D. 2020. Sex-based differences in the response to dexamethasone in bacterial meningitis: analysis of the European dexamethasone in adulthood bacterial meningitis study. Br J Clin Pharmacol 86:386–391. 10.1111/bcp.14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, 3rd, St Sauver JL, Wilson WR, Baddour LM. 2007. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med 167:834–839. 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 246.Green MS, Schwartz N, Peer V. 2020. Sex differences in campylobacteriosis incidence rates at different ages: a seven country, multi-year, meta-analysis. A potential mechanism for the infection. BMC Infect Dis 20:625. 10.1186/s12879-020-05351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Strachan NJ, Watson RO, Novik V, Hofreuter D, Ogden ID, Galan JE. 2008. Sexual dimorphism in campylobacteriosis. Epidemiol Infect 136:1492–1495. 10.1017/S0950268807009934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Jansen A, Stark K, Schneider T, Schöneberg I. 2007. Sex differences in clinical leptospirosis in Germany: 1997–2005. Clin Infect Dis 44:e69–e72. 10.1086/513431. [DOI] [PubMed] [Google Scholar]
- 249.Traxler RM, Callinan LS, Holman RC, Steiner C, Guerra MA. 2014. Leptospirosis-associated hospitalizations, United States. Emerg Infect Dis 20:1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hjertqvist M, Ahlm C, Klingström J. 2010. Sex patterns in diagnoses of tularaemia, Sweden 1997–2008. J Infect 60:186–187. 10.1016/j.jinf.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 251.Zheng R, Xie S, Lu X, Sun L, Zhou Y, Zhang Y, Wang K. 2018. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int 2018:5712920. 10.1155/2018/5712920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Castro Á. 2016. Sexual behavior and sexual risks among Spanish university students: a descriptive study of gender and sexual orientation. Sex Res Soc Policy 13:84–94. 10.1007/s13178-015-0210-0. [DOI] [Google Scholar]
- 253.European Centre for Disease Prevention and Control. 2019. Syphilis and congenital syphilis in Europe: a review of epidemiological trends (2007–2018) and options for response. ECDC, Stockholm, Sweden. [Google Scholar]