ABSTRACT
TORC1 is a critical controller of cell growth in eukaryotes. In yeast (Saccharomyces cerevisiae), the presence of nutrients is signaled to TORC1 by several upstream regulatory sensors that together coordinate TORC1 activity. TORC1 localizes to both vacuolar and endosomal membranes, where differential signaling occurs. This localization is mimicked by Pib2, a key upstream TORC1 regulator that is essential for TORC1 reactivation after nutrient starvation or pharmacological inhibition. Pib2 has both positive and negative effects on TORC1 activity, but the mechanisms remain poorly understood. Here, we pinpoint the Pib2 inhibitory function on TORC1 to residues within short, conserved N-terminal regions. We also show that the Pib2 C-terminal regions, helical region E and tail, are essential for TORC1 reactivation. Furthermore, the Pib2 FYVE domain plays a role in vacuolar localization, but it is surprisingly unnecessary for recovery from rapamycin exposure. Using chimeric Pib2 targeting constructs, we show that endosomal localization is not necessary for TORC1 reactivation and cell growth after rapamycin treatment. Thus, a comprehensive molecular dissection of Pib2 demonstrates that each of its conserved regions differentially contribute to Pib2-mediated regulation of TORC1 activity.
Keywords: TORC1, Pib2, Rapamycin, FYVE
Summary: Vacuolar localization of S. cerevisiae Pib2, a key TORC1 regulator that both inhibits and activates TORC1 activity via conserved regions, is key for recovery and cell growth following rapamycin exposure.
INTRODUCTION
The target of rapamycin complex I (TORC1) is a conserved kinase complex essential for the regulation of cell growth. In yeast (Saccharomyces cerevisiae), TORC1 responds to and integrates a diverse range of nutritional cues to promote anabolic processes, such as protein synthesis, while inhibiting catabolic processes, such as macroautophagy (hereafter autophagy). Inhibition of TORC1, pharmacologically or through nutrient starvation, triggers autophagy and reduces protein and ribosome synthesis (González and Hall, 2017; Loewith and Hall, 2011). In metazoans, growth factor-mediated signaling pathways also converge on TORC1 to provide an additional layer of cell growth regulation. Given its central role in regulating cell growth, TORC1 has been implicated in a variety of diseases including cancer and diabetes, as well as aging (González and Hall, 2017; Saxton and Sabatini, 2017).
Yeast TORC1 consists of two copies each of the serine/threonine kinase Tor1 (or Tor2), Kog1, Lst8 and Tco89 (Loewith et al., 2002; Reinke et al., 2004; Wedaman et al., 2003). TORC1 has several downstream effectors that regulate protein and ribosome synthesis and autophagy. Sch9 is a primary TORC1 effector, which promotes protein synthesis (Hatakeyama et al., 2019; Urban et al., 2007). Major effectors of autophagy include Atg13 and Vps27 (Hatakeyama et al., 2019; Kamada et al., 2010). TORC1 localizes to the vacuolar membrane as well as perivacuolar puncta (Binda et al., 2009; Kira et al., 2016; Sturgill et al., 2008). Through assessing colocalization with endosomal markers, these puncta have been identified as signaling endosomes where TORC1, along with some regulatory components and effectors, colocalize (Chen et al., 2021; Hatakeyama and De Virgilio, 2019; Hatakeyama et al., 2019). It has recently been reported that active vacuolar or endosomal TORC1 serve two different functions; TORC1 activity at the vacuole results in phosphorylation of Sch9 to promote protein synthesis, and phosphorylation of Atg13 and Vps27 by the endosomal pool of TORC1 inhibits autophagy (Hatakeyama et al., 2019). Whether there are differences in the regulatory mechanisms at these distinct subcellular locations remains unclear.
In yeast, TORC1 is ultimately regulated by amino acids via several upstream proteins. A fundamental activator of TORC1 is the exit from rapamycin-induced growth arrest (EGO) complex (Dubouloz et al., 2005), which consists of the GTPases Gtr1 and Gtr2, and the docking proteins Ego1, Ego2, and Ego3 (Powis et al., 2015). Through the EGO complex, the amino acids glutamine and leucine are predominant activators of yeast TORC1 (Binda et al., 2009; Bonfils et al., 2012; Peli-Gulli et al., 2015). Several upstream GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) control the nucleotide-binding status of the Gtr GTPases (Binda et al., 2009; Panchaud et al., 2013; Peli-Gulli et al., 2015). Together, under nutrient rich conditions, these GAPs and GEFs help to maintain an active EGO complex (wherein Gtr1 is GTP bound and Gtr2 is GDP bound) to promote TORC1 activation through direct interaction with TORC1 components (Binda et al., 2009; González and Hall, 2017; Jeong et al., 2012; Nakashima et al., 1999; Panchaud et al., 2013; Peli-Gulli et al., 2015).
Phosphatidylinositol 3-phosphate (PI3P) and Vps34, the kinase that synthesizes it, are also key TORC1 regulators. In mammalian cell models, this connection has been well described; amino acids activate the phosphoinositide 3-kinase (PI3K) Vps34, which generates PI3P and results in mTORC1 activation through a mechanism parallel to the Rag GTPases, the metazoan homologs of the Gtrs (Byfield et al., 2005; Nobukuni et al., 2005; Yoon et al., 2011, 2016). In yeast, Vps34 deletion strains show a loss of both PI3P and phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2] and are hypersensitive to the pharmacological TORC1 inhibitor rapamycin (Bridges et al., 2012). PI3P is a precursor for the synthesis of PI(3,5)P2, which has also been shown to affect TORC1 localization and activity in both yeast and mammalian cells (Bridges et al., 2012; Chen et al., 2021). This highlights the role of these phosphoinositides in TORC1 regulation.
Phosphatidylinositol 3-phosphate-binding protein 2 (Pib2) is a master regulator of TORC1 signaling in yeast (Hatakeyama, 2021). Pib2 is unique in that it was identified in screens for both rapamycin sensitivity (Dubouloz et al., 2005; Parsons et al., 2004) and rapamycin resistance (Michel et al., 2017). Interestingly, Pib2 has two opposing functions in its regulation of TORC1 – an inhibitory effect mediated by its N-terminal regions, and an activation effect mediated by its C-terminal domains (Michel et al., 2017; Sullivan et al., 2019). It has been demonstrated that Pib2 interacts with Tor1 and Kog1, as well as EGO complex components (Michel et al., 2017; Sullivan et al., 2019; Tanigawa and Maeda, 2017; Tarassov et al., 2008; Ukai et al., 2018). Pib2 is essential for reactivation of TORC1 following rapamycin exposure and in response to glutamine and leucine following nitrogen starvation (Varlakhanova et al., 2017). Recently, Pib2 has been implicated as a glutamine sensor that directly interacts with TORC1 in a glutamine-dependent manner, to promote TORC1 activity (Tanigawa and Maeda, 2017; Tanigawa et al., 2021; Ukai et al., 2018). However, the molecular mechanisms of regulatory interactions of Pib2 remain poorly understood.
Pib2 was named based on the presence of a FYVE domain, a common structural module known to interact with PI3P, near its C terminus (Shin et al., 2001). Pib2 localizes to the vacuole and endosomes in a Vps34-dependent manner (Kim and Cunningham, 2015). In addition to the FYVE domain, Pib2 also contains a conserved and predicted α-helical region termed helical region E and a conserved C-terminal tail motif, as well as some shorter regions of conservation, termed regions A–D, embedded in the otherwise low-complexity Pib2 N-terminal sequence. Pib2 induces lysosomal membrane permeabilization (LMP) and subsequent cell death through increased TORC1 activation in cells treated with ER stressors or calcineurin inhibitors (Kim and Cunningham, 2015). In this function, the C-terminal FYVE domain is essential for localization of Pib2 at vacuolar and endosomal membranes, and both the FYVE domain and tail motif are necessary for TORC1 activation and promotion of LMP (Kim and Cunningham, 2015).
Here, we performed a molecular dissection of Pib2 and show that each of its conserved regions differentially contribute to Pib2 localization and regulation of TORC1 activity. We demonstrate that conserved regions A and B within the Pib2 N-terminus are responsible for the inhibitory effect on TORC1 reactivation following rapamycin exposure, whereas the C-terminal α-helical E region and tail motif are essential for TORC1 reactivation. We assessed the role of Pib2 in TORC1 reactivation and cell growth at the endosome and the vacuole, showing that vacuolar localization of Pib2 is essential for recovery of cell growth. We also demonstrate that the alpha-helical E region and the FYVE domain play key roles in the vacuolar localization of Pib2. Further, the helical E region contains residues which are essential for TORC1 reactivation but not Pib2 localization, showing that vacuolar localization is necessary but not sufficient for TORC1 reactivation.
RESULTS
Conserved Pib2 regions differentially regulate TORC1 reactivation
Previous studies have shown that the N- and C-terminal regions of Pib2 function in inhibiting and activating TORC1, respectively (Michel et al., 2017; Sullivan et al., 2019). We sought to identify the precise regions responsible for these dual functions. Through multiple sequence alignments (MSAs) using sequences of Pib2 homologs from 15 ascomycete fungi, seven conserved regions are apparent (Fig. 1A; Fig. S1). The regions identified include the N-terminal regions A–D, a FYVE domain and a C-terminal tail motif. Previous alignments defined a smaller region E (Kim and Cunningham, 2015), which we extended to include other well-conserved residues (298–418). The AlphaFold2 prediction of Pib2 structural elements (Jumper et al., 2021), as well as other bioinformatic approaches, suggest this region has α-helical secondary structure, hence the name helical E region (helE) (Fig. 1A; Fig. S1C).
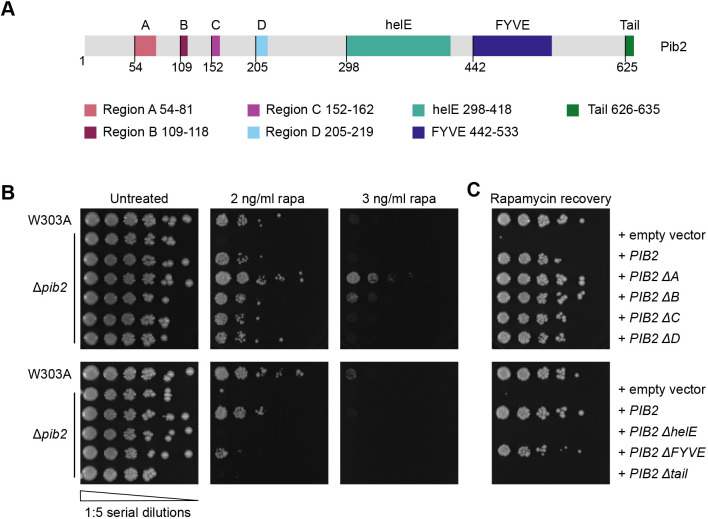
Fig. 1.
TORC1 reactivation is differentially regulated by conserved Pib2 regions. (A) The primary structure of Pib2, highlighting its conserved regions. The starting residue number of each region is shown below the schematic. Deletion constructs were generated by replacing the indicated regions with a short AGAGA linker. (B) Rapamycin exposure assay assessing growth of W303A and Δpib2 cells expressing the indicated constructs on YPD or YPD supplemented with the indicated low concentration of rapamycin (rapa). The left-most spots correspond to 2 µl of a OD600=0.5 culture, followed by 2 µl of sequential 1:5 dilutions. (C) Rapamycin recovery assay assessing growth of W303A and Δpib2 expressing the indicated constructs on YPD following rapamycin exposure. Exponentially growing cells were treated with 200 ng/ml rapamycin in YPD at 30°C for 4 h. After washing, cells were plated on YPD and incubated at 30°C for 3 days. Cells were plated as in B. Images are representative of three experiments.
To elucidate the functions of these Pib2 regions, we generated deletion constructs for each of the seven conserved Pib2 regions (Fig. 1A). Using Δpib2 cells and plasmid-expressed Pib2 region deletion constructs, we performed two types of rapamycin growth assays. We assessed growth of cells in the presence of a low concentration of rapamycin (2 and 3 ng/ml) and on nutrient-rich plates following a 4-h exposure to a high (200 ng/ml) concentration of rapamycin. These two approaches assay growth in an ongoing stress state and recovery from the stressor, respectively. Δpib2 cells expressing Pib2 ΔhelE and Pib2 ΔTail constructs did not grow on rapamycin-containing plates (Fig. 1B) and were unable to recover from rapamycin treatment (Fig. 1C). Unexpectedly, expression of the Pib2 construct lacking its FYVE domain (Pib2 ΔFYVE) was able to mediate growth on rapamycin-containing plates and recovery from exposure to rapamycin, although at a much slower rate than isogenic wild-type (W303A) cells or Δpib2 cells expressing wild-type Pib2 (Fig. 1B,C). In contrast, deletion of Pib2 regions A or B resulted in enhanced growth in both rapamycin exposure assays (Fig. 1B,C). Deletion of Pib2 regions C or D did not influence cell growth on rapamycin plates nor the ability to recover following rapamycin exposure (Fig. 1B,C). These results suggest that the N-terminal regions A and B are key for the inhibitory function of Pib2, whereas the C-terminal helE region and tail motif are essential for TORC1 reactivation. Although previous studies used more extensive region deletions, our results support these observations, showing that the Pib2 N-terminal regions have an inhibitory function, and the C-terminal regions are needed for TORC1 reactivation (Michel et al., 2017; Sullivan et al., 2019). As a control, the growth of the Pib2 deletion constructs did not differ on YPD plates without rapamycin exposure (Fig. 1B).
To ensure that the differences in rapamycin recovery were not due to changes in protein expression levels, we used Pib2 region deletion constructs tagged at the N-terminus with yEGFP and western blotting to confirm that these mutants are expressed at equal levels under logarithmic phase growth conditions (Fig. S2A,B). As a further control, we used GFP–Atg8 to assess the induction of autophagy in response to both rapamycin exposure and nutrient starvation (Guan et al., 2001; Shintani and Klionsky, 2004). No differences were observed between W303A and Δpib2 cells in either condition (Varlakhanova et al., 2017) (Fig. S2C,D). Additionally, without these stressors, no differences in autophagy induction were observed at steady state in Δpib2 cells compared to W303A; thus, Pib2 does not influence the repressive function of TORC1 on autophagy (Varlakhanova et al., 2017) (Fig. S2C,D). In addition, as the rapamycin assays used plasmid-based expression of Pib2, we generated genomic strains of PIB2 ΔA, PIB2 ΔB, and PIB2 ΔhelE, as well as wild-type PIB2, where the only source of PIB2 is its native genomic context, to demonstrate that plasmid expression was representative of endogenous expression. Using these genomically modified strains, we repeated the rapamycin growth assays and observed that the genomic PIB2 region deletion mutants showed the same growth patterns as the plasmid-expressed deletion constructs (Fig. S2E).
Conserved Pib2 regions differentially affect Pib2 subcellular localization
TORC1 localizes to the both the vacuolar and endosomal membranes, where differential TORC1 signaling occurs (Binda et al., 2009; Hatakeyama et al., 2019; Kira et al., 2016; Sturgill et al., 2008). As localization is likely an important aspect of the ability of Pib2 to reactivate TORC1, we sought to determine how the Pib2 regions contribute to its subcellular localization. To this end, we generated the same Pib2 region deletion constructs N-terminally tagged with yEGFP. We first confirmed, using genomically yEGFP-tagged PIB2, that this tag does not interfere with the ability of Pib2 to activate TORC1 and promote cell growth under these conditions (Fig. S3A). Thus, we used yEGFP–Pib2 region deletion constructs to assess localization of the Pib2 deletions via confocal microscopy (Fig. 2A,B). Wild-type Pib2 localized primarily to the vacuolar membrane with occasional perivacuolar puncta (Fig. 2A,C). This vacuolar localization phenotype was consistent for most of the Pib2 deletion constructs. However, we observed that deletion of the FYVE domain resulted in a mixed phenotype, with some vacuolar localization and a large cytosolic component (Fig. 2A,B), as previously described (Ukai et al., 2018). We also observed a similar phenotype with the ΔhelE construct (Fig. 2A,B), suggesting that both the FYVE and helE domains might act as a potentially redundant dual vacuolar recruitment mechanism.
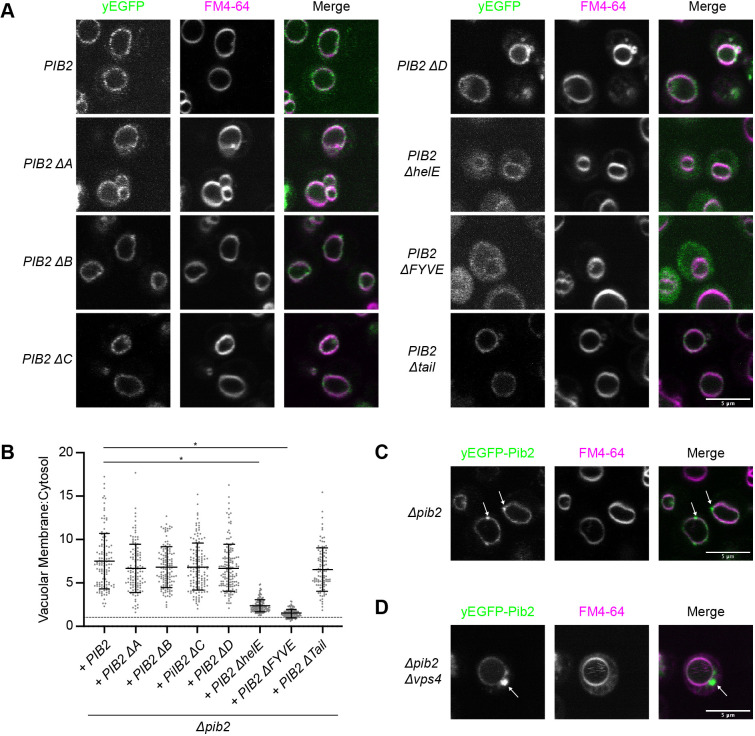
Fig. 2.
Pib2 localization is determined by its conserved regions. (A) Localization of Δpib2 cells expressing the indicated N-terminally yEGFP-tagged Pib2 constructs. For vacuolar visualization, cells were stained with FM4-64 prior to imaging. Representative fields of view are shown. (B) Quantification (mean±s.d.) of the data presented in A. Vacuolar localization is determined as the ratio of vacuolar yEGFP signal to cytosolic yEGFP signal. Following a ROUT outlier analysis (Q=0.1%), a one-way ANOVA was conducted to determine differences in vacuolar localization (F=121.6, P<0.0001). Constructs significantly different from the localization of Pib2, as assessed by Tukey multiple post-hoc comparisons test, are indicated (*P<0.0001). A total of 117–143 cells were quantified for each construct. (C) yEGFP–Pib2 expressed in a Δpib2 strain with perivacuolar puncta (arrows). (D) yEGFP–Pib2 expressed in Δpib2 Δvps4 cells showing Pib2 localization to the Class E compartment (arrow). Images in C,D are representative of three experiments.
To further assess the subcellular localization of Pib2, we expressed yEGFP–Pib2 in a Δpib2 Δvps4 strain. Vps4 is an ATPase that is essential for endosomal morphology and endosome to vacuole transport (Babst et al., 1997, 1998). The vacuolar protein sorting (Vps) genes are divided into phenotypic classes based on vacuolar morphology defects caused by their deletion (Coonrod and Stevens, 2010; Raymond et al., 1992). Vps4 is a Class E mutant; these mutants have enlarged prevacuolar endosomal structures termed Class E compartments (Babst et al., 1997, 1998; Raymond et al., 1992). Using this deletion strain, we observed that the perivacuolar Pib2 puncta correspond to endosomal structures as evidenced by the presence of Pib2 on the Class E compartments (Fig. 2D). Vacuolar localization was not affected. These results agree with previous studies showing that Pib2 localizes to signaling endosomes (Hatakeyama et al., 2019).
N-terminal Pib2 regions A and B display a TORC1 inhibitory function
Previous studies have demonstrated a TORC1 inhibitory function for Pib2 as it pertains to TORC1 reactivation following rapamycin exposure (Michel et al., 2017). Studies using large truncations of its N-terminal region, residues 1–50 or 1–164, have shown that in the absence of the N-terminus, Pib2 is better able to reactivate TORC1 and promote cell growth (Michel et al., 2017; Ukai et al., 2018). In our rapamycin assays (Fig. 1B), we demonstrated that Regions A (residues 54–81) and B (residues 109–118) were central to the Pib2 inhibitory function. As the Pib2 ΔA construct showed enhanced growth in the rapamycin exposure assays, we sought to determine whether the increased growth rate and TORC1 activity was specific to TORC1 reactivation. For this, we assessed growth of select Pib2 constructs in liquid culture. In nutrient replete growth conditions (YPD), we found that the Pib2 ΔA construct grew at the same rate as wild-type Pib2 (Fig. S3B). This suggests the difference in growth rates between wild-type Pib2 and Pib2 ΔA, as observed in rapamycin exposure assays, is specific to TORC1 reactivation. Furthermore, we expressed the Pib2 ΔA construct in a Δgtr1Δgtr2 strain. Pib2 ΔA was unable to rescue the rapamycin sensitivity of this strain, as it did not allow for growth on rapamycin plates or recovery from rapamycin exposure (Fig. S3C). To further uncover the inhibitory mechanism of Pib2, we used our MSAs to select highly conserved residues within regions A and B that might be involved in TORC1 inhibition. Region A contains a series of conserved lysine residues, lysines 59–61 (Fig. 3A). As these lysine residues would be susceptible to post-translational modifications (PTMs) we generated a triple lysine 59–61 to alanine mutant, denoted Pib2 KA. Pib2 KA grew on rapamycin-containing plates and recovered from rapamycin exposure at a similar rate to the Pib2 ΔA construct, and this mutation did not affect Pib2 subcellular localization (Fig. 3B–D). Furthermore, as the positive charge of these lysine residues could form part of a binding interface and result in interactions with negatively charged residues in a binding partner, we also mutated these residues to arginine (Pib2 KR) to maintain a positive charge or glutamate (Pib2 KE) for a charge reversal. Pib2 KE grew at a similar rate to the Pib2 ΔA and Pib2 KA mutants (Fig. 3B). The Pib2 KR mutant, however, grew at a similar rate to wild-type Pib2 (Fig. 3B). As mutation from lysine to either alanine or glutamate residues had a similar effect, and the lysine to arginine mutant had no effect, on cell growth, we propose that the positive charge of the lysine residues in this region are key to its TORC1 inhibitory function.
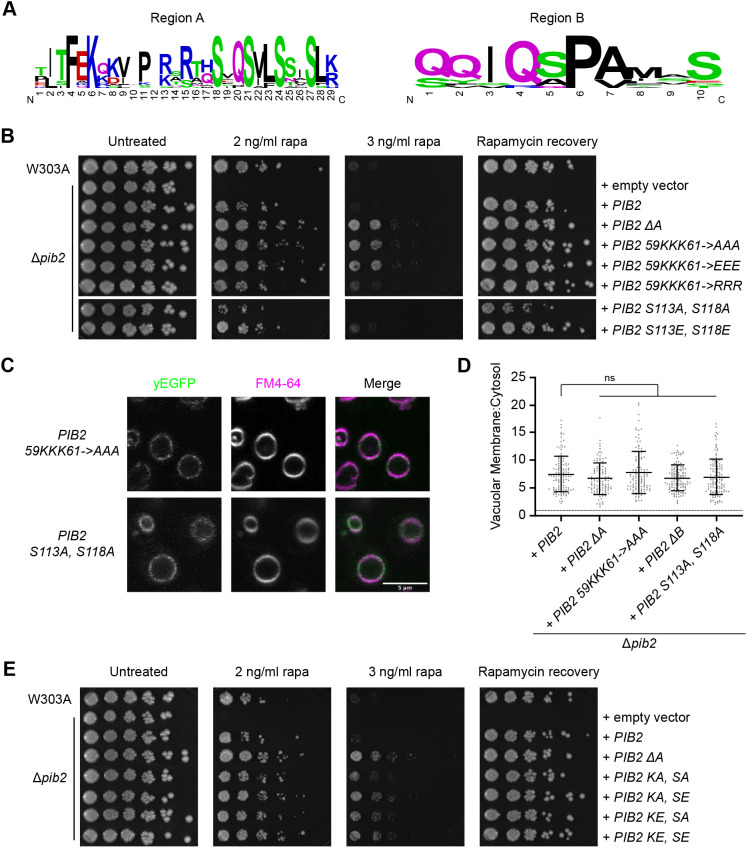
Fig. 3.
Key residues in Pib2 regions A and B are involved in the TORC1 inhibitory mechanism. (A) Sequence logos illustrating the residues conserved in Pib2 regions A and B (15 ascomycete fungi sequences used for alignment). Residue label size is proportional to conservation. (B) Rapamycin exposure and recovery assays of Δpib2 cells expressing the indicated constructs. These were performed as in Fig. 1. (C) Vacuolar localization of indicated yEGFP–Pib2 mutant constructs. Vacuoles were stained with FM4-64. (D) Quantification (mean±s.d.) of the data presented in C. Data for Pib2, Pib2 ΔA, and Pib2 ΔB are the same as in Fig. 2B. Following a ROUT outlier analysis (Q=0.1%), a one-way ANOVA was conducted to determine differences in vacuolar localization (F=3.040, P=0.0169). There were no significant differences from Pib2 localization as determined by Tukey multiple comparisons test. A total of 117–126 cells was quantified for each construct. (E) Rapamycin exposure and recovery assays of Δpib2 cells expressing the indicated constructs. These were performed as in Fig. 1. Images in B,E are representative of three experiments.
In region B, we identified conserved serine residues S113 and S118 (Fig. 3A). Bioinformatic analyses of the Pib2 sequence showed that S113 is a predicted phosphorylation site (Ingrell et al., 2007). We therefore assessed phospho-dead and phospho-mimetic mutants for growth on rapamycin plates and recovery from rapamycin exposure. The phospho-dead mutant, Pib2 S113A, S118A (Pib2 SA), showed reduced growth compared to wild-type Pib2 but did maintain vacuolar localization (Fig. 3B–D). However, the phosphomimetic mutant, Pib2 S113E, S118E (Pib2 SE), grew better than wild-type Pib2 in these rapamycin exposure assays (Fig. 3B). This is most notable on the 2 ng/ml rapamycin plate, as the effects diminished with increased rapamycin concentration (Fig. 3B). This suggests that phosphorylation at one or both of these sites might be involved in the TORC1 inhibitory mechanism of Pib2.
A potential model of TORC1 inhibition by Pib2 could involve intramolecular interactions. To determine whether regions A and B work together to inhibit TORC1 activity, we generated four combinatorial mutants of these key region A and B residues. These included Pib2 KA/SA, Pib2 KA/SE, Pib2 KE/SA and Pib2 KE/SE. Each of these mutants showed improved growth over wild-type cells in both rapamycin exposure assays (Fig. 3E). As the lysine mutations in region A were able to override the activity of the region B serine mutations, this suggests region A is dominant to region B in this mechanism. To determine whether the Pib2 inhibitory regions interact with Pib2 C-terminal activation regions we generated compound deletion mutations. Deletion of region A in combination with one of the C-terminal activation regions (helical E, FYVE or tail) enabled mild recovery following rapamycin exposure compared to deletion of the C-terminal regions alone (Fig. S3D). Although this was a less robust recovery, this indicates that the N- and C-terminal mechanisms for Pib2-mediated inhibition and activation of TORC1 might be separable, as also implicated in a previous study (Michel et al., 2017).
Pib2 vacuolar localization is essential for TORC1 reactivation and cell growth
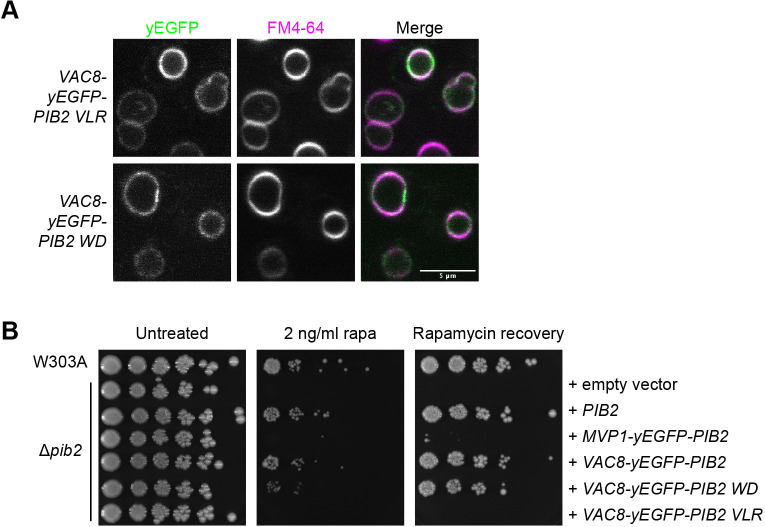
Pib2 localizes to both the vacuole and endosomes (Hatakeyama et al., 2019; Kim and Cunningham, 2015). As Pib2 localization is similar to that of TORC1, we set out to investigate which cellular localization of Pib2 is essential for TORC1 reactivation. We therefore generated several chimeric Pib2 constructs targeted to the vacuole and endosome. These constructs consisted of a targeting protein linked to Pib2 via a yEGFP bridge (Fig. 4A,B). To target Pib2 specifically to the vacuole we used Vac8, which is localized to the vacuole through N-terminal lipid modifications (myristoylation and palmitoylation) (Subramanian et al., 2006) and has been shown to interact with the TORC1 component Tco89 (Jeong et al., 2017). To target Pib2 specifically to endosomes we used the yeast SNX-BAR, Mvp1, which uses its PX and BAR domains to specifically bind endosomal membranes, which are enriched in PI3P (Sun et al., 2020). The Vac8–yEGFP–Pib2 construct localized to the vacuolar membrane with scarce endosomal puncta (Fig. 4A; Fig. S4A). In contrast, the Mvp1–yEGFP–Pib2 construct localized primarily to endosomal puncta with minimal vacuolar localization (Fig. 4A, Fig. S4A). In response to rapamycin exposure assays, Δpib2 cells containing Vac8–yEGFP–Pib2 were able to grow on rapamycin-containing plates and recover from rapamycin exposure, as expected (Fig. 4C). Growth of Δpib2 cells containing Mvp1–yEGFP–Pib2, however, was significantly compromised in response to rapamycin exposure (Fig. 4C).
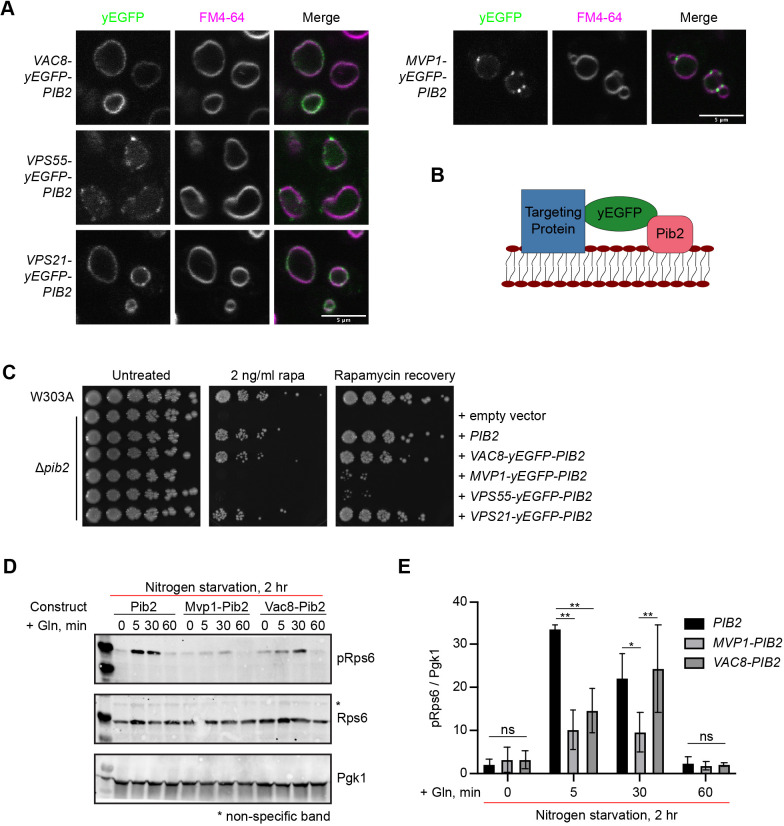
Fig. 4.
Pib2 vacuolar targeting promotes TORC1 reactivation. (A) Localization of the indicated targeting constructs. Vacuoles were stained with FM4-64. (B) Schematic of chimeric Pib2 vacuolar and endosomal targeting constructs. Each construct consists of the targeting protein and yEGFP fused to the N-terminus of Pib2. (C) Rapamycin exposure and recovery assay of Δpib2 cells expressing the indicated targeting constructs. These were performed as in Fig. 1. (D) Representative western blot from a glutamine response assay (n=4). Cells were nitrogen starved for 2 h in SD −N. For stimulation, cells were treated with SD −N supplemented with 3 mM glutamine and incubated for indicated times prior to lysis. TORC1 activation was assessed by phosphorylation of Rps6 under the indicated conditions. Total Rps6 and Pgk1 are shown as loading controls. (E) Quantification of D (mean±s.d.; n=4). Values were normalized to the corresponding Pgk1 loading control. Differences were assessed by two-way ANOVA (P<0.0001). Differences between constructs at each time point as determined by Tukey's post-hoc multiple comparisons are indicated (*P=0.0007, **P<0.0001). Images in A,C are representative of three experiments.
Pib2 has been suggested to be a glutamine sensor (Tanigawa and Maeda, 2017; Tanigawa et al., 2021; Ukai et al., 2018). Thus, we additionally assessed cell growth after nutrient starvation and stimulation with glutamine using phosphorylation of Rps6 at S232/S233 as a readout of TORC1 activity (González et al., 2015). We demonstrated that the endosomal Mvp1–yEGFP–Pib2 construct is deficient in reactivating TORC1 signaling following nitrogen starvation and glutamine stimulation compared to the vacuolar Vac8–yEGFP–Pib2 construct and wild-type Pib2 (Fig. 4D,E). Glutamine is a preferred nitrogen source in yeast and has been shown to result in both a rapid, transient activation of TORC1 and a sustained TORC1 activation that is independent of the Gtrs (Stracka et al., 2014). The Vac8–yEGFP–Pib2 construct was slower to respond to glutamine than wild-type Pib2, exhibiting sustained TORC1 activity, as determined from phosphorylation of Rps6 at 30 min, but not the rapid activation seen at 5 min (Fig. 4D,E). Rps6 is an indirect effector of TORC1, it is phosphorylated by the direct TORC1 substrate Ypk3 (González et al., 2015; Yerlikaya et al., 2016). As only vacuolar localization of Pib2 resulted in Rps6 phosphorylation, this might suggest that Ypk3 is a substrate of vacuolar TORC1.
To confirm that all the targeting constructs were expressed at similar levels, we quantified total GFP fluorescence in cells expressing these constructs and compared it to the fluorescence from cells expressing only yEGFP–Pib2 (Fig. S4B). This method was used due to concerns over differential western blotting transfer efficiencies. All constructs showed comparable fluorescence intensities (Fig. S4B). As a control, we expressed yEGFP alone, using a stronger promoter (VPS1). As expected, we observed elevated fluorescence from yEGFP alone compared to the constructs using PIB2 and MVP1 promoters (data not shown).
As Mvp1 multimerizes (Sun et al., 2020), we reasoned that this may preclude Pib2 from mediating reactivation of TORC1. Thus, we generated two additional constructs to assess localized TORC1 reactivation: Vps55–yEGFP–Pib2 and Vps21–yEGFP–Pib2. Although Vps21 itself is endosomal, fusions to its C-terminus affect the lipidation required for its membrane association and were therefore deliberate to determine the effects on Pib2 localization of a nonfunctional protein. Accordingly, as previously observed, Vps21–yEGFP is cytosolic (data not shown; Dubreuil et al., 2019). By contrast, like wild-type Pib2, Vps21–yEGFP–Pib2 localized to the vacuole with a few perivacuolar puncta (Fig. 4A; Fig. S4A). Hence, Pib2 can direct localization of this chimeric construct. Conversely, Vps55, an integral membrane protein involved in endosome to vacuole trafficking localizes at endosomes (Belgareh-Touze et al., 2002; Schluter et al., 2008; Suzuki et al., 2021). Based on a topology analysis of leptin receptor gene-related protein and leptin receptor overlapping transcript-like 1 (LEPROT and LEPROTL1; also known as endospanin-1 and endospanin-2, respectively) (Seron et al., 2011), close human homologs of Vps55, both the N- and C-terminus of Vps55 are cytosolic. Hence, Pib2 remains in the cytosol in this targeting construct. The Vps55–yEGFP–Pib2 construct localized primarily to perivacuolar puncta with less vacuolar presence (Fig. 4A; Fig. S4A). Like the Mvp1–yEGFP–Pib2 construct, Vps55–yEGFP–Pib2 was unable to rescue growth in the rapamycin exposure assays (Fig. 4C). The wild-type-like Vps21–yEGFP–Pib2 construct, however, was able to rescue growth in these assays, demonstrating that the N-terminal addition of this protein does not affect Pib2 activity (Fig. 4C). Together, these data suggest that vacuolar Pib2 is necessary for the reactivation of TORC1 and resumed cell growth following rapamycin exposure.
Pib2 vacuolar localization is dependent on its helE and FYVE domains
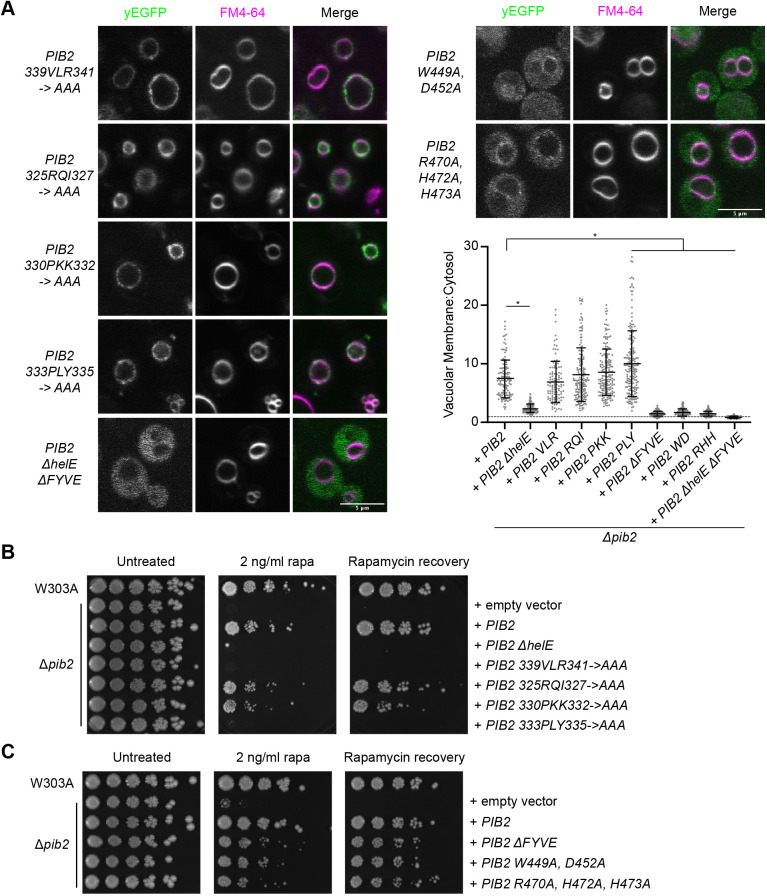
The vacuolar localization of Pib2 has been shown to depend on PI3P and Vps34 (Kim and Cunningham, 2015). PI3P-binding motifs within FYVE domains include WxxD and R+HHCRxCG (where x is any amino acid) (Burd and Emr, 1998; Stenmark et al., 2002). As these are both conserved across Pib2 FYVE domains (Fig. S1A), we generated two mutants, one for each of these motifs – Pib2 W449A, D452A (Pib2 WD) and Pib2 R470A, H472A, H473A (Pib2 RHH). Both mutants showed compromised localization and were primarily cytosolic (Fig. 5A). Mutation of highly conserved residues in the helE region, 325RQI327→AAA (Pib2 RQI), 330PKK332→AAA (Pib2 PKK), 333PLY335→AAA (Pib2 PLY) and 339VLR341→AAA (Pib2 VLR), did not influence vacuolar localization (Fig. 5A). Simultaneous deletion of both the helE region and FYVE domain resulted in a primarily cytosolic phenotype as expected (Fig. 5A).
Fig. 5.
The Pib2 helE region and FYVE domain are essential for TORC1 reactivation. (A) Localization of the indicated yEGFP–Pib2 mutants. Vacuoles were stained with FM4-64. Quantification (mean±s.d.) of the data. Data for Pib2, Pib2 ΔhelE, and Pib2 ΔFYVE were the same as in Fig. 2B. Following a ROUT outlier analysis (Q=0.1%), a one-way ANOVA was conducted to determine differences in vacuolar localization (F=180.9, P<0.0001). Constructs significantly different from WT Pib2, as assessed by Tukey post-hoc multiple comparisons test, are indicated (*P<0.0001). A total of 107–181 cells were quantified for each construct. (B) Rapamycin exposure and recovery assays of Δpib2 cells expressing the indicated Pib2 helE region mutants. These were performed as in Fig. 1. (C) Rapamycin exposure and recovery assays of Δpib2 cells expressing the indicated Pib2 FYVE domain mutants. These were performed as in Fig. 1. Images in B,C are representative of three experiments.
As the FYVE domain alone was not sufficient to direct Pib2 localization to the vacuole, we investigated the ability of the Pib2 FYVE domain to bind to PI3P using isothermal titration calorimetry (ITC). We expressed and purified Pib2 442–625, which included the start of the FYVE domain to the beginning of the tail motif. We also purified Pib2 419–625, which starts 23 residues before the core of the FYVE domain. While the AlphaFold2 Pib2 model does not show any structural elements here, some canonical FYVE domains include an α-helix immediately before the FYVE core that is involved in dimerization. ITC with Pib2 442–625 showed a weak binding interaction with the PI3P headgroup, inositol 1,3-bisphosphate, with a mean KD=212 µM, as well as the short chain lipid, PI3P diC4 (mean KD=736 µM) (Fig. S5A, Table S1). A mutant version of this construct, Pib2 442–625 RHH, did not bind to the lipid headgroup, as expected (Table S1). ITC with Pib2 419–625 also showed a low affinity interaction with the PI3P headgroup (KD>290 µM; Table S1). To validate the assay, we purified two EEA1 FYVE domain constructs, EEA1 1287–1411 and 1347–1411. The boundaries of these constructs were defined using the PDB structures 1JOC (Dumas et al., 2001) and 1HYI (Kutateladze and Overduin, 2001), respectively. The shorter EEA1 FYVE construct, 1347–1411, showed a weak binding affinity (KD>300 µM) that was difficult to fit (Table S1). The longer EEA1 FYVE construct, EEA1 1287–1411, showed a higher affinity for the PI3P headgroup (KD=∼95.8 µM; Table S1). Although not necessarily representative of lipid binding affinity in the membrane, these affinities are comparable to previously published values, which demonstrate micromolar affinities of the purified EEA1 FYVE domain for the PI3P headgroup (Gaullier et al., 2000). Furthermore, we used PIP strips to assess FYVE domain binding to various lipids. Using 5 µg/ml purified protein, we detected an interaction of EEA1 1287–1411 with PI3P. However, there was no PI3P interaction with Pib2 419–625 at that concentration (Fig. S5B). When the protein concentrations were increased to 25 µg/ml, we observed an interaction between Pib2 419–625 and PI3P, but not EEA1 1347–1411 (Fig. S5C). Combined, this supports the lower affinity for PI3P of the Pib2 FYVE domain compared to EEA1 1287–1411.
Although many FYVE domains are monomeric, some are known to dimerize to facilitate PI3P binding (Kutateladze et al., 1999). We therefore used size exclusion chromatography coupled with multi-angle light scattering (SEC-MALS) to determine the oligomeric state of the purified FYVE domains. Pib2 419–625 and Pib2 442–625 were predominantly monomeric (Fig. S6A,B). As these constructs included predicted low complexity sequence downstream of the FYVE domain, we expressed and purified a smaller construct, Pib2 437–542. This was again largely monomeric (Fig. S6B). We also assessed the oligomeric state of the two EEA1 constructs. The crystal structure of EEA1 1287–1411 is a dimer (Dumas et al., 2001), where, in addition to the contacts between the core of the FYVE, there are significant interactions between the N-terminal α-helices (Fig. S6C). Accordingly, this construct was largely dimeric, as assessed by SEC-MALS (Fig. S6C). By contrast, and consistent with existing NMR models (Kutateladze and Overduin, 2001), EEA1 1347–1411, which lacks the N-terminal α-helix, was largely monomeric (Fig. S6C). The AlphaFold2 model of Pib2 lacks an α-helix before the FYVE domain (Fig. S6D) and is, accordingly, monomeric with a PIP3-binding affinity consistent with monomeric EEA1 1347–1411. As the binding affinities of the Pib2 constructs were relatively weak, it further supports that both the helE and FYVE domain are needed for appropriate subcellular localization of Pib2.
Pib2 C-terminal regions are essential for TORC1 reactivation following rapamycin exposure
Previous studies have shown that truncation of the Pib2 C-terminal tail, and larger C-terminal portions, impedes the reactivation of TORC1 following rapamycin exposure (Michel et al., 2017; Ukai et al., 2018). As shown in Fig. 1B,C, the helE and tail motif were essential for TORC1 reactivation under rapamycin exposure conditions. The FYVE domain also influenced TORC1 reactivation, although less so than the other two domains. To determine what aspects of these conserved regions might be key for TORC1 reactivation, we generated point mutations of highly conserved residues within the helE region and FYVE domain to assess their effects on TORC1 activation. The helE region mutants Pib2 VLR and PLY were not able to mediate growth on rapamycin plates and Δpib2 cells expressing these did not recover from rapamycin exposure, similar to what was found for the helE region deletion construct (Fig. 5B). Two other helE mutants, Pib2 RQI and PKK, were able to mediate growth on and recovery from rapamycin although at a slower rate than W303A cells or Δpib2 cells expressing wild-type Pib2 (Fig. 5B). As shown above, unlike the helE region deletion construct, these mutants localized predominately to the vacuole, resembling WT Pib2 localization (Fig. 5A). The helE region of Pib2 is a putative Kog1-binding region (Michel et al., 2017; Sullivan et al., 2019), thus it is possible that these residues might be required for that interaction.
We also assessed growth of the PI3P-binding motif mutations within the Pib2 FYVE domain, Pib2 WD and Pib2 RHH. In addition to their altered localization described above (Fig. 5A), these mutants phenocopied deletion of the FYVE domain in that they mediated recovery at a slower rate than seen in Δpib2 cells expressing wild-type Pib2 (Fig. 5C).
To determine whether vacuolar localization of Pib2 was sufficient for TORC1 reactivation, we incorporated two of the previously described Pib2 mutations, Pib2 VLR and Pib2 WD, into the Vac8–yEGFP–Pib2 construct. As expected, due to the presence of Vac8, these mutants localized to the vacuole (Fig. 6A). The Vac8–yEGFP–Pib2 WD mutant was able to rescue growth under rapamycin exposure conditions just as well as the wild-type Vac8-yEGFP-Pib2 construct (Fig. 6B). As vacuolar localization of the Pib2 WD mutant with this targeting construct was able to rescue growth rate, this suggests that vacuolar localization of Pib2 is necessary for TORC1 reactivation in these conditions. The Vac8–yEGFP–Pib2 VLR mutant, however, was still unable to rescue growth (Fig. 6B). These results support the hypothesis that these VLR residues within the helE region of Pib2 are essential for TORC1 reactivation and might be involved in glutamine sensing (Tanigawa et al., 2021) and/or direct interactions with TORC1 components. Overall, these results demonstrate that vacuolar localization of Pib2 is necessary but not sufficient for TORC1 reactivation following rapamycin exposure in vivo.
Fig. 6.
Vacuolar localization of Pib2 is necessary but not sufficient for TORC1 reactivation. (A) Localization of the indicated vacuolar targeting constructs. Vacuoles stained with FM4-64. (B) Rapamycin exposure and recovery assays of Δpib2 cells expressing the indicated targeting constructs. These were performed as in Fig. 1. Images are representative of three experiments.
DISCUSSION
In this work, we explored the roles of the conserved Pib2 regions in regulating TORC1 activity. We demonstrated that each conserved region has a distinct function in TORC1 reactivation or inhibition, and/or Pib2 subcellular localization. Pib2 N-terminal regions A and B displayed a TORC1 inhibitory function, as demonstrated by enhanced growth of Pib2 ΔA, Pib2 ΔB, and related mutants over Δpib2 cells expressing wild-type Pib2 in rapamycin exposure assays. C-terminal Pib2 regions, notably helE and the tail motif were essential for TORC1 reactivation, as demonstrated by the lack of growth and recovery in rapamycin exposure assays. Furthermore, the C-terminal helE and FYVE regions were key for vacuolar localization of Pib2. Interestingly, we discovered that although both of these regions mediate vacuolar localization of Pib2, only the helE region is necessary for Pib2 function in TORC1 reactivation. This suggests that vacuolar localization driven by the FYVE domain might have a function that is unrelated to TORC1 regulation.
Pib2 C-terminal regions have been shown to be essential for TORC1 reactivation. Deletion mutants of the tail motif or Kog1-binding domains (found within the conserved helE region) exhibit increased rapamycin sensitivity (Michel et al., 2017; Sullivan et al., 2019). We demonstrated here that helE-specific VLR and PLY sequences, and the tail motif are required for the ability of Pib2 to mediate growth on rapamycin-containing plates and recovery following rapamycin exposure (Fig. 1B,C). The mechanism through which the tail motif acts in TORC1 reactivation is unclear. However, several hyperactive tail mutations have been recently reported and it was proposed that the tail directly interacts with TORC1 to promote its activation (Tanigawa et al., 2021).
Pib2 interaction with TORC1 components, Kog1 and Tor1, have previously been demonstrated by western blotting and yeast two-hybrid experiments (Michel et al., 2017; Sullivan et al., 2019; Tanigawa and Maeda, 2017; Ukai et al., 2018). The helE region has been identified as a putative binding region for these interactions (Michel et al., 2017; Sullivan et al., 2019). As confocal imaging of our Pib2 ΔhelE mutant showed, this region is partially responsible for Pib2 localization at the vacuole (Fig. 2A,B). However, the helE mutants Pib2 PLY and Pib2 VLR demonstrated that these residues were essential for TORC1 reactivation following rapamycin exposure, even though they have no effect on vacuolar localization. It has recently been reported that these residues, particularly R341, might be essential for glutamine-induced TORC1 activation (Tanigawa et al., 2021), which could be a part of the TORC1 interaction and activation mechanism. However, further experiments are needed to define how Pib2 interacts with TORC1 components or other TORC1 regulators. One potential model is that these residues are crucial for the interaction between Pib2 and TORC1 components needed for TORC1 reactivation. We were unable to reliably detect an interaction between Pib2 and Kog1 using yeast two-hybrid experiments, so potential effects of the helE mutations on this interaction could not be confirmed.
Previous studies have shown that truncations of the Pib2 N-terminus result in increased resistance to rapamycin exposure (Michel et al., 2017; Ukai et al., 2018), suggesting these regions have inhibitory effects on TORC1 reactivation. One study implicated Pib2 residues 1–50 in its inhibitory function (Ukai et al., 2018). Our results, however, showed that regions A (residues 54–81) and B (residues 109–118) are required for this inhibitory role. We did not investigate the inhibitory role of residues 1–50, however, it is possible this discrepancy might be due to interactions between these residues and regions A and B or other intramolecular interactions. Deletion of regions C and D had no effect on TORC1 reactivation with the readouts used here. Pib2 MSAs showed fewer conserved residues in these regions compared to in regions A and B so they might not be vital for this function (Fig. S1A). Regions C and D could play a role in other Pib2 functions, such as LMP (Kim and Cunningham, 2015). Mutation of conserved residues within regions A and B highlighted a series of lysine and serine residues involved in the inhibitory mechanism. Based on our results and bioinformatic PTM predictions, the positive charge of the lysine residues and the phosphorylation status of the serine residues are likely part of this mechanism. Other groups have reported phosphorylation of Pib2 by the TORC1 downstream kinase Npr1 (Brito et al., 2019; MacGurn et al., 2011), and there are several predicted phosphorylation sites within the Pib2 N-terminus that could affect its function (Fig. S1A). Another candidate Pib2 kinase includes Cdk1, which has previously been shown to phosphorylate Pib2 at S113 (Holt et al., 2009). As the AlphaFold2 prediction shows, these N-terminal regions are found in low complexity sequence, which not only makes them more accessible and susceptible to PTMs but also protein interactions that could play a role in TORC1 regulation. Further studies are needed to elucidate this inhibitory mechanism.
The Gtr-dependent pathway of TORC1 regulation relies on nutrient sensing by upstream GAPs and GEFs to control TORC1 activation. Whether Pib2 activation of TORC1 is a Gtr-dependent or Gtr-independent mechanism has attracted considerable discussion. Simultaneous deletion of Pib2 and EGO subunits results in synthetic lethality (Kim and Cunningham, 2015). Furthermore, Pib2 has been shown to interact with EGO subunits and both Pib2 and EGO are required for TORC1 activation by amino acids (Kim and Cunningham, 2015; Tarassov et al., 2008; Varlakhanova et al., 2017). However, some studies have described a parallel, Gtr-independent activation pathway via glutamine (Tanigawa and Maeda, 2017; Tanigawa et al., 2021; Ukai et al., 2018). Expression of our Pib2 ΔA construct, which confers rapamycin resistance, in a Δgtr1Δgtr2 strain did not allow for growth on rapamycin plates or recovery from rapamycin exposure (Fig. S3C), supporting the idea that Pib2 and the Gtrs might work in the same pathway to promote TORC1 reactivation. A model of dual-phase activation has been suggested in which Pib2 and the Gtrs work cooperatively in some instances and independently in others to promote TORC1 activity (Hatakeyama, 2021). It is possible to generate both Δpib2 and Δgtr1Δgtr2 strains independently, which implies that under nutrient replete, unstressed conditions both pathways are not necessary for TORC1 activity. However, the inability of these strains to recover from stressors like rapamycin suggests that under stress both the Pib2 and Gtr pathways are required for TORC1 activation.
Subcellular localization has been shown to be an important aspect of TORC1 regulation and activity (Hatakeyama et al., 2019; Prouteau et al., 2017). Recently, two spatially distinct pools of TORC1 have been identified that direct different processes, with vacuolar TORC1 promoting cell growth and protein synthesis, and endosomal TORC1 negatively regulating autophagy (Hatakeyama et al., 2019). The Pib2 FYVE domain has been shown to be key for Pib2 localization at the vacuolar membrane (Kim and Cunningham, 2015). FYVE domains are most notably known as PI3P-binding domains and typically display endosomal localization (Stenmark et al., 2002). As that is not the case with Pib2, it is possible that the Pib2 FYVE domain might favor other interactions to help direct it to the vacuole. Indeed, our measurements indicate weak affinities for both the headgroup and a soluble PI3P. As these purified Pib2 FYVE constructs are predominately monomeric, regulation of assembly into a dimeric form might also be required to facilitate interaction with its preferred ligand. Furthermore, we show here that the FYVE domain alone is not sufficient for Pib2 vacuolar localization. Our data showed that whereas the Pib2 ΔFYVE construct had a mostly cytosolic cellular distribution, as previously described (Ukai et al., 2018), it did still have some enrichment at the vacuolar membrane and still enabled recovery from rapamycin exposure, although at a slower rate (Fig. 1B,C; Fig. 2A,B). The Pib2 helE deletion construct, like the FYVE deletion construct, showed a mostly cytosolic phenotype with some vacuolar enrichment; however, it did not allow for recovery from rapamycin exposure (Fig. 1B,C; Fig. 2A,B). As the Pib2 helE region is a TORC1 interaction site (Michel et al., 2017; Sullivan et al., 2019), TORC1 and PI3P, via the FYVE domain, might act as a dual recruitment mechanism to bring Pib2 to the vacuole.
Here, we used endosomal and vacuolar targeting constructs to identify the role of Pib2 at these subcellular localizations. The Vps55–yEGFP–Pib2 construct, which has an endosomal distribution, showed that endosomal Pib2 is not sufficient for TORC1 reactivation and cell growth. Furthermore, vacuolar targeting with the Vac8–yEGFP–Pib2 WD construct rescued the growth deficit seen with the largely cytosolic Pib2 WD mutant. This suggests vacuolar localization of Pib2 is essential for cell growth and recovery following rapamycin exposure. This proposed role of vacuolar Pib2 in reactivating TORC1 and promoting cell growth is in keeping with the role of vacuolar TORC1 in promoting cell growth through phosphorylation of Sch9 (Hatakeyama et al., 2019; Urban et al., 2007). Although vacuolar localization of Pib2 appears to be essential for cell growth following rapamycin exposure, it is not sufficient to promote TORC1 activity. The helE region PLY and VLR mutants, which maintain vacuolar localization of Pib2, still did not support TORC1 reactivation (Figs 5B, 6B). This implies that other interactions at the vacuolar membrane are necessary for Pib2 to reactivate TORC1. Future experiments could use a light-inducible dimerization system to alter the distribution of Pib2 or other TORC1 regulators and assess the role of Pib2 localization in TORC1 activation.
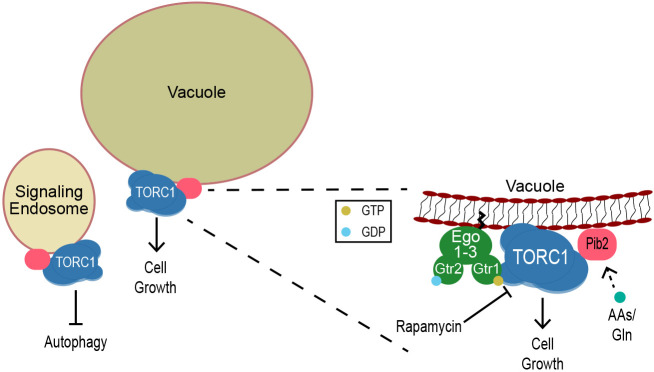
In summary, we have shown that Pib2 has two opposing functions in TORC1 regulation, and each conserved region plays a distinct role in inhibiting or reactivating TORC1. Additionally, using endosomal and vacuolar targeting constructs, we have shown that Pib2 localization at the vacuole is essential but not sufficient for promoting TORC1 activity and cell growth. We propose that an interaction between TORC1 and Pib2 at the vacuole is necessary for TORC1 reactivation and cell growth following exposure to a stressor (Fig. 7). Future work will investigate the mechanisms of TORC1 inhibition by Pib2, as well as implications of the localization of Pib2 and its dependence on other TORC1 regulatory proteins.
Fig. 7.
Proposed model of Pib2 regulation of TORC1. TORC1 and Pib2 localize to both the vacuole and signaling endosomes. Localization of Pib2 at the vacuole is required for interaction with and activation of TORC1 to promote cell growth following exposure to the stressor rapamycin. AAs, amino acids.
MATERIALS AND METHODS
Multiple sequence alignments
MSAs were made using MUSCLE (Edgar, 2004). Alignments shown were made using the output of ESPRIPT 3.0 (Robert and Gouet, 2014) using the AlphaFold2 Pib2 prediction (PDB AF-P53191-F1-model_v1) (Jumper et al., 2021) as an input for secondary structure assignments. The AlphaFold2 structure prediction was rendered using PyMOL (https://pymol.org/).
Media
YPD (1% yeast extract, 2% peptone, 2% glucose, supplemented with L-tryptophan and adenine) was used for routine growth. Synthetic complete (SC; yeast nitrogen base, ammonium sulfate, 2% glucose, amino acids) or synthetic defined (SD; yeast nitrogen base, ammonium sulfate, 2% glucose, appropriate amino acid dropout) media were used as indicated prior to microscopy or to maintain plasmid selection. For sporulation, cells were successively cultured in YPA (1% yeast extract, 2% peptone, 2% potassium acetate) and SPO (1% potassium acetate, 0.1% yeast extract, 0.05% glucose). For nitrogen starvation, cells were grown in SD –N (0.17% yeast nitrogen base without amino acids and ammonium sulfate, 2% glucose).
Yeast genetic manipulation and cloning
Yeast strains used in this work are listed in Table S2. W303A GFP-N10-PIB2::HIS3 (PY_262) was generated by complete replacement of the kanMX cassette in W303A Δpib2::KAN (PY_126) (Varlakhanova et al., 2017). The replacement cassette, which includes ∼40 nucleotide regions from the PIB2 promoter and terminator sequences immediately upstream and downstream of the PIB2 ATG and STOP codons to enable site-specific reintegration, was generated by splicing by overlap extension PCR using a GFP-N10-PIB2 fragment amplified from pRS316 yEGFP-N10-PIB2+UTRs (Varlakhanova et al., 2017) and an ADH1 terminator-His3MX6 fragment, amplified from pFA6a-link-yEGFP-SpHIS5 (pKT0128) (Sheff and Thorn, 2004). The full-length replacement cassette was gel purified and transformed into PY_126. Cells were plated onto SD −HIS and incubated for 3 days at 30°C. A mixture of recombinants was expected: replacement of the kan resistance gene by conversion into HIS prototrophy, without integration of GFP-N10-PIB2 (due to recombination between the Ashbya gossipii TEF promoter and terminators sequences flanking the kanamycin resistance gene in Δpib2::KAN and the S. pombe his5 gene in the replacement cassette) and complete replacement of kanMX6 with the GFP-N10-PIB2::HIS replacement cassette, by recombination in the PIB2 promoter and terminator regions. Colony PCR indicated about one-third of the resulting HIS prototrophs contained the GFP-N10-PIB2 coding sequence at the correct genomic locus. Loss of kanamycin resistance was also verified. Candidate colonies were backcrossed to W303α and re-sporulated to ensure the expected pattern of segregation. The knock-in strain was further verified by sequencing, imaging and functional assays.
W303A PIB2::KAN, W303A PIB2 ΔA::KAN, W303A PIB2 ΔB::KAN, and W303A PIB2 ΔhelE::KAN were generated using a similar approach, but by complete replacement of the His3MX6 cassette in the Δpib2::HIS3 strain (PY_128). The replacement cassettes were generated by splicing by overlap extension using the appropriate PIB2 construct amplified from pRS316 S cer. PIB2+UTRs (Varlakhanova et al., 2017), pRS316 S cer. PIB2 ΔA+UTRs, pRS316 S cer. PIB2 ΔB+UTRs, pRS316 S cer. PIB2 ΔhelE+UTRs and a ADH1 terminator-kanMX6 fragment amplified from pFA6a-link-yEGFP-KanR (pKT0127) (Sheff and Thorn, 2004). After verification of correct reintroduction of the appropriate PIB2 sequence by sequencing and loss of HIS prototrophy, the strains were further validated after backcrossing to W303α and resporulation.
Plasmids used in this work are listed in Table S3. Pib2 mutant constructs were cloned by splicing by overlap extension (SOE) PCR at the site of the mutation using appropriate primers followed by Gibson assembly into the target vector. For Pib2 deletion constructs, the deleted regions were replaced with an AGAGA linker. N-terminal yEGFP–Pib2 constructs were generated with an N10 linker (NSSSNNNNNNNNNNLGIE). Targeting constructs were generated by amplification of the targeting protein from genomic DNA isolated from W303A/α diploids using the Yeast DNA Extraction kit (Thermo Fisher Scientific) or an existing plasmid. The targeting protein was then fused to the appropriate yEGFP-PIB2 construct by SOE with a short linker between the targeting protein and yEGFP (GRRIPGLIN for MVP1-yEGFP constructs or GDGAGLIN for all others). These constructs also contain both the PIB2 promoter (175 bp) and terminator (150 bp) and were fully assembled using Gibson assembly. All plasmids were verified by sanger and/or nanopore sequencing.
Growth analysis
Cells were grown overnight in YPD, SC, or SD with the appropriate dropout for plasmid maintenance. Cells were then diluted and regrown to mid-logarithmic phase [optical density at 600 nm (OD600) of 0.5–0.8] in YPD at 30°C. Cells were diluted to 0.5 OD600/ml and 1:5 serial dilutions were made in water. Each dilution (2 µl) was spotted onto the indicated plates. Where relevant, cells were incubated with 200 ng/ml of rapamycin in YPD at 30°C for the indicated times. After several washes the cells were resuspended in fresh YPD and plated on YPD. All plates were incubated at 30°C and imaged on days 2 and 3.
Preparation of yeast for microscopy
Cells were grown overnight in YPD or the appropriate SD medium. Cells were diluted in YPD and grown to mid-logarithmic phase. Vacuolar membranes were stained with 10 µM FM4-64 (Thermo Fisher Scientific) in YPD for 1 h, followed by washing and incubation in SC medium without dye for 1 h. Cells were plated on no. 1.5 glass-bottomed cover dishes (MatTek Corporation, Ashland) and treated with 15 µl of 2 mg/ml concanavalin-A (Sigma-Aldrich).
Microscopy and image analysis
Confocal images were acquired on a Nikon (Melville, NY) A1 confocal microscope, with a 100× Plan Apo oil objective. NIS Elements imaging software was used to control image acquisition. Images were further processed using the Fiji distribution of ImageJ (Schindelin et al., 2012). GraphPad Prism was used for statistical analyses.
Vacuolar localization of yEGFP–Pib2 constructs was quantified using Fiji. For each cell, vacuolar membrane and cytosolic regions of interest (ROIs) of equal area were determined and the fluorescence within those ROIs was measured. FM4-64 fluorescence was used to determine the location of the vacuolar membrane ROIs. Within each image, an average background fluorescence was determined and subtracted from the vacuolar and cytosolic intensity measurements. The localization was then expressed as a ratio of vacuolar membrane fluorescence to cytosolic fluorescence. For statistical analyses, a ROUT outliers test (Q=0.1%) was used and data were further assessed by one-way ANOVA with Tukey multiple comparisons.
To determine expression levels of targeting constructs, cells were imaged in widefield using a Nikon Ti Microscope with an S Plan Fluor ELWD 20× objective. NIS Elements imaging software was used to control image acquisition. Images were processed in NIS Elements imaging software using a custom macro. GraphPad Prism was used for statistical analyses.
Western blotting
Protein extracts were prepared as previously described (Millen et al., 2009). Briefly, cells were lysed on ice by resuspension in 1 ml cold H2O supplemented with 150 µl 1.85 M NaOH and 7.5% (v/v) β-mercaptoethanol. After a 10-min incubation on ice, the protein was precipitated by addition of 150 µl 50% (w/v) trichloroacetic acid. Pellets were washed twice with acetone, resuspended in 100 µl 1× SDS-PAGE buffer, and boiled for 5 min at 95°C. Primary antibodies were incubated overnight at 4°C and were as follows: anti-GFP (1:1000, ab290, Abcam), anti-PGK1 (1:1000, ab113687, Abcam), anti-Rps6 (1:1000, ab40820, Abcam), anti-phospho-Rps6 (1:1000, 4858, Cell Signaling Technology, Danvers). Secondary antibodies were incubated for 1 h at room temperature and were as follows: IRDye 680RD goat anti-rabbit-IgG antibody (926-68171, Li-Cor, Lincoln) and IRDye 680RD goat anti-mouse-IgG antibody (926-68070, Li-Cor). These were detected using the ChemiDoc MP Imaging System (Bio-Rad). Bands were integrated and quantified using Fiji. See Fig. S7 for original blots.
Size-exclusion chromatography coupled to multi-angle light scattering
After filtration through a 0.22 µm cellulose acetate membrane, the Pib2 FYVE constructs and mutants were subjected to size-exclusion chromatography using a Superdex 75 10/300 equilibrated in SEC-MALS buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl and 1.93 mM β-mercaptoethanol) at room temperature. 500 µl of each protein was loaded onto the column, at a concentration of 50 or 100 µM as indicated. The column was coupled to a static 18-angle light scattering detector (DAWN HELEOS-II) and a refractive index detector (Optilab T-rEX) (Wyatt Technology). Data were collected continuously at a flow rate of 0.3 ml/min, with the flow cells in the scattering and refractive index detectors set to 25°C. Data analysis was performed using the program Astra VII. Monomeric BSA (2.0 mg/ml) (Sigma) was used for data quality control.
Isothermal titration calorimetry
ITC was used to determine the thermodynamics of the interaction of the Pib2 FYVE domain (using Pib2 442–625) with inositol 1,3-bisphosphate [Ins(1,3)P2] or short-chain PI3P. The titrations were performed at 25°C using a PEAQ-ITC instrument (Malvern Analytical), in 20 mM Tris-HCl pH 7.4, 150 mM NaCl and 1.93 mM β-mercaptoethanol. 4 mM Ins(1,3)P2 or 2 mM diC4 PI3P was titrated into 100–150 µM protein. Integrated peaks for each titration were fit to a single-site binding model using the MicroCal PEAQ-ITC software provided by the manufacturer.
FYVE domain binding to PIP strip
PIP strips (Echelon Biosciences) were used to assess binding of purified FYVE domains to indicated lipids. Membrane was incubated for 1 h at room temperature in Tris-buffer saline with 0.1% Tween 20 (TBST) plus 3% fatty-acid (FA)-free BSA (Akron Biotechnology). The indicated concentration of protein in TBST plus 3% FA-free BSA was incubated at room temperature for 1 h. Membrane was washed three times in TBST plus 3% FA-free BSA for 10 min each between protein and antibody incubations. The primary antibody was incubated at 4°C overnight: rabbit anti-His6 (1:1000, ab137839, Abcam). The secondary antibody was incubated for 1 h at room temperature: IRDye 680RD goat anti-rabbit-IgG antibody (926-68171, Li-Cor, Lincoln). Membranes were imaged using the ChemiDoc MP Imaging System (Bio-Rad).
Supplementary Material
Acknowledgements
The authors would like to thank Simon Watkins and Callen Wallace of the Center for Biologic Imaging for assistance with imaging and an image quantification pipeline (University of Pittsburgh School of Medicine). The isothermal titration calorimeter used in this work was purchased under NIH 1S10OD023481 (Angela Gronenborn).
Footnotes
Author contributions
Conceptualization: K.K.T., N.V.V., M.G.J.F.; Methodology: K.K.T., N.V.V., M.G.J.F.; Validation: K.K.T., M.G.J.F.; Formal analysis: K.K.T.; Investigation: K.K.T., N.V.V., B.A.T., R.R.; Resources: M.G.J.F.; Data curation: K.K.T.; Writing - original draft: K.K.T.; Writing - review & editing: K.K.T., N.V.V., R.R., M.G.J.F.; Visualization: K.K.T.; Supervision: M.G.J.F.; Project administration: M.G.J.F.; Funding acquisition: M.G.J.F.
Peer review history
The peer review history is available online at https://journals.biologists.com/jcs/lookup/doi/10.1242/jcs.259994.reviewer-comments.pdf.
References
- Babst, M., Sato, T. K., Banta, L. M. and Emr, S. D. (1997). Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16, 1820-1831. 10.1093/emboj/16.8.1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., Wendland, B., Estepa, E. J. and Emr, S. D. (1998). The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17, 2982-2993. 10.1093/emboj/17.11.2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgareh-Touze, N., Avaro, S., Rouillé, Y., Hoflack, B. and Haguenauer-Tsapis, R. (2002). Yeast Vps55p, a functional homolog of human obesity receptor gene-related protein, is involved in late endosome to vacuole trafficking. Mol. Biol. Cell 13, 1694-1708. 10.1091/mbc.01-12-0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda, M., Péli-Gulli, M. P., Bonfils, G., Panchaud, N., Urban, J., Sturgill, T. W., Loewith, R. and De Virgilio, C. (2009). The Vam6 GEF controls TORC1 by activating the EGO complex. Mol. Cell 35, 563-573. 10.1016/j.molcel.2009.06.033 [DOI] [PubMed] [Google Scholar]
- Bonfils, G., Jaquenoud, M., Bontron, S., Ostrowicz, C., Ungermann, C. and De Virgilio, C. (2012). Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol. Cell 46, 105-110. 10.1016/j.molcel.2012.02.009 [DOI] [PubMed] [Google Scholar]
- Bridges, D., Fisher, K., Zolov, S. N., Xiong, T., Inoki, K., Weisman, L. S. and Saltiel, A. R. (2012). Rab5 proteins regulate activation and localization of target of rapamycin complex 1. J. Biol. Chem. 287, 20913-20921. 10.1074/jbc.M111.334060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, A. S., Soto Diaz, S., Van Vooren, P., Godard, P., Marini, A. M. and Boeckstaens, M. (2019). Pib2-dependent feedback control of the TORC1 signaling network by the Npr1 kinase. iScience 20, 415-433. 10.1016/j.isci.2019.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, C. G. and Emr, S. D. (1998). Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell 2, 157-162. 10.1016/S1097-2765(00)80125-2 [DOI] [PubMed] [Google Scholar]
- Byfield, M. P., Murray, J. T. and Backer, J. M. (2005). hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J. Biol. Chem. 280, 33076-33082. 10.1074/jbc.M507201200 [DOI] [PubMed] [Google Scholar]
- Chen, Z., Malia, P. C., Hatakeyama, R., Nicastro, R., Hu, Z., Peli-Gulli, M. P., Gao, J., Nishimura, T., Eskes, E., Stefan, C. J.et al. (2021). TORC1 determines Fab1 lipid kinase function at signaling endosomes and vacuoles. Curr. Biol. 31, 297-309.e8. 10.1016/j.cub.2020.10.026 [DOI] [PubMed] [Google Scholar]
- Coonrod, E. M. and Stevens, T. H. (2010). The yeast vps class E mutants: the beginning of the molecular genetic analysis of multivesicular body biogenesis. Mol. Biol. Cell 21, 4057-4060. 10.1091/mbc.e09-07-0603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouloz, F., Deloche, O., Wanke, V., Cameroni, E. and De Virgilio, C. (2005). The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol. Cell 19, 15-26. 10.1016/j.molcel.2005.05.020 [DOI] [PubMed] [Google Scholar]
- Dubreuil, B., Sass, E., Nadav, Y., Heidenreich, M., Georgeson, J. M., Weill, U., Duan, Y., Meurer, M., Schuldiner, M., Knop, M.et al. (2019). YeastRGB: comparing the abundance and localization of yeast proteins across cells and libraries. Nucleic Acids Res. 47, D1245-D1249. 10.1093/nar/gky941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, J. J., Merithew, E., Sudharshan, E., Rajamani, D., Hayes, S., Lawe, D., Corvera, S. and Lambright, D. G. (2001). Multivalent endosome targeting by homodimeric EEA1. Mol. Cell 8, 947-958. 10.1016/S1097-2765(01)00385-9 [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792-1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaullier, J. M., Rønning, E., Gillooly, D. J. and Stenmark, H. (2000). Interaction of the EEA1 FYVE finger with phosphatidylinositol 3-phosphate and early endosomes. Role of conserved residues. J. Biol. Chem. 275, 24595-24600. 10.1074/jbc.M906554199 [DOI] [PubMed] [Google Scholar]
- González, A. and Hall, M. N. (2017). Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 36, 397-408. 10.15252/embj.201696010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González, A., Shimobayashi, M., Eisenberg, T., Merle, D. A., Pendl, T., Hall, M. N. and Moustafa, T. (2015). TORC1 promotes phosphorylation of ribosomal protein S6 via the AGC kinase Ypk3 in Saccharomyces cerevisiae. PLoS One 10, e0120250. 10.1371/journal.pone.0120250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, J., Stromhaug, P. E., George, M. D., Habibzadegah-Tari, P., Bevan, A., Dunn, W. A., Jr. and Klionsky, D. J. (2001). Cvt18/Gsa12 is required for cytoplasm-to-vacuole transport, pexophagy, and autophagy in Saccharomyces cerevisiae and Pichia pastoris. Mol. Biol. Cell 12, 3821-3838. 10.1091/mbc.12.12.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama, R. (2021). Pib2 as an emerging master regulator of yeast TORC1. Biomolecules 11, 1489. 10.3390/biom11101489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama, R. and De Virgilio, C. (2019). A spatially and functionally distinct pool of TORC1 defines signaling endosomes in yeast. Autophagy 15, 915-916. 10.1080/15548627.2019.1580107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama, R., Péli-Gulli, M.-P., Hu, Z., Jaquenoud, M., Garcia Osuna, G. M., Sardu, A., Dengjel, J. and De Virgilio, C. (2019). Spatially distinct pools of TORC1 balance protein homeostasis. Mol. Cell 73, 325-338.e8. 10.1016/j.molcel.2018.10.040 [DOI] [PubMed] [Google Scholar]
- Holt, L. J., Tuch, B. B., Villén, J., Johnson, A. D., Gygi, S. P. and Morgan, D. O. (2009). Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science 325, 1682-1686. 10.1126/science.1172867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrell, C. R., Miller, M. L., Jensen, O. N. and Blom, N. (2007). NetPhosYeast: prediction of protein phosphorylation sites in yeast. Bioinformatics 23, 895-897. 10.1093/bioinformatics/btm020 [DOI] [PubMed] [Google Scholar]
- Jeong, J.-H., Lee, K.-H., Kim, Y.-M., Kim, D.-H., Oh, B.-H. and Kim, Y.-G. (2012). Crystal structure of the Gtr1p(GTP)-Gtr2p(GDP) protein complex reveals large structural rearrangements triggered by GTP-to-GDP conversion. J. Biol. Chem. 287, 29648-29653. 10.1074/jbc.C112.384420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, H., Park, J., Kim, H. I., Lee, M., Ko, Y. J., Lee, S., Jun, Y. and Lee, C. (2017). Mechanistic insight into the nucleus-vacuole junction based on the Vac8p-Nvj1p crystal structure. Proc. Natl. Acad. Sci. USA 114, E4539-E4548. 10.1073/pnas.1701030114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., Tunyasuvunakool, K., Bates, R., Zidek, A., Potapenko, A.et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583-589. 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada, Y., Yoshino, K., Kondo, C., Kawamata, T., Oshiro, N., Yonezawa, K. and Ohsumi, Y. (2010). Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell. Biol. 30, 1049-1058. 10.1128/MCB.01344-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, A. and Cunningham, K. W. (2015). A LAPF/phafin1-like protein regulates TORC1 and lysosomal membrane permeabilization in response to endoplasmic reticulum membrane stress. Mol. Biol. Cell 26, 4631-4645. 10.1091/mbc.E15-08-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira, S., Kumano, Y., Ukai, H., Takeda, E., Matsuura, A. and Noda, T. (2016). Dynamic relocation of the TORC1-Gtr1/2-Ego1/2/3 complex is regulated by Gtr1 and Gtr2. Mol. Biol. Cell 27, 382-396. 10.1091/mbc.e15-07-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutateladze, T. and Overduin, M. (2001). Structural mechanism of endosome docking by the FYVE domain. Science 291, 1793-1796. 10.1126/science.291.5509.1793 [DOI] [PubMed] [Google Scholar]
- Kutateladze, T. G., Ogburn, K. D., Watson, W. T., de Beer, T., Emr, S. D., Burd, C. G. and Overduin, M. (1999). Phosphatidylinositol 3-phosphate recognition by the FYVE domain. Mol. Cell 3, 805-811. 10.1016/S1097-2765(01)80013-7 [DOI] [PubMed] [Google Scholar]
- Loewith, R. and Hall, M. N. (2011). Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189, 1177-1201. 10.1534/genetics.111.133363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewith, R., Jacinto, E., Wullschleger, S., Lorberg, A., Crespo, J. L., Bonenfant, D., Oppliger, W., Jenoe, P. and Hall, M. N. (2002). Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457-468. 10.1016/S1097-2765(02)00636-6 [DOI] [PubMed] [Google Scholar]
- MacGurn, J. A., Hsu, P. C., Smolka, M. B. and Emr, S. D. (2011). TORC1 regulates endocytosis via Npr1-mediated phosphoinhibition of a ubiquitin ligase adaptor. Cell 147, 1104-1117. 10.1016/j.cell.2011.09.054 [DOI] [PubMed] [Google Scholar]
- Michel, A. H., Hatakeyama, R., Kimmig, P., Arter, M., Peter, M., Matos, J., De Virgilio, C. and Kornmann, B. (2017). Functional mapping of yeast genomes by saturated transposition. Elife 6, e23570. 10.7554/eLife.23570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen, J. I., Krick, R., Prick, T., Thumm, M. and Goldfarb, D. S. (2009). Measuring piecemeal microautophagy of the nucleus in Saccharomyces cerevisiae. Autophagy 5, 75-81. 10.4161/auto.5.1.7181 [DOI] [PubMed] [Google Scholar]
- Nakashima, N., Noguchi, E. and Nishimoto, T. (1999). Saccharomyces cerevisiae putative G protein, Gtr1p, which forms complexes with itself and a novel protein designated as Gtr2p, negatively regulates the Ran/Gsp1p G protein cycle through Gtr2p. Genetics 152, 853-867. 10.1093/genetics/152.3.853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobukuni, T., Joaquin, M., Roccio, M., Dann, S. G., Kim, S. Y., Gulati, P., Byfield, M. P., Backer, J. M., Natt, F., Bos, J. L.et al. (2005). Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl. Acad. Sci. USA 102, 14238-14243. 10.1073/pnas.0506925102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchaud, N., Peli-Gulli, M. P. and De Virgilio, C. (2013). Amino acid deprivation inhibits TORC1 through a GTPase-activating protein complex for the rag family GTPase Gtr1. Sci. Signal. 6, ra42. 10.1126/scisignal.2004112 [DOI] [PubMed] [Google Scholar]
- Parsons, A. B., Brost, R. L., Ding, H., Li, Z., Zhang, C., Sheikh, B., Brown, G. W., Kane, P. M., Hughes, T. R. and Boone, C. (2004). Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nat. Biotechnol. 22, 62-69. 10.1038/nbt919 [DOI] [PubMed] [Google Scholar]
- Peli-Gulli, M. P., Sardu, A., Panchaud, N., Raucci, S. and De Virgilio, C. (2015). Amino acids stimulate TORC1 through Lst4-Lst7, a GTPase-activating protein complex for the rag family GTPase Gtr2. Cell Rep. 13, 1-7. 10.1016/j.celrep.2015.08.059 [DOI] [PubMed] [Google Scholar]
- Powis, K., Zhang, T., Panchaud, N., Wang, R., De Virgilio, C. and Ding, J. (2015). Crystal structure of the Ego1-Ego2-Ego3 complex and its role in promoting Rag GTPase-dependent TORC1 signaling. Cell Res. 25, 1043-1059. 10.1038/cr.2015.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouteau, M., Desfosses, A., Sieben, C., Bourgoint, C., Lydia Mozaffari, N., Demurtas, D., Mitra, A. K., Guichard, P., Manley, S. and Loewith, R. (2017). TORC1 organized in inhibited domains (TOROIDs) regulate TORC1 activity. Nature 550, 265-269. 10.1038/nature24021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, C. K., Howald-Stevenson, I., Vater, C. A. and Stevens, T. H. (1992). Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3, 1389-1402. 10.1091/mbc.3.12.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke, A., Anderson, S., McCaffery, J. M., Yates, J., 3rd, Aronova, S., Chu, S., Fairclough, S., Iverson, C., Wedaman, K. P. and Powers, T. (2004). TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. J. Biol. Chem. 279, 14752-14762. 10.1074/jbc.M313062200 [DOI] [PubMed] [Google Scholar]
- Robert, X. and Gouet, P. (2014). Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42, W320-W324. 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton, R. A. and Sabatini, D. M. (2017). mTOR signaling in growth, metabolism, and disease. Cell 168, 960-976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B.et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676-682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter, C., Lam, K. K., Brumm, J., Wu, B. W., Saunders, M., Stevens, T. H., Bryan, J. and Conibear, E. (2008). Global analysis of yeast endosomal transport identifies the vps55/68 sorting complex. Mol. Biol. Cell 19, 1282-1294. 10.1091/mbc.e07-07-0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seron, K., Couturier, C., Belouzard, S., Bacart, J., Monté, D., Corset, L., Bocquet, O., Dam, J., Vauthier, V., Lecœur, C.et al. (2011). Endospanins regulate a postinternalization step of the leptin receptor endocytic pathway. J. Biol. Chem. 286, 17968-17981. 10.1074/jbc.M111.224857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, M. A. and Thorn, K. S. (2004). Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21, 661-670. 10.1002/yea.1130 [DOI] [PubMed] [Google Scholar]
- Shin, M. E., Ogburn, K. D., Varban, O. A., Gilbert, P. M. and Burd, C. G. (2001). FYVE domain targets Pib1p ubiquitin ligase to endosome and vacuolar membranes. J. Biol. Chem. 276, 41388-41393. 10.1074/jbc.M105665200 [DOI] [PubMed] [Google Scholar]
- Shintani, T. and Klionsky, D. J. (2004). Cargo proteins facilitate the formation of transport vesicles in the cytoplasm to vacuole targeting pathway. J. Biol. Chem. 279, 29889-29894. 10.1074/jbc.M404399200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., Aasland, R. and Driscoll, P. C. (2002). The phosphatidylinositol 3-phosphate-binding FYVE finger. FEBS Lett. 513, 77-84. 10.1016/S0014-5793(01)03308-7 [DOI] [PubMed] [Google Scholar]
- Stracka, D., Jozefczuk, S., Rudroff, F., Sauer, U. and Hall, M. N. (2014). Nitrogen source activates TOR (target of rapamycin) complex 1 via glutamine and independently of Gtr/Rag proteins. J. Biol. Chem. 289, 25010-25020. 10.1074/jbc.M114.574335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill, T. W., Cohen, A., Diefenbacher, M., Trautwein, M., Martin, D. E. and Hall, M. N. (2008). TOR1 and TOR2 have distinct locations in live cells. Eukaryot. Cell 7, 1819-1830. 10.1128/EC.00088-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, K., Dietrich, L. E., Hou, H., LaGrassa, T. J., Meiringer, C. T. and Ungermann, C. (2006). Palmitoylation determines the function of Vac8 at the yeast vacuole. J. Cell Sci. 119, 2477-2485. 10.1242/jcs.02972 [DOI] [PubMed] [Google Scholar]
- Sullivan, A., Wallace, R. L., Wellington, R., Luo, X. and Capaldi, A. P. (2019). Multilayered regulation of TORC1-body formation in budding yeast. Mol. Biol. Cell 30, 400-410. 10.1091/mbc.E18-05-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, D., Varlakhanova, N. V., Tornabene, B. A., Ramachandran, R., Zhang, P. and Ford, M. G. J. (2020). The cryo-EM structure of the SNX-BAR Mvp1 tetramer. Nat. Commun. 11, 1506. 10.1038/s41467-020-15110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, S. W., Oishi, A., Nikulin, N., Jorgensen, J. R., Baile, M. G. and Emr, S. D. (2021). A PX-BAR protein Mvp1/SNX8 and a dynamin-like GTPase Vps1 drive endosomal recycling. Elife 10, e69883. 10.7554/eLife.69883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa, M. and Maeda, T. (2017). An in vitro TORC1 kinase assay that recapitulates the Gtr-independent glutamine-responsive TORC1 activation mechanism on yeast vacuoles. Mol. Cell. Biol. 37, e00075-17. 10.1128/MCB.00075-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa, M., Yamamoto, K., Nagatoishi, S., Nagata, K., Noshiro, D., Noda, N. N., Tsumoto, K. and Maeda, T. (2021). A glutamine sensor that directly activates TORC1. Commun. Biol. 4, 1093. 10.1038/s42003-021-02625-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov, K., Messier, V., Landry, C. R., Radinovic, S., Serna Molina, M. M., Shames, I., Malitskaya, Y., Vogel, J., Bussey, H. and Michnick, S. W. (2008). An in vivo map of the yeast protein interactome. Science 320, 1465-1470. 10.1126/science.1153878 [DOI] [PubMed] [Google Scholar]
- Ukai, H., Araki, Y., Kira, S., Oikawa, Y., May, A. I. and Noda, T. (2018). Gtr/Ego-independent TORC1 activation is achieved through a glutamine-sensitive interaction with Pib2 on the vacuolar membrane. PLoS Genet. 14, e1007334. 10.1371/journal.pgen.1007334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, J., Soulard, A., Huber, A., Lippman, S., Mukhopadhyay, D., Deloche, O., Wanke, V., Anrather, D., Ammerer, G., Riezman, H.et al. (2007). Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 26, 663-674. 10.1016/j.molcel.2007.04.020 [DOI] [PubMed] [Google Scholar]
- Varlakhanova, N. V., Mihalevic, M. J., Bernstein, K. A. and Ford, M. G. J. (2017). Pib2 and the EGO complex are both required for activation of TORC1. J. Cell Sci. 130, 3878-3890. 10.1242/jcs.207910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedaman, K. P., Reinke, A., Anderson, S., Yates, J., 3rd, McCaffery, J. M. and Powers, T. (2003). Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 1204-1220. 10.1091/mbc.e02-09-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerlikaya, S., Meusburger, M., Kumari, R., Huber, A., Anrather, D., Costanzo, M., Boone, C., Ammerer, G., Baranov, P. V. and Loewith, R. (2016). TORC1 and TORC2 work together to regulate ribosomal protein S6 phosphorylation in Saccharomyces cerevisiae. Mol. Biol. Cell 27, 397-409. 10.1091/mbc.e15-08-0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, M. S., Du, G., Backer, J. M., Frohman, M. A. and Chen, J. (2011). Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J. Cell Biol. 195, 435-447. 10.1083/jcb.201107033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, M. S., Son, K., Arauz, E., Han, J. M., Kim, S. and Chen, J. (2016). Leucyl-tRNA synthetase activates Vps34 in amino acid-sensing mTORC1 signaling. Cell Rep. 16, 1510-1517. 10.1016/j.celrep.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.