Abstract
Aims
Phospholamban (PLN) p.Arg14del mutation carriers are at risk of developing malignant ventricular arrhythmias (VAs) and/or heart failure. Currently, left ventricular ejection fraction (LVEF) plays an important role in risk assessment for VA in these individuals. We aimed to study the incremental prognostic value of left ventricular mechanical dispersion (LVMD) by echocardiographic deformation imaging for prediction of sustained VA in PLN p.Arg14del mutation carriers.
Methods and results
We included 243 PLN p.Arg14del mutation carriers, which were classified into three groups according to the ‘45/45’ rule: (i) normal left ventricular (LV) function, defined as preserved LVEF ≥45% with normal LVMD ≤45 ms (n = 139), (ii) mechanical LV dysfunction, defined as preserved LVEF ≥45% with abnormal LVMD >45 ms (n = 63), and (iii) overt LV dysfunction, defined as reduced LVEF <45% (n = 41). During a median follow-up of 3.3 (interquartile range 1.8–6.0) years, sustained VA occurred in 35 individuals. The negative predictive value of having normal LV function at baseline was 99% [95% confidence interval (CI): 92–100%] for developing sustained VA. The positive predictive value of mechanical LV dysfunction was 20% (95% CI: 15–27%). Mechanical LV dysfunction was an independent predictor of sustained VA in multivariable analysis [hazard ratio adjusted for VA history: 20.48 (95% CI: 2.57–162.84)].
Conclusion
LVMD has incremental prognostic value on top of LVEF in PLN p.Arg14del mutation carriers, particularly in those with preserved LVEF. The ‘45/45’ rule is a practical approach to echocardiographic risk stratification in this challenging group of patients. This approach may also have added value in other diseases where LVEF deterioration is a relative late marker of myocardial dysfunction.
Keywords: phospholamban, genetic cardiomyopathy, ventricular arrhythmia, risk stratification, deformation imaging, mechanical dispersion
Graphical Abstract
Graphical Abstract.
Introduction
Phospholamban (PLN) is a phosphoprotein in the cardiomyocyte that plays a key role in the regulation of intracellular calcium homeostasis.1 Various cardiomyopathy-related mutations have been described in the gene (PLN) that encodes for this protein. One particular mutation is p.Arg14del, which was found in a large subset of arrhythmogenic right ventricular cardiomyopathy (ARVC) patients and dilated cardiomyopathy (DCM) patients in the Netherlands.2,3 Besides the Netherlands, this mutation has also been identified in several other European countries and North America.4–6
Carriers of the PLN p.Arg14del mutation are at risk of developing malignant ventricular arrhythmias (VA) and/or end-stage heart failure.7–9 To prevent sudden cardiac death in these mutation carriers, an implantable cardioverter-defibrillator (ICD) can be implanted. However, due to incomplete penetrance and variable disease expressivity, the risk of developing malignant VA is variable among mutation carriers. To be able to prevent sudden cardiac death, timely identification of mutation carriers who are at higher risk is of paramount importance.
Risk stratification in PLN p.Arg14del mutation carriers is currently guided by the prediction model by van Rijsingen et al.8 This prediction model classifies mutation carriers as being at high risk for malignant VA if they have a history of (non-)sustained VA and/or a left ventricular ejection fraction (LVEF) below 45%.8 The latter is deemed to be a surrogate of myocardial dysfunction. However, since LVEF may be preserved during the early stages of the disease, deterioration of LVEF may be a relatively late sign of myocardial dysfunction and may thus lack sensitivity to predict VA.10,11
Echocardiographic deformation imaging has enabled a more accurate assessment of myocardial function by allowing quantification of intrinsic myocardial mechanics.11–20 One deformation parameter that is frequently used is global longitudinal strain (GLS), which is currently applied in various cardiac diseases for the early detection of global myocardial dysfunction.13 Another parameter that has gained interest is left ventricular mechanical dispersion (LVMD), which has the ability to reveal regional heterogeneity in left ventricular (LV) contraction, even in electrical disorders where structural disease is absent.14–17
Patients with the PLN p.Arg14del mutation exhibit a typical regional pattern of disease.20,21 In a recent study, we demonstrated that LVMD reveals regional contraction heterogeneity in PLN p.Arg14del mutation carriers who are in a very early stage of the disease.20 Since LVMD was previously reported to provide important predictive value for VA in several diseases,15–17 we hypothesized that this parameter has great potential to improve risk prediction models for VA in PLN p.Arg14del mutation carriers.
In this study, we aimed to investigate the incremental prognostic value of LVMD on top of LVEF for the prediction of sustained VA in PLN p.Arg14del mutation carriers. In addition, we aimed to introduce a practical approach to echocardiographic risk stratification in these individuals.
Methods
This observational retrospective longitudinal cohort study was approved by the institutional medical ethics committee of the University Medical Center Utrecht (UMCU) and complied with the principles of the Declaration of Helsinki and the European General Data Protection Regulation.
Study population
Study participants were derived from a national registry that includes all carriers of mutations associated with arrhythmogenic cardiomyopathy, regardless of their phenotype.22 The present cohort was described previously and consists of 301 PLN p.Arg14del mutation carriers (≥18 years) with an echocardiogram of sufficient image quality at the UMCU (n = 62), Amsterdam University Medical Center (n = 83) or University Medical Center Groningen (n = 156) between 2006 and 2019.20 These mutation carriers were both index patients and relatives who underwent genetic cascade screening. Twenty subjects were excluded because they had other relevant cardiovascular disorders (Supplementary data online). The first available echocardiogram of sufficient image quality was selected for this study. Subjects were only included in this study if they had follow-up data available after the date of the echocardiogram.
Baseline cardiac evaluation
We evaluated the history of PLN p.Arg14del mutation carriers until the date of the echocardiogram with regard to the occurrence of (non-)sustained VA and clinical heart failure [according to New York Heart Association (NYHA) functional classification]. Non-sustained VA was defined as a ventricular tachycardia of at least three beats (>100 bpm) lasting not more than 30 s (typically found by Holter recordings). Sustained VA was defined as sudden cardiac arrest/ventricular fibrillation, appropriate ICD intervention or any recorded sustained ventricular tachycardia lasting more than 30 s.
In addition, Holter recordings, 12-lead electrocardiograms and cardiovascular magnetic resonance imaging (CMR) exams were evaluated (details provided in Supplementary data online). CMR exams were evaluated for the presence of late gadolinium enhancement (LGE).
On the basis of all examinations up until the echocardiogram, we evaluated whether subjects fulfilled definite ARVC or DCM diagnosis according to current diagnostic criteria.23,24 In accordance with our previous study, subjects were considered presymptomatic if they had no history of VA, premature ventricular complex (PVC) count <500/24 h, and LVEF ≥45%.20
Baseline echocardiography
All echocardiograms were performed with Vivid 7, E9, and E95 (GE Healthcare, Horten, Norway) as part of routine clinical care. All measurements were performed by one experienced operator (K.T.) who was blinded for clinical data during the measurements. For assessment of inter- and intraobserver agreement, the measurements were blindly repeated by a second operator (R.H.A.C.M.d.B.-B.) and by the first operator in a random sample of 25 subjects.
Conventional echocardiographic measurements were performed in accordance with current recommendations.25 LVEF was measured in all subjects by 2D Simpson’s Biplane method.
Longitudinal strain analysis was performed by 2D speckle tracking with EchoPAC version 203 (GE Healthcare, Horten, Norway). Strain measurements and post-processing methods are described in more detail in the Supplementary data online and in other studies.26 In brief, for measurement of GLS and LVMD, apical four-, two- and three-chamber views were used.27 Images with a frame rate <50/s, foreshortened images or images requiring exclusion of more than one segment were not used for analysis. GLS was defined as the average global peak strain in the three apical views (in %, reported as absolute values). LVMD was defined as the standard deviation of time to peak longitudinal strain (in ms) of the 18 LV segmental deformation curves. A representative example of these measurements is provided in Supplementary data online, Figure S1.
LV function classification: the ‘45/45’ rule
For LVEF, a cut-off value of 45% was used according to the current PLN risk prediction model.8 For LVMD, a cut-off value of 45 ms was used according to a study in desmosomal gene mutation carriers.17 These pre-defined cut-off values were used to categorize mutation carriers into three distinct LV function classes:
Normal LV function: preserved LVEF (≥45%) with normal LVMD (≤45 ms);
Mechanical LV dysfunction: preserved LVEF (≥45%) with abnormal LVMD (>45 ms); and
Overt LV dysfunction: reduced LVEF (<45%), regardless of LVMD.
Follow-up data
Follow-up data after the echocardiogram were derived from an electronic research data platform.22 The primary outcome variable was sustained VA, which was defined as sudden cardiac arrest/ventricular fibrillation, appropriate ICD intervention, or any recorded sustained ventricular tachycardia (>100 bpm) lasting more than 30 s.
Statistical analyses
Data are expressed as means ± standard deviations or medians (interquartile ranges) as appropriate. Continuous variables were compared between two groups using an independent samples t-test or Mann–Whitney U-test. Proportions were compared between groups using a Chi-square test or Fisher’s exact test. Correlations were tested using Pearson’s correlation coefficient. Inter- and intraobserver agreement was studied using intraclass correlation coefficient (ICC) and Bland–Altman analysis (for LVEF and LVMD), or linear weighted kappa analysis (for LV function classification). LVMD was tested in receiver operating characteristic curve (ROC curve) analysis and by Cox proportional hazards analysis for prediction of sustained VA to internally validate the pre-defined cut-off value (using Youden’s index and the optimal Harrell’s C-statistic, respectively). Kaplan–Meier analysis with log-rank testing was used to compare event-free survival between groups. Kaplan–Meier estimates at 3 years follow-up were used to calculate positive predictive values (PPVs) and negative predictive values (NPVs). Cox proportional hazards analyses were used to investigate whether the LV function classes could independently predict sustained VA, taking into account the following pre-defined clinical variables in four separate models: (i) age and sex, (ii) history of (non-)sustained VA, (iii) low QRS voltages and number of leads with T-wave inversion, and (iv) PVC count per 24 h. Due to non-linearity, a log-linear conversion was performed for PVC count. LV function classes were included in the Cox proportional hazards analyses as dummy variable, with the normal LV function class as a reference group. Hazard ratios are provided with 95% confidence intervals (CIs). Two-tailed P-values of <0.05 were considered to indicate statistical significance. In case of multiple testing, P-values were adjusted by Bonferroni correction. Statistical analyses were performed using IBM SPSS Statistics Version 25.0 (IBM Corporation, Armonk, NY, USA) and R Studio Version 1.3.1093 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Baseline clinical and echocardiographic characteristics are presented in Table 1. The final study population consisted of 243 PLN p.Arg14del mutation carriers [median age 41 (30–55) years, 110 (45%) males] who had follow-up data available after the echocardiogram. Fifty-six subjects (23%) were probands and 187 (77%) were relatives. A history of VA was present in 89 subjects (37%), which was sustained in 41 (17%) and non-sustained in 48 (20%). An ICD was present in 56 subjects (23%) at baseline. Thirty subjects (12%) had a history of heart failure, of whom 11 (5%) were previously hospitalized for heart failure. ARVC diagnosis was present in 43 subjects (18%), whereas DCM diagnosis was present in 39 (16%). One hundred and fourteen subjects (47%) were considered presymptomatic.
Table 1.
Baseline characteristics
| Total (n = 243) | Normal LV function (n = 139) | Mechanical LV dysfunction (n = 63) | Overt LV dysfunction (n = 41) | P-value normal function vs. mechanical dysfunction | P-value mechanical dysfunction vs. overt dysfunction | |
|---|---|---|---|---|---|---|
| Age (years) | 41 (30–55) | 34 (24–43) | 53 (41–62) | 51 (40–65) | <0.001a | 1.0 |
| Males | 110 (45) | 60 (43) | 31 (49) | 19 (46) | 0.848 | 1.0 |
| Probands | 56 (23) | 11 (8) | 15 (24) | 30 (73) | 0.004a | <0.001a |
| ARVC diagnosis | 43 (18) | 7 (5) | 18 (29) | 18 (44) | <0.001a | 0.168 |
| DCM diagnosis | 39 (16) | 2 (1) | 3 (5) | 34 (83) | 0.334 | <0.001a |
| VA history | 89 (37) | 25 (18) | 32 (51) | 32 (78) | <0.001a | 0.010a |
| Sustained VA | 41 (17) | 5 (4) | 10 (16) | 26 (63) | ||
| Non-sustained VA | 48 (20) | 20 (14) | 22 (35) | 6 (15) | ||
| HF history (NYHA ≥ II) | 30 (12) | 2 (1) | 3 (5) | 25 (61) | 0.354 | <0.001a |
| Presymptomatic | 114 (47) | 98 (71) | 16 (25) | 0 (0) | <0.001a | <0.001a |
| ICD | 56 (23) | 12 (9) | 14 (22) | 30 (73) | 0.014a | <0.001a |
| Cardiac medication | 99 (41) | 24 (17) | 35 (56) | 40 (98) | <0.001a | <0.001a |
| ACEi/ARB | 59 (24) | 10 (7) | 18 (29) | 31 (76) | ||
| Antiarrhythmic | 29 (12) | 4 (3) | 6 (10) | 19 (46) | ||
| Betablocker | 57 (24) | 12 (9) | 20 (32) | 25 (61) | ||
| Diuretic | 38 (16) | 6 (4) | 7 (11) | 25 (61) | ||
| PVC count (n/24 h)b | 187 (4–1967) | 11 (2–250) | 1096 (334–3637) | 2652 (1111–5336) | <0.001a | 0.016a |
| T-wave inversion in ≥2 leads | 66/229 (29) | 19/131 (15) | 33/61 (54) | 14/37 (38) | <0.001a | 0.292 |
| Low QRS voltages | 79/238 (33) | 24/135 (18) | 25/63 (40) | 30/40 (75) | 0.002a | <0.001a |
| LGE | 43/113 (38) | 21/77 (27) | 17/31 (55) | 5/5 (100) | 0.014a | 0.268 |
| LVEF (%) | 55 (49–60) | 57 (55–61) | 54 (50–59) | 32 (35–39) | <0.001a | <0.001a |
| LVEDV (mL/m2) | 57 (49–66) | 54 (47–60) | 58 (51–62) | 82 (67–109) | 0.302 | <0.001a |
| LVESV (mL/m2) | 25 (20–33) | 22 (19–27) | 25 (21–30) | 58 (45–74) | 0.022a | <0.001a |
| GLS (%) | 19 (16–21) | 20 (19–21) | 18 (16–20) | 9 (7–13) | <0.001a | <0.001a |
| LVMD (ms) | 41 (30–55) | 31 (27–37) | 55 (48–62) | 65 (54–76) | <0.001a | <0.001a |
| RVFAC (%) | 42 (36–49) | 46 (41–51) | 40 (36–47) | 28 (23–34) | 0.002a | <0.001a |
| RVEDA (cm2/m2) | 10 (9–12) | 9 (8–11) | 10 (9–12) | 14 (12–16) | 0.164 | <0.001a |
| RVESA (cm2/m2) | 6 (5–7) | 5 (4–6) | 6 (5–8) | 11 (8–12) | 0.012a | <0.001a |
| RVFWS (%) | 22 (19–26) | 25 (21–28) | 21 (19–24) | 14 (10–16) | 0.002a | <0.001a |
Values are presented as median (IQR) or n (%).
ACEi, ACE-inhibitor; ARB, angiotensin-II receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; DCM, dilated cardiomyopathy; GLS, global longitudinal strain; HF, heart failure; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; LVEDV/LVESV, left ventricular end-diastolic/systolic volume; LVEF, left ventricular ejection fraction; LVMD, left ventricular mechanical dispersion; NYHA, New York Heart Association; PVC, premature ventricular complex; RVEDA/RVESA, right ventricular end-diastolic/systolic area; RVFAC, right ventricular fractional area change; RVFWS, right ventricular free-wall strain; VA, ventricular arrhythmia.
Statistical significant difference (P < 0.05). P-values are adjusted for multiple testing by Bonferroni correction.
Holter recordings were available in 215 subjects (28 with VA during follow-up).
LV function classification
Median baseline LVEF was 55 (49–60)%. Median baseline LVMD was 41 (30–55) ms. There was a significant inverse correlation between LVEF and LVMD [r = −0.668 (95% CI: −0.732 to −0.592), P < 0.001]. Inter- and intraobserver agreement were good for both LVEF [ICC 0.86 (95% CI: 0.71–0.94) and 0.89 (0.76–0.95), respectively] and LVMD [ICC 0.91 (95% CI: 0.83–0.96) and 0.96 (0.92–0.98), respectively]. Bland–Altman plots are presented in Supplementary data online, Figures S2–S5.
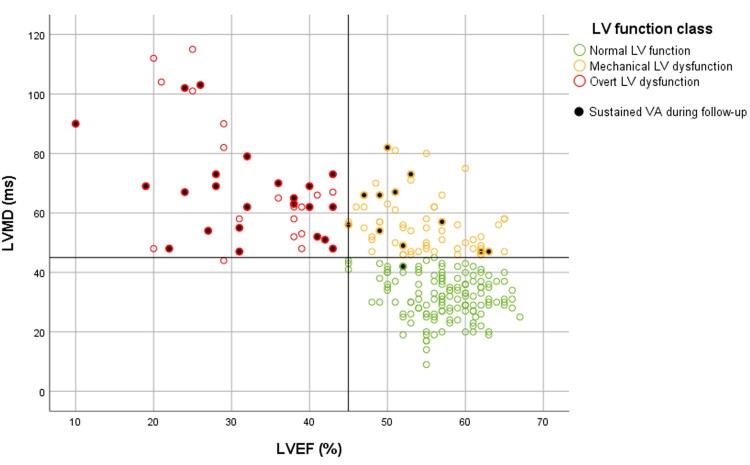
On the basis of the ‘45/45’ rule, mutation carriers were classified as follows: 139 (57%) had normal LV function (i.e. preserved LVEF with normal LVMD), 63 (26%) had mechanical LV dysfunction (i.e. preserved LVEF with abnormal LVMD), and 41 (17%) had overt LV dysfunction (i.e. reduced LVEF) (Figure 1). Only one subject in the overt LV dysfunction class had an LVMD below the cut-off value of 45 ms (i.e. 44 ms). Inter- and intraobserver agreement for LV function classification was good; 24/25 subjects were classified similarly by two independent observers and by the first observer when measurements were blindly repeated [linear weighted kappa 0.93 (95% CI: 0.79–1.00)]. An alternative method for LV function classification, with GLS instead of LVMD, is provided in Supplementary data online, Figure S6.
Figure 1.
Distribution of LVEF and LVMD values in PLN p. Arg14del mutation carriers. The vertical black line represents the cut-off value for LVEF (45%), whereas the horizontal black line represents the cut-off value for LVMD (45 ms). According to these cut-off values, mutation carriers were classified into three subgroups: normal LV function (preserved LVEF and normal LVMD, in green), mechanical LV dysfunction (preserved LVEF and abnormal LVMD, in yellow) and overt LV dysfunction (reduced LVEF, in red). Mutation carriers who experienced sustained VA during follow-up are marked with a black dot. LV, left ventricular; LVEF, LV ejection fraction; LVMD, LV mechanical dispersion; VA, ventricular arrhythmia.
Clinical characteristics of subjects in the three LV function classes are provided in Table 1. CMR was available in 113 subjects (38%). In the normal LV function class, LGE was present in 21/77 subjects (27%). In the mechanical LV dysfunction class, LGE was more common, being present in 17/31 subjects (55%, P = 0.014). In the overt LV dysfunction class, five subjects underwent CMR who all exhibited LGE.
Follow-up
The median follow-up duration was 3.3 (1.8–6.0) years. During follow-up, sustained VA occurred in 35 subjects (14%). This was sudden cardiac arrest/ventricular fibrillation in six, an appropriate ICD intervention in 20 (median cycle length: 280 ms) and a recorded sustained ventricular tachycardia in 9 (median cycle length 281 ms). Characteristics of subjects with sustained VA during follow-up versus subjects without sustained VA are shown in Supplementary data online, Table S1. In order to internally validate the pre-defined cut-off value for LVMD of 45 ms, we performed ROC curve analysis and Cox proportional hazards analysis. Using both methods, the optimal cut-off value for LVMD in the current cohort was found to be 46 ms for prediction of sustained VA [sensitivity 97%, specificity 70% and C-statistic 0.81 (95% CI: 0.77–0.85)].
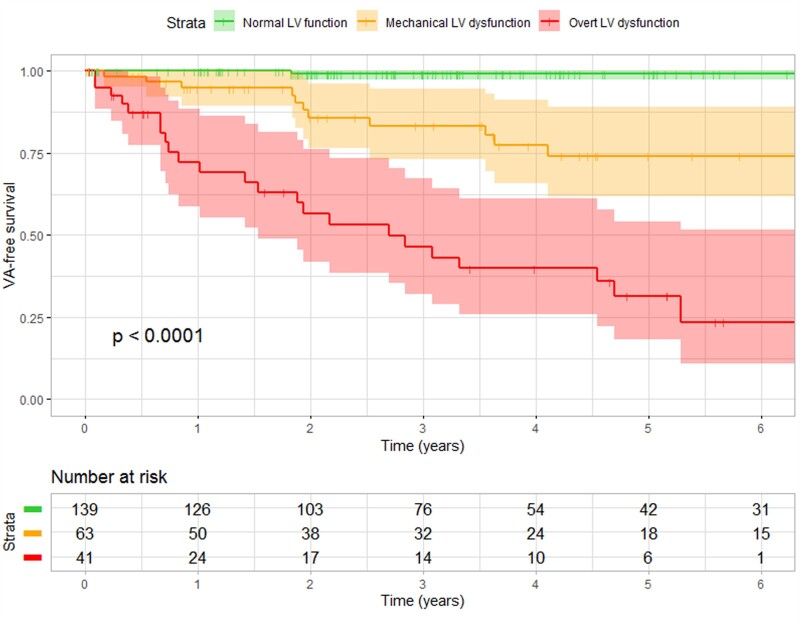
Survival curves for the three LV function classes are shown in Figure 2. Subjects with normal LV function had a favourable prognosis; sustained VA occurred in only one subject (appropriate ICD intervention in a subject with LVMD 42 ms). The NPV of having normal LV function for sustained VA within 3 years was 99% (95% CI: 92–100%). Subjects with mechanical LV dysfunction had a worse prognosis; sustained VA occurred in 11 subjects (18%). The PPV of having mechanical LV dysfunction for sustained VA within 3 years was 20% (95% CI: 15–27%). Subjects with overt LV dysfunction had the worst prognosis; sustained VA occurred in 23 subjects (56%). The PPV of having overt LV dysfunction for sustained VA within 3 years was 56% (95% CI: 43–69%).
Figure 2.
VA-free survival of PLN p.Arg14del mutation carriers in different LV function classes. VA-free survival differed significantly between the three LV function classes. Mutation carriers with a normal function had the most favourable survival, whereas mutation carriers with mechanical dysfunction and overt dysfunction had worse survival. LV, left ventricular; VA, ventricular arrhythmia.
Results of Cox proportional hazards analyses are shown in Table 2. Having mechanical LV dysfunction and having overt LV dysfunction were both significant predictors for sustained VA in all four multivariable models. Additional survival analyses with LVMD as a continuous variable are provided in Supplementary data online, Table S2.
Table 2.
Cox proportional hazards analyses for prediction of sustained VA
| Mechanical LV dysfunction | Overt LV dysfunction | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Univariable (n = 243) | 26.19 (3.38–202.87) | 0.002a | 114.36 (15.43–847.66) | <0.001a |
| Adjusted for age and sex (n = 243) | 29.30 (3.64–235.69) | 0.001a | 132.63 (17.25–1019.75) | <0.001a |
| Adjusted for VA history (n = 243) | 20.48 (2.57–162.84) | 0.004a | 78.31 (10.0–613.47) | <0.001a |
| Adjusted for low QRS voltages and T-wave inversion (n = 238) | 13.79 (1.75–108.54) | 0.013a | 47.89 (6.17–371.84) | <0.001a |
| Adjusted for PVC count/24 h, log-linear conversion (n = 198) | 12.97 (1.50–112.62) | 0.020a | 42.20 (4.76–373.97) | 0.001a |
The LV function classes were included in the Cox proportional hazards analysis as dummy variable, with the normal LV function class as reference group. Due to non-linearity, a log-linear conversion was performed for PVC count.
CI, confidence interval; LV, left ventricular; PVC, premature ventricular complex; VA, ventricular arrhythmia.
P-values <0.05 are considered to indicate statistical significance.
A subgroup analysis was performed in subjects who did not experience a sustained VA before (n = 202, 83%). As demonstrated in Supplementary data online, Figure S7, 134 of these subjects were classified as having normal LV function, 53 were classified as having mechanical LV dysfunction and 15 were classified as having overt dysfunction. After a median follow-up of 3.3 years (1.8–5.6), 14 (7%) developed sustained VA, of whom 8 had mechanical LV dysfunction and 6 had overt dysfunction. None of the subjects with normal LV function at baseline in this subgroup developed sustained VA during follow-up.
Incremental value on top of the current prediction model
The baseline data of the cohort were assessed with the current VA prediction model by van Rijsingen et al.,8 which yielded a C-statistic of 0.77 (95% CI: 0.72–0.82). According to this model, 145 subjects had no risk factors at baseline, 66 subjects had one risk factor and 32 subjects had two risk factors. Among the 35 subjects that developed sustained VA during follow-up, 4 subjects had no risk factors at baseline, 12 had one risk factor, and 19 had two risk factors.
In subjects without risk factors or with one risk factor, LVMD had a high NPV for sustained VA within 3 years (Take home figure). All four subjects without any risk factors who developed sustained VA during follow-up had an increased LVMD at baseline.
In 42 out of 66 subjects with one risk factor, the risk factor was a history of non-sustained VA. Four of these 42 subjects developed sustained VA during follow-up, who all had an increased LVMD at baseline (Supplementary data online, Table S3). None of the subjects with a history of non-sustained VA with normal LVMD at baseline (n = 20) developed a sustained VA during follow-up [median follow-up duration 3.0 (1.3–6.1) years].
Discussion
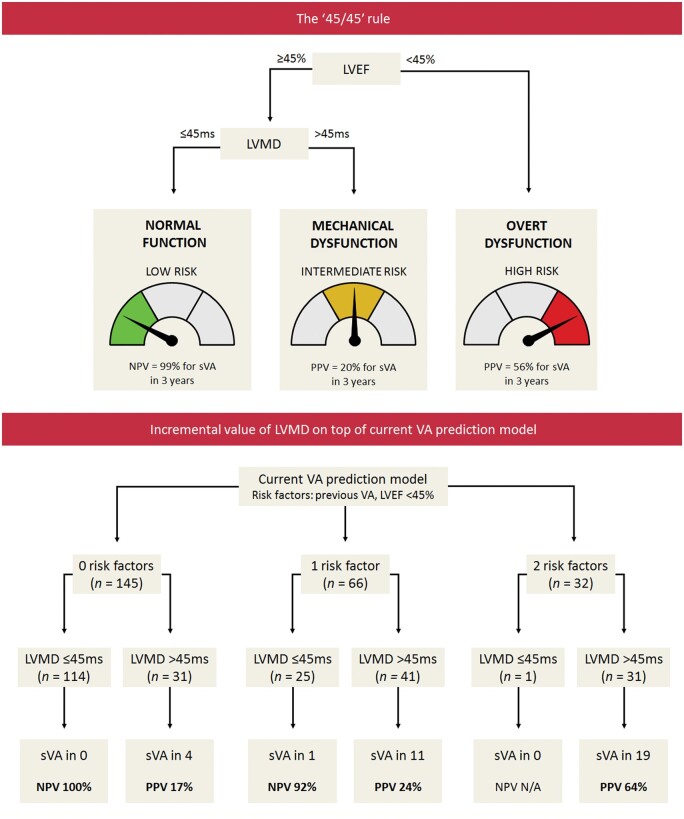
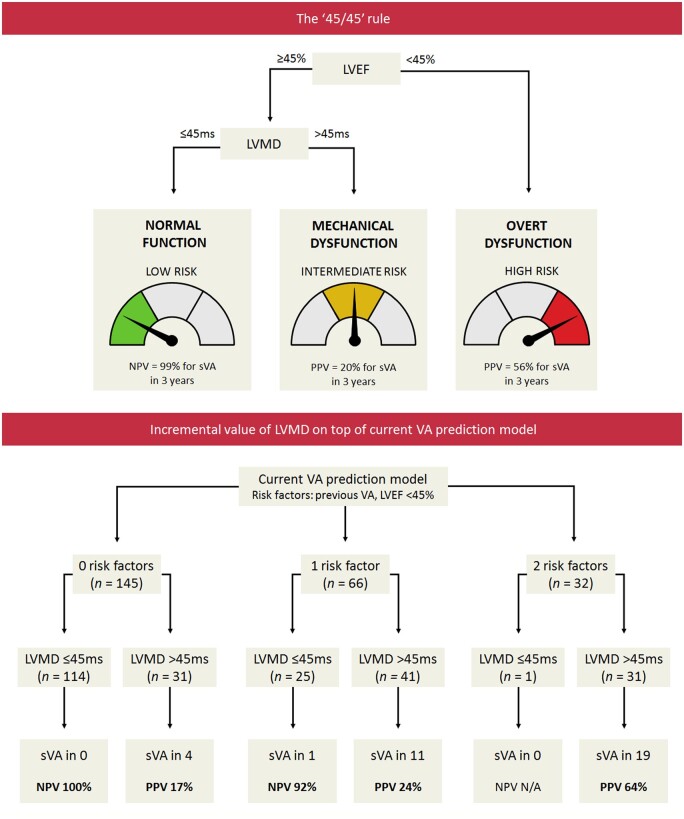
In this study, we aimed to investigate whether measurement of LVMD in PLN p.Arg14del mutation carriers has incremental prognostic value on top of measurement of LVEF, with respect to the development of sustained VA. The key finding of this study is that normal LVMD values exclude the occurrence of sustained VA within 3 years with a high NPV. This is particularly helpful in subjects who have normal LVEF since a normal LVEF does not completely exclude the occurrence of sustained VA. Accordingly, mutation carriers with increased LVMD despite preserved LVEF were found to be at substantially higher risk for sustained VA (Take home figure).
Take home figure.
Optimal echocardiographic assessment of myocardial dysfunction for arrhythmic risk stratification in PLN p.Arg14del mutation carriers. The ‘45/45’ rule can be used to stratify PLN p.Arg14del mutation carriers into three echocardiographic risk categories. Subjects with LVEF < 45% are classified as having overt LV dysfunction, and are considered to be at high risk to develop sustained VA. In subjects with LVEF ≥45%, LVMD should be additionally measured. In case of normal LVMD (i.e. normal LV function), the risk of developing sustained VA is low. However, in case of LVMD >45 ms (i.e. mechanical LV dysfunction), the risk of developing sustained VA is higher, despite normal LVEF. When LVMD is added on top of the current VA risk prediction model, it provides a high NPV, particularly in the lower risk categories. LVEF, left ventricular ejection fraction; LVMD, left ventricular mechanical dispersion; NPV, negative predictive value; PLN, phospholamban; PPV, positive predictive value; (s)VA, (sustained) ventricular arrhythmia.
Risk stratification by LVEF
For arrhythmic risk stratification in PLN p.Arg14del mutation carriers, the prediction model of van Rijsingen et al.8 is currently used, which includes two clinical risk factors: history of (non-)sustained VA and LVEF <45%. Mutation carriers without these risk factors are assumed to be at low risk for malignant VAs, whereas mutation carriers with one or two risk factors are assumed to be at higher risk. This model is currently being used to decide on ICD implantation in these mutation carriers. Therefore, we decided to also use this cut-off value of 45% for LVEF in this study.
The results of this study confirm that LVEF <45% is an important prognostic marker for sustained VA. Mutation carriers with reduced LVEF (in this study classified as overt LV dysfunction) were at significant higher risk of developing sustained VA than mutation carriers with an LVEF ≥45%. More than half of the mutation carriers with reduced LVEF developed sustained VA, and thus the current cut-off value of 45% seems to be appropriate. However, it should be noted that there were also individuals with preserved LVEF who suffered from sustained VA during follow-up, implying that LVEF <45% lacks sensitivity.
LVMD as prognostic marker
Previously, we have shown that LVMD values may be increased in very early stages of disease in PLN p.Arg14del mutation carriers, even before the onset of symptoms.20 On a mechanical level, this is caused by regional contraction duration prolongation (i.e. post-systolic shortening), which contributes to heterogeneity in LV contraction. Since these mechanical alterations were already observed before the presence of overt structural disease by CMR (i.e. myocardial fibrosis), we assumed that increased LVMD values in these subjects reflect an (electro-)mechanical substrate caused by disturbed calcium handling. However, LVMD values were found to be even higher in more advanced stages of the disease, implying that myocardial fibrosis may also contribute to heterogeneity in LV contraction. Because of the typical regional pattern of disease in PLN p.Arg14del mutation carriers, we decided to add LVMD rather than GLS on top of LVEF in this study.
Several previous studies have investigated LVMD as a prognostic marker, in particular for arrhythmic events. A recent meta-analysis by Kawakami et al.16 studied the association between LVMD and VAs in 3198 patients from 12 studies and demonstrated that LVMD has superior prognostic value over LVEF and GLS. However, the results and the proposed cut-off values in the meta-analysis were not directly applicable to the mutation carriers in our study because the study population in the meta-analysis was rather heterogeneous. In addition, we were particularly interested in the performance of LVMD in mutation carriers with a normal LVEF, since risk prediction is often challenging in this group.
In our study, we found LVMD to have considerable incremental value for risk stratification in mutation carriers with a preserved LVEF. Using the ‘45/45’ rule (Take home figure), we could subdivide mutation carriers with a preserved LVEF into two risk groups. Mutation carriers with normal LVMD were found to be at very low risk for sustained VA within 3 years; VA occurred in <1%. On the other hand, mutation carriers with increased LVMD were found to be at unequivocal higher risk for sustained VA, despite a preserved LVEF. By adding LVMD as an extra layer to the risk prediction model that is currently used to guide ICD implantation,8 LVMD could exclude the occurrence of sustained VA with high NPV (particularly in the lower risk categories), and identify subjects who develop sustained VA despite having no risk factors or only one risk factor such as non-sustained VA (Take home figure). Thus, LVMD has great potential to improve future risk prediction models, which may possibly lead to more patient-tailored therapeutic interventions. This does not only hold true for PLN mutation carriers but probably also for other patient populations in which deterioration of LVEF is a relative late marker of regional myocardial dysfunction.
Clinical implications
With regard to risk stratification in PLN p.Arg14del mutation carriers, echocardiograms are currently only assessed for LVEF.8 On the basis of our results, we recommend quantification of LVMD in PLN p.Arg14del mutation carriers, at least in those who have a normal LVEF. Importantly, the prognostic effect of LVMD was not only independent of LVEF but also of VA history and other clinical variables. We propose that mutation carriers with an increased LVMD should have stricter follow-up regimens than mutation carriers with normal LVMD values. Although it seems that patients with increased LVMD may benefit from early ICD implantation or anti-arrhythmic medication to prevent potentially life-threatening VA, we cannot provide direct LVMD-based therapeutic recommendations on the basis of these retrospective observational data. This remains to be investigated in future prospective interventional studies.
In this study, we focused on the prognostic value of LVMD because several studies showed this parameter to be promising for VA prediction16,17 and because of the typical regional pattern of functional and structural abnormalities in PLN mutation carriers.20,21 Based on this study, GLS does not seem to provide additional prognostic information on top of LVEF and LVMD within this specific patient population. However, other deformation imaging parameters such as right ventricular strain should not be neglected on the basis of this study. These parameters should also be investigated in future studies.
Limitations
In this study, we investigated an endpoint of sustained VA, which also included appropriate ICD interventions and sustained ventricular tachycardia. However, it should be noted that these arrhythmias are not always life-threatening and may therefore not necessarily require ICD therapy.
We classified the LV function using categorical data because this approach is most suitable for a practical clinical algorithm. However, valuable information may be lost when using categorical data instead of continuous variables. In future studies, LVMD may potentially be used as a continuous variable to allow fine-tuning of risk prediction. This would translate best into a multi-modality risk model where the amount of LVMD rather than a cut-off value provides additional information on arrhythmogenic risk.
Due to the relatively small number of events in this study, we lacked power to include all variables of interest in one multivariable model. To create a complete multivariable risk prediction model, a larger number of events is needed.
The majority of the subjects who developed VA in this study already had a history of sustained or non-sustained VA. Still, the presence of mechanical LV dysfunction could predict the occurrence of sustained VA when it was corrected for VA history in multivariable analysis. In addition, all subjects who developed the outcome without having a history of sustained VA had increased LVMD at baseline. Nevertheless, longer follow-up intervals would be desirable in future studies, to increase the number of events in mutation carriers who did not have a previous arrhythmic event.
Conclusion
LVMD has incremental prognostic value in PLN p.Arg14del mutation carriers on top of LVEF, particularly in those with preserved LVEF. Normal LVMD values identify mutation carriers who are at very low risk of developing sustained VA within 3 years. Mutation carriers with increased LVMD are at higher risk of developing sustained VA, even in case of preserved LVEF. The proposed ‘45/45’ rule is a practical approach to echocardiographic risk stratification in these mutation carriers and seems to be of additional value on top of the current method for risk assessment. Measurement of LVMD should be investigated in future multi-modality risk prediction models and prospective interventional studies. Moreover, the ‘45/45’ rule should also be investigated in other patient populations in which LVEF is a relative late marker of myocardial dysfunction.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
This work was supported by the PLN Genetic Heart Disease Foundation, Netherlands Heart Institute, the Dutch Heart Foundation (CVON2015-12 eDETECT and CVON2018-30 PREDICT2) and the Leducq Foundation (CURE-PLaN coordinated by P.A.D.). W.P.T.R. is supported by the Dutch Heart Foundation (CVON PREDICT Young Talent Program) and the Leducq Foundation (CURE-PLaN Postdoctoral Fellowship). F.W.A. is supported by UCL Hospitals NIHR Biomedical Research Centre.
Supplementary Material
Contributor Information
Karim Taha, Department of Cardiology, University Medical Center Utrecht, Utrecht, The, Netherlands; Netherlands Heart Institute, Utrecht, The, Netherlands.
Tom E Verstraelen, Heart Center, Department of Cardiology, Amsterdam University Medical Center, Location Academic Medical Center, Amsterdam, The, Netherlands.
Remco de Brouwer, Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The, Netherlands.
Rianne H A C M de Bruin-Bon, Heart Center, Department of Cardiology, Amsterdam University Medical Center, Location Academic Medical Center, Amsterdam, The, Netherlands.
Maarten J Cramer, Department of Cardiology, University Medical Center Utrecht, Utrecht, The, Netherlands.
Wouter P Te Rijdt, Netherlands Heart Institute, Utrecht, The, Netherlands; Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The, Netherlands; Department of Genetics, University Medical Center Groningen, University of Groningen, Groningen, The, Netherlands.
Berto J Bouma, Heart Center, Department of Cardiology, Amsterdam University Medical Center, Location Academic Medical Center, Amsterdam, The, Netherlands.
Rudolf A de Boer, Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The, Netherlands.
Pieter A Doevendans, Department of Cardiology, University Medical Center Utrecht, Utrecht, The, Netherlands; Netherlands Heart Institute, Utrecht, The, Netherlands; Central Military Hospital, Utrecht, The, Netherlands.
Folkert W Asselbergs, Department of Cardiology, University Medical Center Utrecht, Utrecht, The, Netherlands; Institute of Cardiovascular Science, Faculty of Population Health Sciences, University College London, London, UK; Health Data Research UK and Institute of Health Informatics, University College London, London, UK.
Arthur A M Wilde, Heart Center, Department of Cardiology, Amsterdam University Medical Center, Location Academic Medical Center, Amsterdam, The, Netherlands.
Maarten P van den Berg, Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The, Netherlands.
Arco J Teske, Department of Cardiology, University Medical Center Utrecht, Utrecht, The, Netherlands.
References
- 1. MacLennan DH, Kranias EG.. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 2003;4:566–77. [DOI] [PubMed] [Google Scholar]
- 2. van der Zwaag PA, van Rijsingen IAW, Asimaki A, Jongbloed JDH, van Veldhuisen DJ, Wiesfeld ACPet al. . Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail 2012;14:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Zwaag PA, van Rijsingen IAW, de Ruiter R, Nannenberg EA, Groeneweg JA, Post JGet al. . Recurrent and founder mutations in the Netherlands-Phospholamban p.Arg14del mutation causes arrhythmogenic cardiomyopathy. Neth Heart J 2013;21:286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RAet al. . A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci USA 2006;103:1388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posch MG, Perrot A, Geier C, Boldt L-H, Schmidt G, Lehmkuhl HBet al. . Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Heart Rhythm 2009;6:480–6. [DOI] [PubMed] [Google Scholar]
- 6. DeWitt MM, MacLeod HM, Soliven B, McNally EM.. Phospholamban R14 deletion results in late-onset, mild, hereditary dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1396–8. [DOI] [PubMed] [Google Scholar]
- 7. Hof IE, van der Heijden JF, Kranias EG, Sanoudou D, de Boer RA, van Tintelen JPet al. . Prevalence and cardiac phenotype of patients with a phospholamban mutation. Neth Heart J 2019;27:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Rijsingen IAW, van der Zwaag PA, Groeneweg JA, Nannenberg EA, Jongbloed JDH, Zwinderman AHet al. . Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet 2014;7:455–65. [DOI] [PubMed] [Google Scholar]
- 9. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JDHet al. . Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–55. [DOI] [PubMed] [Google Scholar]
- 10. Cikes M, Solomon SD.. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J 2016;37:1642–50. [DOI] [PubMed] [Google Scholar]
- 11. Donal E, Delgado V, Bucciarelli-Ducci C, Galli E, Haugaa KH, Charron P, et al. . Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2019;20:1075–93. [DOI] [PubMed] [Google Scholar]
- 12. Amzulescu MS, De Craene M, Langet H, Pasquet A, Vancraeynest D, Pouleur ACet al. . Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging 2019;20:605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Potter E, Marwick TH.. Assessment of left ventricular function by echocardiography. JACC Cardiovasc Imaging 2018;11:260–74. [DOI] [PubMed] [Google Scholar]
- 14. Verdugo-Marchese M, Coiro S, Selton-Suty C, Kobayashi M, Bozec E, Lamiral Zet al. . Left ventricular myocardial deformation pattern, mechanical dispersion, and their relation with electrocardiogram markers in the large population-based STANISLAS cohort: insights into electromechanical coupling. Eur Heart J Cardiovasc Imaging 2020;21:1237–45. [DOI] [PubMed] [Google Scholar]
- 15. Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP.. Left ventricular mechanical dispersion by tissue Doppler imaging: a novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J 2008;30:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawakami H, Nerlekar N, Haugaa KH, Edvardsen T, Marwick TH.. Prediction of ventricular arrhythmias with left ventricular mechanical dispersion. JACC Cardiovasc Imaging 2020;13:562–72. [DOI] [PubMed] [Google Scholar]
- 17. Lie ØH, Rootwelt-Norberg C, Dejgaard LA, Leren IS, Stokke MK, Edvardsen Tet al. . Prediction of life-threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: a primary prevention cohort study. JACC Cardiovasc Imaging 2018;11:1377–86. [DOI] [PubMed] [Google Scholar]
- 18. Mast TP, Taha K, Cramer MJ, Lumens J, van der Heijden JF, Bouma BJet al. . The prognostic value of right ventricular deformation imaging in early arrhythmogenic right ventricular cardiomyopathy. JACC Cardiovasc Imaging 2019;12:446–55. [DOI] [PubMed] [Google Scholar]
- 19. Taha K, Mast TP, Cramer MJ, van der Heijden JF, Asselbergs FW, Doevendans PAet al. . Evaluation of disease progression in arrhythmogenic cardiomyopathy: the change of echocardiographic deformation characteristics over time. JACC Cardiovasc Imaging 2020;13:631–4. [DOI] [PubMed] [Google Scholar]
- 20. Taha K, Wp Te R, Verstraelen TE, Cramer MJ, de Boer RA, de Bruin-Bon Ret al. . Early mechanical alterations in phospholamban mutation carriers: identifying subclinical disease before onset of symptoms. JACC Cardiovasc Imaging 2021;14:885–896. [DOI] [PubMed] [Google Scholar]
- 21. Te Rijdt WP, Sande JN, Gorter TM, van der Zwaag PA, van Rijsingen IA, Boekholdt SMet al. . Myocardial fibrosis as an early feature in phospholamban p.Arg14del mutation carriers: phenotypic insights from cardiovascular magnetic resonance imaging. Eur Heart J Cardiovasc Imaging 2019;20:92–100. [DOI] [PubMed] [Google Scholar]
- 22. Bosman LP, Verstraelen TE, van Lint FHM, Cox MGPJ, Groeneweg JA, Mast TP, et al. . The Netherlands Arrhythmogenic Cardiomyopathy Registry: design and status update. Neth Heart J 2019;27:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DAet al. . Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm Met al. . Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016;37:1850–8. [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–70. [DOI] [PubMed] [Google Scholar]
- 26. Teske AJ, De Boeck BWL, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJM.. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound 2007;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voigt J-U, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann Ret al. . Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.