Abstract
The ompA gene, encoding the 42-kDa major antigenic outer membrane protein OmpA of Riemerella anatipestifer, the etiololgical agent of septicemia anserum exsudativa, was cloned and expressed in Escherichia coli. Recombinant OmpA displayed a molecular mass similar to that predicted from the nucleotide sequence of the ompA gene but lower than that observed in total cell lysates of R. anatipestifer. The ompA gene showed a conserved C-terminal region comprising the OmpA-like domain and a variable N-terminal region. This structure is similar to those of the analogous outer membrane proteins of several gram-negative bacteria. However, OmpA of R. anatipestifer contains six EF-hand calcium-binding domains and two PEST regions, which distinguish it from other outer membrane proteins. The occurrence of these motifs in OmpA suggests a possible role in virulence for this protein. The ompA gene is present in the R. anatipestifer type strain and in all serotype reference strains. However, it exhibits some minor genetic heterogeneity among different serotypes, which seems not to affect the strong antigenic characteristics of the protein. OmpA is a conserved and strong antigenic determinant of R. anatipestifer and hence is suggested to be a valuable protein for the serodetection of R. anatipestifer infections, independent of their serotype.
Riemerella anatipestifer is a gram-negative, nonmotile, non-spore-forming, rod-shaped bacterium (37). It belongs to the family Flavobacteriaceae in rRNA superfamily V, based on 16S rRNA gene sequence analyses (40). It is the etiological agent of septicemia anserum exsudativa, an enzootic, contagious, often primary septicemic disease of domesticated ducklings (2, 21). The disease causes a serious problem in the duck industry and has a worldwide distribution (18). Endemic infections are restricted to commercial duck and turkey flocks, but other poultry species such as chicken and geese are also susceptible to the infection. In Singapore and other countries of southeast Asia, R. anatipestifer infection has been a continued problem in the intensive production of meat ducks since 1982 (41). Mortality and morbidity rates are usually between 10 and 30% but mortality of as high as 75% has been recorded in infected duck farms.
Slide and tube agglutination tests with antisera differentiate 21 serotypes of R. anatipestifer (23, 28). Serotypes 1, 2, 3, 5, and 15 are most prevalent in outbreaks of septicemia anserum exsudativa (6, 20, 28, 35, 41). The occurrence of more than one R. anatipestifer serotype in infected ducks at any one time and changes in serotypes from year to year within a single farm have been observed (41). Strong variations of virulence as assessed by mortality and morbidity rates in outbreaks have been reported for the different serotypes of R. anatipestifer. In addition, differences in virulence were also observed within a given serotype (8). However, the molecular bases for these differences are unknown, since no virulence factors of R. anatipestifer have been yet found. Thus far, fibrinolytic enzymes, hemolysins, and a lipopolysaccharide have been postulated for R. anatipestifer (5, 8), but the presence of these factors has not yet been established. In addition, very little knowledge about the immunogenic factors of R. anatipestifer exists. Vaccines based on inactivated bacteria were shown to confer some protection against infection with homologous strains or serotypes, but only very poor protection was observed when heterologous strains were used for challenge (34).
Knowledge of the predominant immunogenic components of an infectious agent is essential for the analysis of the molecular mechanisms of virulence, the study of the route of infection, the serological diagnosis of the disease, and the development of strategies for efficient immune protection and eradication of the disease. Outer membrane proteins of pathogenic bacteria are generally very immunogenic (10, 11, 17). They play an important role in virulence of and immunity to bacterial diseases (43). In this study, we report the cloning and analysis of an immunogenic 42-kDa outer membrane protein, OmpA, of R. anatipestifer which seems to be a predominant, specific antigen of the species R. anatipestifer.
MATERIALS AND METHODS
Bacterial strains, cloning vectors, and growth conditions.
The type strain, serotype reference strains (23), and field isolate of R. anatipestifer used in this study are listed in Table 1. All of the strains were grown on Columbia agar plates at 37°C in air enriched with 5% CO2 for 24 h. For gene cloning and expression, we used the following Escherichia coli strains: XL1-Blue {E. coli K-12 recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)]} (Stratagene, La Jolla, Calif.), XL1-Blue MRF′ {E. coli K-12, Δ(mcrA) 183 Δ(mcrCB-hsdSMR-mrr) 173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)]} (Stratagene), XLOLR {E. coli K-12, Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] λr, Su−)} (Stratagene), and BL21(DE3) [E. coli B F− dcm ompT hsdS(rB−mB−) gal λ(DE3 T7pol)] (Stratagene). Plasmid vectors used for cloning and expression were pBluescript II (SK−) (Stratagene) and pBK-CMV (Stratagene). For expression of polyhistidine-tailed proteins, we used plasmid pETHIS-1, which is a ColE1-derived high-copy-number expression vector containing the bla gene (ampicillin resistance) for selection and a specific promoter sequence for the T7 polymerase-dependent expression of cloned genes. It allows the expression of fusion proteins with an N-terminal histidine hexamer and/or a C-terminal histidine decamer (35a) (GenBank/EMBL accession number AF012911). The E. coli strains were grown in Luria-Bertani broth medium. For selection of transformants and maintaining of plasmids, the medium was supplemented with 100 μg of ampicillin per ml for pBluescript II (SK−) and pETHIS-1 or with 50 μg of kanamycin per ml for cloning vector pBK-CMV. Induction of cloned genes on expression vector pETHIS-1 in strain BL21(DE3) was done by the addition of 0.3 mM (final concentration) isopropyl-β-d-thiogalactopyranoside (IPTG) at mid-exponential growth phase and incubation of the cultures for a further 3 h.
TABLE 1.
R. anatipestifer strains
| Straina | Serotype |
|---|---|
| ATCC 11845T | NDb |
| HPRS1795R | 1 |
| HPRS2527R | 2 |
| HPRS2554R | 3 |
| HPRS2550R | 5 |
| CVL389/89R | 6 |
| DRL27179R | 7 |
| HPRS1785R | 9 |
| CCUG25055-890822R | 11 |
| DRL28020R | 11 |
| CCUG25012 890804R | 13 |
| CVL664/83R | 14 |
| CVL743/85R | 15 |
| DRLS-4801R | 16 |
| CVL977/83R | 17 |
| CVL540/86R | 18 |
| CVL30/90R | 19 |
| CVL110/89C | 15 |
ATCC, American Type Culture Collection; HPRS, Houghton Poultry Research Station, Houghton, United Kingdom; CVL, Central Veterinary Laboratory, Singapore; DRL, Duck Research Laboratory, New York, N.Y.; CCUG, Culture Collection, University of Göteborg, Göteborg, Sweden. T, type strain; C, field strain; R, serotype reference strain.
ND, not determined.
Construction and screening of genomic libraries.
Genomic DNA was extracted by the rapid guanidium thiocyanate method (29). R. anatipestifer serotype 15 strain CVL110/89 was used as the host for establishing the phage library. This strain was responsible for a severe outbreak with 25% mortality in duck farms in Singapore. The gene library was made by cloning selected fragments of 1.5 to 4 kb of partially Sau3A-digested genomic DNA (3) into BamHI-digested and dephosphorylated bacteriophage λ ZAP Express vector (Stratagene) and packaged with Gigapack II Gold Packaging Extract (Stratagene). The gene library was plated by standard protocols using the E. coli strain XL1-Blue MRF′. Screening of the library was performed with serum obtained from a duck experimentally infected with a R. anatipestifer serotype 15 field isolate. In vivo excisions of selected clones on plasmid vector pBK-CMV from the phage plaques were made by selection from kanamycin-resistant colonies after infection with the helper phage M13 according to the supplier's protocol. Plasmid pBluescript II (SK−) was used for establishing a library of HindIII fragments of genomic DNA from R. anatipestifer serotype 15 strain 110/89. Ligation products were transformed into XL1-Blue cells by the calcium chloride procedure (33).
For screening of recombinant E. coli clones, colonies were transferred from the plates with solid medium to nitrocellulose filters (Schleicher & Schuell, Dassel, Germany). The recombinants were lysed in situ, and DNA was cross-linked to the membrane by baking at 80°C for 2 h. The filters were then preincubated with hybridization buffer (5× SSC [1× SSC is 150 mM NaCl plus 15 mM trisodium citrate, pH 7.7], 0.1% N-laurylsarcosine, 0.02% sodium dodecyl sulfate [SDS], and 1% blocking reagent [Boehringer Mannheim]) at 68°C for 2 h and then hybridized with hybridization buffer containing 1 μg of digoxigenin-labeled ompA gene probe for 18 h at 68°C. The membrane was washed twice for 15 min with 0.2× SSC containing 0.1% SDS at 68°C. Digoxigenin-labeled DNA probes were detected using phosphatase-labeled antidigoxigenin antibodies (Boehringer Mannheim) according to the producer's instructions.
PCR and production of labeled probes.
The oligonucleotide primers used in this study and their annealing temperatures are listed in Table 2. The PCRs were carried out in a DNA thermal cycler (GeneAmp 9600; Perkin-Elmer Cetus) in a 50-μl reaction mix (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 1.5 mM MgCl2, 170 μM each deoxynucleoside triphosphate, 20 pmol of each primer, 5 ng of plasmid DNA or 200 ng of genomic DNA, and 1.5 U of Taq polymerase [Boehringer Mannheim]). The PCR thermal parameters used were 35 cycles of amplification with 30 s at 94°C, 30 s at the corresponding annealing temperature (Table 2), and 1 min at 72°C. When DNA fragments were produced by PCR for subsequent cloning and expression or for DNA sequence analysis, the elongation steps were increased to 2 min at 72°C and 2.5 U of Taq-Pwo polymerase mix (Boehringer Mannheim) was used instead of Taq polymerase. In addition, an extension step of 7 min at 72°C was added at the end of the last cycle in order to ensure full-length synthesis of the different fragments.
TABLE 2.
Oligonucleotides used in PCRa
| Primer | Sequence | Tmb (°C) |
|---|---|---|
| RA6OMPA-L | GCAACAGGAGCATTACAAGGTA | 50 |
| RA6OMPA-R | CTGTCTTTCATTCTCTCTTTC | 50 |
| RAOMPAH1-R | cgctatggatccTTATTTTCTTTTCTT | 48 |
| RAOMPAH1A-R | tacggatccTTTTCTTTTCTTTTTTACTAC | 48 |
| RAOMPAH1-L | cgcagccatATGGGTTAAAGAATTT | 48 |
| RAOMPAF1 | GACTGGCAAACTTCAGTAGG | 55 |
| RAOMPAF2 | TGGTCTTGGTATCCAAGGGG | 55 |
| RAOMPAF3 | AATAACGGTTGCCCTTGGCC | 55 |
| RAOMPAF4 | GCTGCTTTAGAAGCTAGAGG | 55 |
| RAOMPAR1 | CAATGAAGCTGACGCTTGCC | 55 |
| RAOMPAR2 | GCCCAGGAACTGTAGGACAC | 55 |
| RAOMPAR3 | GTAGCTTCAGCAGAACCAAC | 55 |
| RAOMPAR4 | CAACGAGCCATGCTTAGAGGC | 55 |
| PETHIS1-3′ | CGTCTTCAAGCTCATGTTTG | 55 |
| T7 | TAATACGACTCACTATAGGG | 55 |
| T3B-S | GCGCGCAATTAACCCTCACTAAAG | 73 |
Uppercase letters correspond to the nucleotides of the ompA sequence; lowercase letters specify nucleotides that were added to create restriction enzyme recognition sites for cloning. Primers pETHIS1-3′ and T7 match the segments flanking the codons of the multiple cloning site in vector pETHIS-1 and were used for verifying the correct fusions of the genes cloned in pETHIS-1.
Tm, annealing temperature.
Digoxigenin-labeled DNA probes were made by supplementing the PCR mixture with a final concentration of 50 μM digoxigenin-11-dUTP (Boehringer Mannheim).
DNA sequence analysis.
DNA sequence analysis was done using an AmpliTaq FS dye terminator kit (Perkin-Elmer Cetus) with reaction mixtures containing approximately 500 ng of plasmid DNA and 5 pmol of oligonucleotide primer. The ends of cloned DNA fragments in vectors pBK-CMV, pETHIS-1, and pBluescript II (SK−) were sequenced with primers T3B-S, T7, and PETHIS1-3′ (Table 2), matching the sequences flanking the vectors' multiple cloning sites. The complete nucleotide sequences of cloned fragments were determined by primer walking. Sequences were assembled and edited by using the Sequencher 3.0 program (GeneCodes, Ann Arbor, Mich.) to obtain contiguous sequences. Comparison of the nucleotide sequences in search of related sequences was performed using the National Center for Biotechnology Information BLASTN and BLASTX programs (1). The DNA and amino acid sequences were analyzed using the PCGENE programs PROSITE (4) and PSORT (26) and the Genetics Computer Group programs.
Purification of polyhistidine-tailed fusion proteins.
Polyhistidine-tailed OmpA fusion proteins were expressed by IPTG induction of E. coli host strain BL21(DE3) harboring the corresponding plasmids with the ompA fusion constructs. Following induction, the cells were harvested, washed in TES buffer (10 mM Tris, 1 mM EDTA, 0.8% NaCl, pH 8.0), and dissolved in 50 mM phosphate buffer (pH 8.0) supplemented with 6 M guanidine hydrochloride. The fusion proteins were purified from these cell extracts using Ni2+ chelate affinity chromatography (Qiagen, GmbH, Hilden, Germany) according to the manufacturer's instructions. The bound fusion proteins were eluted by slowly decreasing the pH from 8.0 to 4.5 with 50 mM phosphate buffer–300 mM NaCl–6 M guanidine hydrochloride. The polyhistidine-tailed fusion proteins were eluted at pH 5.0 and subsequently dialyzed against 50 mM phosphate buffer–300 mM NaCl, pH 8.0.
Production of antisera and immunological methods.
Monospecific polyclonal antisera directed against the polyhistidine-tailed fusion protein (His6-OmpA) was obtained by immunization of mice with 330 μg of the purified recombinant protein mixed 1:1 with complete Freund's adjuvant (Difco Laboratories, Detroit, Mich.) in a total volume of 200 μl followed by a booster immunization 2 weeks later with 330 μg of purified protein and incomplete Freund's adjuvant. The serum was collected 7 days after the second immunization. R. anatipestifer serotype 15 strain CVL110/89 was used to prepare killed antigen by heating bacteria at a concentration of 105 CFU/ml at 100°C for 1 h. Four 8-day-old ducklings were immunized subcutaneously with 1 ml of the killed antigen preparation. A second immunization with an equal volume of the antigen preparation was given 11 days after the first. Sera were collected from the ducklings 10 days after the second immunization and pooled. Purified recombinant protein and total cell preparations were mixed with an equal volume of SDS sample buffer (62.2 mM Tris-HCl [pH 6.8], 2% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.005% bromophenol blue) and boiled for 10 min. Proteins were separated by electrophoresis on SDS–10% polyacrylamide gels, and immunoblot analysis was performed as described previously (3). Mouse and duck sera were used at a dilution of 1:2,000. Bound antibodies were visualized on immunoblots by using phosphatase-labeled goat antibodies directed against mouse immunoglobulin G (IgG) and IgM (KPL no. 0751806) or against duck IgG and IgM (KPL no. 052506) (Kirkegaard & Perry Inc., Gaithersburg, Md.).
Ca2+-binding assay.
Proteins were separated on SDS-polyacrylamide gels and transferred to nitrocellulose membranes as described above. The membranes were then soaked in calcium-binding buffer (60 mM KCl, 5 mM MgCl2 and 10 mM imidazole hydrochloride, pH 7.2) for 10 min. Subsequently the membranes were incubated in binding buffer supplemented with 1.0 μCi of 45Ca2+ per ml (0.02 mCi of 45CaCl2+ per μg; Amersham Corp.) for 20 min and then rinsed twice with deionized water for 5 min and dried at room temperature, and bound 45Ca2+ was visualized by autoradiography.
Nucleotide sequence accession numbers.
The GenBank/EMBL DNA sequence accession number of the cloned 2.2-kb fragment of strain CVL110/89 containing the R. anatipestifer ompA gene and flanking gene segments is AF104936. That of ompA of the R. anatipestifer type strain ATCC 11845 is AF104937.
RESULTS
Cloning and sequence analysis of ompA.
A gene library of R. anatipestifer serotype 15 strain CVL110/89 made in the bacteriophage λ ZAP Express cloning vector was screened with pooled convalescent-phase sera from ducks experimentally infected with an R. anatipestifer serotype 15 strain. A strongly immunoreactive clone was retained and converted to a plasmid designated pJFFRA6. This contained a partial open reading frame of 859 bp showing significant similarity to the gene for Bordetella avium outer membrane protein A (OmpA). We therefore designated the corresponding gene on plasmid pJFFRA6 ompA. A digoxigenin-labeled probe for the R. anatipestifer ompA gene segment was produced by PCR using plasmid pJFFRA6 DNA as template and the primers RA6OMPA-L and -R (Table 2), which were derived from the sequence of the cloned fragment of ompA. This probe was used to screen a plasmid vector-based gene library of HindIII-digested genomic DNA of R. anatipestifer serotype 15 strain 110/89 cloned into vector pBluescript II (SK−). Plasmid pJFFRaOmpA15, containing a 2.2-kb HindIII fragment, was retained for further analysis.
The DNA sequence of the insert in plasmid pJFFRaOmpA15 was determined on both strands and shown to contain the entire ompA gene plus flanking gene fragments, as shown schematically in Fig. 1 and in detail in Fig. 2. The DNA sequence of the coding part of the ompA gene from R. anatipestifer type strain ATCC 11845 was established by PCR amplification of the DNA fragment containing ompA, using the oligonucleotide primers RAOMPAF1 and RAOMPAR4 (Table 2) and genomic DNA of strain ATCC 11845 followed by direct sequence determination of the amplified fragment with primers RAOMPAF1 and RAOMPAR4 and internal primers RAOMPAF2 to -4 and RAOMPAR2 to -4 (Table 2), which were deduced from the DNA sequence of ompA from strain CVL110/89. The nucleotide sequence of ompA of the type strain ATCC 11845 shows a total of 35 differences from that of strain CVL110/89. All differences are located in the 3′ half of the gene and result in a total of seven amino acid changes.
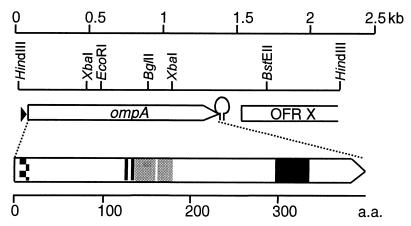
FIG. 1.
Structure of the 2.2-kb insert in plasmid pJFFRaOmpA15. The upper part gives the localization of ompA and the partial open reading frame ORFX, encoding a protein with high similarity to the spore coat protein of B. subtilis. The promoter sequence upstream of ompA is indicated by a triangle. The inverted-repeat structure at the end of ompA, representing a potential transcription stop signal, is indicated by a hairpin. The different domains of OmpA are shown in the lower part. The inside-to-outside transmembrane helix-spanning domain (aa 5 to 22) is represented by a checkered box. The six EF-hand calcium-binding domains (aa 129 to 141) are shown by a vertically hatched box. The two PEST regions (aa 139 to 164 and 166 to 187) are represented by gray boxes, and the OmpA-like domain, consisting of a 45-aa stretch in the C-terminal half of the protein, is indicated by a black box.
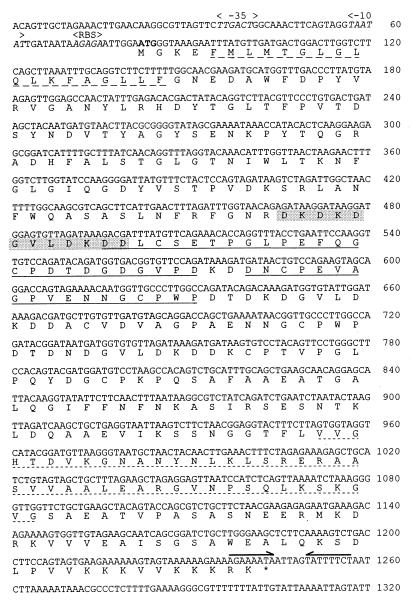
FIG. 2.
DNA sequence of the ompA gene and derived amino acid sequence. The first 1,320 bp of the DNA sequence of the insert in plasmid pJFFRaOmpA15 containing the entire ompA gene and its regulative sequences is shown. The nucleotides are numbered as deposited in the GenBank/EMBL database (accession number AF104936). The −10 and −35 promoter sequences and the ribosome binding site (RBS) are in italic. The start codon ATG is in boldface. The nucleotide sequences forming a stem-loop structure of a potential rho-independent transcription termination signal are indicated by two arrows above the DNA sequence. The amino acids forming an inside-to-outside transmembrane helix structure are underlined with a dashed line. The calcium-binding domains are shown in a gray box. The two PEST regions of OmpA are underlined, and the characteristic OmpA-like domain of 45 aa is underlined with a dotted line.
The sequence data for the insert of plasmid pJFFRaOmpA15 revealed an open reading frame of 1,163 bp, encoding the OmpA protein of 387 amino acids (aa), a deduced molecular mass of 41,696 Da, and a calculated pI of 4.91. It is preceded by a consensus sequence for a ribosome binding site (nucleotides [nt] 71 to 75) 6 nt upstream the ATGMet start codon (Fig. 2). Upstream of the ribosome binding site, there is a canonical promoter sequence with a −10 box (TAATAT) (nt 57 to 62) and a −35 box (TTGACT) (nt 35 to 40) optimally spaced by 16 nt, which is characteristic of promoters recognized by E. coli ς32 RNA polymerases (Fig. 2). The short segment further upstream of the promoter region did not show any homology to known nucleotide or amino acid sequences in the databases. At the end of ompA there is a stem-loop structure (nt 1238 to 1257), representing a rho-independent transcription termination signal. Downstream of ompA, there is a partial open reading frame, ORFX (Fig. 1), showing similarity to the 17-kDa Bacillus subtilis spore coat protein.
Analysis of the OmpA amino acid sequence deduced from the ompA gene sequences of strain CVL110/89 and strain ATCC 11845 revealed the presence of six EF-hand calcium-binding domains between aa 129 and 141 and two PEST regions (aa 139 to 164 and 166 to 187) (Fig. 1 and 2). The N-terminal region (aa 4 to 22) is highly hydrophobic, and aa 5 to 22 form an inside-to-outside transmembrane helix locating the N terminus of OmpA on the inside of the cell (Fig. 1 and 2). The remaining part of the protein, in particular aa 125 to 229, is predominantly hydrophilic. The C-terminal part encompasses the OmpA-like domain, a stretch of 45 aa found in many outer membrane proteins of gram-negative bacteria. The OmpA proteins of serotype 15 strain CVL110/89 and type strain ATCC 11845 differ in only seven amino acids, which lie outside these characteristic structures and are located clustered between aa 228 and 255.
Comparison of the deduced amino acid sequence of the 42-kDa OmpA with the SwissProt data bank by using the National Center for Biotechnology Information BLASTX program revealed 38% identical amino acids with OmpA of B. avium (accession no. Q05146), 28% identical amino acids with OmpA of Serratia marcescens OmpA (accession no. P04845), 28% identical amino acids with OmpA of Enterobacter aerogenes (accession no. P09146), and 29% identical amino acids with the porin protein OprF of Pseudomonas aeruginosa (accession no. P13794). Alignment of these amino acid sequences with OmpA of R. anatipestifer revealed significant similarities in the C-terminal halves of the different OmpA proteins, which contain the OmpA-like domain, showing 33 to 37% identical and 47 to 59% similar (identical plus similar) amino acids. The N-terminal half of OmpA of R. anatipestifer showed no similarity to other proteins in the SwissProt and GenBank/EMBL databases.
Expression and purification of recombinant OmpA.
To obtain purified recombinant R. anatipestifer OmpA antigen, the ompA gene was amplified in vitro with Taq-Pwo polymerase mix and oligonucleotide primers RAOMPAH1-L and RAOMPAH1-R (Table 2), with genomic DNA of R. anatipestifer serotype 15 strain CVL110/89 as the template. The purified PCR product was digested with NdeI and BamHI and cloned into the expression vector pETHIS-1 in order to obtain plasmid pJFFOMPA, which resulted in an in-frame fusion of six histidine codons at the 5′ end of ompA. A second plasmid, pJFFOMP13, was constructed analogously using primers RAOMPAH1-L and RAOMPAH1A-R in order to have the coding frame of ompA fused 5′ terminally to 6 histidine codons and 3′ terminally to 10 histidine codons. The cloned gene constructs in plasmids pJFFOMPA and pJFFOMP13 were verified by DNA sequence analysis. For expression of the polyhistidine-tailed ompA genes, the plasmids pJFFOMPA and pJFFOMP13 were transformed into E. coli BL21(DE3), and the fusion proteins His6-OmpA and His6-OmpA1His10, respectively, were purified from IPTG-induced cultures by Ni2+ chelate chromatography. A second plasmid, pJFFOMP17, which is identical to pJFFOMP13 was constructed independently. Its gene product, His6-OmpA1His10, showed the same characteristics as that obtained from pJFFOMP13 throughout the study.
Immunoreactivity and Ca2+ binding of OmpA.
Immunoreactions of total cell lysates of R. anatipestifer serotype 15 strain CVL110/89 and of purified recombinant His6-OmpA-1His10 were studied on immunoblots using monospecific polyclonal anti-His6-OmpA hyperimmune serum and convalescent-phase sera from ducks infected with a R. anatipestifer serotype 15 strain (Fig. 3). Immunoblots of total cell lysates of R. anatipestifer serotype 15 strain CVL110/89 revealed three bands of 55, 53, and 51 kDa reacting with serum directed against His6-OmpA. Recombinant His6-OmpA1His10 protein revealed three bands with somewhat lower molecular masses of 46, 44, and 42 kDa when reacted with the same serum (Fig. 3A). Immunoblots of total R. anatipestifer cell lysate which was reacted with convalescent-phase sera from ducks experimentally infected with R. anatipestifer serotype 15 revealed the same OmpA triplet of 55, 53, and 51 kDa, besides a few additional immunoreactive proteins (Fig. 3B). This serum also reacted with recombinant His6-OmpA1His10, showing the characteristic triplet at 46, 44, and 42 kDa (Fig. 3B), similar to polyclonal anti-OmpA antibodies. Sera from ducks that were immunized with total cell antigen of strain CVL110/89 gave the same results on immunoblots as convalescent-phase serum.
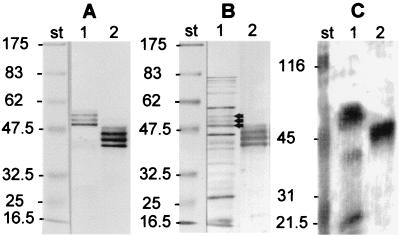
FIG. 3.
Immunological reactions and Ca2+ binding of OmpA. (A) Immunoblot reacted with monospecific polyclonal anti-OmpA antibodies. (B) Immunoblot reacted with sera from convalescent ducks that were infected experimentally with R. anatipestifer serotype 15. (C) Autoradiography of the blot reacted with 45Ca2+. Lanes 1, total cell lysate of R. anatipestifer strain CVL110/89 (serotype 15); lanes 2, purified recombinant His6-OmpA1His10 protein. As molecular mass standards (lanes st) we used broad-range prestained protein markers (New England Biolabs, Beverly, Mass.; no. 7708S) for the immunoblots (A and B) and Bio-Rad (Hercules, Calif.) prestained broad-range standards for the Ca2+ blot (C), which were shown to bind 45Ca2+ (15). The arrows show the position of the OmpA triplet. Numbers on the left of each panel are molecular masses in kilodaltons.
Ca2+-binding experiments with total antigens of R. anatipestifer showed a major band of 51 to 55 kDa corresponding to the OmpA triplet at 55, 53, and 51 kDa and three minor bands of 40, 32, and 30 kDa which bound 45Ca2+ (Fig. 3C). Recombinant His6-OmpA1His10 protein strongly bound 45Ca2+, showing a band in the range of 42 to 46 kDa corresponding to the triplet of His6-OmpA1His10 seen in the immunoblot (Fig. 3C). The individual bands of the triplet which were seen on immunoblots could not be differentiated in these blots due to the lower separation capability of autoradiography.
Presence and expression of ompA in R. anatipestifer strains.
PCR analysis using the primers RAOMPAH1-L and RAOMPAH1A-R (Table 2) showed the presence of 1,177-bp amplification products in all R. anatipestifer type and serotype reference strains analyzed. However, restriction fragment length polymorphism analysis of the PCR products with the frequently cutting enzyme AluI indicated some heterogeneities in the ompA gene, showing three different profiles grouping the type strain ATCC11845 together with serotypes 1, 2, 3, 5, 7, and 17 in one group; serotypes 6, 13, 14, 16, and 19 in a second group; and serotypes 9, 11, 15, and 18 in a third group. Analysis of the positions of the variable AluI restriction sites revealed that the differences are located in the 3′ half of the ompA gene, corresponding to the domain where we detected the nucleotide differences between the ompA sequences of strains CVL110/89 and ATCC 11845.
In immunoblots of total cell lysates of the type strain and different serotype reference strains of R. anatipestifer, all reacted with anti-His6-OmpA polyclonal serum, showing the characteristic triplet bands at 55, 53, and 51 kDa (Fig. 4) as was found for R. anatipestifer serotype 15 strain CVL110/89.
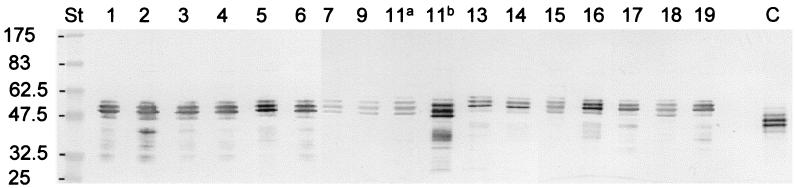
FIG. 4.
Expression of OmpA by the different R. anatipestifer serotypes. Immunoblots of total cell lysates of the R. anatipestifer type strain and the different serotype reference strains reacted with monospecific polyclonal anti-OmpA-His6 mouse serum are shown. The numbers indicate the serotype (Table 1) a, strain CCUG 25055 890822; b, strain DRL 28020. C, purified recombinant His6-OmpA1His10 used as a control; st, prestained broad-range protein markers (New England Biolabs). Molecular masses of the protein markers are given in kilodaltons.
DISCUSSION
The outer membranes of gram-negative bacteria contain a limited number of major outer membrane proteins present in very high copy numbers (19). Of these, outer membrane protein A (OmpA), which is found in many gram-negative bacteria and which was studied most extensively in E. coli, is necessary for maintenance of structural integrity of cell envelopes (39). It is also involved in bacterial conjugation (36), in bacterial attachment, as receptors for certain bacteriophages (12), in colicin uptake (24), and in porin activity (27). OmpA is also known to stimulate a strong antibody response (30). In the present study we have cloned, expressed, and characterized the ompA gene and its gene product, OmpA, from R. anatipestifer.
Nucleotide sequence analysis of the R. anatipestifer ompA gene revealed that it encodes a protein of 387 amino acids with a molecular mass of 42 kDa. The C-terminal half contains the characteristic OmpA-like domain, a stretch of 45 amino acids which shows high homology to outer membrane proteins of many gram-negative bacteria. The rest of the protein, especially the N-terminal amino acid sequence, shows no similarity to other outer membrane proteins. This is also characteristic of OmpA proteins. They are generally heterogeneous at their N-terminal parts. OmpA of R. anatipestifer showed highest similarity to OmpA of B. avium, the etiological agent of turkey bordetellosis, a highly contagious upper respiratory disease of turkeys (22) which is characterized by signs and symptoms similar to those caused by R. anatipestifer in ducks.
Immunoblots of total R. anatipestifer antigens revealed three distinct bands of 55, 53, and 50 kDa. This may be due to the protein being detected at different stages of processing as was reported for OmpA of E. coli and B. avium (16), where multiple bands were identified. They are interpreted to represent (i) an OmpA precursor which contains the signal peptide and is located in the cytoplasm or is associated with the cytoplasmatic membrane (pro-OmpA), (ii) immature processed OmpA without the signal peptide that is found in the periplasm or attached to the inner face of the outer membrane (imp-OmpA), and (iii) mature OmpA (14). The molecular masses of the protein bands of recombinant OmpA were approximately 10 kDa smaller and corresponded better to the calculated molecular mass of OmpA than those from R. anatipestifer endogenous OmpA. The difference in molecular masses could be due to further posttranslational modifications of OmpA in R. anatipestifer, such as additions of glycosaminoglycan chains, which seem not to occur when recombinant OmpA is expressed in E. coli.
The functional domains of OmpA of E. coli are well characterized and contains a transmembrane segment stretching from amino acid 1 to 177 (7, 9, 13, 25). In contrast, OmpA of R. anatipestifer shows no analogous region at the N-terminal part but contains a short inside-to-outside transmembrane helix comprising 17 amino acids. The vast stretches of hydrophilic residues suggest that a large part of OmpA of R. anatipestifer is surface exposed, resulting in these areas being antigenic. This would explain its strong antigenic nature. The absence of alanine-proline- or proline-rich regions in OmpA of R. anatipestifer is remarkable, since such domains are found generally in OmpA of other bacterial species, where they separate the periplasmic domains from the transmembrane domains of the proteins (22).
It is known that calcium-binding proteins play a central role in intracellular signal transduction pathways and are associated with a wide range of effects on disease production (38). The finding of six EF-hand calcium-binding domains in OmpA of R. anatipestifer is notable, since other OmpA proteins do not contain such domains. They might be involved in the strong Ca2+-binding capacity, as shown experimentally for OmpA of R. anatipestifer. Adjacent to the calcium-binding domains are two PEST regions, which are peptide motifs that target proteins for destruction through a yet-unknown mechanism (32). PEST sequences are found in key metabolic enzymes, transcription factors, protein kinases, protein phosphatases, and cyclins, and they are also abundant among proteins that give rise to immunogenic peptides presented on major histocompatibility complex class I molecules. It was postulated that rapidly degraded proteins are more likely to generate immunogenic peptides (31). This would support our finding that OmpA is highly immunogenic in infected ducks. While PEST sequences are often present as carboxy-terminal extensions of proteins (31), they are located in the middle of the OmpA of R. anatipestifer. The presence of two PEST regions adjacent to the EF-hand calcium-binding domains is a good indication that a protein is a preferred calpain substrate (31, 42).
PCR analysis of genomic DNA of the type strain of R. anatipestifer and serotype reference strains and subsequent restriction analysis of the amplified DNA segments show that the ompA gene is common to the species but shows a certain degree of variation among the different serotypes. The variable regions were found at the 3′ end of ompA, downstream of the segment encoding the 45-aa OmpA domain. This part generally shows the highest divergence of the core ompA sequence. The 5′ ends of ompA genes often show totally different structures among different bacterial species but are relatively conserved within a given species. It has to be noted that different serotypes of R. anatipestifer also show variations in their rrs genes, as was described previously (40).
In conclusion, the immunogenic outer membrane protein OmpA is common to different serotypes of R. anatipestifer. Its gene, ompA, shows minor intraspecies variations in its 3′ half which are predominantly silent mutations not affecting the phenotype. The presence of calcium-binding domains and PEST regions and the absence of proline-rich regions suggest that OmpA may have roles in addition to and different from those commonly associated with outer membrane proteins. The N-terminal part of OmpA does not show homology to any protein in the databases. The high immunoreactivity of this protein makes OmpA an interesting candidate for development of specific serological diagnostic tools to detect R. anatipestifer infections of all serotypes. In addition, it might be considered as an antigen for designing new vaccines against contagious septicemia anserum exudativa of ducklings.
ACKNOWLEDGMENTS
We thank J. Nicolet, Head, Institute for Veterinary Bacteriology of the University of Bern, and N.-H. Chua, Head, Laboratory of Plant Molecular Biology, Rockefeller University, New York, N.Y., for their support and encouragement. We also thank Y. Schlatter for her technical assistance and Jos Cox, Department of Biochemistry, University of Geneva, for advice with Ca2+-binding experiments.
This work is part of a collaborative program of the Swiss Asia Foundation between the Institute of Molecular Agrobiology of the National University of Singapore and the Institute for Veterinary Bacteriology of the University of Bern. It is supported by the Priority Programme Biotechnology of the Swiss National Foundation (grant 5002-038920) and the National Science and Technology Board, Singapore.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Asplin F D. A septicaemic disease of ducklings. Vet Rec. 1955;67:854–858. [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 4.Bairoch A, Bucher P, Hofmann K. The PROSITE database, its status in 1995. Nucleic Acids Res. 1995;24:189–196. doi: 10.1093/nar/24.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangun A, Tripathy D N, Hanson L E. Studies of Pasteurella anatipestifer: an approach to its classification. Avian Dis. 1981;25:326–337. [PubMed] [Google Scholar]
- 6.Bisgaard M. Antigenic studies on Pasteurella anatipestifer, species incertae sedis, using slide and tube agglutination. Avian Pathol. 1982;11:341–350. doi: 10.1080/03079458208436109. [DOI] [PubMed] [Google Scholar]
- 7.Bremer E, Cole S T, Hindennach I, Henning U, Beck E, Kurz C, Schaller H. Export of a protein into the outer membrane of Escherichia coli K12. Stable incorporation of the OmpA protein requires less than 193 amino-terminal amino-acid residues. Eur J Biochem. 1982;122:223–231. doi: 10.1111/j.1432-1033.1982.tb05870.x. [DOI] [PubMed] [Google Scholar]
- 8.Brogden K A. Pasteurella anatipestifer infection. In: Adlam C, Rutter J M, editors. Pasteurella and pasteurellosis. London, United Kingdom: Academic Press; 1989. pp. 115–129. [Google Scholar]
- 9.Chen R, Schmidmayr W, Kramer C, Chen S U, Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci USA. 1980;77:4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng X, Nicolet J, Miserez R, Kuhnert P, Krampe M, Pilloud T, Abdo E M, Griot C, Frey J. Characterization of the gene for an immunodominant 72 kDa lipoprotein of Mycoplasma mycoides subsp. mycoides small colony type. Microbiology. 1996;142:3515–3524. doi: 10.1099/13500872-142-12-3515. [DOI] [PubMed] [Google Scholar]
- 11.Chiang Y W, Young T F, Rapp Gabrielson V J, Ross R F. Improved protection of swine from pleuropneumonia by vaccination with proteinase K-treated outer membrane of Actinobacillus (Haemophilus) pleuropneumoniae Vet. Microbiol. 1991;27:49–62. doi: 10.1016/0378-1135(91)90062-k. [DOI] [PubMed] [Google Scholar]
- 12.Datta D B, Arden B, Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977;131:821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freudl R, Klose M, Henning U. Export and sorting of the Escherichia coli outer membrane protein Omp A. J Bioenerg Biomembr. 1990;22:441–449. doi: 10.1007/BF00763176. [DOI] [PubMed] [Google Scholar]
- 14.Freudl R, MacIntyre S, Degen M, Henning U. Cell surface exposure of the outer membrane protein OmpA of Escherichia coli K-12. J Mol Biol. 1986;188:491–494. doi: 10.1016/0022-2836(86)90171-3. [DOI] [PubMed] [Google Scholar]
- 15.Frey J, Meier R, Gygi D, Nicolet J. Nucleotide sequence of the hemolysin I gene from Actinobacillus pleuropneumoniae. Infect Immun. 1991;59:3026–3032. doi: 10.1128/iai.59.9.3026-3032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry-Weeks C R, Hultsch A L, Kelly S M, Keith J M, Curtiss R. Cloning and sequencing of a gene encoding a 21-kilodalton outer membrane protein from Bordetella avium and expression of the gene in Salmonella typhimurium. J Bacteriol. 1992;174:7729–7742. doi: 10.1128/jb.174.23.7729-7742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlach G F, Klashinsky S, Anderson C, Potter A A, Willson P J. Characterization of two genes encoding distinct transferrin-binding proteins in different Actinobacillus pleuropneumoniae isolates. Infect Immun. 1992;60:3253–3261. doi: 10.1128/iai.60.8.3253-3261.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glunder G, Hinz K H. Isolation of Moraxella anatipestifer from embryonated goose eggs. Avian Pathol. 1989;18:351–355. doi: 10.1080/03079458908418608. [DOI] [PubMed] [Google Scholar]
- 19.Hancock R E W. Bacterial outer membranes: evolving concepts. ASM News. 1991;57:175–182. [Google Scholar]
- 20.Harry E G. Pasteurella (Pfeifferella) anatipestifer serotypes isolated from cases of anatipestifer septicaemia in ducks. Vet Rec. 1969;84:673. doi: 10.1136/vr.84.26.673. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson J M, Hilbert K F. A new and serious septicaemic disease of young ducks with a description of the causative organisms, Pfeiferella anatipestifer, N.S. Cornell Vet. 1932;22:239–252. [Google Scholar]
- 22.Leyh R, Griffith R W. Characterization of the outer membrane proteins of Bordetella avium. Infect Immun. 1992;60:958–964. doi: 10.1128/iai.60.3.958-964.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh H, Teo T P, Tan H C. Serotypes of Pasteurella anatipestifer isolates from ducks in Singapore: a proposal of new serotypes. Avian Pathol. 1992;21:453–459. doi: 10.1080/03079459208418863. [DOI] [PubMed] [Google Scholar]
- 24.Lugtenberg B, van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983;737:51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 25.Morona R, Klose M, Henning U. Escherichia coli K-12 outer membrane protein (OmpA) as a bacteriophage receptor: analysis of mutant genes expressing altered proteins. J Bacteriol. 1984;159:570–578. doi: 10.1128/jb.159.2.570-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in Gram-negative bacteria. Protein Struct Funct Genet. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 27.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathanasophon P, Tanticharoenyos T, Sawada T. Physiological characteristics, antimicrobial susceptibility and serotypes of Pasteurella anatipestifer isolated from ducks in Thailand. Vet Microbiol. 1994;39:179–185. doi: 10.1016/0378-1135(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 29.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 30.Puohiniemi R, Karvonen M, Vuopio V J, Muotiala A, Helander I M, Sarvas M. A strong antibody response to the periplasmic C-terminal domain of the OmpA protein of Escherichia coli is produced by immunization with purified OmpA or with whole E. coli or Salmonella typhimurium bacteria. Infect Immun. 1990;58:1691–1696. doi: 10.1128/iai.58.6.1691-1696.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 32.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sandhu T S. Immunization of white pekin ducklings against Pasteurella anatipestifer infection. Avian Dis. 1979;23:662–669. [PubMed] [Google Scholar]
- 35.Sandhu T S, Harry E G. Serotypes of Pasteurella anatipestifer isolated from commercial White Peking ducks in the United States. Avian Dis. 1981;25:497–502. [PubMed] [Google Scholar]
- 35a.Schaller A, Kuhn R, Kuhnert P, Nicolet J, Anderson T J, MacInnes J I, Segers R P A N, Frey J. Characterization of apxIVA, a new RTX determinant of Actinobacillus pleuropneumoniae. Microbiology. 1999;145:2105–2116. doi: 10.1099/13500872-145-8-2105. [DOI] [PubMed] [Google Scholar]
- 36.Schweizer M, Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977;129:1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segers P, Mannheim W, Vancanneyt M, De Brandt K, Hinz K H, Kersters K, Vandamme P. Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the flavobacterium-cytophaga rRNA homology group. Int J Syst Bacteriol. 1993;43:768–776. doi: 10.1099/00207713-43-4-768. [DOI] [PubMed] [Google Scholar]
- 38.Skelton N J, Kordel J, Akke M, Forsen S, Chazin W J. Signal transduction versus buffering activity in Ca(2+)-binding proteins. Nat Struct Biol. 1994;1:239–245. doi: 10.1038/nsb0494-239. [DOI] [PubMed] [Google Scholar]
- 39.Sonntag I, Schwarz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136:280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramaniam S, Chua K L, Tan H M, Loh H, Kuhnert P, Frey J. Phylogenetic position of Riemerella anatipestifer based on 16S rRNA gene sequences. Int J Syst Bacteriol. 1997;47:562–565. doi: 10.1099/00207713-47-2-562. [DOI] [PubMed] [Google Scholar]
- 41.Teo T P, Tan H C, Loh H. Protective efficacy of a bivalent Pasteurella anatipestifer broth-grown bacterin in ducklings. Singapore J Pri Ind. 1992;20:53–60. [Google Scholar]
- 42.Wang K K, Roufogalis B D, Villalobo A. Characterization of the fragmented forms of calcineurin produced by calpain I. Biochem Cell Biol. 1989;67:703–711. doi: 10.1139/o89-105. [DOI] [PubMed] [Google Scholar]
- 43.Weiser J N, Gotschlich E C. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun. 1991;59:2252–2258. doi: 10.1128/iai.59.7.2252-2258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]