ABSTRACT
Paratuberculosis is a chronic enteritis of ruminants caused by the facultative intracellular pathogen Mycobacterium avium subsp. paratuberculosis. The Th1 response inhibits the proliferation of M. avium subsp. paratuberculosis during the early subclinical stage. However, we have previously shown that immune inhibitory molecules, such as prostaglandin E2 (PGE2), suppress M. avium subsp. paratuberculosis-specific Th1 responses as the disease progresses. To date, the mechanism underlying immunosuppression during M. avium subsp. paratuberculosis infection has not been elucidated. Therefore, in the present study, we investigated the function of cytotoxic T-lymphocyte antigen 4 (CTLA-4) expressed by peripheral blood mononuclear cells (PBMCs) from cattle with paratuberculosis because CTLA-4 expression is known to be elevated in T cells under an M. avium subsp. paratuberculosis experimental infection. M. avium subsp. paratuberculosis antigen induced CTLA-4 expression in T cells from cattle experimentally infected with M. avium subsp. paratuberculosis. Interestingly, both PGE2 and an E prostanoid 4 agonist also induced CTLA-4 expression in T cells. In addition, a functional assay with a bovine CTLA-4-immunogobulin fusion protein (CTLA-4-Ig) indicated that CTLA-4 inhibited gamma interferon (IFN-γ) production in M. avium subsp. paratuberculosis-stimulated PBMCs, while blockade by anti-bovine CTLA-4 monoclonal antibody increased the secretion of IFN-γ and tumor necrosis factor alpha production in these PBMCs. These preliminary findings show that PGE2 has immunosuppressive effects via CTLA-4 to M. avium subsp. paratuberculosis. Therefore, it is necessary to clarify in the future whether CTLA-4-mediated immunosuppression facilitates disease progression of paratuberculosis in cattle.
KEYWORDS: CTLA-4, PGE2, EP4, Johne’s disease, cattle
INTRODUCTION
Johne’s disease, which is also known as paratuberculosis, is a chronic enteric disease of ruminants caused by the facultative intracellular pathogen Mycobacterium avium subsp. paratuberculosis (1). Johne’s disease is common worldwide; indeed, no country or region is reported to be free of M. avium subsp. paratuberculosis (2). During the early stage of infection, the production of Th1 cytokines, such as gamma interferon (IFN-γ), is strongly induced by the pathogenic bacteria, which is killed by macrophages activated by IFN-γ (3–5). In contrast, M. avium subsp. paratuberculosis-specific Th1 responses are gradually suppressed during late subclinical and clinical stages (6–8). Our previous studies revealed one mechanism of this suppression, which was observed as the disease progresses (9–11). Programmed death 1 (PD-1) and PD-ligand 1 (PD-L1) are immunoinhibitory molecules that contribute to T-cell dysfunction, i.e., exhaustion, in humans and several animals, including cattle (12, 13). We showed previously that the expression levels of PD-1 and PD-L1 are upregulated in cattle infected with M. avium subsp. paratuberculosis (9). In addition, we found that blockade of the PD-1/PD-L1 pathway using anti-bovine PD-L1 antibody activates M. avium subsp. paratuberculosis-specific Th1 responses both in vitro and in vivo (10, 11). In addition, we demonstrated that prostaglandin E2 (PGE2) was associated with the suppression of Th1 responses in M. avium subsp. paratuberculosis-infected animals (10). In cattle, PGE2 induces PD-L1 expression in bovine immune cells (14) and suppresses Th1 responses via its receptor, E prostanoid 4 (EP4) (10, 15). Serum PGE2 concentrations are upregulated in cattle with Johne’s disease, and treatment with a COX-2 inhibitor, which blocks PGE2 production, activates M. avium subsp. paratuberculosis-specific Th1 responses in vitro (10). Collectively, these results suggest that PGE2 is involved in the progression of Johne’s disease. However, the mechanisms underlying the suppression of Th1 responses have yet to be fully elucidated.
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), also known as CD152, is an immunoinhibitory molecule that is expressed in T cells and an essential negative regulator of T-cell responses (16). CTLA-4 binds to CD80 (B7-1) and CD86 (B7-2), which are expressed in antigen-presenting cells, and suppresses host immunity (17, 18). In humans, CTLA-4 has been associated with the progression of chronic diseases caused by human immunodeficiency virus (HIV) and hepatitis C virus (19, 20). In the veterinary field, CTLA-4 has been associated with the progression of bovine leukemia virus (BLV) infection (a bovine chronic infection); specifically, CTLA-4 expression in T cells is increased in BLV-infected cattle, and the blockade of CTLA-4 using anti-bovine CTLA-4 monoclonal antibody (MAb) activates BLV-specific Th1 responses in vitro (21–23). In addition, the involvement of CTLA-4 in cattle infected with M. avium subsp. paratuberculosis has been shown using anti-human CTLA-4 MAb (24). However, the involvement of CTLA-4 in M. avium subsp. paratuberculosis-infected cattle has yet to be investigated using anti-bovine CTLA-4 MAb.
Therefore, in the present study, we analyzed CTLA-4 expression in T cells in M. avium subsp. paratuberculosis-infected cattle using anti-bovine CTLA-4 MAb, which was established in our previous study (23). In addition, we investigated PGE2/EP4 signaling, finding that it was involved in CTLA-4 upregulation in T cells from cattle. Furthermore, we established a bovine CTLA-4-immunogobulin fusion protein (CTLA-4-Ig), using it and anti-bovine CTLA-4 MAb to examine whether CTLA-4 suppresses Th1 responses to antigens of M. avium subsp. paratuberculosis.
RESULTS
Expression analysis of CTLA-4 in M. avium subsp. paratuberculosis-infected cattle.
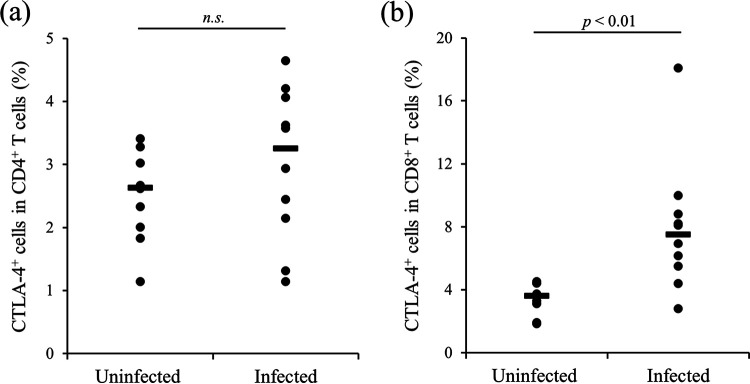
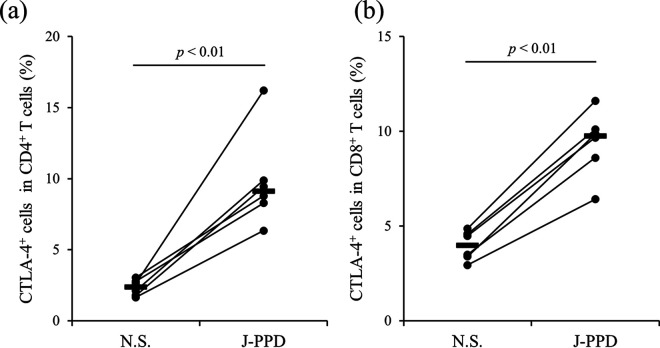
In our previous studies, we demonstrated that CTLA-4 is associated with disease progression in BLV-infected cattle (21, 22). Here, we performed expression analysis of CTLA-4 in cattle infected with M. avium subsp. paratuberculosis using anti-bovine CTLA-4 MAb (4C2-D9). The gating strategy and representative plots of CTLA-4 expression analysis are shown in Fig. S1 in the supplemental material. Compared to cattle not infected with M. avium subsp. paratuberculosis, CTLA-4 expression in CD8+ T cells but not CD4+ T cells was significantly increased in M. avium subsp. paratuberculosis-infected cattle (Fig. 1). Peripheral blood mononuclear cells (PBMCs) from M. avium subsp. paratuberculosis-infected cattle were cultivated with or without J-PPD, and flow cytometric analysis revealed that stimulation with J-PPD significantly upregulated CTLA-4 expression in both CD4+ and CD8+ T cells (Fig. 2). Thus, CTLA-4 expression is apparently upregulated by antigen stimulation in vitro in M. avium subsp. paratuberculosis-infected cattle.
FIG 1.
Expression analysis of CTLA-4 in T cells from M. avium subsp. paratuberculosis-infected cattle. The expression of CTLA-4 in CD4+ T cells (CD3+ IgM− CD4+) (a) and CD8+ T cells (CD3+ IgM− CD8+) (b) was analyzed using flow cytometry (uninfected [cattle not infected with M. avium subsp. paratuberculosis], n = 10; infected [M. avium subsp. paratuberculosis-infected cattle], n = 10; n.s., not significant [the P value was obtained using Mann-Whitney U test]).
FIG 2.
Upregulation of CTLA-4 expression through stimulation with J-PPD. PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis (n = 6) were cultured with J-PPD, and the expression of CTLA-4 in CD4+ T cells (a) and CD8+ T cells (b) was assayed using flow cytometry. N.S., no stimulation; J-PPD, Johnin-purified protein derivative. P values were obtained using Mann-Whitney U tests.
Effects of PGE2 on CTLA-4 upregulation.
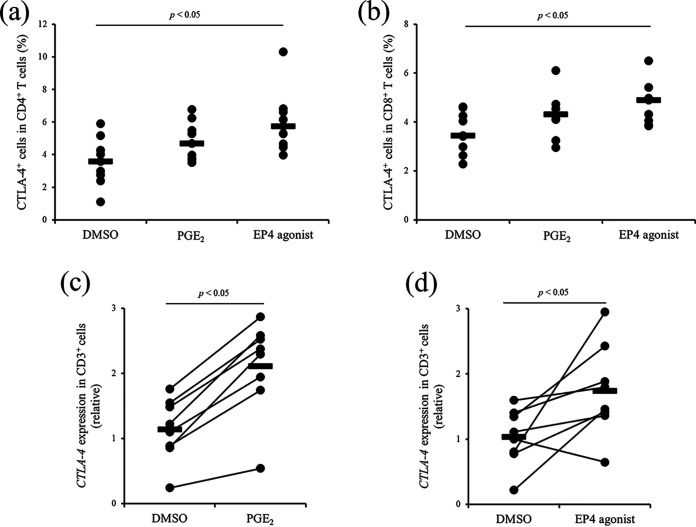
We previously showed that PGE2 production from the PBMCs of M. avium subsp. paratuberculosis-infected cattle was increased by J-PPD stimulation (10). In the present study, we identified the major source of PGE2 production under antigen stimulation. PBMCs and CD14+ cell-deleted PBMCs from M. avium subsp. paratuberculosis-infected cattle were cultured with or without J-PPD, and PGE2 concentrations in culture supernatants were measured using enzyme-linked immunosorbent assay (ELISA). PGE2 production was significantly induced from PBMCs following J-PPD treatment in vitro, whereas PGE2 production was not induced from CD14+ cell-deleted PBMCs (see Fig. S2). Therefore, in M. avium subsp. paratuberculosis-infected cattle, CD14+ cells could be a major source of PGE2 production under antigen stimulation. To determine whether CTLA-4 expression is induced by PGE2 signaling, PBMCs from uninfected cattle were cultivated with PGE2 or an EP4 agonist, and the expression of CTLA-4 was measured using flow cytometry. Treatment with PGE2 tended to induce CTLA-4 expression in CD4+ and CD8+ T cells (Fig. 3a and b), and treatment with the EP4 agonist significantly promoted CTLA-4 expression in both CD4+ and CD8+ cells (Fig. 3a and b). In further tests, CD3+ cells were sorted from uninfected cattle and cultured with PGE2 or the EP4 agonist. The gene expression of CTLA-4 in CD3+ cells was significantly increased by culture with PGE2 and the EP4 agonist (Fig. 3c and d). Collectively, these data suggest that J-PPD-induced PGE2 is involved in the upregulation of CTLA-4 expression, at least in part, via its receptor EP4.
FIG 3.
Upregulation of CTLA-4 expression via treatment with PGE2 or an EP4 agonist. (a and b) First, PBMCs of cattle not infected with M. avium subsp. paratuberculosis were cultured with PGE2 or the EP4 agonist, and dimethyl sulfoxide (DMSO) was used as a vehicle control. Subsequently, CTLA-4 expression in CD4+ T cells (a) and CD8+ T cells (b) was measured by using flow cytometry (n = 10; P values were obtained using Mann-Whitney U tests). (c and d) CD3+ cells were sorted from the PBMCs of cattle not infected with M. avium subsp. paratuberculosis cattle and cultured with PGE2 (c) or the EP4 agonist (d), and DMSO was used as a vehicle control. The gene expression of CTLA-4 was quantitated using qPCR (n = 8; P values were obtained using Steel-Dwass tests).
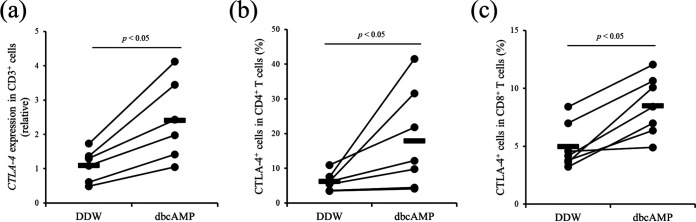
The PGE2/EP4 pathway is known to stimulate the production of intracellular cyclic AMP (cAMP) (25). In the present study, we examined whether the upregulation of intracellular cAMP is involved in upregulation of CTLA-4 expression in cattle. Treatment with dibutyryl cAMP (dbcAMP), a cell-permeable analog of cAMP that activates cAMP-dependent protein kinases, significantly induced the gene expression of CTLA-4 in T cells (Fig. 4a). In addition, flow cytometric analysis revealed that treatment with dbcAMP upregulated CTLA-4 expression in both CD4+ and CD8+ T cells (Fig. 4b and c), suggesting that CTLA-4 expression is induced by the PGE2/EP4/cAMP pathway in cattle.
FIG 4.
Upregulation of CTLA-4 expression via treatment with dbcAMP. (a) CD3+ cells were sorted from the PBMCs of cattle not infected with M. avium subsp. paratuberculosis (n = 6) and cultured with dbcAMP. Double-distilled water (DDW) was used as a vehicle control. Gene expression of CTLA-4 was quantitated using qPCR. (b and c) PBMCs of cattle not infected with M. avium subsp. paratuberculosis (n = 7) were cultured with dbcAMP, DDW was used as a vehicle control, and CTLA-4 expression in CD4+ T cells (b) and CD8+ T cells (c) was assayed by using flow cytometry. All P values were obtained using Mann-Whitney U tests.
Establishment and functional analysis of CTLA-4-Ig.
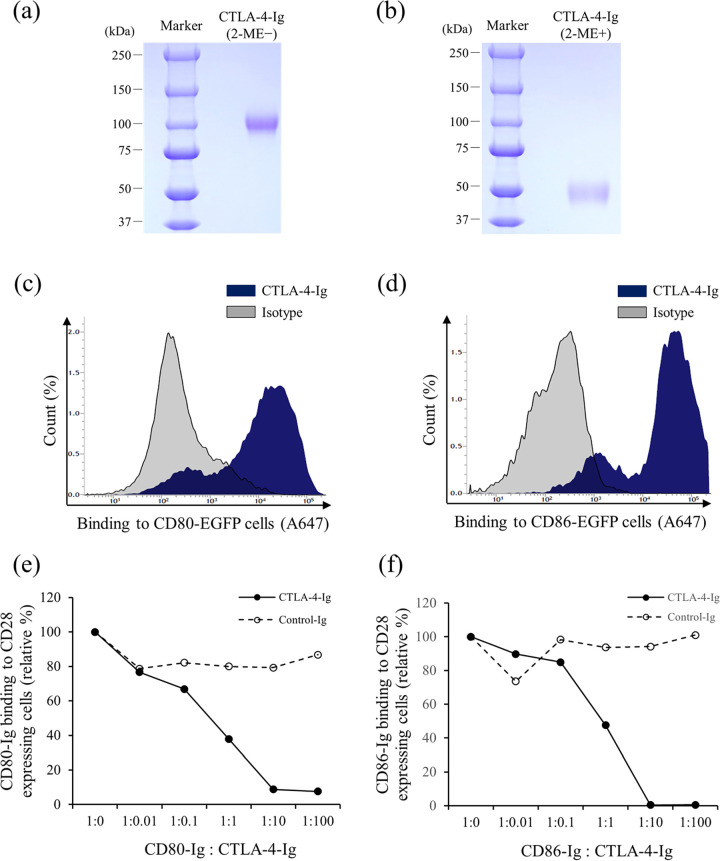
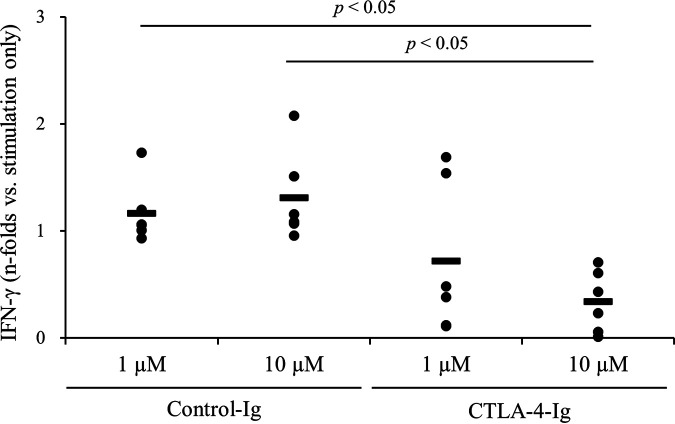
To investigate whether CTLA-4 is involved in the suppression of M. avium subsp. paratuberculosis-specific immune responses, we generated an Fc-fusion protein of bovine CTLA-4, namely, CTLA-4-Ig, which was expressed using a pDC62c5-U533 expression vector and CHO-DG44 cell expression system in vitro. After purification, SDS-PAGE was performed under nonreducing (Fig. 5a) and reducing (Fig. 5b) conditions. Flow cytometric analysis revealed that CTLA-4-Ig bound to both bovine CD80 and CD86 (Fig. 5c and d). In addition, flow cytometric analysis showed that CTLA-4-Ig inhibited the binding of CD80-Ig or CD86-Ig to CD28-expressing cells on the cell surface (Fig. 5e and f). We also used CTLA-4-Ig to assess the involvement of CTLA-4 in the suppression of M. avium subsp. paratuberculosis-specific immune responses. PBMCs from M. avium subsp. paratuberculosis-infected cattle were cultured with CTLA-4-Ig in the presence of J-PPD, and CTLA-4-Ig treatment significantly downregulated the production of IFN-γ from PBMCs (Fig. 6). Therefore, CTLA-4 may be associated with the immune suppression in M. avium subsp. paratuberculosis-infected cattle.
FIG 5.
Establishment and functional analyses of CTLA-4-Ig. (a and b) Purified CTLA-4-Ig was confirmed via SDS-PAGE under nonreducing (a) and reducing (b) conditions. (c and d) Binding of CTLA-4-Ig to bovine CD80 (c) and bovine CD86 (d) was determine using flow cytometry. (e and f) CTLA-4-Ig inhibited the binding of bovine CD80 (e) or bovine CD86 (f) to bovine CD28. 2ME, 2-mercaptoethanol; isotype: purified bovine IgG1; control-Ig, purified rabbit IgG.
FIG 6.
Suppression of IFN-γ production via CTLA-4-Ig. PBMCs from M. avium subsp. paratuberculosis-infected cattle (n = 6) were cultured with CTLA-4-Ig or Control-Ig (39) in the presence of J-PPD. IFN-γ production was measured using ELISA. P values were obtained using Steel-Dwass tests.
Activation of J-PPD-stimulated immunity via CTLA-4 blockade.
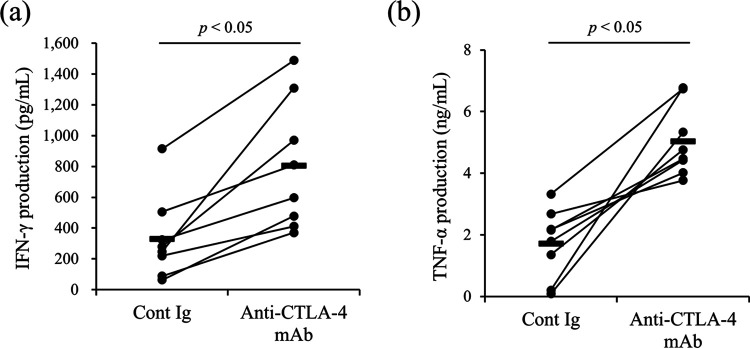
As described previously, the blockade of CTLA-4 activates immune responses in BLV-infected cattle in vitro (22, 23). To examine the effects of CTLA-4 blockade on immune responses in M. avium subsp. paratuberculosis-infected cattle, PBMCs derived from the infected animals were cultured with anti-bovine CTLA-4 MAb (4C2-D9) (23) in the presence of J-PPD. The production of Th1 cytokines, i.e., IFN-γ and tumor necrosis factor alpha (TNF-α), was significantly increased in the group treated with anti-bovine CTLA-4 MAb (Fig. 7).
FIG 7.
Activation of Th1 responses via treatment with anti-CTLA-4 MAb. PBMCs from M. avium subsp. paratuberculosis-infected cattle (n = 8) were cultured with anti-CTLA-4 MAb in the presence of J-PPD, and the concentrations of IFN-γ (a) and TNF-α (b) in culture supernatants were measured using ELISA. P values were obtained using Mann-Whitney U tests. Control-Ig, mouse IgG1 isotype control.
DISCUSSION
In the present study, as well as a previous study (10), we have shown that antigen stimulation activates PGE2 production from the PBMCs of M. avium subsp. paratuberculosis-infected cattle. Furthermore, we showed here that PGE2 upregulated CTLA-4 expression in T cells via the EP4/cAMP pathway (see Fig. S3). cAMP is known to play a critical role in upregulating CTLA-4 expression in T cells (26) and has been associated with the progression of chronic infections, such as HIV (27). However, information was previously lacking on the relationship between antigen stimulation and cAMP-induced CTLA-4; thus, the present study provides new insights in this regard. CTLA-4 expression in T cells is upregulated in many other chronic infections, including HIV (19), hepatitis C virus (20), and BLV (21, 22) infections. Therefore, further studies will be needed to investigate whether antigen stimulation is involved in CTLA-4 expression via the PGE2/EP4/cAMP pathway in bovine chronic infections. In the present study, M. avium subsp. paratuberculosis-experimentally infected cattle showed high expression of CTLA-4 but did not exhibit clear clinical signs because they might be in the latent period. The detailed involvement of CTLA-4 in the process leading to the development of granulomas in Johne’s disease should also continue to be investigated.
CTLA-4 is expressed in regulatory T cells (Tregs) and is known as a Treg-associated molecule (28, 29). In M. avium subsp. paratuberculosis-infected cattle, Tregs have been associated with immune responses to antigen stimulation (30). However, the mechanism underlying Treg proliferation in M. avium subsp. paratuberculosis-infected cattle is still unclear. Our previous studies showed that PGE2 concentrations in sera are increased in infected cattle and that PGE2 induces the expression of the Treg-associated molecules Foxp3 and TGF-β1 in cattle (10, 31). Thus, in M. avium subsp. paratuberculosis-infected cattle, antigen stimulation-induced PGE2 might contribute to increasing the Treg population via its receptor EP4.
The cell-mediated immunity plays an essential role in controlling the progression of Johne’s disease. However, M. avium subsp. paratuberculosis-specific immune responses, especially IFN-γ production, are gradually suppressed in M. avium subsp. paratuberculosis-infected cattle, contributing to progression to the clinical stage (6–8, 32). In the present study, we found that CTLA-4-Ig treatment reduced IFN-γ production from the PBMCs of M. avium subsp. paratuberculosis-infected cattle. In addition, anti-bovine CTLA-4 MAb increased anti-bacterial cytokine productions in vitro. These data suggest that CTLA-4 is involved in the suppression of anti-bacterial responses in M. avium subsp. paratuberculosis-infected cattle.
On farms, calves become infected with M. avium subsp. paratuberculosis mainly via the fecal-oral route (1). Therefore, M. avium subsp. paratuberculosis-infected cattle that exhibit bacterial fecal shedding can be considered a major infection source. Our previous study demonstrated that treatment with anti-bovine PD-L1 antibody significantly activated Th1 responses in M. avium subsp. paratuberculosis-infected cattle in vivo, although the inhibitory effect of anti-PD-L1 antibody alone on bacterial shedding was limited (11). Thus, to reduce bacterial load, the development of a novel control method that induces potent antibacterial responses is desirable. The upregulation of PGE2 is one possible mechanism underlying the inhibition of anti-bacterial responses during PD-1 blockade (15). Our previous study revealed that PGE2 concentrations in sera were increased in BLV-infected cattle after treatment with anti-bovine PD-L1 antibody (15). In the present study, we revealed the role played by PGE2 in the upregulation of CTLA-4 expression in cattle. Therefore, CTLA-4 levels might increase after PD-1 blockade, and CTLA-4 could be a target molecule for inducing potent immune responses. Indeed, in cancer research, the dual blockade of PD-1/PD-L1 and CTLA-4 using specific antibodies enhances therapeutic effects compared to the effects achieved using single blockade of PD-1/PD-L1 or CTLA-4 (33). Moreover, combined treatment with anti-PD-1 antibody and anti-CTLA-4 antibody leads to potent antitumor effects in patients with advanced melanoma (34, 35). In our previous study, we found that the dual blockade of PD-L1 and CTLA-4 enhances antiviral cytokine production from the PBMCs of BLV-infected cattle in vitro (23). Therefore, combination therapy involving anti-PD-1 and CTLA-4 antibodies could be a novel control strategy for Johne’s disease. Future studies should investigate the synergistic effects of dual blockade in cattle infected with M. avium subsp. paratuberculosis.
MATERIALS AND METHODS
Animals and blood samples.
Blood samples of cattle (adults of the Holstein breed) not infected with M. avium subsp. paratuberculosis, which were tested for specific antibodies against M. avium subsp. paratuberculosis using a commercial ELISA test kit (Kyoritsu Seiyaku Corporation, Tokyo, Japan), were obtained at the Field Science Center for Northern Biosphere, Hokkaido University and from dairy farmers in Hokkaido. Blood samples of cattle experimentally infected with M. avium subsp. paratuberculosis were obtained from the National Institute of Animal Health, Tsukuba, Japan. For the experimental infection, Holstein calves 1 to 4 weeks of age were inoculated orally with intestinal tissue homogenate (6.8 × 106 CFU) from M. avium subsp. paratuberculosis-infected cattle once daily for 20 days (10) or once with a high concentration of homogenate (1.36 or 2.50 × 108 CFU), as described previously (11). All infected cattle were kept in a biosafety level II animal facility at this institute and did not show any clinical symptoms. All experiments using bovine blood samples were approved by the National Institute of Animal Health Ethics Committee (approval numbers 15-001, 18-004, and 18-077) and/or the local committee for animal studies at Hokkaido University (approval number 17-0024).
Cell preparation and culture.
PBMCs from blood samples were purified using density gradient centrifugation on Percoll (GE Healthcare, Little Chalfont, UK) as described previously (36). CD3+ and CD14+ cells were isolated from PBMCs using an autoMACS Pro (Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously (15, 31). All cell cultures were grown in 96-well plates (Corning, Inc., Corning, NY) using RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO) including 10% heat-inactivated fetal calf serum (Thermo Fisher Scientific, Waltham, MA), 100 U/mL of penicillin (Thermo Fisher Scientific), 100 μg/mL of streptomycin (Thermo Fisher Scientific), and 2 mM l-glutamine (Thermo Fisher Scientific).
Flow cytometry.
To analyze CTLA-4 expression, flow cytometric analysis was performed using PBMCs from cattle infected or not infected with M. avium subsp. paratuberculosis. To prevent nonspecific reactions, Fc blocking was performed by incubating PBMCs with phosphate-buffered saline (PBS) including 10% goat serum (Thermo Fisher Scientific) for 15 min at 25°C. The cells were then stained with anti-bovine CTLA-4 MAb (4C2-D9) (23) or mouse IgG1 isotype control (15H6; Southern Biotech, Birmingham, AL) for 20 min at 37°C. After being washed twice with PBS containing 1% bovine serum albumin (BSA; Sigma-Aldrich), the cells were stained with Alexa Fluor 647-conjugated anti-mouse IgG(H+L) F(ab′)2 (Invitrogen, Carlsbad, CA) for 20 min at 25°C. They were then washed twice with PBS containing 1% BSA and stained with the following antibodies for 20 min at 25°C: PerCP/Cy 5.5-conjugated anti-bovine CD3 MAb (MM1A; Washington State University Monoclonal Antibody Center, Pullman, WA), FITC-conjugated anti-bovine CD4 MAb (CC8; Bio-Rad, Hercules, CA), PE-conjugated anti-bovine CD8 MAb (CC63; Bio-Rad), and PE/Cy7-conjugated anti-bovine IgM MAb (IL-A30; Bio-Rad). MM1A and IL-A30 were conjugated using Lightning-Link antibody labeling kits (Innova Biosciences, Cambridge, England). After the final staining, the cells were washed twice with PBS containing 1% BSA and analyzed immediately using FACSVerse (BD Biosciences, San Jose, CA, USA).
Enzyme-linked immunosorbent assay.
To measure the concentrations of IFN-γ, TNF-α, and PGE2 in culture supernatants, ELISAs were performed using a bovine IFN-γ ELISA development kit (Mabtech, Nacka Strand, Sweden), a bovine TNF-α Do-It-Yourself ELISA (Kingfisher Biotech), and a PGE2 Express ELISA kit (Cayman Chemical, Ann Arbor, MI), respectively.
Quantitative PCR.
RNA extraction and cDNA synthesis were performed in accordance with methods reported in a previous study (31). The gene expression of CTLA-4 was quantitated using a thermal cycler (LightCycler 480 System 2; Roche Diagnostic, Mannheim, Germany) and SYBR Premix DimerEraser (TaKaRa Bio, Otsu, Japan) according to the manufacturer’s instructions. β-actin (ACTB) was used as a reference gene. Relative expression levels were calculated using the ΔΔCt method, and the results were represented as changes relative to expression levels in the untreated group. The primers used were as follows: 5′-CCA GAG TCA TGG GAC TTG GT-3′ and 5′-TCA CAT GAG AAG CTG GCA AC-3′ for CTLA-4 and 5′-TCT TCC AGC CTT CCT TCC TG-3′ and 5′-ACC GTG TTG GCG TAG AGG TC-3′ for ACTB.
Establishment of CTLA-4-Ig.
To examine the effects of CTLA-4 on M. avium subsp. paratuberculosis-specific Th1 responses, we generated bovine CTLA-4-Ig using Chinese hamster ovary (CHO)-DG44 cells. The amino acid sequence of CTLA-4-Ig consisted of an extracellular domain fragment of bovine CTLA-4 and bovine IgG1, triggering reduced Fc-mediated effector functions (ADCC−) (37). A plasmid vector encoding CTLA-4-Ig (pUCIDT-Kan-CTLA-4-Ig) was digested using AscI and AsiSI (both New England Biolabs, Ipswich, MA), after which the fragment was inserted into pDC62c5-U533 (38) using a DNA ligation kit (Mighty Mix; TaKaRa Bio). The vector (pDC62c5-U533-CTLA-4-Ig) was then transfected into CHO-DG44 cells using Lipofectamine LTX (Life Technologies, Carlsbad, CA). Transfectants (5 × 105 cells/mL) were shaken for 14 days on a rotary shaker at 125 rpm and 37°C under 8% CO2 using CD OptiCHO Medium (Thermo Fisher Scientific). Subsequently, the supernatants were collected and filtered using Centricon Plus-70 (Merck Millipore, Billerica, MA), and CTLA-4-Ig was purified using Ab-Capture ExTra (ProteNova, Tokushima, Japan) according to the manufacturer’s protocol. After purification, SDS-PAGE was performed using a 12% SDS-polyacrylamide gel under reducing and nonreducing conditions. The gel was stained using a Quick-CBB kit (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and Precision Plus Protein All Blue prestained protein standards (Bio-Rad) were used as a marker.
Functional analysis of CTLA-4 Ig.
To confirm the binding of CTLA-4-Ig to bovine CD80 and CD86, Cos-7 cells expressing bovine CD80 or CD86 (22) were incubated with 10 μg/mL of CTLA-4-Ig for 30 min at 25°C. Purified bovine IgG1 (10 μg/mL; Bethyl Laboratories, Montgomery, TX) was used as a negative control. After being washed, the cells were stained with Alexa Fluor 647-conjugated AffiniPure goat anti-bovine IgG (H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA) for 20 min at 25°C and analyzed immediately using FACSVerse. Competitive and blocking assays were also performed to confirm whether CTLA-4-Ig inhibits interactions with CD28 and CD80 or CD86. CTLA-4-Ig was incubated with CD80-Ig or CD86-Ig (22) for 30 min at 25°C. Purified rabbit IgG (Southern Biotech) was used as a negative control of CTLA-4-Ig. Cos-7 cells expressing bovine CD28 were then incubated with a reaction mixture of Fc-fusion proteins for 30 min at 25°C. After incubation, the cells were stained with Alexa Fluor 647-conjugated anti-rabbit IgG(H+L) (Thermo Fisher Scientific) and analyzed immediately using FACSVerse.
Cell cultures.
PBMCs or CD14+ cell-depleted PBMCs from cattle experimentally infected with M. avium subsp. paratuberculosis were incubated with 1 μg/mL of Johnin-purified protein derivative (J-PPD). After 24 h, CTLA-4 expression in T cells was analyzed using flow cytometry as described above. After 72 h, PGE2 concentrations in culture supernatants were measured using ELISA.
To determine whether the PGE2/EP4/cAMP pathway upregulates CTLA-4 expression, PBMCs from cattle not infected with M. avium subsp. paratuberculosis were cultured with 2.5 μM PGE2 (Cayman Chemical), 1 μg/mL of Rivenprost (an EP4 agonist; Cayman Chemical), or 400 μM dbcAMP (an analog of cAMP; Sigma-Aldrich). After 24 h, the cells were collected, and CTLA-4 expression was analyzed as described above.
Purified CD3+ cells of cattle not infected with M. avium subsp. paratuberculosis were cultured with 250 nM PGE2, 1 μg/mL of Rivenprost, or 400 μM dbcAMP. After 24 h, the gene expression of CTLA-4 was measured using quantitative PCR (qPCR).
To examine the effects of CTLA-4-Ig on Th1 responses, PBMCs of M. avium subsp. paratuberculosis-infected cattle were cultured with 10 or 1 μM CTLA-4-Ig or with Control-Ig (39) in the presence of 1 μg/mL of J-PPD. After 24 h, IFN-γ concentrations in culture supernatants were measured using ELISA.
To determine whether anti-bovine CTLA-4 MAb activates M. avium subsp. paratuberculosis-specific Th1 responses, PBMCs from M. avium subsp. paratuberculosis-infected cattle were cultivated with 10 μg/mL of anti-bovine CTLA-4 MAb (4C2-D9) in the presence of 1 μg/mL of J-PPD. After 120 h, the concentrations of IFN-γ and TNF-α in culture supernatants were measured using ELISA.
Statistical analysis.
Statistical significance was determined using Mann-Whitney U tests and Steel-Dwass tests. MEPHAS (http://www.gen-info.osaka-u.ac.jp/MEPHAS/) was used to conduct statistical analyses. Statistical significance was set at P < 0.05.
ACKNOWLEDGMENTS
We are grateful to Hideyuki Takahashi and Tomio Ibayashi for valuable advice and discussions. We thank Enago (http://www.enago.jp) for the English language review.
This study was supported by JSPS KAKENHI grant 22H02503 (to S.K.); grants from the Project of the NARO, Bio-oriented Technology Research Advancement Institution (Research Program on Development of Innovative Technology grant 26058 BC [to S.K.] and Special Scheme Project on Regional Developing Strategy grant 16817557 [to S.K.]); regulatory research projects for food safety, animal health, and plant protection (JPJ008617.17935709) funded by the Ministry of Agriculture, Forestry, and Fisheries of Japan; and AMED under grants JP20am0101078 and JP22ama121008 (to Y.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Satoru Konnai, Email: konnai@vetmed.hokudai.ac.jp.
Sabine Ehrt, Weill Cornell Medical College.
REFERENCES
- 1.Rathnaiah G, Zinniel DK, Bannantine JP, Stabel JR, Gröhn YT, Collins MT, Barletta RG. 2017. Pathogenesis, molecular genetics, and genomics of Mycobacterium avium subsp. paratuberculosis, the etiologic agent of Johne’s disease. Front Vet Sci 4:187. 10.3389/fvets.2017.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, Katani R, Schilling M, Kapur V. 2016. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis on dairy farms. Annu Rev Anim Biosci 4:155–176. 10.1146/annurev-animal-021815-111304. [DOI] [PubMed] [Google Scholar]
- 3.Coussens PM. 2004. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect Immun 72:3089–3096. 10.1128/IAI.72.6.3089-3096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stabel JR. 2006. Host responses to Mycobacterium avium subsp. paratuberculosis: a complex arsenal. Anim Health Res Rev 7:61–70. 10.1017/S1466252307001168. [DOI] [PubMed] [Google Scholar]
- 5.Begg DJ, de Silva K, Carter N, Plain KM, Purdie A, Whittington RJ. 2011. Does a Th1 over Th2 dominancy really exist in the early stages of Mycobacterium avium subspecies paratuberculosis infections? Immunobiology 216:840–846. 10.1016/j.imbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Bassey EO, Collins MT. 1997. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect Immun 65:4869–4872. 10.1128/iai.65.11.4869-4872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrells C, Clarke CJ, Colston A, Kay JM, Porter J, Little D, Sharp JM. 1999. Interferon-gamma and interleukin-2 release by lymphocytes derived from the blood, mesenteric lymph nodes and intestines of normal sheep and those affected with paratuberculosis (Johne’s disease). Vet Immunol Immunopathol 68:139–148. 10.1016/S0165-2427(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 8.Weiss DJ, Evanson OA, Souza CD. 2006. Mucosal immune response in cattle with subclinical Johne’s disease. Vet Pathol 43:127–135. 10.1354/vp.43-2-127. [DOI] [PubMed] [Google Scholar]
- 9.Okagawa T, Konnai S, Nishimori A, Ikebuchi R, Mizorogi S, Nagata R, Kawaji S, Tanaka S, Kagawa Y, Murata S, Mori Y, Ohashi K. 2016. Bovine immunoinhibitory receptors contribute to suppression of Mycobacterium avium subsp. paratuberculosis-specific T-cell responses. Infect Immun 84:77–89. 10.1128/IAI.01014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sajiki Y, Konnai S, Okagawa T, Nishimori A, Maekawa N, Goto S, Ikebuchi R, Nagata R, Kawaji S, Kagawa Y, Yamada S, Kato Y, Nakajima C, Suzuki Y, Murata S, Mori Y, Ohashi K. 2018. Prostaglandin E2 induction suppresses the Th1 immune responses in cattle with Johne’s disease. Infect Immun 86:e00910-17. 10.1128/IAI.00910-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajiki Y, Konnai S, Nagata R, Kawaji S, Nakamura H, Fujisawa S, Okagawa T, Maekawa N, Kato Y, Suzuki Y, Murata S, Mori Y, Ohashi K. 2021. The enhancement of Th1 immune response by anti-PD-L1 antibody in cattle infected with Mycobacterium avium subsp. paratuberculosis. J Vet Med Sci 83:162–166. 10.1292/jvms.20-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wherry EJ. 2011. T cell exhaustion. Nat Immunol 12:492–499. 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 13.Konnai S, Murata S, Ohashi K. 2017. Immune exhaustion during chronic infections in cattle. J Vet Med Sci 79:1–5. 10.1292/jvms.16-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto S, Konnai S, Hirano Y, Kohara J, Okagawa T, Maekawa N, Sajiki Y, Watari K, Minato E, Kobayashi A, Gondaira S, Higuchi H, Koiwa M, Tajima M, Taguchi E, Uemura R, Yamada S, Kaneko MK, Kato Y, Yamamoto K, Toda M, Suzuki Y, Murata S, Ohashi K. 2020. Upregulation of PD-L1 expression by prostaglandin E2 and the enhancement of IFN-γ by anti-PD-L1 antibody combined with a COX-2 inhibitor in Mycoplasma bovis infection. Front Vet Sci 7:12. 10.3389/fvets.2020.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sajiki Y, Konnai S, Cai Z, Takada K, Okagawa T, Maekawa N, Fujisawa S, Kato Y, Suzuki Y, Murata S, Ohashi K. 2020. Enhanced immunotherapeutic efficacy of anti-PD-L1 antibody in combination with an EP4 antagonist. Immunohorizons 4:837–850. 10.4049/immunohorizons.2000089. [DOI] [PubMed] [Google Scholar]
- 16.Vandenborre K, Van Gool SW, Kasran A, Ceuppens JL, Boogaerts MA, Vandenberghe P. 1999. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology 98:413–421. 10.1046/j.1365-2567.1999.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alegre ML, Frauwirth KA, Thompson CB. 2001. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 1:220–228. 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 18.Wing K, Yamaguchi T, Sakaguchi S. 2011. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol 32:428–433. 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 8:1246–1254. 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 20.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Kaminski M, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang KM. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 5:e1000313. 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki S, Konnai S, Okagawa T, Ikebuchi R, Nishimori A, Kohara J, Mingala CN, Murata S, Ohashi K. 2015. Increased expression of the regulatory T cell-associated marker CTLA-4 in bovine leukemia virus infection. Vet Immunol Immunopathol 163:115–124. 10.1016/j.vetimm.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Watari K, Konnai S, Maekawa N, Okagawa T, Suzuki Y, Murata S, Ohashi K. 2019. Immune inhibitory function of bovine CTLA-4 and the effects of its blockade in IFN-γ production. BMC Vet Res 15:380. 10.1186/s12917-019-2082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watari K, Konnai S, Maekawa N, Okagawa T, Sajiki Y, Kato Y, Suzuki Y, Murata S, Ohashi K. 2022. Enhancement of IL-2 production by bovine peripheral blood mononuclear cells treated with the combination of anti-PD-L1 and CTLA-4 chimeric monoclonal antibodies. J Vet Med Sci 84:6–15. 10.1292/jvms.21-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leite FL, Eslabão LB, Pesch B, Bannantine JP, Reinhardt TA, Stabel JR. 2015. ZAP-70, CTLA-4 and proximal T cell receptor signaling in cows infected with Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol 167:15–21. 10.1016/j.vetimm.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Regan JW. 2003. EP2 and EP4 prostanoid receptor signaling. Life Sci 74:143–153. 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Vendetti S, Riccomi A, Sacchi A, Gatta L, Pioli C, De Magistris MT. 2002. Cyclic adenosine 5′-monophosphate and calcium induce CD152 (CTLA-4) upregulation in resting CD4+ T lymphocytes. J Immunol 169:6231–6235. 10.4049/jimmunol.169.11.6231. [DOI] [PubMed] [Google Scholar]
- 27.Moreno-Fernandez ME, Rueda CM, Velilla PA, Rugeles MT, Chougnet CA. 2012. cAMP during HIV infection: friend or foe? AIDS Res Hum Retroviruses 28:49–53. 10.1089/AID.2011.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133:775–787. 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322:271–275. 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 30.Coussens PM, Sipkovsky S, Murphy B, Roussey J, Colvin CJ. 2012. Regulatory T cells in cattle and their potential role in bovine paratuberculosis. Comp Immunol Microbiol Infect Dis 35:233–239. 10.1016/j.cimid.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Sajiki Y, Konnai S, Okagawa T, Nishimori A, Maekawa N, Goto S, Watari K, Minato E, Kobayashi A, Kohara J, Yamada S, Kaneko MK, Kato Y, Takahashi H, Terasaki N, Takeda A, Yamamoto K, Toda M, Suzuki Y, Murata S, Ohashi K. 2019. Prostaglandin E2-induced immune exhaustion and enhancement of antiviral effects by anti-PD-L1 antibody combined with COX-2 inhibitor in bovine leukemia virus infection. J Immunol 203:1313–1324. 10.4049/jimmunol.1900342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohal JS, Singh SV, Tyagi P, Subhodh S, Singh PK, Singh AV, Narayanasamy K, Sheoran N, Singh SK. 2008. Immunology of mycobacterial infections: with special reference to Mycobacterium avium subspecies paratuberculosis. Immunobiology 213:585–598. 10.1016/j.imbio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Callahan MK, Postow MA, Wolchok JD. 2014. CTLA-4 and PD-1 pathway blockade: combinations in the clinic. Front Oncol 4:385. 10.3389/fonc.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. 2013. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133. 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor DR, Salama AK, Taylor MH, Ott PA, Horak C, Gagnier P, Jiang J, Wolchok JD, Postow MA. 2016. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558–1568. 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sajiki Y, Konnai S, Goto S, Okagawa T, Ohira K, Shimakura H, Maekawa N, Gondaira S, Higuchi H, Tajima M, Hirano Y, Kohara J, Murata S, Ohashi K. 2020. The suppression of Th1 response by inducing TGF-β1 from regulatory T cells in bovine mycoplasmosis. Front Vet Sci 7:609443. 10.3389/fvets.2020.609443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okagawa T, Konnai S, Nishimori A, Maekawa N, Ikebuchi R, Goto S, Nakajima C, Kohara J, Ogasawara S, Kato Y, Suzuki Y, Murata S, Ohashi K. 2017. Anti-bovine programmed death-1 rat-bovine chimeric antibody for immunotherapy of bovine leukemia virus infection in cattle. Front Immunol 8:650. 10.3389/fimmu.2017.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki Y, Nakagawa M, Kameda Y, Konnai S, Okagawa T, Maekawa N, Goto S, Sajiki Y, Ohashi K, Murata S, Kitahara Y, Yamamoto K. November 2020. Novel vector and use thereof. US patent application 17/054,936.
- 39.Fujisawa S, Konnai S, Okagawa T, Maekawa N, Tanaka A, Suzuki Y, Murata S, Ohashi K. 2019. Effects of bovine tumor necrosis factor alpha decoy receptors on cell death and inflammatory cytokine kinetics: potential for bovine inflammation therapy. BMC Vet Res 15:68. 10.1186/s12917-019-1813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S3. Download iai.00210-22-s0001.pdf, PDF file, 0.6 MB (579.8KB, pdf)