Abstract
Healthcare is a major global industry accounting for a significant proportion of government spending. Drug and medical device manufacturers are publicly traded companies with a responsibility to their shareholders to maximise profits by increasing sales. In order to achieve this, industry exerts influence over every part of healthcare including academic research, medical education, clinical guideline development, physician prescribing and through direct interactions with patients. In contrast, healthcare services seek to provide effective, safe and evidence-based treatments. This article examines interactions with industry across these domains and seeks to identify mutually beneficial relationships and potential conflict leading to patient harms. Case studies are used to illustrate these interactions.
There is no single solution for improving healthcare's relationship with industry, although increased transparency has raised awareness of this issue. We briefly discuss some successful interventions that have been tried at national and regulatory level. While industry influence is widespread in healthcare and this has benefits for shareholders, healthcare practitioners have an ethical obligation to prioritise their patients’ best interests. Industry interactions with healthcare professionals have a valid role in product development and distribution, but industry sponsorship of healthcare education and practice, guideline development or regulatory decision-making can have harmful consequences for patients. Healthcare practitioners need to carefully consider these issues when deciding whether to collaborate with industry.
Educational aims
To explore the many areas where industry influences healthcare and the subsequent effects on patient care. Case studies are used to illustrate examples of beneficial and harmful effects of this influence.
To raise awareness of the effects of industry influence and for readers to consider their own potential conflicts of interest.
To suggest potential ways to improve the current system with a focus on solutions which have successfully been trialled already.
Short abstract
Industry influence is present across all areas of healthcare and while some interactions may be mutually beneficial, the overall aim of industry is to maximise profits by increasing sales. Patients may come to harm from these interactions. https://bit.ly/3NTw4C2
Introduction
Healthcare systems funded through public systems or insurance organisations strive to deliver medical care that is safe, effective, evidence based and cost-effective in order to confer the greatest benefit to the greatest proportion of their population. In contrast, industry, defined here as profit-making corporations that manufacture, distribute or market products or processes for the prevention, diagnosis or management of health conditions or health states, has an obligation to their shareholders to maximise their profits. Healthcare is a major industry accounting for a total spend of USD 3.3 trillion in the USA in 2016 [1], 17.8% of gross domestic product (GDP). Pharmaceutical companies enjoy larger profit margins than similarly sized companies in non-pharmaceutical sectors. 35 large pharmaceutical companies reported a combined revenue of USD 11.5 trillion between 2000 and 2018 with an income of USD 1.9 trillion [2]. In addition to significant returns on investment, pharmaceutical and biotechnology companies are also included in the portfolios of most “ethical funds” despite many having a history of ethically questionable or illegal activity.
The influence of industry is present across all aspects of medicine including academic research, medical education, clinical guideline and formulary selection as well as to patients directly. This influence is present at numerous interconnected levels as mapped by Chimonas et al. [3]. It is important to note that the majority of new medical products and drugs are developed by industry and while individual researchers may have altruistic intentions, these companies do so in order to make a profit.
There are examples of successful partnerships between industry and both the academic community and patient advocacy groups that have resulted in beneficial outcomes for patients. The development of ivacaftor, the first cystic fibrosis transmembrane conductance regulator (CFTR) modulator, was possible due to industry collaboration with the CF Foundation and has paved the way for individualised treatment based on a person's specific gene mutation. The story of ivacaftor is discussed in more detail in later sections. A more recent example is the funding struggles described by Professor Dame Sarah Gilbert, in her delivery of the 44th Dimbleby lecture, in her quest to secure funding and upscaling for her coronavirus disease 2019 (COVID-19) vaccine research, eventually resulting in co-production between the University of Oxford and AstraZeneca (AZ).
The development of new drugs and medical devices is a long and expensive process with an estimated cost of almost USD 1 billion for a new medicine when the cost of unsuccessful trials is factored in [4]. Profits must cover discovery, early and late phase clinical trials as well as the losses from drugs and therapeutics that fail to receive regulatory approval. Therefore, drug development areas are chosen carefully and significant sums are spent on marketing and promotion of products. This leads to an industry focus on diseases with a high prevalence in the developed world and encourages ongoing development of drugs for diseases with existing treatments rather than development of drugs based on disease burden or global prevalence [5]. As such, new drugs for cardiovascular disease and neoplasms are constantly being produced with limited development into other high disease burden (but less lucrative) areas such as neonatal health or into conditions exclusively found in the developing world, for example, the neglected tropical diseases identified by the World Health Organization (WHO) [6]. The last novel class of antibiotic was developed in the 1980s. The lack of innovation since then is due to the high cost of developing new drugs and the limited profits due to antimicrobial resistance restricting prescribing [7]. An exception to this pattern of drug development has been the focus on rare conditions driven in part by “orphan drug” legislation in Europe and the USA. This legislation sought to encourage development of drugs that would not otherwise have been financially viable by rewarding pharmaceutical companies with lucrative market exclusivity post-approval. This has been exploited by pharmaceutical companies to reap massive profits even though studies have shown that many of these drugs would have been profitable without this assistance [8].
The medical device industry is similarly adept at exploiting legislation to obtain regulatory approval and generate profits. A US study found that 43% of devices were approved prior to the publication of any supporting clinical studies. Where regulatory approval was granted, 90% of supporting clinical studies were low-level evidence case series [9]. The majority of devices were approved via the 510(k) pathway, designed for devices which are “substantially equivalent” to existing devices.
Industry marketing activities can themselves have significant impacts on population health, through driving medical excess, especially if marketing is not appropriately regulated. The negative effects of industry involvement at multiple levels of the healthcare ecosystem are perhaps most clearly illustrated by the opioid epidemic in the USA [10]. Between 2000 and 2016 an estimated 300 000 people died in the USA due to opioid overdose, with prescription opioids involved in 40% of cases. Oxycontin was released by Purdue Pharmaceuticals in 1996 and trials demonstrated that it showed no superiority over other opioids apart from a reduced dosing schedule. Despite this, an aggressive marketing campaign for use in patients with non-cancer related chronic pain led to sales worth USD 1.1 billion in 2000. Purdue Pharmaceuticals identified physicians with high rates of opioid prescribing and targeted them with financial incentives such as meals and free conferences. There was also direct patient advertising. Marketing materials regularly minimised the potential for addiction while exaggerating effectiveness. In 2007, this resulted in criminal charges of misbranding leading to a USD 600 million fine and further legal action is ongoing. Oxycontin is no better or worse than other available opioids, yet it became a major drug of abuse and led to hundreds of thousands of deaths due to industry efforts to increase sales at all costs.
Another recent concerning example of inappropriate industry involvement is the takeover of Vectura, a British pharmaceutical company specialising in medical inhaler devices, by Phillip Morris International (PMI) [11]. The fact that one of the world's largest tobacco companies now owns a company responsible for producing treatments for patients whose lung damage may be caused by their tobacco products, undermines the trust of healthcare providers and patients in these products. The company now stands to supplement its traditional revenue from selling tobacco with additional income from selling treatments for conditions directly caused by their harmful products. This creates a situation where a tobacco company now benefits financially from the harm they cause to people's health. As a tobacco company, PMI's financial incentives are all directly opposed to improving public health. This takeover serves as an example of a traditionally harmful industry seeking to improve its image by associating with a healthcare-related industry in an attempt to be seen as “part of the solution” to the harms caused by the ongoing action of the majority share of its business. The response from the healthcare community has been swift, with Vectura being removed as sponsor for several upcoming medical conferences and the deal being denounced by many medical societies. Due to the accumulated evidence of tobacco industry interference in research and public policy, most journals already refuse to publish work by investigators with ties to the tobacco industry [12] and the European Respiratory Society prohibits involvement with those with a history of working with the tobacco industry any time after 1 January 2000.
This review looks at industry influence on the domains of medical research, medical education, guideline development, physician prescribing and formulary selection, and direct interaction with patients. The available evidence is summarised with illustrative examples and suggestions on how we might improve our relationship with industry.
Methods
Searches were carried out in December 2021 on PubMed for “Industry sponsorship”, “Industry sponsorship” AND “research”, “Industry sponsorship” AND “education”, “Industry sponsorship” AND “guideline”, “Industry sponsorship” AND “prescribing”, “Industry sponsorship” AND “patient”. Articles were excluded if non-clinical or not directly focused on funding discussions. Articles around sponsorship of sport, or sponsorship and advertising strategies by tobacco, food or drink industries and service delivery, dental, and in non-English languages were also excluded. Articles were then sorted into general topic themes and used as a resource to aid discussion of the described sections used for this review.
Results
Our search found 1373 articles, with 351 meeting inclusion criteria sorted into themes (see figure 1). Of these, 167 were general overviews, and the rest split into the following themes: education (n=35), oncology (n=31), patient (n=11), nutrition (n=11), cardiology & vasculature (n=11), psychiatry (n=10), surgery (n=10), orthopaedics (n=9), dermatology (n=6), guidelines (n=5), paediatrics (n=5), pain (n=5), food & beverage (n=4), infectious diseases (n=4), respiratory (n=4), other (n=23).
FIGURE 1.
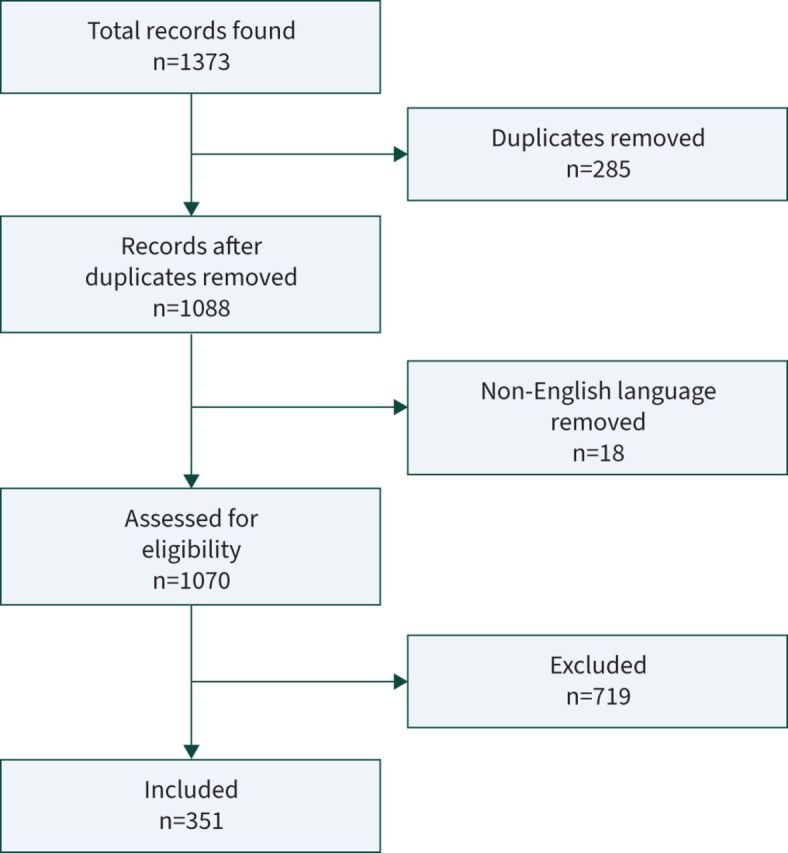
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram.
Industry and academic research
Obtaining funding for research is always an uphill struggle in academia. There are many brilliant research proposals but limited pots of money from not-for-profit research funders and charity organisations. Well designed and well powered clinical trials are expensive to fund. Collaborations and sponsorship from within industry are often the only option to be able to undertake such research with the majority of clinical trials being funded by industry [13]. Medical devices developed by industry alone, or in partnership with academia have been shown to be more likely to receive regulatory approval than devices solely developed by academia [9].
In the age of evidence-based medicine, a randomised controlled trial published in a leading journal is often held as the gold standard of evidence to guide clinical practice. The benefit to industry of such high-profile publications is the potential for increased physician prescribing as well as laying the groundwork for regulatory approval. For example, an industry-sponsored trial investigating the natural history of people with the R117H CFTR mutation determined that this mutation was associated with a delayed but comparable decline in lung function to people with a homozygous F508 mutation [14]. This paper had several limitations including the exclusion of 68% of patients with the R117H mutation from their analysis and makes little mention of the fact that most people with this mutation never develop CF. Despite this, the European Medicines Agency (EMA) have included the R117H mutation as an indication for treatment with ivacaftor. A Cochrane study found that clinical trials which are funded by industry were more likely to report favourable efficacy results and overall outcomes, as well as to downplay potential harms when compared to non-industry funded studies [15]. Even in studies that were independently funded, the presence of a principal investigator with ties to industry was associated with more favourable outcomes [16].
There are many potential sources of bias in industry-sponsored trials as well as ways that clinical trials can be manipulated to favour a particular outcome [17]. These include not reporting negative studies as well as reporting only specific subgroups or centres among others [18, 19]. Jefferson [20] provides striking examples of potential sources of bias in high-profile industry-sponsored trials. A study of reviewers for a prominent journal found no difference in reviewer scoring between papers with completed conflict of interest statements and those without [21]. These examples call into question the current model of editorial and peer review of research.
Yet despite evidence that industry-sponsored trials are inherently biased, ∼60% of clinical trials in the USA are industry funded [13]. Many of these trials are conducted by industry but published in the names of senior academics in order to lend respectability to the study [22]. Industry-sponsored clinical trials make up between two-thirds and three-quarters of studies published in major journals such as JAMA, Lancet and New England Journal of Medicine [23]. Many medical journals are dependent on revenue from industry advertising as well as content (in the form of industry-sponsored trials) to drive subscriptions. This leads to a conflict of interest where the journal's financial survival depends on continued engagement with industry. Industry's influence on academia may undermine the academic process leading to an “illusion of evidence-based medicine” [22].
The EMA is responsible for reviewing and approving medical products for use in European Union (EU) member states. User fees paid by companies wishing to have their product approved accounted for 86% of the EMA's funding in 2021 [24]. The remaining 14% of the EMA funding comes from the EU and covers public health measures, paediatric and orphan drugs that are not financially lucrative for industry to submit for review. The majority funding of a major regulatory body by the pharmaceutical companies represents a potential conflict of interest. Even if all decisions made by the EMA are considered free from bias, the regulatory agenda itself is being largely dictated to them by the interests of the pharmaceutical industry.
Industry partnership with academia plays an essential role in the development of new drugs and therapies, but commercial interests are currently harming patients by directing the research agenda towards repeated drug development in a narrow group of diseases which are highly prevalent in the developed world and neglecting other high disease burden areas. The integrity of medical research is potentially being compromised by industry involvement when the real efficacy and potential harms of new drugs are concealed. There are however some positives, for example, with the University of Oxford–AZ collaboration resulting in the promise of vaccine supply being not-for-profit for the duration of the COVID-19 pandemic and ensuring delivery to low-income countries. Patients’ come to harm when physicians are no longer able to determine the true value of new medications and are unable to offer truly evidence-based treatments. The case study in box 1 illustrates the potential harm to patients from industry involvement in clinical research.
BOX 1 A case study of industry interaction with academic research
Depression is common in children with an estimated prevalence of 2–6% of adolescents and is one of the leading causes of death in this age group [25]. Selective serotonin reuptake inhibitors (SSRIs) were the first drugs licensed for use in this age group due to concerns regarding side-effects of older classes of anti-depressants. Initial studies showed modest benefits with few adverse outcomes and an overall favourable risk–benefit ratio. However, in the following years concerns were raised about a possible association with suicidal ideation and suicide attempts in teenagers taking SSRIs. Further placebo-controlled trials confirmed this, and a black box warning was issued by the FDA in 2004. A study comparing published with unpublished clinical trials of SSRIs in children called into question the efficacy of several agents including citalopram, paroxetine and venlafaxine. As well as efficacy concerns, unpublished trials demonstrated an increase in serious adverse events including suicidality in groups treated with each agent [25].
In this case, there was a clinical need for treatment options for a disease with high prevalence and mortality in adolescents. Industry-sponsored clinical trials in this age group resulted in the approval of several medications, but selective publication resulted in an exaggeration of clinical benefit and the deliberate concealing of potentially fatal side-effects.
Potential benefits of industry involvement in academic research
Industry–academic partnerships fund discovery research.
Industry funding facilitates expensive large clinical trials.
Assessment of new drugs and medical devices by regulatory bodies, such as the EMA and US Food and Drug Administration (FDA), is funded by service users in industry, resulting in a saving of taxpayer money.
Potential harmful consequences of industry involvement in academic research
Research focus on high-return areas with other areas neglected.
Industry may abandon a candidate drug if potential financial benefits are small.
Manipulation of study design to minimise harms or exaggerate effectiveness.
Suppression of negative or unfavourable studies.
Loss of integrity of scientific journals.
Potential conflict of interest with regulators.
Industry and medical education
Continuing Medical Education (CME) is a requirement of most medical licensing organisations. The costs associated with attending events such as conferences and medical courses are significant and therefore many are industry sponsored, especially in resource-poor developing countries [26]. In the USA, over half of all CME education is industry funded [27]. Studies have shown that physicians who attend industry-sponsored CME programmes hold more positive views and have higher prescribing of the sponsored product [28, 29]. Conversely, physicians who did not attend industry-sponsored CME were more likely to prescribe less expensive generic medications [30].
Industry has an obligation to maximise profits through promotion of its product. In direct contrast, CME seeks to ensure physicians remain up to date with medical advances so they can practice in an evidence-based way to most benefit their patients. As discussed in greater detail later in this review, even small incentives such as free meals result in a significant change in physician prescribing behaviour. The involvement of industry in CME programmes introduces bias and risks them becoming marketing exercises. Physicians are generally aware of the risk of bias, but studies have demonstrated a lack of enthusiasm for removal of industry sponsorship if it results in higher registration fees [31].
Undergraduate education represents another potential interaction with industry. Most medical students will come into contact with industry representatives during their training. A Canadian study reported 81% of students being willing to meet with representatives and 75% willing to accept small gifts such as lunches [32]. In Europe, studies have shown that few teaching hospitals in France and almost no medical schools in Germany have comprehensive conflict of interest policies in place [33, 34]. In Germany, no medical school reported teaching on conflicts of interest as part of its medical curriculum [34]. In contrast, the American Medical Student Association (AMSA) started the Just Medicine Campaign which advocates for medical education free from conflicts of interest. They rate individual medical schools on their conflict of interest policies and advocate for teaching curricula to include training how to interact with industry and avoid conflicts of interest.
Industry influence on medical education introduces bias and this is well recognised by physicians. Despite this, there is reluctance to remove this financial subsidisation of educational events. Our primary duty as doctors is to our patients. Can we justify continued participation in activities that we know influence our behaviour to the detriment of those patients? The case study in box 2 illustrates the potential harms caused by industry sponsorship of medical education.
BOX 2 A case study of industry interaction with medical education
Industry involvement in medical education serves to alter prescribing patterns to increase sales of their drug. A Canadian medical school partnered with a pharmaceutical company that produced opioids to develop and deliver a week-long course on pain management to undergraduate medical students [35]. The guest speaker had ties to industry, but this conflict of interest was not disclosed. Reference textbooks were provided free of charge to all medical students and these too were developed by industry with inconsistent declarations of conflicts. Furthermore, the content of the lectures was closely aligned with industry interests and provided potentially dangerous information misclassifying oxycodone as a weak opioid rather than strong. The benefits of opioids in treating non-cancer pain were exaggerated while harms were minimised. Alternative agents such as paracetamol were portrayed as having significant potential adverse events. While efforts have since been made to improve the accuracy of information provided, the potential for previous attendees of this course to become unwitting contributors to the opioid epidemic is present.
Fully qualified physicians have been shown to be unable to identify bias in industry marketing. Undergraduates cannot be expected to perform any better and calls by the AMSA to include training on industry relationships as part of undergraduate medical curricula should be supported.
Potential benefits of industry involvement in medical education
CME ensures physicians remain up to date regarding changes to medical practice and industry funding supports the provision of many of these programmes.
Physicians benefit financially from industry sponsorship of CME as these programmes are often expensive to attend.
Industry funding of undergraduate courses supports medical schools financially.
Industry funding of CME events in developing countries facilitates delivery of education where there otherwise would not be the resources to do so.
Potential harmful consequences of industry involvement in medical education
Industry funding of CME alters physician prescribing in favour of the sponsor's products rather than evidence-based alternatives.
Biased risk–benefit information at undergraduate level risks patients coming to harm once those students graduate.
Risk of prompting overdiagnosis or medicalising normal life.
Risk of causing harm through unnecessary or unsafe medical interventions.
Industry and clinical guideline development
Clinical guidelines are an important decision-making tool used by healthcare professionals directly treating patients around the world. They should be based on a systematic review of the available literature in order to present an evidence-based guidance that has been tailored to the specific healthcare setting and ensure standardised, high-quality and patient-focused care. Clinical guidelines should be free from bias to ensure that their intended goal of maximum benefit for the maximum number of patients is not influenced by industry's goal of maximising profits. High profile guidelines are often adopted internationally and therefore represent a target for industry influence.
A study of guidelines published by the American Academy of Dermatology found that 81.6% of authors received payments from industry with a mean payment of USD 157 177 [36]. Payments were predominantly from companies with products relevant to the author's guideline. In the case of an atopic dermatitis guideline, the study authors noted a 473% increase in industry payments to the guideline authors in the year prior to guideline publication. This was followed by a further 97% increase in payments the year following publication. Across all guidelines reviewed the study found that 55% of authors did not accurately disclose their relationships with industry.
A study of American Oncologists found that those selected to join national guideline committees had more ties to industry and received more financial payments than their peers with fewer industry ties [37]. A more recent review of 223 American clinical guidelines found that only 10.7% of authors had accurate conflict of interest disclosures. General payments were more likely to be inaccurately reported than research funding [38].
Nursing guidelines are also open to conflicts of interest. The Royal College of Nursing (RCN) website claims to “Value our partnerships with industry” and lists examples of mutually beneficial cooperation including the involvement of the pharmaceutical company Besins Healthcare in the update of the RCN Menopause guideline. Besins Healthcare is a French company specialising in hormone replacement therapy medications for the treatment of menopause symptoms and therefore has a financial interest in the pharmaceutical management of menopause symptoms. No conflict of interest nor mention of industry involvement is included in the guideline itself [39].
In France, the national health authority was taken to court over undisclosed conflicts of interest amongst the authors of guidelines related to diabetes and Alzheimer's disease [40]. The court ruled against the health authority and the guidelines were withdrawn, followed by the voluntary withdrawal of a further six guidelines due to similar issues. Since then, further cases have been brought against the French Health Authority due to ongoing involvement of medical societies and institutions with ties to industry in the development of national guidelines.
Clinical guidelines are an essential resource and benefit patients by aiding decision making, standardising care and improving healthcare system efficiency by aligning services and ensuring evidenced-based treatments are promoted. Industry involvement is widespread and influences the development of these guidelines to favour prescribing of their products. This harms patients by delivering care that is not based on best evidence and the presence of conflicts of interest may serve to harm the trust patients have in their healthcare providers. Increased transparency, as seen in the USA, can serve to highlight these conflicts of interest but this does not prevent industry's involvement. More active measures including regulation and as demonstrated in France, legal action would better protect patients from the harmful effects of industry involvement in clinical guideline development. The case study in box 3 illustrates how industry involvement in guideline development leads to overdiagnosis and over-prescription of unnecessary treatments.
BOX 3 A case study of industry interaction with guideline development
Cow's milk allergy (CMA) is relatively common in infancy, with a challenge-proven prevalence of up to 1% [41]. Birth cohort studies from the USA and Australia suggest that this number has remained stable [42, 43], despite a significant rise in prescriptions for specialised infant formulas [44]. Many clinical guidelines exist to aid physicians in diagnosing and managing CMA; however, they risk attributing normal infant behaviour such as crying and vomiting to CMA leading to overdiagnosis [44, 45]. A recent study examining guideline-diagnosed CMA in a large birth-cohort study found that 74% of participants were reported to experience ≥2 mild-moderate symptoms and 9% reported ≥2 severe symptoms in at least 1 month of the study period [46]. This study found no difference in symptoms at 6 months between the infants directly consuming cow's milk and those exclusively breastfed. Many symptoms of CMA listed in guidelines, such as colic, vomiting and loose stools, are part of normal infancy and as these may be distressing to the child's parents, there is often pressure to “treat” these symptoms.
Overdiagnosis leads to over-treatment with strict maternal exclusion diets that may result in decreased breastfeeding or the use of unnecessary and expensive hydrolysed or amino acid-based formulas. A review of UK, European and Middle Eastern guidelines reveals that the writing of many of these guidelines was directly supported by the formula industry and 80% of all authors report conflicts of interest linked to formula companies [44]. In the decade from 2006 to 2016, prescriptions for specialised formula for CMA in England increased by 500% and costs by 700% to an annual spend of GBP 60 million despite no evidence for an associated increase in disease prevalence.
Potential benefits of industry involvement in guideline development
Consensus building between different stakeholders is a time-consuming and expensive process and industry funding facilitates guideline development.
Potential harmful consequences of industry involvement in guideline development
Overdiagnosis of medical conditions with a medicalisation of normal phenomena.
Over-treatment with unnecessary medications at a cost to the patient or healthcare system and the potential for adverse drug events.
The substitution of best-evidence treatments with a specific company's drug of choice.
Industry and physician interaction
Industry marketing budgets are frequently higher than their research and development budgets [47]. A study by the Institute for Health and Socio-Economic Policy found that in 2015, the top 100 pharmaceutical companies spent an average of just 8.32% of their revenue on research and development with over a quarter of companies having a marketing budget more than 10 times the amount spent on research and development [48]. Marketing to physicians remains the largest spend (68%) with an estimated spend of USD 20 billion in 2016 [49]. Drug promotion has been found to correlate poorly with health value with the top-promoted drugs less likely to be advantageous over the top prescribed drugs which are often available as generics [50]. They were also less likely to be recognised as first-line treatments in national guidelines or to be included on the WHO list of essential medicines.
Physician prescribing
Industry aims to court physicians as the primary prescribers in healthcare settings. Gifts are a common strategy with medical samples, promotional materials, free lunches and dinner invitations, scientific journals and invitations to CME sessions [28]. Most physicians consider themselves immune from industry influence but consider their colleagues to be more susceptible to marketing [28]. A study of Canadian psychiatry trainees found an association between the number of gifts received and the belief that discussions with industry representatives would not influence their prescribing [51]. Accepting drug samples and meals is also associated with higher prescription of branded rather than generic medications [30, 52].
A large cross-sectional study of prescribing in the USA focused on oral anticoagulants and non-insulin diabetes medication. It found that payments from industry were associated with increased prescribing of their branded medication in the corresponding geographic area. Payments to specialists and payments for speaking or consulting fees were associated with a larger effect on regional prescribing. A single payment of USD 13 was associated with an additional 3 months of branded medication being prescribed [53]. As discussed earlier, marketing of opioids to physicians in the USA has also been associated with increased opioid prescribing and an associated increase in overdose deaths [54]. Rosiglitazone is an oral antihyperglycaemic agent which was subsequently withdrawn due to cardiovascular side-effects in patients with diabetes. A review of literature published around that time demonstrated that authors with financial ties to either the manufacturer of Rosiglitazone or to other antihyperglycaemic medications were more likely to downplay the cardiovascular side-effects and promote ongoing prescribing both before and after the FDA issued a warning [55].
Formulary selection
Physicians who have contact with industry, including meeting with representatives as well as financial benefits such as sponsored research and paid speaking at educational sessions, are more likely to request that drugs from that company be included in their hospital formulary [56]. Industry payments to physicians were associated with increased prescribing of the company's drug as well as lower overall prescribing of generics and higher prescribing cost, with higher payments associated with higher branded prescribing [57, 58]. Exposure to information directly from industry has been associated with higher rates of prescribing that is of lower quality and higher cost [59, 60]. A Danish study of general practitioners involved in industry-sponsored clinical trials relating to asthma demonstrated that while there was no overall increase in prescribing of inhaled corticosteroids there was a formulary substitution with increased prescribing of the sponsoring company's product [61].
Regulation of industry marketing
Regulation of industry representatives varies between countries. France has significantly stricter rules than the USA and Canada, with French doctors significantly less likely to receive free samples or meals than their North American colleagues. Information provided by industry representatives in France was more likely to include information on potential harmful effects of the treatment compared with their colleagues in North America. However, serious adverse events were rarely mentioned in either region and a majority of physicians positively rated the quality of information provided and expressed a willingness to prescribe the medication [62].
While industry marketing is frequently inaccurate it is rarely challenged. Between 1997 and 2016 there were a total of 103 financial settlements in the USA where pharmaceutical companies were found to have engaged in false or misleading advertising with a cumulative total of USD 11 billion in fines over the time period [49]. While this appears to be a significant sum on money, it pales in comparison to a net income measured in the trillions of dollars over the same time period. The case study in box 4 illustrates the effect of industry influence on prescribers.
BOX 4 A case study of industry interaction with prescribers
Industry sponsorship of clinical meetings and the providing of meals is a well-documented occurrence in both hospital and primary care settings. This represents a form of financial payment to physicians and the Open Payments system in the USA allows for scrutiny of these interactions. A large cross-sectional study examined prescribing habits of over a quarter of a million US physicians with regard to four drugs across different classes. The chosen drugs were all patent-protected at the time of study and each had generic alternatives available with no clear superiority of the chosen drugs over these alternatives. Physicians who had received a single meal with a value less than USD 20 were found to be more likely to prescribe the promoted drug than others in its class. Furthermore, there was a dose–response effect, with higher value and more frequent meals associated with higher relative prescribing rates [63]. This study serves to highlight the small investment required by industry to alter physician prescribing habits to choose more expensive branded medications, which are less likely to be first line in clinical guidelines and represent a potential harm to patients by increasing prescribing costs which has knock-on effects in other areas.
Potential benefits of industry interaction with physicians
Alerting prescribers to new therapeutic options.
Opportunities to network with other prescribers at sponsored events.
Potential harmful consequences of industry interaction with physicians
Changing prescription habits to be less evidence-based and/or more expensive with reduced prescribing of generic medications.
Decreased healthcare equality due to irrational prescribing and knock-on effects of more expensive prescribing.
Loss of public trust in their healthcare provider's impartiality.
Direct industry interaction with patients
Direct-to-consumer advertising
Between 1997 and 2016 there was a shift in focus to direct-to-consumer advertising (DTCA) in the USA. DTCA budgets have increased from 11.9% to 32% of marketing spend with a move to advertising high-tech medications such as biologics and anti-cancer treatments [49]. In contrast, EU legislation prohibits DTCA of prescription drugs (Directive 2001/83/EC).
In countries where DTCA of prescription medications is banned (everywhere except the USA and New Zealand), industry sponsorship of advocacy groups has the potential to become DTCA through the back door by promoting their treatments amongst patients while downplaying potential harms. Industry is increasingly using sponsorship of advocacy groups as a means to increase revenue with a yearly increase in total payments seen [64, 65]. Patient groups receiving industry funding were also more likely to support legislation pushing for reform of EMA rules to allow DTCA advertising in Europe [66].
Advocacy groups
Patient advocacy groups play an important role in raising awareness, educating patients and the public as well as fundraising for services. They also advocate for their members/patients access to healthcare including regulatory approval of newly developed drugs and therapies. Public funding for advocacy groups is often scarce and many receive industry funding [67]. The prevalence of industry funding of advocacy groups ranges from 20% to 83%, with just 27% disclosing this on their websites. Industry-funded groups generally supported their sponsor's interests [65, 68]. An example of this is the finding that organisations with ties to opioid manufacturers were more likely to oppose the US Centers for Disease Control and Prevention 2016 guidelines on the use of opioids for chronic pain on the grounds of both dose and duration of treatment [69]. Ties to opioid manufacturers were seen in 28.5% of organisations commenting on the draft guideline, although none declared this in their submission.
Industry funding of patient groups is distributed to maximise financial return. Companies will give larger sums to advocacy groups for conditions in which they have medications seeking regulatory approval [70]. Sponsorship also focuses on high-return areas, such as groups supporting patients with common conditions and those with high-tech treatments. In the UK, the groups receiving the highest amount of sponsorship were focused on patients with cancer, followed by endocrine and metabolic conditions including diabetes [64, 65]. Sponsorship funding was predominantly directed towards engagement with the public such as educational campaigns as well as “policy engagement”. Patient support and organisational maintenance received just 5.9% and 2.8% of funding, respectively [64]. The concentration of funds to groups for which new drugs are seeking approval is essentially DTCA by another route. This risks turning passionate and well-meaning patient advocacy groups into industry lobbyists seeking regulatory approval of drugs with exaggerated effectiveness and minimised risks.
Sponsored nurses
A more recent phenomenon is the introduction of industry-sponsored nurses delivering patient support programmes to aid with initiation and support when commencing new medications. This may be another disguised route of direct-to-patient advertising/promotion of products. An example of this is the “ambassador” nurses hired by AbbVie to teach patients how to administer adalimumab (Humira), complete related paperwork, answer questions and promote adherence [71]. A lawsuit filed in 2018 alleged that such ambassadors provided unbalanced information especially around downplaying serious side-effects [72]. These ambassadors saved time and resources for the prescribing physician, but were only available for those patients who were prescribed AbbVie's drug [71]. The case was settled out of court with AbbVie paying California USD 24 million but with no admission of wrongdoing [72]. Other pharmaceutical companies with paid nurse roles include, amongst others, AZ, Lilly, Gilead Sciences, Bayer, Pfizer, and more recently, to support with commencement of CFTR modulators, “Patient Support Specialists” from Vertex.
The case study in box 5 illustrates the potential benefits and harms of industry involvement with patient groups.
BOX 5 A case study of industry interaction with patient groups
Ivacaftor (Kalydeco) was licensed in 2012 and was the first of a new generation of drugs to treat cystic fibrosis (CF) by focusing on the underlying genetic mutation. It was developed by a pharmaceutical company in partnership with the CF Foundation, a non-profit organisation established in the 1950s by parents of children with CF. In this “venture-philanthropic” relationship, the CF foundation funded the preclinical and part of phase 1 clinical trials, an estimated 40% of development costs, in exchange for a proportion of the profits [73]. The CF Foundation also played an essential role in raising awareness amongst the CF patient community about the drug and was involved in patient recruitment for the clinical trials [74]. The pharmaceutical company paid the remaining 60%, estimated at about USD 2.5 billion after tax reliefs for orphan drug development are considered. Ivacaftor was then released with an initial price tag of around USD 300 000 annually, which was found to be far in excess of the amounts considered to be justifiable using the typical pharmacoeconomic measure of quality-adjusted life-years (QALY) [74]. Despite this, and thanks to significant lobbying by patient advocacy groups, ivacaftor was approved in most countries with developed healthcare systems and high prevalence of CF [74]. Ivacaftor only benefits patients with the relatively uncommon G551D mutation and the total number of eligible patients and therefore the cost was manageable. Ivacaftor was followed by other CFTR modulators such as ivacaftor/lumacaftor (Orkambi), tezacaftor/ivacaftor (Symkevi) and elexacaftor/lumacaftor/ivacaftor (Kaftrio), which target the more common F508 mutation and therefore represent a higher total healthcare cost due to the higher number of eligible patients and need for lifelong therapy.
As a result of this partnership, a new class of treatments targeting the underlying cause of CF has been developed which is of critical importance to these patients and gives hope to other groups of patients with rare diseases. Another group to benefit is the CF Foundation who sold their royalty stake for USD 3.3 billion, as well as the pharmaceutical company (now Vertex Pharmaceuticals) who currently have a monopoly on these drugs and have made billions of dollars in profit [73]. In this case, the partnership between industry and a patient charity distributed the financial risk of drug development as well as assisting in study recruitment and regulatory lobbying with a mutually beneficial outcome. If this model proves reproducible for other rare diseases, will our healthcare systems be able to fund an increasing number of individualised medicines?
Potential benefits of industry involvement with patients directly
Financial support for patient advocacy groups.
Mutually beneficial lobbying for regulatory approval of new therapies.
Potential harmful consequences of industry involvement with patients directly
Unequal distribution of industry funding marginalises certain groups with conditions perceived by industry to lack financial return.
Majority of industry funding supports marketing and lobbying rather than supporting patients.
DTCA may overstate benefits while minimising potential risks.
Industry may seek to manipulate advocacy groups through misinformation promoting unrealistic expectations in order to pressure policymakers to approve their drug.
Conclusions
Industry has a vital role to play in the development of new medical devices and drugs. Collaboration between academic institutes and industry, such as venture philanthropy for CFTR modulators, represents a potentially beneficial model for future cooperation. Beyond this, industry influence in the healthcare system provides little value for patient care. Industry has a fiduciary duty to their shareholders and this may have a detrimental effect on healthcare decision making. Any post-authorisation engagement between industry and prescribers should be considered a form of advertising as even relatively minor interactions have been shown to influence the behaviour of health professionals and to introduce bias favouring the industry product. This inherently biased relationship between service providers and industry would not be tolerated in other fields and medicine should be no different.
The evolution of our current healthcare model has resulted in significant proportions of medical research, education and patient advocacy groups being financially dependent on relationships with industry. While one side may enter these relationships in good faith and with patient welfare as their priority, there is a growing and overwhelming body of evidence suggesting that in many cases these relationships benefit industry shareholders rather than patients. The move to increased transparency of conflicts of interest has slowly gathered pace with the Open Payments database established in the USA in 2010 as part of the Affordable Care Act. Data have been publicly searchable since 2014 and the resulting research serves to highlight the web of industry influence in healthcare. Similar “Sunshine Legislation” exists in some European countries, such as Denmark, France, Greece, Latvia, Portugal and Romania, although the specific types and value of financial incentives requiring registration varies [75]. The current model adopted by the European Federation of Pharmaceutical Industries and Associations (EFPIA) relies on self-regulation for disclosure of payments to healthcare providers. The effectiveness of this varies considerably with few countries having centralised databases [76]. The disclosure rates of many individual pharmaceutical companies have been found to vary significantly between countries. This raises concerns about the industry's ability to self-regulate and highlights the need for Europe-wide legislation with mandated reporting to centralised, searchable databases. In the UK, the Cumberlege Report recommended expanding industry declarations and making it mandatory for healthcare providers names to be listed [77]. Establishing a mandatory centralised database under the aegis of an independent regulator such as the General Medical Council would serve to improve transparency and patient confidence in their doctor's impartiality. Such a register enjoys broad support from professionals’ organisations in the UK [78]. At a regulatory level, the EMA has mandated public declarations of conflict of interest by all members of their scientific committees. Degrees of interest are described ranging from direct involvement to indirect conflicts of interest with the potential for members to have corresponding restrictions placed on their committee involvement [79].
How can we improve things?
Transparency alone is not enough to remove harmful industry influence. There is a growing movement towards a financial uncoupling from industry with calls for solutions such as increased (and enforced) legislation to limit industry interactions with physicians as well as independent drug evaluation to minimise bias [80].
Solutions exist to many of these problems. For example, the Italian Medicines Agency (AIFA) has funded independent trials financed by a 5% tax on industry promotional budgets [81]. In its first 3 years this programme raised EUR 78 million and funded 151 studies. Conditions for approval include full researcher control of the study including design, recruitment and data analysis. There is also mandatory reporting of all results and studies may not form part of the drug approval process. The programme focuses on areas such as orphan drugs and comparative trials including head-to-head studies (which pharmaceutical companies may not wish to sponsor). AIFA seek input from a wide range of stakeholders including medical and scientific societies, government institutions and the general public as well as industry organisations.
Regulatory bodies such as the EMA are already partially funded through the EU, increasing this would reduce dependence on user fees and allow a more representative range of stakeholders to have influence on the regulatory agenda. Both the EMA and FDA have introduced legislation mandating clinical trials be performed in children where a new drug is likely to benefit them. Further legislation could mandate industry performs head-to-head trials comparing their new drug to the current best in class, in addition to placebo controlled or non-inferiority trials (which are favoured by industry). This would ensure new drugs offer clinical superiority rather than fill the market with clinically indistinguishable branded alternatives.
Industry-sponsored clinical trials have been shown to be at high risk of bias. Strategies to improve transparency of these trials may include full data depository at the time of reporting in addition to the steps already discussed to mandate author conflict of interest declarations. If these or similar measures were unsuccessful then further measures could be considered. Journals already refuse to publish studies sponsored by the tobacco industry. Discussions need to be had about whether journals need to expand this policy to include trials sponsored by the pharmaceutical industry, which evidence suggests are inherently biased. Systemic change takes time.
Table 1 details some suggested changes that can be made by prescribers, policymakers and patients. For the individual prescriber, studies have clearly demonstrated that doctors are easily influenced by gifts, meals and financial payments from industry to change our prescribing habits in favour of branded alternatives with negative impacts on healthcare costs. We are also poor at identifying limitations and bias in information provided by industry. Industry needs to invest relatively little to change prescribing habits that can lead to massive financial benefits thanks to even small increases in their market share. It is in patients’ best interests that we neither rely on industry to self-regulate their interactions with physicians, nor rely on our own ability to remain immune to influence when this has proven to be impossible. Independent evaluation of medication efficacy and safety, coupled with bias-free clinical guideline development is in the best interests of patients. Direct interactions between industry representatives and physicians serve only to fill industry coffers and doctors’ stomachs. As individual healthcare providers, we are answerable to our patients rather than to shareholders and are therefore the only step in the chain potentially free from any conflict of interests.
TABLE 1.
Guidance for prescribers, policymakers and patients when engaging with industry
| Physicians and other prescribers |
|
|
|
| Policymakers |
|
|
|
|
| Patients |
|
|
Key points
Industry influence is present across all areas of healthcare and while some interactions may be mutually beneficial, the overall aim of industry is to maximise their profits by increasing sales.
Industry plays an important role in the development of new therapies but involvement with medical education, guideline development, prescribers and patients provides little benefit to patient care.
Increased transparency has highlighted the extent of industry influence but more needs to be done to reduce conflicts of interest.
Self-evaluation questions
1. Which of the following are ways that industry influences clinical trial outcomes in its favour?
a) Not reporting negative trials
b) Comparing against placebo instead of the main competitor drug
c) Using multiple end-points and selectively publishing the end-point with the most favourable outcome
d) Selectively publishing favourable subgroup analysis
e) All of the above
2. The largest portion of pharmaceutical industry marketing budgets in Europe targets which group?
a) Patients through direct-to-consumer advertising
b) Regulators
c) Prescribers
d) Nurse specialists
e) Medical students
3. Physician prescribing habits have been shown to be influenced by which marketing tactics?
a) Free meals
b) Subsidised/free educational sessions
c) Free samples of medications
d) Consulting/speaker fees
e) All of the above
4. Which of the following would be considered a valid and safe way of interacting with industry, where the risk of adverse outcomes for patients from a conflict of interest is low?
a) Industry pays for a healthcare practitioner to travel to a conference and present their own work, without any conditions or interference in the presented work from industry
b) Industry provides active and placebo capsules for an investigator-initiated trial where industry have an opportunity to comment on the manuscript before submission but are not directly involved in the conduct or reporting of the research project
c) Industry supports a private practice website for a healthcare professional who frequently prescribes their products
d) Industry part-support a specialist nurse salary to help improve a diagnostic service in primary care
e) None of the above
Suggested answers
1. e.
2. c.
3. e.
4. e.
Footnotes
Conflict of interest: A.R. Smyth has received grants or contracts from Vertex Pharmaceuticals; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Vertex Pharmaceuticals; and support for attending meetings and/or travel from Vertex Pharmaceuticals, all outside the submitted work. A.R. Smyth has patents planned, issued or pending (Camara M, Williams P, Barrett D, Halliday N, Knox A, Smyth A, Fogarty A, Barr H, Forrester D. Alkyl quinolones as biomarkers of Pseudomonas aeruginosa infection and uses thereof (US-2016131648-A1; https://pubchem.ncbi.nlm.nih.gov/patent/US-2016131648-A1)), outside the submitted work. A.R. Smyth reports participation on a Data Safety Monitoring Board or Advisory Board for North American Cystic Fibrosis Foundation Therapeutic Development Network Data Safety Monitoring Board, outside the submitted work. R.J. Boyle has received consulting fees from Cochrane, John Wiley and Sons, British Society for Allergy and Clinical Immunology, and Prota Therapeutics, outside the submitted work; and has received payment for expert testimony from Taus and Cebulash, outside the submitted work. The remaining authors have nothing to disclose.
References
- 1.Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA 2018; 319: 1024–1039. doi: 10.1001/jama.2018.1150 [DOI] [PubMed] [Google Scholar]
- 2.Ledley FD, McCoy SS, Vaughan G, et al. Profitability of large pharmaceutical companies compared with other large public companies. JAMA 2020; 323: 834–843. doi: 10.1001/jama.2020.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chimonas S, Mamoor M, Zimbalist SA,et al. Mapping conflict of interests: scoping review. BMJ 2021; 375: e066576. doi: 10.1136/bmj-2021-066576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 2020; 323: 844–853. doi: 10.1001/jama.2020.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrenho E, Miraldo M, Smith PC. Does global drug innovation correspond to burden of disease? The neglected diseases in developed and developing countries. Health Econ 2019; 28: 123–143. doi: 10.1002/hec.3833 [DOI] [PubMed] [Google Scholar]
- 6.Fourteenth Meeting of the Strategic and Technical Advisory Group for Neglected Tropical Diseases, 22–24 June 2021. Geneva, World Health Organization, 2021. https://apps.who.int/iris/handle/10665/346696 [Google Scholar]
- 7.Plackett B. Why big pharma has abandoned antibiotics. Nature 2020; 586: S50–S52. doi: 10.1038/d41586-020-02884-3 [DOI] [Google Scholar]
- 8.Marselis D, Hordijk L. From blockbuster to “nichebuster”: how a flawed legislation helped create a new profit model for the drug industry. BMJ 2020; 370: m2983. doi: 10.1136/bmj.m2983 [DOI] [PubMed] [Google Scholar]
- 9.Marcus HJ, Payne CJ, Hughes-Hallett A,et al. Regulatory approval of new medical devices: cross sectional study. BMJ 2016; 353: i2587. doi: 10.1136/bmj.i2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Zee A. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am J Public Health 2009; 99: 221–227. doi: 10.2105/AJPH.2007.131714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burki TK. Philip Morris International purchases Vectura. Lancet Respir Med 2021; 9: e122. doi: 10.1016/S2213-2600(21)00445-8 [DOI] [PubMed] [Google Scholar]
- 12.Godlee F, Malone R, Timmis A, et al. Journal policy on research funded by the tobacco industry. BMJ 2013; 347: f5193. doi: 10.1136/bmj.f5193 [DOI] [PubMed] [Google Scholar]
- 13.Moses H, Matheson DHM, Cairns-Smith S,et al. The anatomy of medical research: US and international comparisons. JAMA 2015; 313: 174–189. doi: 10.1001/jama.2014.15939 [DOI] [PubMed] [Google Scholar]
- 14.Wagener JS, Millar SJ, Mayer-Hamblett N,et al. Lung function decline is delayed but not decreased in patients with cystic fibrosis and the R117H gene mutation. J Cyst Fibrosis 2018; 17: 503–510. doi: 10.1016/j.jcf.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundh A, Lexchin J, Mintzes B,et al. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017; 2: MR000033. doi: 10.1002/14651858.MR000033.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn R, Woodbridge A, Abraham A, et al. Financial ties of principal investigators and randomized controlled trial outcomes: cross sectional study. BMJ 2017; 356: i6770. doi: 10.1136/bmj.i6770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lexchin J, Bero LA, Davis C, et al. Achieving greater independence from commercial influence in research. BMJ 2021; 9: n370. doi: 10.1136/bmj.n370 [DOI] [PubMed] [Google Scholar]
- 18.Wieseler B, Wolfram N, McGauran N,et al. Completeness of reporting of patient-relevant clinical trial outcomes: comparison of unpublished clinical study reports with publicly available data. PLoS Med 2013; 10: e1001526. doi: 10.1371/journal.pmed.1001526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith R. Medical journals are an extension of the marketing arm of pharmaceutical companies. PLoS Med 2005; 2: e138. doi: 10.1371/journal.pmed.0020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferson T. Sponsorship bias in clinical trials: growing menace or dawning realisation? J R Soc Med 2020; 113: 148–157. doi: 10.1177/0141076820914242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John LK, Loewenstein G, Marder A, et al. Effect of revealing authors’ conflicts of interests in peer review: randomized controlled trial. BMJ 2019; 367: l5896. doi: 10.1136/bmj.l5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jureidini J, McHenry LB. The illusion of evidence based medicine. BMJ 2022; 376: o702. doi: 10.1136/bmj.o702 [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Bartlett C, Jüni P. Are randomised controlled trials in the BMJ different? BMJ 2001; 323: 1253–1254. doi: 10.1136/bmj.323.7323.1253a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.European Medicines Agency . Funding. www.ema.europa.eu/en/about-us/how-we-work/governance-documents/funding. Date last accessed: 4 January 2022.
- 25.Whittington CJ, Kendall T, Fonagy Pet al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004; 363: 1341–1345. doi: 10.1016/S0140-6736(04)16043-1 [DOI] [PubMed] [Google Scholar]
- 26.Jayasinghe S. Pharmaceutical industry sponsorship of academic conferences: ethics of conflict of interest. J Med Ethics 2021; 47: e33. doi: 10.1136/medethics-2020-106224 [DOI] [PubMed] [Google Scholar]
- 27.Fletcher S. Pharma and CME: view from the US. BMJ 2008; 337: a1023. doi: 10.1136/bmj.a867 [DOI] [PubMed] [Google Scholar]
- 28.Fickweiler W, Urbach E. Interactions between physicians and the pharmaceutical industry generally and sales representatives specifically and their association with physicians’ attitudes and prescribing habits: a systematic review. BMJ Open 2017; 7: e016408. doi: 10.1136/bmjopen-2017-016408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieperink ME, Drogemuller L. Industry-sponsored grand rounds and prescribing behavior. JAMA 2001; 285: 1443–1444. doi: 10.1001/jama.285.11.1443 [DOI] [PubMed] [Google Scholar]
- 30.Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One 2014; 9: e110130. doi: 10.1371/journal.pone.0110130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabas JA, Boscardin C, Jacobsen DM,et al. Clinician attitudes about commercial support of continuing medical education: results of a detailed survey. Arch Intern Med 2011; 171: 840–846. doi: 10.1001/archinternmed.2011.179 [DOI] [PubMed] [Google Scholar]
- 32.Barfett J, Lanting B, Lee J,et al. Pharmaceutical marketing to medical students: the student perspective. McGill J Med 2004; 8: 21–27. doi: 10.26443/mjm.v8i1.376 [DOI] [Google Scholar]
- 33.Guy-Coichard C, Perraud G, Chailleu A,et al. Inadequate conflict of interest policies at most French teaching hospitals: a survey and website analysis. PLoS One 2019; 14: e0224193. doi: 10.1371/journal.pone.0224193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grabitz P, Friedmann Z, Gepp S, et al. Quantity and quality of conflict of interest policies at German medical schools: a cross-sectional study and survey. BMJ Open 2020; 10: e039782. doi: 10.1136/bmjopen-2020-039782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persaud N. Questionable content of an industry-supported medical school lecture series: a case study. J Med Ethics 2014; 40: 414–418. doi: 10.1136/medethics-2013-101343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Checketts JX, Sims MT, Vassar M. Evaluating industry payments among dermatology clinical practice guidelines authors. JAMA Dermatol 2017; 153: 1229–1235. doi: 10.1001/jamadermatol.2017.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell AP, Mishra A, Dey Pet al. Personal payments from pharmaceutical companies to authors of oncology clinical practice guidelines. Oncologist 2021; 26: e1897. doi: 10.1002/onco.13983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andreatos N, Zacharioudakis IM, Zervou FN,et al. Discrepancy between financial disclosures of authors of clinical practice guidelines and reports by industry. Medicine (Baltimore) 2017; 96: e5711. doi: 10.1097/MD.0000000000005711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Royal College of Nursing . Menopause: RCN Guidance for Nurses, Midwives and Health Visitors. 2nd Edn. London, Royal College of Nursing, 2020. [Google Scholar]
- 40.Lenzer J. French guidelines are withdrawn after court finds potential bias among authors. BMJ 2011; 342: d4007. doi: 10.1136/bmj.d4007 [DOI] [PubMed] [Google Scholar]
- 41.Schoemaker AA, Sprikkelman AB, Grimshaw KE, et al. Incidence and natural history of challenge-proven cow's milk allergy in European children – EuroPrevall birth cohort. Allergy 2015; 70: 963–972. doi: 10.1111/all.12630 [DOI] [PubMed] [Google Scholar]
- 42.Peters RL, Koplin JJ, Allen KJ,et al. The prevalence of food sensitization appears not to have changed between 2 Melbourne cohorts of high-risk infants recruited 15 years apart. J Allergy Clin Immunol Pract 2018; 6: 440–448.e2. doi: 10.1016/j.jaip.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 43.McGowan EC, Peng RD, Salo PM,et al. Changes in food-specific IgE over time in the national health and nutrition examination survey (NHANES). J Allergy Clin Immunol Pract 2016; 4: 713–720. doi: 10.1016/j.jaip.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munblit D, Perkin MR, Palmer DJ,et al. Assessment of evidence about common infant symptoms and cow's milk allergy. JAMA Pediatr 2020; 174: 599–608. doi: 10.1001/jamapediatrics.2020.0153 [DOI] [PubMed] [Google Scholar]
- 45.Smith TDH, Townsend R, Hussain HS,et al. Milk allergy guidelines for infants in England promote over-diagnosis: a cross-sectional survey. Clin Exp Allergy 2022; 52: 188–191. doi: 10.1111/cea.14053 [DOI] [PubMed] [Google Scholar]
- 46.Vincent R, MacNeill SJ, Marrs T,et al. Frequency of guideline-defined cow's milk allergy symptoms in infants: secondary analysis of EAT trial data. Clin Exp Allergy 2022; 52: 82–93. doi: 10.1111/cea.14060 [DOI] [PubMed] [Google Scholar]
- 47.Gagnon M, Lexchin J. The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med 2008; 5: e1. doi: 10.1371/journal.pmed.0050001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Institute for Health and Socio-Economic Policy . The R&D Smokescreen. The Prioritization of Marketing & Sales in the Pharmaceutical Industry. Date last updated: 20 October 2016. Date last accessed: 18 January 2022. https://nurses.3cdn.net/e74ab9a3e937fe5646_afm6bh0u9.pdf
- 49.Schwartz LM, Woloshin S. Medical marketing in the United States, 1997–2016. JAMA 2019; 321: 80–96. doi: 10.1001/jama.2018.19320 [DOI] [PubMed] [Google Scholar]
- 50.Greenway T, Ross JS. US drug marketing: how does promotion correspond with health value? BMJ 2017; 357: j1855. doi: 10.1136/bmj.j1855 [DOI] [PubMed] [Google Scholar]
- 51.Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. Can Med Assoc J 1995; 153: 553–559. [PMC free article] [PubMed] [Google Scholar]
- 52.Pedan A, Wu H. Asymmetric responsiveness of physician prescription behavior to drug promotion of competitive brands within an established therapeutic drug class. Health Mark Q 2011; 28: 133–154. doi: 10.1080/07359683.2011.545341 [DOI] [PubMed] [Google Scholar]
- 53.Fleischman W, Agrawal S, King M, et al. Association between payments from manufacturers of pharmaceuticals to physicians and regional prescribing: cross sectional ecological study. BMJ 2016; 354: i4189. doi: 10.1136/bmj.i4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadland SE, Rivera-Aguirre A, Marshall BDL, et al. Association of pharmaceutical industry marketing of opioid products with mortality from opioid-related overdoses. JAMA Netw Open 2019; 2: e186007. doi: 10.1001/jamanetworkopen.2018.6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang AT, Mccoy CP, Murad MH, et al. Association between industry affiliation and position on cardiovascular risk with rosiglitazone: cross sectional systematic review. BMJ 2010; 340: c1344. doi: 10.1136/bmj.c1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chren M, Landefeld CS. Physicians’ behaviour and their interactions with drug companies: a controlled study of physicians who requested additions to a hospital drug formulary. JAMA 1994; 271: 684–689. doi: 10.1001/jama.1994.03510330062035 [DOI] [PubMed] [Google Scholar]
- 57.Mitchell AP, Trivedi NU, Gennarelli RL,et al. Are financial payments from the pharmaceutical industry associated with physician prescribing? A systematic review. Ann Intern Med 2021; 174: 353–361. doi: 10.7326/M20-5665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue K, Tsugawa Y, Mangione CM, et al. Association between industry payments and prescriptions of long-acting insulin: an observational study with propensity score matching. PLoS Med 2021; 18: e1003645. doi: 10.1371/journal.pmed.1003645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spurling GK, Mansfield PR, Montgomery BD,et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010; 7: e1000352. doi: 10.1371/journal.pmed.1000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brax H, Fadlallah R, Al-Khaled L,et al. Association between physicians’ interaction with pharmaceutical companies and their clinical practices: a systematic review and meta-analysis. PLoS One 2017; 12: e0175493. doi: 10.1371/journal.pone.0175493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen M, Kragstrup J, Søndergaard J. How conducting a clinical trial affects physicians’ guideline adherence and drug preferences. JAMA 2006; 295: 2759–2764. doi: 10.1001/jama.295.23.2759 [DOI] [PubMed] [Google Scholar]
- 62.Mintzes B, Lexchin J, Sutherland JM,et al. Pharmaceutical sales representatives and patient safety: a comparative prospective study of information quality in Canada, France and the United States. J Gen Intern Med 2013; 28: 1368–1375. doi: 10.1007/s11606-013-2411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeJong C, Aguilar T, Tseng C,et al. Pharmaceutical industry–sponsored meals and physician prescribing patterns for medicare beneficiaries. JAMA Intern Med 2016; 176: 1114–1122. doi: 10.1001/jamainternmed.2016.2765 [DOI] [PubMed] [Google Scholar]
- 64.Ozieranski P, Rickard E, Mulinari, S. Exposing drug industry funding of UK patient organisations. BMJ 2019; 365: l1806. doi: 10.1136/bmj.l1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulinari S, Vilhelmsson A, Rickard E, et al. Five years of pharmaceutical industry funding of patient organisations in Sweden: cross-sectional study of companies, patient organisations and drugs. PLoS One 2020; 15: e0235021. doi: 10.1371/journal.pone.0235021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bero LA, Parker L. Risky business? Pharmaceutical industry sponsorship of health consumer groups. Austr Prescr 2021; 44: 74–76. doi: 10.18773/austprescr.2021.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rose SL, Highland J, Karafa MT, et al. Patient advocacy organizations, industry funding, and conflicts of interest. JAMA Intern Med 2017; 177: 344–350. doi: 10.1001/jamainternmed.2016.8443 [DOI] [PubMed] [Google Scholar]
- 68.Fabbri A, Parker L, Colombo C,et al. Industry funding of patient and health consumer organisations: systematic review with meta-analysis. BMJ 2020; 368: l6925. doi: 10.1136/bmj.l6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin DH, Lucas E, Murimi IB,et al. Financial conflicts of interest and the centers for disease control and prevention's 2016 guideline for prescribing opioids for chronic pain. JAMA Intern Med 2017; 177: 427–428. doi: 10.1001/jamainternmed.2016.8471 [DOI] [PubMed] [Google Scholar]
- 70.Fabbri A, Swandari S, Lau E,et al. Pharmaceutical Industry funding of health consumer groups in Australia: a cross-sectional analysis. Int J Health Serv 2019; 49: 273–293. doi: 10.1177/0020731418823376 [DOI] [PubMed] [Google Scholar]
- 71.Grundy Q, Ladd E. “Nurse ambassadors”: a new “fulcrum” of pharmaceutical marketing. Health Affairs Blog. Date last updated: 4 January 2019. https://www.healthaffairs.org/do/10.1377/forefront.20181227.402878/full/
- 72.Yang YT, Mason DJ. Problematic promotion of medications by nurse ambassadors: legal and ethical issues. JAMA 2021; 325: 345–346. doi: 10.1001/jama.2020.24509 [DOI] [PubMed] [Google Scholar]
- 73.Hollis A. Orphan drug pricing and costs: a case study of kalydeco and orkambi. Healthc Policy 2019; 15: 70–80. doi: 10.12927/hcpol.2019.25937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen D, Raftery J. Paying twice: the charitable drug with a high price tag. BMJ 2014; 348: 18–19. doi: 10.1136/bmj.g18 [DOI] [PubMed] [Google Scholar]
- 75.Fabbri A, Al Santos, Mezinska S,et al. Sunshine policies and murky shadows in Europe: disclosure of pharmaceutical industry payments to health professionals in nine European countries. Int J Health Policy Manag 2018; 7: 504–509. doi: 10.15171/ijhpm.2018.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mulinari S, Martinon L, Jachiet PA,et al. Pharmaceutical industry self-regulation and non-transparency: country and company level analysis of payments to healthcare professionals in seven European countries. Health Policy 2021; 125: 915–922. doi: 10.1016/j.healthpol.2021.04.015 [DOI] [PubMed] [Google Scholar]
- 77.First Do No Harm. The report of the Independent Medicines and Medical Devices Safety Review. Date last accessed: 18 January 2022. Date last updated: 8 July 2020. https://www.immdsreview.org.uk/Report.html [DOI] [PubMed]
- 78.Rimmer A. Nine in 10 professional organisations say doctors should have to register their financial interests. BMJ 2021; 373: n933. doi: 10.1136/bmj.n933 [DOI] [PubMed] [Google Scholar]
- 79.European Medicines Agency . European Medicines Agency policy on the handling of competing interests of scientific committees' members and experts. Date last accessed: 15 January 2022. Date last updated: 11 June 2020. www.ema.europa.eu/en/documents/other/policy-44-european-medicines-agency-policy-handling-competing-interests-scientific-committees_en-0.pdf
- 80.Moynihan R, Bero L, Hill S, et al. Pathways to independence: towards producing and using trustworthy evidence. BMJ 2019; 367: l6576. doi: 10.1136/bmj.l6576 [DOI] [PubMed] [Google Scholar]
- 81.Italian Medicines Agency (AIFA) Research and Development Working Group . Feasibility and challenges of independent research on drugs: the Italian Medicines Agency (AIFA) experience. Eur J Clin Invest 2010; 40: 69–86. doi: 10.1111/j.1365-2362.2009.02226.x [DOI] [PubMed] [Google Scholar]


