Abstract
The respiratory microbiome and its impact in health and disease is now well characterised. With the development of next-generation sequencing and the use of other techniques such as metabolomics, the functional impact of microorganisms in different host environments can be elucidated. It is now clear that the respiratory microbiome plays an important role in respiratory disease. In some diseases, such as bronchiectasis, examination of the microbiome can even be used to identify patients at higher risk of poor outcomes. Furthermore, the microbiome can aid in phenotyping. Finally, development of multi-omic analysis has revealed interactions between the host and microbiome in some conditions. This review, although not exhaustive, aims to outline how the microbiome is investigated, the healthy respiratory microbiome and its role in respiratory disease.
Educational aims
To define the respiratory microbiome and describe its analysis.
To outline the respiratory microbiome in health and disease.
To describe future directions for microbiome research.
Short abstract
The respiratory microbiome encompasses bacterial, fungal and viral communities. In health, it is a dynamic structure and dysbiotic in disease. Dysbiosis can be related to disease severity and may be utilised to predict patients at clinical risk. https://bit.ly/3pNSgnA
Introduction
The respiratory microbiome and its impact on health and disease is increasingly well characterised. Further work on microbial function in the lower airways is ongoing. With the advent of culture-independent techniques and next-generation sequencing (NGS), we have a broader understanding of the microbiome, as well as investigating its functional capacity and therefore, potential direct impact on the host in health and disease. In this review, we will discuss how the microbiome is investigated, the healthy lung microbiome and its role in respiratory disease. In the past, traditional culture-based techniques were used to assess the microbial universe of different environments, however, these techniques are selective and don't provide the full picture [1]. NGS has opened a new door to understanding the interplay between microorganisms and humans in different microenvironments.
Sources and search strategy
References used for this review were identified from PubMed Central and MEDLINE searches. Search teams used included “microbiome”, “microbiota”, “metabolome”, “virome”, “mycobiome”, “metagenomic”, in conjunction with specific disorders, including “lung”, “respiratory”, “oropharyngeal”, “upper respiratory tract”, “asthma”, “COPD/chronic obstructive pulmonary disease”, “interstitial lung disease”, “IPF/idiopathic pulmonary fibrosis”, “cystic fibrosis”, “bronchiectasis”, “lung cancer”. Publications in high-impact journals, specifically targeting the lower airways were prioritised where possible.
What is the microbiome?
Two commonly used terms from culture-independent techniques include the microbiome and microbiota. In practice they are used interchangeably but do have differences. The microbiome is defined as the combined genomes (omic) of all the microorganisms inhabiting a specific environment [2], such as the gut, genitourinary tract or even the lung. That is to include the microorganisms and their genes. Whereas the microbiota refers to the community of microorganisms themselves [3]. Although most research has focused on bacteria at present, NGS allows us to explore other microorganisms such as viruses, fungi and archaea.
How is the microbiome assessed?
16S rRNA gene sequencing
During the late 1970s, sequencing of a small subunit ribosomal gene, referred to as 16S rRNA was pioneered to assess bacterial phylogeny [4, 5]. This gene is of particular use, as it is a conserved component of the transcriptional apparatus of all DNA-based lifeforms [6]. It consists of nine constant regions and nine hypervariable regions (V1–V9) [7]. It is the examination of these hypervariable regions that allows for discrimination between different bacteria, generally one or two hypervariable regions are selected. NGS of 16S rRNA via gene amplicon sequencing allows us to analyse the bacterial composition of many samples at the same time and at low cost. The sequences amplified can then be mapped to a database, leading to quantitative estimates of bacteria present in a sample.
Ecologically, microorganisms are classified taxonomically into Kingdom, Class, Order, Phylum, Genus and Species. However, amplicon sequences often do not correspond to the classically determined taxonomies. As a consequence, clusters of similar sequences are grouped into operational taxonomic units (OTUs). The main disadvantage of the use of OTUs is that errors in a reference database will be reflected in the analysis and novel taxa cannot be detected.
Amplicon sequence variant (ASV) is a newer approach, which determines exactly what sequences were detected and their frequencies. Comparison of similar frequencies determines the probability a particular read is not due to a sequencing error. As ASVs are exact sequences, they can be compared to reference databases at a higher resolution.
Fungal internal transcribed spacer sequencing
NGS of the internal transcribed spacer (ITS) region of the nuclear ribosomal RNA (rRNA) is utilised to assess fungal phylogeny, and from that assessment of the mycobiome [8]. Due to the read length limitations of some sequencing platforms, at times only parts of the ITS region is used, i.e. ITS1 or ITS2, similar to selection of variable regions in 16S sequencing. This selection should be made with care, as some primers can bias toward different phyla [8].
Whole genome sequencing (metagenomics)
16S rRNA and ITS sequencing describe the abundance of bacteria and fungi present in a specific environment. The advancement of NGS has led to the development of whole genome sequencing, also referred to as metagenomics (meta referring to microorganisms as opposed to human genomics) [9]. With this new technology, we can sequence for the abundance of bacteria as well as viruses (the virome), fungi (the mycobiome) and archaea all at once. Functional capacity of the community can then be deduced from the presence of particular genes; however, the expression patterns of particular genes cannot be inferred with this technique [10].
RNA metatranscriptomics
Metagenomics provides information on functional potential of microorganisms, while RNA metatranscriptome sequencing allows us to truly interrogate what microorganisms are actively transcribing. The metatranscriptome is a measure of the total mRNA in a sample, providing a representation of active gene expression in a sample at that moment in time [10].
Resistome
With the discovery of antibiotics, emergence and dissemination of resistance followed soon after. This resistance is mediated mainly by antibiotic resistance genes (ARGs) produced by antibiotic producing and non-pathogenic bacteria [11]. Metagenomics and metatranscriptomics, using NGS, has been applied to the human microbiome to assess the resistome. The resistome of the human microbiome is considered an important reservoir for dissemination of clinical ARGs. Analysis of the resistome of the respiratory microbiota has revealed a core respiratory resistome, even in healthy airways [12].
Ecological terminology
The majority of terms used to describe the human microbiome are derived from ecology. Commonly used metrics in microbiome analysis include alpha diversity and beta diversity. Alpha diversity describes the ecological diversity within a sample. This can refer to the richness (number of species), or evenness (distribution of species). There are many metrics used to calculated alpha diversity, the most simple being a count of all the different OTUs present. The most commonly used is the Shannon diversity index, an account of abundance and evenness of species present. Beta diversity is a measure of the similarity or dissimilarity of two communities. Again, many indices are available to describe beta diversity. Bray–Curtis distance is a statistical tool which quantifies the compositional dissimilarity between two samples, giving a value ranging from 0 to 1, 0 meaning the groups share all species and 1 meaning that they share none. Weighted UniFrac distance estimates differences between samples based on phylogenetic distance, while also incorporating abundance values. The use of phylogenetic distance aims to reduce the contribution of rare species. Another commonly used term is dysbiosis, this refers to a change in the composition of the microorganisms in the microbiome, which results in a reduction in their ability to function normally.
Metabolomics and proteomics
Metabolomics is a developing field in microbiome analyses aimed at understanding the by-products of organisms. Metabolomic analysis, through mass spectrometry, can identify and quantify the metabolites of any given sample, such as fructose–rhamnose and cellobiose, carbohydrates synthesised by bacteria which can represent active bacterial metabolism [13]. The metabolome can reflect the health of an environment or show evidence of dysbiosis [14]. Assessment of metabolites can identify metabolically active organisms which produce metabolically active metabolites with immunomodulatory capabilities, such as short-chain fatty acids [13]. Proteomics is a similar technique using mass spectrometry to study the expression, structure, function and interactions of a set of proteins produced by the host and microbiome (table 1).
TABLE 1.
Summary of definitions
| Microbiome | The combined genomes of all the microorganisms inhabiting a particular environment |
| Microbiota | Community of microorganisms in an environment |
| Metagenomics | The study of the whole genome of a community of microorganisms, allowing assessment of the functional potential of the microorganisms |
| RNA metatranscriptomics | The measurement of the total mRNA in a community, to allow representation of active gene expression at that time |
| Resistome | Collection of antibiotic resistance genes in a community |
| Metabolomics | Assessment of the metabolites of a given sample, allowing identification of metabolically active organisms |
| Proteomics | Assessment of proteins produced by the host and microbiota |
| Alpha diversity | The diversity of a sample, including the richness (number of species) or evenness (distribution of species) |
| Beta diversity | The measure of the similarity or dissimilarity of two communities |
Although providing a vast amount of information about the microbial composition of different environments, there are some limitations to NGS. Most bacteria are only identified at a genus level, and furthermore sequencing cannot always distinguish between differing strains. Contamination can occur at the time of sampling, during DNA extraction or in the course of PCR amplification. Measures can be undertaken to minimise the influence of contamination, for example, concern over contamination from bronchoscopes can be overcome with simultaneous sequencing of background bronchoscopy and bronchoalveolar lavage (BAL) samples, allowing identification of any taxa that are possible contaminants [16]. To overcome contamination from DNA extraction kits and reagents, concurrent sequencing of negative controls can be used [17]. In addition, statistical packages, such as the decontam package in R, can also be used to help identify contaminants. It is important to understand there are areas of the human body which have low amounts of bacteria, such as the lung, joints and breasts. The paucity of organisms can be a further challenge to NGS.
The combination of all the above techniques is referred to as multi-omics, and can be applied to the respiratory microbiome to describe taxonomic composition, functional and metabolic activity of the microbiome. With these techniques, the modern study of the human microbiome came to the forefront in 2007, with the inception of the Human Microbiome Project [18]. This was designed to characterise the human microbiome in the nasal passages, oral cavity, skin, gastrointestinal tract and urogenital tract. Through this integrated analysis it is now clear the gut microbiome has a role in modulating systemic immune tone and drug effectiveness [19, 20]. Furthermore, the role of the gut microbiome in disease is becoming apparent, with recognition of the gut–brain axis in mental health disorders, and its role in metabolic conditions, including obesity and type two diabetes among others [20]. Currently, most studies investigating the respiratory microbiome are observational and the functional potential remains under-investigated. Here we will review some of the literature using the above techniques in evaluating the respiratory microbiome in both health and in specific respiratory diseases.
The development of the respiratory microbiome
The respiratory microbiome is established at birth and appears essential for normal lung development [21]. It then develops rapidly in the first few weeks of life. Many factors influence the development including breastfeeding [22], antibiotic use, vaccination, siblings, smoke exposure, environment and childhood infections. The childhood microbiome can also affect the development of respiratory diseases in later life, including asthma [23].
The respiratory microbiome in health
The respiratory microbiome exists along a gradient, with microbial diversity highest in the upper respiratory tract and decreasing towards the lower respiratory tract [24]. The upper respiratory tract provides a variety of niches for bacterial colonisation and the microbiome varies by location [25].
The upper airway microbiome is complex, as each surface presents a different ecological niche, each with unique conditions suitable for variable organisms. Hence, the microbiome will vary between the hard and soft tissue, saliva, tongue and plaque [26]. Nevertheless, in general the healthy upper airway microbiome is dominated by Streptococci, followed by Neisseria, Prevotella, Rothia and Haemophilus. Of note, anaerobes are uniquely represented in plaque specimens [27].
The upper airway virome is rich and complex, including several phages of the Siphoviridae, Myoviridae and Podoviridae families, which are directed against the most abundant bacterial species in the oral microbiome. Additionally, viruses of the Herpesviridae family are abundant [27]. The upper airway mycobiome is mainly Candida and Saccharomycetales [27].
Sputum is often used as a surrogate for the lower airway microbiome. However, it is important to recognise that expectorated sputum exists as a combination of secretions in the lower respiratory tract and upper airways, including the oral microbiome. Direct comparison between sputum and BAL has shown that sputum contains a bacterial load comparable to that of the upper airways, with significant differences in beta diversity between all samples. Sputum samples are most similar to supraglottic samples, indicating that sputum is not an accurate representative of the lower airway microbiome [28]. Sputum may be a representative sample in diseases with high sputum volume, such as cystic fibrosis (CF), but investigators should be aware of the drawbacks.
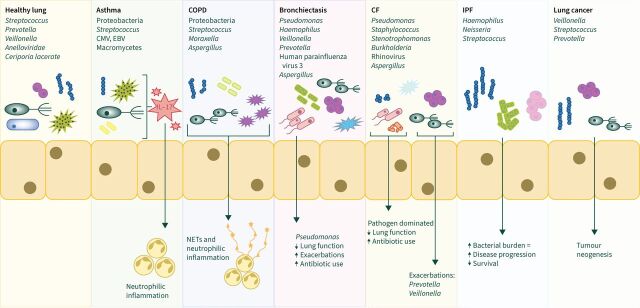
The lower respiratory tract has significantly reduced bacterial concentration and differing diversity compared with the upper airway. The microbiome is characterised by Bacteroidetes, Proteobacteria, Firmicutes and at genus level, Streptococcus, Prevotella and Veillonella [29]. Anelloviridae form the majority of the lower airway virome with herpes viruses and human papillomavirus [30]. The mycobiome consists of Ceriporia lacerate, Saccharomyces cerevisiae and Penicillium brevicompactum [31].
It is important to appreciate that the healthy respiratory microbiome is transient, with microbes moving into the lung by inhalation, micro-aspiration and direct mucosal spread from the upper airway, and then being removed by the mucociliary system and innate immune system. Next, we will review the changes in the respiratory microbiome in several common respiratory diseases (table 2).
TABLE 2.
Summary of the respiratory microbiome in health and disease
| Respiratory microbiome | Micobiome | Virome | Mycobiome |
| In health | Bacteroidetes Proteobacteria Firmicutes Genus: Streptococcus, Prevotella, Veillonella |
Anelloviridae Human herpes viruses Human papillomavirus |
Ceriporia lacerate
Saccharomyces cerevisiae Penicillium brevicompactum |
| Asthma | Proteobacteria Streptococcus Reduction in Prevotella and Veillonella |
Cytomegalovirus Epstein–Barr virus |
Psathyrella candolleana
Termitomyces clypeatus Grifola sordulenta Malassezia pachydermatis |
| COPD | Proteobacteria Haemophilus Streptococcus |
Picornaviruses Influenza Parainfluenza Respiratory syncytial viruses Adenoviruses |
Alternaria Aspergillus Cladosporium Cryptococcus Mycosphaerella Penicillium Trametes Wickerhamomyces |
| Bronchiectasis |
Pseudomonas
Haemophilus Veillonella Prevotella |
Siphoviridae
Caudovirales Myoviridae Phycodnaviridae |
Aspergillus
Cryptococcus Clavispora |
| CF |
Pseudomonas
Staphylococcus Stenotrophomonas Burkholderia |
Influenza Respiratory syncytial virus |
Aspergillus
Candida |
| IPF |
Haemophilus
Neisseria Streptococcus |
Influenza A Human herpesvirus |
Not yet established |
| Lung cancer |
Veillonella
Streptococcus Prevotella |
Not yet established | Not yet established |
The respiratory microbiome in disease
Asthma
Asthma is a heterogeneous condition characterised by airway inflammation and hyperreactivity which, at an immunological level, can be divided into T-helper cell (Th)2 high or Th2 low phenotypes. A long-standing hypothesis in the pathogenesis of asthma relates to hygiene and exposure to irritants. Indeed, further exposure to irritants, such as pollen may increase symptoms in patients with asthma. One area of interest is understanding the respiratory microbiome in asthma and how it relates to different phenotypes and symptom control.
Overall asthmatic patients display a higher bacterial burden compared with healthy controls when assessed via bronchial epithelial brushings [32]. There is a loss of low-level commensal organisms on oropharyngeal swabs [33], with an expansion in Proteobacteria [29, 32, 33], and Streptococcus in severe asthma [29, 32, 34] with a concurrent reduction in the abundance of Prevotella and Veillonella [35]. These findings are independent of inhaled corticosteroid use, with a similar microbial diversity reported in mild asthmatic patients [36]. In fact, Proteobacteria is one of the most prevalent bacterial phyla found in asthma patients, and is associated with worsening symptomology, steroid responsiveness and Th17 and interleukin (IL)-17 driven inflammation [34]. All which promote neutrophil recruitment and neutrophilic airway inflammation [34]. Interestingly, individuals with Th2 inflammation display a higher bacterial burden when compared to Th2-low asthma [36]. Furthermore, expansion in Proteobacteria, sphingomonadaceae and oxalobacteraceae on bronchial brushings are associated with bronchial hyperresponsiveness [32]. Of note, sphingomonadaceae have cell membrane glycosphingolipids, which can activate invariant natural killer T-cells, inducing IL-4 and IL-13, demonstrating a possible relationship between microbiota and local immunological tone.
In asthma there can also be an expansion in potentially pathogenic bacteria which are carried in the airway microbiome of the healthy population [33]. This suggests a diverse microbial community in health may protect against asthma, by inhibition of potential pathobionts; the loss of commensal organisms leads to a loss of local or systemic immune tone which then signals to downregulate immune responses coupled with intermittent mucosal damage by potentially pathogenic bacteria, which are in excess in asthmatic airways.
The mainstay of treatment in asthma, particularly Th2-high asthma, is inhaled corticosteroids. However, some patients have been found to be corticosteroid resistant. Treating these patients can be quite challenging. The respiratory microbiome might provide some new therapeutic options. In corticosteroid-resistant asthma prior studies have found an abundance of Gram-negative lipopolysaccharide (LPS) producing bacteria in BAL with concurrent elevation of IL-8 and LPS, suggesting microbial stimulation [34]. Continuous airway stimulation by LPS has been shown to suppress Th2 activation [36]. In vitro, exposure to Haemophilus parainfluenzae leads to high levels of p38 MAPK activation and reduced cellular response to corticosteroids in peripheral blood monocytes and BAL macrophages. Additionally, lack of response of pulmonary macrophages to corticosteroids leads to persistent airway inflammation in corticosteroid-resistant asthma [37].
Patients with uncontrolled asthma tend to have frequent exacerbations, with worsening of symptoms (such as shortness of breath and wheeze) frequently requiring systemic corticosteroids and sometimes antibiotics. While exacerbations are associated with Moraxella catarrhalis, Streptococcus pneumoniae and Klebsiella species [38], H. influenzae is the most common pathogenic bacteria isolated in induced sputum from asthmatic patients [39]. Interestingly, early childhood colonisation by potential pathogens, S. pneumoniae, M. catarrhalis or H. influenzae isolated from hypopharyngeal aspirates, confers an increased risk for recurrent wheeze or asthma in childhood [23]. The bacterial burden of H. influenzae in induced sputum has been shown to predict treatment response to azithromycin [40], a commonly used antibiotic in respiratory diseases. In an interesting study examining the effects of azithromycin on the respiratory microbiome, following treatment there is a reduction in bacterial richness and altered airway microbiota, leading to Enterococcus becoming the dominant microorganism in asthmatic BAL [41]. While Pseudomonas, Haemophilus and Staphylococcus decrease in abundance in the BAL [41], all potentially pathogenic genera. Interestingly, increasing relative abundance of the Firmicutes phylum on bronchial brushing is negatively correlated with symptoms [34], indicating both an antibiotic and immunomodulatory effect of azithromycin. Thus, specific pathogenic bacteria may influence the response to pharmacotherapy and colonisation by pathogenic bacteria may be potential targets for intervention.
Severe asthma is often linked with fungal sensitisation. Analysis of induced sputum from asthmatic patients has revealed asthmatic individuals have a distinct mycobiome to that of healthy controls [42]. Of interest, three of the four commonest species identified where from the macromycetes (mushroom) group: Psathyrella candolleana, Termitomyces clypeatus and Grifola sordulenta; the remaining species, Malassezia pachydermatis, is a yeast associated with atopic dermatitis. Asthma is associated with damp environments, yet this is the first reported association between macromycetes and asthma [42]. Additionally, analysis of the BAL mycobiome has shown that inhaled corticosteroid treatment is associated with an increased fungal burden of Aspergillus [43].
Viruses also play a key role in the development of asthma and subsequent exacerbations, notably rhinoviruses, influenza and respiratory syncytial viruses [44]. The respiratory virome in asthma patients is distinct to that of healthy controls, with an abundance of Herpes viruses, predominantly Cytomegalovirus and Epstein–Barr virus seen in sputum. Notably, their abundance was associated with more severe disease, increased symptomology and decreased lung function [45].
Individuals with asthma are frequently prescribed antimicrobial therapy both acutely for exacerbations and chronically for symptom control. Given the global burden of antimicrobial resistance, selection pressure leading to antibiotic resistance is a key concern. Analysis of the resistome in the sputum of individuals with asthma has identified a core macrolide resistome, similar to that seen in healthy airways and with less resistance genes in comparison to COPD or bronchiectasis [12]. Notably, long-term exposure to azithromycin increases the abundance of several macrolide resistance genes, and has been associated with clinical resistance to H. influenzae [46].
It is now apparent that in asthma there are significant alterations to the respiratory microbiome. This dysbiosis has been linked to specific phenotypes and exacerbations. The functional effects of the respiratory microbiome and potential areas for interventions, as well as longitudinal studies are required to fully elucidate the role of the microbiome and develop therapeutic targets.
COPD
COPD is characterised by airway inflammation, chronic bronchitis, emphysema and fixed airflow obstruction usually associated with cigarette smoke exposure. Chronic symptoms include shortness of breath, wheeze and a daily cough. Patients are also prone to exacerbations of COPD with worsening of symptoms usually precipitated by a bacterial or viral infection. The respiratory microbiome has been studied both to understand the pathogenesis of COPD, but also to understand disease progression.
Not surprisingly, with chronic airways inflammation the respiratory microbiome differs in COPD when compared with healthy controls, with a predominance of Proteobacteria and Streptococcus in COPD [47–49]. With disease progression and parenchymal destruction (notably so in emphysema), there is a decline in microbial diversity with a further expansion of Proteobacteria, particularly Haemophilus with a reduction in Firmicutes [47].
Reductions in alpha diversity have been associated with poorer lung function, more severe disease and a reduction in survival. These changes produce a measurable host response in lung tissues, as demonstrated by upregulation of host interferon and proinflammatory signalling pathways, neutrophilic infiltration and formation of neutrophil extracellular traps (NETs) associated with Haemophilus and Moraxella [47, 50, 51]. This suggests that a persistent low-level inflammatory immune response associated with the progression of COPD [52] may be driven by bacteria which expand on diminishing bronchiolar and alveolar surfaces [53]. In stable COPD, the sputum microbiome has been shown to be related to long-term survival with a clear relationship between the microbiome and clinical phenotypes. For example, a Proteobacteria-dominated microbiome is associated with chronic bronchitis, eosinopenia, neutrophilic inflammation, poorer lung function and decreased survival [50, 54]. While a Streptococcus-dominant microbiome is associated with raised peripheral blood eosinophils and absence of chronic bronchitis.
As with asthma, inhaled therapy is the cornerstone of treatment in COPD. However, as with asthma, long-term therapy can also induce alterations in the microbiome; inhaled corticosteroids lead to an increase in Proteobacteria (Haemophilus) [55] and expansion of Strepococcus [56], and long-acting β-agonist (LABA) monotherapy leads to an increase in Bacteroidetes (Prevotella) [57].
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of COPD includes evaluating how frequently patients have exacerbations, as this has been shown to be associated with prognosis and helps direct treatment. Frequent exacerbators (GOLD C or D) have lower biodiversity, even during periods of stability, with an increase in Streptococcus, Haemophilus and Moraxella [58]. During exacerbations, there is a further reduction in biodiversity with a dominance of pathogenic taxa [48, 50, 59, 60]. It remains unclear whether these profiles are as a result of disease or intervention; meaning treatment during exacerbations, predominately steroids and antibiotics, further alter the respiratory microbiome [50]. It is likely a combination of the two.
The respiratory mycobiome in COPD, assessed by sputum, exhibits greater diversity in stable COPD compared with healthy controls [61]. Notably, the mycobiome composition is unchanged in differing GOLD stages or lung function. However, it does vary by exacerbation frequency. Two distinct patient clusters were identified based on mycobiome signatures: one cluster characterised by increased symptoms and predominance of Saccharomyces and another cluster characterised by exacerbations, increased mortality and Aspergillus, Curvularia and Penicillium. This cluster also exhibits a systematic immune response to fungi [61].
Viral infections have long been a major driver of acute exacerbations in COPD; however, the virome is less well studied. There is evidence that viral infections lead to alterations in the bacterial microbiome, with consequent bacterial overgrowth and infection [62]. The predominant pathogens detected during exacerbations are picornaviruses, followed by influenza [63]. When the COPD virome was examined via nasopharyngeal swabs during COPD exacerbations, this revealed reduced abundance of bacteriophages in COPD patients suggesting skewing of the virome during infections and a possible mechanism for bacterial overgrowth [64].
Antibiotics are frequently prescribed both acute and chronically for the management of COPD and exacerbations. There is a concern that individuals with COPD may become a reservoir for ARGs. When the sputum of COPD patients was compared with healthy individuals, ARGs were 37% more prevalent in COPD sputum than in healthy controls. This excess was related to the excess bacterial burden in sputum [65]. Patients with COPD also harbour the highest diversity of resistance genes [12]. Microbial taxa associated with ARGS include Actinomyces, Streptococcus and Prevotella. Interestingly, resistance genes identified are associated with those found in the gut microbiota, suggesting seeding of the airway resistome may occur through aspiration of gut microbes [12].
We now have a consistent body of evidence showing the role of the microbiome in COPD, leading to considerations on how the microbiome could be targeted. For example, H. influenzae may be amenable to treatment with antibiotics; however, the reduction in one microorganism can lead to an increase or dominance of another [51, 59]. In bronchiectasis, another chronic lung disease, a reduction in Haemophilus leads to a concurrent increase in Pseudomonas [66]. Future interventional studies are key.
Bronchiectasis
Non-cystic fibrosis bronchiectasis is characterised by irreversible dilation of bronchi, leading to chronic mucus production and poor mucus clearance. This results in a vicious cycle of persistent bacterial colonisation, inflammation, airway obstruction and progressive lung destruction. Traditional culture-based techniques have reported colonisation with H. influenzae, Pseudomonas aeruginosa and Streptococcus pneumoniae [67–69]. However, in up to 20% of patients, no pathogens are isolated from purulent sputum [70, 71]. NGS provides some insight into the microbiology of these patients.
There is a complex respiratory microbiome in bronchiectasis [72] and the structure within the community is extremely variable between patients [73]. Generally speaking, there is limited bacterial diversity, with no detected relationship between total bacterial load and disease state [74]. Importantly, the majority of microbiome studies in bronchiectasis have been performed on sputum samples.
Some studies have used the respiratory microbiome to phenotype and better understand disease processes. In a study which used the respiratory microbiome of patients with bronchiectasis to aide in phenotyping, the authors identified three main groups: Pseudomonas-dominant, Haemophilus-dominant and other taxa-dominant. The other taxa group mostly consisted of Veillonella and Prevotella and the majority of this group were negative on conventional cultures. Those in the Pseudomonas-dominant group had the lowest lung function, the most exacerbations and greatest antibiotic use. The Haemophilus group was associated with fewer exacerbations, but Haemophilus bacterial load was associated with inflammatory activation both in sputum and systemically. This suggests the host inflammatory response to Haemophilus may contain the infection and prevent excerbations [74]. Notably, Pseudomonas was detected in 22 out of the 34 patients in the Haemophilus predominant group; however, they displayed no significant differences to the members without Pseudomonas, suggesting relative abundance is of more significance than detection. This is supported by the finding that simple PCR detection of pathogens does not influence disease severity [74].
Nontuberculous mycobacteria (NTM) are ubiquitous environmental organisms which can cause respiratory disease in patients with pre-existing lung damage, predominantly bronchiectasis. The incidence of NTM is increasing, although the cause is unclear [75]. Despite this, the role of NTM in the respiratory microbiome is not well elucidated. Mycobacteria are not accurately identified by 16S rRNA gene sequencing and although this improves with a Mycobacterium-biased sequencing, a poor correlation between culture positive NTM and sequencing results remains. Additionally, BAL samples from NTM-positive patients have a distinct inflammatory pattern. When microbial signatures were assessed, in NTM-positive individuals oral commensals such as Prevotella, Veillonella and Leptotrichia tended to co-occur, with a distinct inflammatory signature. Notably, Mycobacterium was in a separate cluster with no correlation to inflammatory biomarkers. This suggests micro-aspiration or failure to clear aspirated microbes may contribute to the inflammatory endotype in NTM disease [28].
The fungal mycobiome also influences clinical state in bronchiectasis. The mycobiome assessed via sputum in bronchiectasis is unique and dominated by Aspergillus, Cryptococcus and Clavispora [76]. A high proportion of patients have detectable Aspergillus fumigatus and/or Aspergillus terreus, which is not detected in healthy controls. Interestingly, geographical alterations have been demonstrated, with A. fumigatus detected in Singapore and A. terreus in Scotland. Notably, a significant number of individuals had Aspergillus-associated disease, ranging from colonisation to sensitisation to allergic bronchopulmonary aspergillosis (ABPA), and each had its own distinct mycobiome profile, which may be a target for future phenotyping. Furthermore, Aspergillus-associated disease was associated with more severe bronchiectasis, worse lung function and increased exacerbations, even in apparently clinically stable patients [76].
The virome in bronchiectasis displays a significantly higher viral load in sputum than in healthy controls and human parainfluenza virus 3 was the most prevalent. Notably, again geographic differences were observed, with higher occurrence in Asian patients compared with European patients. Analysis of bacteriophage profiles revealed a predominance of Siphoviridae, Caudovirales, Myoviridae and Phycodnaviridae families. Furthermore, when patients were clustered by exacerbation risk, patients with frequent exacerbations had a distinct bacteriophage profile [77].
Individuals with bronchiectasis frequently develop acute exacerbations, defined as an increase in symptoms for at least 48 h [78]. The mainstay of treatment is antimicrobials, with the current understanding that exacerbations are due to bacterial overgrowth [79]. The microbiome in exacerbations of bronchiectasis is highly individualised and the role of the microbiome remains unclear. Exacerbations are complex events; the microbiome is stable during exacerbations, with little change in bacterial load, diversity or abundant taxa, suggesting changes in the microbiome or bacterial overgrowth does not account for exacerbations [72, 73]. Another study, applying a multi-omic approach clustered patients into two groups. Each cluster contained a range of discriminant bacterial, fungal and viral taxa. Cluster 1 had greater microbial diversity and improved clinical outcomes, cluster 2 were more likely to be frequent exacerbators [77].
The mainstay of treatment for bronchiectasis is airway clearance and antibiotics. Chronic bacterial infections are common in bronchiectasis and are generally treated with recurrent broad-spectrum antibiotics. This in turn facilitates the development of multidrug-resistant pathogens. Similarly, long-term macrolides induce increased rates of macrolide resistance [80].
Even with all this information on the respiratory microbiome in bronchiectasis, interventional targets remain elusive. Further integrated, multi-omic and interventional studies will hopefully provide answers and improve patient outcomes.
Cystic fibrosis
CF is a genetic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. CF is a chronic condition associated with chronic colonisation by respiratory pathogens and understanding these microorganisms and their impact on patients is crucial.
The respiratory microbiome in CF develops over time. Until 2 years of age, the microbiome is rich in oropharyngeal taxa [81]. Eventually, the respiratory microbiome development falls into two main groups: polymicrobial (characterised by oral commensals, similar to that seen in health) or dominated by CF pathogens. Common pathogens include Pseudomonas, Staphylococcus, Stenotrophomonas or Burkholderia. Patients in the pathogen dominated group have decreased microbial diversity, worse lung function, higher degrees of inflammation and greater antibiotic use [82–85]. Assessment of the respiratory microbiome in CF has predominantly been carried out on sputum samples.
CFTR modulators have led to a major shift in CF management. Given their mechanism of action it is not surprising that they effect the respiratory microbiome. Studies examining the effect of CFTR modulators, predominantly ivacaftor, on the microbial community have been diverse, but do suggest a positive effect. A reduction in the relative abundance of Pseudomonas [86] with an increase in the relative abundance of oral commensals [87], leading to an increase in microbial diversity and richness with an overall reduction in total bacterial load [88] have all been demonstrated. This has been replicated with the newer, combination CFTR modulator, elexacaftor/tezacaftor/ivacaftor [89]. The exact pathogenesis is unclear, improved mucus clearance disrupting the viscous cycle of bronchiectasis, a direct anti-inflammatory or antibiotic effect of the modulators, or alterations to the environment have all been postulated [90].
The disease course of CF is regularly punctuated with acute exacerbations. Exacerbations are defined as a requirement for antibiotics due to a change in symptoms [91]. During an exacerbation of CF, the relative abundance of anaerobic genera (Prevotella, Veillonella, Gemella) increases with a subsequent increase in microbial diversity [92, 93]. During treatment, alpha diversity of the microbiome decreases, as does the relative abundance of anaerobes, and recovers back to baseline on recovery from the exacerbation [93]. Hence, there appears to be a stable microbiome dominated by CF pathogens and an exacerbation microbiome, dominated by anaerobic bacteria.
As with other chronic lung diseases, the virome plays an important role in the clinical course of CF. Rhinovirus is associated with worsening lung function, more exacerbations and increased bacterial colonisation [94]. Influenza and respiratory syncytial virus have also been shown to be associated with an increased risk of exacerbation [95]. The role of the mycobiome in CF is also well studied using culture-based methods, with Aspergillus colonisation, sensitisation and development of ABPA negatively correlated to outcomes [96]. When assessed using RNA sequencing, CF patients have a lower fungal diversity and a higher fungal burden compared with non-CF bronchiectasis, and the most prominent species was Candida parapsilosis.
During the lifetime of a CF patient, aggressive antibiotic therapies will be used to combat bacterial colonisation and acute exacerbations. This leads to antimicrobial resistance over time, with up to 45% of CF patients reported as colonised with a multidrug-resistant organisms [97]. The resistome of CF patients with severe disease is enhanced with ARGs compared to controls [97]. Interestingly, bacteriophage (phage) therapy, the use of lytic phages to kill infectious bacteria, has been used to treat CF patients with resistant P. aeruginosa, Staphylococcus aureus, Burkholderia dolosa and Mycobacterium abscessus with a subsequent reduction in sputum bacterial density and improved clinical status [98].
The development and now widespread use of CFTR modulators has transformed care and patient outcomes in CF. There is now a clear body of evidence that microbial dysbiosis plays a role in the pathophysiology of CF. Further studies to understand the functional potential, role of newer CFTR modulators and interventional targets are required to ultimately improve patient care.
Interstitial lung disease
The interstitial lung diseases (ILDs) are a varying group of conditions, manifesting with impaired lung function and various patterns of lung fibrosis and inflammation. Some are associated with underlying conditions (such as connective tissue disease) while others are idiopathic. The most studied of these conditions is idiopathic pulmonary fibrosis (IPF). While the pathogenesis of IPF remains unclear, the underlying mechanism is thought to be a result of episodes of alveolar injury in those with defective alveolar wound-healing mechanisms [99]. One hypothesis regarding the pathogenesis of IPF has revolved around microbial insult or dysbiosis.
With this, several studies have used NGS to explore the respiratory microbiome in IPF, which has been shown to have a significantly higher bacterial burden in BAL compared with COPD or healthy controls [100, 101], where bacterial burden is negatively correlated with disease progression and survival [100, 101]. Interestingly, this association is independent of radiological features and disease extent [101]. Furthermore, the microbiome has less diverse bacterial communities and is more likely to harbour potential pathogens such as Haemophilus, Neisseria and Streptococcus [100]. Moreover, an increased relative abundance of Streptococcus is significantly associated with a reduction in progression-free survival [100]. Understanding the direct effect these microorganisms have on individuals is particularly important in understanding disease progression in IPF, however, this can be challenging. Using network analysis with bulk RNA sequencing (host transcriptome) and microbial data, genes involved in host defence response in conjunction with bacterial burden have been shown to be strongly associated with IPF, suggesting bacterial communities of the lower airways may act as a persistent stimuli and consequently lead to worse outcomes [102].
Acute exacerbations of IPF (AE-IPF) occur commonly in the disease process, with an annual incidence of 4–20% annually [103]. They are defined as an acute, clinically significant respiratory deterioration of unidentifiable cause [104]. The exact pathogenesis of AE-IPF remains unclear, however, it may represent an exaggerated lung injury in response to infection. Acute exacerbations are associated with an increase in bacterial burden in BAL samples [105]. There is a higher relative abundance of Campylobacter and Stenotrophomonas [105]. Of interest, Campylobacter is generally found in the gastrointestinal tract and its presence in the lower airways may suggest silent micro-aspiration of gastric contents. This strengthens the argument associating reflux and aspiration with AE-IPF.
The role of the respiratory virome in IPF is less well understood. RNA sequencing has been applied to lung tissue samples with no significant difference in the abundance of viral RNA between IPF samples and controls [106]. Viral nucleic acid has been detected in the BAL of 44% of patients with an exacerbation, compared to none with stable disease [107]. The most common viruses identified during exacerbation, via nasopharyngeal swab, are influenza A and human herpesvirus 6 [100]. To date, the respiratory mycobiome in IPF has not been studied.
There is a clear association between bacterial burden and prognosis in IPF, however, studies are lacking on the interaction between the respiratory microbiome and immunological tone, particularly in those ILDs associated with immune system activation.
Lung cancer
Although lung cancers are a heterogenous group of malignancies, overall prognosis is poor as many present with advanced disease. Thus, lung cancer is the leading cause of cancer-related deaths worldwide. Given its wide prevalence, high mortality rate, and often delayed diagnosis, there is a need to identify biomarkers and mechanisms of progression to help diagnose, prognosticate and improve treatment options for these patients. The link between the microbiome and malignancy is an area of significant interest and prior studies have shown that distinct microbial signatures in both blood and tissues can help differentiate between cancer types and stages [108]. Thus, the relationship of the respiratory microbiome in lung cancer is of particular interest.
Several studies have shown that the lower airway microbiota differs significantly among those with lung cancer compared with healthy controls. This has been demonstrated in both human subjects as well as mouse models [109]. For example, in a mouse model of lung adenocarcinoma, where cancer was induced through deletion of p53 in lung epithelial cells, there was a significant increase in bacterial abundance and reduced microbial diversity in those with lung cancer compared with controls [110]. In human studies, we have seen that taxa commonly considered as oral commensals are differentially enriched in the lungs of patients with lung cancer. Veillonella, Streptococcus and Prevotella being the most abundant [111]. There are also compositional differences between different stages of lung cancer [109, 112]. For instance, the oral commensals Haemophilus, Fusobacterium, Gemella, Prevotella and Granulicatella are enriched in stage IIIB–IV nonsmall cell lung cancer (NSCLC) compared with stage I–IIIA NSCLC [112].
We now understand that the host immune system has an important role in cancer development and progression. Importantly, through stimulation of the immune system microorganisms can also lead to tumourigenesis. Where individuals with enrichment of oral microbes in their lower airways have a distinct host immune phenotype with upregulation of the Th17 pathway and increased PD-1+ and gamma delta (γδ) T-cells [110–112], all which produce pro-inflammatory cytokines [13]. Moreover, specific pathways known to be important for tumour development and progression, such as the phosphoinositide 3-kinase (PI3K) and ERK pathways, have been shown to be upregulated in mouse models with lower airway dysbiosis [112]. With this relationship efforts are being made in treatment development. For instance, it has been shown that by giving antibiotics to specific pathogen-free mice with lung adenocarcinoma, tumour growth and progression can be suppressed [110]. In another study tumour burden was decreased when mice (with increased IL-17 inflammatory tone from dysbiosis) were given anti-IL-17 antibodies [112]. There is also the potential to augment treatment through the microbiome. For instance, specific taxa within the gut microbiota of patients with advanced cancers on anti-PD-1 immunotherapy were found to be overrepresented in responders when compared with non-responders [113, 114].
Thus, the lung microbiome provides a unique opportunity to identify new biomarkers in lung cancer, to better understand the role of inflammation and dysbiosis in the development and progression of malignancy, and has the potential to be used to augment and develop treatments.
Future directions in the respiratory microbiome
Clinical applications of the microbiome are emerging, although these are limited for the respiratory microbiome. Clinical microbiologists have utilised 16S rRNA gene sequencing as an adjunct for pathogen identification from traditional culture negative isolates. This has been successfully employed to diagnose infective endocarditis, bone and joint infections and meningitis among others. It is particularly useful for slow growing, fastidious or unusual pathogens. Interestingly, this approach has also allowed the identification of novel species [115]. Furthermore, metagenomics in real time has been applied to identify viruses, fungi and manage outbreaks, most prominently identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the emergence of variants of concern [116]. As of yet, real time RNA transcriptomics have not been utilised.
Manipulation of the microbiome is an additional emerging clinical tool, at present limited to the gastrointestinal tract. The role of faecal microbiota transplantation (FMT) has been shown to be successful in the management of recurrent Clostridioides difficile infection, with emerging evidence of successfully inducing remission in ulcerative colitis [117]. Furthermore, altering the microbiome at birth with probiotics reduces the incidence of necrotising enterocolitis in low birthweight preterm infants. Interestingly, maternal FMT has been demonstrated to restore a normal gastrointestinal microbiome in infants delivered by Caesarean-section, although clinical benefit remains to be demonstrated [117]. Altering the respiratory microbiome at birth or in childhood has not yielded any clinical benefit, but is a focus of future research.
As highlighted in this review, the majority of the work evaluating the respiratory microbiome has focused on sputum samples, a poor surrogate of the lower airways. More research will be required using bronchoscopy samples and/or lung tissue samples to fully evaluate the lower airways microbiome in disease. With newer NGS techniques we can now not only identify the presence of microorganisms, but also evaluate their function and their direct effect on the host. More studies using whole genome metagenomics and in particular RNA metatranscriptomic data are required in respiratory diseases. Finally, to better understand disease progression and the role of the respiratory microbiome more longitudinal studies are key.
Conclusion
The development of culture-independent techniques has led to considerable insights into the respiratory microbiome. A concrete body of evidence describing the respiratory microbiome now exists for most disease states (figure 1), where pheno-endotyping with microbiome data can lead to the identification of high-risk patients. The emergence of multi-omic analysis has revealed interactions between the host and microbiome in some conditions, such as bronchiectasis. These interactions are a potential target for future microbiome research, with the aim of identification of treatable traits in the area of host–microbiome interaction. Further expansion of multi-omic analysis into the development of interventional studies should be a key focus of research going forward with the aim of identifying new treatment targets.
FIGURE 1.
The respiratory microbiome. CMV: cytomegalovirus; EBV: Epstein–Barr virus.
Key points
The respiratory microbiome encompasses bacterial, fungal and viral communities. In health, it is a dynamic structure existing across a microbial gradient from highest density in the upper respiratory tract and lowest in the lower respiratory tract.
In health, the respiratory microbiome is characterised by Bacteroidetes, Proteobacteria and Firmicutes. Anelloviridae form the majority of the lower airway virome and the mycobiome consists of Ceriporia lacerate, Saccharomyces cerevisiae and Penicillium brevicompactum.
The respiratory microbiome is dysbiotic in all chronic respiratory diseases studied, including asthma, COPD, ILD, bronchiectasis, CF and lung cancer.
Dysbiosis can be related to disease severity and may be used to predict patients at risk of clinical deterioration.
Further work is required to identify the functional protentional of the microbiome and potential treatable traits.
Self-assessment questions
1) What is the respiratory microbiome?
2) What do the terms Alpha and Beta diversity refer to?
3) How is the microbiome investigated?
4) How is the respiratory microbiome affected in disease states?
Suggested answers
1) The microbiome is defined as the combined genomes (omic) of all the microorganisms inhabiting a specific environment such as the lung. That is to include the microorganisms and their genes.
2) Alpha diversity describes the mean diversity of a single sample. This can refer to the richness (number of species) or evenness (distribution of species). Beta diversity is a measure of the similarity or dissimilarity of two communities at distinct sites.
3) The microbiome and its functional characteristic are investigated with a combination of 16S rRNA gene sequencing, fungal internal transcribed spacer (ITS) sequencing, whole genome sequencing, RNA metatranscriptomics and metabolomics.
4) The respiratory microbiome is dysbiotic in respiratory disease, with each pathology exhibiting expansions in unique pathological taxa.
Footnotes
Conflict of interest: C.D. Campbell reports receiving grants or contracts from Charitable Infirmary Charitable Trust, and Kieran Taaffe Bursary, outside the submitted work.
Conflict of interest: C. Barnett has nothing to disclose.
Conflict of interest: I. Sulaiman reports receiving grants or contracts from Charitable Infirmary Charitable Trust, and Kieran Taaffe Bursary, outside the submitted work.
References
- 1.Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Lung Cell Mol Physiol 2015; 309: L1047–L1055. doi: 10.1152/ajplung.00279.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson RL, de Koff EM, Bogaert D. Characterising the respiratory microbiome. Eur Respir J 2019; 53: 1801711. doi: 10.1183/13993003.01711-2018 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Singh R, Miller BE, et al. Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: an analysis of the COPDMAP study. Thorax 2018; 73: 331–338. doi: 10.1136/thoraxjnl-2017-210741 [DOI] [PubMed] [Google Scholar]
- 4.Fox GE, Magrum LJ, Balch WE, et al. Classification of methanogenic bacteria by 16S ribosomal RNA characterization. Proc Natl Acad Sci USA 1977; 74: 4537–4541. doi: 10.1073/pnas.74.10.4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woese CR, Fox GE. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci USA 1977; 74: 5088–5090. doi: 10.1073/pnas.74.11.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moffatt MF, Cookson WO. The lung microbiome in health and disease. Clin Med (Lond) 2017; 17: 525–529. doi: 10.7861/clinmedicine.17-6-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravorty S, Helb D, Burday M, et al. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods 2007; 69: 330–339. doi: 10.1016/j.mimet.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med 2013; 5: 63. doi: 10.1186/gm467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome 2015; 3: 31. doi: 10.1186/s40168-015-0094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguiar-Pulido V, Huang W, Suarez-Ulloa V, et al. Metagenomics, metatranscriptomics, and metabolomics approaches for microbiome analysis. Evol Bioinform Online 2016; 12: Suppl. 1, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright GD. Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol 2010; 13: 589–594. doi: 10.1016/j.mib.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 12.Mac Aogáin M, Lau KJ, Cai Z, et al. Metagenomics reveals a core macrolide resistome related to microbiota in chronic respiratory disease. Am J Respir Crit Care Med 2020; 202: 433–447. doi: 10.1164/rccm.201911-2202OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segal LN, Clemente JC, Tsay JC, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 2016; 1: 16031. doi: 10.1038/nmicrobiol.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernini P, Bertini I, Luchinat C, et al. Individual human phenotypes in metabolic space and time. J Proteome Res 2009; 8: 4264–4271. doi: 10.1021/pr900344m [DOI] [PubMed] [Google Scholar]
- 15.Sulaiman I, Wu BG, Li Y, et al. Functional lower airways genomic profiling of the microbiome to capture active microbial metabolism. Eur Respir J 2021; 58: 2003434. doi: 10.1183/13993003.03434-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sulaiman I, Chung M, Angel L, et al. Microbial signatures in the lower airways of mechanically ventilated COVID-19 patients associated with poor clinical outcome. Nat Microbiol 2021; 6: 1245–1258. doi: 10.1038/s41564-021-00961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salter SJ, Cox MJ, Turek EM, et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol 2014; 12: 87. doi: 10.1186/s12915-014-0087-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature 2007; 449: 804–810. doi: 10.1038/nature06244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vivarelli S, Salemi R, Candido S, et al. Gut microbiota and cancer: from pathogenesis to therapy. Cancers (Basel) 2019; 11: 38. doi: 10.3390/cancers11010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol 2021; 19: 55–71. doi: 10.1038/s41579-020-0433-9 [DOI] [PubMed] [Google Scholar]
- 21.Yun Y, Srinivas G, Kuenzel S, et al. Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS One 2014; 9: e113466. doi: 10.1371/journal.pone.0113466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosch AATM, Levin E, van Houten MA, et al. Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine 2016; 9: 336–345. doi: 10.1016/j.ebiom.2016.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007; 357: 1487–1495. doi: 10.1056/NEJMoa052632 [DOI] [PubMed] [Google Scholar]
- 24.Dickson RP, Erb-Downward JR, Freeman CM, et al. Bacterial topography of the healthy human lower respiratory tract. mBio 2017; 8: e02287-16. doi: 10.1128/mBio.02287-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumpitsch C, Koskinen K, Schöpf V, et al. The microbiome of the upper respiratory tract in health and disease. BMC Biol 2019; 17: 87. doi: 10.1186/s12915-019-0703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005; 43: 5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caselli E, Fabbri C, D'Accolti M, et al. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol 2020; 20: 120. doi: 10.1186/s12866-020-01801-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulaiman I, Wu BG, Li Y, et al. Evaluation of the airway microbiome in nontuberculous mycobacteria disease. Eur Respir J 2018; 52: 1800810. doi: 10.1183/13993003.00810-2018 [DOI] [PubMed] [Google Scholar]
- 29.Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One 2010; 5: e8578. doi: 10.1371/journal.pone.0008578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young JC, Chehoud C, Bittinger K, et al. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. Am J Transplant 2015; 15: 200–209. doi: 10.1111/ajt.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui L, Lucht L, Tipton L, et al. Topographic diversity of the respiratory tract mycobiome and alteration in HIV and lung disease. Am J Respir Crit Care Med 2015; 191: 932–942. doi: 10.1164/rccm.201409-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 2011; 127: 372–381.e1-3. doi: 10.1016/j.jaci.2010.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turek EM, Cox MJ, Hunter M, et al. Airway microbial communities, smoking and asthma in a general population sample. EBioMedicine 2021; 71: 103538. doi: 10.1016/j.ebiom.2021.103538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YJ, Nariya S, Harris JM, et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol 2015; 136: 874–884. doi: 10.1016/j.jaci.2015.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goleva E, Jackson LP, Harris JK, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med 2013; 188: 1193–1201. doi: 10.1164/rccm.201304-0775OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durack J, Lynch SV, Nariya S, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol 2017; 140: 63–75. doi: 10.1016/j.jaci.2016.08.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Kumar RK, Hansbro PM, et al. Emerging roles of pulmonary macrophages in driving the development of severe asthma. J Leukoc Biol 2012; 91: 557–569. doi: 10.1189/jlb.0711357 [DOI] [PubMed] [Google Scholar]
- 38.Kloepfer KM, Lee WM, Pappas TE, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 2014; 133: 1301–1307.e3. doi: 10.1016/j.jaci.2014.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green BJ, Wiriyachaiporn S, Grainge C, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 2014; 9: e100645. doi: 10.1371/journal.pone.0100645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor SL, Ivey KL, Gibson PG, et al. Airway abundance of Haemophilus influenzae predicts response to azithromycin in adults with persistent uncontrolled asthma. Eur Respir J 2020; 56: 2000194. doi: 10.1183/13993003.00194-2020 [DOI] [PubMed] [Google Scholar]
- 41.Slater M, Rivett DW, Williams L, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax 2014; 69: 673–674. doi: 10.1136/thoraxjnl-2013-204517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Woerden HC, Gregory C, Brown R, et al. Differences in fungi present in induced sputum samples from asthma patients and non-atopic controls: a community based case control study. BMC Infect Dis 2013; 13: 69. doi: 10.1186/1471-2334-13-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraczek MG, Chishimba L, Niven RM, et al. Corticosteroid treatment is associated with increased filamentous fungal burden in airways. J Allergy Clin Immunol 2018; 142: 407–414. doi: 10.1016/j.jaci.2017.09.039 [DOI] [PubMed] [Google Scholar]
- 44.Rantala A, Jaakkola JJ, Jaakkola MS. Respiratory infections precede adult-onset asthma. PLoS One 2011; 6: e27912. doi: 10.1371/journal.pone.0027912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi S, Sohn K-H, Jung J-W, et al. Lung virome: new potential biomarkers for asthma severity and exacerbation. J Allergy Clin Immunol 2021; 148; 1007–1015.e9. doi: 10.1016/j.jaci.2021.03.017 [DOI] [PubMed] [Google Scholar]
- 46.Taylor SL, Leong LE, Mobegi FM, et al. Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med 2019; 200: 309–317. doi: 10.1164/rccm.201809-1739OC [DOI] [PubMed] [Google Scholar]
- 47.Sze MA, Dimitriu PA, Suzuki M, et al. Host response to the lung microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192: 438–445. doi: 10.1164/rccm.201502-0223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Z, Maschera B, Lea S, et al. Airway host–microbiome interactions in chronic obstructive pulmonary disease. Respir Res 2019; 20: 113. doi: 10.1186/s12931-019-1085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pragman AA, Lyu T, Baller JA, et al. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome 2018; 6: 7. doi: 10.1186/s40168-017-0381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J 2016; 47: 1082–1092. doi: 10.1183/13993003.01406-2015 [DOI] [PubMed] [Google Scholar]
- 51.Dicker AJ, Crichton ML, Pumphrey EG, et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2018; 141: 117–127. doi: 10.1016/j.jaci.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hogg JC, Paré PD, Hackett TL. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev 2017; 97: 529–552. doi: 10.1152/physrev.00025.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011; 365: 1567–1575. doi: 10.1056/NEJMoa1106955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dicker AJ, Huang JTJ, Lonergan M, et al. The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2021; 147: 158–167. doi: 10.1016/j.jaci.2020.02.040 [DOI] [PubMed] [Google Scholar]
- 55.Leitao Filho FS, Takiguchi H, Akata K, et al. Effects of inhaled corticosteroid/long-acting β2–agonist combination on the airway microbiome of patients with chronic obstructive pulmonary disease: a randomized controlled clinical trial (DISARM). Am J Respir Crit Care Med 2021; 204: 1143–1152. doi: 10.1164/rccm.202102-0289OC [DOI] [PubMed] [Google Scholar]
- 56.Singanayagam A, Glanville N, Cuthbertson L, et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci Transl Med 2019; 11: eaav3879. doi: 10.1126/scitranslmed.aav3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Contoli M, Pauletti A, Rossi MR, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J 2017; 50: 1700451. doi: 10.1183/13993003.00451-2017 [DOI] [PubMed] [Google Scholar]
- 58.Pragman AA, Knutson KA, Gould TJ, et al. Chronic obstructive pulmonary disease upper airway microbiota alpha diversity is associated with exacerbation phenotype: a case-control observational study. Respir Res 2019; 20: 114. doi: 10.1186/s12931-019-1080-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayhew D, Devos N, Lambert C, et al. Longitudinal profiling of the lung microbiome in the AERIS study demonstrates repeatability of bacterial and eosinophilic COPD exacerbations. Thorax 2018; 73: 422–430. doi: 10.1136/thoraxjnl-2017-210408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouquet J, Tabor DE, Silver JS, et al. Microbial burden and viral exacerbations in a longitudinal multicenter COPD cohort. Respir Res 2020; 21: 77. doi: 10.1186/s12931-020-01340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tiew PY, Dicker AJ, Keir HR, et al. A high-risk airway mycobiome is associated with frequent exacerbation and mortality in COPD. Eur Respir J 2021; 57: 2002050. doi: 10.1183/13993003.02050-2020 [DOI] [PubMed] [Google Scholar]
- 62.Molyneaux PL, Mallia P, Cox MJ, et al. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 188: 1224–1231. doi: 10.1164/rccm.201302-0341OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan A, Chandra S, Agarwal D, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: a systematic review. Respirology 2010; 15: 536–542. doi: 10.1111/j.1440-1843.2010.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Rijn AL, van Boheemen S, Sidorov I, et al. The respiratory virome and exacerbations in patients with chronic obstructive pulmonary disease. PLoS One 2019; 14: e0223952. doi: 10.1371/journal.pone.0223952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramsheh MY, Haldar K, Bafadhel M, et al. Resistome analyses of sputum from COPD and healthy subjects reveals bacterial load-related prevalence of target genes. Thorax 2020; 75: 8–16. doi: 10.1136/thoraxjnl-2019-213485 [DOI] [PubMed] [Google Scholar]
- 66.Rogers GB, Bruce KD, Martin ML, et al. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med 2014; 2: 988–996. doi: 10.1016/S2213-2600(14)70213-9 [DOI] [PubMed] [Google Scholar]
- 67.Nicotra MB, Rivera M, Dale AM, et al. Clinical, pathophysiologic, and microbiologic characterization of bronchiectasis in an aging cohort. Chest 1995; 108: 955–961. doi: 10.1378/chest.108.4.955 [DOI] [PubMed] [Google Scholar]
- 68.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000; 162: 1277–1284. doi: 10.1164/ajrccm.162.4.9906120 [DOI] [PubMed] [Google Scholar]
- 69.Angrill J, Agustí C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax 2002; 57: 15–19. doi: 10.1136/thorax.57.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.King PT. The pathophysiology of bronchiectasis. Int J Chron Obstruct Pulmon Dis 2009; 4: 411–419. doi: 10.2147/COPD.S6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King PT, Holdsworth SR, Freezer NJ, et al. Microbiologic follow-up study in adult bronchiectasis. Respir Med 2007; 101: 1633–1638. doi: 10.1016/j.rmed.2007.03.009 [DOI] [PubMed] [Google Scholar]
- 72.Tunney MM, Einarsson GG, Wei L, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013; 187: 1118–1126. doi: 10.1164/rccm.201210-1937OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cox MJ, Turek EM, Hennessy C, et al. Longitudinal assessment of sputum microbiome by sequencing of the 16S rRNA gene in non-cystic fibrosis bronchiectasis patients. PLoS One 2017; 12: e0170622. doi: 10.1371/journal.pone.0170622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers GB, Zain NM, Bruce KD, et al. A novel microbiota stratification system predicts future exacerbations in bronchiectasis. Ann Am Thorac Soc 2014; 11: 496–503. doi: 10.1513/AnnalsATS.201310-335OC [DOI] [PubMed] [Google Scholar]
- 75.Cassidy PM, Hedberg K, Saulson A, et al. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009; 49: e124–e129. doi: 10.1086/648443 [DOI] [PubMed] [Google Scholar]
- 76.Mac Aogáin M, Chandrasekaran R, Lim AYH, et al. Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J 2018; 52: 1800766. doi: 10.1183/13993003.00766-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mac Aogáin M, Narayana JK, Tiew PY, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med 2021; 27: 688–699. doi: 10.1038/s41591-021-01289-7 [DOI] [PubMed] [Google Scholar]
- 78.Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017; 49: 1700051. doi: 10.1183/13993003.00051-2017 [DOI] [PubMed] [Google Scholar]
- 79.Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the ‘frequent exacerbator phenotype’ in bronchiectasis. Am J Respir Crit Care Med 2018; 197: 1410–1420. doi: 10.1164/rccm.201711-2202OC [DOI] [PubMed] [Google Scholar]
- 80.Altenburg J, de Graaff CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non–cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013; 309: 1251–1259. doi: 10.1001/jama.2013.1937 [DOI] [PubMed] [Google Scholar]
- 81.Muhlebach MS, Zorn BT, Esther CR, et al. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog 2018; 14: e1006798. doi: 10.1371/journal.ppat.1006798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zemanick ET, Wagner BD, Robertson CE, et al. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur Respir J 2017; 50: 1700832. doi: 10.1183/13993003.00832-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Flight WG, Smith A, Paisey C, et al. Rapid detection of emerging pathogens and loss of microbial diversity associated with severe lung disease in cystic fibrosis. J Clin Microbiol 2015; 53: 2022–2029. doi: 10.1128/JCM.00432-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boutin S, Graeber SY, Weitnauer M, et al. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLoS One 2015; 10: e0116029. doi: 10.1371/journal.pone.0116029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coburn B, Wang PW, Diaz Caballero J, et al. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 2015; 5: 10241. doi: 10.1038/srep10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. doi: 10.1164/rccm.201404-0703OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ronan NJ, Einarsson GG, Twomey M, et al. CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with ivacaftor. Chest 2018; 153: 395–403. doi: 10.1016/j.chest.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 88.Graeber SY, Boutin S, Wielpütz MO, et al. Effects of lumacaftor-ivacaftor on lung clearance index, magnetic resonance imaging, and airway microbiome in Phe508del homozygous patients with cystic fibrosis. Ann Am Thorac Soc 2021; 18: 971–980. doi: 10.1513/AnnalsATS.202008-1054OC [DOI] [PubMed] [Google Scholar]
- 89.Sosinski LM, H CM, Neugebauer KA, et al. A restructuring of microbiome niche space is associated with elexacaftor-tezacaftor-ivacaftor therapy in the cystic fibrosis lung. J Cyst Fibros 2021; in press [ 10.1016/j.jcf.2021.11.003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi B, Dalpke AH, Boutin S. Changes in the cystic fibrosis airway microbiome in response to CFTR modulator therapy. Front Cell Infect Microbiol 2021; 11: 548613. doi: 10.3389/fcimb.2021.548613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med 1994; 331: 637–642. doi: 10.1056/NEJM199409083311003 [DOI] [PubMed] [Google Scholar]
- 92.Tunney M, Klem E, Fodor A, et al. Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. Thorax 2011; 66: 579–584. doi: 10.1136/thx.2010.137281 [DOI] [PubMed] [Google Scholar]
- 93.Carmody LA, Caverly LJ, Foster BK, et al. Fluctuations in airway bacterial communities associated with clinical states and disease stages in cystic fibrosis. PLoS One 2018; 13: e0194060. doi: 10.1371/journal.pone.0194060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Budden KF, Shukla SD, Rehman SF, et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med 2019; 7: 907–920. doi: 10.1016/S2213-2600(18)30510-1 [DOI] [PubMed] [Google Scholar]
- 95.Somayaji R, Goss CH, Khan U, et al. Cystic fibrosis pulmonary exacerbations attributable to respiratory syncytial virus and influenza: a population-based study. Clin Infect Dis 2017; 64: 1760–1767. doi: 10.1093/cid/cix203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amin R, Dupuis A, Aaron SD, et al. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010; 137: 171–176. doi: 10.1378/chest.09-1103 [DOI] [PubMed] [Google Scholar]
- 97.McCaughey G, Gilpin D, Elborn J, et al. The future of antimicrobial therapy in the era of antibiotic resistance in cystic fibrosis pulmonary infection. Expert Rev Respir Med 2013; 7: 385–396. doi: 10.1586/17476348.2013.814411 [DOI] [PubMed] [Google Scholar]
- 98.Chan BK, Stanley G, Modak M, et al. Bacteriophage therapy for infections in CF. Pediatric Pulmonology 2021; 56: S4–S9. [DOI] [PubMed] [Google Scholar]
- 99.Maher T, Wells A, Laurent G. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J 2007; 30: 835–839. doi: 10.1183/09031936.00069307 [DOI] [PubMed] [Google Scholar]
- 100.Spagnolo P, Molyneaux PL, Bernardinello N, et al. The role of the lung's microbiome in the pathogenesis and progression of idiopathic pulmonary fibrosis. Int J Mol Sci 2019; 20: 5618. doi: 10.3390/ijms20225618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Invernizzi R, Barnett J, Rawal B, et al. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur Respir J 2020; 55: 1901519. doi: 10.1183/13993003.01519-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Molyneaux PL, Willis-Owen SAG, Cox MJ, et al. Host-microbial interactions in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 195: 1640–1650. doi: 10.1164/rccm.201607-1408OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 104.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Molyneaux PL, Cox MJ, Wells AU, et al. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res 2017; 18: 29. doi: 10.1186/s12931-017-0511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yin Q, Strong MJ, Zhuang Y, et al. Assessment of viral RNA in idiopathic pulmonary fibrosis using RNA-seq. BMC Pulm Med 2020; 20: 81. 10.1186/s12890-020-1114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 1698–1702. doi: 10.1164/rccm.201010-1752OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poore GD, Kopylova E, Zhu Q, et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020; 579: 567–574. doi: 10.1038/s41586-020-2095-1 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 109.Greathouse KL, White JR, Vargas AJ, et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol 2018; 19: 123. doi: 10.1186/s13059-018-1501-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin C, Lagoudas GK, Zhao C, et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell 2019; 176: 998–1013.e16. doi: 10.1016/j.cell.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsay JJ, Wu BG, Badri MH, et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am J Respir Crit Care Med 2018; 198: 1188–1198. doi: 10.1164/rccm.201710-2118OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsay JJ, Wu BG, Sulaiman I, et al. Lower airway dysbiosis affects lung cancer progression. Cancer Discov 2021; 11: 293–307. doi: 10.1158/2159-8290.CD-20-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. doi: 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 114.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. doi: 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Church DL, Cerutti L, Gürtler A, et al. Performance and application of 16S rRNA gene cycle sequencing for routine identification of bacteria in the clinical microbiology laboratory. Clin Microbiol Rev 2020; 33: e00053-19. doi: 10.1128/CMR.00053-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oude Munnink BB, Worp N, Nieuwenhuijse DF, et al. The next phase of SARS-CoV-2 surveillance: real-time molecular epidemiology. Nat Med 2021; 27: 1518–1524. doi: 10.1038/s41591-021-01472-w [DOI] [PubMed] [Google Scholar]
- 117.Waller KMJ, Leong RW, Paramsothy S. An update on fecal microbiota transplantation for the treatment of gastrointestinal diseases. J Gastroenterol Hepatol 2022; 37: 246–255. doi: 10.1111/jgh.15731 [DOI] [PubMed] [Google Scholar]