Abstract
Immunoglobulin A (IgA) deficiency occurs more frequently in patients with celiac disease (CD) than in the general population and can lead to false-negative results in the best serologic test for CD, endomysial IgA (EMA). To evaluate the impact of IgA deficiency on serologic detection of CD in a reference laboratory setting, IgA levels were measured in 510 consecutive serum specimens submitted for testing for EMA; 510 consecutive serum specimens submitted for Helicobacter pylori IgG testing served as a gastrointestinal symptom control group. The frequency of IgA deficiency was significantly higher among the specimens submitted for testing for EMA (5.1%) than among the specimens from the symptom control group (1.4%). Three subsets of sera from the group of specimens submitted for testing for EMA were then tested by additional serologic assays for CD; these subsets were EMA-positive sera (n = 25), EMA-negative, IgA-deficient sera (n = 26), and control sera (from EMA-negative, IgA-nondeficient patients age matched to IgA-deficient patients; n = 26). The proportions of EMA-positive sera positive by other assays for CD were 92% for transglutaminase IgA (TG-IgA), 80% for gliadin IgA, 84% for gliadin IgG, 60% for endomysial IgG (EMG), and 32% for transglutaminase IgG (TG-IgG). Very low proportions (0 to 8%) of IgA-deficient sera and control sera were positive for TG-IgA, gliadin IgA, EMG, and TG-IgG. Eight of 26 (31%) IgA-deficient serum samples were positive for gliadin IgG, whereas 3 of 26 (12%) control serum samples were positive for gliadin IgG, but this difference was not statistically significant. Physicians supplied clinical data for 18 of 26 patients with IgA deficiency; only 4 patients had undergone small-bowel biopsy, and 0 of 4 patients showed villous atrophy. These findings show that IgA deficiency is found more frequently among sera submitted for testing for EMA in a reference laboratory setting, but there was no clear-cut serologic or clinical evidence of CD in EMA-negative, IgA-deficient patients.
Celiac disease (CD) reflects intolerance to gliadin (a component of gluten) from wheat and related proteins from rye and barley. Children typically present with failure to thrive, diarrhea, and malabsorption; adults, in contrast, may exhibit a vast array of symptoms, including dermatitis herpetiformis, recurrent abdominal pain, and anemia (6, 17, 18). CD is histologically identified by detection of villous atrophy in small-bowel biopsy specimens (6, 17). Patients with CD are successfully treated by removing gluten from their diets (12, 17).
Most CD patients produce immunoglobulin G (IgG) and IgA antibodies that recognize gliadin (7, 23, 24). Via processes only beginning to be understood (12, 19, 25, 28), gliadin exposure in CD patients also leads to the production of autoantibodies that recognize endomysium, an intermyofibril substance found in primate smooth-muscle connective tissue (8). Recent studies have identified transglutaminase as the major autoantigenic component of endomysium (9).
Although biopsy remains the “gold standard” for the diagnosis of CD, serologic testing is valuable as a screen for CD. The single best serologic test for CD is endomysial IgA (EMA) detection, on the basis of its high (>95%) sensitivity and specificity (6, 29). Preliminary studies that have measured transglutaminase IgA (TG-IgA) demonstrate sensitivity and specificity values approaching those of tests for EMA, but kits cleared by the U.S. Food and Drug Administration have only recently become available (1, 10, 26). For reasons that remain unclear, tests for neither endomysial IgG (EMG) nor transglutaminase IgG (TG-IgG) are as sensitive or specific as tests for EMA for the detection of CD (1, 2, 11, 26). Tests for gliadin IgG and gliadin IgA offer good sensitivity and specificity, respectively, but neither assay is as efficient as an assay for EMA (6, 7, 17, 24).
Although EMA measurement is considered the best serologic test for CD, the assay does have limitations. In children less than 2 years old, EMA detection is less than 90% sensitive (27, 29). Another limitation to the use of EMA detection for serologic detection of CD is the increased frequency of IgA deficiency in patients with CD. Approximately 3% of CD patients exhibit IgA deficiency, whereas only 0.3% of the general population has IgA deficiency (5, 13). Thus, sera from CD patients who are also IgA deficient may give a false-negative result for EMA (1, 2, 5, 22, 27). This limitation has led some investigators to recommend that IgA levels be measured in all sera screened for CD by use of the assay for EMA (5). Although other serologic tests for CD may be positive for CD patients with IgA deficiency, it is not clear which assay or combination of assays is the most diagnostically useful.
In a reference laboratory setting, many sera are submitted for testing for EMA only. Since the IgA levels in these sera are not routinely measured, the potential number of false-negative results for EMA due to IgA deficiency is unknown. We therefore assessed the frequency of IgA deficiency in 510 serum specimens submitted for testing for EMA and then measured the levels of other CD-associated antibodies in the 26 IgA-deficient serum specimens identified. Lastly, to assess the diagnostic value of these additional serologic test results, we sought clinical information for the patients with IgA deficiency.
MATERIALS AND METHODS
Patient sera.
All sera submitted for testing for EMA over a 3-month period (n = 510) were selected for study. An equal number of consecutive serum specimens submitted for Helicobacter pylori IgG testing served as a gastrointestinal symptom control group. IgA levels were measured within 2 days after completion of testing for EMA or H. pylori IgG; sera were then frozen at −85°C until the end of the accrual period. Three subsets of sera from the group submitted for testing for EMA were then thawed and tested by additional serologic assays for CD; these three subsets were (i) EMA-positive sera (n = 25), (ii) EMA-negative, IgA-deficient sera (n = 26), and (iii) EMA-negative, IgA-nondeficient sera (n = 26) from patients age matched to the EMA-negative, IgA-deficient patients (control sera).
IgA measurement.
Serum IgA levels were measured by nephelometry; all reagents and instrumentation were purchased from Beckman Coulter, Inc. (Fullerton, Calif.). IgA levels were considered deficient when they were less than the lower limit of established age-dependent reference intervals. These limits were 70 mg/dl for teenagers and adults, 23 mg/dl for children 3 to 12 years old, and 17 mg/dl for children <3 years old.
IgG measurement.
IgG levels were measured in EMA-negative, IgA-deficient sera by nephelometry (Beckman Coulter, Inc.). IgG levels were considered deficient when they were less than the lower limit of established age-dependent reference intervals. These limits were 680 mg/dl for teenagers and adults and 353 mg/dl for children 2 to 12 years old.
Testing for endomysial antibodies.
EMA and EMG antibodies were detected by indirect immunofluorescence with monkey esophagus as the substrate (The Binding Site, Inc., San Diego, Calif.). EMA was detected by using fluorescein-tagged sheep anti-human IgA, and EMG was detected by using fluorescein-tagged, monkey-absorbed, sheep anti-human IgG (The Binding Site). Sera were screened at a 1:5 dilution as described previously (2, 14); titers were then determined for positive sera.
TG-IgA, TG-IgG, gliadin IgA, and gliadin IgG testing.
TG-IgA and gliadin IgA levels in selected sera were measured with commercially available enzyme-linked immunosorbent assay (ELISA) kits supplied by INOVA Diagnostics (San Diego, Calif.); for both assays, values of ≥20 units were considered positive. TG-IgG was measured by using the TG-IgA ELISA kit in a modified procedure in which IgG-specific conjugate (INOVA) was substituted for the kit-supplied IgA-specific conjugate. On the basis of a preliminary evaluation of sera from 110 healthy adults, optical density (OD) values of 0.20 or greater by the TG-IgG ELISA were considered positive. Gliadin IgG was measured by an in-house ELISA essentially identical to a previously described ELISA (21, 24); values of ≥15 units were considered positive.
Statistics.
Differences among proportions were evaluated by two-by-two contingency table (chi-square) analysis with StatMost software (DataMost Corp., Salt Lake City, Utah). Significance was defined as a P value of <0.01.
RESULTS
Frequency of IgA deficiency.
The age distribution of patients whose sera were evaluated for IgA deficiency and the frequency of IgA deficiency observed are shown in Table 1. The number of individuals per age group were similar in the group tested for EMA and the symptom control group except for children <3 years of age; in this age group, the proportion in the group tested for EMA (56 of 510; 11%) versus the symptom control group was significantly higher (3 of 510; 0.6%). Evaluation of the proportion of sera that exhibited IgA deficiency within each age group showed no significant differences between the group tested for EMA and the symptom control group. However, when all sera were considered, the frequency of IgA deficiency was significantly higher in the group tested for EMA (26 of 510; 5.1%) than in the symptom control group (7 of 510; 1.4%). The range of observed IgA levels was similar in the group tested for EMA (<7 to 1,100 mg/dl) and the symptom control group (<7 to 1,141 mg/dl).
TABLE 1.
Patient age distribution and frequency of IgA deficiency
| Age (yr) | No. of patients
|
|||
|---|---|---|---|---|
| EMA testing group
|
Symptom control group
|
|||
| Total | IgA deficient | Total | IgA deficient | |
| <3 | 56a | 1 | 3 | 0 |
| 3–12 | 94 | 4 | 83 | 1 |
| 13–20 | 56 | 10 | 44 | 3 |
| 21–40 | 73 | 1 | 90 | 1 |
| 41–60 | 113 | 6 | 135 | 1 |
| >60 | 99 | 4 | 119 | 1 |
| Unknown | 19 | 0 | 36 | 0 |
| Total | 510 | 26a | 510 | 7 |
Significantly different from the comparable symptom control group value.
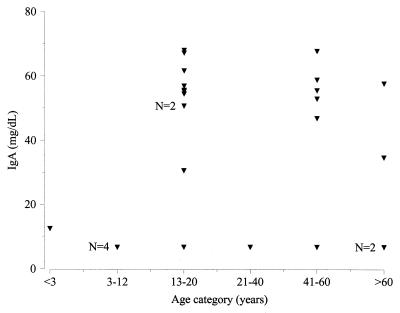
In Fig. 1, IgA values are plotted as a function of age category for the 26 IgA-deficient serum samples from the group tested for EMA. Notably, nearly all (13 of 14) serum samples with marginal IgA deficiency (45 to 70 mg/dl) clustered within either the 13- to 20-year-old age group or the 41- to 60-year-old age group. In contrast, sera with more marked IgA deficiency (<35 mg/dl) were distributed across all age categories.
FIG. 1.
IgA levels as a function of patient age category for IgA-deficient sera. Unless otherwise noted, each triangle represents the result for a single serum specimen.
Relationship of IgA deficiency to results of testing for EMA.
Of the 510 serum samples in the group tested for EMA, 25 were EMA positive, with titers ranging from 1:5 to ≥1:1,280. The EMA-positive subset and the IgA-deficient subset were mutually exclusive; thus, all 26 IgA-deficient serum samples were EMA negative, and all 25 EMA-positive serum samples had normal IgA levels.
Further serologic characterization of subsets of sera from group tested for EMA.
To evaluate alternative testing strategies for the identification of CD patients with IgA deficiency, we next tested EMA-negative, IgA-deficient sera and control sera in other serologic assays for CD. We also took the opportunity to evaluate EMA-positive sera from the group tested for EMA by these other serologic assays for CD.
As shown in Table 2, most EMA-positive sera were positive by assays for TG-IgA, gliadin IgA, and gliadin IgG, with many having very high levels. Similarly, the majority of EMA-positive sera were also positive for EMG. TG-IgG was detected in 8 of 25 (32%) EMA-positive serum samples, and 7 of these 8 TG-IgG-positive serum samples were also positive for EMG; 8 serum samples in the EMA-positive group were EMG positive (titer range, 1:5 to 1:160) but TG-IgG negative.
TABLE 2.
Frequency of positive results and range of positive values in other serologic tests for CD for three subsets of sera from the group tested for EMA
| Assay criteriona | EMA-positive sera (n = 25)
|
IgA-deficient sera (n = 26)
|
Control sera (n = 26)
|
|||
|---|---|---|---|---|---|---|
| No. (%) of serum samples | Range | No. (%) of serum samples | Range | No. (%) of serum samples | Range | |
| TG-IgA, ≥20 | 23 (92) | 20–>200 | 1 (4) | 21 | 1 (4) | 35 |
| Gliadin IgA, ≥20 | 20 (80) | 25–>200 | 0 (0) | 1 (4) | 35 | |
| Gliadin IgG, ≥15 | 21 (84) | 17–>140 | 8 (31) | 18–57 | 3 (12) | 19–26 |
| EMG, ≥1:5 | 15 (60) | 1:5–≥1:1,280 | 2 (8) | 1:5–1:20 | 0 (0) | |
| TG-IgG, ≥0.20 | 8 (32) | 0.22–2.59 | 1 (4) | 1.07 | 0 (0) | |
Units of measure: TG-IgA, units; gliadin IgA, units; gliadin IgG, units; EMG, titer; TG-IgG, OD.
TG-IgA was detected in only 1 of 26 IgA-deficient serum samples (Table 2); the TG-IgA level in this serum sample was only 2 units over the cutoff, and the IgA level was only marginally deficient (67.9 mg/dl). None of the 26 IgA-deficient serum samples were positive for gliadin IgA. One of 26 serum samples in the control group was TG-IgA positive, and 1 of 26 was gliadin IgA positive (Table 2); the single samples with positive values by these assays were only moderately positive (35 units). The TG-IgA-positive result and the gliadin IgA-positive result in the control group were found in different serum samples.
Eight of 26 IgA-deficient serum samples were positive for gliadin IgG (Table 2; Table 3); 7 of 8 gliadin IgG-positive serum samples were only moderately positive, with levels between 15 and 40 units. Three of 26 control serum samples were positive for gliadin IgG (Table 2), and all 3 samples had levels between 15 and 30 units. When comparing the IgA-deficient group and the control group, the difference in the proportion of the group that was gliadin IgG positive (8 of 26 versus 3 of 26, respectively) did not reach statistical significance (P = 0.17).
TABLE 3.
Laboratory and clinical findings for patients with IgA deficiencya
| Patient no. | Age (yr) | IgA level (mg/dl) | IgG level (mg/dl) | Gliadin IgG level (units) | EMG titer | TG-IgG OD | Biopsy findings | Clinical findings |
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | <7 | 782 | 22 | <1:5 | 0.11 | Not done | Asymptomatic; the mother has CD |
| 2 | 4 | <7 | 1,140 | 26 | <1:5 | 0.02 | Not done | Upper respiratory infections |
| 3 | 7 | <7 | 1,440 | 2 | <1:5 | 0.03 | Not done | Type 1 diabetes |
| 4 | 7 | <7 | 1,680 | 18 | <1:5 | 0.05 | NVAb | Inflammatory bowel disease |
| 5 | 13 | <7 | 1,320 | 10 | 1:20 | 1.07 | Unknown | Unknown |
| 6 | 32 | <7 | 1,432 | 1 | 1:5 | 0.05 | Not done | Chronic bloating, gas |
| 7 | 45 | <7 | 1,810 | 13 | <1:5 | 0.04 | Unknown | Unknown |
| 8 | 61 | <7 | 346 | 1 | <1:5 | 0.01 | NVA | Diarrhea, malabsorption |
| 9 | 67 | <7 | 2,110 | 57 | <1:5 | 0.12 | Unknown | Unknown |
| 10 | 2 | 12.5 | 618 | 19 | <1:5 | 0.03 | Not done | Chronic diarrhea |
| 11 | 15 | 30.5 | 691 | 37 | <1:5 | 0.03 | Not done | Irritable bowel syndrome |
| 12 | 77 | 34.5 | 1,500 | 1 | <1:5 | 0.03 | NVA | Diarrhea, colitis |
| 13 | 47 | 46.7 | 411 | 1 | <1:5 | 0.05 | Not done | Diarrhea, anemia |
| 14 | 16 | 50.5 | 964 | 5 | <1:5 | 0.02 | Not done | Diarrhea, weight loss |
| 15 | 56 | 52.7 | 1,170 | 8 | <1:5 | 0.01 | Not done | Decreased vitamin D levels |
| 16 | 19 | 54.3 | 560 | 13 | <1:5 | 0.02 | Not done | Irritable bowel syndrome |
| 17 | 15 | 55.1 | 1,390 | 3 | <1:5 | 0.18 | Unknown | Unknown |
| 18 | 48 | 55.3 | 854 | 7 | <1:5 | 0.03 | Not done | Family history of CD |
| 19 | 15 | 55.4 | 770 | 6 | <1:5 | 0.13 | Unknown | Unknown |
| 20 | 13 | 56.7 | 905 | 30 | <1:5 | 0.04 | Not done | Diarrhea, abdominal pain |
| 21 | 68 | 57.4 | 690 | 11 | <1:5 | 0.04 | See note*c | Known CD on gluten-free diet; low urine calcium levels |
| 22 | 47 | 58.6 | 2,230 | 3 | <1:5 | 0.05 | Unknown | Unknown |
| 23 | 14 | 61.5 | 800 | 11 | <1:5 | 0.03 | Not done | Type I diabetes |
| 24 | 13 | 67.1 | 609 | 32 | <1:5 | 0.03 | NVA | Poor weight gain |
| 25 | 47 | 67.6 | 459 | 1 | <1:5 | 0.01 | Unknown | Unknown |
| 26 | 13 | 67.9 | 1,050 | 4 | <1:5 | 0.09d | Unknown | Unknown |
Boldface type indicates abnormal results for IgG (decreased), gliadin IgG (increased), EMG (increased), or TG-IgG (increased).
NVA, no villous atrophy.
Biopsy showed villous atrophy when CD was diagnosed many years earlier; no biopsy was performed recently.
This patient's serum exhibited a slightly increased TG-IgA level (21 units).
Two IgA-deficient serum samples were positive for EMG (Table 2; Table 3), and both serum samples had undetectable levels of IgA (<7 mg/dl). One IgA-deficient serum specimen was positive for TG-IgG; this specimen was also positive for EMG at a titer of 1:20 (Table 3). None of the control serum specimens were EMG positive or TG-IgG positive.
To determine if patients with IgA deficiency also exhibited IgG deficiency, IgG levels were measured in EMA-negative, IgA-deficient sera (Table 3). A total of five serum specimens, all from teenagers or adults, showed IgG deficiency; notably, four of five IgG-deficient serum specimens exhibited only marginal IgA deficiency (IgA level, 45 to 70 mg/dl).
Clinical findings.
The physicians of the 26 IgA-deficient patients were informed by mail of their patient's IgA deficiency and were asked to supply basic clinical information regarding symptoms or diagnosis and biopsy results, if available. Responses were received for 18 patients; multiple attempts to contact the nonresponding physicians by telephone and facsimile were unsuccessful. Although most patients exhibited gastrointestinal symptoms, only four patients had undergone small-bowel biopsy, and none of these four patients showed villous atrophy (Table 3).
DISCUSSION
Our findings demonstrate an increased frequency of IgA deficiency in sera submitted to a reference laboratory for testing for EMA. Subsequent measurement of other CD-associated antibodies in EMA-negative, IgA-deficient sera, as well as limited clinical feedback, did not provide any strong evidence of CD in these patients. It appears more likely that the increased frequency of IgA deficiency in the group tested for EMA reflects the similarity of gastrointestinal symptoms observed in patients with selective IgA deficiency and CD (4). Thus, for patients with gastrointestinal manifestations of IgA deficiency a test for EMA is often ordered as part of a systematic gastrointestinal disease evaluation.
The quantitative IgA values observed in IgA-deficient sera segregated into two distinct groups; one group had markedly decreased IgA levels (<35 mg/dl), whereas the second group showed marginally decreased IgA levels (45 to 70 mg/dl). Notably, all except one of the patients in the second group were either teenagers or 41 to 60 years old. Since the lower limit of the IgA reference interval shifts upward at age 13, the teenagers with marginally decreased IgA levels may simply represent individuals whose age-related IgA increase is slightly behind the norm (20). Possible explanations for the cluster among 41 to 60 year olds are less straightforward, and the results for these patients require more investigation.
Although the primary focus of our study was characterization of sera testing negative for EMA, we capitalized on the availability of EMA-positive specimens to evaluate the co-occurrence of other CD-associated antibodies. High proportions of EMA-positive sera were also positive for TG-IgA, gliadin IgA, and gliadin IgG, consistent with published values (6, 10, 24, 26). Likewise, the values of 60% EMG positivity and 32% TG-IgG positivity agreed with published values of 54 and 23%, respectively (1, 11). However, in light of reports (9, 26) that have identified transglutaminase as the target antigen of endomysial antibodies, the relatively large number of EMG-positive, TG-IgG-negative sera among the EMA-positive group (8 of 25) is confusing. Further studies are needed to clarify the antigenic specificities of EMG antibodies and to optimize assays for the measurement of EMG and TG-IgG.
Because none of the serologic assays for CD are 100% specific, we were not surprised to find that a small number of serum specimens from the control group had positive results by some assays. Our value of 12% gliadin IgG positivity for the control group agreed well with published values, which generally range from 10 to 25% (1, 7). Similarly, our values of 4% TG-IgA positivity and 4% gliadin IgA positivity agreed with published values (1).
Several investigators have evaluated alternative testing strategies for the serologic detection of CD in patients with IgA deficiency. An early description of an IgA-deficient CD patient demonstrated EMG reactivity at a high titer, leading the investigators to suggest that EMG production may represent a compensatory response in CD patients when EMA production is impaired (2). A later study likewise demonstrated high titers of EMG in two IgA-deficient CD patients (14); however, a third study found EMG (titer not given) in only one of three IgA-deficient CD patients (27). On the basis of the identification of transglutaminase as the antigen recognized by endomysial antibodies, Sulkanen et al. (26) tested 14 IgA-deficient CD patients for TG-IgG and found that all were positive. Taken together, these findings suggest that EMG and/or TG-IgG detection may represent the best alternative serologic approach for the identification of CD in IgA-deficient patients, even though neither assay is suitable for patients with normal IgA levels. In our study, EMG was detected in two EMA-negative, IgA-deficient serum specimens, one of which was also strongly positive for TG-IgG. Unfortunately, biopsy data were not available for either patient; it thus remains unknown if EMG and TG-IgG detection in these IgA-deficient patients heralded CD. Evaluation of assays that detect EMG and TG-IgG in a large cohort of IgA-deficient CD patients is needed to clarify their value as alternative serologic assays for CD.
Other investigators have focused on gliadin IgG as an alternative serologic assay for CD in patients with IgA deficiency. In one study, gliadin IgG was detected in one of two IgA-deficient CD patients (22). In a second study of a large cohort, gliadin IgG was detected in 51 of 54 IgA-deficient CD patients (5). These data strongly suggested that gliadin IgG was the most appropriate alternative serologic test for CD detection in patients with IgA deficiency. However, the recent findings of Lock and Unsworth (16) were inconsistent with that view. In an approach similar to ours, they measured IgA in sera from 482 patients with suspected CD and identified 7 patients with IgA deficiency. Gliadin IgG was increased in only one of seven IgA-deficient serum specimens, and biopsy of the patient demonstrated no villous atrophy. In our study, gliadin IgG was increased in 8 of 26 IgA-deficient serum specimens, including 6 of 12 serum specimens from patients with marked IgA deficiency; however, only one patient with marked IgA deficiency and elevated gliadin IgG levels was biopsied, and no villous atrophy was observed. Our findings thus follow the same trends observed by Lock and Unsworth (16), providing little support for an assay for gliadin IgG as a sensitive alternative assay for CD in patients with IgA deficiency.
Our efforts to recover clinical data for IgA-deficient patients met with only limited success. Most patients for whom data were available exhibited gastrointestinal symptoms suggestive of CD; however, only four patients had undergone small-bowel biopsy, and all four showed normal villi. Thus, there was no strong clinical evidence of CD in IgA-deficient patients.
Our findings indicate that, in a reference laboratory setting, identification of IgA deficiency in sera tested for EMA, followed by tests for detection of other CD-associated antibodies in these sera, offers no appreciable advantage over testing for EMA alone for the serologic detection of CD. This view concurs with that of Lock and Unsworth (16), who found that the identification of IgA deficiency in samples from patients with suspected CD did not help in the serologic diagnosis of CD. Thus, until a sensitive alternative serologic approach for the identification of CD in patients with IgA deficiency is available, routine measurement of IgA levels in sera submitted to a reference laboratory for testing for EMA is not recommended. However, in situations in which CD is strongly suspected in a patient negative for EMA antibodies, the physician should consider measuring IgA levels before ruling out CD.
ACKNOWLEDGMENTS
We thank Suzanne Rivas, Nimfa Burgos, Naomi Adlaon, and Zakera Gandhi for expert technical assistance.
REFERENCES
- 1.Bazzigaluppi E, Lampasona V, Barera G, Venerando A, Bianchi C, Chiumello G, Bonifacio E, Bosi E. Comparison of tissue transglutaminase-specific antibody assays with established antibody measurements for coeliac disease. J Autoimmun. 1999;12:51–56. doi: 10.1006/jaut.1998.0253. [DOI] [PubMed] [Google Scholar]
- 2.Beutner E H, Kumar V, Chorzelski T P, Szaflarska-Czerwionka M. IgG endomysial antibodies in IgA-deficient patient with coeliac disease. Lancet. 1989;i:1261–1262. doi: 10.1016/s0140-6736(89)92352-0. [DOI] [PubMed] [Google Scholar]
- 3.Burgin-Wolff A, Hadziselimovic F H. Screening test for coeliac disease. Lancet. 1997;349:1843–1844. doi: 10.1016/S0140-6736(05)61732-1. [DOI] [PubMed] [Google Scholar]
- 4.Burgio G R, Duse M, Monafo V, Ascione A, Nespoli L. Selective IgA deficiency: clinical and immunological evaluation of 50 pediatric patients. Eur J Pediatr. 1980;133:101–106. doi: 10.1007/BF00441577. [DOI] [PubMed] [Google Scholar]
- 5.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza G R. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Gut. 1998;42:362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Challacombe D N. Screening tests for coeliac disease. Arch Dis Child. 1995;73:3–7. doi: 10.1136/adc.73.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chartrand L J, Argulnik J, Vanounou T, Russo P A, Baehler P, Seidman E G. Effectiveness of antigliadin antibodies as a screening test for celiac disease in children. Can Med Assoc J. 1997;157:527–533. [PMC free article] [PubMed] [Google Scholar]
- 8.Chorzelski T P, Beutner E H, Sulej J, Tchorzewska H, Jablonska S, Kumar V, Kapuscinska A. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984;111:395–402. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- 9.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken E O, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 10.Dieterich W, Laag E, Schopper H, Volta U, Ferguson A, Gillett H, Riecken E O, Schuppan D. Autoantibodies to tissue transglutaminase as predictors for celiac disease. Gastroenterology. 1998;115:1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- 11.Fasano A. Tissue transglutaminase: the holy grail for the diagnosis of celiac disease, at last? J Pediatr. 1999;134:134–135. doi: 10.1016/s0022-3476(99)70402-6. [DOI] [PubMed] [Google Scholar]
- 12.Godkin A, Jewell D. The pathogenesis of celiac disease. Gastroenterology. 1998;115:206–210. doi: 10.1016/s0016-5085(98)70382-8. [DOI] [PubMed] [Google Scholar]
- 13.Klemola T. IgA deficiency. Ann Clin Res. 1987;19:248–257. [PubMed] [Google Scholar]
- 14.Korponay-Szabo I R, Kovacs J B, Czinner A, Goracz G, Vamos A, Szabo T. High prevalence of silent celiac disease in preschool children screened with IgA/IgG antiendomysium antibodies. J Pediatr Gastroenterol Nutr. 1999;28:26–30. doi: 10.1097/00005176-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Leonard J N, Chorzelski T P, Beutner E H, Sulej J, Griffiths C E M, Kumar V J, Fry L. IgA anti-endomysial antibody detection in the serum of patients with dematitis herpetiformis following gluten challenge. Arch Dermatol Res. 1985;277:349–351. doi: 10.1007/BF00509231. [DOI] [PubMed] [Google Scholar]
- 16.Lock R J, Unsworth D J. Identifying immunoglobulin-A-deficient children and adults does not necessarily help the serologic diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 1999;28:81–83. doi: 10.1097/00005176-199901000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Maki M, Collin P. Coeliac disease. Lancet. 1997;349:1755–1759. doi: 10.1016/S0140-6736(96)70237-4. [DOI] [PubMed] [Google Scholar]
- 18.McMillan S A, Watson R P G, McCrum E E, Evans A E. Factors associated with serum antibodies to reticulin, endomysium, and gliadin in an adult population. Gut. 1996;39:43–47. doi: 10.1136/gut.39.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molberg O, McAdam S N, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin K E A, Sjostrom H, Sollid L M. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 20.Noroski L M, Shearer W T. Screening for primary immunodeficiencies in the clinical immunology laboratory. Clin Immunol Immunopathol. 1998;86:237–245. doi: 10.1006/clin.1997.4469. [DOI] [PubMed] [Google Scholar]
- 21.Not T, Horvath K, Hill I D, Partanen J, Hammed A, Maguzzu G, Fasano A. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494–498. doi: 10.1080/00365529850172052. [DOI] [PubMed] [Google Scholar]
- 22.Rittmeyer C, Rhoads J M. IgA deficiency causes false-negative endomysial antibody results in celiac disease. J Pediatr Gastroenterol Nutr. 1996;23:504–506. doi: 10.1097/00005176-199611000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Rujner J, Socha J, Barra E, Gregorek H, Madalinski K, Wozniewicz B, Giera B. Serum and salivary antigliadin antibodies and serum IgA anti-endomysium antibodies as a screening test for coeliac disease. Acta Paediatr. 1996;85:814–817. doi: 10.1111/j.1651-2227.1996.tb14157.x. [DOI] [PubMed] [Google Scholar]
- 24.Sacchetti L, Ferrajolo A, Salerno G, Esposito P, Lofrano M M, Oriano G, Micillo M, Paparo F, Troncone R, Auricchio S, Salvatore F. Diagnostic value of various serum antibodies detected by diverse methods in childhood celiac disease. Clin Chem. 1996;42:1838–1842. [PubMed] [Google Scholar]
- 25.Sollid L M, Molberg O, McAdam S, Lundin K E A. Autoantibodies in coeliac disease: tissue transglutaminase—guilt by association? Gut. 1997;41:851–852. doi: 10.1136/gut.41.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulkanen S, Halttunen T, Laurila K, Kolho K-L, Korpanay-Szabo I R, Sarnesto A, Savilahti E, Collin P, Maki M. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–1328. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 27.Valdimarsson T, Franzen L, Grodzinsky E, Skogh T, Strom M. Is small bowel biopsy necessary in adults with suspected celiac disease and IgA anti-endomysium antibody? 100% positive predictive value for celiac disease in adults. Dig Dis Sci. 1996;41:83–87. doi: 10.1007/BF02208588. [DOI] [PubMed] [Google Scholar]
- 28.Van der Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–1588. [PubMed] [Google Scholar]
- 29.Vogelsang H, Genser D, Wyatt J, Lochs H, Ferenci P, Granditsch G, Penner E. Screening for celiac disease: a prospective study on the value of noninvasive tests. Am J Gastroenterol. 1995;90:394–398. [PubMed] [Google Scholar]