Abstract
Treatment of obstructive sleep apnoea in adults is evolving, from a “one treatment fits all” to a more individualised approach. The spectrum of treatment options is broad and heterogeneous, including conservative, technological and pharmaceutical modalities. This raises the questions of which patients these modalities might be useful for, and if there are specific criteria for single or combined treatment. The most commonly used non-CPAP treatment is a mandibular advancement device. Furthermore, it appears from the available evidence that upper airway surgery, bariatric surgery, and maxillomandibular advancement can be effective in particular patient groups and should be indicated more readily in clinical practice. Technically, a tracheotomy is the most effective surgical treatment, but is not socially acceptable and is associated with major side-effects. Other treatment options are emerging, like positional therapy, hypoglossal nerve stimulation, and myofunctional exercises. Drug therapy is also promising when pathophysiological traits are considered.
The range of currently available treatment options will be discussed in this review, with emphasis on the selection of appropriate patients, therapeutic efficacy and compliance, and reference to recent guidelines. In the selection process, routine application of drug-induced sleep endoscopy to assess the site(s) of collapse during sleep can increase the success rate of both surgical interventions and oral appliance therapy.
Educational aims
To outline recommendations concerning the proper management of obstructive sleep apnoea (OSA) patients that cannot be treated adequately with continuous positive airway pressure (CPAP) due to intolerance, poor adherence or compliance, or CPAP refusal.
To provide information about the selection of appropriate patients for alternative non-CPAP treatment options.
To better understand the different aspects of OSA treatment with noninvasive approaches, such as oral appliances, positional therapy, drug treatment and myofunctional therapy, including indications, contraindications, and expected short- and long-term results.
To discuss the different surgical options for the treatment of OSA and to provide information on the important issue of proper patient selection for surgery, as most OSA surgical outcomes are associated with the pre-operative assessment of the level(s) of upper airway collapse.
Short abstract
Treatment of obstructive sleep apnoea in adults is evolving, from a “one treatment fits all” to a more individualised approach. Proper patient selection and use of specific criteria are crucial for a beneficial outcome. https://bit.ly/3Avydju
Introduction
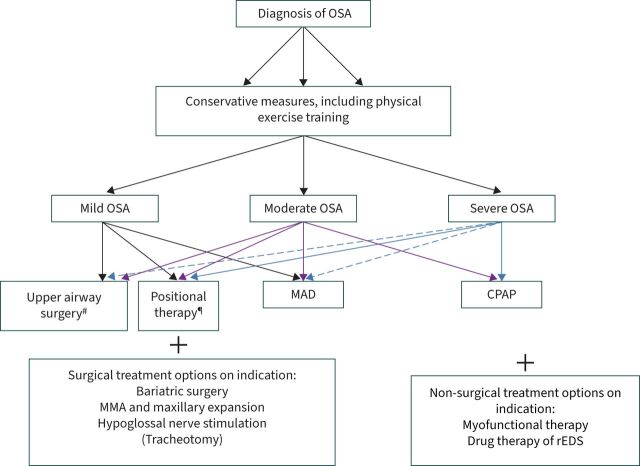
Currently, several treatment modalities are available for patients diagnosed with obstructive sleep apnoea (OSA). In general, therapeutic strategies for patients with OSA may be categorised into three main groups: behaviour modifications, indicated for all patients with a modifiable risk factor; surgical treatment; and non-surgical treatment options (figure 1). Overall, it is of paramount importance to select the most effective therapeutic option for the individual patient. The final goal is to establish a stable ventilatory pattern and oxygen saturation curve, eliminate sleep fragmentation, abolish snoring and establish symptomatic control, which is in the patient's direct interest. The standard treatment for moderate-to-severe OSA is application of nasal continuous positive airway pressure (CPAP). Its effectiveness is potentially high, but accounting for the rather low acceptance and compliance, its actual effect and use remains relatively low, encouraging the search for reliable alternatives to CPAP in selected groups [1–4]. Today, oral appliances are widely prescribed for the treatment of mild and moderate OSA. Positional therapy, bariatric surgery, maxillofacial surgery, upper airway surgery including hypoglossal nerve stimulation, myofunctional therapy and drug therapy are growing in popularity. The aim of this review is to discuss these non-CPAP treatment modalities for OSA.
FIGURE 1.
Current treatment modalities in obstructive sleep apnoea (OSA). Dashed arrows: mandibular advancement device (MAD) therapy or upper airway surgery indicated in patients who do not tolerate continuous positive airway pressure (CPAP). MMA: maxillomandibular advancement; rEDS: residual excessive daytime sleepiness. #: in selected patients (drug-induced sleep endoscopy); ¶: if position dependent.
Lifestyle modification (behavioural therapy)
First-line treatment of OSA starts with the avoidance of aggravating factors, such as intake of alcohol, sedatives and muscle relaxants before bedtime, weight gain, lack of exercise, and smoking, which may influence both anatomical and non-anatomical traits, and thus the severity of OSA. Alcohol selectively reduces upper airway muscle tone, and increases the frequency of apnoeas and hypopnoeas during sleep. Alcohol also prolongs respiratory event duration by delaying arousal and is an important source of calories. Active treatment of OSA can be significantly affected by the amount of alcohol intake [5, 6]. Sedatives, like benzodiazepines and Z-drugs can have adverse effects in some OSA patients, but do not necessarily increase the apnoea–hypopnoea index (AHI), while improving sleep efficiency and inducing no adverse consequences on alertness the day after [7]. Obesity and being overweight are major risk factors for the development of OSA. Successful dietary weight loss may improve the AHI in obese OSA patients [1, 2, 8]. Weight loss is recommended in more than 80% of cases and leads to a decrease in upper airway collapsibility, and consequently decreases the severity of OSA [9]. In a clinical cohort, Georgoulis et al. [10] found that even a <5% weight loss can reduce respiratory events, but a ≥5%, and ideally ≥10%, weight loss was necessary for reducing the prevalence of severe OSA. Unfortunately, only a minority of overweight subjects can lose and keep off weight. This problem is exacerbated when patients have daytime sleepiness, tend to snack to stay awake, and have a passive lifestyle. Some patients even report a weight gain, which could be related to altered energy expenditure at night when receiving a conventional therapy. It must be highlighted that weight loss is always the first option in all OSA patients that are overweight or obese. On top of this, other OSA treatment can be initiated, and follow-up of such patients is of paramount importance to reassess OSA severity. The risks of insufficient treatment should also be discussed with the patient if limited weight loss is achieved. It is also important to emphasise that OSA can recur, even if weight loss is maintained. Moderate intensity exercise training is an inexpensive and healthy conservative intervention that may help individual patients with OSA to improve their AHI, quality of sleep and quality of life. It has been shown to improve sleep quality, but also reduces anxiety levels in those with insomnia, even in the absence of weight reduction [11]. A reduction in rostral fluid shift from the lower body, improvement in upper airway function and changes in body composition could be involved as causal factors [12]. An association between smoking and sleep apnoea is well-known from previous studies [13]. A recent meta-analysis confirmed that even second-hand smoke exposure is significantly associated with OSA [14]. Cigarette smoking may increase the severity of OSA through alterations in sleep architecture, upper airway neuromuscular function, arousal mechanisms, and upper airway inflammation [15]. One potential adverse consequence of smoking cessation is weight gain. Median weight gain after cessation is close to 2 kg. However, about 10% of quitters experience a 13 kg increase in body weight [15]. This is counterproductive to OSA and may also serve to heighten the individual's anxiety about his or her appearance, thus thwarting quit attempts.
Oral appliances
Oral appliance therapy represents an appealing first-line treatment for selected patients with mild-to-moderate OSA. It is also recommended in adult patients with more severe OSA who are intolerant to CPAP, do not respond to CPAP, fail treatment attempts with CPAP or prefer alternative therapy [1, 2, 16].
The most frequently prescribed type of oral appliance is the mandibular advancement device (MAD), which represents the main noninvasive CPAP alternative for patients with OSA. These devices are worn intraorally at night to keep the lower jaw anteriorly positioned during sleep. The mechanism of action of the MAD is usually assumed to be an enlargement of the cross-sectional upper airway dimensions by anterior displacement of the mandible and the attached tongue, resulting in improved upper airway patency. Furthermore, the MAD aims at resisting the downward rotation of the mandible during sleep and the accompanying retrusion of the mandible, compromising upper airway patency [17].
Most of these appliances are a bespoke dentally produced device, constructed in a lab using dental impressions from plaster casts of patients’ teeth and construction bites obtained by the dentist. Potential disadvantages of this personalised method are the costs and time required to construct the device. However, standard self-moulded devices can be adapted immediately at the outpatient clinic. They are constructed of a thermoplastic material that becomes mouldable when warmed by immersion in hot water. The patient takes a mould of their teeth by biting into the softened material that then sets on cooling [18]. Different studies have found that a thermoplastic MAD could reduce AHI. However, thermoplastic MADs were less effective since they were poorly tolerated and fell out easily, so overall adherence was lower [19, 20].
Currently, custom-made titratable MADs are the recommended type of oral appliances. They consist of two parts covering the teeth of the upper and lower jaw that are dynamically interconnected, allowing for fine-tuning of the mandibular advancement through incremental advancement of the mandible, a process called titration [21].
One major limitation of MAD treatment is its dependence on oral health and the fact that it takes time to become accustomed to the device. Oral devices are attached to the teeth, and therefore this therapy is highly dependent on a healthy dentition [22–24]. Patients must have a minimal number of healthy teeth for an MAD. A minimum of 6–10 teeth in each arch is recommended, although the location rather than the number of teeth may be more important, in particular posterior teeth provide retention that is more adequate. In the absence of natural teeth, osseointegrated dental implants can effectively anchor an MAD. Currently, there are also “edentulous” options [25]. In addition, mouth opening and protrusion ability have to be assessed.
Common side-effects are discomfort of the temporomandibular joint, teeth pain, dryness of the mouth or hypersalivation (table 1). However, these are generally mild and reported mainly during the initial adaptation to MAD therapy. In the longer term, bite changes become more common, but these could well depend on the type of MAD and are usually minor, and can even be beneficial, and do not disturb patients who are satisfied with the treatment outcome in terms of symptomatic control. Generally, each candidate for MAD therapy should be examined by a qualified dentist to determine whether an MAD is feasible and safe from the dental perspective.
TABLE 1.
Potential side-effects during mandibular advancement device treatment
| Transient | Persistent |
| Discomfort or pain in the mandibular joint complex | Occasional damage to teeth and prosthetic restorations |
| Stiff masticatory muscles | Tooth shifting with undesirable occlusion changes |
| Dry mouth | Periodontal problems |
| Excessive salivation | |
| Tooth sensitivity | |
| Gum irritation |
In general, MAD therapy is efficacious in reducing snoring and the severity of OSA. However, the efficacy is patient dependent. Approximately one-third of patients under MAD therapy showed a complete resolution of the OSA disease obtaining an AHI <5 events·h−1 under MAD therapy, another third of patients showed a decrease in AHI of 50% or more, while the last one-third of patients only showed a negligible improvement in OSA severity [26, 27]. Therefore, there is a great interest in prospective studies to validate the predictive value of clinical characteristics or of the location of airway occlusion during sleep, making use of diagnostic tests such as sleep endoscopy to mention just the most frequently used technique. These tests examine whether simulating advancement of the lower jaw leads to improved patency of the upper airway during (artificially induced) sleep. With drug-induced sleep endoscopy (DISE), it was found that complete concentric collapse at the palatal level and a complete lateral oropharyngeal collapse were associated with nonresponse to MAD treatment, while tongue base collapse could be used as a positive predictor of MAD responsiveness [28–30]. Moreover, the use of a simulation bite in maximal comfortable protrusion (MCP) of the mandible, as used during DISE, tends to be effective in predicting treatment response of MAD treatment [31]. A model of the upper airway using combined upper airway imaging and computational fluid dynamics can be used to evaluate the influence of the MAD on upper airway volume and resistance and could be implemented in routine MAD outcome prediction [32, 33]. Regarding pathophysiological traits, patients with a lower collapsibility and more stable ventilatory control tend to have increased odds of responding to MAD treatment [29, 34, 35]. Phenotyping and endotyping of patients will therefore become an important tool in improving the selection process for MAD therapy.
Randomised crossover trials have shown that, compared with CPAP, MAD treatment is effective but reduces the AHI less on average. However, the reported therapy adherence appears to be higher with MAD treatment [4, 27]. Furthermore, MAD treatment is often considered by patients to be a more acceptable treatment option compared with CPAP. However, not all patients have a clinically meaningful response to oral appliances. The variety of device designs may explain some of the variability in outcome between patients and studies. The advantages over CPAP are that they are less obtrusive, make no noise, and do not need a power source.
To achieve maximum therapeutic effect, MADs with an integrated titratable mechanism allowing for gradual mandibular protrusion are to be preferred [21]. Due to the progressively applied advancement, the protrusion can be tailored individually in terms of positive effects on breathing efficacy and tolerability. However, it must be emphasised that a reduction of efficacy can occur when advancing the mandible too much. It is believed that in some cases, the airway dimension changes from a wide lateral diameter to a narrow lateral diameter if titration is overdone. Currently, the optimal titration of the MAD is most often based on “trial and error”, with a custom-made device titrated from an initial 50% of maximum mandibular advancement. The conventional method relies on subjective improvement in symptoms, although this may not provide the most accurate indicator for efficient titration. Therefore, relying on objective criteria in the titration process should be advantageous. In analogy with CPAP, titration of the most optimal mandibular protrusion could be performed using a remotely controlled mandibular positioner (RCMP) during an overnight titration polysomnography [36, 37]. Recently, it was shown that titration under direct visualisation of upper airway patency and collapsibility is feasible using the RCMP during DISE [38]. Self-reported short-term compliance with MAD treatment is high, ranging from 76% to 95% of the patients. Objective compliance monitoring is feasible by inserting a thermosensitive microsensor into the device [4, 27], but this approach is currently not widely available. Two parameters were identified that correlate with higher objective compliance during oral appliance therapy, namely a more pronounced decrease in snoring promotes adherence, while the presence of dry mouth during MAD treatment demotivates adherence [27]. Interruption of treatment is usually related to discomfort and a lack of effect on the pattern of complaints, particularly with respect to snoring [39].
Positional treatment
Positional therapy is a treatment modality for OSA that has been studied since the 1980s. It is aimed at preventing sleep in the most adverse sleeping position, usually the supine position [40]. Positional therapy is not recommended for patients who, for any reason (shoulder problems or any other physical disability that interferes with their sleep in the lateral position), cannot avoid the supine posture during sleep. This therapy is also not an optimal solution for positional OSA patients who continue to snore loudly and perhaps have events of flow limitation while sleeping in the lateral posture. The most widely used technique to avoid the supine position involves strapping a bulky object to the back of the patient, the so-called tennis ball technique. This technique has been shown to be effective in reducing supine sleep time (and inherently less respiratory events) but is often uncomfortable for patients and results in disturbed sleep and disappointingly low long-term compliance [41]. These drawbacks have partly been solved by the introduction of neck-worn and chest-worn devices, correcting the supine sleeping position by activating a subtle vibration alarm and stimulus [42]. This novel concept has shown promising results, with a higher compliance, although adherence to treatment is still a concern [43]. Positional therapy can also be used as part of a multimodality therapeutic approach [44]. Not surprisingly, sleeping in an upright position is very effective in reducing the AHI, but has an underestimated value and is not well documented in the literature [45, 46]. It could be considered as a salvage solution in case conventional treatments fail, as a combination therapy or temporarily when travelling in remote areas. Follow-up of OSA patients using positional therapy is imperative, since an increase in weight may convert positional into non-positional OSA patients.
Surgery of the upper airway
In severe forms of OSA, surgery can be indicated in case of an unfavourable outcome with non-surgical therapies, such as CPAP or MAD therapy. Surgery can also be considered as a first-line treatment in mild OSA. At the same time, there are also patients who do not want to be dependent on a lifelong aid. Such patients are often motivated to undergo surgery. In all cases, surgical treatment of OSA aims to correct anatomical abnormalities in the upper airway that contribute to its collapse during sleep. Surgery may also be considered to correct anatomic deficiencies that compromise other therapies or to improve acceptance and tolerance of other OSA treatments [1]. Various surgical modifications of the upper airway have been proposed to manage, and in some cases, treat OSA. When DISE shows obstruction at the level of the palate-tonsil many surgical techniques are indicated, such as radiofrequency thermotherapy (RFTT), uvulopalatopharyngoplasty (UPPP) and some modifications [47–50]. Tonsillectomy is only recommended for adult patients with tonsillar hypertrophy or as part of UPPP. In UPPP (including tonsillectomy) the posterior and anterior pharyngeal arches are attached, giving greater anteroposterior and lateral dimensions. However, this technique is accompanied by considerable morbidity [51, 52]. This intervention is painful and there is temporary velopharyngeal insufficiency, post-operative bleeding, nasopharyngeal stenosis, voice changes and alterations in speech. This usually resolves within a month of surgery. Chances of success of UPPP (50% reduction in AHI and AHI <20 events·h−1) for OSA seem to be ∼40% in unselected patients, or a ∼30% chance of a post-operative AHI below 10 events·h−1, but this rises to 70–80% if the location of the upper airway obstruction is determined with DISE [53, 54]. Palatal reconstruction techniques have been recently developed, such as barbed reposition pharyngoplasty (BRP) and expansion sphincter pharyngoplasty (ESP), and are the procedures that are currently being performed [49, 50]. These surgical procedures are designed to remodel but not to resect the pharyngeal tissues. These new modalities of pharyngeal surgery are less often accompanied by the disadvantages of UPPP and seem to be superior compared with UPPP in reducing the AHI [55]. RFTT of the tongue base results in some volume reduction and stiffening of the tongue base [47]. RFTT is considered where a hypertrophic tongue is a causal factor of OSA. This treatment can also be applied in combination with hyoidthyroidpexia (HTP). HTP consists of hyoid advancement through fixation of the hyoid bone to the thyroid cartilage [56]. It aims to increase the lower pharyngeal lumen, but is generally not considered as a standalone therapy for most OSA cases. Transoral robotic surgery (TORS) may be indicated for patients with severe OSA and total collapse of the base of tongue after other therapies have failed [57]. Use of a robot makes it possible to remove a considerably more posteriorly and deeply located part of the base of the tongue. TORS has been shown to be an effective option to treat tongue base obstruction, and to lower the AHI. Tracheotomy is considered as the last resort in an emergency and/or when no improvement is seen after several surgical interventions [53].
Given the fact that every specific surgical technique targets a specific pharyngeal level, OSA patients should be selected by investigating the pre-operative pattern of upper airway obstruction. With DISE, the upper airway is evaluated endoscopically, and the degree, pattern and level(s) of collapse are scored [58]. Several studies demonstrate that there are many sites in the upper airway that may contribute to pharyngeal collapse during sleep. Therefore, the concept of one obstruction site/one surgical treatment has been replaced by multilevel surgery addressing several airway segments. The selection of the appropriate treatment for a particular patient becomes more complex and an in-depth knowledge of the different surgical options, their results and possible complications is required. The success criteria employed by Sher et al. [59] are a reduction in the AHI of at least 50% and a post-operative AHI <20 events·h−1. These criteria are frequently employed in the current literature on OSA surgery, but their validity might be questioned given the recent data that even milder forms of OSA might be associated with cardiovascular morbidity [60]. Others propose considering treatment success as an AHI <15, <10 or even <5 events·h−1. The level of AHI is currently under debate, given the poor correlation between AHI and the severity of symptoms [61]. Apart from these considerations, patients should also be advised about the success of surgical procedures and side-effects, as well as the success rate of alternative treatment options. For those requiring a stepped procedure approach, patients should be clearly informed that multiple surgical procedures may be needed. Appropriate clinical follow-up is also required since results tend to deteriorate over time.
An overview of the currently applied upper airway surgical techniques is presented in table 2. A detailed description is outside of the scope of this review.
TABLE 2.
Current upper airway and maxillofacial surgeries for obstructive sleep apnoea (OSA)
| Intervention site | Main procedures | Main indications |
| Nose | Septoplasty Turbinoplasty |
For subjects with persistent nasal obstruction, mainly to improve feasibility of CPAP or MAD |
| Nasopharynx | Adenoidectomy | Adenoid hypertrophy |
| Oropharynx | Tonsillectomy Barbed reposition pharyngoplasty (BRP) Expansion sphincter pharyngoplasty (ESP) Soft palate RFITT |
Tonsillar hypertrophy; in adults, mainly with other procedures Retropalatal obstruction Retropalatal obstruction Retropalatal obstruction in mild OSA |
| Hyoid bone | Hyoidthyroidpexia | Hypopharyngeal obstruction |
| Tongue | Tongue RFITT Transoral robotic surgery (TORS) Tongue suspension |
Moderate macroglossia and retrolingual obstruction, mainly in mild-to-moderate OSA, or as part of multilevel surgery |
| Maxilla/mandible | Maxillomandibular advancement Maxillary expansion |
Mandibular deficiency, severe OSA with obstruction at multiple sites |
| Larynx | Epiglottopexy | Obstruction at the epiglottic level |
| Trachea | Tracheostomy | Only in emergency situations; rarely performed when other treatments are not feasible in severe OSA |
| Multisite | Variable combined procedures | Obstruction at multiple sites in moderate-to-severe OSA |
CPAP: continuous positive airway pressure; MAD: mandibular advancement device; RFITT: radiofrequency interstitial thermotherapy.
Maxillomandibular surgery
Maxillomandibular advancement (MMA) surgery has been performed for OSA since the 1980s [62]. Using this technique, the maxilla and mandible are displaced ∼10 mm in the forward direction, sometimes combined with a rotation, following intraoral osteotomy (a Le Fort I osteotomy of the maxilla and bilateral sagittal split osteotomy of the mandible). Finally, the maxillofacial complex is stabilised with plates. This intervention affects the configuration of the attached soft tissues, with enhanced tension of the pharyngeal dilator musculature, resulting in an expansion of the pharyngeal lumen. Transient (77%) or persistent (18%) facial paraesthesia is a frequent complication, which resolves in 6–12 months in most cases [63]. In addition, facial change after MMA is inevitable. MMA is the most obvious form of treatment in patients with maxillomandibular deficiency, micrognathia or retrognathia, but this procedure does not require that patients have these features. Overall, this type of surgery has the best record of success as a treatment for OSA, and response rates of 86% have been published, while cure (AHI <5 events·h−1) is present in about 43% [53, 54]. It should be mentioned that the ultimate effect of CPAP depends on therapy adherence, and that this aspect does not apply to surgical interventions, either MMA or the previously mentioned surgical interventions. Therefore, the mean disease alleviation of MMA might be superior to CPAP. It appears that patients with a substantial reduction in AHI (at least 50%) with an MAD are good candidates for MMA surgery. Other predictors of success are younger age, lower pre-operative weight and AHI, and greater degree of mandibular advancement. Complete lateral wall collapse and complete concentric palatal collapse have also been found not to hamper MMA efficacy, and MMA is likely to eliminate complete concentric palatal collapse [64]. It seems that maxillomandibular surgery is underutilised in the treatment of OSA, but orthodontic pre- and post-operative treatment (1 year before and 1 year after MMA) is usually performed, depending on the dentoskeletal characteristics of the patient. This relatively long treatment trajectory and the invasive nature of MMA is a major drawback, and requires a well informed and motivated patient.
Maxillary expansion
Maxillary expansion was initially described as a solution to maxillary transverse deficiency, and its effect has been demonstrated in paediatric OSA [65]. Maxillary expansion has also been investigated in adults with OSA, using both surgically assisted and nonsurgical approaches. In 2016, a meta-analysis reported an impressive change in AHI from 24 to 10 events·h−1 [66], but more evidence is necessary to understand the role of maxillary expansion in the management of adult OSA.
Bariatric surgery
Bariatric surgery is an effective means to achieve major weight loss and is indicated in individuals with a body mass index (BMI) ≥40 kg·m−2 or those with a BMI ≥35 kg·m−2 with important comorbidities (arterial hypertension, diabetes, OSA) and in whom dietary attempts at weight control have been ineffective [67]. Mild-to-moderate OSA is not usually an indication for bariatric surgery. The aims of bariatric surgery are to reduce caloric intake and to alter the hormonal milieu involved in nutrient absorption. Procedures can be classified into restrictive (gastric banding, sleeve gastroplasty) and malabsorptive interventions (Scopinaro technique) or a combination of both (Roux-en-Y gastric bypass (RYGB)). Overall, gastric bypass is more efficacious than gastric banding or gastroplasty, but with higher complication rates. At present, these techniques can be performed as a laparoscopic procedure. Different studies have shown the beneficial effects of bariatric surgery on OSA, with a reduction in AHI of 77% and cure of OSA in 64–86% of the cases [1]. However, a substantial number of patients have to continue CPAP, and no randomised controlled trial (RCT) is available that compared RYGB to CPAP. This reflects a significant inter-individual variability and emphasises the need for ongoing clinical and polysomnographic follow-up of these patients. Recently, international scientific societies (American Thoracic Society, European Respiratory Society (ERS)) have recommended performing gastric bypass surgery in obese patients [2, 8].
Hypoglossal nerve stimulation
Electrical stimulation of the hypoglossal nerve (i.e. the motor nerve of the genioglossus muscle) causes tongue protrusion and stiffening of the anterior pharyngeal wall, and is therefore an attractive potential therapeutic target for OSA. The most widely and commercially available implanted system makes use of a stimulation electrode placed on a branch of the right hypoglossal nerve that induces protrusion of the ipsilateral part of the tongue, a breathing-sensing lead between the internal and external intercostals muscles to detect ventilatory effort, and an implantable pulse generator (IPG) located at the mid-infraclavicular region (Inspire Medical Systems Inc., Minneapolis, MN, USA). The breathing sensor detects the breathing effort, and next, the IPG calculates the start of inspiration, after which electrical pulses are continuously given between the start and end of inspiration [68]. The results of the pivotal STAR trial showed a significant reduction in OSA severity (AHI −68%) without disrupting the patient's sleep [69]. Inclusion criteria were a BMI ≤32 kg·m−2, an AHI between 15 and 50 events·h−1, and exclusion of a complete circular collapse at the palatal level during DISE. Results from the global ADHERE registry clearly support that the indication for hypoglossal nerve stimulation could be broadened for patients with an AHI above 65 events·h−1, which, to date, is not common practice [70]. More recently, a novel device called the GENIO system (Nyxoah, Mont-Saint-Guibert, Belgium), delivering bilateral hypoglossal nerve stimulation via a small implanted electrode and activated by a unit worn externally, was introduced for moderate-to-severe OSA. At 6 months, the AHI decreased 45% (from 23.7 to 12.9 events·h−1) [71]. The ERS recommends use of these technologies as a salvage treatment when standard therapy cannot be tolerated. In recent years, transcutaneous approaches have also been tested. The ongoing TESLA trial evaluates transcutaneous electrical stimulation of the upper airway dilator muscles in a crossover design and will determine whether electrical stimulation can be a treatment modality for a wider range of patients with OSA [72].
Both anatomical (complete concentric collapse at the level of the palate) and physiological parameters are associated with treatment outcome [73, 74]. Therefore, upfront patient selection based on these parameters can increase response rates.
Drug treatment
Today, there are no medications that are licensed for pharmaceutical treatment of OSA [1, 2, 75]. Much effort has been made to improve upper airway patency pharmacologically, but a large body of studies are not of a very high quality. Some agents that produce an improvement have a direct or indirect effect on sleep and respiration. More recent approaches have focused on the selective norepinephrine reuptake inhibitor atomoxetine [76]. Atomoxetine has only moderate effects on the AHI when administered alone, but combined with the antimuscarinic oxybutynin a reduction of the AHI by 62% was reached. Finally, in a recent 4-week RCT of the carbonic anhydrase inhibitor sulthiame in 68 patients with moderate-to-severe OSA the AHI was reduced by 41% [77]. Frequent arousals that occur during minor airway collapse are believed to facilitate cyclic breathing events. The available studies suggest a neutral response or an improvement after hypnotics (benzodiazepines, Z-drugs, trazodone) in subjects with moderate OSA, and indicate a high inter-individual variability. However, there were only limited effects on the AHI in controlled trials, and no approved therapy is available based on this rationale. However, drug treatment can be expected (given promising preliminary data) in the future, based on the pathophysiological traits. Comorbid disorders are also a potential target for drug therapy in OSA, including weight-reducing drugs and antihypertensives. Limited studies have been performed with phentermine, orlistat (a gastrointestinal lipase inhibitor), liraglutide (a glucagon-like peptide-1 agonist), metformin added to liraglutide, and empagliflozin (a sodium-glucose co-transporter 2 inhibitor) [78–82]. Added metabolic benefits may be achieved with weight reduction, including improvements in insulin resistance, lipoproteins, visceral and subcutaneous abdominal fat, which are associated with a reduction in cardiovascular risk. Residual excessive daytime sleepiness (rEDS) is a relatively common condition in OSA, which may require use of wake-promoting drugs, after excluding any specific cause, namely suboptimal objective adherence to treatment, ill-fitting masks (in case of CPAP therapy), suboptimal titration, insufficient sleep, poor sleep hygiene and concomitant sleep disorders, such as narcolepsy, periodic leg movements, restless legs, and depression. There has been recent interest in the use of modafinil as a symptomatic treatment for OSA [83]. However, the indication of OSA was repealed by the European Medicines Agency (EMA), because of severe skin reactions as well as psychiatric and cardiovascular side-effects. Pitolisant is a selective H3-receptor antagonist/inverse agonist that increases histamine synthesis and release, promoting wakefulness. The drug has been evaluated in two recent, placebo-controlled RCTs (HAROSA I, II) that included patients with moderate-to-severe OSA with rEDS [84, 85]. Both studies showed significant improvement in sleepiness and fatigue based on patient-reported outcomes. Side-effects were not different from the placebo group. Pitolisant is approved for clinical use in OSA by EMA. Solriamfetol is a dopamine and norepinephrine reuptake inhibitor, which has also shown beneficial results in OSA [86, 87]. It is a recent US Food and Drug Administration-approved drug, but headache was moderately common in ∼10% of studied patients. These drugs open new therapeutic windows for patients with the phenotype of rEDS, and may improve quality of life and daily performance at work and on the road, especially if administered in well-selected patients.
Myofunctional therapy
Myofunctional therapy is a relatively new approach towards non-CPAP therapy, training the movement, strength, endurance and neuromuscular activation of the upper airway, pharyngeal and tongue muscles to facilitate a state in which snoring and OSA are diminished when asleep. The current recommendation in the ERS guidelines found a very low level of evidence of data from RCTs. Myofunctional therapy should not be considered when CPAP therapy is available; however, it may be considered if there is no other treatment that can be offered [2]. Recently, a systematic review and meta-analysis reported an improvement of ∼50% in the AHI that was complemented by a symptomatic improvement [88].
Conclusions
Efficacious alternative therapies to CPAP include oral appliance therapy, positional therapy, different surgical procedures, hypoglossal nerve stimulation, drug treatment and orofacial exercise. Given the clinical heterogeneity of OSA, patient selection is mandatory for the assessment of treatment success of these non-CPAP therapies. Moreover, it has been shown that by adding DISE to the diagnostic workup of OSA patients, the success rate of different interventions can be significantly increased. However, the concept of targeting treatment on the different pathophysiological traits remains challenging in routine clinical practice.
Key points
Although weight loss is highly efficacious in OSA, only 5% of overweight individuals are able to lose and keep off weight.
In mild-to-moderate OSA, oral appliances have a similar effectiveness to CPAP therapy in terms of mean disease alleviation, while MAD therapy can be effective in selected cases with severe OSA.
Positional therapy may be efficacious for positional OSA, but weight gain may convert positional into non-positional OSA patients.
Successful sleep surgery is most commonly defined as a >50% reduction in AHI and a post-operative AHI <20 events·h−1 (“Sher” definition).
Bariatric surgery and maxillomandibular osteotomy can be highly effective in selected cases.
There is limited research and data on the use of maxillary expansion in adult OSA.
The ERS recommends use of hypoglossal nerve stimulation as a salvage treatment when standard therapy cannot be tolerated.
Availability of innovative drugs opens new avenues based on pathophysiological phenotyping.
Myofunctional therapy should not be considered when CPAP therapy is available. However, it may be considered if there is no other treatment that can be offered.
Upfront patient selection based on DISE can increase response rates.
Regarding non-CPAP therapies, clinicians should manage OSA patients with care and in a multidisciplinary setting.
Self-evaluation questions
- Regarding lifestyle modification, which statement relating to alcohol and OSA is not true?
- Alcohol is a minor source of calories
- Alcohol selectively reduces upper airway muscle tone
- Alcohol increases the frequency of respiratory events during sleep
- Alcohol also prolongs apnoea or hypopnoea by delaying arousal
- Regarding oral devices, which statement relating to OSA and MADs is not true?
- MADs are the most common class of oral appliances used for the treatment of OSA
- MADs are not usually indicated for severe OSA
- Compared with CPAP, oral appliances have in general a similar efficacy in terms of changes in AHI
- Compared with CPAP, oral appliances have in general a lower patient and partner satisfaction
- Regarding positional treatment, which statement relating to supine OSA is not true?
- Patients with supine position OSA tend to have a lower AHI, to be younger and to be less obese
- Supine position OSA patients have a non-pathological level of AHI in the non-supine position (AHI <5 events·h−1)
- Weight gain in OSA may convert positional into non-positional patients
- In follow-up studies, long-term compliance with positional therapy is excellent
- At present, successful upper airway surgery is most commonly defined as:
- A significant reduction in 24 h arterial blood pressure
- A >50% reduction in AHI and a post-operative AHI <20 events·h−1
- An AHI <5 events·h−1
- A reduction in the Epworth Sleepiness score to values <10
- Which of the following statements relating to bariatric surgery and OSA is not true?
- Bariatric surgery is not indicated in individuals with mild-to-moderate OSA
- Bariatric surgery is indicated in individuals with a BMI ≥35 kg·m−2
- Bariatric surgery is indicated in individuals with a BMI ≥40 kg·m−2
- Bariatric surgery is indicated in individuals with a BMI ≥35 kg·m−2 with important comorbidities (arterial hypertension, diabetes, OSA)
- Which of the following statements relating to drug treatment and OSA is true?
- No evidence that any drug is likely to benefit an unselected patient with OSA
- Some drugs described in the literature consistently reduce the severity of the condition by more than 50%
- We can expect that a single drug therapy will fit all OSA patients
- Intranasal steroids as a single intervention are recommended for treatment of adult OSA
Suggested answers
a.
d.
d.
b.
b.
a.
Footnotes
Conflict of interest: All authors have seen and approved this manuscript. J. Verbraecken reports institutional fees and educational grants from AirLiquide, AstraZeneca, Bekaert Deslee Academy, Bioprojet, Desitin, Ectosense, Epilog, Fisher & Paykel, Heinen & Löwenstein, Idorsia, Inspire, Jazz Pharmaceutics, Medidis, Mediq Tefa, OSG, Philips, ResMed, Sefam, SomnoMed, Total Care, UCB Pharma, Vivisol, and Westfalen Medical. M. Dieltjens holds a Postdoctoral Fellowship at the Research Foundation Flanders (FWO: 12H4520N). S. Op de Beeck holds a Postdoctoral Fellowship at the Research Foundation Flanders (FWO). A. Vroegop has nothing to disclose. M. Braem reports a research grant from SomnoMed at the Antwerp University Hospital and sits on the advisory board of ResMed and SomnoMed. O. Vanderveken reports research support outside the submitted work from Philips, SomnoMed, Inspire Medical Systems and Nyxoah at the Antwerp University Hospital, consultancy for Galvani and Liva Nova, and is an advisory board member for Zephyr and SomnoMed; he holds a Senior Clinical Investigator Fellowship from the Research Foundation Flanders (FWO: 1833517 N). W. Randerath reports personal fees and travel grants from Weinmann, Heinen & Löwenstein, Resmed, Philips, Inspire and Bioprojet.
References
- 1.Randerath WJ, Verbraecken J, Andreas S, et al. . Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J 2011; 37: 1000–1028. doi: 10.1183/09031936.00099710 [DOI] [PubMed] [Google Scholar]
- 2.Randerath W, Verbraecken J, de Raaff CAL, et al. . European Respiratory Society guideline on non-CPAP therapies for obstructive sleep apnoea. Eur Respir Rev 2021; 30: 210200. doi: 10.1183/16000617.0200-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grote L, Hedner J, Grunstein R, et al. . Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J 2000; 16: 921–927. doi: 10.1183/09031936.00.16592100 [DOI] [PubMed] [Google Scholar]
- 4.Vanderveken OM, Dieltjens M, Wouters K, et al. . Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax 2013; 68: 91–96. doi: 10.1136/thoraxjnl-2012-201900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry 1982; 45: 353–359. doi: 10.1136/jnnp.45.4.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Guo X, Liu W, et al. . Alcohol as an independent risk factor for obstructive sleep apnea. Ir J Med Sci 2022; 191: 1325–1330. doi: 10.1007/s11845-021-02671-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messineo L, Eckert DJ, Lim R, et al. . Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. J Physiol 2020; 598: 4681–4692. doi: 10.1113/JP280173 [DOI] [PubMed] [Google Scholar]
- 8.Hudgel DW, Patel SR, Ahasic AM, et al. . The role of weight management in the treatment of adult obstructive sleep apnea. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e70–e87. doi: 10.1164/rccm.201807-1326ST [DOI] [PubMed] [Google Scholar]
- 9.Peppard PE, Young T, Palta M, et al. . Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284: 3015–3021. doi: 10.1001/jama.284.23.3015 [DOI] [PubMed] [Google Scholar]
- 10.Georgoulis M, Yiannakouris N, Kechribari I, et al. . Dose–response relationship between weight loss and improvements in obstructive sleep apnea severity after a diet/lifestyle interventions: secondary analyses of the ‘MIMOSA’ randomized clinical trial. J Clin Sleep Med 2022; 18: 1251–1261. doi: 10.5664/jcsm.9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passos GS, Poyares D, Santana MG, et al. . Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med 2010; 6: 270–275. doi: 10.5664/jcsm.27825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendelson M, Marillier M, Bailly S, et al. . Maximal exercise capacity in patients with obstructive sleep apnoea syndrome: a systematic review and meta-analysis. Eur Respir J 2018; 51: 1702697. doi: 10.1183/13993003.02697-2017 [DOI] [PubMed] [Google Scholar]
- 13.Kim KS, Kim JH, Park SY, et al. . Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med 2012; 8: 367–374. doi: 10.5664/jcsm.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CW, Chang CH, Chuang HY, et al. . What is the association between secondhand smoke (SHS) and possible obstructive sleep apnea: a meta-analysis. Environ Health 2022; 21: 58. doi: 10.1186/s12940-022-00868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan V, Dixon-Williams S, Thornton JD. Where there is smoke…there is sleep apnea: exploring the relationship between smoking and sleep apnea. Chest 2014; 146: 1673–1680. doi: 10.1378/chest.14-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramar K, Dort LC, Katz SG, et al. . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med 2015; 11: 773–827. doi: 10.5664/jcsm.4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vroegop AV, Vanderveken OM, Van de Heyning PH, et al. . Effects of vertical opening on pharyngeal dimensions in patients with obstructive sleep apnoea. Sleep Med 2012; 13: 314–316. doi: 10.1016/j.sleep.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 18.Basyuni S, Barabas M, Quinnell T. An update on mandibular advancement devices for the treatment of obstructive sleep apnoea hypopnoea syndrome. J Thorac Dis 2018; 10: Suppl. 1, S48–S56. doi: 10.21037/jtd.2017.12.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderveken OM, Devolder A, Marklund M, et al. . Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med 2008; 178: 197–202. doi: 10.1164/rccm.200701-114OC [DOI] [PubMed] [Google Scholar]
- 20.Quinnell TG, Bennett M, Jordan J, et al. . A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO). Thorax 2014; 69: 938–945. doi: 10.1136/thoraxjnl-2014-205464 [DOI] [PubMed] [Google Scholar]
- 21.Dieltjens M, Vanderveken OM, Heyning PH, et al. . Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med Rev 2012; 16: 177–185. doi: 10.1016/j.smrv.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 22.Marklund M, Braem MJA, Verbraecken J. Update on oral appliance therapy. Eur Respir Rev 2019; 28: 190083. doi: 10.1183/16000617.0083-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doff MH, Hoekema A, Pruim GJ, et al. . Effects of a mandibular advancement device on the upper airway morphology: a cephalometric analysis. J Oral Rehabil 2009; 36: 330–337. doi: 10.1111/j.1365-2842.2009.01946.x [DOI] [PubMed] [Google Scholar]
- 24.Bartolucci ML, Bortolotti F, Martina S, et al. . Dental and skeletal long-term side effects of mandibular advancement devices in obstructive sleep apnea patients: a systematic review with meta-regression analysis. Eur J Orthod 2019; 41: 89–100. doi: 10.1093/ejo/cjy036 [DOI] [PubMed] [Google Scholar]
- 25.Banu F, Jeyapalan K, V AK. Custom-made dual-functional oral appliance for management of obstructive sleep apneic completely edentulous patient. Cureus 2021; 13: e16656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips CL, Grunstein RR, Darendeliler MA, et al. . Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med 2013; 187: 879–887. doi: 10.1164/rccm.201212-2223OC [DOI] [PubMed] [Google Scholar]
- 27.Dieltjens M, Verbruggen AE, Braem MJ, et al. . Determinants of objective compliance during oral appliance therapy in patients with sleep-disordered breathing: a prospective clinical trial. JAMA Otolaryngol Head Neck Surg 2015; 141: 894–900. doi: 10.1001/jamaoto.2015.1756 [DOI] [PubMed] [Google Scholar]
- 28.Vroegop AV, Vanderveken OM, Verbraecken JA. Drug-induced sleep endoscopy: evaluation of a selection tool for treatment modalities for obstructive sleep apnea. Respiration 2020; 99: 451–457. doi: 10.1159/000505584 [DOI] [PubMed] [Google Scholar]
- 29.Op de Beeck S, Dieltjens M, Verbruggen AE, et al. . Phenotypic labelling using drug-induced sleep endoscopy improves patient selection for mandibular advancement device outcome: a prospective study. J Clin Sleep Med 2019; 15: 1089–1099. doi: 10.5664/jcsm.7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marques M, Genta PR, Azarbarzin A, et al. . Structure and severity of pharyngeal obstruction determine oral appliance efficacy in sleep apnoea. J Physiol 2019; 597: 5399–5410. doi: 10.1113/JP278164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vroegop AV, Vanderveken OM, Dieltjens M, et al. . Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res 2013; 22: 348–355. doi: 10.1111/jsr.12008 [DOI] [PubMed] [Google Scholar]
- 32.Van den Bossche K, Op de Beeck S, Dieltjens M, et al. . Multimodal phenotypic labelling using drug-induced sleep endoscopy, awake nasendoscopy and computational fluid dynamics for the prediction of mandibular advancement device treatment outcome: a prospective study. J Sleep Res 2022; in press [ 10.1111/jsr.13673]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Gaver H, Op de Beeck S, Dieltjens M, et al. . Functional imaging improves patient selection for mandibular advancement device treatment outcome in sleep-disordered breathing: a prospective study. J Clin Sleep Med 2022; 18: 739–750. doi: 10.5664/jcsm.9694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamagoos AA, Cistulli PA, Sutherland K, et al. . Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc 2019; 16: 1422–1431. doi: 10.1513/AnnalsATS.201903-190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards BA, Andara C, Landry S, et al. . Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2016; 194: 1413–1422. doi: 10.1164/rccm.201601-0099OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dieltjens M, Braem MJ, Op de Beeck S, et al. . Remotely controlled mandibular positioning of oral appliance therapy during polysomnography and drug-induced sleep endoscopy compared with conventional subjective titration in patients with obstructive sleep apnea: protocol for a randomized crossover trial. Trials 2019; 20: 615. doi: 10.1186/s13063-019-3698-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazemeini E, Op de Beeck S, Vroegop A, et al. . A pilot study on comparison of subjective titration versus remotely controlled mandibular positioning during polysomnography and drug-induced sleep endoscopy, to determine the effective protrusive position for mandibular advancement device therapy. Sleep Breath 2022; in press [ 10.1007/s11325-022-02569-3]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kastoer C, Dieltjens M, Op de Beeck S, et al. . Remotely controlled mandibular positioning during drug-induced sleep endoscopy toward mandibular advancement device therapy: feasibility and protocol. J Clin Sleep Med 2018; 14: 1409–1413. doi: 10.5664/jcsm.7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attali V, Chaumereuil C, Arnulf I, et al. . Predictors of long-term effectiveness to mandibular repositioning device treatment in obstructive sleep apnea patients after 1000 days. Sleep Med 2016; 27–28: 107–114. doi: 10.1016/j.sleep.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 40.Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep 1984; 7: 110–114. doi: 10.1093/sleep/7.2.110 [DOI] [PubMed] [Google Scholar]
- 41.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. . Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med 2009; 5: 428–430. doi: 10.5664/jcsm.27597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eijsvogel MM, Ubbink R, Dekker J, et al. . Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. J Clin Sleep Med 2015; 11: 139–147. doi: 10.5664/jcsm.4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyers J, Dieltjens M, Kastoer C, et al. . Evaluation of a trial period with a sleep position trainer in patients with positional sleep apnea. J Clin Sleep Med 2018; 14: 575–583. doi: 10.5664/jcsm.7048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dieltjens M, Vroegop AV, Verbruggen AE, et al. . A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath 2015; 19: 637–644. doi: 10.1007/s11325-014-1068-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McEvoy RD, Sharp DJ, Thornton AT. The effects of posture on obstructive sleep apnea. Am Rev Respir Dis 1986; 133: 662–666. doi: 10.1164/arrd.1986.133.4.662 [DOI] [PubMed] [Google Scholar]
- 46.Neill AM, Angus SM, Sajkov D, et al. . Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 1997; 155: 199–204. doi: 10.1164/ajrccm.155.1.9001312 [DOI] [PubMed] [Google Scholar]
- 47.Powell NB, Riley RW, Troell RJ, et al. . Radiofrequency volumetric tissue reduction of the palate in subjects with sleep-disordered breathing. Chest 1998; 113: 1163–1174. doi: 10.1378/chest.113.5.1163 [DOI] [PubMed] [Google Scholar]
- 48.Fujita S, Conway W, Zorick F, et al. . Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg 1981; 89: 923–934. doi: 10.1177/019459988108900609 [DOI] [PubMed] [Google Scholar]
- 49.Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg 2007; 137: 110–114. doi: 10.1016/j.otohns.2007.03.014 [DOI] [PubMed] [Google Scholar]
- 50.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscopy 2003; 113: 1961–1968. doi: 10.1097/00005537-200311000-00020 [DOI] [PubMed] [Google Scholar]
- 51.Franklin KA, Anttla H, Axelsson S, et al. . Effects and side-effects of surgery for snoring and OSA: a systematic review. Sleep 2009; 32: 27–36. [PMC free article] [PubMed] [Google Scholar]
- 52.Varendh M, Berg S, Andersson M. Long-term follow-up of patients operated with uvulopalatopharyngoplasty from 1985 to 1991. Respir Med 2012; 106: 1788–1793. doi: 10.1016/j.rmed.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 53.Caples SM, Rowley JA, Prinsell JR, et al. . Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep 2010; 33: 1396–1407. doi: 10.1093/sleep/33.10.1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aurora RN, Casey KR, Kristo D, et al. . Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep 2010; 33: 1408–1413. doi: 10.1093/sleep/33.10.1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maniaci A, Di Luca M, Lechien JR, et al. . Lateral pharyngoplasty vs. traditional uvulopalatopharyngoplasty for patients with OSA: systematic review and meta-analysis. Sleep Breath 2022; in press [ 10.1007/s11325-021-02520-y]. [DOI] [PubMed] [Google Scholar]
- 56.den Herder C, van Tinteren H, de Vries N. Hyoidthyroidpexia: a surgical treatment for sleep apnea syndrome. Laryngoscope 2005; 115: 740–745. doi: 10.1097/01.mlg.0000156464.37681.BF [DOI] [PubMed] [Google Scholar]
- 57.Vicini C, Montevecchi F, Gobbi R, et al. . Transoral robotic surgery for obstructive sleep apnea syndrome: principles and technique. World J Otorhinolaryngol Head Neck Surg 2017; 3: 97–100. doi: 10.1016/j.wjorl.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Vito A, Carrasco Llatas M, Ravesloot MJ, et al. . European position paper on drug-induced sleep endoscopy: 2017 update. Clin Otolaryngol 2018; 43: 1541–1552. doi: 10.1111/coa.13213 [DOI] [PubMed] [Google Scholar]
- 59.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep 1996; 19: 156–177. doi: 10.1093/sleep/19.2.156 [DOI] [PubMed] [Google Scholar]
- 60.Bouloukaki I, Grote L, McNicholas WT, et al. . Mild obstructive sleep apnea increases hypertension risk, challenging traditional severity classification. J Clin Sleep Med 2020; 16: 889–898. doi: 10.5664/jcsm.8354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pevernagie DA, Gnidovec-Strazisar B, Grote L, et al. . On the rise and fall of the apnea–hypopnea index: a historical review and critical appraisal. J Sleep Res 2020; 29: e13066. doi: 10.1111/jsr.13066 [DOI] [PubMed] [Google Scholar]
- 62.Riley RW, Powell NB, Guilleminault C, et al. . Maxillary, mandibular, and hyoid advancement: an alternative to tracheostomy in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg 1986; 94: 584–588. doi: 10.1177/019459988609400509 [DOI] [PubMed] [Google Scholar]
- 63.Ravesloot MJL, de Raaff CAL, van de Beek MJ, et al. . Perioperative care of patients with obstructive sleep apnea undergoing upper airway surgery: a review and consensus recommendations. JAMA Otolaryngol Head Neck Surg 2019; 145: 751–760. doi: 10.1001/jamaoto.2019.1448 [DOI] [PubMed] [Google Scholar]
- 64.Kastoer C, Op de Beeck S, Dom M, et al. . Drug-induced sleep endoscopy upper airway collapse patterns and maxillomandibular advancement. Laryngoscope 2020; 130: E268–E274. doi: 10.1002/lary.28022 [DOI] [PubMed] [Google Scholar]
- 65.Cistulli PA, Palmisano RG, Poole MD. Treatment of obstructive sleep apnea syndrome by rapid maxillary expansion. Sleep 1998; 21: 831–835. doi: 10.1093/sleep/21.8.831 [DOI] [PubMed] [Google Scholar]
- 66.Abdullatif J, Certal V, Zaghi S, et al. . Maxillary expansion and maxillomandibular expansion for adult OSA: a systematic review and meta-analysis. J Craniomaxillofac Surg 2016; 44: 574–578. doi: 10.1016/j.jcms.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 67.de Raaff CAL, Gorter-Stam MAW, de Vries N, et al. . Perioperative management of obstructive sleep apnea in bariatric surgery: a consensus guideline. Surg Obes Relat Dis 2017; 13: 1095–1109. doi: 10.1016/j.soard.2017.03.022 [DOI] [PubMed] [Google Scholar]
- 68.Vanderveken OM, Beyers J, Op de Beeck S, et al. . Development of a clinical pathway and technical aspects of upper airway stimulation therapy for obstructive sleep apnea. Front Neurosci 2017; 11: 523. doi: 10.3389/fnins.2017.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strollo PJ Jr, Soose RJ, Maurer JT, et al. . Upper-airway stimulation for obstructive sleep apnea. N Engl J Med 2014; 370: 139–149. doi: 10.1056/NEJMoa1308659 [DOI] [PubMed] [Google Scholar]
- 70.Bosschieter PFN, de Vries N, Mehra R, et al. . Similar effect of hypoglossal nerve stimulation for obstructive sleep apnea in 5 disease severity categories. J Clin Sleep Med 2022; 18: 1657–1665. doi: 10.5664/jcsm.9956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eastwood PR, Barnes M, MacKay SG, et al. . Bilateral hypoglossal nerve stimulation for treatment of adult obstructive sleep apnoea. Eur Respir J 2020; 55: 1901320. doi: 10.1183/13993003.01320-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pengo MF, Xiao S, Ratneswaran C, et al. . Randomised sham-controlled trial of transcutaneous electrical stimulation in obstructive sleep apnoea. Thorax 2016; 71: 923–931. doi: 10.1136/thoraxjnl-2016-208691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanderveken OM, Maurer JT, Hohenhorst W, et al. . Evaluation of drug-induced sleep endoscopy as a patient selection tool for implanted upper airway stimulation for obstructive sleep apnea. J Clin Sleep Med 2013; 9: 433–438. doi: 10.5664/jcsm.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Op de Beeck S, Wellman A, Dieltjens M, et al. . Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea. Am J Respir Crit Care Med 2021; 203: 746–755. doi: 10.1164/rccm.202006-2176OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaisl T, Haile SR, Thiel S, et al. . Efficacy of pharmacotherapy for OSA in adults: a systematic review and network meta-analysis. Sleep Med Rev 2019; 46: 74–86. doi: 10.1016/j.smrv.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 76.Taranto-Montemurro L, Messineo L, Sands SA, et al. . The combination of atomoxetine and oxybutynin greatly reduces obstructive sleep apnea severity. A randomized, placebo-controlled, double-blind crossover trial. Am J Respir Crit Care Med 2019; 199: 1267–1276. doi: 10.1164/rccm.201808-1493OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hedner J, Stenlöf K, Zou D, et al. . A randomized controlled clinical trial exploring safety and tolerability of sulthiame in sleep apnea. Am J Respir Crit Care Med 2022; 205: 1461–1469. doi: 10.1164/rccm.202109-2043OC [DOI] [PubMed] [Google Scholar]
- 78.Winslow DH, Bowden CH, DiDonato KP, et al. . A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep 2012; 35: 1529–1539. doi: 10.5665/sleep.2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blackman A, Foster GD, Zammit G, et al. . Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes 2016; 40: 1310–1319. doi: 10.1038/ijo.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Svendsen M, Tonstad S. Orlistat after initial dietary/behavioural treatment: changes in body weight and dietary maintenance in subjects with sleep related breathing disorders. Nutr J 2011; 10: 21. doi: 10.1186/1475-2891-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trenson L, Trenson S, van Nes F, et al. . Liraglutide for weight management in the real world: significant weight loss even if the maximal daily dose is not achieved. Obes Facts 2022; 15: 83–89. doi: 10.1159/000520217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neeland IJ, Eliasson B, Kasai T, et al. . The impact of empagliflozin on obstructive sleep apnea and cardiovascular and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020; 43: 3007–3015. doi: 10.2337/dc20-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuan YC, Wu D, Huang KW, et al. . Effects of modafinil and armodafinil in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials. Clin Ther 2016; 38: 874–888. doi: 10.1016/j.clinthera.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 84.Dauvilliers Y, Verbraecken J, Partinen M, et al. . Pitolisant for daytime sleepiness in patients with obstructive sleep apnea who refuse continuous positive airway pressure treatment. A randomized trial. Am J Respir Crit Care Med 2020; 201: 1135–1145. doi: 10.1164/rccm.201907-1284OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pépin JL, Georgiev O, Tiholov R, et al. . Pitolisant for residual excessive daytime sleepiness in OSA patients adhering to CPAP: a randomized trial. Chest 2021; 159: 1598–1609. doi: 10.1016/j.chest.2020.09.281 [DOI] [PubMed] [Google Scholar]
- 86.Schweitzer PK, Rosenberg R, Zammit GK, et al. . Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3). A randomized controlled trial. Am J Respir Crit Care Med 2019; 199: 1421–1431. doi: 10.1164/rccm.201806-1100OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Strollo PJ Jr, Hedner J, Collop N, et al. . Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest 2019; 155: 364–374. doi: 10.1016/j.chest.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 88.Camacho M, Certal V, Abdullatif J, et al. . Myofunctional therapy to treat obstructive sleep apnea: a systematic review and meta-analysis. Sleep 2015; 38: 669–675. doi: 10.5665/sleep.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]