Abstract
Paracoccidioidomycosis (PCM) is the most prevalent systemic mycosis in Latin America. Patients with PCM show a wide spectrum of clinical and pathological manifestations depending on both host and pathogen factors. Two clinical forms of the disease are recognized: the acute or juvenile form and the chronic or adult form. The major antigenic component of the parasite is a glycoprotein of 43 kDa (gp43). All patient sera present antibodies against gp43 (anti-gp43) and, as demonstrated before by our group, spontaneous anti-idiotypic (anti-Id) antibodies (Ab2) can be detected in patient sera with high titers of anti-gp43. Since it has been postulated that anti-Id antibodies may have a modulating function, we decided to purify and characterize anti-Id antibodies in this system. The possible correlation of Ab2 titers with different clinical forms of disease was also verified. Results showed that purified human anti-Id antibodies (human Ab2) recognized specifically the idiotype of some murine monoclonal anti-gp43 (17c and 3e) but not others (40.d7, 27a, and 8a). Spontaneous anti-Id antibodies were found in all clinical forms of disease. The majority of patients (88%, n = 8) with the acute form of PCM had high titers of Ab2. However, among patients with the multifocal chronic form of the disease, only 29% (n = 14) had high titers of Ab2; 70% (n = 10) of patients with the unifocal chronic form had low titers of Ab2. A correlation between Ab2 titers and anti-gp43 titers was observed before and during antimycotic treatment. Our results suggest that titers of anti-Id antibodies correlate with the severity of PCM in humans.
Paracoccidioidomycosis (PCM) is a systemic mycosis restricted to Latin America, with large areas of endemicity in Brazil, Colombia, Venezuela, and Argentina (6, 31). The etiologic agent of the disease is a thermal dimorphic fungus, Paracoccidioides brasiliensis, which grows as a characteristic multiple budding yeast in the host or at 37°C or as mycelium at 25°C (6). In spite of the lack of knowledge of the habitat of P. brasiliensis, it is assumed that humans become infected via inhalation of conidia present in the environment. Depending on both host and pathogen factors, a wide spectrum of clinical and pathological manifestations can be observed in these patients (25). In areas of endemicity, up to 60% of the population acquires only asymptomatic infection, with positive paracoccidioidin skin tests, while some individuals develop disease (15, 25). Two main clinical forms of the disease are recognized: the acute or juvenile form (AF) and the chronic or adult form (CF). The AF affects mainly children and young adults of both sexes. This form is characterized by a rapid evolution and by marked involvement of the mononuclear-phagocytic system (spleen, liver, lymph nodes, and bone marrow). The CF occurs mainly in adult males (approximately 80 to 90%) more than 35 years old. The disease progresses slowly and may take months or even years to become fully established. The CF can be restricted to only one organ (unifocal CF [UCF]) or disseminated to several organs or systems (multifocal CF [MCF]). Most frequently, lesions occur in the lungs, oral and laryngeal mucous membranes, skin, lymph nodes, and adrenal glands (6, 13, 31).
Diagnosis currently relies on direct microscopic examination, the ability to grow the fungus in the laboratory, and serologic tests (15, 22, 27). Some of the recent serological assays use the 43-kDa surface glycoprotein (gp43) (10, 11). This glycoprotein is the major antigenic component of P. brasiliensis, since 100% of PCM patient sera display antibodies against it. gp43 is a high-mannose concanavalin A-binding glycoprotein (30, 32, 33, 39, 43). Several biological functions have been proposed for this molecule. Our group has characterized gp43 as a laminin-binding protein implicated in fungal pathogenesis in vivo (40). Others have shown that gp43 expresses immunodominant epitopes eliciting T-cell-dependent delayed hypersensitivity reactions, inducing a CD4+ T-lymphocyte proliferation response in humans and experimental animals (2, 37).
According to the idiotypic network hypothesis proposed by Jerne (20), different antigenic determinants within variable domains of immunoglobulins can be recognized and can elicit an immune response in the same individual (5, 20). These antigenic determinants are known collectively as idiotypes (Ids). The potential immunoregulatory role of Id–anti-Id interactions has been intensively investigated by several groups (4, 21, 42).
Our group demonstrated that mice immunized with anti-gp43 monoclonal antibodies (MAbs) (Ab1), shown to induce the idiotypic cascade in the gp43 system, produced both anti-Id antibodies (Ab2) and anti-anti-Id antibodies (Ab3). Moreover, we found for the first time that PCM patient sera also displayed significant amounts of Ab2. Our results showed spontaneous modulation of the idiotypic cascade in the gp43 system of P. brasiliensis in both mice and humans (35).
Although we have demonstrated Ab2 in patients with PCM (35), the possible correlation between the levels of Ab1 and Ab2 in the different clinical forms of the disease has not been investigated. Since anti-Id antibodies may have immunomodulating functions, we decided to characterize the human anti-Id antibodies in this system and verify their possible correlation with different clinical forms of the disease. The results presented here demonstrate that all clinical forms of the disease have spontaneous anti-Id antibodies and suggest a correlation with the different clinical forms of the disease. Also, our data suggest that anti-Id antibodies correlate with anti-gp43 titers.
MATERIALS AND METHODS
Human serum specimens.
Individual serum specimens from 32 patients with PCM (8 from patients with the AF, 10 from patients with the UCF, and 14 from patients with the MCF) were selected based on clinical diagnosis of the disease (6) and confirmed by positive direct examination of characteristic multiple budding yeast forms either in histopathologic sections or in biological fluids. All patient sera were found positive for anti-gp43 antibodies by both the immunodiffusion test (9) and a capture enzyme immunoassay (EIA), which was shown to be more sensitive and specific (10). These sera were collected before antimycotic therapy. Five patients were given antimycotic therapy (three with itraconazole, one with fluconazole, and one with a sulfamide derivative), and none was considered cured during the time span of this study. All serum specimens, from healthy individuals or from PCM patients, were obtained from Instituto de Medicina Tropical de São Paulo or Hospital São Paulo, Federal University of São Paulo.
MAbs.
The anti-gp43 MAbs 17c, 40.d7, 27a, 3e, and 8a (all immunoglobulin G2b [IgG2b], κ light chain) (16, 29) and the anti-anti-carcinoembryonic antigen MAb 6.C4 (IgG2b) (26) were used in EIAs. The anti-gp43 MAbs 17c and 8a were also used for the purification of human anti-Id antibodies (Ab2) as described below.
Purification of anti-gp43 antibodies from human PCM sera.
Patient sera with high titers of anti-gp43 antibodies were selected for purification by affinity chromatography. For this purpose, CNBr-activated Sepharose 4B (Pharmacia, Uppsala, Sweden) coupled to gp43 according to the manufacturer's instructions was used. Briefly, 5 ml of filtered patient sera adjusted to pH 8.2 was applied to the column for 3 h. After a wash with 50 mM Tris base–0.15 M NaCl buffer (pH 8.2), antibodies were eluted with 50 mM glycine–0.15 M NaCl buffer (pH 2.8). The protein concentration was determined at 280 nm. All purified human anti-gp43 antibodies were tested for their ability to bind to gp43 in EIAs and immunoblotting tests as described below.
Purification of anti-Id antibodies (Ab2) from human PCM sera.
Human anti-Id antibodies were purified by affinity chromatography with CNBr-activated Sepharose 4B coupled to both murine anti-gp43 MAbs 17c and 8a according to the manufacturers' instructions. For this purpose, patient sera with high titers of anti-Id antibodies were selected.
Binding of purified human Ab1 to gp43 in the EIA.
The EIA was performed as described before (11). Briefly, polyvinyl microplates (Costar Corp., Cambridge, Mass.) were coated with 2 μg of purified gp43 per ml in phosphate-buffered saline (PBS, 50 μl/well) for 1 h at 37°C. Free sites were blocked with PBS containing 1% bovine serum albumin (BSA) for 1 h at 37°C, followed by treatment of the wells with 0 to 20 μg of purified human Ab1 per ml or 10 μg of an IgG pool obtained from 20 healthy volunteers per ml (negative control) in a final volume of 50 μl/well for 1 h at 37°C. After incubation, wells were washed three times with PBS containing 0.5% gelatin (Difco Laboratories, Detroit, Mich.) and 0.05% Tween 20 (Sigma Chemical Co., St Louis, Mo.) (PBS-Tw-G) and then treated for 1 h at 37°C with 50 μl of goat anti-human IgG–horseradish peroxidase conjugate (Bio-Rad Laboratories, Hercules, Calif.). Microplates were then washed as described before, and reactions were developed with o-phenylenediamine (Sigma) in 0.1 M acetate-phosphate buffer (pH 5.8) and interrupted with 4 N H2SO4; results were read with a Titertek Multiscan EIA reader at 492 nm. Each datum point represents experiments done at least twice, always in duplicate.
Binding of purified human Ab2 to murine anti-gp43 MAbs.
Murine anti-gp43 MAbs 17c, 40.d7, 27a, 3e, and 8a were used in binding assays with purified human Ab2. MAb 6.C4 (26) was used as an irrelevant MAb. Briefly, polyvinyl microplates were coated with anti-gp43 MAbs and the irrelevant MAb (10 μg/ml, 100 μl/well) diluted in PBS for 1 h at 37°C. The remaining sites of wells were blocked as described above, and 10 μg of purified human Ab2 per ml was added and incubated for 1 h at 37°C. After three washes, wells were treated as described before.
Inhibition by purified human Ab1 of binding of affinity-purified Ab2 to anti-gp43 MAb 17c.
Purified human Ab1 were used for the inhibition of binding of purified human Ab2 to anti-gp43 MAb 17c in an EIA. Microplate wells were coated with 20 μg of purified human Ab2 per ml (100 μl/well) and incubated for 1 h at 37°C. After blocking of free sites with PBS–1% BSA, 0 to 10 μg of murine anti-gp43 MAb 17C per ml (50 μl/well) in the presence or absence of purified human Ab1 (0.25, 2.5, or 10 μg/ml) was added. After incubation for 1 h at 37°C, wells were exhaustively washed and treated for 1 h at 37°C with 100 μl of anti-murine IgG coupled to peroxidase (Bio-Rad). Wells were further treated as described above.
Detection of anti-gp43 antibodies in human PCM sera.
PCM patient sera were diluted 1:25,000 (vol/vol) in PBS and assayed by a capture EIA as described before (11, 36). Briefly, polyvinyl microplates were coated with 20 μg of anti-gp43 MAb 17c per ml in PBS (100 μl/well) for 1 h at 37°C. Free sites were blocked with PBS–1% BSA, followed by treatment of the wells with 10 μg of affinity-purified gp43 per ml (100 μl/well) for 1 h at 37°C. Wells were then exhaustively washed with PBS-Tw-G and incubated overnight at 4°C with diluted human PCM sera. After incubation, wells were treated as described above.
Detection of anti-Id antibodies (Ab2) in human PCM sera.
All human PCM sera or sera from healthy volunteers were serially diluted (volume/volume) in PBS containing 0.1% BSA, and the presence of Ab2 was determined as described before (35). Briefly, microplate wells were coated with 20 μg of murine anti-gp43 MAb 17c per ml (100 μl/well) for 1 h at 37°C and blocked as described above. Wells were then incubated overnight at 4°C with diluted patient sera. Afterward, the plates were treated with the conditions already mentioned.
SDS-PAGE and immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on vertical slab gels of 10% acrylamide, always under reducing conditions (23). Immunoblotting was performed as described elsewhere (38).
Statistical analysis.
Data were analyzed by the Student-Newman-Keuls multiple-comparisons test or by a one-way analysis of variance.
RESULTS
Characterization of purified human anti-gp43 antibodies.
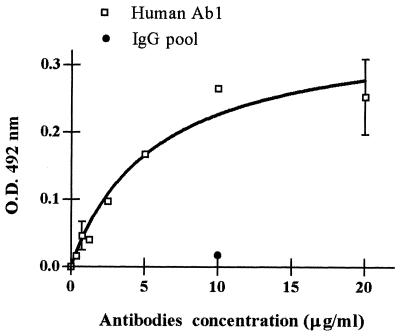
Affinity-purified human Ab1 were characterized for their ability to bind to gp43 in both EIAs and immunoblotting tests. They were able to bind to gp43 in an EIA with a saturation tendency. A pool of IgG obtained from 20 healthy volunteers and purified in Sepharose-protein A was used as a negative control (Fig. 1). With immunoblotting, it was demonstrated that purified human Ab1 recognized gp43 in both its integral and its deglycosylated forms, thus showing that binding occurs through peptidic epitopes of the molecule (data not shown).
FIG. 1.
Binding of purified human anti-gp43 antibodies to gp43 in an EIA. gp43 was recognized by purified human anti-gp43 antibodies at concentrations ranging from 0 to 20 μg/ml. A pool of purified IgG from healthy individuals was used as a negative control. Error bars show standard deviations. O.D., optical density.
Characterization of purified anti-Id antibodies (Ab2).
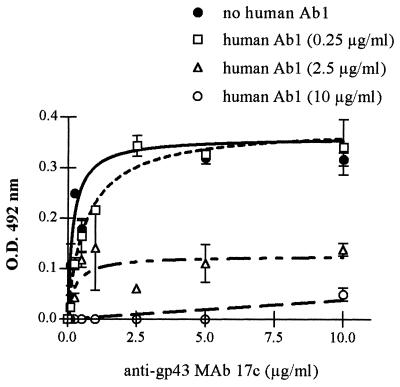
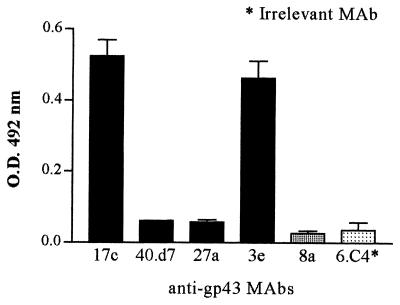
In order to characterize anti-Id antibodies, inhibition of the binding of affinity-purified human Ab2 to murine anti-gp43 MAb 17c (Ab1) by purified human anti-gp43 antibodies (Ab1) was investigated. The binding of anti-gp43 MAb 17c to purified human Ab2 showed a typical saturation curve that was inhibited by purified human Ab1 in a dose-dependent pattern. Moreover, at a purified human Ab1 concentration of 10 μg/ml, binding inhibition was nearly 100% (Fig. 2). Purified human Ab2 reacted with the idiotypes of MAbs 17c and 3e. However, idiotypes of MAbs 40.d7 and 27a were not recognized. Although human Ab2 were purified by affinity chromatography in CNBr-activated Sepharose 4B coupled to MAbs 17c and 8a, they did not recognize the MAb 8a idiotype. Finally, as expected, there was no significant binding to the irrelevant MAb 6.C4 (Fig. 3).
FIG. 2.
Inhibition of the binding of purified human anti-Id antibodies (Ab2) to anti-gp43 MAb 17c by purified human anti-gp43 antibodies. Binding of 0.25 to 10 μg of anti-gp43 MAb 17c per ml to purified human anti-Id antibody (Ab2)-coated plates was measured in the absence or presence of purified human anti-gp43 antibodies. Data were obtained from a representative patient. Assays were done in duplicate. Error bars show standard deviations. O.D., optical density.
FIG. 3.
Binding of purified human anti-Id antibodies (Ab2) to anti-gp43 MAbs. Plates coated with anti-gp43 MAbs 17c, 40.d7, 27a, 3e, and 8a were assayed for recognition by purified human anti-Id antibodies (Ab2). Positive reactions were only seen for MAbs 17c and 3e. MAb 6.C4 was used as a control. This plot is representative of three independent experiments. Error bars show standard deviations. O.D., optical density.
Detection of anti-gp43 antibodies (Ab1) and anti-Id antibodies (Ab2) in PCM sera from humans with different clinical forms of PCM.
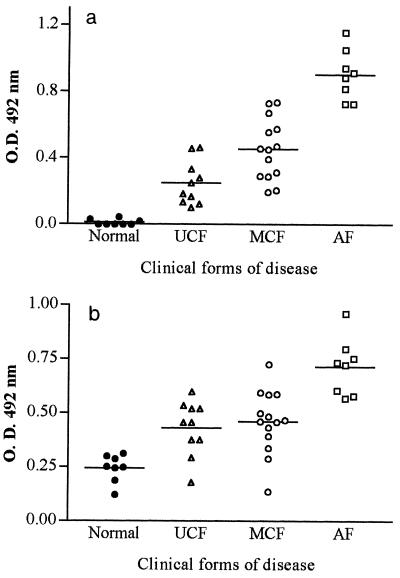
Figure 4a shows the anti-gp43 titers in sera of patients with different clinical forms of PCM (all diluted 1:25,000). Confirming previous results (9), patients with the AF of PCM had higher titers of anti-gp43 antibodies than patients with the MCF and UCF (P, <0.001). When the MCF and the UCF were compared, the first showed higher anti-gp43 titers (P, <0.01).
FIG. 4.
Detection of anti-gp43 antibodies by a capture EIA (a) and anti-Id antibodies (b) in sera of patients with different clinical forms of PCM. Sera of healthy individuals were used as negative controls. Both assays were performed with MAb 17c bound to the solid phase. Each datum point represents the result for each patient. O.D., optical density. Means for both Ab1 and Ab2 are indicated (horizontal lines).
In order to verify whether the anti-Id pattern was similar to the anti-gp43 pattern in the different clinical forms of PCM, patient sera diluted 1:500 were used in EIAs as described in Materials and Methods (Fig. 4b). Similar to the results found for anti-gp43 antibodies, the anti-Id levels were higher in the AF than in the CF of PCM (P, <0.001). However, with this serum dilution, no statistical difference was detected between the MCF and the UCF (P, >0.05). All sera were previously tested and did not react against BSA in the solid phase.
To corroborate these results, determination of titers of anti-Id antibodies (Ab2) was carried out (Table 1). The majority of AF patients (88%) had high titers (≥1:8,000) of Ab2, while only 29% of MCF patients and 0% of UCF patients did. Seventy percent of patients with the UCF of PCM displayed low titers (≤1:2,000) of Ab2. Taken together, these results strongly suggest a correlation between spontaneous anti-Id antibodies and the severity of disease.
TABLE 1.
Presence of anti-Id antibodies in sera from untreated patients with different clinical forms of PCM
| Clinical form | Classification of Ab2 titersa | No. (%) of Ab2-positive patients |
|---|---|---|
| AF (n = 8) | High | 7 (88) |
| Medium | 1 (12) | |
| Low | 0 (0) | |
| MCF (n = 14) | High | 4 (29) |
| Medium | 2 (14) | |
| Low | 8 (57) | |
| UCF (n = 10) | High | 0 (0) |
| Medium | 3 (30) | |
| Low | 7 (70) |
High, ≥1:8,000; medium, 1:4,000; low, ≤1:2,000.
Correlation between Ab1 and Ab2 in patients with different clinical forms of PCM.
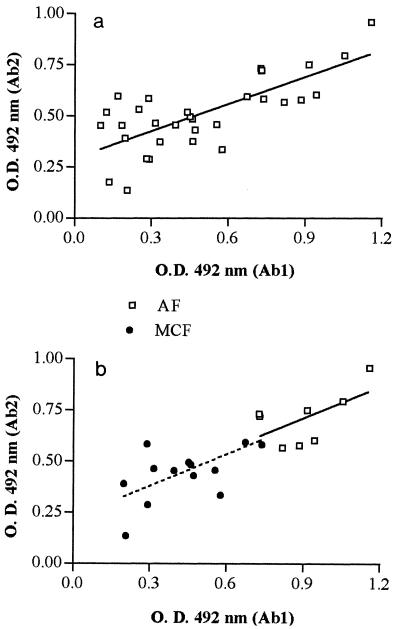
The correlation between anti-gp43 antibodies (Ab1) and spontaneous anti-Id antibodies (Ab2) in human PCM sera (before antimycotic treatment) was also investigated (Fig. 5). Figure 5a shows the results obtained for all patient sera regardless of the clinical form of the disease. The correlation coefficient for Ab1 and Ab2 (r, 0.7301) was considered extremely significant (P, <0.0001). However, analysis of the clinical forms of PCM disclosed some particularities in that correlation. As observed in Fig. 5b, there was a correlation between Ab1 and Ab2 in the AF (r, 0.5761, P, 0.0195) as well as in the MCF of the disease (r, 0.6434, P, 0.0002). Strikingly, in the UCF, no significant correlation was observed even when lower dilutions (1:10,000) were used for the Ab1 assay (data not shown).
FIG. 5.
Correlation between anti-gp43 antibodies (Ab1) and anti-Id antibodies (Ab2) in sera of patients with PCM before antimycotic therapy. Analysis of the correlation was performed for all patient sera regardless of clinical forms (n = 32) (a) or with consideration of clinical forms: AF (n = 8) and MCF (n = 14). O.D., optical density.
Follow-up of anti-gp43 and anti-Id antibodies in patients given antimycotic therapy.
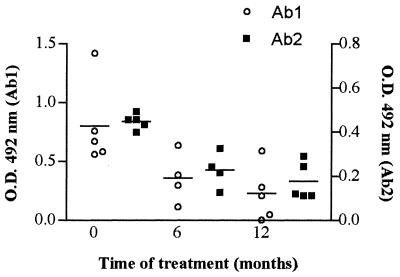
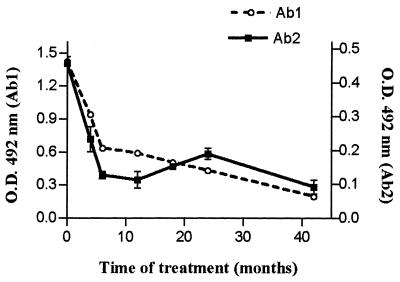
Some PCM patients were serologically monitored along the course of treatment. Figure 6 shows the anti-gp43 and anti-Id titers in five patients with the AF of the disease after 0, 6, and 12 months of antimycotic therapy. Results showed that both anti-gp43 and anti-Id levels decayed in parallel along the treatment. Even after 12 months of adequate treatment, there were significant amounts of both anti-gp43 and anti-Id antibodies. Similar results were observed in Fig. 7, which presents data obtained from a representative patient during 42 months of antimycotic therapy.
FIG. 6.
Follow-up of anti-gp43 antibody (Ab1) and anti-Id antibody (Ab2) titers in patients given antimycotic therapy. Sera of patients with the AF of PCM were assayed for the presence of anti-gp43 and anti-Id antibodies at 0, 6, and 12 months after antimycotic treatment. Datum points represent optical density (O.D.) values minus the average O.D. (0.174) obtained for normal sera diluted 1:1,000 for Ab2 tests. Lines indicate mean values for both Ab1 and Ab2.
FIG. 7.
Follow-up of anti-gp43 antibody (Ab1) and anti-Id antibody (Ab2) titers in a representative patient given antimycotic therapy. Sera of patients with the AF of PCM were assayed for the presence of anti-gp43 and anti-Id antibodies at 0, 4, 6, 12, 18, 24, and 42 months after antimycotic treatment. Datum points represent optical density (O.D.) values minus the average O.D. (0.149) obtained for normal sera diluted 1:1,000 for Ab2 tests. Error bars indicate standard deviations.
DISCUSSION
In 1974, Jerne (20) postulated a hypothesis in which the idiotypic network could have influence in immunoregulation. According to Jerne's hypothesis, lymphocyte antigen receptors (i.e., immunoglobulin on B cells and T-cell receptor on T cells) express antigenic determinants which are potentially immunogenic; i.e., humoral and cellular immune responses in an individual can be induced against antigen receptors expressed by the lymphocytes of that same individual. These antigenic determinants are known collectively as Ids. Jerne's network theory predicts that anti-Id antibodies (Ab2), bearing the internal image of the corresponding antigen, are able to induce immunity to the original antigen in hosts not previously exposed to it (19, 20, 28). It has been suggested that the immune response can be modulated at the level of recognition of idiotype determinants via the idiotype–anti-idiotype network (4, 21). While there is substantial evidence indicating that anti-Id antibodies are normal components of the immune response, their significance in its regulation remains unclear (29, 34). Although it is generally assumed that spontaneous anti-Id antibodies, which arise in an intact network, will decrease immune responses associated with a particular Id, anti-Id antibodies may also have the capacity to increase immune responses (12, 14).
Recent studies showed that the course of PCM is under genetic control (7). It was shown that an autosomal dominant gene controls resistance or susceptibility to the disease. It is assumed that in resistant mice, which carry the Pbr (P. brasiliensis resistance) gene, the immune response is directed to Th1 activation, with consequent resolution of the disease. Susceptible mice, which carry the P. brasiliensis susceptibility (Pbs) gene, would mount predominantly a Th2 type of immune response, leading to progressive disease (7, 8). It has been demonstrated by our group and by others (24) that PCM patients show high levels of antibodies directed against gp43 (Ab1), the major antigenic component of the fungus. We also recently demonstrated the existence of significant titers of anti-Id antibodies (Ab2) in those patients (35).
Since spontaneous anti-Id antibodies can play a significant role in the immune response against several pathogens and also because anti-Id antibodies can be responsible for high titers of antibodies in several infections (1, 41), we decided to further characterize human Ab1 and Ab2 in PCM. For this purpose, sera of PCM patients with the AF, MCF, and UCF of the disease were evaluated in this study.
In order to better characterize both human anti-gp43 antibodies (Ab1) and anti-anti-gp43 antibodies (Ab2), sera from untreated PCM patients displaying high titers of anti-gp43 antibodies were affinity purified using Sepharose coupled with purified gp43 or with murine anti-gp43 MAbs, respectively. As expected, affinity-purified human anti-gp43 antibodies recognized specifically the antigen (gp43) in an EIA. It was observed that when increasing amounts of affinity-purified human anti-gp43 antibodies were assayed, a “plateau” was reached after the addition of >5 μg of anti-gp43 antibodies per ml, strongly suggesting saturation (Fig. 1). Moreover, the affinity-purified human anti-gp43 antibodies were able to specifically recognize deglycosylated gp43, suggesting that the majority of them are directed against peptidic regions of the antigen, as observed in mice (35). Considering our previous observation with mice showing that Ab2 could also induce Ab3 production (35), we cannot rule out the possibility that affinity-purified human polyclonal antibodies recognizing specifically gp43 could be a mixture of both Ab1 and Ab3.
Spontaneously produced anti-Id antibodies (Ab2) were also affinity purified and characterized. As expected, results showed that the purified human Ab2 were able to specifically recognize the idiotype of murine anti-gp43 MAb 17c. When >1.0 μg/ml was added, a typical saturation plot was obtained. This binding was inhibited by purified human anti-gp43 antibodies in a dose-dependent pattern. Furthermore, at a concentration of 10 μg of purified human Ab1 per ml, binding inhibition was close to 100% (Fig. 2). These findings suggest that purified human anti-Id antibodies are directed against antigenic determinants on the binding site (idiotopes) of human anti-gp43 antibodies and also that the majority of them probably bear the internal image of the antigen (gp43). Anti-Id antibodies with these properties are classified as Ab2β. It has been assumed that Ab2β are able to elicit immune responses against the original antigen and therefore potentially have an immunoregulatory function (3, 5, 28).
The specificity of purified human anti-Id antibodies was also confirmed through their binding to several murine anti-gp43 MAbs (Fig. 3). Purified human Ab2 recognized MAbs 17c and 3e but not MAbs 40.d7 and 27a although, as demonstrated before, these anti-gp43 antibodies compete with MAb 17c in gp43 peptide recognition (17). These results demonstrated that different antibodies can recognize the same epitope, without sharing the same idiotype, confirming our previous results obtained with mice (35). On the other hand, purified human anti-Id antibodies were not able to recognize MAb 8a, even though they were affinity purified in a column coupled to both MAbs 8a and 17c. Several possibilities can explain this phenomenon, such as the amount and affinity of Ab2 for MAb 8a present in the human sera, the amount of MAb 8a bound to the column, and the lack of a similarly immunogenic MAb 8a idiotype in humans.
The fact that purified human anti-Id antibodies recognize some but not all idiotypes of murine anti-gp43 MAbs strongly suggests that at least some idiotypes are conserved in humans. Other studies have demonstrated that certain idiotypes appear on a large proportion of antibody molecules in several individuals of the same or of different species; such idiotypes are known as public, recurrent, dominant, or cross-reactive (CR) idiotypes (CRI) (21). The importance of CRI in the pathogenesis of disease or in immunoregulation has been demonstrated in several systems (1, 18, 41). Many but not all antibodies bearing CRI in these disease models are encoded by germ line gene sequences (1, 18). The results reported here imply the existence in gp43 of dominant epitopes that induce conserved, possibly germ line gene-encoded, humoral responses in humans and mice, as has been postulated for the antibacterial T15+ Id-bearing antibody responses of mice and humans (18).
After characterization of human anti-Id antibodies (Ab2), the possible correlation of Ab1 and Ab2 titers in patient sera with different clinical forms of PCM was investigated. As demonstrated before by others (10), our results confirmed that anti-gp43 titers correlate with clinical forms of the disease (Fig. 4a). Since anti-gp43 antibodies (Ab1) can elicit an anti-Id antibody response (Ab2) we investigated whether the anti-Id pattern parallels the anti-gp43 pattern. It was observed that anti-Id antibody levels also correlate with the clinical forms of the disease (Fig. 4b). In order to further characterize this correlation, Ab2 titers were determined. The results showed that 88% of patients with the AF of PCM had high titers (≥1:8,000) of spontaneous anti-Id antibodies (Ab2), while only 29% of patients with the MCF and 0% of patients with the UCF did. In contrast, 70% of patients with the UCF of PCM had low titers (≤1:2,000) of Ab2 (Table 1). Since there is a correlation between anti-Id titers and the clinical forms of the disease, analysis of the titers of spontaneous anti-Id antibodies (Ab2) might be of importance for the prognosis of the disease.
Considering that the anti-gp43 pattern and the anti-Id pattern in different clinical forms of PCM are similar (Fig. 4), the possibility of a correlation between them was addressed. When all patient sera were considered regardless of the clinical form, the correlation was extremely significant (Fig. 5a). However, some differences were observed when each of the clinical forms of PCM was individually analyzed. In the AF and MCF, there was a significant correlation between anti-gp43 and anti-Id titers (Fig. 5b). However, no significant correlation was observed for the UCF (data not shown).
Some patients may have persistent significant titers of anti-gp43 antibodies even after prolonged treatment and with clinical and mycological cure (Z. P. Camargo and J. R. Alves, personal communication). The stimulation of the idiotypic cascade could be responsible for the persistence of anti-gp43 antibodies in these patients. To address this question, patients with the AF of PCM were serologically monitored during treatment. Both anti-gp43 and anti-Id titers decayed in parallel with therapy. Nevertheless, even after several months of adequate treatment, there were still significant levels of Ab2 (Fig. 6 and 7). These results suggest that Ab2 can elicit anti-gp43 antibody production after antigen elimination. It seems that spontaneous anti-Id titers also correlate with anti-gp43 titers during treatment.
Studies of patients with schistosome infection showed that those with acute infection or hepatosplenic disease had high levels of antibodies to soluble egg antigen (SEA) and low titers of anti-Id antibodies, whereas patients with the asymptomatic form of disease had low titers of anti-SEA antibodies and high titers of anti-Id antibodies (41). The authors suggested that the failure to modulate the expression of anti-SEA antibodies and produce high levels of immunomodulatory anti-Id antibodies during chronic infection correlates with severe schistosome-induced disease (41).
Because more severe forms of PCM had higher anti-Id titers and because there was a correlation between anti-gp43 and anti-Id antibodies, one can assume that in PCM anti-Id antibodies are not sufficient to modulate the immune response. However, it must be considered that the anti-Id antibodies studied here recognized only idiotypes similar to the idiotypes of murine anti-gp43 MAb 17c. Therefore, we cannot rule out the possibility of the existence in humans of different anti-Id antibodies directed against other anti-gp43 antibodies (Ab1) with a role in the pathogenesis of the infection or in its immunoregulation. Specific studies about the importance of the anti-idiotypic network in fungal pathogenesis are now being carried out in our laboratory.
Spontaneous idiotypic modulation is not a common feature in all systems, as our group has already shown (35). For PCM, we have demonstrated for the first time that the idiotypic network is unleashed after infection by the fungus in both mice and humans (35). In the present work, it was shown that there are spontaneous anti-Id antibodies (Ab2) in humans with different clinical forms of the disease and that there is a correlation between some forms and the severity of disease which could be useful for clinical evaluation.
ACKNOWLEDGMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Financiadora Nacional de Projetos (FINEP), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
We are indebted to Rosana Puccia and Luiz R. Travassos for the generous gift of anti-gp43 MAbs and to Jane Z. Moraes for providing the irrelevant MAb used (6.C4). We are also grateful to Mario Mariano, Maria Aparecida Shikanai-Yasuda, and Roger Chammas for helpful suggestions. We thank Creuza Rosa de Oliveira and Laura Dias Batista for technical assistance.
REFERENCES
- 1.Amano T, Nakazawa M, Oshima T, Bosshardt S C, Colley D G. Cross-reactive idiotypes on rabbit anti-SEA antibodies stimulate anti-idiotype spleen and lymph node cell responses of mice infected with Schistosoma mansoni. Parasite Immunol. 1996;18:21–28. doi: 10.1046/j.1365-3024.1996.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 2.Benard G, Mendes-Giannini M J S, Juvenale M, Miranda E T, Duarte A J S. Immunosuppression in paracoccidioidomycosis: T cell hyporesponsiveness to two Paracoccidioides brasiliensis glycoproteins that elicit strong humoral immune response. J Infect Dis. 1997;175:1263–1267. doi: 10.1086/593694. [DOI] [PubMed] [Google Scholar]
- 3.Betákova T, Varecková E, Kostolanský F, Mucha V, Daniels R S. Monoclonal anti-idiotypic antibodies mimicking the immunodominant epitope of influenza virus haemagglutinin elicit biologically significant immune responses. J Gen Virol. 1998;79:461–470. doi: 10.1099/0022-1317-79-3-461. [DOI] [PubMed] [Google Scholar]
- 4.Bona C A, Köhler H. Anti-idiotypic antibodies and internal images. Recept Biochem Methodol. 1984;14:141–149. [Google Scholar]
- 5.Bona C A. Internal image concept revisited. Proc Soc Exp Biol Med. 1996;213:32–42. doi: 10.3181/00379727-213-44033. [DOI] [PubMed] [Google Scholar]
- 6.Brummer E, Castañeda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6:89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calich V L G, Burger E, Kashino S S, Fazioli R A, Singer-Vermes L M. Resistance to Paracoccidioides brasiliensis in mice is controlled by a single autosomal gene. Infect Immun. 1987;55:1919–1923. doi: 10.1128/iai.55.8.1919-1923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calich V L G, Kashino S S. Cytokines produced by susceptible and resistant mice in the course of Paracoccidioides brasiliensis infection. Braz J Med Biol Res. 1998;31:615–623. doi: 10.1590/s0100-879x1998000500003. [DOI] [PubMed] [Google Scholar]
- 9.Camargo Z P, Unterkircher C, Campoy S, Travassos L R. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol. 1988;26:2147–2151. doi: 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo Z P, Cano L E R. Humoral immunity. In: Franco M, Lacaz C S, Restrepo-Moreno A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 187–201. [Google Scholar]
- 11.Camargo Z P, Gesztesi J-L, Saraiva E C O, Taborda C P, Vicentini A P, Lopes J D. Monoclonal antibody capture enzyme immunoassay for detection of Paracoccidioides brasiliensis antibodies in paracoccidioidomycosis. J Clin Microbiol. 1994;32:2377–2381. doi: 10.1128/jcm.32.10.2377-2381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dang H, Ogawa N, Takei M, Lazaridis K, Talal N. Induction of lupus-associated autoantibodies by immunization with native and recombinant Ig polypeptides expressing a cross-reactive idiotype 4B4. J Immunol. 1993;151:7260–7267. [PubMed] [Google Scholar]
- 13.Del Negro G, Lacaz C S, Zamith U A, Siqueira A M. General clinical aspects: polar forms of paracoccidioidomycosis, the disease in childhood. In: Franco M, Lacaz C S, Restrepo-Moreno A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 225–232. [Google Scholar]
- 14.Fagerberg J, Frodin J-E, Wigzell H, Mellstedt H. Induction of an immune network cascade in cancer patients treated with monoclonal antibodies (ab1). I. May induction of ab1-reactive T cells and anti-anti-idiotypic antibodies (ab3) lead to tumor regression after mAb therapy? Cancer Immunol Immunother. 1993;37:264–270. doi: 10.1007/BF01518521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferri R G. Estudo imunoquímico de antígenos intracelulares. Hospital (Rio de Janeiro) 1961;59:917–924. [PubMed] [Google Scholar]
- 16.Franco M. Host-parasite relationships. J Med Vet Mycol. 1987;25:5–8. doi: 10.1080/02681218780000021. [DOI] [PubMed] [Google Scholar]
- 17.Gesztesi J-L, Puccia R, Travassos L R, Vicentini A P, de Moraes J Z, Franco M F, Lopes J D. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15:415–422. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 18.Halpern R, Kaveri S V, Kohler H. Human anti-phosphorylcholine antibodies share idiotopes and are self-binding. J Clin Investig. 1991;88:476–482. doi: 10.1172/JCI115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herlyn D, Somasundaram R, Li W, Maruyama H. Anti-idiotype cancer vaccines: past and future. Cancer Immunol Immunother. 1996;43:65–76. doi: 10.1007/s002620050305. [DOI] [PubMed] [Google Scholar]
- 20.Jerne N K. Towards a network theory of the immune response. Ann Immunol Paris. 1974;125c:373–389. [PubMed] [Google Scholar]
- 21.Jerne N K, Roland J, Cazenave P A. Recurrent idiotopes and internal images. EMBO J. 1982;1:243–247. doi: 10.1002/j.1460-2075.1982.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacaz C S. Mycological diagnosis. In: Franco M, Lacaz C S, Restrepo-Moreno A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 339–344. [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Mendes-Giannini M J S, Bueno J P, Shikanai-Yasuda M A, Stolf A M S, Masuda A, Neto V A, Ferreira A W. Antibody response to the 43 kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am J Trop Med Hyg. 1990;43:200–206. doi: 10.4269/ajtmh.1990.43.200. [DOI] [PubMed] [Google Scholar]
- 25.Montenegro M R, Franco M. Pathology. In: Franco M, Lacaz C S, Restrepo-Moreno A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 131–150. [Google Scholar]
- 26.Moraes J Z, Carneiro C R W, Buchegger F, Mach J-P, Lopes J D. Induction of an immune response through the idiotypic network with monoclonal anti-idiotype antibodies in the carcinoembryonic antigen system. J Cell Biochem. 1992;50:324–335. doi: 10.1002/jcb.240500313. [DOI] [PubMed] [Google Scholar]
- 27.Moses A. Fixação de complemento na blastomicose. Mem Inst Oswaldo Cruz. 1916;8:68–70. [Google Scholar]
- 28.Nisonoff A, Lamoyi E. Implications of the presence of internal images of the antigen in anti-idiotypic antibodies: possible applications to vaccine production. Clin Immunol Immunopathol. 1981;21:397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- 29.Nisonoff A. Idiotypes: concepts and applications. J Immunol. 1991;147:2429–2438. [PubMed] [Google Scholar]
- 30.Puccia R, Travassos L R. 43-Kilodalton glycoprotein from Paracoccidioides brasiliensis: immunochemical reactions with sera from patients with paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J Clin Microbiol. 1991;29:1610–1615. doi: 10.1128/jcm.29.8.1610-1615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restrepo A. The ecology of Paracoccidioides brasiliensis: a puzzle still unsolved. Sabouraudia. 1985;23:323–334. [PubMed] [Google Scholar]
- 32.Restrepo A, Drouhet E. Étude des anticorps précipitants dans la blastomycose sul-américaine par l'analyse immunoéletrophorétique des antigènes de Paracoccidioides brasiliensis. Ann Inst Pasteur. 1970;119:338–346. [PubMed] [Google Scholar]
- 33.Restrepo A, Moncada L H. Characterization of the precipitin bands detected in the immunodiffusion test for paracoccidioidomycosis. Appl Microbiol. 1974;28:138–144. doi: 10.1128/am.28.1.138-144.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodey G E. Anti-idiotypic antibodies and regulation of immune responses. Transfusion. 1992;32:361–372. doi: 10.1046/j.1537-2995.1992.32492263453.x. [DOI] [PubMed] [Google Scholar]
- 35.Souza A R, Gesztesi J-L, Moraes J Z, Cruz C R B, Sato J, Mariano M, Lopes J D. Evidence of idiotypic modulation in the immune response to gp43, the major antigenic component of Paracoccidioides brasiliensis in both mice and humans. Clin Exp Immunol. 1998;114:40–48. doi: 10.1046/j.1365-2249.1998.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souza M C, Gesztesi J-L, Souza A R, Moraes J Z, Lopes J D, Camargo Z P. Differences in reactivity of paracoccidioidomycosis sera with gp43 isoforms. J Med Vet Mycol. 1997;35:13–18. doi: 10.1080/02681219780000811. [DOI] [PubMed] [Google Scholar]
- 37.Taborda C P, Juliano M A, Puccia R, Franco M, Travassos L R. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun. 1998;66:786–793. doi: 10.1128/iai.66.2.786-793.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Travassos L R. Immunochemistry of Paracoccidioides brasiliensis antigens. In: Franco M, Lacaz C S, Restrepo-Moreno A, del Negro G, editors. Paracoccidioidomycosis. Boca Raton, Fla: CRC Press, Inc.; 1994. pp. 67–86. [Google Scholar]
- 40.Vicentini A P, Gesztesi J-L, Franco M F, de Souza W, de Moraes J Z, Travassos L R, Lopes J D. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun. 1994;62:1465–1469. doi: 10.1128/iai.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisnewski A V, Olds G R, Johnson J H, Ramirez B, Kresina T F. Function and expression of a human idiotypic network in schistosomiasis japonica. Parasite Immunol. 1996;18:439–447. doi: 10.1111/j.1365-3024.1996.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y F, Thanavalla Y A. A comparison of the antibody and T cell response elicited by internal image and noninternal image anti-idiotypes. Clin Immunol Immunopathol. 1995;75:154–158. doi: 10.1006/clin.1995.1065. [DOI] [PubMed] [Google Scholar]
- 43.Yarzábal L A, Bout D, Naquira F, Fruit L, Andrieu S. Identification and purification of the specific antigen of Paracoccidioides brasiliensis responsible for immunoelectrophoretic band E. Sabouradia. 1977;15:9–15. [PubMed] [Google Scholar]