Abstract
Background & Aims
Coronavirus disease 2019 (COVID-19) is associated with long-term gastrointestinal sequelae; however, prospective longitudinal data are sparse. We prospectively studied the frequency, spectrum, and risk factors of post infection functional gastrointestinal disorders/disorders of gut-brain interaction (PI-FGID/DGBI) after COVID-19.
Methods
Three hundred twenty cases with COVID-19 and 2 control groups, group A, 320 healthy spouses/family controls, and group B, 280 healthy COVID serology-negative controls, were prospectively followed up at 1, 3, and 6 months by using validated Rome IV criteria to evaluate the frequency of PI-FGID/DGBI.
Results
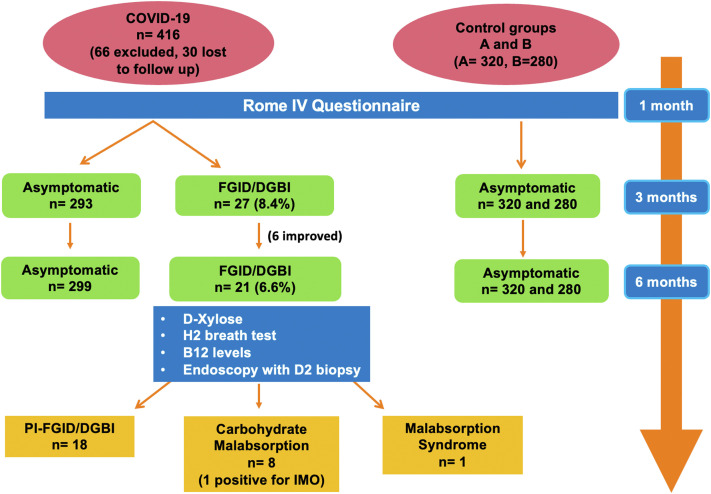
Of 320 cases, at 1 month 36 (11.3%) developed FGID symptoms. Persistent symptoms were noted in 27 (8.4%) at 3 months and in 21 (6.6%) at 6 months. At 3 months, 8 (2.5%) had irritable bowel syndrome, 7 (2.2%) had functional diarrhea, 6 (1.9%) had functional dyspepsia, 3 (0.9%) had functional constipation, 2 (0.6%) had functional dyspepsia–IBS overlap, and 1 (0.3%) had functional abdominal bloating/distention. Among symptomatic individuals at 3 months, 8 (29.6%) were positive for isolated carbohydrate malabsorption, 1 (3.7%) was positive for post infection malabsorption syndrome, and 1 (3.7%) was positive for intestinal methanogen overgrowth. None of the healthy controls developed FGID up to 6 months of follow-up (P < .01). Predictive factors at 3 and 6 months were severity of infection (P < .01) and presence of gastrointestinal symptoms at the time of infection (P < .01).
Conclusions
COVID-19 led to significantly higher number of new onset PI-FGID/DGBI compared with healthy controls at 3 and 6 months of follow-up. If further investigated, some patients can be diagnosed with underlying malabsorption.
Keywords: COVID-19, Functional Gastrointestinal Disorders (FGID), Long COVID, Post Infection-Irritable Bowel Syndrome (PI-IBS)
Abbreviations used in this paper: COVID-19, coronavirus disease 2019; DGBI, disorders of gut-brain interaction; FD, functional dyspepsia; FGID, functional gastrointestinal disorders; GI, gastrointestinal; IBS, irritable bowel syndrome; IMO, intestinal methanogen overgrowth; MAS, malabsorption syndrome; PACS, post-acute COVID-19 syndrome; PI, post infection; SIBO, small intestinal bacterial overgrowth
Graphical abstract
What You Need to Know.
Background
SARS-CoV-2 is primarily a respiratory pathogen but can infect the gastrointestinal tract. Post infection functional gastrointestinal disorders/disorders of gut-brain interaction (FGID/DGBI) can occur after COVID-19. There is paucity of data from prospective studies evaluating the occurrence of post-COVID-19 FGID/DGBI.
Findings
This study shows that post-COVID-19 FGID/DGBI is present in about 7% of patients after 6 months of follow-up. This study explores concomitant malabsorption among FGID patients, finding that around 30% have isolated carbohydrate malabsorption.
Implications for patient care
Familiarizing with this entity will enable clinicians to better recognize and manage post-COVID-19 FGID/DGBI, potentially minimizing unnecessary testing.
Coronavirus disease 2019 (COVID-19) is a multisystem disease with predominantly respiratory involvement. Gastrointestinal (GI) symptoms such as diarrhea, vomiting, and abdominal pain are seen in approximately 12%–20%.1 There is evidence that fecal-oral transmission is possible and that viral RNA can persist in stool samples even after nasopharyngeal samples have become negative.2 A proportion of patients recovering from COVID-19 can have either prolonged systemic symptoms or develop new symptoms termed as long COVID or post-acute COVID-19 syndrome (PACS).3 It is defined as persistence of/ongoing symptoms after recovery beyond 4 weeks of infection that cannot be attributed to any other diagnosis.4
Functional gastrointestinal disorders (FGID), now called disorders of gut-brain interaction (DGBI) by the Rome Foundation, are encountered both in gastroenterology practice and in the community. A Rome Foundation global study in 2021 found the prevalence of FGID to be greater than 40% across 33 countries.5 Studies have shown that irritable bowel syndrome (IBS) may follow an episode of gastroenteritis and is referred to as post infection (PI)-IBS.6 PI-FGID/DGBI are multifactorial conditions driven primarily by an abnormality in gut-brain interaction. In the recent past, a microorganic basis, including small intestinal bacterial overgrowth (SIBO), altered gut permeability, and persistence of low grade of immune activation has been reported.7 After infectious diarrhea by various pathogens, around 10%–30% of patients continue to have symptoms suggestive of IBS.8 On further exploration, several studies have shown that a proportion of these patients have features suggestive of malabsorption and SIBO.9 Because PI-FGID/DGBI is a clinical diagnosis made by Rome criteria, a patient with mild malabsorption syndrome (MAS) could be missed. Hence exclusion of malabsorption by appropriate investigations is important.
Most of the prior studies done on post-COVID-19 FGID lacked recruitment of prospective controls. GI symptoms on follow-up were mainly self-reported without use of validated questionnaires. In addition, none of the studies explored the link of FGID after COVID-19 with PI MAS/SIBO. We prospectively studied the frequency, spectrum, and predictive risk factors of PI-FGID/DGBI after COVID-19 compared with uninfected controls and family members.
Methods
Study Design
This prospective cohort study was conducted from April 2021 to January 2022. It consisted of 2 cohorts, a case group that included patients admitted at All India Institute of Medical Sciences, New Delhi, a dedicated COVID care center. These patients were recruited post discharge in April–May 2021 during the second wave dominated by the delta variant (irrespective of severity), with documented infection by reverse-transcriptase polymerase chain reaction or cartridge based-nucleic acid amplification testing. The other cohort included 2 groups. Group A included age-matched spouses/family members of the case group sharing the same dietary and environmental factors. Group B included COVID serology negative healthcare workers at our institution. Both cases and control groups A and B had no history of COVID-19 or FGID (as per Rome IV criteria). Cases and controls with inflammatory bowel disease, major psychiatric illness, GI malignancies, history of abdominal surgeries, or on immunosuppressive therapy were excluded. Follow-up was carried out either in person or by telephone using a self-administered/interviewer-based questionnaire (Rome IV questionnaire). The questionnaire was made available in RED-Cap (Research Electronic Data Capture) software for online administration. Electronic informed consent was obtained from each study participant. Subjects who fulfilled the criteria of various FGID in the case and control groups at 3 months were further investigated by lab-based and endoscopic methods to assess for MAS/SIBO. The study protocol was approved by the Institute Ethics Committee (reference no: IECPG 766/23.12.2020).
Definitions
Diagnosis of FGID/DGBI was made using the Rome IV criteria.10 Analysis was done in the symptomatic patients at 3 months considering the latest update of Rome IV, which specifies time duration of 6 months as not mandatory to establish diagnosis.11 We performed a hydrogen breath test as a surrogate marker for SIBO. Diagnosis of MAS required demonstration of malabsorption of at least 2 unrelated nutrients.9 A D-Xylose test was performed for evaluation of carbohydrate malabsorption. Relevant blood tests were also done. Severity of COVID-19 was based on the clinical guidelines of the National Institutes of Health.12
Techniques
D-Xylose test
After obtaining basal breath sample after an overnight fast, a 5-g dose of D-Xylose was used.13 Urine was collected for 5 hours, starting from the time the dose was given. The fasting blood, timed blood, and 5-hour urine samples were tested for xylose concentrations. Value less than 1 g/5 g/5 h was considered positive.
Glucose hydrogen breath test
Glucose hydrogen breath test was done by a breath analyzer to detect SIBO.14 Breath hydrogen concentration was measured in the expired air at fasting state and sequentially at 15-minute intervals for 3 hours after 70 g glucose/8 oz of water administration orally. A rise in breath hydrogen concentration above 12 ppm of basal value was considered positive for SIBO. A rise in breath methane concentration above 10 ppm of basal value during the test was considered positive for intestinal methanogen overgrowth (IMO).
Endoscopic duodenal biopsy
Biopsies were taken during esophagogastroduodenoscopy and were subjected to histologic examination after hematoxylin-eosin staining using standard techniques.15 Villous height and crypt depth ratio of >3:1, <3:1, and ≤1:1 was considered as normal, partial, and total villous atrophy, respectively.
Statistical Analysis
Sample size calculation
On the basis of prior studies, the incidence of PI-IBS after episodes of acute gastroenteritis was estimated around 15%. Keeping the risk difference of 0.10, with alpha error of 5% and power of 90%, 187 patients and controls each would be required. Assuming a 40% dropout rate, a total of 262 subjects were required in both case and control groups.
Data analysis
Statistical software SPSS (version 20; SPSS Inc, Chicago, IL) was used for statistical analyses. Normally distributed continuous variables were expressed as mean (standard deviation), and continuous variables with skewed distribution were expressed as median (range). The incidence of FGID/DGBI was calculated as proportion with 95% confidence interval. Shapiro-Wilk test was used to check the normal distribution of the data. Categorical data were presented as proportions. Subgroup analysis by univariate analysis was done. A two-tailed P value <.05 was considered significant.
Results
Baseline Demographic and Clinical Profile
The study included 416 post-COVID-19 recovered patients, of whom 66 were excluded because of non-availability of a COVID-19 negative spouse/family member, and 30 were lost to follow-up. Hence, a total of 320 cases and 320 controls in group A and 280 controls in group B were analyzed. The mean age was 38.02 ± 11.4 years in the case group, 37.94 ± 11.9 years in control group A, and 38.47 ± 11.7 years in control group B. On the basis of COVID disease severity, 238 (74.3%) had mild, 71 (22.1%) had moderate, and 11 (3.4%) had severe disease. The baseline characteristics are shown in Table 1 .
Table 1.
Demographic Characteristics of Cases and Controls
| Parameter | COVID-19 cases | Control group A | Control group B | P value |
|---|---|---|---|---|
| N | 320 | 320 | 280 | |
| Age, mean (y) | 38.02 ± 11.4 | 37.94 ± 11.9 | 38.47 ± 11.7 | .87 (group A) .76 (group B) |
| Gender | ||||
| Male | 163 (50.9%) | 175 (54.6%) | 172 (61.4%) | .88 (group A) |
| Female | 157 (49.0%) | 145 (45.3%) | 108 (38.5%) | |
| Comorbidities | .17 (group A) .33 (group B) |
|||
| Diabetes | 27 (8.4%) | 16 (5.0%) | 12 (4.2%) | |
| Hypertension | 37 (11.5%) | 21 (6.6%) | 21 (7.5%) | |
| CAD | 8 (2.5%) | 5 (1.5%) | 0 | |
| CKD | 4 (1.2%) | 1 (0.3%) | 0 | |
| Test to confirm | ||||
| RT-PCR | 275 (85.9%) | |||
| CB-NAAT | 45 (14.1%) | |||
| Severity of COVID-19 | ||||
| Mild | 238 (74.3%) | — | — | |
| Moderate | 71 (22.1%) | — | — | |
| Severe | 11 (3.4%) | — | — |
CAD, coronary artery disease; CB-NAAT, cartridge based-nucleic acid amplification test; CKD, chronic kidney disease; RT-PCR, reverse transcriptase-polymerase chain reaction.
Baseline Gastrointestinal Symptoms
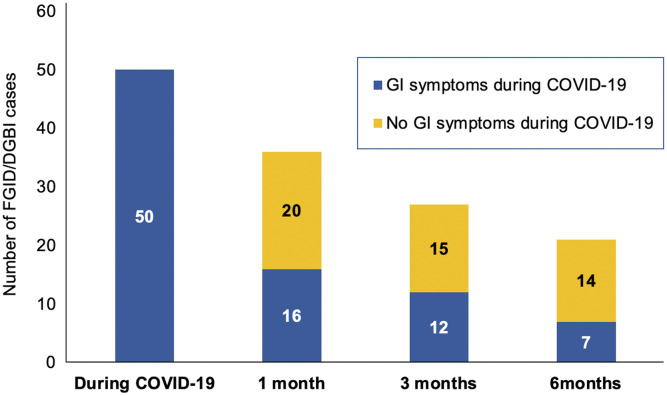
During COVID-19, 50 (15.6%) among the 320 patients developed GI complaints. The predominant symptom was diarrhea in 23 (7.2%), followed by abdominal pain in 16 (5.0%) and nausea with vomiting in 11 (3.4%).
PI-FGID/DGBI in COVID-190 Patients and Healthy Controls
At 1 month, 36 among the 320 cases (11.3%) developed FGID-like symptoms. At 3 months, 27 (8.4%) persisted to have symptoms, and 9 improved. At 6 months, another 6 improved, and 21 (6.6%) had persistent symptoms. No new patients in the case group developed symptoms on follow-up. Of the various reported FGID as per Rome IV questionnaire at 3 months, 8 (2.5%) had IBS, 7 (2.2%) had functional diarrhea, 6 (1.9%) had functional dyspepsia (FD), 3 (0.9%) had functional constipation, 2 (0.6%) had FD-IBS overlap, and 1 (0.3%) had functional abdominal bloating/distention. Among patients with IBS and FD-IBS overlap, IBS-diarrhea predominant (7/10) was most common, followed by IBS-mixed (2/10) and IBS-constipation predominant (1/10). Among healthy controls in both groups A and B, none developed PI-FGID/DGBI at 3 and 6 months of follow-up (P < .01) (Figure 1 ).
Figure 1.
Schematic representation of results.
Predictive Risk Factors
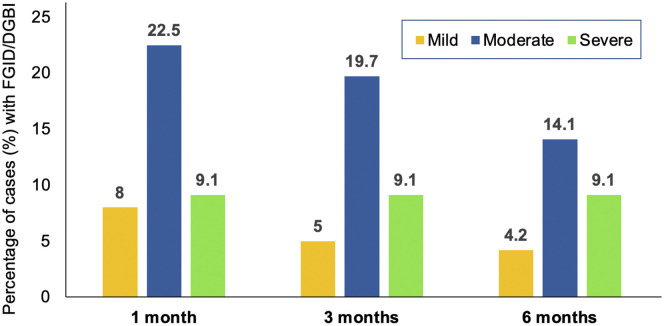
Moderate-severe COVID-19 was associated with development of FGID (P < .01) (Supplementary Figure 1). Presence of GI symptoms during COVID-19 and at 1 month was also a predictive risk factor (P < .01) (Table 2 ). At 3 and 6 months of follow-up among FGID patients, 12 (44.4%) and 7 (33.3%), respectively, had GI symptoms during COVID-19, whereas the remaining did not have GI symptoms at baseline (Figure 2 ).
Supplementary Figure 1.
Bar graph depicting baseline severity of infection during follow-up among FGID/DGBI patients.
Table 2.
Univariate Analysis of Predictive Risk Factors
| Parameter | FGID (n = 27) | No FGID (n = 293) | P value |
|---|---|---|---|
| Age, mean (y) | 35.58 ± 10.8 | 38.33 ± 12 | .19 |
| Gender (female) | 14 (51.9%) | 143 (48.8%) | .7 |
| Presence of comorbidity | 3 (11.1%) | 56 (19.1%) | .3 |
| Severity of COVID-19 | |||
| Mild | 12 (44.4%) | 226 (77.1%) | |
| Moderate | 14 (51.9%) | 57 (19.5%) | <.01 |
| Severe | 1 (3.7%) | 10 (3.5%) | |
| Gastrointestinal symptoms during COVID-19 | 12 (44.4%) | 38 (13%) | <.01 |
FGID, functional gastrointestinal disorders.
Figure 2.
Bar graph depicting gastrointestinal (GI) symptoms during COVID-19 at baseline and follow-up among FGID/DGBI patients.
Tests for MAS/SIBO
Patients who had FGID symptoms at 3 months were followed up until 6 months and underwent additional lab/endoscopic tests. Of the 27 patients who had persistent symptoms at 3 months, 8 (29.6%) had isolated carbohydrate malabsorption (positive D-xylose test), and 1 (3.7%) had 2 positive tests fulfilling criteria for MAS (positive D-Xylose test + low B12). One patient (3.7%) had IMO as documented by methane production on hydrogen breath test. Symptomatic patients who underwent endoscopy with biopsies had normal small intestinal histology (Table 3 ).
Table 3.
Characteristics of Patients With FGID/DGBI
| Subject no. | Age (y)/sex | Type of FGID/DGBI | D-Xylose, g/5 g/5 h (positive) | Hydrogen breath test | Hemoglobin (g/dL) | Albumin (g/dL) | B12 (pg/mL) | UGIE with biopsy |
|---|---|---|---|---|---|---|---|---|
| 1 | 35/M | IBS-C | 0.55 | Negative | 15.5 | 5.3 | 432 | Normal |
| 2 | 68/M | Functional dyspepsia | 0.62 | Negative | 13.1 | 3.5 | 362 | Normal |
| 3 | 42/F | FAB/D | 0.91 | Negative | 13.3 | 4.1 | 214 | Normal |
| 4 (IMO) | 20/F | IBS-M/FD | 0.76 | Methane producer | 13.3 | 4.4 | 224 | Normal |
| 5 | 23/M | Functional diarrhea | 0.88 | Negative | 15.0 | 5.3 | 240 | Normal |
| 6 | 40/F | IBS-D/FD | 0.23 | Negative | 12.3 | 4.3 | 450 | Normal |
| 7 | 36/F | IBS-M | 0.42 | Negative | 12.3 | 4.1 | 1492 | Normal |
| 8 | 46/F | Functional dyspepsia | 0.88 | Negative | 14.0 | 4.9 | 422 | Normal |
| 9 (MAS) | 37/M | Functional dyspepsia | 0.96 | Negative | 13.8 | 4.1 | 186 | Normal |
FAB/D, functional abdominal bloating/distention; FD, functional dyspepsia; IBS-C, irritable bowel syndrome-constipation; IBD-D, irritable bowel syndrome-diarrhea; IBS-M, irritable bowel syndrome-mixed; IMO, intestinal methanogen overgrowth; MAS, malabsorption syndrome; UGIE, upper gastrointestinal endoscopy.
Discussion
This prospective cohort study with 6 months of follow-up showed that (1) patients with COVID-19 have a higher probability of developing FGID/DGBI, (2) moderate and severe forms of infection pose greater risk than mild ones, (3) presence of GI symptoms during COVID-19 is associated with a higher frequency of development of FGID/DGBI, and (4) PI-FGID can be concomitant with underlying MAS/SIBO/IMO that can be detected if appropriate tests are used.
PI-IBS is a common disorder in which symptoms begin after an episode of infective gastroenteritis. As per Rome criteria, PI-IBS is diagnosed if 2 of 4 criteria are present: (1) fever, (2) diarrhea, (3) vomiting, and (4) positive stool culture that suggests acute infectious gastroenteritis, with symptoms developing immediately after resolution with no evidence of IBS before the episode.16 The first formal description of PI-IBS was published in 1962 by Chaudhary and Truelove.17 Various studies have reported incidence of PI-IBS to range between 5% and 32%.8 Similar to above criteria, Schmulson et al18 proposed criteria for post-COVID-19 FGID/DGBI as the following: symptoms fulfilling Rome IV criteria for any FGID/DGBI in the past 3 months, with symptom onset at least 6 months before diagnosis associated with (1) previous COVID-19 infection confirmed by severe acute respiratory syndrome–associated coronavirus-2 real-time polymerase chain reaction, (2) symptom development immediately after resolution of acute infection, and (3) should not meet criteria for FGID before onset of acute illness. A systematic review of 45 studies comprising 21,000 individuals with enteritis followed for 3 months–10 years found a pooled prevalence of IBS at 12 months to be 10.1%.6 Our study found that 36 among the 320 (11.3%) developed FGID-like symptoms at 1 month, 27 (8.4%) and 21 (6.6%) had persistent symptoms at 3 and 6 months, respectively, similar to prior studies.
On the basis of prior experiences with viral gastroenteritis, it was hypothesized that COVID-19 could be followed by the development of FGID/DGBI.18 We showed that at 3 months, 8 (2.5%) had IBS, 7 (2.2%) had functional diarrhea, 6 (1.9%) had FD, 3 (0.9%) had functional constipation, 2 (0.6%) had FD-IBS overlap, and 1 (0.3%) had functional abdominal bloating/distention. Among patients with IBS and FD-IBS overlap, IBS-diarrhea predominant was most common. Our findings are similar to those of recent studies exploring the surge of FGID/DGBI.18 A multicenter study done in India and Bangladesh showed at 6 months after infection, 15 (5.3%), 6 (2.1%), and 5 (1.8%) of the 280 COVID-19 patients developed IBS, uninvestigated dyspepsia, and IBS-uninvestigated dyspepsia overlap, respectively.19 Various studies on post-COVID-19 FGID/DGBI are summarized in Table 4 .3 , 19, 20, 21, 22, 23, 24
Table 4.
Various Studies Done on Post–COVID-19 FGID/DGBI
| Study | No. of patients/controls | Frequency of IBS/FGIDs in cases | Frequency of IBS/FGIDs in controls | Follow-up (mo) | Comments |
|---|---|---|---|---|---|
| Ghoshal et al19 (2021) | 280/264 | IBS: 5.3% UD: 2.1% IBS-UD overlap: 1.8% |
IBS: 0.3% | 6 | Historical controls No investigations done for MAS |
| Velez et al20 (2022) | 200/no controls | IBS: 29% FD: 1% Overlap: 9.5% |
— | 6 | No controls |
| Blackett et al21 (2022) | 1783/no controls | GI symptoms: 29% | — | 6 | No controls |
| Ziyad Al-Aly et al3 (2021) | 33,940 breakthrough infections vs controls | Increased GI disturbances | — | 6 | Self-reported questionnaire No investigations done for MAS |
| Oshima et al22 (2020) | 5157/no controls | FD: 8.5% IBS: 16.6% FD-IBS: 4% |
— | 6 | No controls |
| Nakov et al23 (2021) | 1896/980 | FGID: 36% | 6 | Controls not well-defined No investigations done for MAS |
|
| Noviello et al24 (2021) | 164/183 | IBS: 26.2% | IBS: 25.1% | 5 | Controls not well-defined No investigations done for MAS |
FD, functional dyspepsia; FGID, functional gastrointestinal disorders; GI, gastrointestinal; IBS, irritable bowel syndrome; MAS, malabsorption syndrome; UD, uninvestigated dyspepsia.
The pathophysiology underlying the development of PI-IBS after COVID-19 is not fully understood, although proposed theories include persistent subclinical inflammation, changes in the permeability of the gut barrier, and alteration in gut microflora.25 Prior studies showed inflammation could persist for several months to years, leading to prolonged intestinal dysfunction.26 Persistence of low-grade inflammation with gut dysbiosis appears to be the most important trigger for FGID after infection. A prospective study followed 106 patients with PACS and found that gut microbiota at baseline could predict the occurrence of PACS. Non-PACS COVID-19 patients had recovering gut microbiota as compared with those who developed PACS.27 Although research is in its early stages, preliminary data reveal enrichment of opportunistic pathogens and depletion of commensal flora after infection with COVID-19.27
Recent studies also suggest some patients with IBS could have underlying malabsorption and SIBO.28 Because all 3 entities have overlapping presentations, it is expected that many true cases of MAS and SIBO could be missed.7 In the past after an episode of acute gastroenteritis, around 10%–30% of patients were reported to develop PI-MAS, which is also referred to as tropical sprue.9 Pimentel et al29 have validated novel biomarkers for identifying IBS-diarrhea predominant, especially the post-infectious subtype. They found titers of anti-cytolethal distending toxin B and anti-vinculin antibody levels to be much higher when compared with other causes of diarrhea. Another study also showed higher antibody titers in IBS compared with healthy controls, and titers were higher in IBS-diarrhea and -mixed compared with IBS-constipation.30 In a randomized controlled trial of 80 patients with IBS, 15 (19%) had SIBO on upper gut aspirate culture.31 In one meta-analysis of 12 studies comprising 1921 patients with IBS, pooled prevalence of SIBO was 54%.32 An Indian study done by Rana and Malik33 showed the prevalence of SIBO to be 11.1% in IBS. A prospective cohort study studied the outcomes of 345 patients who had recovered from infectious gastroenteritis. Among those having FGID, 2 of 23 had underlying MAS diagnosed by lab-based tests.34 Because of lack of controls in many studies, the exact causal relationship between PI-IBS and MAS/SIBO could not be effectively demonstrated. Our study showed that among the 27 patients who fulfilled criteria for FGID at 3 months, 8 (29.6%) had 1 positive test for carbohydrate malabsorption (D-Xylose test), and 1 (3.7%) had 2 positive tests. None were positive for SIBO; however, 1 (3.7%) had increased methane production (>10 ppm) on glucose hydrogen breath test and was labeled as IMO. Hence further studies are required to explore this association.
Considering the benign and self-resolving nature of PI-FGID, management options have not been extensively explored and include symptom-based treatment. A recent study showed mast cell activation as one of the important mechanisms of long COVID, and therapies directed against it may benefit symptoms.35 For example, H1/H2 blockers may lead to symptomatic improvement in patients with long COVID.36
The results of our study reconfirm that PI-IBS has a good prognosis, considering the improvement of symptoms in patients over time. Another important observation is that the incidence of PI-IBS was similar to published evidence on COVID-1919 and norovirus.37 , 38 Hence, it is plausible that post-COVID-19 FGID behaves in a manner similar to other viral gastroenteritis, with comparable overall prognosis.
A notable strength of our study is the recruitment of age- and sex-matched controls who are family members of cases and sharing the same environmental and dietary conditions. A second control group B who were serology negative for COVID-19 was also included. Another strength is the use of Rome IV criteria to diagnose FGID/DGBI.
Our study has certain limitations. Patients were infected predominantly by the delta variant; thus, it is not known whether the results can be extrapolated to other COVID variants. Although a COVID antibody negative control group B was recruited, there is still a possibility of asymptomatic infection that could be detected by spike protein and T-cell testing.39 Although psychiatric comorbidities are important risk factors for FGID, we have excluded subjects with major psychiatric illnesses, considering that heightened distress during pandemic times could lead to false-positive results. Another limitation is that the mechanisms of post-COVID-19 FGID were not evaluated.
In conclusion, at 6 months of follow-up, COVID-19 infection led to development of various FGID/DGBI. The long-term course of these symptoms and the underlying microbiome alterations may help unravel the pathophysiological mechanisms.
Acknowledgments
CRediT Authorship Contributions
Rithvik Golla (Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Equal; Writing – original draft: Lead)
Sudheer Vuyyuru (Supervision: Supporting; Writing – review & editing: Supporting)
Bhaskar Kante (Supervision: Supporting; Writing – review & editing: Supporting)
Peeyush Kumar (Supervision: Supporting; Writing – review & editing: Supporting)
David Thomas Mathew (Writing – review & editing: Supporting)
Govind Makharia (Supervision: Supporting; Writing – review & editing: Supporting)
Saurabh Kedia (Formal analysis: Supporting; Investigation: Supporting; Supervision: Equal; Writing – review & editing: Supporting)
Vineet Ahuja (Conceptualization: Lead; Formal analysis: Supporting; Methodology: Equal; Project administration: Lead; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://doi.org/10.1016/j.cgh.2022.10.015.
Supplementary Material
References
- 1.Rokkas T. Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:355–365. doi: 10.20524/aog.2020.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature [Internet. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 4.Greenhalgh T., Knight M., A’Court C., et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 5.Sperber A.D., Bangdiwala S.I., Drossman D.A., et al. Worldwide prevalence and burden of functional gastrointestinal disorders: results of Rome Foundation global study. Gastroenterology. 2021;160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Klem F., Wadhwa A., Prokop L., et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology. 2017;152:1042–1054.e1. doi: 10.1053/j.gastro.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghoshal U.C., Gwee K.A. Post-infectious IBS, tropical sprue and small intestinal bacterial overgrowth: the missing link. Nat Rev Gastroenterol Hepatol. 2017;14:435–441. doi: 10.1038/nrgastro.2017.37. [DOI] [PubMed] [Google Scholar]
- 8.Thabane M., Marshall J.K. Post-infectious irritable bowel syndrome. World J Gastroenterol. 2009;15:3591–3596. doi: 10.3748/wjg.15.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghoshal U.C., Srivastava D., Verma A., et al. Tropical sprue in 2014: the new face of an old disease. Curr Gastroenterol Rep. 2014;16:391. doi: 10.1007/s11894-014-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drossman D.A. Functional gastrointestinal disorders: history, pathophysiology, clinical features, and Rome IV. Gastroenterology. 2016;150:1262–1279.e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Drossman D.A., Tack J. Rome Foundation clinical diagnostic criteria for disorders of gut-brain interaction. Gastroenterology. 2022;162:675–679. doi: 10.1053/j.gastro.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Cascella M., Rajnik M., Aleem A., et al. StatPearls Publishing; Treasure Island, FL: 2022. Features, evaluation, and treatment of coronavirus (COVID-19) [PubMed] [Google Scholar]
- 13.Peled Y., Doron O., Laufer H., et al. D-xylose absorption test: urine or blood? Dig Dis Sci. 1991;36:188–192. doi: 10.1007/BF01300755. [DOI] [PubMed] [Google Scholar]
- 14.Ghoshal U.C. How to interpret hydrogen breath tests. J Neurogastroenterol Motil. 2011;17:312–317. doi: 10.5056/jnm.2011.17.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra S., Jani P.A. An approach to duodenal biopsies. J Clin Pathol. 2006;59:1133–1150. doi: 10.1136/jcp.2005.031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbara G., Grover M., Bercik P., et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156:46–58.e7. doi: 10.1053/j.gastro.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary N.A., Truelove S.C. The irritable colon syndrome: a study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med. 1962;31:307–322. [PubMed] [Google Scholar]
- 18.Schmulson M., Ghoshal U.C., Barbara G. Managing the inevitable surge of post–COVID-19 functional gastrointestinal disorders. Am J Gastroenterol. 2021;116:4–7. doi: 10.14309/ajg.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 19.Ghoshal U.C., Ghoshal U., Rahman M.M., et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: a case–control study. J Gastroenterol Hepatol. 2022;37:489–498. doi: 10.1111/jgh.15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vélez C., Paz M., Silvernale C., et al. Factors associated with chronic de novo post-coronavirus disease gastrointestinal disorders in a metropolitan US county. Clin Gastroenterol Hepatol. 2022;20:e1488–e1492. doi: 10.1016/j.cgh.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackett J.W., Wainberg M., Elkind M.S.V., et al. Potential long coronavirus disease 2019 gastrointestinal symptoms 6 months after coronavirus infection are associated with mental health symptoms. Gastroenterology. 2022;162:648–650.e2. doi: 10.1053/j.gastro.2021.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshima T., Siah K.T.H., Yoshimoto T., et al. Impacts of the COVID-19 pandemic on functional dyspepsia and irritable bowel syndrome: a population-based survey. J Gastroenterol Hepatol. 2021;36:1820–1827. doi: 10.1111/jgh.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakov R., Dimitrova-Yurukova D., Snegarova V., et al. Increased prevalence of gastrointestinal symptoms and disorders of gut-brain interaction during the COVID-19 pandemic: an internet-based survey. Neurogastroenterol Motil. 2022;34 doi: 10.1111/nmo.14197. [DOI] [PubMed] [Google Scholar]
- 24.Noviello D., Costantino A., Muscatello A., et al. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: a controlled cohort study. Neurogastroenterol Motil. 2022:34. doi: 10.1111/nmo.14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbosa da Luz B., de Oliveira N.M.T., França dos Santos I.W., et al. An overview of the gut side of the SARS-CoV-2 infection. Intest Res. 2021;19:379–385. doi: 10.5217/ir.2020.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thabane M., Kottachchi D.T., Marshall J.K. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:535–544. doi: 10.1111/j.1365-2036.2007.03399.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu Q., Mak J.W.Y., Su Q., et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022;71:544–552. doi: 10.1136/gutjnl-2021-325989. [DOI] [PubMed] [Google Scholar]
- 28.Takakura W., Pimentel M. Small intestinal bacterial overgrowth and irritable bowel syndrome: an update. Front Psychiatry. 2020;11:664. doi: 10.3389/fpsyt.2020.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pimentel M., Morales W., Rezaie A., et al. Development and validation of a biomarker for diarrhea-predominant irritable bowel syndrome in human subjects. PLOS One. 2015;10 doi: 10.1371/journal.pone.0126438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezaie A., Park S.C., Morales W., et al. Assessment of anti-vinculin and anti-cytolethal distending toxin B antibodies in subtypes of irritable bowel syndrome. Dig Dis Sci. 2017;62:1480–1485. doi: 10.1007/s10620-017-4585-z. [DOI] [PubMed] [Google Scholar]
- 31.Goyal O., Nohria S., Dhaliwal A.S., et al. Prevalence, overlap, and risk factors for Rome IV functional gastrointestinal disorders among college students in northern India. Indian J Gastroenterol. 2021;40:144–153. doi: 10.1007/s12664-020-01106-y. [DOI] [PubMed] [Google Scholar]
- 32.Ford A.C., Spiegel B.M.R., Talley N.J., et al. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286. doi: 10.1016/j.cgh.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Rana S.V., Malik A. Hydrogen breath tests in gastrointestinal diseases. Indian J Clin Biochem. 2014;29:398–405. doi: 10.1007/s12291-014-0426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman M.M., Ghoshal U.C., Sultana S., et al. Long-term gastrointestinal consequences are frequent following sporadic acute infectious diarrhea in a tropical country: a prospective cohort study. Am J Gastroenterol. 2018;113 doi: 10.1038/s41395-018-0208-3. 1363–1175. [DOI] [PubMed] [Google Scholar]
- 35.Weinstock L.B., Brook J.B., Walters A.S., et al. Mast cell activation symptoms are prevalent in long-COVID. Int J Infect Dis. 2021;112:217–226. doi: 10.1016/j.ijid.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynne P., Tahmasebi N., Gant V., et al. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J Investig Med. 2022;70:61–67. doi: 10.1136/jim-2021-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marshall J.K., Thabane M., Borgaonkar M.R., et al. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol. 2007;5:457–460. doi: 10.1016/j.cgh.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Zanini B., Ricci C., Bandera F., et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]
- 39.Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]