Abstract
Implemented control measures brought about by the coronavirus disease 2019 (COVID-19) pandemic have changed the prevalence of other respiratory viruses, often relegating them to a secondary plan. However, it must not be forgotten that a diverse group of viruses, including other human coronaviruses, rhinoviruses, respiratory syncytial virus, human metapneumoviruses, parainfluenza and influenza, continue to be responsible for a large burden of disease. In fact, they are among the most common causes of acute upper and lower respiratory tract infections globally. Viral respiratory infections can be categorised in several ways, including by clinical syndrome or aetiological agent. We describe their clinical spectrum. Distinctive imaging features, advances in microbiological diagnosis and treatment of severe forms are also discussed.
Educational aims
To summarise the knowledge on the spectrum of disease that respiratory viral infections can cause and recognise how often they overlap.
To learn the most common causes of respiratory viral infections and acknowledge other less frequent agents that may target certain key populations (e.g. immunocompromised patients).
To improve awareness of the recent advances in diagnostic methods, including molecular assays and helpful features in imaging techniques.
To identify supportive care strategies pivotal in the management of severe respiratory viral infections.
Short abstract
Non-COVID-19 respiratory viral infections are a major burden of disease. Emerging molecular-based detection methods and knowledge of viral lower respiratory tract infections’ distinctive features improve diagnosis, treatment and outcome of severe forms. https://bit.ly/3qMqk3T
Introduction
Viral respiratory tract infections (RTIs) are a worldwide leading cause of morbidity and mortality and represent a substantial burden to health services. Acute RTIs are the most frequent ill-health conditions across all age groups [1], but young children and the elderly are at increased risk of severe and life-threatening outcomes, especially in low- and middle-income countries (LMICs). The world population faces a high frequency of RTI every year, with an estimated incidence of 2–5 million cases and 290 000–650 000 deaths [1].
Additionally, it is well established that viral RTIs are a major cause of exacerbations of asthma and COPD, contributing to most of the morbidity, mortality, and healthcare costs associated with these conditions [2].
Several viruses replicate in the respiratory tract and cause RTIs, the most relevant belonging to the Ortho- and Paramyxoviridae, Picornaviridae, Coronaviridae and Adenoviridae families. The aim of this review is to be an educational guide on non-coronavirus disease 2019 (non-COVID-19) respiratory viral infections. To deal with the challenges and possible threats imposed by viral RTI, we will cover the spectrum of disease that non-COVID-19 respiratory viruses can cause, what recent advances in diagnostic method tools are available to aid in early case detection, and what supportive care strategies can be selected in the management of severe respiratory viral infections.
Transmission of respiratory viruses
Viral infections of the respiratory tract can be transmitted when a virus is released and subsequently transferred through the environment from one infected person to a susceptible exposed host. Four main modes of transmission are recognised, which can occur either simultaneously or independently: direct contact, indirect contact, droplet and aerosol (or airborne) [3]. Direct contact implies transmission via close physical contact (e.g. via contaminated hands) between an infector and an infectee. Transmission through the air occurs via large droplets or fine respiratory particles (aerosols) spread from person to person by talking, sneezing or coughing [3]. Despite varying considerably between studies, a commonly accepted cut-off size between droplets and aerosols is 5 µm. Droplets travel <1 m before depositing on the mucosa of contacts or inanimate surfaces, whereas aerosols settle more slowly, thereby remaining suspended in the air for longer and being capable of travelling further [4]. Although varicella-zoster virus (VZV) and measles virus spread by aerosols, most respiratory viruses spread by droplets. Fomite transmission (indirect contact) occurs via contact with intermediate objects and surfaces contaminated with infected respiratory droplets [3]. Some respiratory viruses, including influenza, human coronaviruses (CoVs) and rhinoviruses, infect the gastrointestinal tract cells and have been recovered from faeces samples, suggesting that this route may play a role in transmission [3].
The relative importance of each of these routes in the transmission of respiratory viral infections is still unclear. The chance of transmission is further modified by pathogen, host and environmental factors, such as temperature and humidity [3, 4]. In fact, the conditions that are optimal for transmission of different viruses vary. For example, rhinoviruses and adenoviruses, unlike parainfluenza virus (PIV) and influenza A, survive best when humidity is high [5]. Non-enveloped viruses like rhinovirus can be found on contaminated surfaces for days, whereas enveloped viruses like influenza or respiratory syncytial virus (RSV) are easily susceptible to degradation, rendering them infectious on surfaces for only a few minutes to hours [5].
Seasonality of transmission in humans
Epidemiological studies in temperate regions show a seasonal oscillation in the transmission of respiratory viruses [6]. Influenza, human CoVs and RSV show a peak in incidence during the winter period. Adenovirus, human metapneumovirus (HMPV), PIV and human bocavirus (HBoV) can be found all year round. Although rhinovirus peak rates are detected in spring and autumn, disease severity increases in winter months. Seasonal patterns related to PIV are type-specific, with epidemics of PIV-1 peaking in autumn and of PIV-3 peaking in the spring and summer seasons. The prevalence of some enteroviruses rises in summer [7]. Seasons are reversed between the northern and southern hemispheres, with winter epidemics occurring between November and March in the northern temperate regions. In the tropics, acute viral respiratory infections with RSV and influenza are more common during the rainy seasons [8]. Reasons for the seasonality seen with respiratory viruses include changes in contact rates and the stability of the pathogen in the environment, which is affected by factors such as temperature and humidity [6, 7].
There is usually one dominant virus circulating at any one time, the replication conflicts of which contribute to non-overlapping peak incidences [7]. For example, although both influenza and RSV are prevalent in winter, they do not share peaks in transmission at the same time and it seems there is a negative interaction between rhinovirus and seasonal influenza A. These patterns of interference on transmission might be explained (among other factors) by viral receptor disruption, host interferon response or protective antibody-driven interference [7].
Clinical syndromes
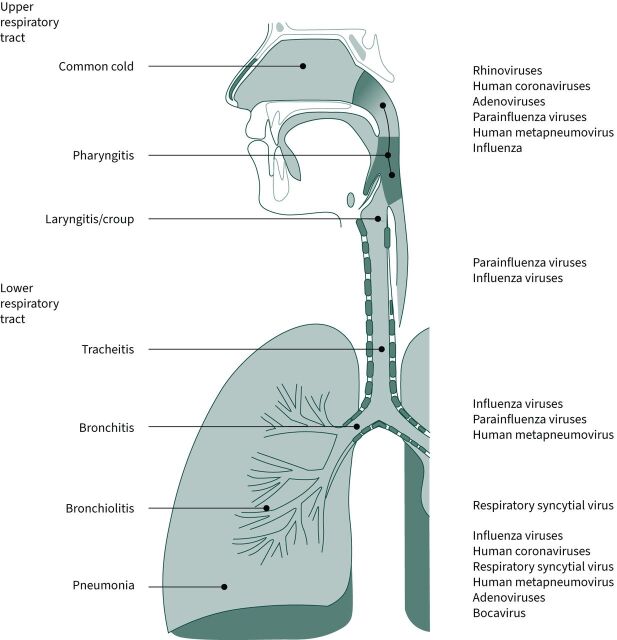
Clinical syndromes caused by respiratory viruses significantly overlap. Acute infections usually start at the upper respiratory tract, the port of entry being the eyes, nose or mouth. Within <5 days, the infection may propagate to the lower airways and lungs. For education purposes, we divide respiratory infections into different clinical syndromes according to their attendant signs and symptoms (table 1 and figure 1) [9, 10].
TABLE 1.
Common viral respiratory syndromes
| Syndrome | Description | Examples of pathogens |
| Common cold (coryza) | Self-limited syndrome of mild to moderate severity Portals of entry: nasal and conjunctival mucosa Symptoms attributed to infection of ciliated nasal epithelium and increased vascular permeability: odynophagia, nasal congestion, rhinorrhoea and cough |
Rhinoviruses are responsible for almost half of cases, but can also be caused by other viruses (e.g. adenovirus, respiratory syncytial virus, influenza and parainfluenza viruses, metapneumovirus and human coronaviruses) |
| Pharyngitis | Although generally a primary disease, it can also be associated with systemic infectious and non-infectious disorders Most cases in winter months Triad of odynophagia, fever and pharyngeal inflammation |
As a group, viruses are the single most common cause, with adenoviruses being commonly identified |
| Acute laryngitis | Characteristic recent onset of hoarseness or husky voice, episodes of dysphonia, and dry cough Usually occurs after an episode of upper respiratory tract infection with odynophagia, cough and nasal discharge |
Nearly all major respiratory viruses have been implicated as causative agents |
| Acute laryngotracheobronchitis (croup) | Primarily affects children aged <6 years Marked swelling of the subglottic laryngeal region Marked by barking cough and inspiratory stridor |
Parainfluenza types 1 and 3 are the most frequent aetiological agents |
| Tracheitis/bronchitis | Self-limited infection of the tracheal and bronchial epithelium without pneumonia signs Acute cough with or without sputum production |
Most common agents are viruses, with <10% caused by bacteria (e.g. Mycoplasma pneumoniae, Chlamydia pneumoniae and Bordetella pertussis) |
| Bronchiolitis | Disease condition of infants and young children Air trapping in the lungs and difficulty in expiration caused by the formation of dense debris plugs that obstruct expiratory airflow Signs and symptoms of wheezing, nostril flaring, use of accessory respiratory muscles and, possibly, cyanosis and apnoea |
Respiratory syncytial virus is the most common implicated agent |
| Pneumonia | Viruses reach the small airways and alveoli through inhalation of tiny particles or contiguous spread from other foci Defined by inflammation of the lung parenchyma leading to gas exchange impairment, usually with concomitant radiological changes Acute generalised illness with fever, headaches, myalgias, gastrointestinal signs and symptoms, cough, sputum production, increased respiratory rate and cyanosis in severe disease Features of other respiratory syndromes (such as rhinitis) may be present Secondary bacterial pneumonia by Staphylococcus aureus or Streptococcus pneumoniae may follow respiratory viral illnesses |
Common agents are influenza, respiratory syncytial virus, human metapneumovirus, adenoviruses and human coronaviruses |
FIGURE 1.
Diagram of the respiratory tract, divided by respiratory syndromes and with some examples of the most commonly implicated viral agents.
Specific viral causes of respiratory disease
Influenza virus
Influenza is known for its potential to cause yearly recurrent epidemics. Influenza viruses are enveloped single-stranded RNA viruses belonging to the family of Orthomyxoviridae. Respiratory disease is mainly caused by influenza A and influenza B viruses [11]. Influenza A is classified into subtypes based on the haemagglutinin (H) and neuraminidase (N) antigens (e.g. H5N1). Influenza has great antigenic variability due to antigenic drift and shift. Antigenic drift is minor, frequently occurring, antigenic variations that occur in the H and N antigens. Antigenic shift may occur infrequently in influenza A and causes major antigenic changes due to reassortment of the H and N antigens (e.g. H3N2 and H5N1 reassorting to H5N2). Since there is less immunity for new antigenic variants, these variants may cause new epidemics. Influenza B currently has only two circulating lineages: “Victoria” and “Yamagata”.
Clinical presentation and prognosis
The incubation period for influenza is 1–4 days [12]. Influenza in adults manifests itself with abrupt onset of fever, malaise, headache, sore throat, dry cough and myalgia. The fever peaks within 24 h and lasts 1–5 days [12]. Older adults may present atypically with fever and confusion without respiratory symptoms, while children may present with high fever and gastrointestinal symptoms. Young children, the elderly and immunocompromised patients are at higher risk for complications. Complications from influenza include viral pneumonia, secondary bacterial pneumonia, myositis, cardiac complications (myocarditis, pericarditis) and central nervous system complications (Guillain–Barré syndrome, transverse myelitis, encephalitis) [13].
Diagnosis
During the influenza season, uncomplicated influenza may be diagnosed clinically in the outpatient setting. Patients who require hospitalisation or are at higher risk of complications should be tested for influenza if they have symptoms [14]. Molecular tests (i.e. reverse transcriptase PCR (RT-PCR) and rapid molecular assays) are more sensitive than antigen tests and are therefore preferred [14]. Suitable specimens for testing include upper respiratory tract samples (nasopharyngeal, nasal and throat swabs). Testing lower respiratory tract samples may be considered for hospitalised patients.
Treatment and prevention
Antiviral therapy is recommended for patients at higher risk of complications or severe disease, and those being hospitalised. In low-risk patients, antivirals that are started within 48 h of symptom onset may reduce duration of symptoms [15]. Effective antivirals include oseltamivir, zanamivir, peramivir and baloxavir [14, 16]. Oseltamivir, zanamivir and peramivir are neuraminidase inhibitors that act by inhibiting the influenza A and B neuraminidase, an enzyme that removes the terminal sialic acid from host cell receptor glycoproteins, a step needed for newly formed viruses to be released from infected cells and subsequently infect new ones [13]. Baloxavir is a molecule that works by inhibiting influenza A and B cap-dependent endonuclease, an enzyme needed for viral transcription priming and viral replication [17]. To date, all circulating influenza viruses exhibit resistance to the adamantanes rimantadine and amantadine, drugs only active against susceptible influenza A viruses and that block the M2 ion channel activity, thus preventing viral uncoating in the early replication stages [13]. Both adamantanes and neuraminidase inhibitors also have a role in household and institutional outbreak chemoprophylaxis in persons at high risk of complications deemed not adequately protected (i.e. due to significant immunocompromise, ineffective vaccine to circulating strains or lack of vaccination, and in children aged <6 months). In institutional outbreaks, prophylaxis should begin as soon as possible and be continued for 14 days or 7 days after the onset of symptoms in the last person infected [14]. Regarding influenza vaccine, its efficacy in preventing disease is estimated to be 59% [18]. Recommendations on which groups to annually vaccinate may vary between countries.
Respiratory syncytial virus
RSV (types A and B) is a common RNA virus causing acute RTI in all ages. It spreads through respiratory droplets and direct contact with contaminated surfaces, causing outbreaks in cold months (northern hemisphere) [19–21].
Clinical presentation and prognosis
RSV causes upper respiratory tract infection (URTI), acute bronchiolitis and pneumonia. Acute bronchiolitis is the main presentation of primary infection occurring in most children under 2 years of age. The initial symptoms are nasal congestion, rhinorrhoea and cough, progressing after 2–3 days to respiratory distress symptoms, crackles, rales and wheezing attributable to infection/inflammation in small airways/bronchioles [20]. Although usually self-limited, acute bronchiolitis is one of the most common causes of child admissions and infant mortality (especially if preterm birth) [22]. Infants with severe bronchiolitis have increased risk of recurrent wheezing during childhood [19, 20]. Reinfections occur, possibly causing exacerbations of pre-existing respiratory conditions like asthma and COPD, and influenza-like illness in elderly and high-risk adults [19].
Diagnosis
Presumptive diagnosis is based on typical symptoms [19, 20]. RSV viral identification is sometimes required, namely in hospitalised and immunocompromised patients. Microbiological confirmation can be attained with RT-PCR or rapid antigen detection tests of respiratory specimens from nasopharyngeal swabs/aspirates, sputum and bronchoalveolar lavage [19].
Treatment and prevention
Treatment of RSV lower respiratory tract infection (LRTI) is mainly supportive: monitoring, hydration and respiratory support, including mechanical ventilation in severe cases [19, 20]. Efficacy of antivirals like ribavirin has not been completely proven and there is no available vaccine to date, one of the challenges being the concerns surrounding the abnormal immune responses to subsequent infections as observed in the trials of formalin-inactivated vaccines [23]. Primary prevention with respiratory hygiene measures is important. Palivizumab, a specific monoclonal antibody against RSV fusion (F) glycoprotein, is used as prophylaxis in high-risk children: for example, in the USA, it might be used in premature infants with <29 weeks' gestation, cardiac disease not surgically corrected, chronic pulmonary diseases or severe immunocompromise [19, 20]. Palivizumab is usually administered in 5-monthly doses of 15 mg·kg−1. Although not preventing infection, immunoprophylaxis reduces clinical severity and need for hospitalisation. In fact, among high-risk infants it reduced the hospitalisation rates by 4.4–5.8% when initiated 1 month before the RSV season [24]. Palivizumab use to reduce RSV mortality after paediatric bone marrow transplantation is still a matter of controversy, with a decision analysis model showing a number needed to treat of 12 children to prevent one death [25]. Newer humanised monoclonal antibodies against the F protein are being evaluated, such as motavizumab (not licensed) and nirsevimab [26, 27]. The latter has a 100-fold in vitro potency when compared with palivizumab and an extended half-life that allows for possible protection of infants during an entire RSV season after a single administration [27].
Adenovirus
The Adenoviridae family is composed of double-stranded DNA viruses classified into seven species (A–G), and over 60 human adenovirus serotypes have been described. Adenoviruses have a worldwide distribution and infections occur without seasonality [28]. Children and immunocompromised patients are at increased risk for infection. Adenoviruses are mainly transmitted by droplets, the faecal–oral route and fomites [29].
Clinical presentation and prognosis
Adenoviruses are most frequently associated with URTI, such as coryza, pharyngitis and, in children, otitis media and bronchiolitis. Pneumonia is an important associated clinical syndrome and serotypes 3, 7, 14, 21 and 55 are associated with severe disease and acute respiratory distress syndrome (ARDS). Severe pneumonia is more often described in newborn, elderly and immunocompromised patients [30]. Ocular manifestations include pharyngoconjunctival fever and epidemic keratoconjunctivitis. Acute gastroenteritis and mesenteric adenitis are also associated with adenoviruses in children. Other clinical manifestations described are acute haemorrhagic cystitis, urethritis, meningitis, encephalitis and myocarditis. The spectrum of clinical disease is wider in immunocompromised patients, including solid organ and haematopoietic transplant recipients. In these groups, adenoviral infection may be acquired de novo or from reactivation of persistent infection. Disseminated infection has been reported and can affect any organ with high mortality [31].
Diagnosis
Viral culture is the gold standard for diagnosis, but a definitive diagnosis may also require histopathological examination [32]. Although rapid, antigen detection assays are less sensitive. PCR is frequently used but a positive test does not necessarily mean active disease (i.e. may only represent viral shedding).
Treatment and prevention
Most infections are self-limited and treatment is usually supportive. Antiviral therapy is reserved for patients with severe infection, with cidofovir being the most frequently used since some studies suggest a survival benefit [33, 34]. Other agents are less well studied. Recovery from infection is associated with neutralising antibodies that protect against the same serotype but not against others. A live oral vaccine against serotypes 4 and 7 is available and used only in USA military camps.
Rhinovirus
Rhinoviruses are single-stranded RNA viruses that belong in the Enterovirus genus of the Picornaviridae family. Comprising three species (RV-A, -B and -C), they are ubiquitous and prevalent respiratory viral pathogens in humans [35]. They cause infections all year round, with a peak incidence in spring and autumn in temperate regions. In the northern hemisphere, the autumn peak occurs in late August and early September, when 80% of URTIs may be attributed to rhinovirus infection, and an increase in asthma exacerbations triggered by this virus and fuelled by low use of asthma medications during summer is seen in children following return to school (i.e. “September asthma epidemic”) [36]. All age groups are susceptible, but infants and young children are more affected. Immunocompromised hosts are frequently infected and present prolonged rhinovirus shedding. Reinfections can occur lifelong, causing predominantly self-limited URTI. Transmission occurs by direct contact hand-to-nose or hand-to-eye and less frequently by droplets or even aerosols [35].
Clinical presentation and prognosis
The common cold is the most frequent clinical presentation associated with rhinovirus. Severity and duration are variable (median 7 days). It can also present as self-limited viral rhinosinusitis. Rhinoviruses are commonly implicated in exacerbations of asthma and chronic bronchitis. Due to frequent infection and shedding, rhinoviruses are often detected in the upper respiratory tract and co-detected with other respiratory pathogens in LRTIs. The pathogenesis of rhinovirus on LRTI remains to be determined. Rhinovirus is the second virus involved in bronchiolitis, but its potential role in pneumonia is uncertain as a sole causative pathogen [37].
Diagnosis
Sensitive and rapid molecular techniques, such as RT-PCR or multiplex PCR, are the mainstay of laboratory diagnosis. Samples can be obtained from nasal aspirates, swabs or washings and from tracheal or bronchial aspiration or bronchoalveolar lavage [37].
Treatment and prevention
Specific antiviral treatment is not available, and supportive therapy is recommended. No vaccine is available. Evidence for infection prevention is limited to hand hygiene [35].
Human coronavirus
CoVs are a large family of enveloped, positive-sense, single-stranded RNA viruses, causing a wide spectrum of animal and human disease [38]. Due to their wide zoonotic distribution and the high rates of genomic nucleotide substitutions and recombination, there is a constant risk of spillover from their usual animal reservoir to intermediate species and humans. Therefore, they are considered a serious potential threat for global health. Six species of CoV capable of infecting humans had been discovered prior to the emergence of severe acute respiratory syndrome (SARS)-CoV-2. HCoV-229E and -NL63 belong to the genus Alphacoronavirus, while HCoV-OC43, -HKU1, SARS-CoV and Middle East respiratory syndrome (MERS)-CoV are classified as Betacoronavirus [38].
Clinical presentation and prognosis
Human endemic CoVs (-229E, -NL63, -OC43 and -HKU1) represent frequent aetiological agents of URTI [39, 40], being responsible for 7–30% of common cold cases. In temperate climates, CoV infections are common during the winter, while in tropical regions they are prevalent throughout the year. After 2–5 days of incubation, typical symptoms are nasal stuffiness, rhinorrhoea, pharyngodynia, sneezing, cough, fever, headache, anorexia and myalgias [39, 40]. However, clinical presentation is non-specific and may range from asymptomatic infection to life-threatening disease. Severe diseases are more commonly observed in the following groups: in children, who may develop laryngeal croup, bronchiolitis and pneumonia [40]; in the elderly with chronic underlying diseases, who may experience pneumonia, COPD exacerbations and attendant congestive heart failure decompensation [39]; and in immunocompromised hosts, who are at risk for severe pneumonia and acute respiratory failure [41]. Gastrointestinal symptoms are also frequently observed and there is some evidence for CoV-related neurological syndromes [39, 40].
Recently, two previously unknown CoVs, SARS-CoV and MERS-CoV, have received great attention because of their epidemic spread and their association with severe pneumonia, respiratory failure and death [42, 43]. Both epidemics came from animal-to-human spillover. The SARS global outbreak (2002–2003), which originated in China, was sustained by high inter-human transmission. After a few days of non-specific symptoms, a significant percentage of patients developed sudden respiratory deterioration and need for respiratory support. Conversely, many cases of MERS were registered after close contact with dromedary camels. Human-to-human transmission, although significant in healthcare settings, was infrequent in the community. Severe cases experienced respiratory, renal and multi-organ failure, with a high mortality rate [42, 43]. Gastrointestinal symptoms were frequently observed in both SARS and MERS.
Diagnosis
The clinical usefulness of aetiological diagnosis may be scarce for mild self-limited infections. However, virological tests have a pivotal role in cases of more serious illness. Direct demonstration of viral infection is usually performed through RT-PCR and requires adequate samples, such as naso-oropharyngeal swabs, sputum or bronchoalveolar lavage [39–43]. Sensitivity is higher at the end of the incubation period and on the first days after symptom appearance, when viral load is higher. Serology becomes positive after several days since disease onset and is mainly used for epidemiological purposes [39, 42, 43].
Treatment and prevention
Treatment of endemic CoVs is usually aimed at controlling symptoms and complications. Several therapeutic interventions have been investigated for SARS and MERS management, with inconclusive results [42, 43]. Antiviral drugs, such as ribavirin, protease inhibitors and interferon, and passive immunisation with convalescent plasma or monoclonal antibodies may be useful during the early stage, in order to quickly decrease viral load. Conversely, immune modulation may be an important tool for treating severe advanced disease [42, 43]. Unfortunately, high-dose steroid therapy did not show any objective clinical benefit in SARS and MERS. Mechanical ventilation and intensive care are frequently needed. Prevention of CoV infections other than SARS-CoV-2 remains limited to transmission-avoiding measures.
Parainfluenza virus
Human PIVs are negative-sense, single-stranded, enveloped RNA viruses from the Paramyxoviridae family and can be classified into four major serotypes (PIV-1, -2, -3 and -4) [44]. Human PIVs are one of the most common causes of RTI worldwide, and the severity of infection correlates with the viral replication site and the infecting serotype. PIV-associated illness is more common in young children; in adults, immunosuppression and older age are associated with more severe disease [45].
Clinical presentation and prognosis
PIV-1 and PIV-2 replicate in the upper airway epithelium, more commonly causing URTI, while lower respiratory tract replication of PIV-3 makes it the most common human PIV serotype causing clinically significant infection. PIVs cause mild URTI, croup, acute bronchitis, bronchiolitis and pneumonia, as well as exacerbations of asthma and COPD. Bacterial and fungal co-infection is not infrequent, and should be ruled out upon diagnosis, as it is associated with increased mortality [44–47].
Diagnosis
The preferred method for diagnosis of PIV is an RT-PCR-based assay, performed as a single assay or as a part of multiplex panels with detection and differentiation of the four PIV serotypes [48].
Treatment and prevention
The cornerstone of treatment for PIV infection is supportive care, as there are no antiviral agents approved yet [45]. The data supporting the efficacy of ribavirin are scarce, and the use of intravenous immunoglobulin is controversial. DAS181 is a promising investigational agent for the treatment of these infections [45]. For adults in healthcare settings, standard and contact precautions are recommended [44]. Vaccine development is underway, but to date there are no licensed vaccines for PIV [46].
Human metapneumovirus
HMPV is a negative-sense, non-segmented, single stranded RNA virus first identified in 2001 as a cause of respiratory symptoms in children in the Netherlands [49]. It is now recognised as a common cause of RTI in children, the elderly and immunosuppressed patients, and was identified as the third most common virus causing community-acquired pneumonia in a study of 2488 adults in the USA [50].
Clinical presentation and prognosis
HMPV can infect and replicate within both the upper and the lower respiratory tract. In children, LRTI can lead to pneumonia, bronchiolitis, asthma exacerbations and croup. In otherwise healthy young adults, HMPV infection is typically a mild URTI, but severity increases with increasing age and comorbidity. HMPV can cause pneumonia and exacerbations of asthma and COPD [49, 51].
Diagnosis
Diagnosis of HMPV is based on PCR assays [52]. Detection of HMPV is incorporated into many commercial multiplex assays for detection of respiratory pathogens.
Treatment and prevention
There are no antiviral therapies available for HMPV that are approved by the European Medicines Agency or US Food and Drug Administration. Treatment is typically supportive. Antipyretics can be used for fever and intravenous fluid hydration may be required for dehydrated patients who cannot tolerate oral hydration. In more severe cases, supplemental oxygen support including high-flow nasal cannula (HFNC) or mechanical ventilation may be necessary. To limit and prevent spread of the virus, patients should be placed on droplet precautions [53]. There are currently no licensed vaccines for HMPV.
Other viruses that infect the respiratory tract
Although less common, other viruses can be responsible for both URTI and LRTI, sometimes even resulting in severe forms of viral pneumonia. Such is the case of infection with measles virus, VZV, Epstein–Barr virus (EBV) and cytomegalovirus (CMV). Measles virus is a paramyxovirus of the genus Morbillivirus that causes a systemic illness known as rubeola, sometimes including severe pneumonia, when infection occurs in immunocompromised and/or unvaccinated adults. It is the most contagious virus in humans [54]. Various herpesviruses cause URTI: herpes simplex virus (HSV), especially HSV-1, is associated with ulcerative stomatitis and pharyngitis; EBV may result in infectious mononucleosis, typically seen in teenagers and young adults, and characterised by acute or subacute exudative pharyngitis, sometimes with severe tonsillar swelling and imminent airway occlusion. EBV, as well as other herpesviruses, such as CMV, VZV, and human herpesviruses 6, 7 and 8, can cause severe pneumonia syndromes in the immunocompromised host [54].
Non-polio enteroviruses have a worldwide distribution and may cause outbreaks of respiratory disease. Of note, coxsackieviruses, associated with hand-foot-and-mouth disease in children, cause oral lesions and herpangina, marked by fever, odynophagia and difficulty in swallowing. Outbreaks typically occur during the summer season. Enterovirus A71 may also cause hand-foot-and-mouth disease in Asia, sometimes leading to severe encephalitis with polio-like features and death. Echovirus 11, another enterovirus belonging to Enterovirus B species, is implicated in croup [54].
A new virus, named HBoV, a member of the Parvoviridae family, was identified in 2005 as a cause of respiratory tract and gastrointestinal infections, primarily in children [55]. Concerns about causality were raised, given the virus was also identified in the respiratory tract samples of asymptomatic individuals and often in the presence of other co-infecting respiratory viruses [55, 56]. However, subsequent studies have shown that HBoV titres are significantly higher in the respiratory samples of symptomatic than asymptomatic children and in monoinfections when compared to co-infections, thus implying a role as sole culprits of both URTI and LRTI, including wheezing in children [55–57]. Human infections have been reported worldwide, especially in the winter season. Infections are mainly seen in those aged 6 months to 2 years, and by the age of 6 years most children have serological evidence of previous contact with this virus [56, 57].
Hantaviruses, which belong to the order Bunyavirales, are zoonotic pathogens transmitted to humans by inhalation of contaminated aerosols from rodent excreta. They are responsible for two disease entities in humans: haemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS), caused by Old World and New World hantaviruses, respectively. The HCPS form is mainly seen in the American continent, particularly in South America, where intermittent outbreaks of infection occur. Compared to HFRS, HCPS is more severe, resulting in higher case fatality rates of 30–50%. Its clinical course progresses through prodromal, cardiopulmonary and convalescent phases, with clinical manifestations varying from mild hypoxaemia to respiratory failure and cardiogenic shock. Treatment is supportive [58].
Hendra and Nipah viruses are emerging zoonotic paramyxoviruses of the Henipavirus genus. The natural reservoir of these viruses is thought to be a fruit bat present in the Pacific region and Australia. Intermediate hosts are responsible for the transmission of infection to humans (pigs in Nipah and horses in Hendra viruses). Although not completely clear, the mode of transmission from the intermediate hosts to humans is probably through the respiratory route. The initial clinical presentation is that of an influenza-like syndrome, which can later progress to encephalitis, with ensuing death in approximately half of the identified cases [54].
Distinctive imaging features of respiratory viral infections
The imaging features of respiratory viral infections are diverse and frequently non-specific, overlapping with those of non-viral infections and inflammatory conditions. However, there are some characteristic imaging patterns of viral pneumonia that radiologists and clinicians should be familiar with.
Viruses within the same family share similar pathogenic mechanisms and usually present with identical patterns on imaging studies. Imaging features characteristic of specific viral infections can at times be encountered on chest radiography (CXR) and computed tomography (CT) scans and must be promptly recognised [59]. Some features that may be encountered with specific aetiologies are listed in table 2. However, not all cases present with these typical imaging patterns and an aetiological diagnosis usually cannot be reached based solely on imaging studies. Laboratory findings, clinical features, such as age and immune status, and the epidemiological context of the infection must be considered to help narrow the differential diagnosis [60].
TABLE 2.
Some common features that may be encountered with specific respiratory viral infections
| Virus | Imaging features |
| Influenza | CXR: bilateral reticulonodular opacities, sometimes associated with poorly defined areas of consolidation, that can become confluent with time CT: GGOs interspersed with patchy consolidations; small and ill-defined centrilobular nodules can be seen [60] |
| Respiratory syncytial virus | Airway-centric pattern of disease characterised by tree-in-bud opacities, bronchial wall thickening and peribronchial consolidations [59, 61] |
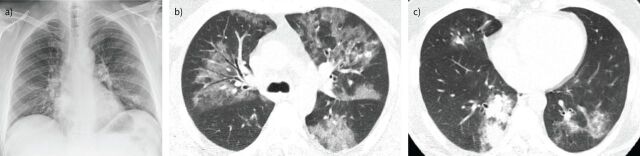
| Adenovirus | CXR: most characteristic pattern is diffuse, bilateral bronchopneumonia associated with hyperinflation, especially in children; lobar atelectasis is also a common finding, with right upper lobe atelectasis more common in infants and left lower lobe atelectasis in older children [60] CT: typically presents as bilateral and multifocal GGOs associated with patchy consolidations (figure 2); occasionally a lobar or segmental pattern of involvement, which resembles that of bacterial infections, may be encountered [59] |
| Rhinovirus | Multiple, bilateral, patchy areas of GGO and consolidation, associated with interlobular septal thickening [59] |
| Human coronaviruses (SARS/MERS) | CXR: may be normal initially but rapid progression to multifocal consolidations commonly seen; predominant lower lobe involvement CT: multiple GGOs and consolidations; in the areas of GGO, thickening of the interlobular septa may be seen, resulting in a “crazy paving” pattern; subpleural and peribronchovascular distribution is typical [59, 62] |
| Parainfluenza | CT: multifocal GGOs is the most common appearance; small peribronchial nodes and patchy appearances can also be found [62] |
| Human metapneumovirus | CXR: generally depicts multilobular infiltrates CT: bilateral, multifocal, patchy GGOs and small ill-defined nodular opacities; consolidations present in <50% of patients [59, 62] |
| Measles | CXR: mixed reticular opacities and consolidations CT: the typical appearance is multifocal GGOs and consolidations; small peribronchial nodular opacities and thickening of the interlobular septa can also be encountered; hilar lymphadenopathies and pleural effusions frequently seen [59, 60] |
| Herpes simplex virus type 1 | CXR: patchy bilateral GGOs and consolidations that can have a lobular, subsegmental or segmental pattern of distribution CT: patchy lobular, segmental or subsegmental consolidations and GGOs, usually with a predominance of GGOs; pleural effusions are not uncommon [59, 62] |
| Varicella-zoster virus | CXR: multiple, small and ill-defined nodules that may become confluent CT: also multiple, small (5–10 mm) nodules, some with a surrounding halo of GGOs; patchy GGOs and areas of confluence of nodules can also be encountered; occasionally lesions may calcify and persist as numerous, randomly distributed, small calcifications [59, 62] |
| Cytomegalovirus | Bilateral, asymmetric and patchy GGOs associated with interlobular septal thickening; centrilobular nodules and consolidations can also be seen [59] |
| Epstein–Barr virus | Most frequent imaging finding is mediastinal lymphadenopathy CT: non-specific and includes diffuse or focal interstitial infiltrates [59, 62] |
| Hantavirus | Interstitial oedema that can rapidly progress to air-space consolidations CT: extensive bilateral GGOs and thickened interlobular septa typical; cardiomegaly and pleuro-pericardial effusions can also be seen [60, 62] |
CXR: chest radiography; CT: computed tomography; GGO: ground-glass opacity; SARS: severe acute respiratory syndrome; MERS: Middle East respiratory syndrome.
FIGURE 2.
Pneumonia due to adenovirus in a 61-year-old man with cough and fever. a) Initial chest radiograph shows bilateral, patchy and ill-defined areas of consolidation and air-space opacities. b and c) Axial chest computed tomography images (3 mm thickness) obtained 3 days later reveal b) extensive, ill-defined areas of ground-glass opacities and c) a focal area of patchy consolidation in the right inferior lobe.
Conventional radiography
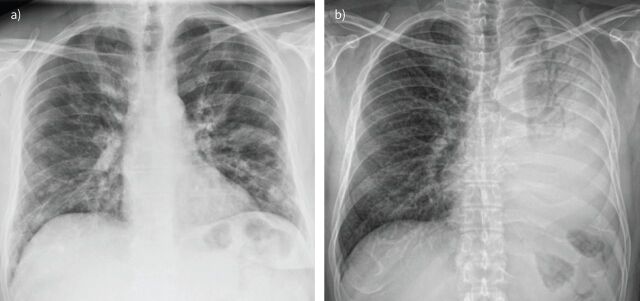
CXR may demonstrate normal findings or reveal patchy areas of consolidation, which can be unilateral or bilateral. Nodular opacities and bronchial thickening are also frequent findings. Lobar consolidations are uncommon and should raise the suspicion of an underlying bacterial infection (figure 3). Pleural effusions are also uncommon and, when present, are generally of small volume [62].
FIGURE 3.
Viral versus bacterial pneumonia. a) Pneumonia due to influenza A virus in a 53-year-old man with cough and dyspnoea. Chest radiography shows bilateral nodular opacities and patchy, focal areas of consolidation, predominantly located in the lower lobes. b) Pneumonia due to Streptococcus pneumoniae in an HIV-infected, 55-year-old man. Chest radiography reveals an extensive consolidation of the left lung with air bronchogram.
Computed tomography
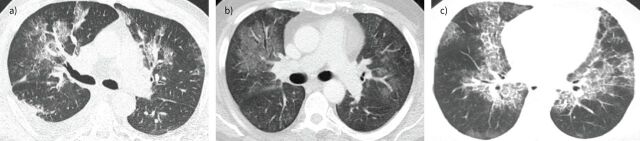
CT findings of viral pneumonia are diverse, also overlapping with other causes of pneumonia, and may be affected by numerous factors, including the immune status of the host and bacterial co-infection [59]. However, there are at least four common patterns, although not specific, of presentation of viral pneumonia that must be recognised: 1) airway-centric disease, with bronchial wall thickening, peribronchial consolidations and tree-in-bud opacities (figure 4a); 2) multifocal pneumonia, with various areas of ground-glass opacity (GGO) with or without associated airspace consolidations (figure 4b); 3) unifocal pneumonia, with a single area of GGO, airspace consolidation or tree-in-bud opacities; and 4) normal, i.e. the absence of imaging findings associated with the infection [61, 62].
FIGURE 4.
Patterns of viral pneumonia. a) Airway-centric disease. Axial chest computed tomography (CT) reveals irregular patchy consolidations and ground-glass opacities (GGOs) distributed predominantly along the bronchovascular bundles, associated with mild bronchial wall thickening and some centrilobular nodules. A small pleural effusion is seen on the right. b) Multifocal pneumonia. Axial chest CT reveals predominantly central, patchy areas of GGO that involve both lungs. c) Crazy paving. Axial chest CT shows multiple areas of GGO associated with smooth interlobular septal thickening, a pattern named “crazy paving”.
Other frequently encountered findings include smooth interlobular septal thickening that can be associated with GGOs, giving rise to a pattern named “crazy paving”, which is characteristic of some viral infections (figure 4c). Patchy inhomogeneities in the attenuation of lung parenchyma (mosaic attenuation pattern) are also a recognised feature in some viral infections [61, 62]. It should be noted that the areas of airspace consolidation, when present, are most commonly patchy and ill-defined, consistent with a pattern of bronchopneumonia. Lobar consolidations are atypical and should always raise suspicion of bacterial co-infection. Pleural effusions, hilar and mediastinal lymphadenopathies, and pneumothorax are also uncommon.
CT patterns generally reflect the pathogenesis of the infection and vary according to the viral family causing the infection (table 2).
Recent advances and perspectives in the detection of respiratory virus infections in humans
As described, most viral respiratory infections exhibit overlapping clinical and radiological features, which are difficult to distinguish without solid laboratory support. Over the past decades, we have assisted in a revolution in viral diagnostic testing. Conventional diagnostic methods, such as viral culture and direct/indirect immunofluorescence assays, which are particularly time-consuming, limited in sensitivity and laborious, have been surpassed by rapid antigen detection, highly sensitive nucleic acid amplification tests (NAATs), and point-of-care tests [63–65].
Depending on the pathogen, several approaches can be followed. Although viral isolation and culture is still the gold standard for diagnosing some viruses, it is seldom used now. Traditional assays like ELISA and immunofluorescence assays gained popularity despite their lower sensitivity when compared with nucleic-acid-based methods. Modified ELISA methods have been developed and immunofluorescence assays can rapidly detect antigens. With the advent of molecular biology, NAATs based on PCR have become strategic [63]. There is a wide variety of NAATs, which includes RT-PCR, loop-mediated isothermal amplification-based assay (LAMP), DNA-microarray-based and sequencing-based tests. They represent a fast, specific and sensitive approach for the detection of low viral titres, subgrouping and quantification of pathogens. Multiplex assays allow the simultaneous rapid detection of several potential viruses and DNA-microarray-based assays offer accurate and rapid diagnosis using multiple oligonucleotides designed to target conserved regions encoding specific viral proteins. Despite these advantages, NAATs are highly dependent on the availability of instruments and trained professionals, and expensive [63]. Emerging technologies such as LAMP, biosensors, nanotechnology-based paper strips and microfluidics offer rapid, low-cost and user-friendly respiratory viral monitoring platforms that can be used as point-of-care tests, making them desirable especially in the LMIC setting [64, 65].
Influenza virus infections impose diagnostic challenges, such as the antigenic drift caused by point mutations in viral genome that needs to be promptly identified. Next-generation sequencing has been a great achievement, allowing this task to be performed at low cost, and it will probably be used massively in the diagnosis and identification of other respiratory pathogens [63].
Treatment of severe respiratory viral infections
Although respiratory viruses mostly cause mild-to-moderate disease, they may also be responsible for severe disease with need of intensive care unit (ICU) admission. Most patients are admitted because of severe respiratory infections resulting in ARDS. Other associated syndromes, such as neurological disease (e.g. Guillain–Barré syndrome or concomitant encephalitis), cardiac disease (myocarditis) or shock (haemorrhagic disease) may also require ICU treatment.
In recent years, respiratory viruses have been more frequently identified in critical illness, probably due to the expanding use of highly sensitive (including multiplex) RT-PCR assays. However, one must be aware that identification by molecular methods could also mean that the detected virus might be a respiratory tract coloniser and not the agent responsible for the patient's symptoms [66]. Common agents of viral pneumonia causing critical illness in immunocompetent patients include influenza, human CoV, HMPV and PIV. Immunocompromised hosts are more susceptible to RSV, CMV, HSV, VZV and adenovirus pneumonia [67, 68].
The role of respiratory viruses in the pathogenesis of severe pneumonia and respiratory failure is not completely elucidated. They may be the direct cause of severe illness (viral pneumonia), a risk factor for superinfection with bacteria, or a trigger for a dysregulated immune response. The role of the ICU in the management of severe viral respiratory infection is mostly through organic support, especially ventilatory support.
Antiviral therapy
Antiviral treatment may be necessary when some aetiologies are suspected or confirmed. The neuraminidase inhibitors oseltamivir and zanamivir are the current first-line agents for the treatment of patients with influenza requiring hospitalisation, and prompt initiation is associated with a reduction of disease duration, but not with mortality reduction [69]. It is plausible to consider their use in critically ill patients. High-dose oseltamivir (i.e. 150 mg·day−1) can be an option in severe patients, although without clear supporting evidence [68]. Treatment of CMV disease in critically ill patients is controversial. There are specific guidelines regarding treatment and prophylaxis in immunocompromised hosts. In immunocompetent patients, curative treatment in confirmed CMV disease is recommended, for example with ganciclovir, valganciclovir, foscarnet and cidofovir, with a positive impact on their outcome [70]. There are some antivirals used for treatment of other viruses, with limited evidence of efficacy (e.g. ribavirin in RSV infection) [66].
Supportive care
Besides antiviral therapy, ventilation management is pivotal in management of severe disease. Oxygenation and ventilatory support can be delivered through HFNC, non-invasive ventilation or invasive mechanical ventilation. Recently, HFNC has gained more importance with its increased usage in hypoxaemic respiratory failure. It can deliver a high fraction of inspired oxygen and give a high flux of gas, allowing some respiratory support. Above all, its humidification allows for a great tolerance of this technique. Non-invasive ventilation provides pressure in the airways through a sealed interface, while the patient is awake or lightly sedated. It may be effective in reducing endotracheal intubation in patients with severe viral pneumonia, especially in those with COPD exacerbations or cardiogenic pulmonary oedema. Its benefit in ARDS is debatable, showing high failure rates and risk of self-inflicted lung injury [66, 67]. Mechanical ventilation is the mainstay of treatment of severe disease. However, it requires deeper sedation, leading to higher risk of prolonged ICU stay and myopathy. Management of patients with ARDS requiring invasive mechanical ventilation should be done with a lung-protective strategy, namely low tidal volumes and controlled plateau and driving pressures [71]. Several studies have shown good outcomes of extracorporeal membrane oxygenation (ECMO) in patients with influenza and MERS [72, 73]. ECMO should be considered in patients in which previous oxygenation strategies have failed, after a well-balanced risk–benefit evaluation considering organ dysfunction and previous functional status.
Importance of public health measures in mitigating the risk of non-COVID-19 respiratory viral infections: lessons from the COVID-19 pandemic
As previously discussed, most respiratory viruses are spread by direct (physical) contact, indirect contact (fomites), (large) droplets and the airborne route [3, 4]. We are used to expecting a seasonal oscillation in their transmission, with influenza and RSV peaking during the winter months, PIV in non-winter months, some enteroviruses in the summer and rhinoviruses in the spring and autumn seasons [7]. But how much did these patterns shift following the COVID-19 pandemic and the adoption of public health measures to control it? To reduce the transmission of COVID-19, several public health measures have been implemented worldwide, including community education, respiratory hygiene and cough etiquette, handwashing, mandatory public use of face masks, social distancing measures and avoidance of mass gatherings, school closures, remote working, border control and lockdown policies, and isolation of positive cases and quarantine of their close contacts, aiming to reduce the frequency and duration of contacts and thus the effective reproduction number (Re or Rt) [74]. Various studies have shown a reduction in the transmission of other respiratory viruses. For example, in Singapore, the number of influenza cases decreased by 76% during the sixth epidemiological week when compared with that of 2016–2019; in the USA, influenza-related hospitalisations were lower during the 2020–2021 season and, in Australia, low levels of influenza activity were reported in 2020 [74, 75]. In a Canadian study, apart from ongoing enterovirus/rhinovirus detection, levels of non-SARS-CoV-2 respiratory viruses (influenza A and B, RSV, HMPV, PIV, adenovirus, seasonal CoV) were dramatically lower during the 2020–2021 season when compared to pre-pandemic levels [74]. However, other studies have shown an upsurge of rhinovirus infections: in both the UK and Hong Kong, an increase in the detection of acute URTIs due to this virus was noted following the reopening of schools in September 2020 [76, 77]. Of note, rhinovirus is a non-enveloped virus that may be more stable on surfaces and more resistant to inactivation by handwashing or disinfectants [74]. Out-of-season unexpected patterns of RSV infections were reported in older infants in Australia during spring [78]. Another end-of-season upsurge of RSV infections was reported in New York, with patients younger than in previous winter seasons being admitted with more severe disease [21]. This was followed by other similar reports from France and Japan [79, 80]. Together, the evidence so far suggests public health measures being used to tackle the COVID-19 pandemic seem to be effective in reducing epidemics of other viral RTIs as well. However, it is difficult to determine whether this observed reduction can also be attributed to a reduction in laboratory testing/reporting for other respiratory pathogens or even to possible negative viral–viral interactions between SARS-CoV-2 and other respiratory viruses (i.e. viral interference) [74, 81]. The recent unexpected out-of-season upsurges in the number of RSV infections is an example of how difficult it might be to predict the pattern of respiratory viruses in the upcoming months, reminding us to be watchful when it comes to surveillance testing of both COVID-19 and non-COVID-19 respiratory viral infections.
Conclusion
The global burden of non-COVID-19 viral respiratory infections is substantial across all age groups. To deal with the challenge of their prevention and treatment it is important to recognise their clinical syndromes and specific viral agents’ behaviour. Regarding their diagnosis, emerging molecular-based detection methods have progressively replaced conventional diagnostic tests, providing more reliable and faster results. The imaging findings of respiratory viral infections are diverse and overlapping. Although a definitive diagnosis cannot be reached based solely on imaging studies, the knowledge of viral LRTI distinctive features improves the accuracy of the diagnosis, avoiding unnecessary treatments and studies. Although non-COVID-19 respiratory viruses mostly cause mild-to-moderate disease, they may also be responsible for severe disease with need of ICU admission for supportive care.
Key points
Although different respiratory viruses usually have a preference for replication in different anatomical areas of the respiratory tract, clinical syndromes caused by respiratory viruses may overlap: the syndrome known as the “common cold” is marked by nasal congestion, rhinorrhoea, cough and odynophagia; laryngitis is characterised by hoarseness/dysphonia; acute tracheobronchitis is accompanied by cough with or without sputum production in the absence of pneumonia evidence; bronchiolitis is characteristically seen in infants and young children with wheezing; and typical clinical features of pneumonia include productive cough, dyspnoea and chest pain, with or without systemic signs and symptoms, such as fever and myalgia.
Influenza viruses, RSV and HMPV are the most common causes of serious lower respiratory tract disease in healthy patients, whereas special populations, such as the immunocompromised host, are susceptible to other less frequent viruses (i.e. HSV, EBV, CMV and adenoviruses) that may cause symptoms after a de novo infection with an opportunistic agent or following reactivation of latent infection.
Not all respiratory viral infections present with typical imaging patterns and an aetiological diagnosis usually cannot be reached based solely on imaging studies: laboratory findings, clinical features and epidemiological context must be taken into account to help narrow the differential diagnosis.
Although respiratory viruses mostly cause mild-to-moderate disease, they may also be responsible for severe disease needing ICU admission, mostly due to ARDS, for which oxygenation and ventilatory support modalities are available (HFNC, non-invasive ventilation or invasive mechanical ventilation).
Self-evaluation questions
- A patient walks into your clinic complaining of odynophagia, nasal congestion, rhinorrhoea and dry cough for the last 3 days. His physical examination is unremarkable except for a slight redness in the oropharynx. He has no fever, tender nodes or any findings in chest examination. What term best describes this respiratory syndrome?
- a) Pharyngitis
- b) Common cold
- c) Croup
- d) Tracheitis
- e) Laryngitis
- Emerging technologies such as LAMP offer rapid, low-cost and user-friendly respiratory viral monitoring platforms that can be used as point-of-care tests, without the need for high technology requirements or highly trained professionals. In which of the following settings would this technology be desirable?
- a) Malawi
- b) Australia
- c) France
- d) USA
- e) Russia
- Which of the following agents is least likely to be found as a culprit of a respiratory infection in the immunocompetent host?
- a) HCoV-HKU1
- b) Adenovirus
- c) Influenza
- d) Rhinovirus
- e) CMV
- Regarding the imaging appearances of respiratory viral infections, select the incorrect statement:
- a) The imaging appearances of viral pneumonias are usually non-specific and can mimic other infectious diseases.
- b) Pleural effusions and mediastinal lymphadenopathies are not a common finding of viral pneumonias.
- c) The typical pattern of adenovirus pneumonia is airway-centric disease with bronchial thickening and peribronchial consolidations.
- d) Adenovirus pneumonia is frequently associated with atelectasis, particularly in children.
- e) VZV pneumonia usually manifests on CXR as multiple, ill-defined nodules.
Suggested answers
1. b.
2. a.
3. e.
4. c.
Footnotes
Conflict of interest: S. Castanhinha reports receiving a grant from Ordem dos Médicos for an ERS Paediatric Bronchoscopy course; consulting fees, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Vertex Pharmaceuticals; support for attending meetings and/or travel from Zambon Pharma; participation on a Data Safety Monitoring Board or Advisory Board for Vertex Pharmaceuticals; and a leadership or fiduciary role in another board, society, committee or advocacy group for Comissão Coordenadora do Tratamento da Doença Fibrose Quística, all outside the submitted work. A. Silva-Pinto reports receiving a grant to his institution from Merck Sharp and Dohme; payment or honoraria for presentations received from Merck Sharp and Dohme, and from Gilead; and support for attending meetings and/or travel received from Merck Sharp and Dohme, ViiV Healthcare, and Gilead, all outside the submitted work. M. Tavares reports receiving support for attending meetings and/or travel from MDS Portugal, outside the submitted work. The remaining authors have nothing to disclose.
References
- 1.Waghmode R, Jadhav S, Nema V. The burden of respiratory viruses and their prevalence in different geographical regions of India: 1970–2020. Front Microbiol 2021; 12: 723850. doi: 10.3389/fmicb.2021.723850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitt R, Farne H, Ritchie A, et al. The role of viral infections in exacerbations of chronic obstructive pulmonary disease and asthma. Ther Adv Respir Dis 2016; 10: 158–174. doi: 10.1177/1753465815618113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol 2021; 19: 528–545. doi: 10.1038/s41579-021-00535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutter JS, Spronken MI, Fraaij PL, et al. Transmission routes of respiratory viruses among humans. Curr Opin Virol 2018; 28: 142–151. doi: 10.1016/j.coviro.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall CB. The spread of influenza and other respiratory viruses: complexities and conjectures. Clin Infect Dis 2007; 45: 353–359. doi: 10.1086/519433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisman D. Seasonality of viral infections: mechanisms and unknowns. Clin Microbiol Infect 2012; 18: 946–954. doi: 10.1111/j.1469-0691.2012.03968.x [DOI] [PubMed] [Google Scholar]
- 7.Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of respiratory viral infections. Annu Rev Virol 2020; 7: 83–101. doi: 10.1146/annurev-virology-012420-022445 [DOI] [PubMed] [Google Scholar]
- 8.Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatr Respir Rev 2003; 4: 105–111. doi: 10.1016/S1526-0542(03)00024-1 [DOI] [PubMed] [Google Scholar]
- 9.van Doorn HR, Yu H. Viral respiratory infections. In: Ryan ET, Hill DR, Solomon T, et al., eds. Hunter's Tropical Medicine and Emerging Infectious Diseases. 10th Edn. Elsevier, 2020; pp. 284–288. [Google Scholar]
- 10.Cohen YZ, Flores AR, Caserta MT, et al. Upper respiratory tract infections. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th Edn. Philadelphia, Elsevier, 2020; pp. 819–875. [Google Scholar]
- 11.Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers 2018; 4: 3. doi: 10.1038/s41572-018-0002-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox NJ, Subbarao K. Influenza. Lancet 1999; 354: 1277–1282. doi: 10.1016/S0140-6736(99)01241-6 [DOI] [PubMed] [Google Scholar]
- 13.Treanor JJ. Influenza viruses, including avian influenza and swine influenza. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th Edn. Philadelphia, Elsevier, 2020; pp. 2143–2168. [Google Scholar]
- 14.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019; 68: e1–e47. doi: 10.1093/cid/ciy866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehnert R, Pletz M, Reuss A, et al. Antiviral medications in seasonal and pandemic influenza. Dtsch Arztebl Int 2016; 113: 799–807. doi: 10.3238/arztebl.2016.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet 2015; 385: 1729–1737. doi: 10.1016/S0140-6736(14)62449-1 [DOI] [PubMed] [Google Scholar]
- 17.Yuan S, Chu H, Singh K, et al. A novel small-molecule inhibitor of influenza A virus acts by suppressing PA endonuclease activity of the viral polymerase. Sci Rep 2016; 6: 22880. doi: 10.1038/srep22880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterholm MT, Kelley NS, Sommer A, et al. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 36–44. doi: 10.1016/S1473-3099(11)70295-X [DOI] [PubMed] [Google Scholar]
- 19.Griffiths C, Drews S. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev 2017; 30: 277–319. doi: 10.1128/CMR.00010-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics . Respiratory syncytial virus. In: Kimberlin DW, Barnett ED, Lynfield R, et al., eds. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. 32nd Edn. Itasca, American Academy of Pediatrics, 2021; pp. 628–636. [Google Scholar]
- 21.Agha R, Avner JR. Delayed seasonal RSV surge observed during the COVID-19 pandemic. Pediatrics 2021; 148: e2021052089. doi: 10.1542/peds.2021-052089 [DOI] [PubMed] [Google Scholar]
- 22.Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol 2017; 52: 556–569. doi: 10.1002/ppul.23570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Canchola J, Brandt C, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89: 422–434. doi: 10.1093/oxfordjournals.aje.a120955 [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics Committee on Infectious Diseases, American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134: e620–e638. doi: 10.1542/peds.2014-1666 [DOI] [PubMed] [Google Scholar]
- 25.Thomas N, Hollenbeak C, Ceneviva G, et al. Palivizumab prophylaxis to prevent respiratory syncytial virus mortality after pediatric bone marrow transplantation: a decision analysis model. J Pediat Hematol Oncol 2007; 29: 227–232. doi: 10.1097/MPH.0b013e3180437ded [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Pfarr D, Johnson S, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol 2007; 368: 652–665. doi: 10.1016/j.jmb.2007.02.024 [DOI] [PubMed] [Google Scholar]
- 27.Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9: eaaj1928. doi: 10.1126/scitranslmed.aaj1928 [DOI] [PubMed] [Google Scholar]
- 28.Xiaoyan L, Joshi A, Flomenberg P. Adenoviruses. In: Kaslow RA, Stanberry LR, LeDuc JW, eds. Viral Infections of Humans. 5th Edn. New York, Springer, 2014; p. 99. [Google Scholar]
- 29.Stephenson KE, Rhee EG, Barouch DH. Adenoviruses. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th Edn. Philadelphia, Elsevier, 2020; p. 1910. [Google Scholar]
- 30.Khanal S, Ghimire P, Dhamoon AS. The repertoire of adenovirus in human disease: the innocuous to the deadly. Biomedicines 2018; 6: 30. doi: 10.3390/biomedicines6010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ison MG. Adenovirus infections in transplant recipients. Clin Infect Dis 2006; 43: 331–339. doi: 10.1086/505498 [DOI] [PubMed] [Google Scholar]
- 32.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011). Transpl Infect Dis 2012; 14: 555–563. doi: 10.1111/tid.12022 [DOI] [PubMed] [Google Scholar]
- 33.Ljungman P, Ribaud P, Eyrich M, et al. Cidofovir for adenovirus infections after allogeneic hematopoietic stem cell transplantation: a survey by the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2003; 31: 481–486. doi: 10.1038/sj.bmt.1703798 [DOI] [PubMed] [Google Scholar]
- 34.Neofytos D, Ojha A, Mookerjee B, et al. Treatment of adenovirus disease in stem cell transplant recipients with cidofovir. Biol Blood Marrow Transplant 2007; 13: 74–81. doi: 10.1016/j.bbmt.2006.08.040 [DOI] [PubMed] [Google Scholar]
- 35.Bizot E, Bousquet A, Charpié M, et al. Rhinovirus: a narrative review on its genetic characteristics, pediatric clinical presentations, and pathogenesis. Front Pediatr 2021; 9: 643219. doi: 10.3389/fped.2021.643219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol 2007; 120: 526–529. doi: 10.1016/j.jaci.2007.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolin R. Rhinovirus. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th Edn. Philadelphia, Elsevier, 2020; pp. 2262–2268. [Google Scholar]
- 38.Fung TS, Liu DX. Human coronavirus: host–pathogen interaction. Annu Rev Microbiol 2019; 73: 529–557. doi: 10.1146/annurev-micro-020518-115759 [DOI] [PubMed] [Google Scholar]
- 39.Walsh EE, Shin JH, Falsey AR. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis 2013; 208: 1634–1642. doi: 10.1093/infdis/jit393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominguez SR, Robinson CC, Holmes KV. Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J Med Virol 2009; 81: 1597–1604. doi: 10.1002/jmv.21541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2017; 64: 1532–1539. doi: 10.1093/cid/cix160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am 2019; 33: 869–889. doi: 10.1016/j.idc.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleibtreu A, Bertine M, Bertin C, et al. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV). Med Mal Infect 2020; 50: 243–251. doi: 10.1016/j.medmal.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev 2003; 16: 242–264. doi: 10.1128/CMR.16.2.242-264.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Branche AR, Falsey AR. Parainfluenza virus infection. Semin Respir Crit Care Med 2016; 37: 538–554. doi: 10.1055/s-0036-1584798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell E, Ison MG. Parainfluenza virus in the hospitalized adult. Clin Infect Dis 2017; 65: 1570–1576. doi: 10.1093/cid/cix528 [DOI] [PubMed] [Google Scholar]
- 47.Russell E, Yang A, Tardrew S, et al. Parainfluenza virus in hospitalized adults: a 7-year retrospective study. Clin Infect Dis 2019; 68: 298–305. doi: 10.1093/cid/ciy451 [DOI] [PubMed] [Google Scholar]
- 48.Charlton CL, Babady E, Ginocchio CC, et al. Practical guidance for clinical microbiology laboratories: viruses causing acute respiratory tract infections. Clin Microbiol Rev 2018; 32: e00042-18. doi: 10.1128/CMR.00042-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 2001; 7: 719–724. doi: 10.1038/89098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373: 415–427. doi: 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Hoogen BG, van Doornum GJ, Fockens JC, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis 2003; 188: 1571–1577. doi: 10.1086/379200 [DOI] [PubMed] [Google Scholar]
- 52.Jeong S, Park MJ, Song W, et al. Advances in laboratory assays for detecting human metapneumovirus. Ann Transl Med 2020; 8: 608. doi: 10.21037/atm.2019.12.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention . Human Metapneumovirus (HMPV) Clinical Features. Date last updated: 5 September 2019. Date last accessed: 26 September 2021. www.cdc.gov/surveillance/nrevss/hmpv/clinical.html
- 54.Crowe JE. Common viral respiratory infections. In: Jameson JL, Kasper DL, Longo DL, et al., eds. Harrison's Principles of Internal Medicine. 20th Edn. New York, McGraw Hill, 2018; pp. 1375–1382. [Google Scholar]
- 55.Guido M, Tumolo MR, Verri T, et al. Human bocavirus: current knowledge and future challenges. World J Gastroenterol 2016; 22: 8684–8697. doi: 10.3748/wjg.v22.i39.8684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jartti T, Hedman K, Jartti L, et al. Human bocavirus – the first 5 years. Rev Med Virol 2012; 22: 46–64. doi: 10.1002/rmv.720 [DOI] [PubMed] [Google Scholar]
- 57.Lüsebrink J, Wittleben F, Schildgen V, et al. Human bocavirus – insights into a newly identified respiratory virus. Viruses 2009; 1: 3–12. doi: 10.3390/v1010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avšič-Županc T, Saksida A, Korva M. Hantavirus infections. Clin Microbiol Infect 2019; 21: e6–e16. doi: 10.1111/1469-0691.12291 [DOI] [PubMed] [Google Scholar]
- 59.Koo H, Lim S, Choe J, et al. Radiographic and CT features of viral pneumonia. Radiographics 2018; 38: 719–739. doi: 10.1148/rg.2018170048 [DOI] [PubMed] [Google Scholar]
- 60.Kim EA, Lee KS, Primack SL, et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 2002; 22: Suppl. 1, S137–S149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137 [DOI] [PubMed] [Google Scholar]
- 61.Miller W, Mickus T, Barbosa E, et al. CT of viral lower respiratory tract infections in adults: comparison among viral organisms and between viral and bacterial infections. AJR Am J Roentgenol 2011; 197: 1088–1095. doi: 10.2214/AJR.11.6501 [DOI] [PubMed] [Google Scholar]
- 62.Franquet T. Imaging of pulmonary viral pneumonia. Radiology 2011; 260: 18–39. doi: 10.1148/radiol.11092149 [DOI] [PubMed] [Google Scholar]
- 63.Souf S. Recent advances in diagnostic testing for viral infections. Biosci Horiz 2016; 9: hzw010. doi: 10.1093/biohorizons/hzw010 [DOI] [Google Scholar]
- 64.Nelson PP, Rath BA, Fragkou PC, et al. Current and future point-of-care tests for emerging and new respiratory viruses and future perspectives. Front Cell Infect Microbiol 2020; 10: 181. doi: 10.3389/fcimb.2020.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu S, Lin S, Zhang H, et al. Methods of respiratory virus detection: advances towards point-of-care for early intervention. Micromachines 2021; 12: 697. doi: 10.3390/mi12060697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah RD, Wunderink RG. Viral pneumonia and acute respiratory distress syndrome. Clin Chest Med 2017; 38: 113–125. doi: 10.1016/j.ccm.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med 2020; 46: 315–328. doi: 10.1007/s00134-020-05943-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelesidis T, Mastoris I, Metsini A, et al. How to approach and treat viral infections in ICU patients. BMC Infect Dis 2014; 14: 321. doi: 10.1186/1471-2334-14-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jefferson T, Jones M, Doshi P, et al. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ 2014; 348: g2545. doi: 10.1136/bmj.g2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nangle S, Mitra S, Roskos S, et al. Cytomegalovirus infection in immunocompetent adults: is observation still the best strategy? IDCases 2018; 14: e00442. doi: 10.1016/j.idcr.2018.e00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan E, Del Sorbo L, Goligher EC, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195: 1253–1263. doi: 10.1164/rccm.201703-0548ST [DOI] [PubMed] [Google Scholar]
- 72.Alshahrani MS, Sindi A, Alshamsi F, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care 2018; 8: 3. doi: 10.1186/s13613-017-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sukhal S, Sethi J, Ganesh M, et al. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: a systematic review and meta-analysis. Ann Card Anaesth 2017; 20: 14–21. doi: 10.4103/0971-9784.197820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Groves HE, Piché-Renaud PP, Peci A, et al. The impact of the COVID-19 pandemic on influenza, respiratory syncytial virus, and other seasonal respiratory virus circulation in Canada: a population-based study. Lancet Reg Health Am 2021; 1: 100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soo RJJ, Chiew CJ, Ma S, et al. Decreased influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis 2020; 26: 1933–1935. doi: 10.3201/eid2608.201229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fong M, Leung N, Cowling BJ, et al. Upper respiratory infections in schools and childcare centers reopening after COVID-19 dismissals, Hong Kong. Emerg Infect Dis 2021; 27: 1525–1527. doi: 10.3201/eid2705.210277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poole S, Brendish NJ, Tanner AR, et al. Physical distancing in schools for SARS-CoV-2 and the resurgence of rhinovirus. Lancet Respir Med 2020; 8: e92–e93. doi: 10.1016/S2213-2600(20)30502-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Foley DA, Yeoh DK, Minney-Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clin Infect Dis 2021; 73: e2829–e2830. doi: 10.1093/cid/ciaa1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casalegno JS, Ploin D, Cantais A, et al. Characteristics of the delayed respiratory syncytial virus epidemic, 2020/2021, Rhône Loire, France. Euro Surveill 2021; 26: 2100630. doi: 10.2807/1560-7917.ES.2021.26.29.2100630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ujiie M, Tsuzuki S, Nakamoto T, et al. Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg Infect Dis 2021; 27: 2969–2970. doi: 10.3201/eid2711.211565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nickbakhsh S, Mair C, Matthews L, et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci USA 2019; 116: 27142–27150. doi: 10.1073/pnas.1911083116 [DOI] [PMC free article] [PubMed] [Google Scholar]