Abstract
The prompt recruitment of neutrophils to the site of infection is essential for the defense of the bovine mammary gland against invading pathogens and is determinant for the outcome of the infection. Escherichia coli is known to induce clinical mastitis, characterized by an intense neutrophil recruitment leading to the eradication of the bacteria, whereas Staphylococcus aureus induces subclinical mastitis accompanied by a moderate neutrophil recruitment and the establishment of chronic mastitis. To elicit the neutrophil recruitment into the udder, inflammatory mediators must be produced after recognition of the invading pathogen. To our knowledge, those mediators have never been studied during S. aureus mastitis, although understanding of the neutrophil recruitment mechanisms could allow a better understanding of the differences in the pathogeneses elicited by E. coli and S. aureus. Therefore, we studied, at several time points, the accumulation of neutrophils and the presence of the chemoattractant complement fragment C5a and of the cytokines interleukin-1β (IL-1β), tumor necrosis factor alpha, and IL-8 in milk after inoculation of E. coli or S. aureus in lactating bovine udders. The low levels of C5a and the absence of cytokines in milk from S. aureus-infected cows, compared to the high levels found in milk from E. coli-infected animals, mirror the differences in the severities of the two inflammatory reactions. The cytokine deficit in milk after S. aureus inoculation in the lactating bovine mammary gland could contribute to the establishment of chronic mastitis. This result could help in the design of preventive or curative strategies against chronic mastitis.
An acute inflammatory reaction is crucial in the defense of host tissue against invading pathogens. Leukocytes, especially neutrophils, are the major contributors to this mechanism of natural defense, and their migration to the site of infection is determinant for the outcome of the infection. Neutrophil migration is elicited by inflammatory mediators which are produced in the infected tissue by cells responding to bacterial toxins or metabolites. The array of known inflammatory mediators is vast and includes complement fragments, arachidonic acid metabolites, vasoactive amines, and cytokines. Cytokines mediate the leukocyte recruitment to inflammatory sites by their chemotactic activity and by activation of adhesion molecules on circulating leukocytes and endothelial cells in adjacent vasculature (5, 22). Several cytokines, including interleukin 1β (IL-1β), IL-8, tumor necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor, and gamma interferon, and the complement fragment C5a are known to be important for the accumulation of leukocytes at sites of inflammation (3, 10, 18, 38, 42). Cytokines can also enhance the bactericidal activity of phagocytes (35, 43).
Inflammation of the mammary gland caused by invading pathogens is common among lactating dairy cows and is a major cause of economic losses (8). The mammary gland is particularly suited to investigations on the inflammatory response to infection at an epithelial surface, because it lends itself to kinetic studies of the appearance of inflammatory cells and mediators in the luminal compartment as a result of the ease of noninvasive and repeated samplings of milk. Bovine mastitis is of two types, i.e., (i) clinical or (ii) subclinical with eventual sporadic clinical episodes. Escherichia coli causes severe mastitis, during which death or extensive damage to mammary tissues may result (11, 20). When the cow survives, the clinical episode is followed by a spontaneous bacteriological cure (19). In contrast, Staphylococcus aureus infection often starts with an acute phase and generally becomes chronic and subclinical (44).
These opposed characteristics are likely to result from the different pathogenic mechanisms used by the two pathogens. Among these mechanisms, the role of bacterial adherence is a controversial topic (2, 14, 17). It has been suggested that adhesion is important for the virulence of S. aureus but not for E. coli, which multiplies rapidly within the milk compartment between milkings (19). Moreover, S. aureus expresses several specific virulence factors such as protein A and a capsule or a pseudocapsule (slime), which are antiphagocytic factors, and toxins (alpha and beta) which appear to play a major role in the staphylococcal virulence (44). On the other hand, E. coli releases endotoxins (lipopolysaccharide [LPS]-protein complexes) that appear to be the primary initiator of inflammation (23). Although LPS is not a chemoattractant for bovine neutrophils (7, 16), it can induce the production of cytokines, one of which, IL-1, mediates many of the biologic responses to LPS, including fever and the acute-phase response (10, 15).
The differences in the proposed pathogenesis and clinical reactions offer the opportunity to contrast two inflammatory responses which have different outcomes: the inflammatory reaction allows the eradication of E. coli and a bacteriological cure, whereas in S. aureus infection, bacteria are persistent in the udder and a chronic infection develops.
This work was designed to compare the early host responses to E. coli and S. aureus intramammary infections by measuring inflammatory markers (bovine serum albumin [BSA] and haptoglobin) and inflammatory mediators involved in neutrophil recruitment (TNF-α, IL-1β, IL-8, and C5a). To achieve this, experimental infections of the bovine udder were carried out. E. coli-induced inflammatory mediators in the bovine mammary gland have been the subject of previous works, in contrast to S. aureus-induced inflammatory mediators, which have never been studied. This research related the moderate cell recruitment during S. aureus infection to an apparent lack of production of the major chemoattractant and inflammatory mediators in the luminal compartment of the mammary gland, contrasting with the intense accumulation of mediators in the case of E. coli infection. These results contribute to a better understanding of subclinical, chronic infections at an epithelial surface.
MATERIALS AND METHODS
Animals.
Eleven clinically healthy Holstein cows in midlactation were experimentally infected in one randomly selected mammary gland. Quarters were determined to be free of bacterial infection prior to challenge upon bacteriological analyses. In experiment 1, five cows were challenged with E. coli. In experiment 2, the other six cows were challenged with S. aureus.
Bacterial challenge.
E. coli strain P4 and S. aureus strain 107-59 were isolated from natural cases of bovine mastitis (6, 31). The bacteria were cultivated in a brain heart infusion medium (Difco Laboratories, Detroit, Mich.) at 37°C for 5 to 6 h to allow them to be in the exponential growth phase. They were then harvested, washed once in pyrogen-free saline (PFS) and suspended in PFS. The optical density at 550 nm was measured, and the number of CFU was determined using a standard curve. After appropriate dilution in PFS, 0.2 ml of the bacterial suspension (∼50 CFU of E. coli and 50 to 100 CFU of S. aureus in experiment 1 and experiment 2, respectively) was injected in the challenged glands via the teat canal immediately after the evening milking.
Samples.
Just before the inoculation of the bacteria, foremilk and blood samples were collected and rectal temperatures were measured. After E. coli infusion, rectal temperatures and blood samples were obtained at 8, 12, 14, 16, 24, 48, and 72 h postinfusion (p.i.) and at 4, 8, and 14 days p.i., and sterile foremilk samples were collected at 4, 6, 8, 10, 12, 14, 16, 18, 24, 39, 48, 63, and 72 hours p.i. and at 4, 8, and 14 days p.i. After S. aureus infusion, rectal temperatures and blood and sterile foremilk samples were obtained once a day from day 1 to 5 p.i. and then at 7, 11, 14, 21, and 28 days p.i.
Aseptically taken foremilk samples (∼30 ml) were used for bacteriological and bacterial concentration analysis. A portion of this milk was also used to determine the somatic cell count (SCC), i.e. the number of cells in milk, as a measure of inflammation (recruitment of leukocytes). Another portion of foremilk was centrifuged; the cream was discarded, and the skim milk was harvested. The cell pellet was suspended in saline buffer, and a portion, after appropriate dilution, was cytocentrifuged for differential cellular count; the rest, after cell numeration, was centrifuged, and then the supernatant was discarded and the pellet was frozen (−20°C) for subsequent cytokine analysis. The skim milk was ultracentrifuged at 90,000 × g for 30 min, and the whey was harvested and stored frozen (−20°C) for subsequent BSA and cytokine analysis.
A portion of the blood samples was used to count under a microscope the total blood cell number after dilution in Hayes liquid. A drop was used for a smear to determine the differential cellular composition. The rest was centrifuged; the plasma was harvested and stored frozen (−20°C) for subsequent haptoglobin analysis.
Assays.
The SCC assays were performed with a Coulter Counter (Coultronics France S.A., Andilly, France). Differential cellular counts were performed under a microscope after May-Grünwald staining. Bacterial concentrations were obtained from cultures of appropriate dilutions of milk samples on sheep blood agar plates for 24 h at 37°C.
The concentration of BSA in milk samples was determined by the radial immunodiffusion technique (30). Haptoglobin in plasma was measured using a colorimetric method, and results were expressed as haptoglobin-binding capacity (Hb-BC mg/100 ml of plasma) (26).
Whey samples were analyzed for TNF-α, IL-1β, and IL-8 concentrations, in addition to the complement fragment C5a concentration. TNF-α and C5a were quantified by enzyme-linked immunosorbent assay (ELISA) as previously described (32, 33). Zymosan (2 mg/ml; Sigma Chemical Co., St. Louis, Mo.) was used in vitro to activate, in the whey samples, the complement that was not consumed in vivo; thereafter, C5a in both activated and nonactivated whey samples was measured by ELISA. IL-1β was measured by ELISA using commercial antibodies against ovine IL-1β (Serotec Ltd., Oxford, England). The coating monoclonal antibody and the detecting antiserum were diluted as suggested by the manufacturer. IL-8 was measured with a human IL-8 ELISA kit as described by the manufacturer (R&D Systems, Minneapolis, Minn.). IL-8 in the milk cell pellets was also measured using the same kit (27). Before ELISA, frozen cells were suspended in saline buffer at a concentration of 106 cells/ml and cells were lysed by two successive cycles of freezing and thawing.
Levels of inflammatory mediators in milk whey are reported as concentrations. No correction was made to account for changes in the volume of milk within the gland at any particular time.
Statistical analysis.
For each experiment (E. coli and S. aureus challenges), infected glands were compared to the same prechallenged glands in a paired t test. E. coli- and S. aureus-infected glands were compared in a two-sample t test. Data for bacterial concentrations and SCC were logarithmically transformed to maintain a normal distribution. Data are expressed as means ± standard errors of the means.
RESULTS
Clinical signs.
In the E. coli-challenged cows there was profound swelling of the infused quarters by 12 h, which increased in severity until 39 to 63 h, depending on the cow, and subsequently declined until quarters were normal by approximately 1 week. At the same times, the milk secretion was grossly abnormal, containing clots and from time to time blood. The S. aureus-infused glands showed no swelling, and there was no visible change in the milk appearance.
A systemic reaction occurred in association with the E. coli mastitic episode. Rectal temperatures increased from 38.3 ± 0.09°C to 41 ± 0.2°C at 16 h p.i. and were maximal at this time. After the S. aureus challenge, rectal temperatures did not increase (data not shown).
Bacteria and somatic cells.
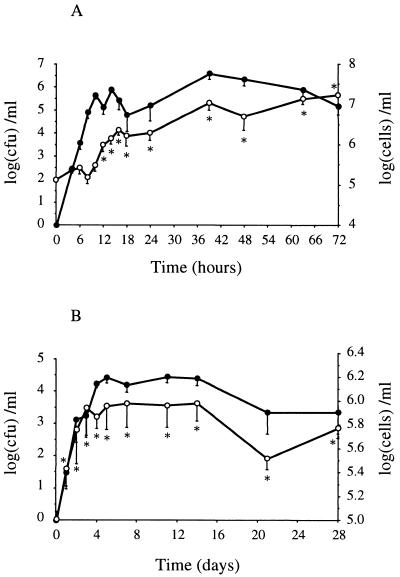
E. coli growth in the gland was exponential until the 10th hour. The bacterial peak concentration averaged 6.6 ± 0.3 log10 CFU/ml. The number of bacteria decreased slowly from the 39th hour until the end of the experiment (Fig. 1A). The SCC increased significantly (P < 0.015) from 5.07 ± 0.03 log10 cells/ml to 5.9 ± 0.2 log10 cells/ml at 12 h after E. coli infusion and reached a maximum of 7.2 ± 0.02 log10 cells/ml at 72 h p.i. (Fig. 1A). S. aureus growth in the udder was much lower than E. coli growth and varied markedly from one cow to another (Fig. 1B). The bacterial peak concentration averaged 4.4 ± 0.1 log10 CFU/ml at 11 days after challenge. The SCC increased significantly (P < 0.04) from 5.01 ± 0.07 log10 cells/ml to 5.4 ± 0.1 log10 cells/ml at 24 h p.i. and reached a plateau at 5.9 ± 0.2 log10 cells/ml from day 3 to day 14 after challenge. SCC kinetics were very different among the animals, and the SCC remained significantly (P < 0.0004) lower than that observed in E. coli-infected glands (Fig. 1B).
FIG. 1.
Bacterial count (●) and SCC (○) in milk samples from quarters experimentally infected with E. coli (A) or S. aureus (B). Data are expressed as means ± standard errors of the means. ∗, significant difference (P < 0.05) compared with preinfection values.
Differential cellular counts indicated that during the inflammatory episode, most of the recruited cells in the mammary gland were polymorphonuclear neutrophils. Before challenge, the percentage of polymorphonuclear neutrophils in milk was 38.8% ± 5.2% of the total cells. After E. coli infusion, it increased dramatically up to 97.3% ± 0.9% at 16 h p.i., but after 3 days, mononuclear cells constituted again the main population (76% ± 5.6% of the total cells). After S. aureus infusion, the neutrophil percentage did not increase so much, reaching 70% ± 6.2% at 2 days p.i. The percentage remained high (74.3% ± 3.7% of the total cells) until the 28th day p.i., the end of the experiment (data not shown).
Foremilk was still collected during the month following the experimental infections for bacteriological analysis and SCC. The results indicated that all of the E. coli-challenged glands were free from bacteria (spontaneous cure) at about 10 days after the intramammary infusion, but the level of somatic cells remained high (more than 400,000 cells/ml) during about 1 month. In the mammary glands infused with S. aureus, a chronic infection was established (data not shown).
BSA.
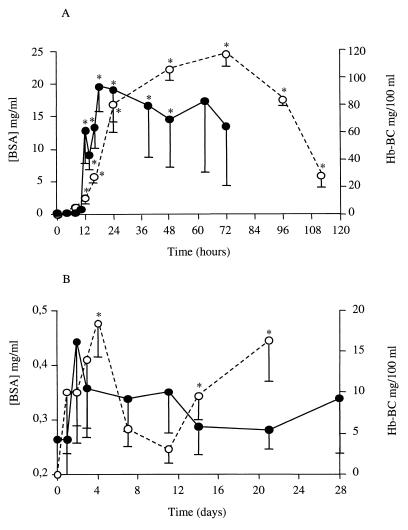
During the E. coli experimental mastitis, milk BSA concentrations were significantly increased (P < 0.047) from the 12th hour p.i. (10.4 ± 3.6 mg/ml) compared to the baseline concentrations (0.23 ± 0.02 mg/ml). The kinetics of the BSA transudation (Fig. 2A) roughly paralleled the somatic cell variations. These results were very different from those obtained for the S. aureus-infected glands in which a slight but not significant increase in BSA concentrations was observed (Fig. 2B).
FIG. 2.
BSA concentration in whey (●) and haptoglobin concentration in plasma (○) obtained from quarters experimentally infected with E. coli (A) or S. aureus (B). Data are expressed as means ± standard errors of the means. ∗, significant difference (P < 0.05) compared with preinfection values.
Haptoglobin.
Haptoglobin, an acute-phase protein that is not constitutive, was detected from the 8th hour p.i. in the sera of four E. coli-infected cows and from the 12th hour in the serum of the other cow. The mean of the maximal concentrations was high (119.6 ± 8.9 Hb-BC mg/100 ml of plasma), and concentrations remained elevated (P < 0.02) for all of the animals from the 16th hour to the 4th day (Fig. 2A). Haptoglobin was detected from 24 h p.i. in the sera of four S. aureus-infected cows and from 48 h p.i. in the sera of the other two cows (Fig. 2B). The increase was less pronounced than during E. coli infections, with a mean of the maximal values of 21.9 ± 6.3 Hb-BC mg/100 ml of plasma.
TNF-α.
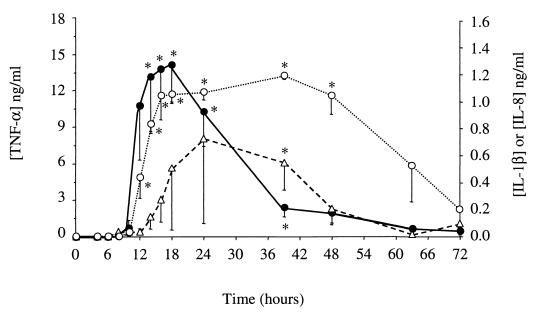
During the E. coli infection, TNF-α concentrations in whey increased dramatically between the 10th and the 39th hours p.i. The peak concentrations (14.1 ± 3.2 ng/ml) were observed between the 14th and the 18th hours p.i. for all of the animals. From 24 h p.i. on, the concentrations decreased rapidly to a low level (Fig. 3). No TNF-α was detected in the whey obtained from the S. aureus-infected animals.
FIG. 3.
TNF-α (●), IL-1β (▵), and IL-8 (○) concentrations in whey obtained from quarters experimentally infected with E. coli. Data are expressed as means ± standard errors of the means. ∗, significant difference (P < 0.05) compared with preinfection values.
IL-1β.
Increases in IL-1β concentrations in whey from E. coli-infected glands began slightly after increases in TNF-α, and maximal concentrations were reached later (Fig. 3). No IL-1β was detected in whey obtained before challenge. In samples from one cow, a very small amount of IL-β was detected only at 48 h p.i. In samples from the other five cows, peak levels ranged from 0.53 to 3.22 ng/ml. No IL-1β was detected in S. aureus-infected samples.
IL-8.
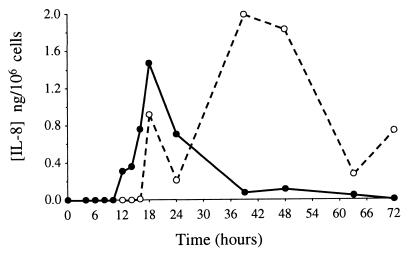
In the whey from the E. coli-infected cows, IL-8 was detected from the 10th hour p.i. for one cow and from the 12th hour p.i. for the others. The concentrations remained high (1.08 ± 0.1 ng/ml) during the first 2 days (P < 0.02) and then decreased rapidly (Fig. 3). IL-8 was not detected in whey from S. aureus-infected cows (the detection limit of the method was 31 pg/ml). IL-8 in the milk cell pellets was also measured. Only samples from one cow infected with E. coli were available and analyzed. However, samples from the six S. aureus-infected cows were analyzed. High concentrations of IL-8 (20 pg/ml to 28.4 ng/ml, corresponding to 15 pg/106 cells to 1.99 ng/106 cells) were detected in cells from the E. coli-infused udder from the 16th hour p.i., so that most IL-8 was cell associated at 40 h and after (Fig. 4). No IL-8 was detected in cells from S. aureus-infected glands.
FIG. 4.
Amount of cell-associated IL-8 per 106 cells (●) and amount of free IL-8 in a volume of whey containing 106 cells (○). Values were obtained from one cow experimentally infected with E. coli.
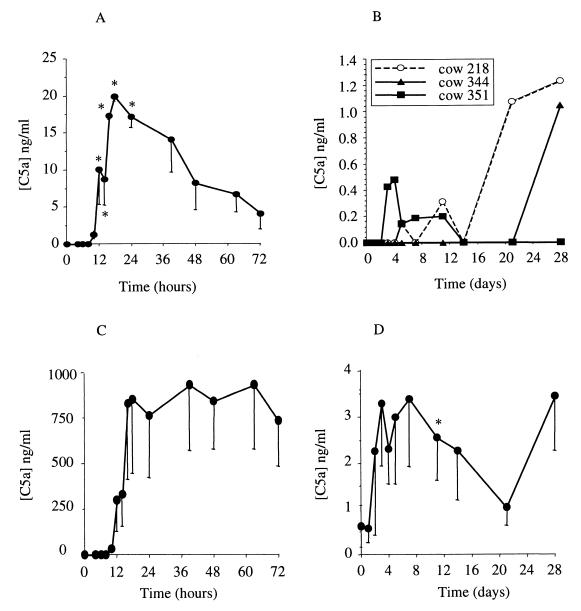
C5a.
No C5a was detected in whey samples collected before intramammary infusions. In whey of E. coli-infected animals, C5a was detected from the 10th hour p.i. for four cows and from the 12th hour p.i. for the fifth cow. The kinetics was comparable to kinetics of TNF-α, but with a longer persistence (Fig. 5A). Some increases at sporadic points were observed in three cows among the six that were infected with S. aureus (Fig. 5B). The ability of milk to generate C5a was also measured in the whey samples after addition of zymosan. The amount of in vitro-generated C5a in E. coli infected animal whey was huge, up to 100-fold the amount of the in vivo-generated C5a (Fig. 5C). In the S. aureus-infected animal whey, C5a was also produced through complement activation. The quantities were not as high as those found in E. coli-infected animal whey (the mean of the maximal concentrations was 6.1 ± 1.9 ng/ml for S. aureus and 1.3 ± 0.4 μg/ml for E. coli during the first 7 days p.i.). However, all of the samples contained unconsumed complement during the studied inflammation time (Fig. 5D).
FIG. 5.
C5a concentration in whey of cows experimentally infected with E. coli (A) or S. aureus (B) and C5a concentration in zymosan-activated whey obtained from cows experimentally infected with E. coli (C) or S. aureus (D). Data are expressed as means ± standard errors of the means, except for panel B, where individual data are shown. ∗, significant difference (P < 0.05) compared with preinfection values.
DISCUSSION
The outcomes of the experimental infections performed in the present study were as expected on the basis of the current forms displayed by naturally occurring infections. Intramammary E. coli infusions provoked clinical mastitis with severe signs associated with a breakdown of the blood-milk barrier, as indicated by a coincident increase in BSA concentrations in milk. S. aureus infusions induced subclinical mastitis, which became chronic. The acute-phase response, which represents the host's reaction to the infection, involved the production and release of acute-phase proteins by the liver, demonstrating that a systemic inflammatory response developed. High levels of haptoglobin, the most reactive acute-phase protein in cattle (12), were measured in plasma of cows infected with E. coli during the first 4 days after challenge, in keeping with a previous report (34). Compared to E. coli mastitis, about 50-fold less haptoglobin was found in the plasma of S. aureus-infected cows, and haptoglobin was detected throughout the 21 days of the experiment, indicating that a systemic reaction developed and persisted, although with a much lower magnitude than in E. coli infections.
Following E. coli intramammary infusion, bacteria grew exponentially until the 10th hour p.i.; at that time, cells began to arrive in the udder and the growth was then controlled. The total eradication of bacteria occurred within 10 days after challenge without any therapeutic treatment. Compared to E. coli, S. aureus grew slower and reached a maximal concentration at 6 days p.i. Moreover, cells arrived later in the udder (between the 1st and the 4th days p.i. depending on the cow), and SCC kinetics were considerably heterogeneous among the cows. All of the challenged quarters became chronically infected. Our results support the hypothesis that the establishment of S. aureus chronic mastitis is the consequence of a low cell recruitment due to a quasiabsence of the major inflammatory mediators at the site of infection, at least in the milk compartment.
In response to intramammary E. coli infection, multiple inflammatory mediators, including TNF-α, interleukins, and C5a, are produced (38, 40). To our knowledge, no results are available concerning S. aureus bovine mastitis. To better understand staphylococcal mastitis, we compared the production of those mediators in milk from E. coli- and S. aureus-infected quarters.
One of the major inflammatory cytokines that mediates the acute-phase reaction is TNF-α. The TNF-α concentrations found following E. coli challenge in the present study are in accordance with those in previous work where biologic tests were used to measure TNF-α activity (37, 38, 40). In contrasting to the case for E. coli infection, no TNF-α was found in the milk from S. aureus-infected glands. The moderate reaction induced by S. aureus could explain the fact that TNF-α was not detected. Indeed, intramammary infusions of a small amount (10 μg) of E. coli endotoxin does not allow the measurement of TNF-α activity in milk (39), in contrast to the case for higher doses (24, 37, 41). Nevertheless, the detection limit of the method was 0.4 ng/ml. Since TNF-α biological activity can be observed at 0.01 ng/ml, it is not excluded that TNF-α was present in those samples at a low but active level.
Another major inflammatory cytokine is IL-8, a powerful chemoattractant for neutrophils (38). In accordance with previous work, no IL-8 was detected in any of the milk samples obtained before challenge (4). Following E. coli injection, we detected IL-8 earlier than Shuster et al. (38) (between the 14th and the 16th hours p.i., versus between the 24th and the 48th hours p.i.). We found the kinetics and concentrations of IL-8 to be very similar among the five challenged glands, in contrast to Shuster et al. (38), who described a heterogeneous production, with three of the eight infected glands not exhibiting obvious increases in IL-8.
No IL-8 was detected in milk from cows infected with S. aureus. In vitro, Barber and Yang (4) found that an IL-8 neutrophil chemotactic activity exists in S. aureus mastitic secretions. Therefore, it cannot be excluded that IL-8 was present in the milk samples of S. aureus-infected cows at concentrations of ≤30 pg/ml (the detection limit of the ELISA kit). At those low concentrations, the chemotactic activity of bovine IL-8 on bovine neutrophils remains unknown, but strong chemotactic activity of recombinant human IL-8 on bovine neutrophils was found at concentrations of ≤50 pg/ml (28, 38). Since IL-8 has the capability to link itself to cell membrane receptor or to be rapidly internalized by cells (27), we also measured IL-8 in the milk cell pellets. High levels of IL-8 were found in the cells from the E. coli-infected cow during the inflammatory period, suggesting that in previous studies, the quantities of IL-8 present in inflammatory milk were underestimated (up to more than 100-fold in some samples). In contrast to the case for the E. coli samples, no IL-8 was detected in the S. aureus cell samples. This result reinforced the results we obtained with the whey samples. The fact that no TNF-α or IL-1β was detected in whey samples of S. aureus-infected cows could explain why IL-8 was not detected either. Indeed, studies showed that IL-1 and TNF-α stimulate IL-8 secretion (25, 28). In contrast, TNF-α and IL-1β must have enhanced IL-8 secretion in E. coli-infected glands.
The last inflammatory mediator we measured was the complement fragment C5a. The role of complement in the recruitment of leukocytes in the udder is still uncertain (9, 28, 38). In the milk of E. coli-infected cows, the presence of C5a was detected from the 12th hour p.i. and was concomitant with the cell arrival and IL-8 increase, so it was not possible to conclude if C5a represented the initial chemoattractive activity, as Shuster et al. suggested (38). In milk from S. aureus-infected animals, no C5a was detected, except sporadically in three animals. At those times, elevations of C5a concentrations were not correlated with increases in cell numbers in milk. Moreover, despite the absence of C5a, cell recruitment did occur following S. aureus infusion. Together, these results make clear that the cell recruitment in the udder was not dependent upon C5a chemoattractant activity.
The quasiabsence of C5a in milk from S. aureus-infected cows could be attributed to an absence of C5 activation or to a lack of C5 or any other complement components playing a role in the complement cascade before the C5a generation. To verify this hypothesis, unconsumed complement was activated by addition of zymosan, a complement activator, in infectious milk samples. The C5a eventually in vitro generated was then quantified. Some was detected from the 4th day to the 14th or the 28th day p.i., depending on the cow. These results showed that the absence of C5a in milk from S. aureus-infected cows was not due to a lack of complement in the udder. Therefore, S. aureus infection, under our experimental conditions, did not permit the activation of the complement. This can be attributed to an insufficient number of bacteria in milk or to an incapacity of S. aureus in activating the complement. Either case would contribute to the persistence of S. aureus, as C5a plays an important role in neutrophil activation (21).
The low cell recruitment during S. aureus infection appears to be the consequence of a lack of production of the chemoattractant or inflammatory mediators. The moderate numbers of S. aureus organisms in milk could explain this phenomenon by being below the threshold necessary for the triggering of an effective inflammatory response. Another explanation could be a repressive effect of S. aureus exerted on the expression of cytokines by the host cells, as has been observed with other bacteria (29, 36).
However, cell influx does exist after S. aureus intramammary infusions, and haptoglobin was detected in the plasma of infected cows, proving that there were local and systemic inflammatory reactions. Therefore, inflammatory mediators should have been synthesized at the site of infection, but none of the expected ones were detected in milk. S. aureus is known to adhere to the mammary epithelium, and several studies have demonstrated that epithelial cells can generate a variety of inflammatory mediators upon interaction with the bacteria (1, 13). Therefore, it could be conceivable that with S. aureus stimulation, the mammary epithelial cells could have secreted inflammatory cytokines directly in the interstitium. This could explain why there were neutrophil recruitment and a systemic inflammatory reaction without any cytokines detectable in the milk of the infected udder.
Further investigations, in particular on the contribution of epithelial cells to leukocyte recruitment, are necessary to understand those mechanisms. This could help in the development of strategies to prevent the installation of chronic mastitis in lactating cows.
ACKNOWLEDGMENTS
We are grateful to the dairy farm staff for assistance in collecting milk and blood samples. We thank Thierry Cochard for assistance with bacteriological studies, Jacques Dufrenoy for technical assistance, and Max Paape and Ted Elsasser (U.S. Department of Agriculture, Beltsville, Md.) for providing the TNF-α ELISA reagents.
REFERENCES
- 1.Agace W W, Hedges S R, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J C. Absence of bacterial adherence in the establishment of experimental mastitis in mice. Vet Pathol. 1978;15:770–775. doi: 10.1177/030098587801500609. [DOI] [PubMed] [Google Scholar]
- 3.Baggiolini M, Walz A, Kunkel S L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber M R, Yang T J. Chemotactic activities in nonmastitic and mastitic mammary secretions: presence of interleukin-8 in mastitic but not nonmastitic secretions. Clin Diagn Lab Immunol. 1998;5:82–86. doi: 10.1128/cdli.5.1.82-86.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner B S, Luscinskas F W, Gimbrone J, Newman W, Sterbinsky S A, Derse A C, Klunk D, Schleimer R P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramley A J. Variations in the susceptibility of lactating and non-lactating bovine udders to infection when infused with Escherichia coli. J Dairy Res. 1976;43:205–211. doi: 10.1017/s0022029900015752. [DOI] [PubMed] [Google Scholar]
- 7.Carroll E J, Mueller R, Panico L. Chemotactic factors for bovine leukocytes. Am J Vet Res. 1982;43:1661–1664. [PubMed] [Google Scholar]
- 8.Christ W L, Harmon R J, Newman L E. How much does mastitis cost? Udder Top. 1983;6:6–9. [Google Scholar]
- 9.Colditz I G, Maas P J. The inflammatory activity of activated complement in ovine and bovine mammary glands. Immunol Cell Biol. 1987;65:433–436. doi: 10.1038/icb.1987.51. [DOI] [PubMed] [Google Scholar]
- 10.Cybulsky M I, McComb D J, Movat H Z. Neutrophil leukocyte emigration induced by endotoxin. Mediator roles of interleukin 1 and tumor necrosis factor alpha 1. J Immunol. 1988;140:3144–3149. [PubMed] [Google Scholar]
- 11.Eberhart R J, Natzke R P, Newbould F H S. Coliform mastitis—a review. J Dairy Sci. 1979;62:1–22. doi: 10.3168/jds.s0022-0302(79)83196-3. [DOI] [PubMed] [Google Scholar]
- 12.Eckersall P D, Conner J G. Bovine and canine acute phase proteins. Vet Res Commun. 1988;12:169–178. doi: 10.1007/BF00362798. [DOI] [PubMed] [Google Scholar]
- 13.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost A J. Selective adhesion of microorganisms to the ductular epithelium of the bovine mammary gland. Infect Immun. 1975;12:1154–1156. doi: 10.1128/iai.12.5.1154-1156.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff J P, Naito Y, Kehrli M E J, Hayes P, Daley M. Physiologic effects of administration of interleukin 1 beta in cows. Am J Vet Res. 1992;53:1983–1987. [PubMed] [Google Scholar]
- 16.Gray G D, Knight K A, Nelson R D, Herron M J. Chemotactic requirements of bovine leukocytes. Am J Vet Res. 1982;43:757–759. [PubMed] [Google Scholar]
- 17.Gudding R, McDonald J S, Cheville N F. Pathogenesis of Staphylococcus aureus mastitis: bacteriologic, histologic, and ultrastructural pathologic findings. Am J Vet Res. 1984;45:2525–2531. [PubMed] [Google Scholar]
- 18.Hamilton J A. Colony stimulating factors, cytokines and monocyte-macrophages—some controversies. Immunol Today. 1993;14:18–24. doi: 10.1016/0167-5699(93)90319-G. [DOI] [PubMed] [Google Scholar]
- 19.Hill A W. Escherichia coli mastitis. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: Cab International; 1994. pp. 117–133. [Google Scholar]
- 20.Hogan J S, Smith K L, Hoblet K H. Field survey of clinical mastitis in low somatic cell count herds. J Dairy Sci. 1989;72:1547–1556. doi: 10.3168/jds.s0022-0302(89)79266-3. [DOI] [PubMed] [Google Scholar]
- 21.Hopken U E, Lu B, Gerard N P, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- 22.Huber A R, Kunkel S L, Todd R F, Weiss S J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 23.Issekutz A C, Bhimji S. Role for endotoxin in the leukocyte infiltration accompanying Escherichia coli inflammation. Infect Immun. 1982;36:558–566. doi: 10.1128/iai.36.2.558-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lahti P, Lilius E M, Zurakowski M J, Paape M J. Tumor necrosis factor-α (TNF-α) responses in blood and mammary secretions after intramammary injection of endotoxin. J Dairy Sci. 1994;11(Suppl. 1):255. [Google Scholar]
- 25.Larsen C G, Anderson A O, Oppenheim J J, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68:31–36. [PMC free article] [PubMed] [Google Scholar]
- 26.Makimura S, Suzuki N. Quantitative determination of bovine serum haptoglobin and its elevation in some inflammatory diseases. Nippon Juigaku Zasshi. 1982;44:15–21. doi: 10.1292/jvms1939.44.15. [DOI] [PubMed] [Google Scholar]
- 27.Marie C, Fitting C, Cheval C, Losser M R, Carlet J, Payen D, Foster K, Cavaillon J M. Presence of high levels of leukocyte-associated interleukin-8 upon cell activation and in patients with sepsis syndrome. Infect Immun. 1997;65:865–871. doi: 10.1128/iai.65.3.865-871.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persson K, Larsson I, Hallen S C. Effects of certain inflammatory mediators on bovine neutrophil migration in vivo and in vitro. Vet Immunol Immunopathol. 1993;37:99–112. doi: 10.1016/0165-2427(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 29.Plitnick L M, Banas J A, Jelley-Gibbs D M, O'Neil J, Christian T, Mudzinski S P. Inhibition of interleukin-2 by Gram-positive bacterium, Streptococcus mutans. Immunology. 1998;95:522–528. doi: 10.1046/j.1365-2567.1998.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poutrel B, Caffin J P, Rainard P. Physiological and pathological factors influencing bovine serum albumin content of milk. J Dairy Sci. 1983;66:535–541. doi: 10.3168/jds.S0022-0302(83)81822-0. [DOI] [PubMed] [Google Scholar]
- 31.Poutrel B, Lerondelle C. Induced staphylococcal infections in the bovine mammary gland. Influence of the month of lactation and other factors related to the cow. Ann Rech Vet. 1978;9:119–128. [PubMed] [Google Scholar]
- 32.Rainard P, Paape M J. Sensitization of the bovine mammary gland to Escherichia coli endotoxin. Vet Res. 1997;28:231–238. [PubMed] [Google Scholar]
- 33.Rainard P, Sarradin P, Paape M J, Poutrel B. Quantification of C5a/C5a(desArg) in bovine plasma, serum and milk. Vet Res. 1998;29:73–88. [PubMed] [Google Scholar]
- 34.Salonen M, Hirvonen J, Pyorala S, Sankari S, Sandholm M. Quantitative determination of bovine serum haptoglobin in experimentally induced Escherichia coli mastitis. Res Vet Sci. 1996;60:88–91. doi: 10.1016/s0034-5288(96)90138-1. [DOI] [PubMed] [Google Scholar]
- 35.Sample A K, Czuprynski C J. Priming and stimulation of bovine neutrophils by recombinant human interleukin-1 alpha and tumor necrosis factor alpha. J Leukoc Biol. 1991;49:107–115. doi: 10.1002/jlb.49.2.107. [DOI] [PubMed] [Google Scholar]
- 36.Schesser K, Spiik A K, Dukuzumuremyi J M, Neurath M F, Pettersson S, Wolf W H. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 37.Shuster D E, Kehrli M E J. Administration of recombinant human interleukin 1 receptor antagonist during endotoxin-induced mastitis in cows. Am J Vet Res. 1995;56:313–320. [PubMed] [Google Scholar]
- 38.Shuster D E, Kehrli M E J, Rainard P, Paape M. Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect Immun. 1997;65:3286–3292. doi: 10.1128/iai.65.8.3286-3292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuster D E, Kehrli M E J, Stevens M G. Cytokine production during endotoxin-induced mastitis in lactating dairy cows. Am J Vet Res. 1993;54:80–85. [PubMed] [Google Scholar]
- 40.Shuster D E, Lee E K, Kehrli M E J. Bacterial growth, inflammatory cytokine production, and neutrophil recruitment during coliform mastitis in cows within ten days after calving, compared with cows at midlactation. Am J Vet Res. 1996;57:1569–1575. [PubMed] [Google Scholar]
- 41.Sordillo L M, Peel J E. Effect of interferon-gamma on the production of tumor necrosis factor during acute Escherichia coli mastitis. J Dairy Sci. 1992;75:2119–2125. doi: 10.3168/jds.S0022-0302(92)77971-5. [DOI] [PubMed] [Google Scholar]
- 42.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 43.Steinbeck M J, Roth J A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989;11:549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- 44.Sutra L, Poutrel B. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J Med Microbiol. 1994;40:79–89. doi: 10.1099/00222615-40-2-79. [DOI] [PubMed] [Google Scholar]