ABSTRACT
Gram-positive bacteria produce small autoinducing peptides (AIPs), which act to regulate expression of genes that promote adaptive traits, including virulence. The Gram-positive pathogen Staphylococcus aureus generates a cyclic AIP that controls expression of virulence factors via the accessory gene regulatory (Agr) system. S. aureus strains belong to one of four Agr groups (Agr-I, -II, -III, and -IV); each group harbors allelic variants of AgrD, the precursor of AIP. In a prior screen for S. aureus virulence factors, we identified MroQ, a putative peptidase. A ΔmroQ mutant closely resembled a Δagr mutant and had significant defects in AIP production in an Agr-I strain. Here, we show that expression of AgrD-I in a ΔmroQ mutant leads to accumulation of an AIP processing intermediate at the membrane that coincides with a loss of secreted mature AIP, indicating that MroQ promotes maturation of AgrD-I. MroQ is conserved in all Agr sequence variants, suggesting either identical function among all Agr types or activity specific to Agr-I strains. Our data indicate that MroQ is required for AIP maturation and activity in Agr-I, -II, and -IV strains irrespective of background. However, MroQ is not required for Agr-III activity despite an identifiable role in peptide maturation. Isogenic Δagr and Δagr ΔmroQ strains complemented with Agr-I to -IV validated the critical role of MroQ in the generation of active AIP-I, -II, and -IV but not AIP-III. These findings were reinforced by skin infection studies with mice. Our data substantiate the prevailing model that MroQ is a mediator of cyclic peptide maturation.
KEYWORDS: Agr, MroQ, Staphylococcus aureus, peptidase, peptide, pheromone, quorum sensing, skin infection, virulence
INTRODUCTION
In Gram-positive pathogens, quorum sensing occurs in response to small signaling peptides called pheromones (1, 2). These pheromones must be processed and released by the bacterium for signaling to occur (3, 4). Following transport outside the bacterial cell, the peptide activates or inhibits gene expression, either upon import back into the bacterial cell followed by direct interaction with a transcription factor or via binding of membrane-embedded sensor kinases at the plasma membrane (5). Quorum sensing is an important means of regulating the expression of a wide array of genes, including those related to virulence, biofilm formation, and motility (2–4, 6). Therefore, understanding how these peptides are processed and transported and how they signal is imperative to better combating pathogenic traits of Gram-positive bacteria.
One class of pheromone, the cyclic autoinducing peptide (AIP), is central to the virulence of several Gram-positive pathogens, including Staphylococcus aureus (4, 7–9). AIP is produced by the accessory gene regulatory (Agr) system to control the expression of virulence genes, including leukotoxins, hemolysins, and tissue-degrading enzymes (4, 6–10). The agrBDCA operon encodes the components of the Agr system, which include AgrC and AgrA, a histidine kinase-response regulator pair, the protease AgrB, and the peptide AgrD, which is posttranslationally processed and exported as AIP to activate AgrC-AgrA (11–13). AgrD contains an N-terminal amphipathic α-helical leader, followed by AIP and a charged C-terminal tail (Fig. 1A). The N-terminal α-helix localizes the precursor peptide to the membrane, where subsequent events in peptide maturation occur (14). The first step in AgrD processing is cleavage of the C-terminal charged tail by AgrB (15–19). This proteolytic event triggers thiolactone ring formation between the C-terminal carbonyl and the sulfur atom of a conserved cysteine side chain (15, 16, 19). Thiolactone ring formation is required to activate AgrC (16, 19). The resulting intermediate, consisting of the leader peptide linked to AIP, then undergoes N-terminal proteolytic processing and export to give rise to mature AIP in the extracellular space (15, 18, 19). These final steps in AIP maturation are yet to be fully understood. One study proposed a role for the canonical signal peptidase, SpsB, in removal of the N-terminal α-helix (20); however, recent publications support a role for additional proteases in this process (21–23). The α-helical leader peptide is separated from the central AIP by a conserved isoleucine/glycine (IG) helix breaker followed by a 3- to 5-amino-acid linker region (Fig. 1), which may facilitate presentation of the peptide cleavage site to the active site of a protease (20).
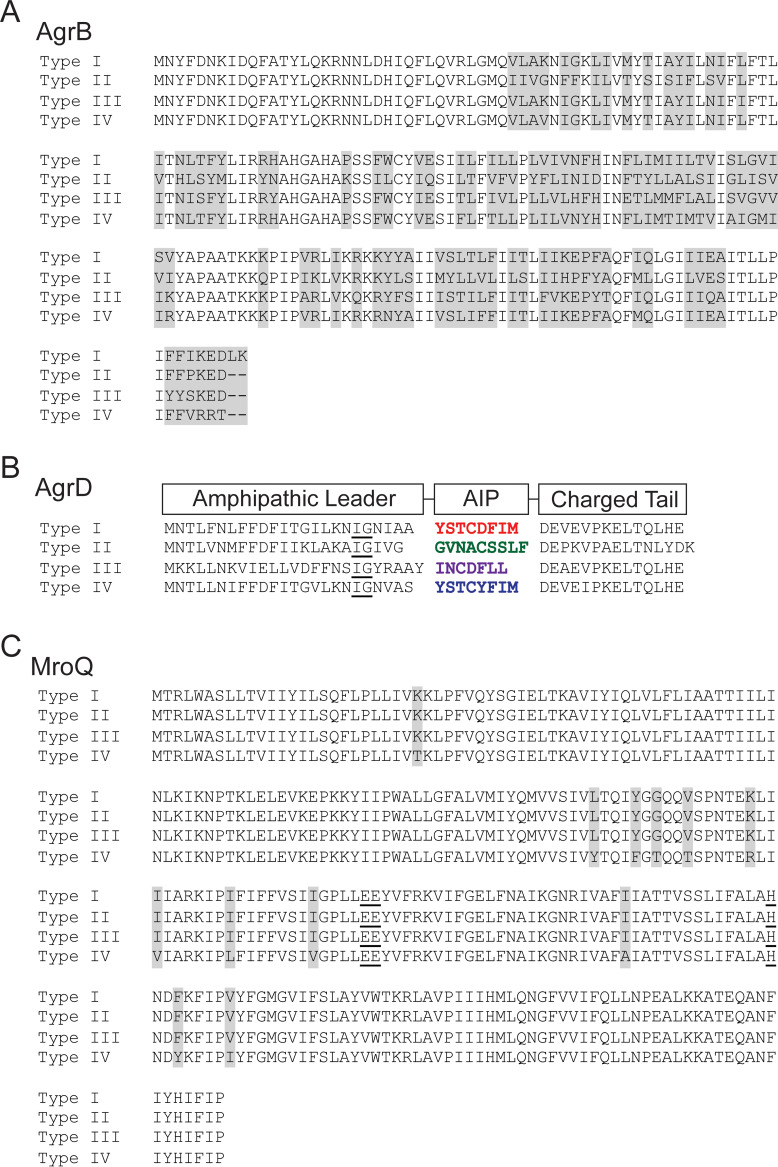
FIG 1.
MroQ is strongly conserved among S. aureus strains harboring Agr allelic variants. Comparison of the amino acid sequences of AgrB (A) and AgrD (B) in LAC (type I), SA502A (type II), MW2 (type III), and RN4850 (type IV). An underlined “IG” shows the conserved “helix breaker”; colored regions correspond to AIPs I to IV. Type I and IV AIPs peptides differ by 1 amino acid. (C) Amino acid sequence alignments of MroQ from LAC (type I), SA502A (type II), MW2 (type III), and RN4850 (type IV). Gray shading shows regions of dissimilarity. Underlined amino acids correspond to conserved active-site residues in MroQ.
S. aureus isolates harbor one of four Agr variants on account of hypervariable regions within the coding sequences of agrB, agrC, and agrD (4, 17, 24–30). Each Agr variant produces a unique AIP that binds to and signals through its cognate histidine kinase (31–34). Furthermore, each AIP inhibits the activity of noncognate Agr systems, via competitive binding to peptide recognition sites on AgrC (13, 25, 26, 31, 34–36). The exception to this phenomenon is AIP-I and AIP-IV, which differ by a single amino acid at position 5 and have been shown to cross-activate AgrC-IV and AgrC-I, respectively (25, 26, 34). AIP production and signaling kinetics vary among variants, with Agr-I, -II, and -IV undergoing activation much sooner than Agr-III in broth culture (23, 26). S. aureus strains containing any of the four Agr variants can cause disease, and at least one study has suggested associations with disease outcome: type I variants are enriched in cases of bacteremia, type II variants are overrepresented in infective endocarditis, and type III variants are increased in menstrual toxic shock syndrome (24, 25, 37). Strains with defective Agr systems are attenuated for pathogenicity, highlighting the importance of the system to infectious disease (10, 38). Though there is an established link between AIP signaling and S. aureus pathogenesis, gaps remain in our understanding of the precise mechanisms behind AgrD processing among all allelic variants.
Our previously published work and that of others identified MroQ as an important mediator of S. aureus pathogenesis in strains harboring a type I Agr variant (21, 22, 39). MroQ is annotated as a putative type II CAAX protease, a family of multipass transmembrane proteins (21, 22, 39). A ΔmroQ mutant phenocopies a Δagr mutant for reduced levels of secreted proteins, decreased toxin production, and attenuated skin and soft tissue infection (21, 22). These shared phenotypes suggest a link between MroQ function and Agr system activation. Furthermore, global transcriptome profiling revealed that all Agr system genes were downregulated in a ΔmroQ mutant (22). We found that loss of MroQ did not impact AgrC-AgrA signaling capacity but rather led to accumulation of full-length C-terminal 6×-His-tagged AgrD in the bacterial cell, highlighting a possible link to AIP maturation (21). In accordance with its annotation as a type II CAAX protease, we observed loss of Agr system function upon mutation of predicted active-site residues E141, E142, and H180 to alanine, suggesting a requirement for catalytic activity in MroQ-mediated AIP maturation (21). Indeed, recent work by Zhao et al. showed that AgrD maturation required only AgrB, MroQ, and AgrD, supporting the model that MroQ functions as a protease that cleaves AgrD (23).
In this work, we investigated the role of MroQ in the maturation and export of each AgrD allelic variant. Furthermore, we examined the relevance of strain background to MroQ activity and its relationship to virulence. We found that MroQ is required for not only AgrD processing but also export or release from the plasma membrane in an Agr-I sequence variant. Furthermore, examination of the impact of an mroQ mutation on Agr system activation in Agr-II and Agr-III variants showed reduced activity in Agr-II but not Agr-III strains. In contrast, visualization of processing of 6×-histidine-tagged AgrD-II and AgrD-III showed an accumulation of intermediates that closely resembled AgrD-I intermediates and suggest a conserved role for MroQ in at least one step of AIP maturation. However, the generation of isogenic strains harboring Agr-I, -II, -III, or -IV demonstrated that while MroQ-mediated AIP maturation is required for the generation of active AIP from Agr-I, -II, and -IV strains, it does not seem to be required for Agr-III system activation. These findings are largely recapitulated in skin and soft tissue infection models. Taken together, these findings lead us to suggest a requirement for MroQ in at least one step of AgrD maturation that is uniform across all sequence variants and is indispensable for the generation of active AIP in three of four variants in vitro. This represents a remarkable conservation of function despite significant diversity among the four Agr systems of S. aureus and may imply that similar conservation exists in other bacteria with Agr peptide signaling systems.
RESULTS
MroQ is conserved among S. aureus strains harboring Agr variants.
We and others previously tested the hypothesis that MroQ is involved in promoting Agr system activation and found that deletion of mroQ disrupted Agr activity, via a presumed defect in peptide maturation that centered on AgrB and AgrD (21, 22). These studies were conducted using S. aureus strain LAC, which contains an Agr-I allele. AgrB has 50% sequence identity among Agr types I to IV (Fig. 1A). The amino acid sequence of AgrD is similarly hypervariable, with 26% identity among Agr types I to IV (Fig. 1B). In contrast, the amino acid sequence of MroQ is well conserved among representative strains harboring each Agr allele (LAC, SA502A, MW2, and RN4850) (Fig. 1C). Strains LAC, SA502A, and MW2 had 100% identity, while strain RN4850 had 95% identity. Our analysis of all sequenced S. aureus strains containing Agr-IV in the NCBI database further indicated that MroQ is identical within these strains; however, all had 95% identity relative to LAC, SA502A, and MW2. Thus, despite significant divergence among AgrB and AgrD alleles, MroQ does not share a similarly hypervariable sequence.
A ΔmroQ mutant is defective for activation of Agr type I and Agr type IV systems.
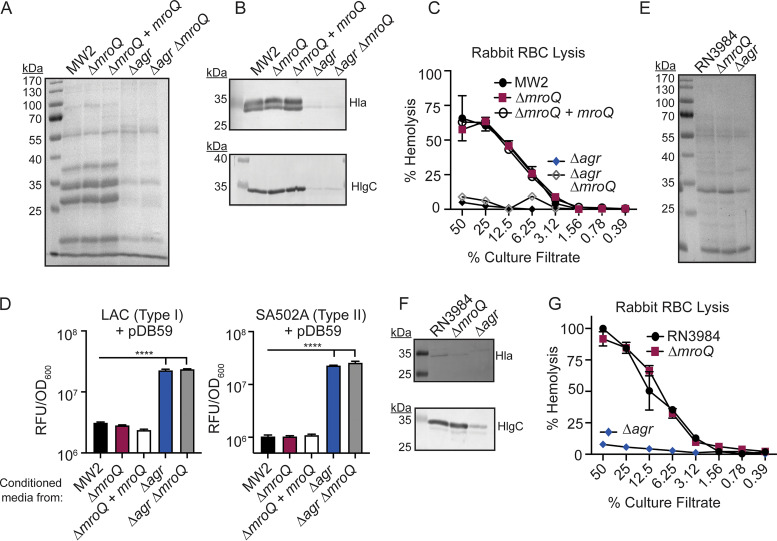
Given the conservation of MroQ among sequenced S. aureus strains but significant variability among proteins in the Agr locus, we reasoned that the role of MroQ in promoting Agr system activation might be restricted to Agr type I and Agr type IV, which share the greatest sequence identity (Fig. 1A and B) (25, 26, 34). To test this hypothesis, we first verified MroQ-dependent defects in Agr system activation in Agr type I strain LAC. LAC (wild type [WT]), ΔmroQ, ΔmroQ + mroQ, (a LAC-derived strain with integrated pJC1112-mroQ for complementation), Δagr::tet, and Δagr::tet ΔmroQ strains were evaluated for total exoprotein secretion, leukotoxin production, and hemolytic activity on rabbit red blood cells (RBCs). As expected, we observed reduced exoprotein abundance in a ΔmroQ mutant compared to that in the WT or the ΔmroQ + mroQ complement strain (Fig. 2A) (21). The decreased exoprotein production phenocopied Δagr::tet, and Δagr::tet ΔmroQ mutants, validating a role for MroQ in Agr system function (Fig. 2A) (21). Immunoblot analysis of trichloroacetic acid (TCA)-precipitated supernatant from a ΔmroQ mutant showed reduced levels of α-hemolysin (Hla) and γ-hemolysin (HlgC) in accordance with established Agr regulation patterns (Fig. 2B) (11, 40). In agreement with decreased hemolysin levels, a ΔmroQ mutant had reduced hemolytic activity against rabbit RBCs (Fig. 2C). To further demonstrate perturbation of Agr system function in a ΔmroQ mutant, we assessed agr P3 promoter activity using the fluorescent transcriptional reporter plasmid pDB59-P3-GFP (41). Mature AIP-I inhibits the activation of Agr-II and Agr-III but activates Agr-IV (25, 26, 34). We found that addition of cell-free supernatant from WT LAC (Agr-I) inhibited the activation of pDB59-P3-GFP in an Agr-II strain (SA502A) and Agr-III strain (MW2), whereas supernatant from a ΔmroQ mutant led to robust activation, as determined by green fluorescent protein (GFP) fluorescence (Fig. 2D). Additionally, supernatant from WT LAC activated an Agr-IV strain (RN4850) containing pDB59-P3-GFP, whereas supernatant from a ΔmroQ mutant did not, suggesting that Agr-I and Agr-IV are likely to be impacted by MroQ in similar ways (Fig. 2E) (25, 26, 34). Altogether, these data validate prior work indicating that Agr-I system activation is defective in the absence of MroQ and established that the highly similar Agr-IV system is activated by AIP-I in a MroQ-dependent manner.
FIG 2.
MroQ contributes to Agr type I and IV activation. (A and B) TCA-precipitated exoproteins (A) and Hla and HlgC immunoblots (B) from LAC, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (C) Rabbit red blood cell lysis of cell-free culture filtrates derived from LAC, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (D) pDB59 reporter activity (relative fluorescence units [RFU]/OD600) in SA502A (left) and MW2 (right) upon addition of conditioned medium from LAC, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (E) pDB59 reporter activity (RFU/OD600) in RN4850 (type IV) upon addition of conditioned medium from LAC, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. Hemolysis and GFP reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots and GelCode blue-stained gels are a representative of at least four replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by one-way analysis of variance (ANOVA) with Tukey’s posttest.
A ΔmroQ mutant is defective for AIP-I export and processing.
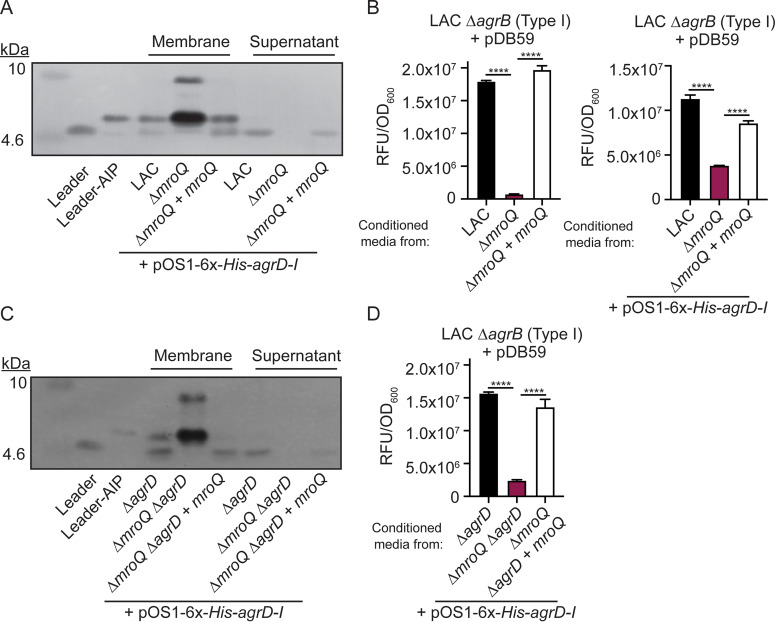
Previous work suggested a role for MroQ in the processing and/or export of AgrD to give rise to AIP (21). To determine if loss of MroQ affects AgrD-I maturation or export, we expressed 6×-His-AgrD-I in the WT (LAC), ΔmroQ, and ΔmroQ + mroQ Agr type I strains and monitored the location and generation of processing intermediates via immunoblotting. We observed species which corresponded to AgrB-processed AgrD-I (leader-AIP) and N-terminally processed AgrD-I (leader peptide alone) in the membrane fraction of WT and the ΔmroQ + mroQ complement strains (Fig. 3A). In addition, the cell-free supernatant contained significant quantities of Leader peptide (Fig. 3A). In contrast, a ΔmroQ mutant showed accumulation of species with molecular weights that approximate full-length AgrD-I and leader-AIP in the membrane fraction and a complete loss of leader peptide in both the membrane and supernatant fractions (Fig. 3A). Complementation of the ΔmroQ mutant fully restored AIP maturation and release of the leader peptide, which correlated with activation of an Agr type I reporter (ΔagrB + pDB59-P3-GFP) and was independent of the pOS1-6×-His-agrD-I plasmid (Fig. 3B). The WT, ΔmroQ, and ΔmroQ + mroQ Agr type I strains containing the 6×-His-AgrD-I expression plasmid also produce AgrD from the native Agr operon. To rule out the possibility that native AgrD might impact the analysis of 6×-His-AgrD-I processing, we generated an in-frame deletion of agrD in the WT, ΔmroQ, and ΔmroQ + mroQ strain backgrounds. 6×-His-AgrD-I processing and activation of the ΔagrB + pDB59-P3-GFP reporter were identical to those of the parental strains (Fig. 3C and D). These observations support a role for MroQ in the processing and export of AgrD-I and are in keeping with recent biochemical evidence for MroQ processing of AgrD (23).
FIG 3.
A ΔmroQ mutant is compromised for AIP-I export and processing. (A) Immunoblots of supernatant and membrane fractions of LAC, ΔmroQ, and ΔmroQ + mroQ strains constitutively expressing 6×-His-AgrD-I (pOS1-PsarA-6×-His-agrD-I) using anti-His monoclonal antibody. 6×-His-leader-AIP-I (AgrB processing intermediate) and 6×-His-leader-I (AgrD-I leader peptide) were isolated from constitutively expressing S. aureus and are included as controls. (B) pDB59 reporter activity (RFU/OD600) in LAC ΔagrB upon addition of conditioned medium from LAC, ΔmroQ, and ΔmroQ + mroQ strains or LAC, ΔmroQ, and ΔmroQ + mroQ strains constitutively expressing 6×-His-AgrD-I. (C) Immunoblots of supernatant and membrane fractions of ΔagrD, ΔmroQ ΔagrD, and ΔmroQ ΔagrD + mroQ strains constitutively expressing 6×-His-AgrD-I (pOS1-PsarA-6×-His-agrD-I) using anti-His monoclonal antibody. (D) pDB59 reporter activity (RFU/OD600) in LAC ΔagrB upon addition of conditioned medium from ΔagrD, ΔmroQ ΔagrD, and ΔmroQ ΔagrD + mroQ strains constitutively expressing 6×-His-AgrD-I. Reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots are representative of at least three replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by one-way ANOVA with Tukey’s posttest.
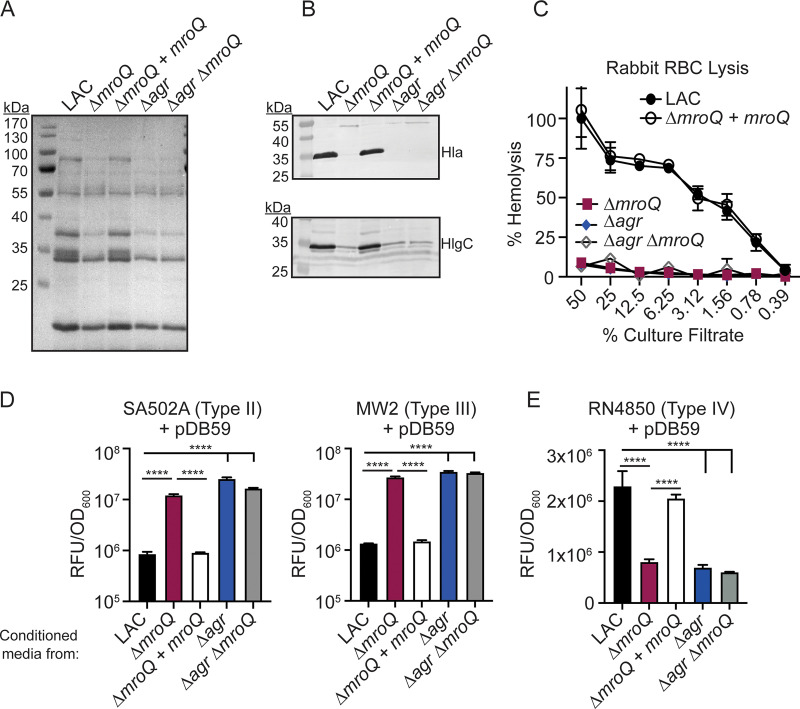
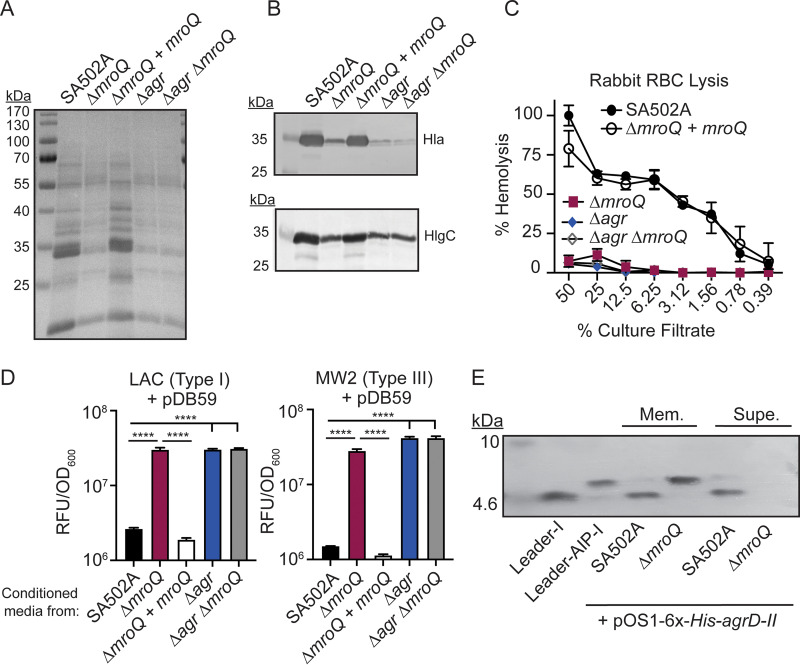
MroQ function is conserved in an Agr-II allelic variant.
To test the role of MroQ in Agr-II peptide processing and export, we generated ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ mutants in the Agr type II strain SA502A and assessed total exoprotein secretion, production of leukotoxins, and hemolytic activity on rabbit RBCs. We observed decreased exoprotein abundance in a ΔmroQ mutant, similar to the case with Δagr and Δagr ΔmroQ mutants (Fig. 4A). Further, we saw defective production of Hla and HlgC in ΔmroQ, Δagr, and Δagr ΔmroQ mutants (Fig. 4B). Consistent with these observations, we observed decreased hemolytic activity against rabbit RBCs for supernatant from a ΔmroQ mutant compared to culture supernatant from WT cells (Fig. 4C). The ΔmroQ + mroQ complementation strain fully restored all mutant phenotypes (Fig. 4A to C). Agr-P3 reporter activity assays demonstrated inhibition of reporter activation by WT and the ΔmroQ + mroQ complement strains, whereas cell-free supernatant from a ΔmroQ mutant led to activation of the Agr system in both Agr-I and Agr-III reporter strains (Fig. 4D). Furthermore, the expression of 6×-His-AgrD-II in SA502A showed leader peptide alone in both the membrane fraction and the secreted fraction (Fig. 4E). In contrast, expression of 6×-His-AgrD-II in a ΔmroQ mutant led to accumulation of leader-AIP in the membrane fraction with no leader peptide in the membrane or supernatant (Fig. 4E). We were unable to monitor 6×-His-AgrD-II maturation in a ΔmroQ + mroQ strain due to the plasmid required for complementation in this strain background (see Materials and Methods). Taken together, these data indicate that MroQ is required for the maturation and export of active AIP-II.
FIG 4.
A ΔmroQ mutant is defective for Agr type II activation and AIP-II maturation and export. (A) TCA-precipitated exoproteins from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (B) Hla and HlgC immunoblots from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (C) Rabbit red blood cell lysis of cell-free culture filtrates derived from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (D) pDB59 reporter activity (RFU/OD600) of LAC (left) and MW2 (right) upon addition of conditioned medium from SA502A, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (E) Immunoblots of supernatant and membrane fractions from SA502A and ΔmroQ strains constitutively expressing 6×-His-AgrD-II (pOS1-PsarA-6×-His-agrD-II) using anti-His monoclonal antibody. 6×-His-leader-AIP-I (AgrB processing intermediate) and 6×-His-leader-I (AgrD-I leader peptide) were isolated from constitutively expressing S. aureus and were included as controls. Hemolysis and reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots and GelCode blue-stained gels are representative of at least four replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by one-way ANOVA with Tukey’s posttest.
MroQ contributes to Agr-III peptide processing but is not required for Agr system activation.
Given the conservation of MroQ function in Agr-I, -II, and presumably -IV strains, we hypothesized that MroQ would also be required for Agr system activity in an Agr-III strain. We tested the role of MroQ in Agr system activity in two Agr type III strains, MW2 and RN3984. ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains were generated in MW2, whereas ΔmroQ and Δagr::tet strains were generated from RN3984. These strains were grown in broth and monitored for exoprotein production, leukotoxin production, and rabbit RBC hemolysis. For strain MW2, we observed similar levels of exoproteins in WT, ΔmroQ, and ΔmroQ + mroQ strains, whereas exoprotein levels were decreased in Δagr::tet and Δagr::tet ΔmroQ strains (Fig. 5A). Consistent with the similar exoprotein production, we saw equivalent levels of Hla and HlgC secreted by WT, ΔmroQ, and ΔmroQ + mroQ strains (Fig. 5B). Furthermore, we noted identical hemolytic activity against rabbit RBCs in the same strains (Fig. 5C). Conditioned medium from a ΔmroQ mutant fully inhibited pDB59-P3-GFP promoter activation in Agr-I and Agr-II reporter strains, whereas addition of conditioned medium from a Δagr::tet or Δagr::tet ΔmroQ mutant to Agr-I or -II reporter strains did not inhibit promoter activation (Fig. 5D). Similar results were obtained with strain RN3984 (Fig. 5E to G). Together, these data indicate that MroQ is not required for Agr-III activation.
FIG 5.
MroQ is not required for Agr type III activation. (A) TCA-precipitated exoproteins and (B) Hla and HlgC immunoblots from MW2, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (C) Rabbit red blood cell lysis of cell-free culture filtrates derived from MW2, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (D) pDB59 reporter activity (RFU/OD600) of LAC (left) and SA502A (right) upon addition of conditioned medium from MW2, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ. (E) TCA-precipitated exoproteins from RN3984, ΔmroQ, and Δagr::tet strains. (F) Hla and HlgC immunoblots of from RN3984, ΔmroQ, and Δagr::tet strains. (G) Rabbit red blood cell lysis of cell-free culture filtrates derived from RN3984, ΔmroQ, and Δagr::tet strains. Hemolysis and reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots and GelCode blue-stained gels are representative of at least four replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by one-way ANOVA with Tukey’s posttest.
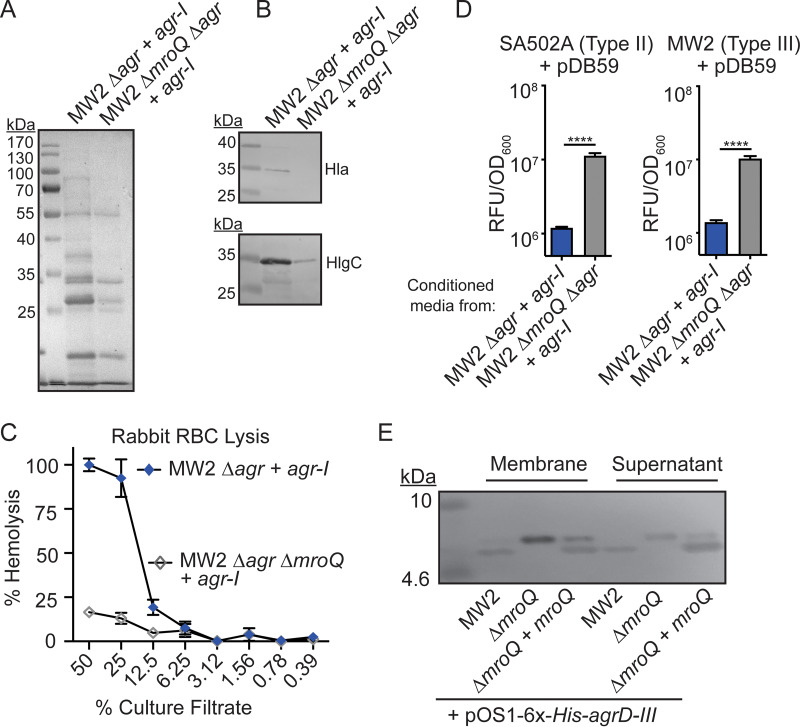
Given the observation that MroQ was not required for Agr-III system activation in MW2 and RN3984, we surmised that MroQ may be dispensable for the generation of AIPs in an Agr type III strain background. To test this hypothesis, we generated MW2 Δagr::tet + pJC1111-Agr-I and MW2 ΔmroQ Δagr::tet + pJC1111-Agr-I strains and monitored Agr system function via exoprotein production, leukotoxin levels, and rabbit RBC hemolysis. We observed decreased exoprotein production from the MW2 ΔmroQ Δagr::tet + pJC1111-Agr-I strain compared to the MW2 Δagr::tet + pJC1111-Agr-I strain (Fig. 6A). The decreased exoprotein production in the MW2 ΔmroQ Δagr::tet + pJC1111-Agr-I strain corresponded with lower levels of toxins and loss of hemolytic activity against rabbit RBCs (Fig. 6B and C), further suggesting that loss of MroQ in this background caused defective Agr-I system activity. These data were corroborated using Agr reporter assays, which showed that conditioned medium from Δagr::tet + pJC1111-Agr-I inhibited pDB59-P3-GFP expression in Agr-II and Agr-III strains, whereas conditioned medium from ΔmroQ Δagr::tet + pJC1111-Agr-I had robust GFP production (Fig. 6D). Thus, MroQ is still required for Agr-I activity when expressed in strain MW2, and strain background presumably does not dictate the requirement for MroQ in AgrD processing. Given this observation, we sought to further explore if MroQ is required for any aspect of AIP-III maturation. To this end, we monitored the expression of 6×-His-AgrD-III in WT, ΔmroQ, and ΔmroQ + mroQ MW2 strains by immunoblotting and noted accumulation of a band that resembled leader-AIP in the membrane fraction of the ΔmroQ strain, whereas WT and ΔmroQ + mroQ strains produced a species that resembled the leader peptide in both the membrane and supernatant (Fig. 6E). Together, these data suggest that MroQ processes AIP in a manner that is independent of strain background or Agr type, yet an active signaling peptide can be generated in the absence of MroQ in Agr-III strains.
FIG 6.
MroQ contributes to AIP-I processing in an Agr type III strain. (A) TCA-precipitated exoproteins from MW2 Δagr::tet + agr-I and MW2 Δagr::tet ΔmroQ + agr-I strains. (B) Hla and HlgC immunoblots from MW2 Δagr::tet + agr-I and MW2 Δagr::tet ΔmroQ + agr-I strains. (C) Rabbit red blood cell lysis of cell-free culture filtrates derived from MW2 Δagr::tet + agr-I and MW2 Δagr::tet ΔmroQ + agr-I strains. (D) pDB59 reporter activity (RFU/OD600) of SA502A (left) and MW2 (right) upon addition of conditioned medium from MW2 Δagr::tet + agr-I and MW2 Δagr::tet ΔmroQ + agr-I strains. (E) Immunoblots of supernatant and membrane fractions from MW2, ΔmroQ, and ΔmroQ + mroQ strains constitutively expressing 6×-His-AgrD-III (pOS1-PsarA-6×-His-agrD-III) using anti-His monoclonal antibody. Hemolysis and reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots and GelCode blue-stained gels are representative of at least four replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by a two-tailed t test.
MroQ is required for virulence in Agr-I strain LAC.
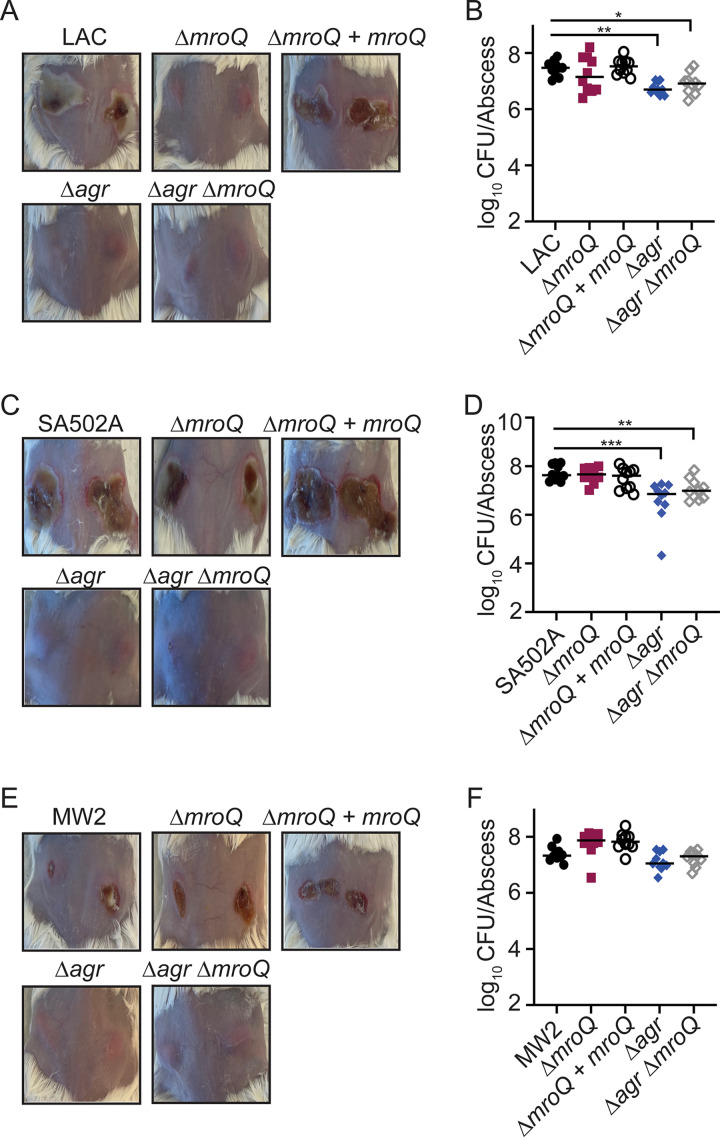
Animals infected intradermally with a ΔmroQ mutant of an Agr-I strain (LAC) have dramatic reductions in abscess pathology and exhibit modest reductions in CFU (~5-fold) (21). To determine if MroQ is required for skin and soft tissue infection of strains with Agr-II and Agr-III alleles, we intradermally infected mice with WT, ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ mutants in LAC (Agr-I), SA502A (Agr-II), and MW2 (Agr-III) and CFU and gross pathology were assessed 96 h postinfection. For strain LAC, we observed no ruptured abscesses after infection with ΔmroQ, Δagr::tet, and Δagr::tet ΔmroQ mutants, although CFU remained high for most infected animals (Fig. 7A and B). Though we observed dramatic reductions in pathology for animals infected with Δagr::tet, and Δagr::tet ΔmroQ mutants from strain SA502A, there was little difference in CFU and abscesses were ruptured and dermonecrotic in animals infected with the SA502A ΔmroQ strain (Fig. 7C and D). In agreement with our in vitro assays, when animals were infected with the MW2 ΔmroQ strain, we observed CFU and pathology similar to those in animals infected with WT MW2 (Fig. 7E and F). This contrasted with Δagr::tet and Δagr::tet ΔmroQ mutants, which had no dermonecrosis and modest reductions in CFU (Fig. 7E and F). These data indicate that MroQ-mediated maturation of AIP is required for virulence in an Agr-I strain but seems largely dispensable in an Agr-II or Agr-III strain.
FIG 7.
MroQ is important for S. aureus skin and soft tissue infection in Agr type I strains. (A) Representative images of skin abscesses at 96 h after infection with LAC (WT), ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (B) Bacterial burden in skin abscesses of mice at 96 h after infection with LAC (WT) (n = 10), ΔmroQ (n = 10), ΔmroQ + mroQ (n = 10), Δagr::tet (n = 10), and Δagr::tet ΔmroQ (n = 10) strains. (C) Representative images of skin abscesses at 96 h after infection with SA502A (WT), ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ strains. (D) Bacterial burden in skin abscesses of mice at 96 h after infection with SA502A (WT) (n = 10), ΔmroQ (n = 10), ΔmroQ + mroQ (n = 10), Δagr::tet (n = 10), and Δagr::tet ΔmroQ (n = 10) strains. (E) Representative images of skin abscesses at 96 h after infection with MW2 (WT), ΔmroQ, ΔmroQ + mroQ, Δagr::tet, and Δagr::tet ΔmroQ. (F) Bacterial burden in skin abscesses of mice at 96 h after infection with MW2 (WT) (n = 10), ΔmroQ (n = 10), ΔmroQ + mroQ (n = 10), Δagr::tet (n = 10), and Δagr::tet ΔmroQ (n = 10) strains. P values were determined by a nonparametric one-way ANOVA (Kruskal-Wallis test) with Dunn’s posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
MroQ-mediated AIP maturation is required for Agr-I, -II, and -IV activation but not Agr-III.
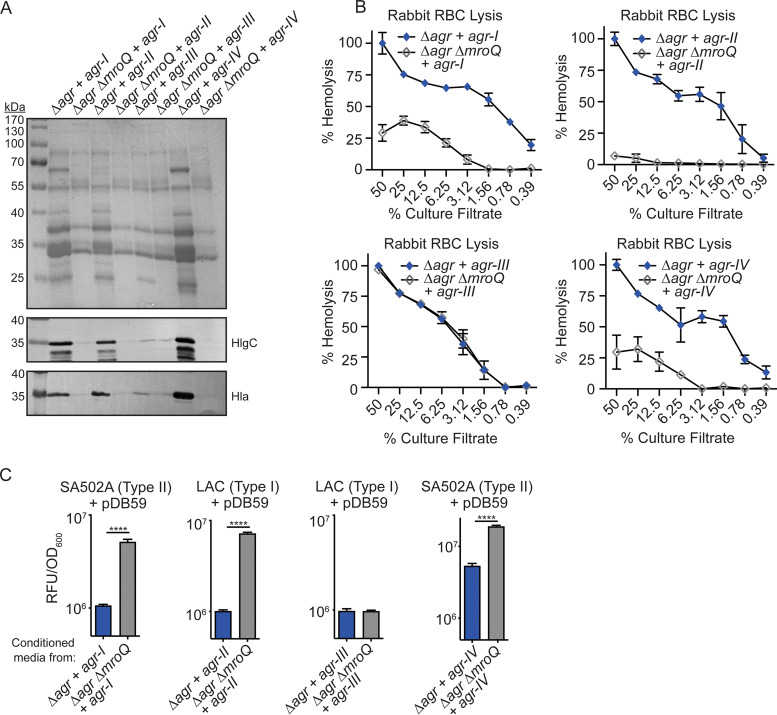
As a complementary approach and to further rule out the possibility that strain background drives dependence on MroQ for AIP maturation, we reconstituted the entire Agr locus from each of the four Agr types into isogenic Δagr and Δagr ΔmroQ mutants in strain LAC using the site-specific integrational plasmids pJC1111-Agr-I, pJC1111-Agr-II, pJC1111-Agr-III, and pJC1111-Agr-IV (26). To explore the consequences of the ΔmroQ mutation in these strains, we assessed exoprotein levels, leukotoxin production, and hemolytic activity on rabbit RBCs. We observed decreased exoprotein levels and corresponding reductions in Hla and HlgC abundance in the Δagr ΔmroQ mutant + pJC1111-Agr-I, the Δagr ΔmroQ mutant + pJC1111-Agr-II, and the Δagr ΔmroQ mutant + pJC1111-Agr-IV but not the Δagr ΔmroQ mutant + pJC1111-Agr-III (Fig. 8A). In agreement with decreased hemolysin production, we also observed a reduction in hemolytic activity on rabbit RBCs upon addition of supernatant from the Δagr ΔmroQ mutant + pJC1111-Agr-I, the Δagr ΔmroQ mutant + pJC1111-Agr-II, and the Δagr ΔmroQ mutant + pJC1111-Agr-IV but not the Δagr ΔmroQ mutant + pJC1111-Agr-III (Fig. 8B). These data suggest that MroQ is required for Agr activity of Agr-I, -II, and -IV variants but not Agr-III variants, regardless of strain background. This conclusion was further supported by data from Agr reporter inhibition assays which showed no reporter inhibition when conditioned media from the Δagr ΔmroQ mutant + pJC1111-Agr-I, the Δagr ΔmroQ mutant + pJC1111-Agr-II, and the Δagr ΔmroQ mutant + pJC1111-Agr-IV were added to noncognate reporter strains. In contrast, conditioned medium from the Δagr ΔmroQ mutant + pJC1111-Agr-III fully inhibited Agr reporter activity (Fig. 8C).
FIG 8.
MroQ is required for Agr activity of isogenic strains containing Agr-I, -II, and -IV but not Agr-III. (A) TCA-precipitated exoproteins and Hla and HlgC immunoblots from Δagr::tet and Δagr::tet ΔmroQ strains in LAC (type I) complemented with the entire Agr locus from each Agr variant (+ agr-I, agr-II, agr-III, or agr-IV). (B) Rabbit red blood cell lysis of cell-free culture filtrates derived from Δagr::tet and Δagr::tet ΔmroQ strains from LAC (type I) reconstituted with the entire Agr locus from each Agr variant (+ agr-I, agr-II, agr-III, or agr-IV). (C) pDB59 reporter activity (RFU/OD600) of SA502A (type II) or LAC (type I) upon addition of conditioned medium from Δagr::tet and Δagr::tet ΔmroQ strains in LAC (type I) reconstituted with the entire Agr locus from each Agr variant (+ agr-I, agr-II, agr-III, or agr-IV). Hemolysis and reporter assay data are from one of at least three experiments conducted in triplicate. Immunoblots and GelCode blue-stained gels are representative of at least four replicates. Means ± SD are shown (n = 3). ****, P < 0.0001 by a two-tailed t test.
DISCUSSION
In this work, we explored how the membrane peptidase MroQ promotes virulence traits and maturation/export AIP in strains of S. aureus that harbor four allelic variants of AgrD. Our data suggest a requirement for MroQ in at least one step of AgrD processing among all four Agr variants. However, while MroQ-dependent AgrD maturation was important for the generation of active AIP in Agr-I, -II, and -IV strains, it was not required for activation of the Agr-III system. These observations were recapitulated in vivo, where virulence was independent of MroQ in an Agr-III strain. Overall, our data argue that MroQ is a mediator of AIP processing and export that promotes quorum sensing and virulence factor gene expression in S. aureus, with implications for MroQ-like proteins in the function of Agr systems in other bacteria.
The final steps of AgrD processing and export have remained elusive. In this study, through use of 6×-His-AgrD expression plasmids, we showed an accumulation of a leader-AIP peptide intermediate in the membrane fraction and a corresponding loss of leader peptide (reflecting reduced generation of mature AIP) in the supernatant fraction of ΔmroQ strains of Agr-I, -II, and -III variants, suggesting that MroQ promotes these final steps of peptide maturation and export (Fig. 3A and C, 4E, and 6E). However, despite an apparent defect in peptide maturation as determined by immunoblotting, ΔmroQ mutant strains from MW2 (Agr-III) and RN3984 (Agr-III) maintained the ability to activate AgrC (Fig. 5A to G). This unusual observation suggests that either an AgrD-III processing intermediate is sufficient to activate the Agr system or the activity of MroQ can be bypassed by an alternative protease to generate sufficient AIP-III for activity. In consideration of the first possibility, Zhao et al. recently showed that recombinant AgrB, MroQ, and AgrD were sufficient for the generation of mature AIP-I and AIP-II but not AIP-III, which contained an intermediate with an additional N-terminal tyrosine (23). In addition, several prior reports established a requirement for a precise AIP-III N-terminal tail length for efficient AgrC-III activation (35, 42). The addition of as little as a single N-terminal tyrosine to AIP-III caused inactivation of AIP-III (35). Thus, there is unlikely to be an AgrD processing intermediate that is capable of efficiently activating Agr-III. In contrast to these biochemical assays, mass spectrometry (MS)-based peptide analysis of supernatant of a ΔmroQ mutant from Agr-III strain MW2 identified significant amounts of mature AIP-III (23). Thus, in keeping with our data, the work of Zhao et al. suggests that MroQ can cleave AgrD-III, yet it is not necessary for the generation of active AIP-III. Taking all these points into consideration, we suspect that an alternative protease exists that is sufficient to drive AIP-III maturation, possibly in conjunction with MroQ.
How and if MroQ contributes to AIP release are unknown. Our data demonstrate that peptide processing intermediates accumulate at the S. aureus membrane and are not appreciably released from the cell in ΔmroQ mutant strains. Translocation of AIP must occur before or after the final N-terminal processing step, and MroQ could play a direct or indirect role in this process. Though the translocation-first model has been favored, the lack of an ATP-binding cassette in either AgrB or MroQ does not support a role for active transport by either protein (20, 23, 27). Another possible mechanism of release could involve the insertion of the N-terminal leader of AgrD into the membrane in a way that positions the peptide for processing by an AgrB-MroQ complex, with subsequent passive diffusion of AIP outside the cell. We favor this hypothesis, and work is under way to interrogate the physical relationship between MroQ and AgrB and its impact on the release of mature AIP.
While this work was being conducted, Zhao et al. provided evidence for a direct role of MroQ in leader peptide cleavage to generate mature AIP-I and AIP-II but not AIP-III (23). This work successfully reconstituted the Agr quorum sensing circuit with purified components and proposed a model for how MroQ generates mature AIP-I and AIP-II via amino acid differences in the linker region between the α-helical leader peptide and AIP. Here, we corroborate these findings by demonstrating that MroQ-mediated processing of AIP-I, -II, and -IV is required for Agr system activation, whereas MroQ-mediated AIP-III processing is dispensable for Agr system activation in Agr-III-containing strains (Fig. 2, 4, and 6). In addition, we expand upon these studies by demonstrating MroQ-dependent impacts on peptide processing and export within live bacteria (Fig. 2, 6, and 8). Furthermore, our work highlights the impact of MroQ on the pathogenesis of Agr-I (LAC), Agr-II (SA502A), and Agr-III (MW2) strains. Infection of mice with a ΔmroQ mutant of strain LAC has substantially reduced abscess pathology compared to that in animals infected with the parental WT strain (Fig. 7A and B). This contrasts with what was seen for animals infected with a ΔmroQ mutant from SA502A (Agr type II) and MW2 (Agr type III), which exhibited pathology similar to that in animals infected with WT SA502A or MW2 (Fig. 7C to F). These data cement a requirement for MroQ in the severity of Agr-mediated skin pathology for a type I Agr system but not type II or type III. This observation suggests that despite decreased Agr system activation of an SA502A ΔmroQ mutant in vitro, the strain has restored Agr activity in vivo, possibly due to host protease-mediated maturation of AIP-II or host-mediated induction of S. aureus proteases capable of facilitating AIP-II maturation. Of note, while we observed similar modest reductions in CFU among animals infected with ΔmroQ and Δagr mutants from the LAC strain background (21, 22), this was not true for all strains and infection conditions, where CFU were largely unchanged. We surmise the contributions of Agr to abscess pathology stem primarily from the well-established role for Hla and phenol-soluble modulins (PSMs) in promoting tissue damage during skin infection, whereas impacts on bacterial burden are less defined (43–46).
To interrogate if MroQ was sufficient for AIP-I-IV maturation in an isogenic strain background, we reconstituted Δagr::tet and Δagr::tet ΔmroQ mutants of LAC (Agr-I) with the entire Agr locus from each variant. We observed a loss of Agr system activation in Δagr::tet ΔmroQ mutant strains expressing the Agr-I, -II, and -IV loci but not a Δagr::tet ΔmroQ mutant expressing the Agr-III locus, supporting a requirement for MroQ-mediated maturation of AIP-I, -II, and -IV but not AIP-III (Fig. 8). Additionally, reconstitution of a Δagr::tet mutant from MW2 (Agr-III) with the Agr-I locus results in production of mature AIP-I, whereas reconstitution of a Δagr::tet ΔmroQ mutant with Agr-I does not, suggesting that MroQ is active in Agr-III-containing strains (Fig. 6A to C). The phenotypic similarities between isogenic mutants reconstituted with agr-I to -IV and native strains containing mroQ mutations argues that MroQ function is conserved in all strain backgrounds. Furthermore, dispensability of MroQ for maturation of AIP-III in strain LAC suggests that whatever alternative protease(s) or factors facilitate processing of AIP-III must also exist in an Agr-I strain background.
MroQ maintains remarkable sequence conservation across strains of S. aureus that harbor each Agr allelic variant, with 100% amino acid identity across Agr-I, -II, and -III-containing strains and 95% identity in Agr-IV-containing strains. The reduced conservation of MroQ from Agr-IV strains represents 12 of 247 amino acids. It remains plausible that MroQ-mediated maturation of AgrD-IV is impacted by these amino acid differences; however, our isogenic reconstitution studies indicate that MroQ from a type I strain can promote maturation of AIP-IV (Fig. 8). The catalytic residues E141, E142, and H180 remain conserved in the sequence of MroQ from Agr-IV-containing strains (21). Further, three-dimensional (3D) modeling revealed a predicted structure identical to that of the MroQ from Agr-I-, -II-, and -III-containing strains, potentially indicating that this limited divergence in sequence identity is not critical for enzymatic activity or structural integrity.
Defining a conserved region or regions that confer MroQ specificity for Agr peptide maturation could give insight into MroQ function in other AIP-containing Gram-positive bacteria. Notable bacteria with Agr systems include Clostridioides difficile, Enterococcus faecalis, Staphylococcus epidermidis, and Listeria monocytogenes (47–53). Studies by Olson et al. revealed a requirement for Agr in S. epidermidis skin infection models, highlighting the relevance of this system across multiple species (54). AIP maturation is believed to occur in a manner similar to that in S. aureus (54, 55). While AgrB has been implicated in this process, whether other proteases facilitate AIP maturation in these bacteria is less clear (52–57). We have identified MroQ homologues in S. epidermidis (63% amino acid identity), L. monocytogenes (37% amino acid identity), and E. faecalis (38% amino acid identity). Given the ability of MroQ to exert a conserved function across widely divergent S. aureus AgrD sequences, it is certainly feasible that this function could at least be maintained in S. epidermidis and/or L. monocytogenes. Conservation of MroQ function across species would provide tremendous insight into peptide processing in Gram-positive organisms.
In conclusion, this work reinforces the prevailing model that MroQ mediates AIP processing and release in S. aureus. Furthermore, this work demonstrates a conservation of function on a range of peptide precursors, a remarkable phenomenon considering the divergent peptide sequence. Our studies add to the rapidly evolving knowledge of MroQ and its role in Agr system activation.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All bacterial strains used in this work are described in Table 1. S. aureus LAC (AH-1263), SA502A, MW2, and RN3984 were used as WT strains for experiments in this study. LAC (AH-1263) is an S. aureus USA300 clinical isolate that harbors a type I Agr system and is cured of its resistance plasmid (58). SA502A (SA502A) is a clinical isolate that harbors a type II Agr system (59). MW2 is a clinical community-acquired methicillin-resistant isolate (60). RN3984 is a naturally occurring toxic shock syndrome strain (61). RN4850 is a type IV Agr strain from an individual with scalded skin syndrome (62). Most recombinant plasmids were maintained in Escherichia coli DH5α or BH10C before transformation into S. aureus strains RN4220 and RN9011 (RN4220 + pRN7023) and subsequent electroporation or transduction into AH-1263, SA502A, MW2, RN3984, or their respective isogenic mutant derivatives. All E. coli strains were grown in lysogeny broth (LB; Amresco), and S. aureus strains were grown in either tryptic soy broth (TSB; Amresco) or RPMI medium (Corning) supplemented with 1% Casamino Acids (Amresco) and 2.4 mM sodium bicarbonate (Amresco). When required, media were supplemented with the following antibiotics: chloramphenicol (Cm; Amresco), 10 μg/mL; ampicillin (Amp; GoldBio) 100 μg/mL; erythromycin (Erm; Amresco) 5 μg/mL; tetracycline (Tet; Amresco), 2 μg/mL; and anhydrous tetracycline (AnTet; Acros Organics), 1 μg/mL. To select for pJC1111 transductants, cadmium chloride (Alfa Aesar) was used at 0.1 to 0.3 mM. Bacterial growth was monitored by measuring optical density at 600 nm (OD600) using a Genesys 10S UV-visible spectrophotometer.
TABLE 1.
Strains used in this study
| Strain | Description | Designation | Reference or source |
|---|---|---|---|
| AH-1263 | S. aureus USA300 CA-MRSA strain LAC, type I Agr | LAC | 58 |
| SA502A | S. aureus clinical isolate SA502A, type II Agr | SA502A | 59 |
| MW2 | S. aureus CA-MRSA strain, type III Agr | MW2 | 61 |
| RN3984 | S. aureus clinical isolate, type III Agr | RN3984 | 61 |
| RN4850 | S. aureus clinical isolate, type IV Agr | RN4850 | 62 |
| BH10C | E. coli strain that restricts plasmid copy no. for cloning of mroQ | BH10C | 66 |
| DH5α | E. coli strain used for cloning | DH5α | |
| RN4220 | Restriction-negative S. aureus | RN4220 | 67 |
| RN9011 | RN4220 with pRN7023 expressing the SaPI-I integrase | RN9011 | 68 |
| FA-S922 | LAC with in-frame deletion of mroQ | ΔmroQ | 21 |
| FA-S982 | FA-S922 with integrated pJC1112-mroQ for complementation | ΔmroQ + mroQ | 21 |
| FA-S1008 | LAC with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet | 21 |
| FA-S995 | FA-S922 with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet ΔmroQ | 21 |
| FA-S2733 | SA502A with in-frame deletion of mroQ | ΔmroQ | This work |
| FA-S2764 | FA-S2733 with pOS1-FLAG-GG-mroQ for complementation | ΔmroQ + mroQ | This work |
| FA-S2766 | SA502A with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet | This work |
| FA-S2741 | FA-S2733 with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet ΔmroQ | This work |
| FA-S2441 | MW2 with in-frame deletion of mroQ | ΔmroQ | This work |
| FA-S2454 | FA-S2441 with integrated pJC1112-mroQ for complementation | ΔmroQ + mroQ | This work |
| FA-S2735 | MW2 with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet | This work |
| FA-S2738 | FA-S2441 with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet ΔmroQ | This work |
| FA-S2730 | RN3984 with in-frame deletion of mroQ | ΔmroQ | This work |
| FA-S2734 | RN3984 with gene replacement of agrBDCA with tetracycline resistance cassette | Δagr::tet | This work |
| FA-S949 | LAC with P3-GFP reporter plasmid pDB59 | LAC + pDB59 | 69 |
| FA-S1972 | AH-1263 with an in-frame deletion of agrB and P3-GFP reporter plasmid pDB59 | ΔagrB + pDB59 | 21 |
| FA-S1881 | SA502A with P3-GFP reporter plasmid pDB59 | SA502A + pDB59 | 69 |
| FA-S2347 | MW2 with P3-GFP reporter plasmid pDB59 | MW2 + pDB59 | 69 |
| FA-S2767 | RN4850 with P3-GFP reporter plasmid pDB59 | RN4850 + pDB59 | 69 |
| FA-S2005 | AH-1263 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | LAC pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | This work |
| FA-S2010 | FA-S922 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | ΔmroQ pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | This work |
| FA-S2008 | FA-S982 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | ΔmroQ + mroQ pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | This work |
| FA-S2422 | SA502A containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-II | SA502A pOS1-PsarA-sodRBS-6×-His-GG-agrD-II | This work |
| FA-S2746 | FA-S2733 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-II | ΔmroQ pOS1-PsarA-sodRBS-6×-His-GG-agrD-II | This work |
| FA-S2383 | MW2 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-III | MW2 pOS1-PsarA-sodRBS-6×-His-GG-agrD-III | This work |
| FA-S2452 | FA-S2441 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-III | ΔmroQ pOS1-PsarA-sodRBS-6×-His-GG-agrD-III | This work |
| FA-S2762 | FA-S2454 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-III | ΔmroQ + mroQ pOS1-PsarA-sodRBS-6×-His-GG-agrD-III | This work |
| FA-S2028 | FA-S1008 with integrated pJC1111-agr-I | Δagr::tet + agr-I | This work |
| FA-S2030 | FA-S995 with integrated pJC1111-agr-I | Δagr::tet ΔmroQ + agr-I | This work |
| FA-S2032 | FA-S1008 with integrated pJC1111-agr-II | Δagr::tet + agr-II | This work |
| FA-S2034 | FA-S995 with integrated pJC1111-agr-II | Δagr::tet ΔmroQ + agr-II | This work |
| FA-S2036 | FA-S1008 with integrated pJC1111-agr-III | Δagr::tet + agr-III | This work |
| FA-S2038 | FA-S995 with integrated pJC1111-agr-III | Δagr::tet ΔmroQ + agr-III | This work |
| FA-S2040 | FA-S1008 with integrated pJC1111-agr-IV | Δagr::tet + agr-IV | This work |
| FA-S2042 | FA-S995 with integrated pJC1111-agr-IV | Δagr::tet ΔmroQ + agr-IV | This work |
| FA-S2728 | FA-S2735 with integrated pJC1111-agr-I | MW2 Δagr::tet + agr-I | This work |
| FA-S2750 | FA-S2738 with integrated pJC1111-agr-I | MW2 Δagr::tet ΔmroQ + agr-I | This work |
| FA-S2530 | LAC with an in-frame deletion of agrD | ΔagrD | This work |
| FA-S2564 | FA-S922 with an in-frame deletion of agrD | ΔmroQ ΔagrD | This work |
| FA-S2718 | FA-S982 with an in-frame deletion of agrD | ΔmroQ ΔagrD + mroQ | This work |
| FA-S2526 | FA-S2530 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | ΔagrD pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | This work |
| FA-S2577 | FA-S2564 containing pOS1-PsarA-sodRBS-6×-His-GG-agrD-I | ΔmroQ ΔagrD pOSI-PsarA-sodRBS-6×-His-GG-agrD-I | This work |
| FA-S2772 | FA-S2718 containing pOSI-PsarA-sodRBS-6×-His-GG-agrD-I | ΔmroQ ΔagrD + mroQ pOSI-PsarA-sodRBS-6×-His-GG-agrD-I | This work |
| FA-S2525 | FA-S2530 containing pOS1-PsarA-sodRBS-6×-His-GG-Leader-I | ΔagrD pOS1-PsarA-sodRBS-6×-His-GG-Leader-I | This work |
| FA-S2583 | FA-S2564 containing pOS1-PsarA-sodRBS-6×-His-GG-Leader-AIP-I | ΔmroQ ΔagrD pOS1-PsarA-sodRBS-6×-His-GG-Leader-AIP-I | This work |
Genetic techniques.
For isolation of genomic DNA from S. aureus, bacterial strains were grown overnight in 5 mL of TSB at 37°C and 220 rpm. The next day, 1.5 mL of culture was centrifuged at 8,000 rpm for 10 min and the resulting pellet was resuspended in TSM buffer (50 mM Tris [pH 7.5], 0.5 M sucrose, 10 mM MgCl2), followed by incubation with lysostaphin (2 mg/mL in 0.5 M Tris [pH 8.0]) for 15 min at 37°C to allow for digestion of the cell wall. Cellular digests were centrifuged at 14,000 rpm for 2 min and supernatants were discarded. Genomic DNA was isolated using the Wizard genomic DNA purification kit (Promega) or DNeasy blood and tissue kit (Qiagen). PCR was performed using Q5 DNA polymerase, GoTaq DNA polymerase, or DreamTaq DNA polymerase (Thermo) and deoxynucleoside triphosphates (Quanta BioSciences). All PCRs were performed in a FlexID Mastercycler (Eppendorf) according to the manufacturer’s suggested protocols. Oligonucleotides were purchased from Eurofins or Integrated DNA Technologies (IDT) and are listed in Table 2. Electrophoresis of DNA samples was carried out in 0.8% or 2% agarose (Amresco) gels. The restriction endonucleases KpnI, SacI, PstI, and EcoRI (New England BioLabs) were used to perform DNA digestions. All reactions were performed according to the manufacturer’s suggested protocol, and all digested plasmids were further treated with shrimp alkaline phosphatase (Amresco). Ligations were performed with T4 DNA ligase (New England Biolabs) and were incubated overnight at 16°C in a ThermoMixer (Eppendorf). DNA gel extraction and PCR purification were performed using Qiagen QIAquick kits. Plasmids were isolated from E. coli using a Qiagen miniprep kit. For plasmid isolation from S. aureus, strains were grown overnight in 5-mL cultures at 37°C with shaking at 220 rpm. Bacterial cells were centrifuged at 3,900 rpm for 5 min, and the resulting pellet was resuspended in TSM plus lysostaphin (2 mg/mL) and incubated for at least 10 min at 37°C. Following treatment, bacterial cells were centrifuged at 13,000 rpm for 2 min and the remaining miniprep was carried out using a Qiagen miniprep kit, with a 5-min incubation following addition of P1 and P2. Plasmid concentrations were measured using a NanoDrop instrument (Thermo).
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence |
|---|---|
| MroQ-1 | CCC-GGTACC(KpnI)-CCATAAATGATAAACCTCCAT |
| MroQ-2 | GTGTGATTCGTTTTTTTTATTA-GGCGCC(KasI)-CATAATTTTCCTCCAAATATT |
| MroQ-3 | AATATTTGGAGGAAAATTATG-GGCGCC(KasI)-TAATAAAAAAAACGAATCACAC |
| MroQ-4 | CCC-GAGCTC(SacI)-ATTTTTAGCCTTGGCAAATG |
| MroQFwd | ATGACAAGATTATGGGCATCAT |
| MroQRev | TTATGGAATAAAAATGTGATAT |
| pOS1UniSOE1 | CCC-CTGCAG(PstI)-CTGATATTTTTGACTAAACCAA |
| PsarA-sodRBS-6×-His-GG-agrD-SOE2 | ACCACCGTGATGGTGATGGTGATGGCTGCTGCCCAT-AAATAATCATCCTCCTAAGGT |
| PsarA-sodRBS-6×-His-GG-agrDI-SOE3 | ATGCATCACCATCACCATC-CCTTAGGAGGATGATTATTT |
| PsarA-sodRBS-6×-His-GG-agrDI-SOE4 | ATAT-GAATTC(EcoRI)-TTATTCGTGTAATTGTGTTAAT |
| PsarA-sodRBS-6×-His-GG-agrDII-SOE3 | ATGGGCAGCAGCCATCACCATCACCATCACGGTGGT-AATACACTTGTTAATATGTTTTTT |
| PsarA-sodRBS-6×-His-GG-agrDII-SOE4 | CCC-GAATTC(EcoRI)-CTATTTGTCGTATAAATTCGTT |
| PsarA-sodRBS-6×-His-GG-agrDIII-SOE3 | CATCACCATCACCATCACGGTGGT-AAAAAATTACTCAACAAAG |
| PsarA-sodRBS-6×-His-GG-agrDIII-SO4 | CCC-CCATGG(NcoI)-TTATTCGTGTAATTGAGTTAATT |
| AgrD-1 | AAA-GGTACC(KpnI)-TCCATTTTACTAAGTCACCG |
| AgrD-2 | CTCTCTATTTAAATTATTCGTGATTCATTTTAAGTCCTCCTTA |
| AgrD-3 | TAAGGAGGACTTAAAATGAATCACGAATAATTTAAATAGAGAG |
| AgrD-4 | AAA-GAGCTC(SacI)-TCGGGTATTTCGATACTAAT |
| T1LeaderREV | AAA-GAATTC(EcoRI)-TTAAGCTGCGATGTTACCAATGT |
| T1Leader-AIPREV | AAA-GAATTC(EcoRI)-TTACATTATGAAGTCACAAGT |
| FLAG-GG-MroQSOE1 | CCC-CTGCAG(PstI)-CTGATATTTTTGACTAAACCAA |
| FLAG-GG-MroQSOE2 | ACCACCCTTGTCGTCATCGTCTTTGTAGTCGCTGCTGCCCAT-AAATAATCATCCTCCTAAGGT |
| FLAG-GG-MroQSOE3 | ACAAGATTATGGGCATCATT-ATGGGCAGCAGCGACTACAAAGACGATGACGACAAGGGTGGT |
| FLAG-GG-MroQSOE4 | CCC-GAATTC(EcoRI)-TTATGGAATAAAAATGTGATATA |
Generation of mroQ and agrD in-frame deletion mutants.
The temperature-sensitive plasmid pIMAY (63) was used to generate ΔmroQ in-frame deletion mutants. To amplify two fragments corresponding to ~500 bp of sequence homology immediately upstream or downstream of mroQ, oligonucleotides MroQ-1, MroQ-2, MroQ-3, and MroQ-4 were used (Table 2). MroQ-1 and MroQ-2 were designed to amplify the region upstream of mroQ, while MroQ-3 and MroQ-4 were designed to amplify the region immediately downstream. To join the fragments, splicing by overlap extension (SOE) PCR was performed using the amplicons from the above-mentioned PCRs as the template along with primers MroQ-1 and MroQ-4. The amplicon was subcloned into the multicloning site of pIMAY after digestion with KpnI and SacI restriction endonucleases. Since mroQ and the regions immediately upstream and downstream of the gene share sequence homology among Agr types I to III, the same plasmid was used to generate in-frame deletions in wild-type strains harboring these allelic variant Agr systems. To generate ΔagrD mutants, primers AgrD-1, AgrD-2, AgrD-3, and AgrD-4 were used to amplify two fragments corresponding to ~500 bp of sequence homology immediately upstream or downstream of agrD. The resulting amplicons were used as the template for SOE PCR, which was performed using the primers AgrD-1 and AgrD-4. The amplicon was subcloned into pIMAY as described above. In each case, mutagenesis was performed according to previously published protocols and mutations were confirmed by PCR and Sanger sequencing (21, 39).
Bacteriophage-mediated generalized transduction.
Transduction was used to transfer stably integrated complementation plasmids between strains and to mobilize marked mutations within the S. aureus chromosome. In this study, S. aureus-specific bacteriophages ϕ11, 80α, and ϕ85 were used. Donor strains were grown overnight in TSB-LB (1:1) supplemented with 5 mM CaCl2 and 5 mM MgSO4, diluted 1:100 in TSB-LB, and grown for 2.5 to 3 h at 37°C and 220 rpm until the OD600 reached approximately 0.3 to 0.9. To package donor DNA, 100 μL of serially diluted bacteriophage stock in TMG buffer (10 mM Tris [pH 7.5], 5 mM MgCl2, 0.1% [vol/vol] gelatin) was incubated with 500 μL of bacterial culture in microcentrifuge tubes for 30 min at room temperature. Melted and cooled CY top agar (3 g/L of Casamino Acids, 3 g/L of yeast extract, 6 g/L of NaCl, 7.5 g/L of agar) supplemented with 5 mM CaCl2 and 5 mM MgSO4 was added to the bacterium-phage mixture and immediately poured onto prewarmed tryptic soy agar (TSA) plates. Plates were incubated at 30°C overnight. The following day, phages were harvested from two to four plates with confluent plaques. Phage stocks were stored at 4°C. To transduce plasmids and marked mutations, recipient strains grown overnight in 20 mL of TSB-LB (1:1) supplemented with 5 mM CaCl2 were centrifuged at 3,900 rpm for 15 min and the resulting pellet was resuspended in 3 mL of TSB-LB plus 5 mM CaCl2. Recipient bacteria were diluted 1:1, 1:10, and 1:100 in fresh TSB-LB plus 5 mM CaCl2 in a final volume of 500 μL. A total of 100 μL of phage stock was added to each bacterial dilution and incubated at room temperature for 30 min, with inversion of the tubes to mix every 10 min. After 30 min, each mixture was supplemented with sodium citrate to a final volume of 40 mM and incubated at room temperature for another 30 min, with inversion every 10 min. Bacterium-phage mixtures were centrifuged at 14,000 rpm for 5 min, and the resulting pellet was washed two times in TSB-LB plus 40 mM sodium citrate and then resuspended in 100 μL of TSB-LB plus 40 mM sodium citrate and plated on TSA plates with 10 mM sodium citrate and any antibiotics necessary for selection. Plates were incubated at 37°C overnight, and any potential transductants were screened for antibiotic resistance and acquired mutations using PCR and DNA sequencing.
Construction of Δagr::tet mutants.
To generate Δagr::tet mutants, a marked deletion mutant of agrBDCA was transduced into AH-1263, SA502A, MW2, and RN3984 by bacteriophage-mediated transduction as described above.
Generation of complementation strains.
To generate single-copy chromosomal complementation strains expressing mroQ under the control of its native promoter, the integrative plasmid pJC1112 was used to generate pJC1112-mroQ as previously described (21, 39). Complementation of the ΔmroQ mutation in SA502A with pJC1112-mroQ under the control of its native promoter was unsuccessful presumably because of altered expression patterns in this strain. To overcome this limitation, we expressed FLAG-GG-mroQ under the control of the constitutive PsarA promoter in plasmid pOS1 (64). To generate this plasmid, the PsarA promoter linked to the S. aureus superoxide dismutase (sod) ribosomal binding site was fused to the coding sequence for mroQ with an N-terminal FLAG tag and diglycine linker. To amplify PsarA-sodRBS, primers FLAG-GG-MroQSOE1 and FLAG-GG-MroQSOE2 were used. FLAG-GG-mroQ was generated using FLAG-GG-MroQSOE3 and FLAG-GG-MroQOE4. Amplicons were spliced together using SOE PCR with primers FLAG-GG-MroQSOE1 and FLAG-GG-MroQSOE4 and cloned into pOS1 using PstI and EcoRI restriction endonucleases. The resulting plasmid was transformed into E. coli BH10C and electroporated into S. aureus RN4220. The complementation vector was then transduced into an SA502A ΔmroQ mutant as described above. To generate complementation strains harboring Agr loci under the control of their native promoters, the integrative plasmid pJC1111 was used. Integrated complementation vectors were transduced into ΔmroQ (pJC1112-mroQ), Δagr (pJC1111-agrI-IV), or Δagr ΔmroQ (pJC1111-agrI-IV) as defined above. Complement strains were verified using PCR and DNA sequencing. Primers are listed in Table 2.
Construction of P3-gfp reporter strains.
Plasmid pDB59 harboring the Agr-regulated P3 promoter driving the expression of gfp was isolated from E. coli, passaged through RN4220, and electroporated into all indicated strains (21, 41, 65).
Construction of pOS1-PsarA-6×-His-GG-agrD expression plasmid.
A 6×-His-GG-agrD expression plasmid for each Agr type was generated by fusing the PsarA promoter linked to the S. aureus superoxide dismutase (sod) ribosomal binding site with the coding sequence for AgrD containing an N-terminal 6×-His tag and diglycine linker. To amplify PsarA-sodRBS, primers pOS1uniSOE1 and PsarA-sodRBS-6×-His-GG-agrD-SOE2 were used. 6×-His-GG-AgrD-I was amplified using PsarA-sodRBS-6×-His-GG-agrDI-SOE3 and PsarA-sodRBS-6×-His-GG-agrDI-SOE4. 6×-His-GG-AgrD-II was amplified using PsarA-sodRBS-6×-His-GG-agrDII-SOE3 and PsarA-sodRBS-6×-His-GG-agrDII-SOE4. 6×-His-GG-Agr-III was amplified using PsarA-sodRBS-6×-His-GG-agrDIII-SOE3 and PsarA-sodRBS-6×-His-GG-agrDIII-SOE4. SOE PCR was used to splice AgrDI to -III to PsarA-sodRBS using the resulting amplicons from the above-described PCRs as the template with primers pOS1uniSOE1 and PsarA-sodRBS-6×-His-GG-agrDI-SOE4, PsarA-sodRBS-6×-His-GG-agrDII-SOE4, and PsarA-sodRBS-6×-His-GG-agrDIII-SOE4. The resulting fusion products were cloned into pOS1 using PstI and EcoRI restriction endonucleases. These products were transformed into DH5α, passaged through RN4220, and electroporated into wild-type, ΔmroQ, and ΔmroQ + mroQ isogenic strains for LAC and MW2 and wild-type and ΔmroQ isogenic strains for SA502A. To generate leader and leader-AIP peptide controls for Agr type I, the primers pOS1uniSOE1 and PsarA-sodRBS-6×-His-GG-agrD-SOE2 were used to amplify PsarA-sodRBS. PsarA-sodRBS-6×-His-GG-agrDI-SOE3 and T1LeaderREV or T1Leader-AIPREV were used to amplify leader or leader-AIP, respectively. SOE PCR was used to splice the leader or leader-AIP to PsarA-sodRBS using the above-described amplicons as the template with primers pOS1uniSOE1 and T1LeaderREV or T1Leader-AIPREV. All primers are listed in Table 2.
Analysis of 6×-His-AgrD maturation by immunoblotting.
Five-milliliter overnight cultures of 6×-His-GG-agrD-expressing strains were subcultured 1:100 in 50 mL to 400 mL of RPMI or TSB at 37°C and 220 rpm for 8 h or overnight. Bacterial cells were centrifuged at 3,900 rpm for 20 min, and supernatants were removed and filter sterilized using a 0.22-μm filter before addition of imidazole (10 mM) and phenylmethylsulfonyl fluoride (PMSF; 1.2 mM), followed by overnight incubation with preequilibrated nickel-nitrilotriacetic acid (Ni-NTA). Bound 6×-His-AgrD was washed two times with 1 mL of 6 M urea buffer, followed by addition of 100 μL of 4× SDS sample buffer and boiling for 10 min. For isolation of membrane fractions, bacterial pellets were resuspended in 10 mL of phosphate-buffered saline (PBS) and treated with lysostaphin for 30 min at 37°C, followed by sonication using a Sonifier sonicator (Branson) at 30% power with 20 s on and 20 s off for a total of 2 min. Cellular debris was removed by centrifugation at 12,000 rpm for 15 min at 4°C. Lysates were ultracentrifuged at 20,000 rpm for 70 min at 4°C, and the resulting pellet was solubilized in PBS plus 1 M NaCl2, 6 M urea, and 1% n-doceyl-β-D-maltoside (DDM) overnight. Equilibrated Ni-NTA was added to the DDM-solubilized samples, which were incubated for 3 h to allow for binding. Bound 6×-His-AgrD was washed two times with 1 mL of 6 M urea buffer and boiled in 4× SDS sample buffer as described above. Samples were resolved on 16% Tris-Tricine gels containing 6 M urea for 2 h at 30 V to allow for migration into the gel and then overnight at 90 V. Proteins were transferred to 0.2-μm-pore-size polyvinylidene difluoride (PVDF) membranes (Immobilon; Roche) in 20% methanol transfer buffer at 100 V for 30 min. Membranes were blocked for 1 h in Tris-buffered saline (TBS)-Tween 20 (TBST; 0.1% Tween 20 [Amresco] in TBS [Corning]) containing 5% bovine serum albumin (BSA; GoldBio), incubated with anti-His6 monoclonal mouse antibody (1:5,000 dilution) (ab18184; Abcam) overnight, and washed three times in 10 to 20 mL of TBST for 5 min each. Blots were then incubated with goat anti-mouse IgG (H+L) conjugated to alkaline phosphatase (Thermo) for 1 h, followed by three washes in 10 to 20 mL of TBST for 5 min each and development using 5-bromo-4-chloro-3-indoyl-phosphate–nitroblue tetrazolium (BCIP/NBT) substrate (GoldBio/VWR).
Exoprotein preparations.
Bacterial strains were subcultured 1:100 in 5 mL of TSB or RPMI for 8 h at 37°C and 220 rpm. The OD600 was measured, cell suspensions were centrifuged at 3,900 rpm for 15 min, and supernatants were removed and filter sterilized through a 0.22-μm filter. A total of 1.3 mL of supernatant was collected in a 1.5-mL microcentrifuge tube, and 150 μL of 100% trichloroacetic acid (TCA) was added. Mixtures were incubated overnight at 4°C, centrifuged at 12,000 rpm for 15 min to pellet precipitated proteins, incubated with 1 mL of 100% ethanol or acetone for at least 30 min at 4°C, and centrifuged again at 12,000 rpm for 15 min. The resulting pellets were allowed to air dry at room temperature, followed by addition of TCA-SDS sample buffer (4% SDS plus 0.5 M Tris-HCl mixed 1:1 with 2× SDS loading buffer) and boiling for 10 min. Samples were stored at −20°C. OD600-normalized exoprotein preparations were resolved by SDS-PAGE using 12% acrylamide gels at 120 V in a Quadra minivertical PAGE/blotting system (CBS Scientific). Gels were fixed in a solution containing methanol-acetic acid-H2O (50:10:4) for 25 min before washing with 10 mL of water three times for 5 min each. Following fixation, gels were stained in Gel-Code blue stain reagent (Pierce) for 1 h at room temperature and subsequently washed in 10 to 20 mL of H2O for 2 to 3 h to allow for destaining.
Immunoblot analysis for secreted virulence factors.
SDS-PAGE-separated proteins were transferred to a 0.2-μm-pore-size PVDF membrane as described above. Membranes were blocked with TBST plus 5% BSA for 1 h before addition of human IgG (Sigma) (1:2,000) in TBST for 1 h to block protein A, followed by washing three times with 10 mL of TBST for 15 min each before addition of Rb-anti-HlgC (1:5,000) or Ms-anti-Hla (1:5,000) antibodies overnight. The following day, membranes were washed three times with 10 mL of TBST and incubated with alkaline phosphatase conjugated goat anti-mouse IgG (H+L) or goat anti-rabbit IgG (H+L) (Invitrogen) for 1 h. Membranes were washed three times with 10 mL of TBST and then developed using BCIP/NBT reagent as described above.
AIP inhibition assays.
Bacterial strains were subcultured 1:100 in TSB or RPMI for 5 h at 37°C and 220 rpm. Cell suspensions were centrifuged at 3,900 rpm for 5 min, and supernatants, designated “conditioned media (CM),” were filter sterilized through a 0.22-μm filter. Overnight cultures of P3-GFP reporter strains were then subcultured 1:100 in 50% CM and allowed to grow for 5 h at 37°C and 220 rpm. Following incubation, 100-μL aliquots were pelleted at 3,900 rpm for 5 min, washed three times with PBS, and resuspended in a final volume of 100 μL of PBS. GFP fluorescence and OD600 were measured using a SpectraMax ID3 plate reader (Molecular Devices). Relative fluorescence was calculated by normalizing fluorescence units to OD600.
AIP activation assay.
Bacterial strains were subcultured 1:100 in 5 mL of TSB for 5 to 8 h at 37°C and 220 rpm. Cell suspensions were centrifuged at 3,900 rpm for 5 min, and supernatants from test strains were collected and filter sterilized through a 0.22-μm filter, whereas bacterial pellets from reporter strains were retained for later application of conditioned medium. The reporter strain pellets were resuspended in 5 mL of fresh TSB, and 250 μL of this resuspension was mixed with 500 μL of test strain supernatant. A total of 200 μL of the suspension was added in triplicate to a v-bottom plate and incubated for 3 h at 37°C and 220 rpm. Cell suspensions were centrifuged at 3,900 rpm for 5 min, followed by 2 washes and resuspension in 200 μL of PBS. GFP fluorescence and OD600 were measured as described above.
Rabbit RBC lysis assay.
Bacterial strains were subcultured 1:50 in 150 μL of TSB or RPMI in a 96-well plate for 6 h for strains LAC, SA502A, MW2 Δagr::tet + pJC1111-Agr-I, and MW2 Δagr::tet ΔmroQ + pJC1111-Agr-I and 16 h for strains from MW2 and RN3984 at 37°C and 220 rpm. Cell suspensions were centrifuged at 3,900 rpm for 5 min, and OD600-normalized cell-free supernatants were serially diluted 1:1 in PBS in a round-bottom 96-well plate followed by 1:1 addition of defibrinated rabbit red blood cells (RBCs; Colorado Serum Company) diluted to 2% packed-cell volume (PCV). Following incubation for 1 h at 37°C, samples were centrifuged at 1,500 rpm for 5 min and supernatants were transferred to flat-bottom 96-well plates followed by measurement of RBC lysis at OD450.
Murine skin and soft tissue infection.
Bacterial strains were grown overnight in TSB (Criterion) at 37°C and 220 rpm and subcultured 1:100 in 15 mL of TSB for 3 h. Strains were washed three times with 5 mL of PBS then normalized to an OD600 of 0.32 to 0.33 (1 × 108 CFU) followed by mixing 1:1 with sterile Cytodex microcarrier beads (Sigma-Aldrich). Mice were anesthetized with 2,2,2-tribromoethanol (Avertin; Sigma) via intraperitoneal injection (250 mg/kg of body weight), and 200 μL of the bacterium-Cytodex bead mixture (1 × 107 CFU) was injected intradermally into each side of the shaved flank region of anesthetized mice. After 4 days, mice were euthanized and abscesses were harvested, homogenized, and plated on TSA for enumeration of CFU. Images of representative abscesses were captured and are displayed in Fig. 7 and 8.
Ethics statement.
All animal experiments followed the ethical standards outlined by the Institutional Animal Care and Use Committee (IACUC) and institutional biosafety committee at Loyola University Chicago, Health Sciences Division. Loyola University Chicago is registered by the USDA (33-R-0024 through 24 August 2023), approved by the Public Health Service (PHS; A3117-01 through 28 February 2026), and fully accredited by AAALAC International (000180 through November 2022). All animal experiments were performed following USDA and PHS policy guidelines on the humane care and use of animals and were carried out in biosafety level 2 facilities with IACUC-approved protocols under the guidance of the Office of Laboratory Animal Welfare (OLAW).
Statistical analysis.
All experiments were repeated at least 3 independent times. For AIP reporter assays, statistical significance was analyzed using GraphPad Prism (version 9.0) with representative data from experiments conducted in triplicate at least three independent times. Statistical tests are specified in the figure legends. For animal studies, statistical analysis was performed by Kruskal-Wallis test with Dunn’s post hoc test for multiple comparisons.
ACKNOWLEDGMENTS
We thank the Alonzo lab for helpful discussions. Several strains and reagents used in this study were kindly provided by Richard P. Novick and Victor J. Torres.
This study was supported by grants NIH R01 AI120994 and NIH R01 AI153059 and by a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award to F.A.
Contributor Information
Francis Alonzo, III, Email: falonzo@uic.edu.
Victor J. Torres, New York University School of Medicine
REFERENCES
- 1.Monnet V, Juillard V, Gardan R. 2016. Peptide conversations in Gram-positive bacteria. Crit Rev Microbiol 42:339–351. 10.3109/1040841X.2014.948804. [DOI] [PubMed] [Google Scholar]
- 2.Waters CM, Bassler BL. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21:319–346. 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Cook LC, Federle MJ. 2014. Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol Rev 38:473–492. 10.1111/1574-6976.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thoendel M, Kavanaugh JS, Flack CE, Horswill AR. 2011. Peptide signaling in the staphylococci. Chem Rev 111:117–151. 10.1021/cr100370n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon GJ, Novick RP. 2004. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides (NY) 25:1389–1403. 10.1016/j.peptides.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J 12:3967–3975. 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novick RP, Muir TW. 1999. Virulence gene regulation by peptides in staphylococci and other Gram-positive bacteria. Curr Opin Microbiol 2:40–45. 10.1016/S1369-5274(99)80007-1. [DOI] [PubMed] [Google Scholar]
- 8.Cheung GYC, Wang R, Khan BA, Sturdevant DE, Otto M. 2011. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect Immun 79:1927–1935. 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saravia-Otten P, Müller HP, Arvidson S. 1997. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol 179:5259–5263. 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery CP, Boyle-Vavra S, Daum RS. 2010. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One 5:e15177. 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol 170:4365–4372. 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S, Novick RP. 1995. Theagr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet 248:446–458. 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 13.Geisinger E, George EA, Chen J, Muir TW, Novick RP. 2008. Identification of ligand specificity determinants in AgrC, the Staphylococcus aureus quorum-sensing receptor. J Biol Chem 283:8930–8938. 10.1074/jbc.M710227200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Lin J, Ji G. 2004. Membrane anchoring of the AgrD N-terminal amphipathic region is required for its processing to produce a quorum-sensing pheromone in Staphylococcus aureus. J Biol Chem 279:19448–19456. 10.1074/jbc.M311349200. [DOI] [PubMed] [Google Scholar]
- 15.Qiu R, Pei W, Zhang L, Lin J, Ji G. 2005. Identification of the putative staphylococcal AgrB catalytic residues involving the proteolytic cleavage of AgrD to generate autoinducing peptide. J Biol Chem 280:16695–16704. 10.1074/jbc.M411372200. [DOI] [PubMed] [Google Scholar]
- 16.Thoendel M, Horswill AR. 2009. Identification of Staphylococcus aureus AgrD residues required for autoinducing peptide biosynthesis. J Biol Chem 284:21828–21838. 10.1074/jbc.M109.031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linsheng Z, Guangyong J. 2004. Identification of a staphylococcal AgrB segment(s) responsible for group-specific processing of AgrD by gene swapping. J Bacteriol 186:6706–6713. 10.1128/JB.186.20.6706-6713.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Gray L, Novick RP, Ji G. 2002. Transmembrane topology of AgrB, the protein involved in the post-translational modification of AgrD in Staphylococcus aureus. J Biol Chem 277:34736–34742. 10.1074/jbc.M205367200. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Zhao A, Novick RP, Muir TW. 2015. Key driving forces in the biosynthesis of autoinducing peptides required for staphylococcal virulence. Proc Natl Acad Sci USA 112:10679–10684. 10.1073/pnas.1506030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kavanaugh JS, Thoendel M, Horswill AR. 2007. A role for type I signal peptidase in Staphylococcus aureus quorum sensing. Mol Microbiol 65:780–798. 10.1111/j.1365-2958.2007.05830.x. [DOI] [PubMed] [Google Scholar]
- 21.Cosgriff CJ, White CR, Teoh WP, Grayczyk JP, Alonzo F, III.. 2019. Control of Staphylococcus aureus quorum sensing by a membrane-embedded peptidase. Infect Immun 87:e00019-19. 10.1128/IAI.00019-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marroquin S, Gimza B, Tomlinson B, Stein M, Frey A, Keogh RA, Zapf R, Todd DA, Cech NB, Carroll RK, Shaw LN. 2019. MroQ is a novel Abi-domain protein that influences virulence gene expression in Staphylococcus aureus via modulation of Agr activity. Infect Immun 87:e00002-19. 10.1128/IAI.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao A, Bodine SP, Xie Q, Wang B, Ram G, Novick RP, Muir TW. 2021. Reconstitution of the S. aureus agr quorum sensing pathway reveals a direct role for the integral membrane protease MroQ in pheromone biosynthesis. bioRxiv. 10.1101/2021.12.29.473670. [DOI] [PMC free article] [PubMed]
- 24.Jarraud S, Lyon GJ, Figueiredo AM, Lina G, Gérard L, Vandenesch F, Etienne J, Muir TW, Novick RP. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J Bacteriol 182:6517–6522. 10.1128/JB.182.22.6517-6522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji G, Beavis R, Novick RP. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027–2030. 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 26.Geisinger E, Chen J, Novick RP. 2012. Allele-dependent differences in quorum-sensing dynamics result in variant expression of virulence genes in Staphylococcus aureus. J Bacteriol 194:2854–2864. 10.1128/JB.06685-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Muir TW. 2016. Regulation of virulence in Staphylococcus aureus—molecular mechanisms and remaining puzzles. Cell Chem Biol 23:214–224. 10.1016/j.chembiol.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright JS, III, Traber KE, Corrigan R, Benson SA, Musser JM, Novick RP. 2005. The agr radiation—an early event in the evolution of staphylococci. J Bacteriol 187:5585–5594. 10.1128/JB.187.16.5585-5594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dufour P, Jarraud S, Vandenesch F, Greenland T, Novick RP, Bes M, Etienne J, Lina G. 2002. High genetic variability of the agr locus in Staphylococcus species. J Bacteriol 184:1180–1186. 10.1128/jb.184.4.1180-1186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tal-Gan Y, Ivancic M, Cornilescu G, Cornilescu CC, Blackwell HE. 2013. Structural characterization of native autoinducing peptides and abiotic analogues reveals key features essential for activation and inhibition of an AgrC quorum sensing receptor in Staphylococcus aureus. J Am Chem Soc 135:18436–18444. 10.1021/ja407533e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. 1999. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA 96:1218–1223. 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDowell P, Affas Z, Reynolds C, Holden MTG, Wood SJ, Saint S, Cockayne A, Hill PJ, Dodd CER, Bycroft BW, Chan WC, Williams P. 2001. Structure, activity and evolution of the group I thiolactone peptide quorum-sensing system of Staphylococcus aureus. Mol Microbiol 41:503–512. 10.1046/j.1365-2958.2001.02539.x. [DOI] [PubMed] [Google Scholar]
- 33.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, Zhao A, Novick RP, Muir TW. 2014. Activation and inhibition of the receptor histidine kinase AgrC occurs through opposite helical transduction motions. Mol Cell 53:929–940. 10.1016/j.molcel.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyon GJ, Wright JS, Muir TW, Novick RP. 2002. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 41:10095–10104. 10.1021/bi026049u. [DOI] [PubMed] [Google Scholar]
- 36.Geisinger E, Muir TW, Novick RP. 2009. agr receptor mutants reveal distinct modes of inhibition by staphylococcal autoinducing peptides. Proc Natl Acad Sci USA 106:1216–1221. 10.1073/pnas.0807760106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X, Etienne J, Vandenesch F. 2002. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun 70:631–641. 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung AL, Eberhardt KJ, Chung E, Yeaman MR, Sullam PM, Ramos M, Bayer AS. 1994. Diminished virulence of a sar-/agr- mutant of Staphylococcus aureus in the rabbit model of endocarditis. J Clin Invest 94:1815–1822. 10.1172/JCI117530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grayczyk JP, Harvey CJ, Laczkovich I, Alonzo F, III.. 2017. A lipoylated metabolic protein released by Staphylococcus aureus suppresses macrophage activation. Cell Host Microbe 22:678–687. 10.1016/j.chom.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandenesch F, Kornblum J, Novick RP. 1991. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol 173:6313–6320. 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malone CL, Boles BR, Lauderdale KJ, Thoendel M, Kavanaugh JS, Horswill AR. 2009. Fluorescent reporters for Staphylococcus aureus. J Microbiol Methods 77:251–260. 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tal-Gan Y, Stacy DM, Foegen MK, Koenig DW, Blackwell HE. 2013. Highly potent inhibitors of quorum sensing in Staphylococcus aureus revealed through a systematic synthetic study of the group-III autoinducing peptide. J Am Chem Soc 135:7869–7882. 10.1021/ja3112115. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, DeLeo FR. 2011. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis 204:937–941. 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. 2011. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17:1310–1314. 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams MR, Costa SK, Zaramela LS, Khalil S, Todd DA, Winter HL, Sanford JA, O’Neill AM, Liggins MC, Nakatsuji T, Cech NB, Cheung AL, Zengler K, Horswill AR, Gallo RL. 2019. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med 11:eaat8329. 10.1126/scitranslmed.aat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuomanen EI, Xiang Q, Singh KV, Weinstock GM, Murray BE. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun 68:2579–2586. 10.1128/IAI.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiro N, Shengmin C, Nozomi O, Kenzo N, Azab EA, Emi T, Reiko K, Kenji S. 2006. Revised model for Enterococcus faecalis fsr quorum-sensing system: the small open reading frame fsrD encodes the gelatinase biosynthesis-activating pheromone propeptide corresponding to staphylococcal AgrD. J Bacteriol 188:8321–8326. 10.1128/JB.00865-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arthur W, Madan BM. 2008. Conservation and evolutionary dynamics of the agr cell-to-cell communication system across Firmicutes. J Bacteriol 190:743–746. 10.1128/JB.01135-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol 10:R102. 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monot M, Boursaux-Eude C, Thibonnier M, Vallenet D, Moszer I, Medigue C, Martin-Verstraete I, Dupuy B. 2011. Reannotation of the genome sequence of Clostridium difficile strain 630. J Med Microbiol 60:1193–1199. 10.1099/jmm.0.030452-0. [DOI] [PubMed] [Google Scholar]
- 52.van Wamel WJB, van Rossum G, Verhoef J, Vandenbroucke-Grauls CMJE, Fluit AC. 1998. Cloning and characterization of an accessory gene regulator (agr)-like locus from Staphylococcus epidermidis. FEMS Microbiol Lett 163:1–9. 10.1111/j.1574-6968.1998.tb13018.x. [DOI] [PubMed] [Google Scholar]
- 53.Autret N, Raynaud C, Dubail I, Berche P, Charbit A. 2003. Identification of the agr locus of Listeria monocytogenes: role in bacterial virulence. Infect Immun 71:4463–4471. 10.1128/IAI.71.8.4463-4471.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson ME, Todd DA, Schaeffer CR, Paharik AE, van Dyke MJ, Büttner H, Dunman PM, Rohde H, Cech NB, Fey PD, Horswill AR. 2014. Staphylococcus epidermidis agr quorum-sensing system: signal identification, cross talk, and importance in colonization. J Bacteriol 196:3482–3493. 10.1128/JB.01882-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riedel CU, Monk IR, Casey PG, Waidmann MS, Gahan CGM, Hill C. 2009. AgrD-dependent quorum sensing affects biofilm formation, invasion, virulence and global gene expression profiles in Listeria monocytogenes. Mol Microbiol 71:1177–1189. 10.1111/j.1365-2958.2008.06589.x. [DOI] [PubMed] [Google Scholar]
- 56.Banerji R, Karkee A, Kanojiya P, Patil A, Saroj SD. 2022. Bacterial communication in the regulation of stress response in Listeria monocytogenes. LWT 154:112703. 10.1016/j.lwt.2021.112703. [DOI] [Google Scholar]
- 57.Gray B, Hall P, Gresham H. 2013. Targeting agr- and agr-like quorum sensing systems for development of common therapeutics to treat multiple gram-positive bacterial infections. Sensors (Basel) 13:5130–5166. 10.3390/s130405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boles BR, Thoendel M, Roth AJ, Horswill AR. 2010. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One 5:e10146. 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shinefield HR, Ribble JC, Boris M, Eichenwald HF. 1963. Bacterial interference—its effect on nursery-acquired infection with Staphylococcus aureus. I. Preliminary observations on artificial colonzation [sic] of newborns. Am J Dis Child 105:646–654. [PubMed] [Google Scholar]
- 60.Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827. 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 61.Chu MC, Kreiswirth BN, Pattee PA, Novick RP, Melish ME, James JF. 1988. Association of toxic shock toxin-1 determinant with a heterologous insertion at multiple loci in the Staphylococcus aureus chromosome. Infect Immun 56:2702–2708. 10.1128/iai.56.10.2702-2708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Reilly M, Dougan G, Foster TJ, Arbuthnott JP. 1981. Plasmids in epidermolytic strains of Staphylococcus aureus. J Gen Microbiol 124:99–107. 10.1099/00221287-124-1-99. [DOI] [PubMed] [Google Scholar]
- 63.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ, Novick RP. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schneewind O, Mihaylova-Petkov D, Model P. 1993. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J 12:4803–4811. 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quave CL, Horswill AR. 2014. Flipping the switch—tools for detecting small molecule inhibitors of staphylococcal virulence. Front Microbiol 5:706–710. 10.3389/fmicb.2014.00706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howell-Adams B, Seifert HS. 2000. Molecular models accounting for the gene conversion reactions mediating gonococcal pilin antigenic variation. Mol Microbiol 37:1146–1158. 10.1046/j.1365-2958.2000.02067.x. [DOI] [PubMed] [Google Scholar]
- 67.Fairweather N, Kennedy S, Foster TJ, Kehoe M, Dougan G. 1983. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun 41:1112–1117. 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen J, Yoong P, Ram G, Torres VJ, Novick RP. 2014. Single-copy vectors for integration at the SaPI1 attachment site for Staphylococcus aureus. Plasmid 76:1–7. 10.1016/j.plasmid.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirchdoerfer RN, Garner AL, Flack CE, Mee JM, Horswill AR, Janda KD, Kaufmann GF, Wilson IA. 2011. Structural basis for ligand recognition and discrimination of a quorum-quenching antibody. J Biol Chem 286:17351–17358. 10.1074/jbc.M111.231258. [DOI] [PMC free article] [PubMed] [Google Scholar]