Abstract
Aims
Permanent transseptal left bundle branch area pacing (LBBAP) is a promising new pacing method for both bradyarrhythmia and heart failure indications. However, data regarding safety, feasibility and capture type are limited to relatively small, usually single centre studies. In this large multicentre international collaboration, outcomes of LBBAP were evaluated.
Methods and results
This is a registry-based observational study that included patients in whom LBBAP device implantation was attempted at 14 European centres, for any indication. The study comprised 2533 patients (mean age 73.9 years, female 57.6%, heart failure 27.5%). LBBAP lead implantation success rate for bradyarrhythmia and heart failure indications was 92.4% and 82.2%, respectively. The learning curve was steepest for the initial 110 cases and plateaued after 250 cases. Independent predictors of LBBAP lead implantation failure were heart failure, broad baseline QRS and left ventricular end-diastolic diameter. The predominant LBBAP capture type was left bundle fascicular capture (69.5%), followed by left ventricular septal capture (21.5%) and proximal left bundle branch capture (9%). Capture threshold (0.77 V) and sensing (10.6 mV) were stable during mean follow-up of 6.4 months. The complication rate was 11.7%. Complications specific to the ventricular transseptal route of the pacing lead occurred in 209 patients (8.3%).
Conclusions
LBBAP is feasible as a primary pacing technique for both bradyarrhythmia and heart failure indications. Success rate in heart failure patients and safety need to be improved. For wider use of LBBAP, randomized trials are necessary to assess clinical outcomes.
Keywords: Conduction system pacing, Left bundle branch pacing, Left ventricular septal pacing, Left bundle fascicular pacing, Distal capture, Complications
Structured Graphical Abstract
Structured graphical abstract.
LBBP, left bundle branch pacing; LBFP, left bundle fascicular pacing; LVSP, left ventricular septal pacing; LBBAP, left bundle branch area pacing; OR, odds ratio.
See the editorial comment for this article ‘Left bundle branch area pacing in perspective’, by Michele Brignole and Richard Sutton, https://doi.org/10.1093/eurheartj/ehac447.
Introduction
The undesirable consequences of right ventricular pacing, when used to treat bradycardia and limitations of biventricular pacing (BiV) as a method to deliver cardiac resynchronization therapy (CRT), prompted the development of more physiological pacing options.
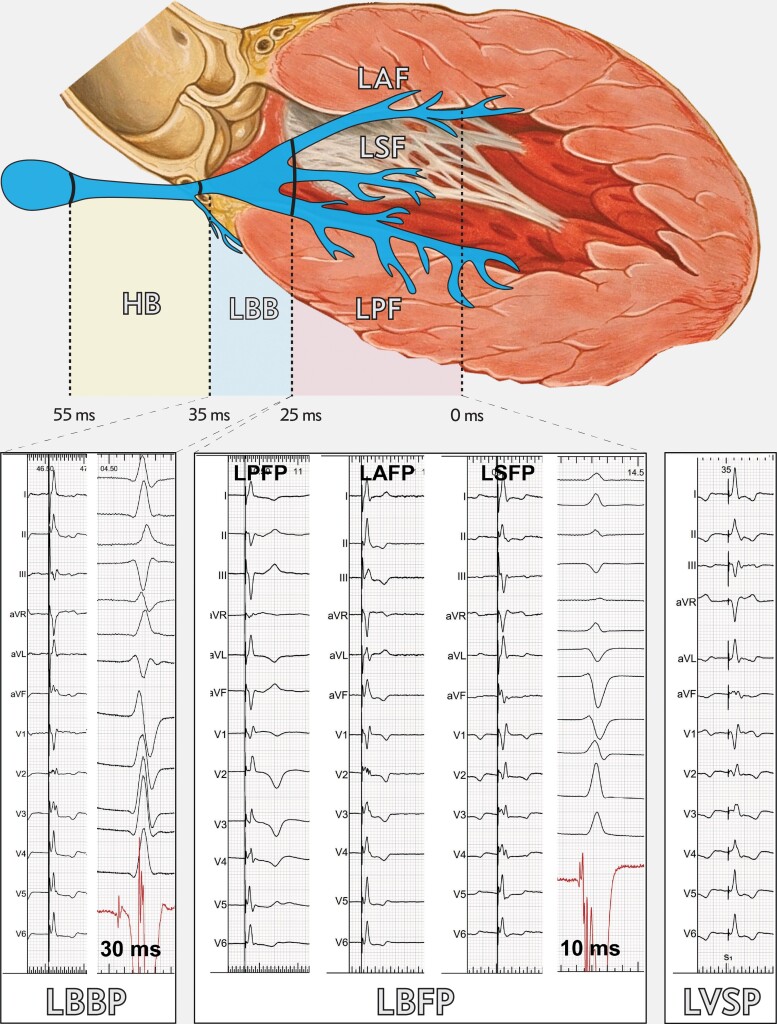
The feasibility of permanent left ventricular septal pacing (LVSP) via the ventricular transseptal route was demonstrated in 2016 by Mafi-Rad et al.1 in the first-in-human study. This technique was modified by Huang et al.2 who demonstrated that direct pacing of the proximal left bundle branch pacing (LBBP) can be achieved using the transseptal approach. Small differences in the paced QRS complex between LVSP and LBBP, the occurrence of intermediate capture types (left bundle fascicular pacing, LBFP), a lack of standard and precise differentiating criteria and scarcity of data regarding differences in clinical outcome, justify the popular use of the term left bundle branch area pacing (LBBAP) as the common descriptor of these new pacing modalities (Figure 1).3–6
Figure 1.
Examples of paced electrocardiogram patterns and endocardial electrograms during left bundle branch area pacing, characterized by left bundle branch potential to QRS interval of 34–25 ms and lead tip position approximately 1.5 cm from the His bundle. LBFP, left bundle fascicular pacing—characterized by potential to QRS of 24–0 ms and lead tip position approximately 1.5–4.5 cm from His bundle. Left bundle fascicular pacing includes: left posterior fascicle pacing; left anterior fascicle pacing; left septal fascicle pacing. LVSP: diagnosed when left bundle branch capture criteria are not met, any distance from His bundle. Heart drawing based on work by Patrick J. Lynch and C. Carl Jaffe, MD/CC-BY 2.5, https://commons.m.wikimedia.org/wiki/File:Heart_anterior_view_coronal_section.jpg.
Within four years of these two landmark publications, several small and medium-sized, mainly single-centre clinical studies have demonstrated the feasibility of LBBAP, in lieu of conventional anti-bradycardia pacing and BiV-CRT.5,7–10 However, valid questions regarding safety and in-depth characterization of this new pacing technique in real-world clinical practice remain.
The Multicentre European Left Bundle Branch Area Pacing Outcomes Study (MELOS) is a registry-based observational study, which was designed to gather data from a large group of patients from 14 centres who were early adopters of LBBAP. Our primary focus was characterization of LBBAP capture types and pacing parameters, learning curve assessment and procedure-related complications at follow-up.
Methods
Study design and population
This is a multicentre observational study based on pooled LBBAP registries maintained in 14 European hospitals (listed in Table 1). Only European centres considered as experienced (>60 LBBAP implants) were invited. The study population comprised all patients who underwent an attempt at LBBAP lead implantation at these centres for any indication.
Table 1.
Multicentre European left bundle branch area pacing outcomes study—participating centres and enrolment details
| Centre | Country | Date of first implant | Number of patients | Number of operators | Enrolment policy per operators | Enrolment per all implants in centre | Registry type | Success rate |
|---|---|---|---|---|---|---|---|---|
| Amsterdam | Netherlands | 02 Dec 2019 | 61 | 3 | 1 | 50% | Mixed | 100% |
| Antwerp | Belgium | 04 Feb 2020 | 89 | 1 | 1 | 32% | Prospective | 80% |
| Eindhoven | Netherlands | 08 Jan 2020 | 100 | 2 | 1 | 41% | Prospective | 80% |
| Geneva | Switzerland | 25 Feb 2020 | 121 | 2 | 1,2 | 46% | Prospective | 84% |
| Gent | Belgium | 27 Nov 2019 | 150 | 1 | 1 | 90% | Prospective | 90% |
| Krakow | Poland | 12 Jun 2018 | 607 | 5 | 1,2 | 62% | Prospective | 86% |
| London | United Kingdom | 23 Nov 2020 | 67 | 4 | 1,2 | N/A | Prospective | 84% |
| Maastricht | Netherlands | 25 Nov 2019 | 120 | 2 | 3,4 | 30% | Mixed | 98% |
| Prague 1 | Czechia | 21 Nov 2019 | 358 | 2 | 1,2 | 39% | Prospective | 92% |
| Prague 2 | Czechia | 28 Apr 2020 | 114 | 1 | 3 | 18% | Mixed | 100% |
| Rome | Italy | 15 Jan 2020 | 125 | 1 | 1,2 | 8% | Prospective | 87% |
| Rovigo | Italy | 20 May 2019 | 202 | 4 | 2 | 35% | Mixed | 99% |
| Valencia | Spain | 16 Jun 2019 | 292 | 1 | 1 | 45% | Prospective | 86% |
| Zwolle | Netherlands | 12 Dec 2019 | 127 | 2 | 1 | 55% | Prospective | 97% |
| SUMMARY | 14 | 12 Jun 2018 | 2533 | 31 | 35% | 87% of cases prospective | 90% |
Enrolment policy: 1—Left bundle branch area pacing (LBBAP) as primary approach for all pacing indications; 2—LBBAP as primary approach for all pacing indications after initial attempt at His bundle pacing; 3—LBBAP only for atrioventricular block and cardiac resynchronization therapy candidates; 4—preselected sick sinus syndrome patients.
The recruitment policy for LBBAP for each centre/operator was investigated to estimate potential selection bias. This was approximated globally by the percentage of all patients with indications for pacing/CRT who underwent attempted LBBAP during the MELOS recruitment period. Additionally, enrolment strategy was categorized per operator (Table 1) because in most centres only some of the operators implant LBBAP devices, and they might do so in all their consecutive, unselected patients.
The study complies with the Declaration of Helsinki, and was approved by the local ethics committees; informed consent was obtained from the subjects.
Left bundle branch area pacing device implantation
We classified the type of LBBAP capture type achieved. LBBAP lead implantation was considered successful when a deep intraseptal lead position was obtained, and the paced QRS complex included a terminal R/r wave in lead V1, indicating a delay in activation of the right ventricle. In rare cases we accepted a QS configuration (lack of terminal R) in V1 provided that a terminal R/r wave in lead V1 appeared during programmed stimulation or other features indicating LBBAP (described below) were present.
LBBAP lead implantation technique generally followed the previously described methods,11 albeit with some modifications. The LBBAP target zone was regarded more liberally and leads were positioned over a wide area on the midseptum, rather than strictly 1.5–2.0 cm from the His bundle in the apical direction as described by Huang et al.2,11 The His bundle was generally not used as an anatomical marker; the LBBAP lead deployment site was determined using the tricuspid ring as a marker, the paced QRS morphology (polarity discordance of leads II and III, and V1 nadir notch) and endocardial electrograms. Lead depth in the interventricular septum during implantation was monitored using progressive change of paced QRS morphology, fixation beats, local endocardial electrogram, fluoroscopy with sheath ventriculography and impedance.3,4,11–13 The number of lead implantation attempts, as well as the final position/capture type were at the discretion of the implanting cardiologist. While evidence of direct LBB capture and R-wave peak time in lead V6 (V6RWPT) <80 ms, were favoured by all,3 the final lead position was dictated by the anatomy and the limitations of currently available delivery sheaths and leads. An electrophysiology digital recording system was used by the majority of operators to record and analyse intracardiac electrograms and surface electrocardiogram (ECG) using digital callipers at a high sweep speed of 100–200 mm/s.
Left bundle branch area pacing capture type categorization
We classified the type of LBBAP capture achieved (Figure 1) using the following steps.
Step 1: Is there evidence of direct left conduction system capture?
We required any of the following criteria to be met to diagnose left conduction system capture:
Diagnostic QRS morphology transition during threshold test.3,11
Diagnostic QRS morphology transition during programmed stimulation.14
Pacing stimulus to V6RWPT <80 ms in patients with narrow QRS/isolated right bundle branch block patients or <90 ms in patients with more advanced ventricular conduction system disease.3,15
LBB potential to V6RWPT interval equal to the stimulus to V6RWPT interval (±10 ms).3
V6-V1 interpeak interval >40 ms.13
Diagnostic QRS morphology transition was defined as a sudden change in QRS morphology, compared to the QRS pattern observed during initial non-selective LBBAP capture (that is simultaneous capture of left conduction system and septal myocardium), with a change to either selective LBBAP or LVSP. A transition to LVSP was considered to have occurred if V6RWPT prolonged by >10 ms. A change to selective LBBAP was diagnosed if any of the following became apparent with a change in pacing output or programmed stimulation: isoelectric line after the pacing stimulus, a discrete local potential on the electrogram recorded from the pacing lead, or there was sudden prolongation in V1RWPT.3,15
Left ventricular septal myocardial capture (LVSP) was diagnosed if LBB capture criteria were not fulfilled, but a terminal R/r in lead V1 was present. Fluoroscopic confirmation of the pacing lead position in basal/mid-septal region was mandatory to exclude presence of R/r wave in V1 due to apical lead position. Moreover, deep septal lead position was assured with additional methods (progressive change of paced QRS morphology with lead rotation, fixation beats, and sheath ventriculography).
LBBAP failure was recognized when neither conduction system capture criteria nor terminal R/r in lead V1 were present.
Step 2: Location of left conduction system capture
In patients where direct left conduction system capture was confirmed we classified the location of capture within the left ventricular (LV) conduction system by assessing the LBB/fascicular Purkinje potential to QRS interval, and QRS polarity in leads II and III.
Proximal LBB capture (LBBP) was diagnosed if all of the following were observed: (i) LBB potential to QRS interval value within the range of 35–25 ms and (ii) inferior or intermediate QRS axis.
Left bundle fascicular pacing (LBFP): (i) Fascicular Purkinje potential to QRS interval within the range of 24–0 ms or absence of a potential.
Additionally LBFP was subdivided into:
Left posterior fascicle pacing (LPFP): superior QRS axis (leads II and III predominantly negative).
Left anterior fascicle pacing (LAFP): inferior QRS axis (leads II and III positive).
Left septal fascicle pacing (LSFP): intermediate QRS axis (lead II predominantly positive, and lead III with negative component).
The flowchart (see Supplementary material online, Figure S1) of LBBAP categorization provides information regarding inclusion/exclusion of patients for this analysis.
The LBBAP lead delivery method was divided into two categories:
The conventional approach using thin (4F), lumenless lead designed for targeting different sites with a dedicated fixed-curve or deflectable delivery sheath.
The stylet-driven approach using variety of 5.6–5.8 F leads originally designed for traditional right ventricular pacing and positioned with a large diameter fixed-curved or deflectable delivery sheath.16
Data collection and endpoints
The same standardized datasheet was used by all centres, this was populated from the data collected from the registries which were maintained at the participating centres. If necessary, additional data were retrieved from patient’s files. Data pooling, cleaning, capture type adjudication and statistical analysis was performed by one core statistical team.
The analysed demographic data, baseline clinical characteristics and procedure related variables are listed in Tables 2 and 3. The reasons for LBBAP lead implantation failure were collected. We recorded all complications, including those which may have occurred as a result of the transseptal lead approach including acute and delayed septal perforation, coronary artery fistula, stroke, acute coronary event—as listed in Table 4.
Table 2.
Basic clinical and electrocardiographical characteristics of the studied group (n = 2533)
| Age [years] | 73.9 ± 11.8 (95% CI 73.5–74.4) |
| Male sex | 1073 (42.4%) |
| Comorbidities | |
| Diabetes mellitus | 738 (29.1%) |
| Coronary heart disease | 773 (30.5%) |
| Heart failure | 1003 (39.6%) |
| Hypertension | 1828 (72.2%) |
| Severe valvular disease | 413 (16.3%) |
| Permanent atrial fibrillation | 672 (26.5%) |
| Pacing indication | |
| Sick sinus syndrome | 373 (14.7%) |
| Atrioventricular block | 1218 (48.1%) |
| Atrial fibrillation with bradycardia | 94 (3.7%) |
| Heart failure | 696 (27.5%) |
| Othera | 152 (6.0%) |
| Baseline QRS duration [ms] | 137.1 ± 35.9 (95% CI 135.7–138.5) |
| Baseline QRS type | |
| Narrow | 831 (32.8%) |
| LAFB/LPFB | 87 (3.4%) |
| RBBB | 265 (10.5%) |
| RBBB + LAFB/LPFB/NIVCD | 237 (9.4%) |
| LBBB | 568 (22.4%) |
| NIVCD | 199 (7.8%) |
| Asystole/escape/paced | 346 (13.7%) |
CI, confidence interval; LAFB, left anterior fascicular block; LPFB, left posterior fascicular block; RBBB, right bundle branch block; NIVCD, non-specific intraventricular conduction disturbance; LBBB, left bundle branch block.
Including atrioventricular node ablation and neurocardiogenic syncope.
Table 3.
Procedure-related, electrocardiographic and electrophysiologic characteristics
| 95% CI | P | ||
|---|---|---|---|
| Fluoroscopy time [min] | 9 (5.5–14.6)a | 8.5–9.2 | |
| LBBAP lead type: lumenless/stylet driven | 1902 (83.8%)/369 (16.2%) | ||
| LBBAP capture threshold at implant [V] | |||
| LBBP | 0.6 (0.5–0.9) | 0.5–0.7 | 0.002c |
| LBFP (LPFP + LAFP + LSFP) | 0.6 (0.5–0.9) | 0.6–0.7 | — |
| LVSP | 0.7 (0.5–1.0) | 0.7–0.75 | — |
| LBBAP sensing at implant [mV] | |||
| LBBP | 10 (6.8–15) | 8–11.3 | 0.56 |
| LBFP (LPFP + LAFP + LSFP) | 10 (7–13.9) | 9.3–10.1 | — |
| LVSP | 10 (6.7–13) | 9–10 | — |
| LBBAP lead impedance at implant [Ohm] | 652.1 (± 234.5) | 642.3–661.8 | |
| Loss of r/R in V1 at follow-up | 54/1357 (4.0%) | ||
| LBB/LPF/LAF/LSF potential at implant | 599/2270 (26.4%) | ||
| LBBP capture subtypes | |||
| LBBP | 121/1345 (9.0%) | ||
| LPFP | 333/1345 (24.8%) | ||
| LAFP | 232/1345 (17.2%) | ||
| LSFP | 370/1345 (27.5%) | ||
| LVSP | 289/1345 (21.5%) | ||
| LBB capture confirmed with:f | |||
| QRS transition at threshold test | 599/2270 (26.4%) | ||
| QRS transition at programmed stimulation | 213/2270 (9.4%) | ||
| V6RWPT < 80/90 ms b | 1384/2270 (61%) | ||
| Potential-V6RWPT = stimulus-V6RWPT | 444/2270 (19.6%) | ||
| V6-V1 interpeak interval > 40 ms | 416/2270 (18.3%) | ||
| Paced V6RWPT per baseline QRS type [ms] | |||
| Narrow QRS/isolated RBBB | 77.7 (± 12.8) | 77.0–78.5 | <0.001 |
| LBBB/NIVCD/RBBB+ | 83.0 (± 15.2) | 82.1–83.9 | — |
| Paced V6RWPT per obtained capture type [ms] | |||
| LBBP | 79.0 (± 12.0) | 76.9–81.2 | <0.001d |
| LBFP (LPFP + LAFP + LSFP) | 74.8 (± 12.3) | 74.0–75.6 | — |
| LVSP | 94.3 (± 11.6) | 93.3–95.4 | — |
| Paced QRS duration per baseline QRS type [ms] | |||
| Narrow QRS/isolated RBBB | 137.5 (± 19.3) | 136.4–138.7 | <0.001 |
| LBBB/NIVCD/RBBB(+) | 145.3 (± 22.5) | 144.0–146.6 | — |
| Paced QRS duration per obtained capture type [ms] | |||
| LBBP | 141.4 (± 16.9) | 138.4–144.4 | <0.001e |
| LBFP (LPFP + LAFP + LSFP) | 139.0 (± 19.0) | 137.8–140.2 | — |
| LVSP | 150.3 (± 22.3) | 148.3–152.3 | — |
DAP, dose/area product; LBBAP, left bundle branch area pacing; LBBP, left bundle branch pacing; LBFP, left bundle fascicular pacing; LAFP, left anterior fascicular pacing; LPFP: left posterior fascicular pacing; LSFP, left septal fascicular pacing; LVSP—left ventricular septal pacing; NIVCD, non-specific intraventricular conduction disturbance; RBBB, right bundle branch block; RBBB(+), right bundle branch block with fascicular block or NIVCD.
Values in parentheses represent quartiles (Q1—Q3) or ± standard deviation as appropriate.
80 ms for narrow QRS/isolated RBBB, 90 ms for LBBB/NIVCD/RBBB+.
In post-hoc analysis differences were present for pairs: LBBP vs. LVSP (P = 0.02) and LBPF vs. LVSP (P = 0.008).
In post-hoc analysis differences were present for pairs: LBBP/LPFP (P = 0.02), LBBP vs. LVSP (P < 0.001) and LPFP vs. LVSP (P < 0.001).
In post-hoc analysis differences were present for pairs: LBBP vs. LVSP (P = 0.001) and LBPF vs. LVSP (P < 0.001).
Often multiple criteria were present in the same person, therefore the percentages do not add up to 100%.
Table 4.
Complications of left bundle branch area pacing (n = 2533)
| Generic device implantation complications | |
|---|---|
| Penumothorax | 14 (0.55%) |
| Pocket/wound infection | 13 (0.51%) |
| Systemic infection/endocarditis | 6 (0.24%) |
| Atrial lead dislodgement | 14 (0.55%) |
| Pocket haematoma | 10 (0.4%) |
| Pericardial effusiona | 12 (0.47%) |
| Large vein thrombosis | 2 (0.08%) |
| Re-intervention for other non-LBBAP lead reasonsb | 15 (0.59%) |
| Subclavian arteriovenous fistula after puncture | 1 (0.04%) |
| Summary | 87 (3.43%) |
| Complications attributed to the transseptal route of the pacing lead | |
|---|---|
| Intraprocedural perforation into the LV cavity | 93 (3.67%) |
| Delayed perforation into the LV cavity | 2 (0.08%) |
| Acute chest pain | 25 (0.98%) |
| Acute ST-segment elevation in multiple leads | 6 (0.24%) |
| Acute coronary syndrome c | 11 (0.43%) |
| Coronary vein fistula | 7 (0.28%) |
| Coronary artery fistula | 2 (0.08%) |
| Painful pacing/chest pain | 4 (0.16%) |
| LBBAP lead unscrewable/trapped/damaged helix | 11 (0.43%) |
| LBBAP lead dislodgement | 38 (1.5%) |
| Threshold rise to an absolute value > 2 V | 17 (0.67%) |
| Threshold rise > 1 V from baseline | 18 (0.71%) |
| Threshold rise leading to re-intervention | 4 (0.16%) |
| Stroke/TIA | 0 (0) |
| Summary | 209 (8.25%) |
In three cases cardiosurgical operation was necessary.
Listed in Supplementary material online.
Acute coronary syndrome was diagnosed when two out of three (ST elevation, troponin release, chest pain) were present.
Acute coronary events were diagnosed when at least two of the following three criteria were present during or after the procedure: acute chest pain, ST-segment elevation and troponin level >320 pg/mL within 12–24 h, (over three standard deviations above the average level observed after uncomplicated LBBAP procedure).16,17
Learning curves
The experience was defined as the number of cases performed by the operator. To characterize the learning process the following parameters were assessed: procedure success, presence of LBB capture, paced V6RWPT, paced QRS duration (measured from the pacing stimulus to the end of the QRS using the 12-lead ECG) and fluoroscopy time. To minimize non-homogeneity and ensure high precision of measurements the learning curves for V6RWPT and QRS duration as endpoints were limited to operators with >200 implants who measured QRS using computer-based electrophysiology system.
Statistical analysis
Comparisons between groups were performed using Student’s t-test for independent variables or the chi-square test. For within-patient changes in LV ejection fraction and LV end-diastolic diameter paired t-tests were performed. Differences between groups were assessed using analysis of variance (parametric and Kruskal–Wallis type if necessary). Univariable and multivariable logistic regressions were performed to describe the effect of potential predictors of procedure success. For success rate assessment (multivariable logistic regression, learning curves), only centres/operators with prospective data, non-preselected patients and a reported failure rate >3% were analysed. To assess the impact of experience, binary logistic regression and polynomial regression models were constructed. For all variables the cubic fit line was chosen as the line of the best fit based on the curve estimation analysis. The results were deemed statistically significant at P < 0.05. Statistical analysis was performed using SPSS statistical software (IBM Statistics 27; Chicago, IL, USA).
Results
Enrolment and baseline characteristics
A total of 2533 patients from 14 centres across Europe (Table 1) were analysed; range of enrolled patients per centre was 61–607, with the first procedure in June 2018 and the last in November 2021, including all consecutive LBBAP cases in each centre. The majority of patients were enrolled on a prospective basis (2203/2533, 87%) using local prospective conduction system pacing implantation registries. LBBAP was undertaken in 35% of all patients admitted for pacemaker/CRT implantation in the MELOS centres during the enrolment period. The enrolment policies are listed in Table 1. LBBAP as a primary approach for all indications, and as secondary approach for all indications after an initial attempt at His bundle lead implantation were the dominant strategies, reported for 60.1% (1524/2533) and 32.5% (823/2533), respectively. There were 31 operators active in the study with median number of procedures per operator of 84 (Q1–Q3: 24–120; 95% CI: 31–100).
Baseline characteristics of the MELOS cohort, including comorbidities, pacing indications and QRS morphology types are presented in Table 2.
Procedural success rate and learning curve
The average LBBAP lead implantation success rate was 89.6% (2270/2533). Success rate for bradyarrhythmia and heart failure indications was 92.4% (1698/1837) and 82.2% (572/696), respectively. The independent preprocedural predictors of failure to implant a LBBAP lead were heart failure, LV end-diastolic diameter and broad baseline QRS. Results of the univariable and multivariable analyses are presented in Table 5. The reported reasons for implantation failure included inability to penetrate deep into the interventricular septum in 41.8% (110/263), inability to reach the target area due to enlarged heart chambers in 19.4% (51/263), unsatisfactory paced QRS in 27.8% (73/263), high capture threshold/unstable lead in 0.8% (2/263), chest pain in 0.8% (2/263) and other reasons in 9.4% (25/263).
Table 5.
Preprocedural determinants of LBBAP lead implantation failure (n = 1809)
| Uni OR (95% CI) | P | Multi OR a (95% CI) | P | |
|---|---|---|---|---|
| Ageb | 0.9 (0.82–0.99) | 0.03 | ||
| Male sex | 1.02 (0.78–1.33) | 0.9 | ||
| LVEFc | 0.7 (0.65–0.77) | <0.001 | ||
| LVEDDd | 1.85 (1.59–2.16) | <0.001 | 1.53 (1.26–1.86) | <0.001 |
| Device upgrade | 2.26 (1.62–3.14) | <0.001 | ||
| Heart failure indication | 2.75 (2.1–3.6) | <0.001 | 1.49 (1.01–2.21) | 0.04 |
| Baseline QRS duratione | 1.15 (1.1–1.19) | <0.001 | 1.08 (1.03–1.14) | 0.002 |
| Baseline QRS typef | 2.38 (1.78–3.19) | <0.001 | ||
| Stylet driven lead | 0.74 (0.48–1.13) | 0.16 |
UNI, univariable logistic regression; MULTI, multivariable logistic regression; OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimeter; LAHB, left anterior hemiblock; LPHB, left posterior hemiblock; RBBB, right bundle branch block.
Adjusted for centre.
Per 10 years increase.
Per 10% increase.
Per 10 mm increase.
Per 10 ms increase.
LBBB, NIVCD, RBBB + LAFB/LPFB/NIVCD.
The learning curve for LBBAP success was gradual, with the steepest part over the first 100 cases (Figure 2). With increasing experience, the proportion of LBBP vs. LVSP did not change (Figure 2). The learning curve based on fluoroscopy time showed a significant decrease over the initial 110 cases and then remained flat. The paced V6RWPT (Figure 2) and paced QRS duration (see Supplementary material online, Figure S2), progressively shortened with increasing experience up to 110 cases and then flattened off.
Figure 2.
Learning curves for the left bundle branch area pacing technique based on the number of procedures performed by the operators. (A) Probability of success of left bundle branch area pacing lead implantation slowly increases until 270 cases (P < 0.001). (B) Decrease in fluoroscopy time over the initial 110 cases (P < 0.001). (C) Despite increase in experience the proportion of left ventricular septal pacing does not decrease (P = 0.5) but remain stable. (D) Decrease of paced V6 R-wave peak time is present over the initial 110 cases (P < 0.001). Curves on (A), (B), and (C) were based on 1809 cases performed by 14 mid-high volume operators, while (D) curve was based on 860 cases performed by 3 high-volume operators—see Methods section.
LBBAP capture types and pacing parameters.
Average paced V6RWPT and global QRS duration for the whole group were 80.4 ± 14.3 ms and 141.5 ± 21.3 ms, respectively. These were significantly influenced by baseline QRS morphology and the type of LBBAP capture which was obtained (Table 3).
In the whole group of patients implanted with an LBBAP lead, LBB capture was diagnosed in 78.5% of cases (1782/2270; see Supplementary material online, Figure S1). In the remaining cases, left conduction system capture criteria were not fulfilled and, therefore, LVSP was diagnosed in 21.5% (488/2270). Direct left conduction system capture was diagnosed during threshold test in 26.4% (599/2270) cases, using the V6RWPT criterion in 61% (1384/2270) cases and other criteria for LBB capture diagnosis were present in 29% (1073/2270) cases (Table 3).
A left conduction system Purkinje potential was observed in 26.4% (599/2270) cases. Potential to QRS interval was reported in 524 of these cases with an average interval of 22.6 ± 6.5 ms, 29.1 ± 3.1 ms and 20.5 ± 5.9 ms for the whole group, LBBP and LBFP, respectively. The distribution of the potential to the QRS interval values is presented in Figure 3.
Figure 3.
Distribution of left bundle branch/Purkinje potential to QRS intervals—attesting to the variety of lead positions and wide target area on the interventricular septum. During proximal left bundle branch pacing, probably already including proximal parts of the major fascicles, the potential to QRS interval is likely in the range of 34–25 ms, this would correspond the main LBB length of 1.5–2.0 cm. Anterior, posterior and septal fascicular pacing is characterized by potential to QRS interval of 24–0 ms, with the values <10 ms indicating pacing of very distal arborization of the left conduction system, close to the Purkinje fibres to myocytes interface.
LBFP was the predominant capture type, observed in 69.5% (935/1345). The proportion of all LBBAP capture types is detailed in Table 3 and categorization flow-chart (see Supplementary material online, Figure S1).
LBBAP QRS was characterized by the presence of a terminal R wave in 92.4% (2097/2270) of successful cases. Patients without terminal r/R in V1 (n = 173) were diagnosed as LBBAP on the basis that V6RWPT was diagnostic of LBB capture (122/173), or the appearance of V1 R/r wave during programmed stimulation (15/60) or a diagnostic QRS transition during threshold test (36/122).
The capture threshold and sensing amplitudes at implant and at final follow-up of mean 6.4 ± 5.7 months were satisfactory and stable: 0.76 ± 0.56 V vs. 0.75 ± 0.51 V (P = 0.55) and 11.3 ± 5.7 mV vs. 11.5 ± 7 mV (P = 0.36), respectively. Pacing parameters for each of the different LBBAP capture types are presented in Table 3. Results regarding echocardiographic response and comparision of lumenless versus stylet-driven LBBAP leads are in Supplementary material.
Complications
No deaths, strokes, or other thromboembolic complications in the period from implantation to hospital discharge were observed. Acute and late complications were observed in 11.7%. Complications related to the transseptal route of the LBBAP lead were identified in 8.3% (209/2533), including, among others, delayed septal perforation and coronary artery damage/spasm—these were listed in Table 4 and illustrated in Figure 4. No further complications were observed following lead repositioning in case of perforations into the LV cavity and lead dislodgements. Acute coronary events were managed conservatively without further sequelae; details concerning this complication are presented in Supplementary material online, Table S1.
Figure 4.
Illustrations of the complications of the transseptal route of the left bundle branch area pacing lead. (A) Coronary venous fistula (arrow points to contrast in great cardiac vein). (B) Coronary artery fistula (arrow points to the blood jet near the lead entry site). (C) Acute ST-segment elevation in leads II, III, aVF and V3-V6 with concomitant chest pain during left bundle branch area pacing lead deployment. (D) Late lead perforation into left ventricular cavity (initial lead position superimposed, arrow indicates leftward displacement from the perforation site). (E) Helix entrapment with subsequent lead break during attempts to unscrew/remove (arrow points to the helix, broken and entrapped in the endocardium). Figure in (B) reproduced with permission from De Pooter J, Calle S, Demulier L et al. Septal coronary artery fistula following left bundle branch area pacing. JACC Clin Elecrtrophysiol. 2020; 6: 1337–1338.
A clinically significant increase (i.e. to an absolute value >2 V at 0.5 ms pulse width) of LBBAP pacing threshold was observed in 0.7% of patients (17/2533), this was on average detected 7.1 ± 5.0 months post implantation, while loss of terminal R/r in V1 was noted in 4.0% (54/1357).
No differences in complication rates were observed between different LBBAP capture types: 12.4%, 8.34% and 6.4% in LBBP, LBFP and LVSP, respectively (P = 0.08).
Discussion
MELOS is to date the largest multicentre evaluation of the LBBAP technique. The primary findings of this study are as follows: (i) when LBBAP is adopted into routine clinical practice, it does not provide homogeneous results. Several distinct capture types are observed as a result of differences in pacing locations, implantation technique and baseline substrate; (ii) in the European experience left bundle fascicular capture is the predominant type of LBBAP; (iii) LBBAP is a feasible primary pacing technique for all-comers regardless of the pacing indication. However, the learning curve is gradual; and (iv) several complications specific to the transseptal route were observed; in the majority of cases these were minor (Structured Graphical Abstract).
Left bundle branch area pacing technique evolution
Initially, two research groups in the Netherlands investigated the ventricular transseptal route for LV pacing.1,18,19 These studies showed feasibility, safety and favourable hemodynamics with this method, first in an animal model and then in humans. In some of the first human cases in the Mafi-Rad et al.1 study, it is likely that direct distal left conduction system capture was achieved, although this was neither pursued nor realized at the time. It was not until the case report by Huang et al.2 with clear demonstration of LBB capture that the full potential of the transseptal pacing technique was appreciated. The current study suggests that contemporary LBBAP lead implantation is based on a technique that preferentially targets fascicles and distal arborizations, and is intermediate between the ‘distal’ technique described by Mafi-Rad et al.1 and the ‘proximal’ approach developed by Huang et al.
Left bundle branch area pacing success rate
The overall success rate of LBBAP lead implantation in our study was 89.6%, which suggests that with currently available tools a deep septal lead deployment can be challenging even for experienced operators. Lead implantation failures were more likely to occur in patients with heart failure, enlarged left ventricle and broad baseline QRS duration (Table 5). Patients with these findings are more likely to have enlargement of the cardiac chambers and septal fibrosis, which were the two major reasons reported by MELOS operators for lead implantation failure. These factors are likely to explain the lower success rate for CRT patients and bundle branch block patients which was also reported by Vijayaraman et al.5 and Padala et al.8 These findings suggests that dedicated implant tools and leads are likely to be required to increase LBBAP lead implantation success rates in this challenging group of patients.
Comparison of success rate between studies is limited due to the lack of standard and precise LBB capture criteria. Our success rate seems similar to that reported in other studies (89.4–97.8%).5,8,9,20 However, we considered LVSP, which constituted 21.5% of our cases, as a success, while in the above referenced studies this was considered as a failure. The higher proportion of LVSP in our study is likely to be explained by our perception that LVSP is a good procedural endpoint and the use of more up-to-date capture criteria in our study.3,6,13,15 Several studies based their capture criteria on the expert recommendations which were published before validated capture criteria became available.7,11,21 These recommendations did not specify V6RWPT (a.k.a. LVAT) cut-off criteria for capture diagnosis and considered the presence of a LBB potential as obligatory, while this was absent in the majority of patients both in the study by Padala et al.8 and in our population.
Learning curves of left bundle branch area pacing
This is the first multicentre study reporting the learning curve for deep septal lead implantation success. Our learning curve showed a slow rise from the initial success rate of approximately 78–97% obtained after 270 cases; the steepest rise was for the initial 100 cases with a more gradual ascent later.
The learning curves for fluoroscopy time, paced V6RWPT, and global paced QRS duration all showed a similar improvement over the initial 110 cases and then a plateau (P < 0.001). With experience paced V6RWPT and global QRS duration shortened from 90 to 79 ms and from 159 to 152 ms, respectively. This is similar to the only other published learning curve for V6RWPT, which showed a plateau after 200 cases (for a single operator).21
Variety of left bundle branch area pacing capture types
Our results stand in contrast to some single centre studies which reject LVSP as a good outcome and promote a strict description of LBBAP using a technique that limits the target to the proximal LBB area located 1.5–2.0 cm from the His bundle.7,9,11,20 The implantation technique recently described by Liu et al.22 and Jiang et al.23 is more consistent with the approach used in our study. The findings of our study suggest that many operators are adopting an approach which targets a wider area on the interventricular septum, compared to that described in the early papers on LBBAP,2,9,11,20 and that a variety of LBBAP capture types are obtained. This is best attested by the bell curve distribution of LBB/fascicular Purkinje potential to QRS intervals (Figure 3) and the proportion of LBBP (9%), LBFP (69.5%) and LVSP (21.5%). Acceptance of a wider target area and various types of capture during LBBAP lead implantation may decrease the need for lead repositioning during the procedure, thereby limiting the septal damage and facilitating implantation.
Left bundle fascicular pacing—novel conduction system pacing modality
The predominant type of LBBAP in our study was LBFP—diagnosed when conduction system capture criteria are present but with a short potential to QRS interval and/or a superior axis (see Supplementary material for more details). These findings are indicative of distal fascicular/arborization capture rather than capture of the predivisional LBB trunk.
This type of capture, which can be obtained over wide mid-septal area, is easier to achieve than the more challenging proximal LBB capture which targets the short, narrower and insulated LBB trunk at the high basal septum. Apart from this anatomical factor, capture of the distal conduction system might be easier, since it does not require close proximity of the pacing lead to the fascicles/Purkinje fibres as they are not insulated at this level—in contrast to the proximal LBB. We observed that LBFP can often be achieved even when a LBB/Purkinje potential is not detected on the electrogram recorded from the lead. This finding suggests that the pacing lead does not need to be in very close proximity to the fascicles/Purkinje fibres in order to achieve LBFP capture. Distant LBB capture was demonstrated by a recent in vivo study.24 It is likely that capture is achieved via a virtual electrode effect and more distant fibres can be captured by adjusting pulse duration.24 This could further simplify LBFP by making it less dependent on precise lead positioning and potentially allow a transition to a more empirical approach of lead implantation, where the lead is deployed deep in the mid to basal septum. In contrast, the presence of a LBB potential is considered by Huang et al.11 as obligatory for proximal LBB capture. In this respect proximal LBBP seems similar to His bundle pacing where even a reversed situation is often observed. i.e. potential is recorded albeit conduction system capture is absent.
Interestingly, LBFP seems to offer faster activation of the LV than LBBP or LVSP—as suggested by shorter paced V6RWPT, and shorter paced QRS duration. The impact of proximal vs. distal LBB capture on V6RWPT observed in the current study is in line with the results of the recently published electrophysiological analysis of LBB pacing.25
The shorter QRS duration which we observed with LBFP compared to proximal LBB capture, is most likely the result of a reduction in the impact of the non-physiological capture of the adjacent septal myocardium, which is always observed during LBBAP, at outputs programmed for chronic pacing (≥2.0 V). Since the potential to QRS interval is short in this location one would expect breakout from the conduction system to occur more rapidly, which limits the amount of the myocardium which is activated by the wavefront initiated by direct local myocardial stimulation. Furthermore, direct septal depolarization occurring closer to the area of the physiological activation of the septal myocardium via the Purkinje system, also brings LBFP closer to physiological activation compared to LBBP.
The favourable physiology, QRS characteristics, trend for lower complication rate and practicalities of distal fascicular/arborization capture suggest that LBFP might be the future of LBBAP.
Left ventricular septal pacing—a simple method for indirect left bundle branch activation
In our experience, LV myocardial-only septal capture (i.e. LVSP) is a common procedural outcome. Even though LBBP/LBFP is preferentially targeted, LVSP was observed in 488 (21.5%) of MELOS patients. The percentage of LVSP in MELOS did not decrease with experience (Figure 2). This suggests the LVSP was perceived as a good procedural endpoint and/or that the current tools make it difficult/impossible to obtain LBB capture in all cases. LVSP may be considered as successful LBBAP for the following reasons: (i) pacing lead position and capture are in the LBB area, (ii) secondary LBB/fascicular engagement via retrograde activation from myocardial capture, while slightly delayed probably still plays a major role in LV depolarization, (iii) QRS morphology and duration are similar with LBBP and LVSP—while both stand in contrast to right ventricular paced QRS, (iv) hemodynamic and electrocardiographic studies of LVSP point to favourable activation/contraction of the ventricles,26–28 (v) distinguishing LBBP/LBFP from LVSP may not always be clear-cut with the currently available criteria.6
Nevertheless, long-term clinical outcomes of LVSP vs. LBBP, especially in heart failure patients, might differ. In the LOT-CRT study, the LBB capture sub-group had better echocardiographic, electrocardiographic and clinical outcomes than LVSP patients.29 Until results of randomized trials comparing capture types are available, it seems reasonable to strive, particularly in heart failure patients, for direct left conduction system capture in order to restore ventricular activation to be as physiological as possible.30
Left bundle branch area pacing capture types in other studies
In a dual-centre study (n = 305) by Padala et al.8 the LBB/Purkinje potential to QRS interval was 23 ± 7.2 ms (vs. 22.6 ± 6.5 ms in the current study) and in the majority of their cases (59%) LBB/fascicular Purkinje potentials were absent. Both findings suggest that LBFP or LVSP, rather than proximal LBBP, were the predominant forms of pacing.8 In the studies by Wang et al.21 (n = 376) and Chen et al.20 (n = 250) paced QRS axis suggested LBFP rather than LBBP in 29.5% and 79.7% of cases, respectively.
Complications related to the ventricular transseptal route of the pacing lead
The overall complication rate observed with LBBAP (11.7%) is comparable with the complication rate reported for BiV-CRT implantations.31 However, the ventricular transseptal route of the pacing lead is a source of new complications and concerns. We identified 209 cases (8.3%) where such complications were present (Table 4). This was in contrast to the previously reported outcome studies, none of which reported acute coronary events, chest pain during pacing, coronary vessel fistulas, lead helix entrapment problems or a significant rate of lead dislodgements.
A total of 0.99% (25/2533) patients experienced periprocedural chest pain, ST-segment elevation or significant troponin release. While acute coronary syndrome was reported in 0.4% (11/2533), the clinical course appeared to be benign, with no significant abnormalities detected on coronary angiography in those in whom this was performed and no significant regional wall motion abnormalities were detected. An acute coronary event during LBBAP implantation might be caused by a direct occlusion of the mid-portion of the septal perforator by the pacing lead. However, coronary artery spasm as a response to mechanical irritation or pacing should be postulated in cases with widespread transient ST segment elevation (Figure 4), since such ECG pattern is unlikely to be caused by the occlusion of a perforator branch.
Acute perforation into the LV cavity is a relatively common complication, reported in 0.3–6.0% by several other studies;7–10,16 a comparable rate (3.67%) was seen in MELOS. We did not observe adverse clinical consequences as a result of this complication. Delayed septal perforation is a potentially serious complication with LBBAP, which we observed in 0.08% of cases, and required repositioning of the lead. We did not observe any strokes associated with this complication. The rate of delayed septal perforation in our study was comparable to that reported in the studies by Su et al.7 (0.15%) and Chen et al.32 (0.33%).
LBBAP lead dislodgement was relatively common in our experience—seen in 38 cases (1.5%), while absent, or rare (0.3–0.9%) in other reports.7–10 The lead displacement rate in our study is lower than that reported for LV leads implanted for BiV pacing and are comparable to those reported with conventional right ventricular pacing leads.31
Efforts should be made to limit the occurrence of LBBAP complications. We believe that with the development of leads specifically designed for LBBAP, including dedicated deep septal fixation mechanisms, it may be possible in the future to reduce lead dislodgement and septal damage/perforation rates and facilitate successful implantation.
Study limitations
Multiple centres and operators were involved in the study, as a result there was a lack of homogeneity with respect to the implantation technique, LBB capture criteria used during implantation, the methods used for QRS duration and interval measurements and enrolment strategy.
The lack of an independent central adjudication committee and partially retrospective retrieval of data might have resulted in underreporting of failures and complications. Nevertheless, these were reported in a higher percentage of patients than in any other study.
Follow-up analysis was limited to the procedural outcomes, complication, echocardiographic response and electrical parameters over an average follow-up of only 6 months. Follow-up 12-lead ECG was available only for 1357 patients, potentially influencing the reported incidence of loss LBBAP. Importantly, neither mortality nor heart failure episodes were analyzed.
Our results might be less applicable to non-European populations.
Conclusions
This is the largest study to date reporting multicentre outcomes of LBBAP. We found that LBBAP is feasible as a primary pacing strategy for any pacing indication, but that with current tools, implantation is more challenging in patients with heart failure, reduced ejection fraction and prolonged QRS duration. Complications of the transseptal lead route are not rare and while most were minor, there is room for further improvement in implant tools and techniques aimed at reducing these complications.
This study redefines LBBAP technique from a proximal to more a straightforward distal conduction system pacing technique via direct left bundle fascicular capture and LVSP with secondary left conduction system activation. QRS duration was shorter with LBFP compared to proximal left bundle capture, which suggests that pacing in this location successfully delivers physiological pacing.
Randomized trials comparing the clinical outcomes of LBBAP vs. the current standard-of-care implantation techniques are warranted to formulate recommendations for clinical use of LBBAP.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank than the folowing persons for help during the study: Marta Olszewska (Krakow), Marco Centoni (Rovigio), Leonardo Calò (Rome), Justin Luermans (Maastricht), and Fenna Daniels (Zwolle).
Contributor Information
Marek Jastrzębski, First Department of Cardiology, Interventional Electrocardiology and Hypertension, Jagiellonian University, Medical College, Jakubowskiego 2, 30-688 Krakow, Poland.
Grzegorz Kiełbasa, First Department of Cardiology, Interventional Electrocardiology and Hypertension, Jagiellonian University, Medical College, Jakubowskiego 2, 30-688 Krakow, Poland.
Oscar Cano, Electrophysiology Section, Cardiology Department, Hospital Universitari i Politècnic La Fe, Valencia, Spain; Centro de Investigaciones Biomédicas en RED en Enfermedades Cardiovasculares (CIBERCV), 28029 Madrid, Spain.
Karol Curila, Cardiocenter, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czechia.
Luuk Heckman, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre+ (MUMC+), Maastricht, the Netherlands.
Jan De Pooter, Heart Center, Ghent University Hospital, Ghent, Belgium.
Milan Chovanec, Department of Cardiology, Homolka Hospital, Prague, Czechia.
Leonard Rademakers, Department of Cardiology, Catharina Ziekenhuis, Eindhoven, the Netherlands.
Wim Huybrechts, Department of Cardiology, University Hospital Antwerp, Antwerp, Belgium.
Domenico Grieco, Division of Cardiology, Policlinico Casilino, Rome, Italy.
Zachary I Whinnett, National Heart & Lung Institute, Imperial College London, London, UK.
Stefan A J Timmer, Department of Cardiology, Noordwest Ziekenhuisgroep, Alkmaar, the Netherlands.
Arif Elvan, Department of Cardiology, Isala Hospital Zwolle, Postbus 10400, 8000 GK Zwolle, the Netherlands.
Petr Stros, Cardiocenter, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czechia.
Paweł Moskal, First Department of Cardiology, Interventional Electrocardiology and Hypertension, Jagiellonian University, Medical College, Jakubowskiego 2, 30-688 Krakow, Poland.
Haran Burri, Cardiac Pacing Unit, Cardiology Department, University Hospital of Geneva, Geneva, Switzerland.
Francesco Zanon, Arrhythmia and Electrophysiology Unit, Cardiology Department, Santa Maria Della Misericordia Hospital, Rovigo, Italy.
Kevin Vernooy, Cardiocenter, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czechia; Department of Cardiology, Radboud University Medical Centre (RadboudUMC), Nijmegen, the Netherlands.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Mafi-Rad M, Luermans JG, Blaauw Y, Janssen M, Crijns HJ, Prinzen FW, et al. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circ Arrhythm Electrophysiol 2016;9:e003344. [DOI] [PubMed] [Google Scholar]
- 2. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–1736.e3. [DOI] [PubMed] [Google Scholar]
- 3. Jastrzebski M, Kielbasa G, Curila K, Moskal P, Bednarek A, Rajzer M, et al. Physiology-based electrocardiographic criteria for left bundle branch capture. Heart Rhythm 2021;18:935–943. [DOI] [PubMed] [Google Scholar]
- 4. Jastrzebski M, Moskal P. Reaching the left bundle branch pacing area within 36 heartbeats. Kardiol Pol 2021;79:587–588. [DOI] [PubMed] [Google Scholar]
- 5. Vijayaraman P, Ponnusamy S, Cano O, Sharma PS, Naperkowski A, Subsposh FA, et al. Left bundle branch area pacing for cardiac resynchronization therapy: results from the international LBBAP collaborative study group. JACC Clin Electrophysiol 2021;7:135–147. [DOI] [PubMed] [Google Scholar]
- 6. Jastrzebski M. ECG and pacing criteria for differentiating conduction system pacing from myocardial pacing. Arrhythm Electrophysiol Rev 2021;10:172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Su L, Wang S, Wu S, Xu L, Huang Z, Chen X, et al. Long-term safety and feasibility of left bundle branch pacing in a large single-center study. Circ Arrhythm Electrophysiol 2021;14:e009261. [DOI] [PubMed] [Google Scholar]
- 8. Padala SK, Master VM, Terricabras M, Chiocchini Andrea, Garg Aatish, Kron Jordana, et al. Initial experience, safety, and feasibility of left bundle branch area pacing: a multicenter prospective study. JACC Clin Electrophysiol 2020;6:1773–1782. [DOI] [PubMed] [Google Scholar]
- 9. Hua W, Fan X, Li X, Niu H, Gu M, Ning X, et al. Comparison of left bundle branch and his bundle pacing in bradycardia patients. JACC Clin Electrophysiol 2020;6:1291–1299. [DOI] [PubMed] [Google Scholar]
- 10. Vijayaraman P, Subzposh FA, Naperkowski A, Panikkath R, John K, Mascarenhas V, et al. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm 2019;16:1774–1782. [DOI] [PubMed] [Google Scholar]
- 11. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm 2019;16:1791–1796. [DOI] [PubMed] [Google Scholar]
- 12. Jastrzebski M, Kielbasa G, Moskal P, Bednarek A, Kusiak A, Sondej T, et al. Fixation beats: a novel marker for reaching the left bundle branch area during deep septal lead implantation. Heart Rhythm 2021;18:562–569. [DOI] [PubMed] [Google Scholar]
- 13. Jastrzebski M, Burri H, Kielbasa G, Curila K, Moskal P, Bednarek A, et al. The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace 2022;24:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jastrzebski M, Moskal P, Bednarek A, Kiełbasa G, Kusiak A, Sondej T, et al. Programmed deep septal stimulation: a novel maneuver for the diagnosis of left bundle branch capture during permanent pacing. J Cardiovasc Electrophysiol 2020;31:485–493. [DOI] [PubMed] [Google Scholar]
- 15. Wu S, Chen X, Wang S, Xu L, Xiao F, Huang Z, et al. Evaluation of the criteria to distinguish left bundle branch pacing from left ventricular septal pacing. JACC Clin Electrophysiol 2021;7:1166–1177. [DOI] [PubMed] [Google Scholar]
- 16. De PJ, Calle S, Timmermans F, Van Heuverswyn F. Left bundle branch area pacing using stylet-driven pacing leads with a new delivery sheath: a comparison with lumen-less leads. J Cardiovasc Electrophysiol 2021;32:439–448. [DOI] [PubMed] [Google Scholar]
- 17. Ponnusamy SS, Patel NR, Naperkowski A, Subzposh FA, Vijayaraman P. Cardiac troponin release following left bundle branch pacing. J Cardiovasc Electrophysiol 2021;32:851–855. [DOI] [PubMed] [Google Scholar]
- 18. Grosfeld MJ, Res JC, Vos DH, De Boer TJ, Bos HJ. Testing a new mechanism for left interventricular septal pacing: the transseptal route; a feasibility and safety study. Europace 2002;4:439–444. [DOI] [PubMed] [Google Scholar]
- 19. Mills RW, Cornelussen RN, Mulligan LJ, Strik M, Rademakers LM, Skadsberg ND, et al. Left ventricular septal and left ventricular apical pacing chronically maintain cardiac contractile coordination, pump function and efficiency. Circ Arrhythm Electrophysiol 2009;2:571–579. [DOI] [PubMed] [Google Scholar]
- 20. Chen X, Jin Q, Bai J, Wang W, Qin S, Wang J, et al. The feasibility and safety of left bundle branch pacing vs. Right ventricular pacing after mid-long-term follow-up: a single-centre experience. Europace 2020;22:ii36–ii44. [DOI] [PubMed] [Google Scholar]
- 21. Wang Z, Zhu H, Li X, Yao Y, Liu Z, Fan X. Comparison of procedure and fluoroscopy time between left bundle branch area pacing and right ventricular pacing for bradycardia: the learning curve for the novel pacing strategy. Front Cardiovasc Med 2021;8:695531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Niu HX, Gu M, Chen X, Hu Y, Cai M, et al. Contrast-enhanced image-guided lead deployment for left bundle branch pacing. Heart Rhythm 2021;18:1318–1325. [DOI] [PubMed] [Google Scholar]
- 23. Jiang H, Hou X, Qian Z, Wang Y, Tang L, Qiu Y, et al. A novel 9-partition method using fluoroscopic images for guiding left bundle branch pacing. Heart Rhythm 2020;17:1759–1767. [DOI] [PubMed] [Google Scholar]
- 24. Niri A, Bhaskaran A, Asta J, Massé S, Lai PFH, Veluppillai A, et al. Stimulation and propagation of activation in conduction tissue: implications for left bundle branch area pacing. Heart Rhythm 2021;18:813–821. [DOI] [PubMed] [Google Scholar]
- 25. Sun W, Upadhyay G, Tung R. Influence of capture selectivity and left-intrahisian block on ors characteristics during left bundle branch pacing. JACC Clin Electrophysiol 2022;8:635–647. [DOI] [PubMed] [Google Scholar]
- 26. Curila K, Jurak P, Vernooy K, Jastrzebski M, Waldauf P, Prinzen F, et al. Left ventricular myocardial septal pacing in close proximity to LBB does not prolong the duration of the left ventricular lateral wall depolarization compared to LBB pacing. Front Cardiovasc Med 2021;8:787414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rademakers LM, van Hunnik A, Kuiper M, Vernooy K, van Gelder B, Bracke FA, et al. A possible role for pacing the left ventricular septum in cardiac resynchronization therapy. JACC Clin Electrophysiol 2016;2:413–422. [DOI] [PubMed] [Google Scholar]
- 28. Heckman LIB, Luermans JGLM, Curila K, Van Stipdonk AMW, Westra S, Smisek R, et al. Comparing ventricular synchrony in left bundle branch and left ventricular septal pacing in pacemaker patients. J Clin Med 2021;10:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jastrzebski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm 2021;19:13–21. [DOI] [PubMed] [Google Scholar]
- 30. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 31. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–3520. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Wei L, Bai J, Wang W, Qin S, Wang J, et al. Procedure-related complications of left bundle branch pacing: a single-center experience. Front Cardiovasc Med 2021;8:645947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.