Abstract
Objectives
This study aims to evaluate the school careers of patients with congenital heart disease (CHD) and microcephaly.
Methods
An exploratory online survey was conducted on patients from a previous study on somatic development in children with CHD in 2018 (n = 2818). A total of 750 patients participated in the online survey (26.6%). This publication focuses on 91 patients (12.1%) diagnosed with CHD and microcephaly who participated in the new online survey.
Results
Microcephaly was significantly associated with CHD severity (p < 0.001). Microcephalic patients suffered from psychiatric comorbidity two times as often (67.0%) as non-microcephalic patients (29.8%). In particular, the percentage of patients with developmental delay, intellectual debility, social disability, learning disorder, or language disorder was significantly increased in microcephalic CHD patients (p < 0.001). A total of 85.7% of microcephalic patients and 47.6% of non-microcephalic patients received early interventions to foster their development. The school enrollment of both groups was similar at approximately six years of age. However, 89.9% of non-microcephalic but only 51.6% of microcephalic patients were enrolled in a regular elementary school. Regarding secondary school, only half as many microcephalic patients (14.3%) went to grammar school, while the proportion of pupils at special schools was eight times higher. Supportive interventions, e.g., for specific learning disabilities, were used by 52.7% of microcephalic patients and 21.6% of non-microcephalic patients.
Conclusion
Patients with CHD and microcephaly are at high risk for impaired educational development. Early identification should alert clinicians to provide targeted interventions to optimize the developmental potential.
Keywords: microcephaly, congenital heart disease, education, school, supportive interventions, development
Introduction
Children with congenital heart disease (CHD) are at risk of impaired somatic growth, especially those with single ventricle physiology (1, 2). The cause of growth retardation is complex and multifactorial, with genetic and hemodynamic factors as essential determinants for the underlying CHD (3, 4).
Most studies analyzing somatic development focus on body weight and length but less often on the head circumference (2, 4, 5). However, head circumference is an important somatic parameter, as microcephaly may indicate impaired neurodevelopmental outcomes from early childhood until adolescence (6–9). Neurodevelopmental deficits may have a broad clinical manifestation and may also affect areas of memory and executive function, visual-spatial imagination, attention, and social skills (10) and therefore impair the child's school career.
To date, the association between microcephaly and education in children with CHD has not been analyzed. Hence, this study aimed to evaluate the scholastic development of CHD patients with microcephaly.
Patients and methods
Study design and setting
This is an exploratory cross-sectional follow-up study using an online survey to evaluate the school careers of patients with CHD. The ethics committee of the Charité—Universitätsmedizin Berlin, Germany, approved this study (no. EA2/190/19), which was conducted in 2020.
Study participants received an invitation to participate in the survey by email or postal letters, and if a response was missing, they were reminded once to participate. The survey could be completed by the patient, a parent, or a third party (legal guardian or caregiver). The corresponding addressee received a slightly different questionnaire. The survey consisted of a maximum of 74 questions, depending on the participant and the option of sequential questions. A total of 92% of the survey's questions were closed multiple-choice questions. Most questions offered the participant the option of adding their own answer if none of the possible answers adequately described their situation in a free-text box. The survey comprised questions about medical data such as further chronic diseases, psychiatric comorbidities (attention deficit disorder, developmental disability, intellectual debility, social disability, emotional disability, depression, anxiety disorder, learning disorder, and language disorder), genetic syndromes, and subjective health conditions. The focus was on questions about the school career: enrollment age, school form (primary and secondary), (early) supportive therapy (physiotherapy, speech therapy, occupational therapy, or psychotherapy), school year repetition, absenteeism from school, and participation in physical education. Furthermore, the educational achievement and qualifications of the parents were elicited. Medical data were supplemented by the database of the National Register for Congenital Heart Defects (NRCHD). The NRCHD is Germany's national repository for medical data on CHD patients. With ~55,000 members, the NRCHD is Europe's largest register for CHD patients and can be regarded as a basis for representative studies (11).
Patient cohort
From 2006 to 2011, the Competence Network for Congenital Heart Defects (Berlin, Germany) conducted the “PAN-study” (Prävalenz angeborener Herzfehler bei Neugeborenen in Deutschland), which analyzed the prevalence of CHD prospectively in newborns in Germany (12). To determine the somatic development (height, length, and head circumference) of the PAN-cohort, the “PANKU-study” (Prävalenz angeborener Herzfehler bei Neugeborenen in Deutschland Kopfumfang) was carried out; a follow-up study analyzed somatic developmental data from the “Kinderuntersuchungsheft” (“child's medical records”), which records the examination results of the mandatory screening for children and adolescents (13). To assess the academic development of this particular cohort, we chose this cohort for the present study “PANKU-Education” (Prävalenz angeborener Herzfehler bei Neugeborenen in Deutschland: Kopfumfang—Education). Of the 2818 families that were contacted, 750 (26.6%) completed the survey.
Inclusion criteria
Participation in the “PAN-study” and “PAN-KU-study”
Availability of complete and up-to-date contact information at the time of the present study
Complete answering the online-survey
Microcephaly
To analyze the prevalence of microcephaly in the presented study, head circumference data at the child's three-month checkup (corresponding to U4 screening between the 3rd and 4th month of life in the German health care system) were used. These data were converted into sex- and age-adjusted percentiles, taking into account the date of measurement, date of birth, sex, and gestational age at birth. The percentiles, according to Braegger et al., commonly used in Germany, served as the basis for the calculation (14). Microcephaly was defined as a head circumference < 3rd percentile.
The german school system
In Germany, school attendance is compulsory until the age of 15 years and is free. Usually, children start elementary school at the age of 5–7 years, mainly, however, at the age of 6 years. If a child has special needs in their educational, developmental, and learning possibilities (e.g., due to a learning or mental/cognitive disability, a sensory and/or physical disability, or less frequently due to a long-term illness), they are introduced to a special school. After finishing primary education (4–6 years, depending on the federal state), there are several options for secondary schooling according to the student's abilities: the highest is the grammar school (“Gymnasium”), where pupils graduate after 8–9 years with a high school diploma, enabling them to study at university. Graduation from secondary school options (usually after 5–7 years) allows for starting an apprenticeship.
Statistical analyses
The cardiac diagnoses were arranged in accordance with the classification of the International Pediatric and Congenital Cardiac Code (IPCCC) (15). Following Warnes et al., the CHD diagnoses were assigned to four groups: simple CHD, moderate CHD, complex CHD, and other/non-classified CHD (16).
For the various research questions, different subgroups were defined. The influence of parental education level was analyzed using descriptive analysis due to limited data.
For this, the parental education level was classified as “high,” “medium,” and “low” according to the International Standard Classification of Education (ISCED) (17). Group differences were analyzed using the chi-square test for nominal variables of independent subsamples. The Mann-Whitney U test was used for ordinal scaled variables, and interval scaled variables were analyzed using the t-test. A p-value of < 0.05 was considered statistically significant. SPSS (Statistics for Mac, Version 27.0, IBM Crop. Armonk, NY) was used for statistical analysis.
Results
Description of the overall cohort
A total of 750/2818 study participants (26.6%) answered all survey questions and were included in the statistical analyses. The mean age of the overall study cohort was 12.16 years (confidence interval: 12.10–12.22), and 50.4% were females (Table 1). Subjective health status was excellent, but psychiatric comorbidity was reported in 34.5%. Most patients had simple CHD (51.3%), with ventricular septal defect (40.7%) and atrial septal defect (11.9%) being the most frequent ones (Table 2).
Table 1.
Characteristics of all patients from our cohort.
| N | Mean | SD | ||
|---|---|---|---|---|
| Median | IQR | |||
| Total participants | 750 | 100% | ||
| Mean age | 12.16 | 0.847 | ||
| Sex | ||||
| Female | 378 | 50.4% | ||
| Male | 372 | 49.6% | ||
| Health status | ||||
| Subjective health status (1 very good- 6 bad) | 1 | 1 | ||
| Epilepsy | 13 | 1.7% | ||
| Optic support | 262 | 34.9% | ||
| Hearing support | 22 | 2.9% | ||
| Walking aid | 11 | 1.5% | ||
| Psychiatric comorbidity | 259 | 34.5% | ||
| Gestational age | ||||
| Preterm | 115 | 15.8% | ||
| Term | 610 | 83.8% | ||
| Post-term | 3 | 0.4% | ||
| Syndromic disorders | 64 | 8.3% | ||
| Alagille syndrome | 1 | 1.6% | ||
| DiGeorge syndrome | 5 | 7.8% | ||
| Goldenhar syndrome | 2 | 3.1% | ||
| Trisomy 18 | 1 | 1.6% | ||
| Trisomy 21 | 38 | 59.4% | ||
| Noonan syndrome | 3 | 4.7% | ||
| Williams syndrome | 3 | 4.7% | ||
| Others | 11 | 17.2% |
Table 2.
CHD diagnoses of the patients in our cohort.
| N | ||
|---|---|---|
| Ventricular septal defect | 305 | 40.7% |
| Atrial septal defect | 89 | 11.9% |
| Tetralogy of Fallot | 48 | 6.4% |
| Univentricular heart | 46 | 6.1% |
| Aortic valve disease | 36 | 4.8% |
| Coarctation of the aorta | 35 | 4.7% |
| Transposition of the great arteries (intact ventricular septum) | 32 | 4.3% |
| Atrioventricular septal defect | 29 | 3.9% |
| Pulmonary valve stenosis | 28 | 3.7% |
| Patent ductus arteriosus | 20 | 2.7% |
| Other | 82 | 10.9% |
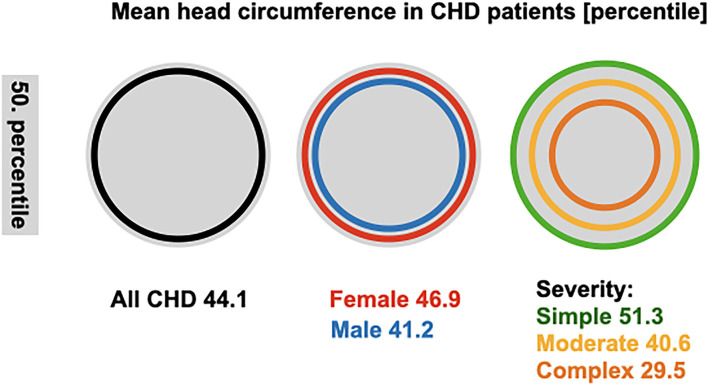
At the age of 3–4 months, the mean head circumference of CHD patients from our cohort (percentile 44.1 ± 33.3) equaled the age-dependent percentile; Figure 1 illustrates the head circumference percentiles. The mean percentile in female patients was 46.9 ± 33.3 and in male patients 41.2 ± 33.1. Patients with simple CHD had a head circumference approximating the 51.3 ± 33.8 percentile, moderate CHD 40.6 ± 33.1, and complex CHD 29.5 ± 33.2.
Figure 1.
Mean head circumference in CHD patients. Head circumference percentiles at 3–4 months in all CHD patients and subgroups. The gray circle represents the 50 percentile, and data of patients and subgroups are denoted as the mean. SD for all, sex and simple severity are 33, moderate severity 32, and complex 31.
Characterization of microcephalic CHD patients
In total, 91 out of 750 patients (12.1%) had a head circumference below the 3rd percentile and thus were classified as microcephalic (Tables 3, 4). This subgroup had a mean age of 12.2 ± 0.85 years; 48.4% were female, and 51.6% were male. Their subjective health was not as good as the non-microcephalic patients' but still very good (51.6% of microcephalic vs. 66.3% of non-microcephalic patients rated their health status as very good) (Table 3). The prevalence of epilepsy and the need for optic support, hearing support, or walking aid was higher among microcephalic patients. In contrast, gestational age was not significantly associated with microcephaly.
Table 3.
Characteristics of microcephalic and non- microcephalic patients.
| Non-microcephalic patients | Microcephalic patients | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||||
| Median | IQR | Median | IQR | ||||||
| Total participants | 621 | 100% | 91 | 100% | |||||
| Mean age | 12.14 | 841 | 12.2 | 0.885 | n.s. | ||||
| Sex | |||||||||
| Female | 317 | 51.0% | 44 | 48.4% | n.s. | ||||
| Male | 304 | 49.0% | 47 | 51.6% | n.s. | ||||
| Health status | |||||||||
| Subjective health status (1 very good- 6 bad) | 1 | 1 | 1 | 1 | + | ||||
| Epilepsy | 6 | 1.0% | 7 | 7.7% | * | ||||
| Optic support | 199 | 32.0% | 44 | 48.4% | + | ||||
| Hearing support | 8 | 1.3% | 11 | 12.1% | * | ||||
| Walking aid | 4 | 0.6% | 7 | 7.7% | * | ||||
| Psychiatric comorbidity | 185 | 29.8% | 61 | 67.0% | * | ||||
| Gestational age | |||||||||
| Preterm | 94 | 15.1% | 16 | 17.6% | n.s. | ||||
| Term | 524 | 84.4% | 75 | 82.4% | n.s. | ||||
| Post-term | 3 | 0.5% | 0 | 0% | n.s. | ||||
| Syndromic disorders | 32 | 5.2% | 28 | 30.8% | * | ||||
| Alagille syndrome | 1 | 3.1% | - | - | |||||
| DiGeorge syndrome | 2 | 6.3% | 2 | 7.1% | |||||
| Goldenhar syndrome | 1 | 3.1% | 1 | 3.6% | |||||
| Trisomy 18 | – | – | 1 | 3.6% | |||||
| Trisomy 21 | 18 | 56.3% | 17 | 60.7% | |||||
| Noonan syndrome | – | – | 1 | 3.6% | |||||
| Williams syndrome | 2 | 6.3% | 1 | 3.6% | |||||
| Others | 8 | 25.0% | 5 | 17.9% | |||||
p *denotes significant difference compared to non-microcephalic p < 0.001, + denotes p < 0.05, n.s. = not significant.
For ordinal scaled variables, median and IQR were given accordingly (printed in italics).
Table 4.
CHD diagnoses of microcephalic patients.
| N | ||
|---|---|---|
| Ventricular septal defect | 29 | 31.9% |
| Atrial septal defect | 7 | 7.7% |
| Tetralogy of Fallot | 6 | 6.6% |
| Univentricular heart | 11 | 12.1% |
| Aortic valve disease | 2 | 2.2% |
| Coarctation of the aorta | 4 | 4.4% |
| Transposition of the great arteries (intact ventricular septum) | 7 | 7.7% |
| Atrioventricular septal defect | 12 | 13.2% |
| Other | 13 | 14.3% |
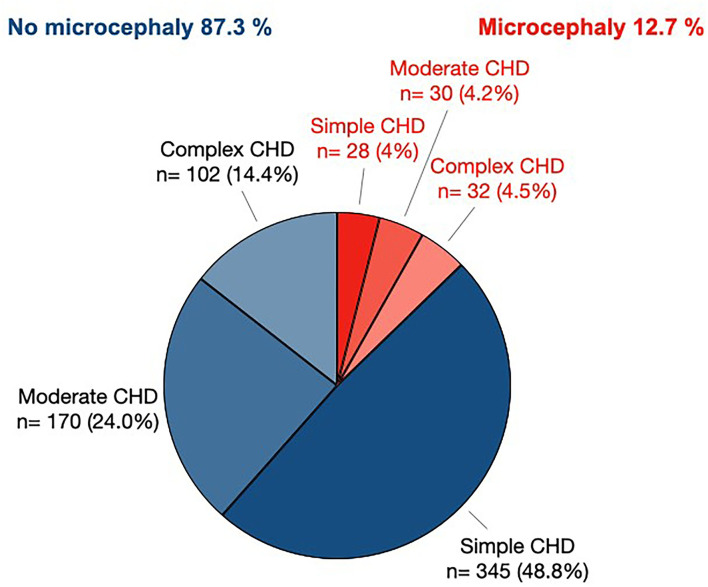
Syndromal diseases existed in 30.8% of the microcephalic patients (Table 3). Ventricular septal defect (31.9%), atrioventricular septal defect (13.2%), and univentricular heart (12.1%) were the most frequent CHDs in this subset (Table 4). Microcephaly was significantly associated with CHD severity (Figure 2). Simple CHD was seen in 55.9% of normocephalic patients, while moderate CHD was seen in 27.5% and complex CHD in 16.6%. In contrast, simple, moderate, and complex CHD was found in equal parts (31.1, 33.3, and 35.6%) in microcephalic patients.
Figure 2.
Prevalence of microcephaly in patients with simple, moderate, or complex CHD. The pie chart denotes the raw n of patients defined by CHD severity classification and head circumference. Only patients with known CHD severity scores and known head circumference are included (n = 707).
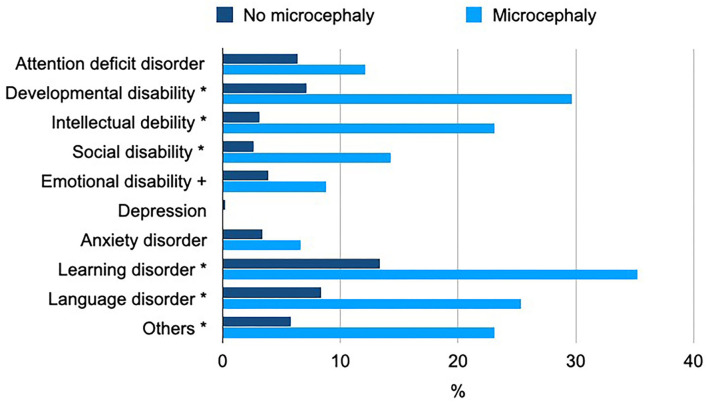
A total of 29.8% of non-microcephalic patients suffered from psychiatric comorbidity, while in microcephalic patients, this proportion was more than two times as high (67.0%) (Table 3). In particular, the percentage of patients with developmental delay, intellectual debility, social disability, learning disorder, or language disorder was significantly increased in microcephalic CHD patients (p < 0.001; Figure 3).
Figure 3.
Psychiatric comorbidity in microcephalic CHD patients. The chart visualizes the percentage of patients with the most prevalent psychiatric disorders among microcephalic or non-microcephalic patients; * denotes a significant difference compared to non-microcephalic, p < 0.001, and + denotes p < 0.05.
Educational status of microcephalic CHD patients
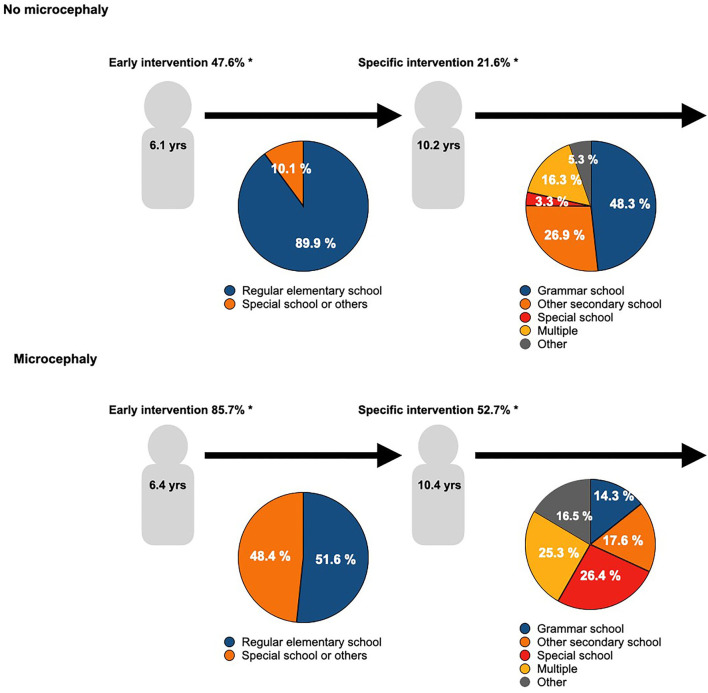
The educational career and usage of support interventions of microcephalic patients compared to CHD patients without microcephaly are visualized in Figure 4. A total of 85.7% of the microcephalic patients but only 47.6% of non-microcephalic patients received early interventions to foster their development. The school enrollment of both groups was similar at approximately six years of age. 89.9% of non-microcephalic but only 51.6% of microcephalic patients were enrolled in a regular elementary school. About four years later, at the age of 10, children from both groups transferred to a new school: 48.3% of the non-microcephalic patients went to grammar school, and 3.3% needed specialized schools. In contrast, only 14.3% of the microcephalic patients went to grammar school, while the proportion of pupils at special schools was eight times higher than in non-microcephalic patients. Supportive interventions, e.g., because of specific learning disabilities, were used by 52.7% of microcephalic patients and 21.6% of non-microcephalic patients.
Figure 4.
Educational development in CHD patients. The graph visualizes the school career of CHD patients with or without microcephaly. The median age of school enrollment and the first change of school are given in the figures. The distribution of different kinds of primary schools in the left pie chart is represented, and the pie chart on the right represents the different kinds of secondary schools. Further, the usage of early and/ or specific interventions to support the school careers is given. Differences in the kind of school are significant < 0.001; additionally * denotes a significant difference compared to non-microcephalic patients, p < 0.001, and + denotes p < 0.05.
Staying down a year was statistically insignificant between microcephalic and non-microcephalic patients (Table 5); school grades were similar in both groups, too. Absenteeism from school added to < 1 month in most microcephalic patients (Table 5). In our cohort, parental educational level did not influence the attended school forms (primary and secondary school), the class repetition rate, or the utilization of (early) supportive measures.
Table 5.
School career of the microcephalic patients.
| Microcephaly (n = 91) | No microcephaly (n = 621) | ||||
|---|---|---|---|---|---|
| N | N | p | |||
| Stayed down? | |||||
| Yes | 14 | 15.4 % | 57 | 9.2 % | n.s. |
| No | 77 | 84.6 % | 564 | 90.8 % | |
| Participating in physical education? | |||||
| Yes | 69 | 75.8 % | 550 | 88.6 % | + |
| No | 22 | 24.2 % | 71 | 11.4 % | |
| Absenteeism from school | |||||
| < 1 month | 73 | 82 % | 552 | 94 % | * |
| 1 month−1 year | 16 | 18 % | 33 | 5.6 % | |
| >1 year | 0 | 0 % | 2 | 0.3 % | |
The table informs about school attendance in microcephalic and non-microcephalic patients. The whole cohort comprises 750, but data on the head circumference are missing in 28 patients. Thus, the sum of microcephalic and non- microcephalic patients is 712. * denotes significant difference compared to non-microcephalic p < 0.001, + denotes p < 0.05, n.s. = insignificant data regarding missed school time was missing in n = 2.
Discussion
Our data demonstrate that microcephaly in early childhood is a crucial risk factor for impaired scholastic development in patients with CHD. These results expand the current state of research, as the educational career of microcephalic vs. non-microcephalic CHD patients has not been evaluated. This is noteworthy as education is a particularly important and objectively measurable characteristic of neurodevelopment. In our study, microcephalic participants had a high demand for early and specific interventions. They frequently attended special schools more frequently and compared to non-microcephalic CHD patients, their likelihood of attending a grammar school was halved. Approximately two-thirds of microcephalic patients were diagnosed with psychiatric disorders such as learning, emotional, or behavioral disorders.
Previous studies have shown that a small head circumference in CHD patients is related to neurodevelopmental outcomes in early childhood (6, 7, 18), preschool age (19), and adolescence (20). This is in line with our study results, as microcephalic CHD patients were confronted with multiple problems in their whole (pre-) scholastic development. The etiology of this phenomenon is complex and thought to be multifactorial.
First, mounting evidence suggests that prenatal factors play a crucial role. It has been proven that intrauterine brain growth is predominantly altered in children with complex CHD and may result in a smaller head circumference and brain volume at birth (21, 22). Hemodynamic factors are assumed as an explanatory variable and include umbilical vein oxygen saturation, altered blood flow, and reduced oxygen content in the ascending aorta (23). Moreover, animal and human studies suggest that prenatal maternal psychosocial stress may adversely affect the developing fetal brain (24, 25). However, for expectant mothers after the diagnosis of CHD in their child, this association has hardly been studied (26). A longitudinal study analyzing this would be even more important as a prenatal CHD diagnosis may be a traumatic situation for the expectant mother.
Second, postnatal growth is often impaired in CHD patients. This has mainly been attributed to disrupted normal feeding behavior in the neonatal and infant period (27). In CHD patients requiring early operative treatment, this problem often persists during the postoperative phase, as patients frequently require a nasogastric feeding tube (28, 29). Hypermetabolic state and malabsorption may be further pathophysiologic mechanisms, while genetic and environmental factors are thought to play a subordinate role (27, 30). These are important considerations when interpreting our study results because more than two-thirds of microcephalic CHD patients suffer from moderate or complex CHD.
Third, social environment in the meaning of parental education and supportive therapy may be a rationale as this is an important determinant in childhood development (31, 32). In our study cohort, almost all microcephalic patients received early supportive therapy, and specific interventions fostered more than half at school age. Supportive therapy may compensate for learning deficits (33) and contribute to the fact that a small proportion of microcephalic patients were able to attend grammar school. Interestingly, the parental educational level did not influence the child's scholastic development in the present analysis. This argues for the assumption that in this specific patient cohort, the neurodevelopmental outcome is predominantly associated with somatic factors. Preterm birth, a known risk factor for altered brain growth (34), did not significantly contribute to microcephaly in our study cohort as more than 80% of study participants were born at full term.
Another noteworthy result of the present analysis is the prevalence of psychiatric comorbidities: nearly one-third of non-microcephalic patients suffered from psychiatric comorbidity, but in microcephalic patients, this proportion was more than two times as high. Numerous studies have observed the clinical manifestation of developmental delay, intellectual debility, social disability, learning disorder, or language disorder (35, 36). Underlying pathophysiologic mechanisms are still under investigation. However, prenatal hemodynamic factors and a genetic link between heart and brain development are assumed to be important rationales (37).
Interestingly, the subjective health status of microcephalic patients was not as good as the non-microcephalic patients' but still very good. This has been reported before (38). A possible explanation could be that, as CHD patients were born with heart disease, they might have learned early in life how to develop a strong “sense of coherence” and select the right coping strategies (39).
Clinical implications for this study
It is important for CHD patients, affected families, and treating physicians to be aware of microcephaly as a risk factor for impaired scholastic development. Routine follow-up examinations should be established to identify developmental deficits. Unfortunately, underlying variables such as prenatal hemodynamic factors or the number and point of time of CHD operations are hardly modifiable. However, supportive therapy seems to be a promising compensation mechanism. Therefore, CHD patients and their families should be given low-threshold access to supportive interventions.
Limitations
Due to the data privacy policy of the NRCHD, a non-responder analysis could not be performed. Our results need to be interpreted in light of a potential selection bias. Highly educated and/or healthier CHD patients might be more inclined to participate in scientific studies than patients with lower educational levels and/or more health problems. However, a proportion of 48% of microcephalic patients attending a particular school speaks against this assumption. Our analysis of parental ISCED and child education supports this assumption that in microcephalic patients, somatic (co-) morbidities have a more significant influence than the parental level of education. However, parents with higher education might be overrepresented compared to the German general population. The response rate of 26.6% is in the middle range and may still be considered valid and quite representative (40). The results cannot be easily applied to other countries, as education and health care systems may differ. Due to the study setting (follow-up study of a patient cohort on the somatic development in children with CHD), we could not assess the highest educational/academic achievement as the mean age of our study cohort was ~12 years of age. Seventy patients in the study were diagnosed with a syndromal disease; among these, 28 were microcephalic. Based on the heterogeneity of diagnoses and small subgroups, we could not correct our results for this or perform subgroup analyses. Our study does not answer whether microcephalic patients with or without CHD differ in their educational perspectives. Microcephaly appears to be a risk factor for impaired educational success in CHD patients.
Conclusion
Patients with CHD and microcephaly are at risk for impaired educational development. Head circumference measurement in infants and children with CHD should be integrated into the serial routine monitoring of somatic parameters in children with CHD. Microcephaly in early childhood should alert clinicians to provide targeted interventions to optimize the developmental potential. Further studies are necessary to evaluate the impact of these interventions and to determine the long-term follow-up of this specific patient cohort at risk.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Charité—Universitätsmedizin Berlin, Germany (No. EA2/190/19). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
CP, H-AK, MP, FB, UB, PH, and KS: study concept/design. CP, PH, and KS: data interpretation. CP: drafting of the original manuscript. LS and AH: data analysis. AH: acquisition of data. LS: interpretation, visualization, and drafting of the original manuscript (results). PH, H-AK, MP, and KS: critical revisions of the manuscript. H-AK and MP: acquisition of data. FB and UB: critical revisions of the manuscript. All authors approved the final version of the manuscript.
Funding
This study received funding from Deutsche Herzstiftung e.V. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. CP is participant in the BIH Charité Clinician Scientist Program funded by the Charité—Universitätsmedizin Berlin and the Berlin Institute of Health. This work was supported by the Competence Network for Congenital Heart Defects (Federal Ministry of Education and Research/grant number 01GI0601) and the National Register for Congenital Heart Defects (Federal Ministry of Education and Research/grant number 01KX2140). We acknowledge financial support by Land Schleswig-Holstein within the funding program Open Access Publikationsfonds.
Conflict of interest
Authors UB and PH were employed by the companies National Register for Congenital Heart Defects and Competence Network for Congenital Heart Defects.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JL declared a shared affiliation with the author LS to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.917507/full#supplementary-material
References
- 1.Daymont C, Neal A, Prosnitz A, Cohen MS. Growth in children with congenital heart disease. Pediatrics. (2013) 131:e236–42. 10.1542/peds.2012-1157 [DOI] [PubMed] [Google Scholar]
- 2.Burch PT, Ravishankar C, Newburger JW, Lambert LM, Pemberton VL, Granger S, et al. Assessment of growth 6 years after the Norwood procedure. J Pediatr. (2017) 180:270–4. 10.1016/j.jpeds.2016.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. (2015) 135:816–25. 10.1542/peds.2014-3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. (2007) 50:1876–83. 10.1016/j.jacc.2007.07.050 [DOI] [PubMed] [Google Scholar]
- 5.Francois K, Bove T, Panzer J, De Groote K, Vandekerckhove K, De Wilde H, et al. Univentricular heart and Fontan staging: analysis of factors impacting on body growth. Eur J Cardiothorac Surg. (2012) 41:e139–45. 10.1093/ejcts/ezs194 [DOI] [PubMed] [Google Scholar]
- 6.Limperopoulos C, Majnemer A, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C, et al. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. (2002) 141:51–8. 10.1067/mpd.2002.125227 [DOI] [PubMed] [Google Scholar]
- 7.Mussatto KA, Hoffmann R, Hoffman G, Tweddell JS, Bear L, Cao Y, et al. Risk factors for abnormal developmental trajectories in young children with congenital heart disease. Circulation. (2015) 132:755–61. 10.1161/CIRCULATIONAHA.114.014521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg CS, Lu M, Sleeper LA, Mahle WT, Gaynor JW, Williams IA, et al. Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. (2014) 165:490-6 e8. 10.1016/j.jpeds.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotermann I, Logoteta J, Falta J, Wegner P, Jung O, Dutschke P, et al. Neuro-developmental outcome in single-ventricle patients: is the Norwood procedure a risk factor? Eur J Cardiothorac Surg. (2017) 52:558–64. 10.1093/ejcts/ezx119 [DOI] [PubMed] [Google Scholar]
- 10.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. (2012) 27:82–91. 10.1097/HCO.0b013e328350197b [DOI] [PubMed] [Google Scholar]
- 11.Helm PC, Koerten MA, Abdul-Khaliq H, Baumgartner H, Kececioglu D, Bauer UM. Representativeness of the German national register for congenital heart defects: a clinically oriented analysis. Cardiol Young. (2016) 26:921–6. 10.1017/S1047951115001547 [DOI] [PubMed] [Google Scholar]
- 12.Lindinger A, Schwedler G, Hense HW. Prevalence of congenital heart defects in newborns in Germany: results of the first registration year of the PAN Study (July 2006 to June 2007). Klin Padiatr. (2010) 222:321–6. 10.1055/s-0030-1254155 [DOI] [PubMed] [Google Scholar]
- 13.Poryo M, Paes LA, Pickardt T, Bauer UMM, Meyer S, Wagenpfeil S, et al. Somatic development in children with congenital heart defects. J Pediatr. (2018) 192:136-43 e4. 10.1016/j.jpeds.2017.09.059 [DOI] [PubMed] [Google Scholar]
- 14.Braegger C JO, Konrad D, Molinari L. Neue Wachstumskurven für die Schweiz. Paediatrica. (2011) 22:9–11. [Google Scholar]
- 15.Franklin RC, Jacobs JP, Krogmann ON, Beland MJ, Aiello VD, Colan SD, et al. Nomenclature for congenital and pediatric cardiac disease: historical perspectives and the international pediatric and congenital cardiac code. Cardiol Young. (2008) 18:70–80. 10.1017/S1047951108002795 [DOI] [PubMed] [Google Scholar]
- 16.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, et al. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. (2001) 37:1170–5. 10.1016/S0735-1097(01)01272-4 [DOI] [PubMed] [Google Scholar]
- 17.Schneider S. The International Standard Classification of Education (ISCED-97): An Evaluation of Content and Criterion Validity for 15 European Countries. Mannheim: MZES. (2008). [Google Scholar]
- 18.Hangge PT, Cnota JF, Woo JG, Hinton AC, Divanovic AA, Manning PB, et al. Microcephaly is associated with early adverse neurologic outcomes in hypoplastic left heart syndrome. Pediatr Res. (2013) 74:61–7. 10.1038/pr.2013.61 [DOI] [PubMed] [Google Scholar]
- 19.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. (2006) 148:72–7. 10.1016/j.jpeds.2005.08.036 [DOI] [PubMed] [Google Scholar]
- 20.Matos SM, Sarmento S, Moreira S, Pereira MM, Quintas J, Peixoto B, et al. Impact of fetal development on neurocognitive performance of adolescents with cyanotic and acyanotic congenital heart disease. Congenit Heart Dis. (2014) 9:373–81. 10.1111/chd.12152 [DOI] [PubMed] [Google Scholar]
- 21.von Rhein M, Buchmann A, Hagmann C, Dave H, Bernet V, Scheer I, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr. (2015) 167:1259-63 e1. 10.1016/j.jpeds.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 22.Owen M, Shevell M, Donofrio M, Majnemer A, McCarter R, Vezina G, et al. Brain volume and neurobehavior in newborns with complex congenital heart defects. J Pediatr. (2014) 164:1121-7 e1. 10.1016/j.jpeds.2013.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun L, Macgowan CK, Sled JG, Yoo SJ, Manlhiot C, Porayette P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. (2015) 131:1313–23. 10.1161/CIRCULATIONAHA.114.013051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. (2012) 109:E1312–9. 10.1073/pnas.1201295109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 curt richter award paper. Psychoneuroendocrinology. (2015) 62:366–75. 10.1016/j.psyneuen.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Andescavage N, Lopez C, Quistorff JL, Donofrio MT, du Plessis AJ, et al. Maternal mental distress and cortisol levels in pregnancies with congenital heart disease. Cardiol Young. 2021:1–5. 10.1017/S1047951121003504 [DOI] [PubMed] [Google Scholar]
- 27.Schwalbe-Terilli CR, Hartman DH, Nagle ML, Gallagher PR, Ittenbach RF, Burnham NB, et al. Enteral feeding and caloric intake in neonates after cardiac surgery. Am J Crit Care. (2009) 18:52–7. 10.4037/ajcc2009405 [DOI] [PubMed] [Google Scholar]
- 28.Mitchell IM, Logan RW, Pollock JC, Jamieson MP. Nutritional status of children with congenital heart disease. Br Heart J. (1995) 73:277–83. 10.1136/hrt.73.3.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron JW, Rosenthal A, Olson AD. Malnutrition in hospitalized children with congenital heart disease. Arch Pediatr Adolesc Med. (1995) 149:1098–102. 10.1001/archpedi.1995.02170230052007 [DOI] [PubMed] [Google Scholar]
- 30.Burnham N, Ittenbach RF, Stallings VA, Gerdes M, Zackai E, Bernbaum J, et al. Genetic factors are important determinants of impaired growth after infant cardiac surgery. J Thorac Cardiovasc Surg. (2010) 140:144–9. 10.1016/j.jtcvs.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassedy A, Drotar D, Ittenbach R, Hottinger S, Wray J, Wernovsky G, et al. The impact of socio-economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes. (2013) 11:99. 10.1186/1477-7525-11-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCusker CG, Doherty NN, Molloy B, Rooney N, Mulholland C, Sands A, et al. A randomized controlled trial of interventions to promote adjustment in children with congenital heart disease entering school and their families. J Pediatr Psychol. (2012) 37:1089–103. 10.1093/jpepsy/jss092 [DOI] [PubMed] [Google Scholar]
- 33.Pfitzer C, Buchdunger LA, Helm PC, Blickle MJ, Rosenthal LM, Ferentzi H, et al. Education of children with cyanotic congenital heart disease after neonatal cardiac surgery. Ann Thorac Surg. (2021) 112:1546–52. 10.1016/j.athoracsur.2020.07.072 [DOI] [PubMed] [Google Scholar]
- 34.de Kieviet JF, Zoetebier L, van Elburg RM, Vermeulen RJ, Oosterlaan J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: a meta-analysis. Dev Med Child Neurol. (2012) 54:313–23. 10.1111/j.1469-8749.2011.04216.x [DOI] [PubMed] [Google Scholar]
- 35.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. (2008) 121:e759–67. 10.1542/peds.2007-1066 [DOI] [PubMed] [Google Scholar]
- 36.Bellinger DC, Watson CG, Rivkin MJ, Robertson RL, Roberts AE, Stopp C, et al. neuropsychological status and structural brain imaging in adolescents with single ventricle who underwent the Fontan procedure. J Am Heart Assoc. (2015) 4:1–16. 10.1161/JAHA.115.002302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. (2015) 350:1262–6. 10.1126/science.aac9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amedro P, Dorka R, Moniotte S, Guillaumont S, Fraisse A, Kreitmann B, et al. Quality of life of children with congenital heart diseases: a multicenter controlled cross-sectional study. Pediatr Cardiol. (2015) 36:1588–601. 10.1007/s00246-015-1201-x [DOI] [PubMed] [Google Scholar]
- 39.Antonovsky A. The life cycle, mental health and the sense of coherence. Isr J Psychiatry Relat Sci. (1985) 22:273–80. [PubMed] [Google Scholar]
- 40.Taylor BV, Palmer A, Simpson S, Jr, Lucas R, Simmons RD, Mason D, et al. Assessing possible selection bias in a national voluntary MS longitudinal study in Australia. Mult Scler. (2013) 19:1627–31. 10.1177/1352458513481511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.