Abstract
Introduction
Real-world data on COVID-19 vaccine effectiveness are needed to validate evidence from randomized clinical trials. Accordingly, this study aims to evaluate, in a real-world setting in Brazil, the effectiveness of Pfizer-BioNTech BNT162b2 against symptomatic COVID-19 and COVID-19-related complications across diverse populations.
Materials and methods
A test-negative case-control study with follow-up of cases is currently being conducted in Toledo, a city in southern Brazil, following a mass COVID-19 vaccination campaign with BNT162b2. The study is being conducted among patients aged 12 years or older seeking care in the public health system with acute respiratory symptoms and tested for SARS-CoV-2 on reverse transcription polymerase chain reaction (RT-PCR). Cases are RT-PCR positive and controls RT-PCR negative. Test-positive cases are prospectively followed through structured telephone interviews performed at 15 days post-enrollment, and at 1, 3, 6, 9 and 12 months. Baseline demographic, clinical, and vaccination data are being collected by means of structured interviews and medical registry records reviews at the time of enrollment. All RT-PCR-positive samples are screened for mutations to identify SARS-CoV-2 variants.
Ethics and dissemination
The study protocol has been approved by the research ethics committee of all participant sites. Study findings will be disseminated through peer-reviewed publications and conference presentations.
Trail registration
Clinicatrials.gov: NCT05052307.
Introduction
COVID-19, caused by SARS-CoV-2 infection, has affected millions of people around the world. In Brazil, the number of cases has surpassed 28 million, with more than 640,000 deaths from COVID-19 by February 2022 [1]. Therefore, mass-vaccination campaigns using approved COVID-19 vaccines have become a health priority [2].
The Pfizer-BioNTech mRNA COVID-19 vaccine BNT162b2 was approved on June 11, 2021 by the Brazilian National Health Surveillance Agency (ANVISA) for persons 12 years of age and older [3]. A randomized, controlled trial involving 43,448 participants aged 16 years or older showed that a two-dose 21-days apart regimen of BNT162b2 demonstrated 95% efficacy (95% confidence interval [CI], 90% to 98%) against laboratory-confirmed SARS-CoV-2 infection [4]. Related adverse events were reported by 21% of participants (mainly pain at the injection site, fatigue and headache), and were mostly mild-to-moderate in severity. A subsequent randomized, controlled trial involving 2,260 adolescents aged 12 to 16 years showed that a two-dose 21-days apart regimen of the BNT162b2 vaccine demonstrated 100% efficacy (95% CI, 75% to 100%) against laboratory-confirmed SARS-CoV-2 infection, with a safety profile characterized mainly by mild-to-moderate injection-site pain, fatigue and headache [5]. However, evidence regarding effectiveness of BNT162b2 in Brazil, especially against new variants of concern such as Omicron (currently the most prevalent lineage) [6] is limited; likewise, the long-term protection conferred by BNT162b2 vaccination and impact on COVID-19 severity and prolonged symptoms are not yet available.
Objectives
Primary objective
The primary aim of this study is to assess the effectiveness of BNT162b2 in preventing symptomatic COVID-19 in a real-world setting in Brazil.
Secondary objectives
The secondary objectives are to assess the impact of BNT162b2 on: (1) symptomatic SARS-CoV-2 infection caused by circulating variants (e.g., Delta, and Omicron); (2) duration of COVID-19 symptoms; (3) hospitalization for COVID-19; (4) need for ICU admission; (5) need for invasive mechanical ventilation; (6) death from COVID-19; (7) health-related quality of life; (8) persistent COVID-19 symptoms; and (9) symptomatic SARS-CoV-2 reinfection.
Materials and methods
This study protocol was registered at Clinicaltrials.gov before the enrollment of the first participant (NCT05052307).
Study setting
This study has been conducted in Toledo (24° 42′ 50″ S, 53° 44′ 34″ W), a city in the state of Paraná, southern Brazil. The population of Toledo was estimated at 144,601 people in 2021. As of 05 February 2022, COVID-19 has affected 38,614 people with 478 deaths. The city secretary of health initiated an age-based COVID-19 vaccination campaign on 20 January 2021, starting with adults 16 years of age and older. A mass vaccination campaign using BNT162b2 for all nonimmunized individuals aged ≥ 12 years was initiated on 27 August 2021 using a two-dose schedule administered 21 days apart. A third dose (i.e., booster dose) of BNT162b2 has been recommended for healthy (according to an age-based prioritization strategy) and immunocompromised individuals aged ≥ 12 years since 15 September 2021. Other available COVID-19 vaccines in Toledo include CoronaVac (Sinovac), ChAdOx1 nCoV-19 (AstraZeneca), and Ad26.COV2.S (Janssen); around 50% of the vaccine-eligible individuals immunized against COVID-19 have received BNT162b2.
Toledo was chosen due to the city standard of care, which provides free healthcare access to a wide variety of COVID-19 diagnosis and management services, and due to the feasibility of accessing information on people who were tested for SARS-CoV-2 through the city surveillance system.
Study design
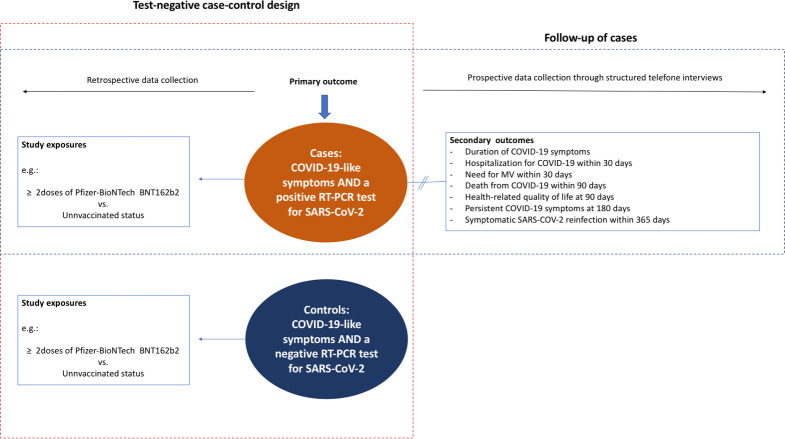
The present study is a test-negative case-control study with follow-up of cases (Fig 1). In this design, the same clinical case definition is used for both cases (i.e., participants with symptoms consistent with COVID-19) and controls (i.e., participants without the outcome) [11]. Reverse transcription polymerase chain reaction (RT-PCR) test for SARS-CoV-2 is prospectively used to distinguish cases from controls. Vaccination status and potential confounders are retrospectively ascertained among cases and controls through structured interviews and medical records review. Test-positive cases are prospectively followed through structured telephone interviews performed at 15 days, and at 1, 3, 6, 9 and 12 months.
Fig 1. Study design.
MV, mechanical ventilation; RT-PCR, reverse transcriptase polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Participants
The present study population is composed of consecutive individuals aged 12 years or older who seek the public healthcare system of Toledo with symptoms suggestive of COVID-19.
Inclusion criteria are (1) age ≥ 12 years old; (2) to be a resident of Toledo; (3) seeking care in the public healthcare system of Toledo with symptoms suggestive of COVID-19 defined as follows: acute respiratory infection symptoms (nasal congestion, rhinorrhea, anosmia, sore throat, hoarseness, new or increased-from-baseline cough, sputum production, dyspnea, wheezing, myalgia) OR admitting diagnosis suggestive of acute respiratory infection symptoms (pneumonia, upper respiratory infection, bronchitis, influenza, cough, asthma, viral respiratory illness, respiratory distress, respiratory failure), and (4) having a nasopharyngeal (NP) or nasal sample for SARS-CoV-2 diagnosis obtained as standard of care.
Patients with history of remdesivir treatment in the past 30 days, convalescent plasma treatment in the past 90 days, treatment with monoclonal antibodies for COVID-19 in the past 90 days (e.g., sotrovimab, casirivimab-imdevimab, bamlanivimab, etesivumab), and those who refused to provide informed consent are excluded. Participants are allowed to re-screen; however, the follow-up is performed considering only the first enrollment of each individual for the study.
Exposures
The primary exposure is the receipt of two or more doses of BNT162b2 according to the Brazilian National Immunization Program (BNIP) recommendations (with the receipt of the second dose 7 days or more before the onset of symptoms of COVID-19-like illness).
Secondary exposures are as follows: (1) receipt of one or more doses of BNT162b2 (with the receipt of the first dose 14 days or more before the onset of COVID-19-like symptoms); (2) receipt of one dose of BNT162b2 (with the receipt of dose, 14 days or more before the onset of COVID-19-like symptoms); (3) receipt of two doses of BNT162b2 (with the receipt of the second dose 7 days or more before the onset of COVID-19-like symptoms); (4) receipt of three doses of BNT162b2 (with the receipt of the third dose 7 days or more before the onset of COVID-19-like symptoms); (5) complete vaccination with other available COVID-19 vaccines in Toledo (CoronaVac [Sinovac], ChAdOx1 nCoV-19 [AstraZeneca], and Ad26.COV2.S [Janssen]) according to Brazil National Immunization Program (BNIP) recommendations.
The reference exposure group is composed of participants who had not received a first vaccine dose before the date of sample collection.
Outcomes
The primary outcome is symptomatic COVID-19, defined by the presence of at least one COVID-19-like symptom as listed in Participants section and confirmed by a positive RT-PCR result for SARS-CoV-2 infection from participants’ NP or nasal swabs.
Secondary outcomes are: (1) symptomatic SARS-CoV-2 infection associated with circulating variants (e.g., Delta, and Omicron); (2) duration of COVID-19 symptoms; (3) hospitalization for COVID-19 within 30 days from enrollment among patients with confirmed COVD-19; (4) need for ICU admission within 30 days from enrollment among patients with confirmed COVD-19; (5) need for invasive mechanical ventilation within 30 days from enrollment among patients with confirmed COVD-19; (6) death from COVID-19 within 90 days from enrollment among patients with confirmed COVD-19; (7) health-related quality of life at 90 days from enrollment among patients with confirmed COVD-19, measured by the three-level version of the EuroQol five-dimensional descriptive system (range, -0.17 [worst] to 1.0 [best]; minimal important difference, 0.03) [7, 8]; (8) persistent COVID-19 symptoms at 180 days from enrollment among patients with confirmed COVD-19; (9) symptomatic SARS-CoV-2 reinfection within 365 days from enrollment among patients with confirmed COVD-19, defined as recurrence of COVID-19-like symptoms confirmed by a positive RT-PCR or antigen test for SARS-CoV-2 more than 90 days after primary infection.
Safety data including adverse events and serious adverse events will be assessed upon inclusion of cases and controls and over the 12-month follow-up for cases, under the standard of care procedures for the city of Toledo’s surveillance program per Brazilian regulatory authority’s requirements.
Sample size
The sample size was calculated based on formulas published by O’Neill RT [9]. Assuming the recruitment of two controls per case, a vaccine coverage rate of 90% among controls, a sample of 622 cases and 1,244 controls will be required to detect a vaccine effectiveness of 80% with a precision width of 10%. Considering that around 50% of enrolled participants will fulfil criteria for the primary analysis (≥ 2 doses of BNT162b2 or unvaccinated) and increasing the sample size by 20% (due to parameter uncertainties, potential COVID-19 misclassifications, and need for covariate adjustment), the study will need to include 1,500 cases and 3,000 controls.
Data collection
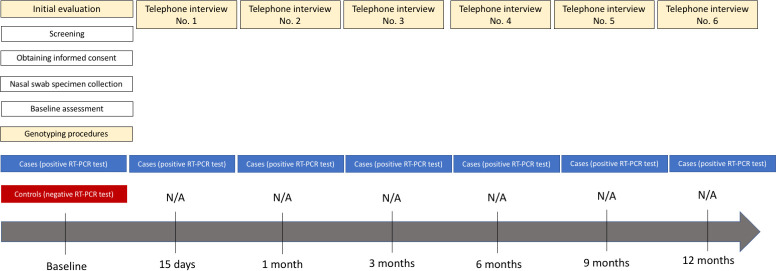
The schedule of enrollment and assessment is detailed in Fig 2. Controls are evaluated at a single point in time (baseline). Cases are evaluated at baseline and then followed up for 12 months by means of structured telephone interviews at 15 days, and at 1, 3, 6, 9 and 12 months after inclusion in the study.
Fig 2. Schedule of enrollment and assessment.
N/A. Not appliable; RT-PCR, reverse transcriptase polymerase chain reaction.
Baseline assessment is performed by trained researchers at each participating site at the time an eligible participant with symptoms of COVID-19-like illness seeks care through the public healthcare system. The vaccination status is assessed through structured interviews and adjudicated through review of city medical registry records reviews. Telephone follow-up is conducted via a single central telephone system located in the study coordinating centre (Hospital Moinhos de Vento). Therefore, all participants who are eligible for telephone follow-up (cases), regardless of the site of inclusion, are followed up by researchers trained in good clinical practices and in standardized telephone interview methods. The reference date for scheduling telephone interviews is the date of inclusion in the study. Interviews are considered lost if the participants’ telephone lines are disconnected or non-existent or after 10 attempts at different times on several days within a 5-day time window (5 days after the estimated interview date) for the 15-day interview, and 15-day time window (15 days after the estimated interview date) for the 1-, 3-,6-,9-, and 12-month interviews. Regardless of whether an interview is lost or not, contact is attempted at the subsequent scheduled interviews. This follow-up methodology has been used in previous observational studies [10, 11].
The data are collected using smartphone-, tablet- or computer-based electronic case report forms available on the Otus platform (https://site.otus-solutions.com.br/). Access to databases on this platform are granted only by the principal investigator to research team members through a personal, non-transferable username and password. An encrypted digital certificate is used to ensure security in data handling. Platform users (team members) have specific permissions related to their role in the study.
The following procedures have been performed to ensure the quality of the data collected: (1) all researchers have received training in good clinical practices and study procedures, including data collection, before the start of the study; (2) trained personnel at the participating sites are able to contact the study coordinating centre to resolve any questions or problems that they might have; (3) the database is backed up automatically every 12 hours; (4) telephone interviews are recorded and audited to verify data consistency, and the audio files are stored on a server following the same security standards as for the Otus platform database; (5) data cleaning is conducted periodically to identify potential inconsistencies in data sets, in which case the researchers are notified for correction; and (6) the coordinating centre have been performing remote monitoring of data quality, revision of detailed reports on screening, inclusion, follow-up, data consistency and completeness on a monthly basis, taking immediate action to resolve any issues, and have been using statistical techniques for identifying fraud throughout the study.
Collection of biological samples, and diagnostic procedures
A NP or nasal swab sample is collected from all participants for SARS-CoV-2 RT-PCR test and identification of the implicated genetic variant of SARS-CoV-2 at the time of enrollment. This collection is performed by a trained healthcare provider as part of the local policy for surveillance of individuals with suspected or confirmed diagnosis of COVID-19.
All biological materials collected in the study are transported, stored and processed by the Federal University of Paraná (UFPR) according to standardized protocols. NP or nasal swabs are stored in 15 mL falcon tubes containing viral transport medium (VTM) at -80°C, until RT-PCR testing for SARS-CoV-2 and identification of the genetic variants of SARS-CoV-2 are performed.
RT-PCR procedures
Total RNA is extracted from the specimens using a viral RNA and DNA kit (MVXA-P096FAST) in an automatic nucleic acid extractor (Extracta 32, Loccus Biotecnologia, Brazil), using magnetic beads. The specimens are then tested to confirm the presence of SARS-CoV-2 genetic material using the BIOMOL OneStep/COVID-19 KIT (IBMP, Curitiba, Brazil) or using the Centers for Disease Control and Prevention 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel (https://www.fda.gov/media/134922/download). This approach has demonstrated high specificity (100%) and sensitivity (88% to 98%) for COVID-19 diagnosis [12, 13].
Variant identification
All confirmed positive samples are screened for mutations by RT-PCR to detect SARS-CoV-2 variants as follows: Initially, the multiplex RT-PCR method is performed to detect the Spike Δ69–70 and ORF1a Δ3675–3677 deletions for distinguishing Alpha, Beta/Gamma, wild type/Delta, and Omicron variants (Table 1) [14, 15]. Secondly, the predicted variant is confirmed by two allelic discrimination TaqMan assays (ThermoFisher, USA), which include: K417T, K417N, N501Y, P681R, G339D (Table 2). These assays allow to identify the variants of concern: Alpha (N501Y), Beta (K417N, N501Y), Gamma (K417T, N501Y), Delta (P681R), Omicron BA.1 (N501Y, G339D), and Omicron BA.2 (N501Y, G339D, K417T/N).
Table 1. Interpretation of the multiplex RT-PCR for Spike del69/70, Orf1a and N gene.
| Spike Del69/70 | Orf1a Del3675/3677 | N gene | Putative variant |
|---|---|---|---|
| ND | ND | + | Alpha |
| + | ND | + | Beta/Gamma/BA.2 |
| + | + | + | Wild-type/Delta |
| ND | + | + | Omicron BA.1 |
ND: non detected (cycle threshold [CT] value ˃ 40.0); +: positive (CT value ≤ 40.0).
Table 2. Identification of SARS-CoV-2 variants by two allelic discrimination assays.
| Multiplex assay indication | Two allelic discrimination TaqMan assay | RT-PCR Results | Variant detected |
|---|---|---|---|
| Alpha | K417T | K417T: wt | Alpha |
| N501Y | N501Y: mut | ||
| Beta/Gamma/Omicron BA.2 | G339D | G339D: wt | Beta |
| K417T/N | K417N: mut | ||
| N501Y | N501Y: mut | ||
| G339D: wt | Gamma | ||
| K417T: mut | |||
| N501: mut | |||
| G339D: mut | Omicron BA.2 | ||
| K417T/N: wt | |||
| N501Y: mut | |||
| Wild/Delta | N501Y | N501Y: wt | Wild-type |
| P618R | P681R: wt | ||
| N501Y: wt | Delta | ||
| P681R: mut | |||
| Omicron BA.1 | G339D | G339D: mut | Omicron BA.1 |
| N501Y | N501Y: mut |
wt: wild-type allele amplification; mut: mutated allele amplification. Any different profile will be classified as “inconclusive”.
Assays are performed using the GoTaq Probe 1-Step RT-qPCR System (Promega) in a 7500 Fast or QuantStudio5 real-time thermocycler (ThermoFisher Scientific Inc., USA), as described by Adamoski et al [16].
Statistical analysis plan
Overall principles
Analysis will start once all data for the last included participant have been obtained, the database has been cleaned and locked, and the study protocol has been submitted for publication. All analyses will be carried out using the R statistical software (R Development Core Team–version to be determined at time of analysis) [17]. Estimate effects will be reported with 95% CIs. A significance level of 0.05 will be used for all statistical comparisons. Secondary analyses and subgroup analyses will not be adjusted for multiple comparisons. They should, therefore, be interpreted as exploratory.
Handling missing data
No data imputation will be performed for main exposure (vaccination status) and for the study outcome. Main analysis will consist of observed data, without imputation. For sensitivity analysis we will perform multiple imputation if missing data on core variables is > 5%, under the assumption that the missingness pattern is missing at random conditional on the observed data. Core variables are defined as the covariates to be used in the primary analysis: age, gender, Charlson comorbidity index [18], current smoking status, and body mass index (BMI) category.
Participant flow
The flow of participants will be displayed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) flow diagram (STROBE 2007 statement) [19]. This description will include information about the number of screened for enrollment, reasons for non-participation, number of actually enrolled, and number of analyzed.
Baseline characteristics
The baseline characteristics of participants will be presented for the entire study population, test-positive cases, and test-negative controls. Categorical variables will be described as absolute and relative frequencies. Continuous variables will be expressed as mean (standard deviation) for variables with symmetric distribution, and as median (interquartile range) for variables with asymmetric distribution. The symmetry of continuous variables will be checked using graphical analysis of histograms and by the Shapiro-Wilk test.
Primary outcome analysis
The primary study analysis compares the vaccine effectiveness for symptomatic SARS-CoV-2 infection of participants vaccinated with two or more doses of BNT162b2 mRNA and participants never vaccinated. Logistic regression adjusted by age, gender, Charlson comorbidity index, BMI, and current smoking status will be used to estimate the odds ratio of vaccination among test positive cases and test negative controls. We also will incorporate in the model the time (number of days) elapsed between the study start and date of RT-PCR sample collection as a proxy of temporal trend. The reference group for vaccination status will be those who had not received a first vaccine dose before the date of sample collection. The estimate of vaccine effectiveness will be provided by 1 –odds ratio x 100 under the assumptions of a test negative design [20]. 95% CIs will be calculated using the Wald method.
Sensitivity analyses for the primary outcome
The following sensitivity analyses will be conducted to assess consistency and risk of bias: (1) performing multiple imputation for missing core variables data (see handling missing data section); (2) considering only the first enrollment of each individual for the study; (3) excluding as controls those with less than 5 days of symptom onset.
Subgroup analyses for the primary outcome
Subgroup analyses, by means of interaction tests, will be conducted to explore potential differences in vaccine effectiveness for symptomatic SARS-CoV-2 infection by age group (12–17, 18–64, and ≥ 65 years), BMI categories, cardiovascular disease (composite of heart failure, ischemic heart disease, cerebrovascular disease, and peripheral arterial disease), and Charlson Comorbidity index ≥ 2.
Secondary outcome analyses
The association between the exposition variables with symptomatic SARS-CoV-2 infection caused by circulating variants as well as the association between secondary exposition variables with symptomatic SARS-CoV-2 infection will be assessed using the same statistical model used for the primary outcome.
Crude and adjusted generalized linear models will be used to assess the impact of exposures on duration of COVID-19 symptoms, hospitalisation for COVID-19, need for ICU admission, need for invasive mechanical ventilation, death from COVID-19, health-related quality of life, persistent COVID-19 symptoms, symptomatic SARS-CoV-2 reinfection among participants with a positive RT-PCR result for SARS-CoV-2, and to test associations between length of time after receipt of the second or third dose of BNT162b2 and the risk of symptomatic SARS-CoV-2 infection, according to the probability distribution of each outcome. Quantitative outcomes will be expressed as mean difference. Categorical outcomes will be expressed as hazard ratio or relative risk as appropriate.
Ethics and dissemination
The study complies with Brazilian National Health Council Resolution no. 466/12 [21]. The study protocol has been approved by the National Commission of Research Ethics (CONEP; CAAE, 50293121.9.0000.5330; opinion number, 4.911,499) and research ethics committee of all participant sites. All participants provide informed consent to participate prior to inclusion in the study. Participants younger than 18 years also provide written informed assent. For persons who lack the cognitive or physical conditions necessary to provide consent, consent to participation is obtained from family proxies.
Results from this study will be disseminated through peer-reviewed publications and conference presentations, addressing primary objective and secondary objectives. Authorship will be defined according to the International Committee of Medical Journal Editors (ICMJE) recommendations [22].
Study status
The study design and protocol were finalised in October 2021. The first participant was included on November 3, 2021. At study start, approximately 2/3 of the target population was fully vaccinated. Two thousand and thirty-four participants were enrolled up to February 2, 2022. Currently, this study is recruiting participants in 3 referral COVID-19 sites in Toledo. We anticipate that the recruitment will be completed in July 2022.
Protocol review and amendments
Sample size review
According to the original sample size estimation, 2,979 participants (993 cases and 1,986 controls) would be required to detect an odds ratio ≤ 0.70 for the primary outcome analysis, assuming the recruitment of two controls per case, a rate of complete vaccination with the Pfizer-BioNTech BNT162b2 mRNA vaccine of 60% in the control group, a two-tailed alpha of 5%, a power of 80%, and an increased sample size by 30% (due to parameter uncertainties and need for covariate adjustment). In order to achieve adequate power to assess the impact of BNT162b2 on symptomatic SARS-CoV-2 infection associated with the Gamma variant (predominant SARS-COV-2 lineage at the time of study protocol development), considering a rate of 32.5% of genotyping tests with inconclusive results and a prevalence of 90% of the Gamma variant, the original sample size was determined to be 4,500 participants (1,500 cases and 3,000 controls).
In January 2022, the study sample size was revised, given the frequent variation of COVID-19 pandemic factors that could impact sample size estimation (e.g., emergence of Omicron variant, scarcity of new COVID-19 cases due to Gamma variant, increase in vaccine coverage, and vaccine effectiveness variations for distinct SARS-CoV-2 variants). Revision was made based solely on the number of recruited patients, number of cases and proportion of positive cases in the whole sample, without any additional analysis. The original study sample size (4,500 participants) was judged to provide an appropriate power to assess the primary outcome analysis as described in the sample size section.
Change in exposure definitions
Given the third vaccine dose of BNT162b2 e became part of standard of care in COVID-19 vaccination in Brazil after study approval, the primary exposure definition was changed from receipt of two doses of BNT162b2 (with the receipt of the second dose 7 days or more before the onset of symptoms of COVID-19-like illness) to receipt of two or more doses of BNT162b2 (with the receipt of the second dose 7 days or more before the onset of symptoms of COVID-19-like illness). We also added the receipt of three doses of Pfizer-BioNTech BNT162b2 mRNA vaccine (with the receipt of the third dose 7 days or more before the onset of symptoms of COVID-19-like illness) as a secondary exposure variable. No data was analysed for this protocol amendment.
Discussion
The present study has been designed as the first real-world mass vaccination city-wide study with the Pfizer-BioNTech BNT162b2 COVID-19 vaccine in Brazil. Results of this study will allow healthcare professionals, researchers, and policy makers to better understand the real-world effectiveness of BNT162b2 against prevalent variants of concern like Delta and Omicron. In addition, the present study will explore the vaccine effectiveness on relevant, but less explored, outcomes such as persistence of COVID-19 symptoms (i.e., “long COVID-19”) and occurrence symptomatic SARS-CoV-2 re-infections.
The test-negative design was chosen due to its feasibility and reliability of findings in a real-world mass vaccination context. Test-negative design studies have been extensively used to estimate vaccine effectiveness against symptomatic respiratory infections [23], including COVID-19 [24–26], while controlling for potential biases such as healthcare seeking behaviour and misclassification of COVID-19 status. Strengths of our study include the prospective ascertainment of infection status to guarantee the inclusion of case and controls with similar disease manifestation—preventing sample bias; the use of adjudication procedures to avoid recall bias of exposure factors (i.e., vaccination status); the genotyping procedures of all COVID-19 cases—allowing the assessment of vaccine effectiveness against specific SARS-CoV-2 variants of concern; and the prospective follow-up of cases to allow the evaluation of vaccine impact on COVID-19-related complications.
Our study has some limitations. First the present study design is susceptible to confounding due to differences between vaccinated and unvaccinated persons that may influence the occurrence of COVID-19. In order to minimize this potential bias, relevant risk factors for COVID-19 (both person- and time-related variables) will be used for covariate adjustment in the primary analyses. Moreover, sensitivity analyses will be conducted to assess consistency of results. Second, the sample size calculation may be inaccurate, given premises used to calculate the number of participants are highly variable based on the prevalence of COVID-19 in the study period. Third, misclassifications of cases and controls are possible, given the accuracy of RT-PCR for COVID-19 is not perfect. Nevertheless, the study protocol includes actions aimed to optimize diagnosis accuracy (e.g., exclusion of individuals at increased risk for false negative tests, use of validated RT-PCR test procedures with high sensitivity and specificity, and sensitivity analyses to assess consistency of findings under distinct test accuracy scenarios). Fourth, modifications in the standard of care regarding COVID-19 vaccination demanded changes in the definition of exposure variables to reflect the real-world practices while keeping the study procedures feasible.
Evidence regarding effectiveness of BNT162b2 in Brazil and in the southern hemisphere is limited and this study will help fill this important epidemiological gap. These data can broaden the evidence-base from clinical trials help guide effective COVID-19 vaccination policies.
Supporting information
(PDF)
Acknowledgments
The authors would like to thank the city secretary of health of Toledo, and the Brazilian National Immunization Program for their support in conducting the study.
Data Availability
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding Statement
This study has been conducted as a collaboration between Hospital Moinhos de Vento and Pfizer. Hospital Moinhos de Vento is the study sponsor. Pfizer is funding the project under its Global Corporative Policy for research grant, internal register process subscribe number 68771211. The funder contributed to the design and to the writing of the manuscript.
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 2.Hasan T, Beardsley J, Marais BJ, Nguyen TA, Fox GJ. The Implementation of Mass-Vaccination against SARS-CoV-2: A Systematic Review of Existing Strategies and Guidelines. Vaccines. 2021;9(4):326. doi: 10.3390/vaccines9040326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministério da Saúde/Agência Nacional de Vigilância Sanitária/2ª Diretoria/Gerência-Geral de Medicamentos e Produtos Biológicos. Resolução RE n° 2.324, de 10 de junho de 2021. https://www.in.gov.br/web/dou/-/resolucao-re-n-2.324-de-10-de-junho-de-2021-325295063
- 4.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385(3):239–250. doi: 10.1056/NEJMoa2107456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcantara LCJ, Nogueira E, Shuab G, Tosta S, Fristch H, Pimentel V, et al. SARS-CoV-2 epidemic in Brazil: how the displacement of variants has driven distinct epidemic waves. Virus Res. 2022. Jul 2;315:198785. doi: 10.1016/j.virusres.2022.198785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos M, Cintra MACT, Monteiro AL, Santos B, Gusmão-Filho F, Andrade MV, et al. Brazilian Valuation of EQ-5D-3L Health States: Results From a Saturation Study. Med Decis Making. 2016;36(2):253–63. doi: 10.1177/0272989X15613521 [DOI] [PubMed] [Google Scholar]
- 8.Coretti S, Ruggeri M, McNamee P. The minimum clinically important difference for EQ-5D index: a critical review. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):221–33. doi: 10.1586/14737167.2014.894462 [DOI] [PubMed] [Google Scholar]
- 9.O’Neill RT. On sample sizes to estimate the protective efficacy of a vaccine. Stat Med. 1988;7:1279–88. doi: 10.1002/sim.4780071208 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira C, Rosa RG, Sganzerla D, Sanchez EC, Robinson CC, Dietrich C, et al. The Burden of Mental Illness Among Survivors of Critical Care-Risk Factors and Impact on Quality of Life: A Multicenter Prospective Cohort Study. Chest. 2021;160(1):157–164. doi: 10.1016/j.chest.2021.02.034 [DOI] [PubMed] [Google Scholar]
- 11.Rosa RG, Falavigna M, Robinson CC, Sanchez EC, Kochhann R, Schneider D, et al. Early and Late Mortality Following Discharge From the ICU: A Multicenter Prospective Cohort Study. Crit Care Med. 2020;48(1):64–72. doi: 10.1097/CCM.0000000000004024 [DOI] [PubMed] [Google Scholar]
- 12.Freire-Paspuel B, Garcia-Bereguiain MA. Analytical sensitivity and clinical performance of a triplex RT-qPCR assay using CDC N1, N2, and RP targets for SARS-CoV-2 diagnosis. Int J Infect Dis. 2021;102:14–16. doi: 10.1016/j.ijid.2020.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukasawa LO, Sacchi CT, Gonçalves MG, Lemos APS, Almeida SCG, Caterino-de-Araujo A. Comparative performances of seven quantitative Reverse-Transcription Polymerase Chain Reaction assays (RT-qPCR) for detecting SARS-CoV-2 infection in samples from individuals suspected of COVID-19 in São Paulo, Brazil. J Clin Virol Plus. 2021;1(1):100012. doi: 10.1016/j.jcvp.2021.100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogels CBF, Breban MI, Ott IM, Alpert T, Petrone ME, Watkins AE, et al. Multiplex qPCR discriminates variants of concern to enhance global surveillance of SARS-CoV-2. PLOS Biol. 2021;19(5):e3001236. doi: 10.1371/journal.pbio.3001236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–46. doi: 10.1016/S0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamoski D, Oliveira JC, Bonatto AC, Wassem R, Nogueira MB, Raboni SM, et al. Large-Scale Screening of Asymptomatic Persons for SARS-CoV-2 Variants of Concern and Gamma Takeover, Brazil. Emerg Infect Dis. 2021;27(12):3124–3127. doi: 10.3201/eid2712.211326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. https://www.r-project.org/ [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19.Vandenbroucke JP, Elm EV, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Plos Med. 2007;4(10):e297. doi: 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol. 2016;184:345–53. doi: 10.1093/aje/kww064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conselho Nacional de Saúde (Brasil). Resolução n o 466, de 12 de dezembro de 2012. Brasília, DF, 2012. https://bvsms.saude.gov.br/bvs/saudelegis/cns/2013/res0466_12_12_2012.hml
- 22.International Committee of Medical Journal Editors. Defining the role of authors and contributors. http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.htm
- 23.Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, et al. Influenza Vaccine Effectiveness in the United States during the 2015–2016 Season. N Engl J Med. 2017;377:534–43. doi: 10.1056/NEJMoa1700153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyams C, Marlow R, Maseko Z, King J, Ward L, Fox K, et al. Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: a test-negative, case-control study. Lancet Infect Dis. 2021;21:1539–48. doi: 10.1016/S1473-3099(21)00330-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez Bernal J, Andrews N, Gower C, Robertson C, Stowe J, Tessier E, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021; 373:n1088. doi: 10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranzani OT, Hitchings MDT, Dorion M, D’Agostini TL, de Paula RC, de Paula OFP, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:n2015. doi: 10.1136/bmj.n2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.