Abstract
Background
The contemporaneous presence of immune defects and heart diseases in patients with 22q11.2 deletion syndrome (22q11.3DS) might represent risk factors for severe coronavirus 2019 disease (COVID-19).
Objective
To analyze severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outcome in 22q11.2DS patients and immunogenicity of different doses of mRNA SARS-CoV-2 vaccine.
Methods
Longitudinal observational study on SARS-CoV-2 outcome in 60 adults with 22q11.2DS (March 2020–June 2022). Anti-Spike, and anti–receptor binding domain (RBD) antibody responses, generation of Spike-specific memory B cells (MBCs) and Spike-specific T cells at different time points before and after the mRNA BNT162b2 vaccination were evaluated in 16 22q11.2DS patients.
Results
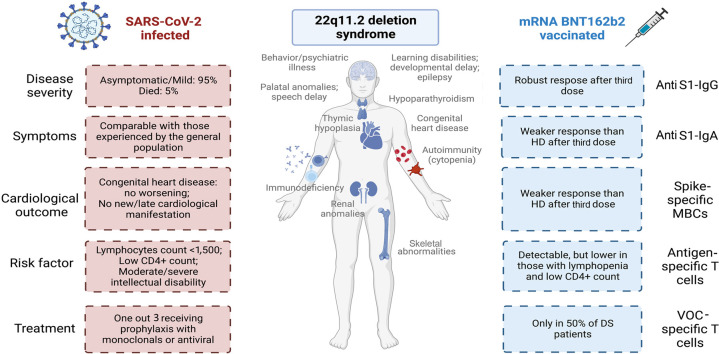
We recorded a 95% rate of vaccination, with almost all patients being immunized with the booster dose. Twenty-one patients had SARS-CoV-2 infection. Three patients were infected before vaccine availability, 6 after receiving 2 doses of vaccine, and 12 after one booster dose. The SARS-CoV-2- infection had a mild course, except in one unvaccinated patient with several comorbidities who died from acute respiratory distress syndrome (fatality rate 5%). Infected patients had more frequently moderate/severe intellectual disability, lymphopenia, and lower CD4+ count. Despite major congenital heart diseases, COVID-19 did not impact cardiological conditions. The BNT162b2 vaccine induced S1–immunoglobulin G (IgG) responses, low serum S1-IgA, and slightly impaired specific MBCs response. Specific T-cell responses observed were related to lymphocytes and CD4+ T cell counts.
Conclusions
The SARS-CoV-2 infection had a mild course in most patients with 22q11.2DS, even in patients with major cardiovascular diseases. Immunization induced Spike-specific IgG responses and generated specific MBCs and memory T cells. The weaker memory responses in patients with lymphopenia suggested the need for additional doses.
Key words: SARS-CoV-2 infection, COVID-19, 22q11.2 deletion syndrome, Cardiovascular disease, mRNA BNT162b2 vaccine, Spike antibody response, Specific memory B cells, Specific T cells, Lymphopenia, CD4 T cells
VISUAL SUMMARY
What is already known about this topic? At present, data on the course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in 22q11.2DS patients are scarce and limited mainly to pediatric case reports. Data on immunological response to immunization are lacking.
What does this article add to our knowledge? The SARS-CoV-2 infection in 22q11.2 deletion syndrome (22q11.2DS) had a mild course in most patients, even in those with major cardiovascular diseases. Lymphopenia represents a risk factor for becoming infected. The mRNA BNT162b2 vaccine induced Spike-specific immunoglobulin G responses and generated specific memory B and T cells.
How does this study impact current management guidelines? The weaker memory responses in patients with lymphopenia suggested the need for periodic reassessment of serology to identify patients needing additional recall dose administration. Fatal course in one unvaccinated person highlights the importance of immunization to protect this population from severe coronavirus 2019 (COVID-19).
Introduction
Despite being prioritized in vaccination campaigns, patients with inborn errors of immunity became frequently infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), showing higher inpatient mortality at a younger age than the general population.1, 2, 3, 4 Studies have been run to assess the efficacy of immunization strategies in inborn errors of immunity.5, 6, 7, 8 However, data on coronavirus 2019 disease (COVID-19) and immunological correlates of SARS-CoV-2 immunization in people with 22q11.2 deletion syndrome (22q11.2DS) are sporadic and refer primarily to pediatric patients9, 10, 11, 13, 14, 12 or self-completing surveys.15 Individuals with 22q11.2DS can present with a wide range of features, including immune deficiency, congenital heart diseases (CHDs), palatal abnormalities, learning difficulties, and neuropsychiatric disorders.16, 17, 18 Immunological defects are variable, ranging from the absence of the thymus with a severe combined immunodeficiency–like phenotype to less-severe impairment with T-lymphocytopenia and autoimmunity.19, 20, 21 In adults, homoeostatic expansion and reconstitution of the T-cell compartment have been described, with the early conversion of naive to memory T cells, shorter telomeres, and lower T-cell recombination excision circles, possibly leading to perturbation in T-cell function.22 , 23 In addition, hypogammaglobulinemia and abnormal B-cell function might contribute to infection recurrence24 , 25 and reduced immunogenicity of vaccines.26, 27, 28 The presence of immune defects and heart diseases,29 2 conditions implicated in severe COVID-19, puts this population at high risk in the pandemic, since adults and children with previous or preexisting cardiovascular conditions had an increased risk for severe COVID-19.30 , 31 In addition, COVID-19 has been associated with developing several new cardiovascular pathologies.32 In the 22q11.2DS population, cardiac consequences of COVID-19 remain largely unknown, in patients both with and without CHDs. In this study, we assessed the clinical course of SARS-CoV-2 infection by a longitudinal study of a monocentric cohort of 60 22q11.2DS adults, aiming to analyze whether 22q11.2DS-associated conditions might affect the COVID-19 severity (and vice versa). As secondary objectives, we analyzed the antibody- and B-cell/T-cell–specific responses after 2 and 3 doses of the BNT162b2 mRNA-based SARS-CoV-2 vaccine.
Methods
Study on COVID-19 infection
The observational-longitudinal study was carried out on 60 adults with 22q11.2DS followed up at Policlinico Umberto I, Sapienza University of Rome, between 1 March 2020 and 30 June 2022. All patients harbored a 22q11.2 microdeletion, verified through multicolor fluorescent in situ hybridization or by Comparative Genomic Hybridization-array at the time of diagnosis.
Soon after the pandemic began, we informed all patients about the pandemic risk, prevention measures, and the need to contact the hospital in case of SARS-CoV-2 infection. Patients were tested for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction on the nasopharyngeal swab every time they attended a hospital site, in case of positive household contacts irrespective of symptoms, and upon onset of symptoms possibly related to COVID-19. In SARS-CoV-2–positive patients, we evaluated the duration of the viral shedding by recording the dates of the first positive and first negative nasopharyngeal swab, COVID-19 severity (scored according to World Health Organization [WHO] stage,33 hospitalization, vaccination status, and SARS-CoV-2–specific treatments and cardiological outcome. Cardiological outcome was evaluated by chart reviews and self-reports during acute infection for out-patients, by medical files for hospitalized patients, and after recovery, by transthoracic Doppler echocardiography and electrocardiography (ECG) at rest. For both infected and noninfected patients, we collected clinical characteristics, including neuropsychiatric, cardiovascular, and immunological diseases. Cardiovascular conditions included major CHDs such as tetralogy of Fallot (ToF), pulmonary atresia and ventricular septal defect, truncus arteriosus, interrupted aortic arch, and isolated ventricular septal defects (VSDs). In addition, we evaluated the presence of acquired conditions including arrhythmias, cardiac function impairment, and previous heart surgery. We also retrieved known risk factors for severe COVID-19 courses, such as overweight, obesity, hypertension, diabetes mellitus, intellectual and developmental disabilities, mental health disorders, smoking, and use of corticosteroids or other immunosuppressive medications.34 Overweight and obesity were defined as having a body mass index of 24.9 to 29.9 kg/m2 and greater than 30kg/m2, respectively. Immunological laboratory data included complete blood count and immunoglobulin (Ig) serum levels.
Prospective study on immunological response to immunization
In 6 22q11.2DS adults naive to SARS-CoV-2 infection, we carried out a pilot study on immunological response to BNT162b2 immunization after 2 vaccine doses. Then, we extended the study to 16 22q11.2DS adults naive to SARS-CoV-2 infection who received 3 doses of the BNT162b2 vaccine. The vaccine was administered in 2 doses, 21 days apart; the third dose was administered 6 months after completing the full immunization schedule. We obtained blood samples for serological and cellular immunity assessment before the first dose (T0), 1 week after the second dose, 6 months after the second dose on the day of the third dose administration, and 1 week after the third dose. Twenty-four age-matched healthy donors (HDs) were included as controls. Eligible patients were informed about the study and subscribed to informed consent for vaccination and the immunological study. The Ethical Committee of the Sapienza University of Rome approved the study (Prot. 0521/2020, July 13, 2020). The study was performed following the Good Clinical Practice guidelines, the International Conference on Harmonization guidelines, and the most recent version of the Declaration of Helsinki.
Enzyme-linked immunosorbent assay for specific IgG and IgA detection
A semi-quantitative in vitro determination of anti–SARS-CoV-2 IgG and IgA was performed on serum samples using the anti–SARS-CoV-2 Spike enzyme-linked immunosorbent assay (EUROIMMUN), according to the manufacturer's instructions and previously reported.5 Results are reported as the ratio between the optical density (OD) sample and the OD calibrator. The ratio interpretation was as follows: negative, less than .8; borderline, less than or equal to .8 to less than 1; positive, greater than or equal to 1.1.
Cell isolation and cryopreservation
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll Paque Plus 206 (Amersham PharmaciaBiotech) density-gradient centrifugation and immediately frozen and stored in liquid nitrogen until use. The freezing medium contained 90% Fetal Bovine Serum (FBS) and 10% dimethyl sulfoxide (DMSO).
Detection of antigen-specific B cells
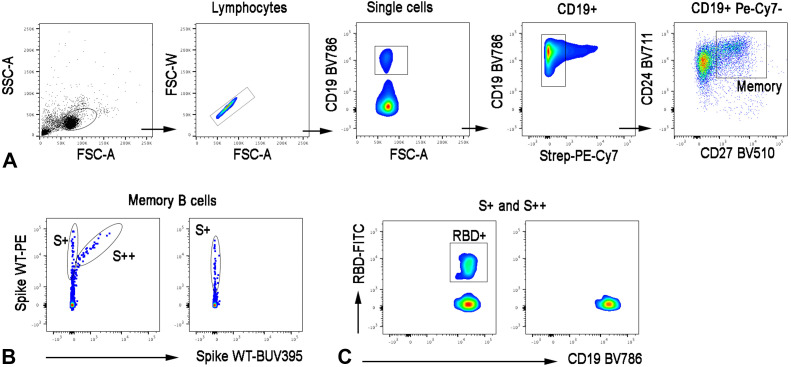
Recombinant biotinylated SARS-CoV-2 Spike (S1+S2; aa16-1211, R&D systems) was individually multimerized with streptavidin BUV395 (BD Bioscience) and streptavidin PE (BD Bioscience) at 25:1 ratio and 20:1 ratio, respectively at 4°C for 1 hour. Biotinylated receptor binding domain (RBD) (kindly provided by Takis) was mixed with streptavidin-FITC (BD Bioscience) at 2.5:1 ratio. Nonspecific streptavidin-binding B-cells were gated out with streptavidin PE-Cy7 (BD Bioscience). Several 5 × 106 previously frozen PBMC samples were stained with a 100-ng Spike per probe (total 200 ng), 27.5 ng of RBD, and 20 ng of streptavidin-PE-Cy7 at 4°C for 30 minutes. After the cells were washed, surface staining was performed in a brilliant buffer at 4°C for 30 minutes. Memory B cells (MBCs) were defined as CD19+CD24+CD27+ (Figure E1 ; available in this article’s Online Repository at www.jaci-inpractice.org). The MBCs specific for SARS-CoV-2 were distinguished by their ability to bind biotin-labeled recombinant Spike into S+ (PE single positive) or S++ (PE-BUV395 double positive). Among Spike-specific MBCs, we were able to identify RBD-specific MBCs. Stained PBMC samples were acquired by FACS LSR Fortessa (BD Bioscience). At least 4 × 106 cells were acquired and analyzed using FlowJo10.7.1 (BD Bioscience). Phenotype analysis of antigen-specific B cells was performed only when at least 10 cells were detected in the respective antigen-specific gate.
FIGURE 1.
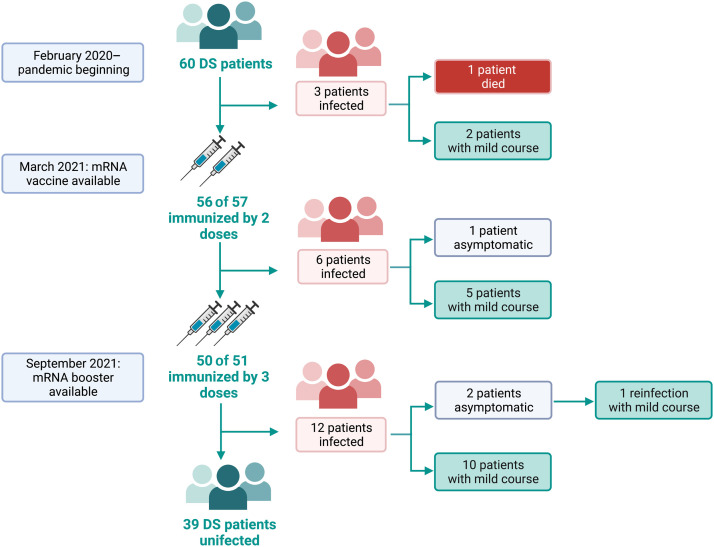
The SARS-Cov-2 infection and immunization coverage in a cohort of 60 patients with 22q11.2DS over the period March 2020 to the end of June 2022. Data on SARS-CoV-2 infection are reported according to the availability of immunization.
Detection of SARS-CoV-2–specific T-cell response
The frequency of Spike-specific T cells before and after vaccination was assessed by standard interferon-gamma (IFN-γ) ELISpot assay, as previously described.35 , 36 The PBMCs were thawed and rested overnight at 37°C in R10 medium (RPMI 1640 [Sigma Aldrich] supplemented with 10% heat-inactivated highly defined FBS-HyClone, 2 mmol/L L-glutamine, 10 mmol/L HEPES buffer [N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid, Sigma Aldrich], 100 U/mL penicillin, and 100 μg/mL streptomycin [Gibco]). The PBMCs were plated at 3 × 10 cells/well in ELISpot plates (Human IFN-γ ELISpot plus kit; Mabtech) and stimulated for 18-20 hours at 37°C (5% CO2) with a pool of peptides (Miltenyi Biotec) spanning the whole spike protein of the wild-type SARS-CoV-2, or with a pool of peptides spanning the mutated portion of the Omicron Spike protein and, as a control, with a pool of peptides spanning the same region of the wild-type Spike protein. A superantigen was used as a positive control. At the end of incubation, the ELISpot assay was developed according to the manufacturer's instructions. Results are expressed as spot-forming cells/106 PBMCs in stimulating cultures after subtracting the background. The cut-off value was set by calculating the mean of the background plus 2 SD (25 SFC).
Statistical analysis
The primary analysis of the observational study was to investigate clinical and laboratory characteristics in 2 groups defined as SARS-CoV-2–infected versus –uninfected. Continuous variables were described using median and interquartile ranges (IQR), categorical variables using frequencies and percentages. Secondary analysis was performed to ascertain risk factors associated with SARS-CoV-2 infection in 22q11.2DS. To evaluate infection prediction performance of lymphopenia (lymphocytes < 1,500/mm3), a simple logistic regression model was developed, and odds ratio and 95% confidence intervals (CI) were measured. For the study on immunization, patients have been compared with controls. Immunological and clinical variables were compared between the different study times. Values were compared by the nonparametric Kruskal-Wallis test, and if not significant, the Wilcoxon matched-pair signed-rank test or the two-tailed Mann-Whitney U test were used. Differences were deemed significant when P was less than .05. Statistical analysis was performed with SPSS 18.0 soft-ware for Windows (SPSS, Chicago, IL).
Results
Patients
Sixty adults (median age 28 years [IQR 24–35]; females 42%) with 22q11.2DS were included in the study. A CHD was recorded in 38% of patients, with VSDs and ToF being the most common condition (25% and 14%, respectively). Previous heart surgery was recorded for 30% of patients. Psychiatric conditions were recorded in 65% of participants, neurological conditions in 20%, learning issues in 76%, and autoimmune disorders in 42% (with 3% of patients being under immunosuppressive treatment). The analysis of known risk factors for severe COVID-19 identified 56% of patients being overweight, with 25% being obese; moreover, 9% of patients had diabetes mellitus type 2 and 15% hypertension (Table I ). Baseline immunological evaluation (Table II ) showed lymphopenia in 38% of patients, with 17% having less than 400 CD4+ cells/mm3. Moreover, 8% of patients had low IgG serum levels, 16% an IgM deficiency (<35 mg/dL) and 4% an IgA deficiency (<68 mg/dL).
TABLE I.
Demographics, clinical characteristics of 60 patients with 22q11.2DS
| Demographics | Value |
|---|---|
| Female, n (%) | 25 (42) |
| Age (y), median (IQR) | 27 (24–35) |
| Clinical data | |
| BMI (kg/m2) | |
| >25 (overweight) | 33 (56) |
| >30 (obesity) | 15 (25) |
| Cardiovascular conditions, n (%) | |
| Previous heart surgery | 18 (30) |
| Major CHD | 23 (38) |
| Hypertension | 9 (15) |
| DMT | 4 (9) |
| Dyslipidemia | 13 (22) |
| Smoking habit | 11 (19) |
| Psychiatric conditions, n (%) | 39 (65) |
| Neurological conditions, n (%) | 12 (20) |
| Learning issues, n (%) | 45 (76) |
| Autoimmune disorders, n (%) | 25 (42) |
| Thyroiditis | 14 (24) |
| Psoriasis | 5 (8) |
| Autoimmune cytopenias | 3 (5) |
| Alopecia | 2 (3) |
| Arthritis | 1 (2) |
| Immunosuppressive treatment, n (%) | 2 (3) |
| Steroids | 1 (1,5) |
| Tocilizumab | 1 (1,5) |
BMI, Body mass index; DMT, diabetes mellitus.
TABLE II.
Immunological data on 60 patients with 22q11.2DS
| Laboratory data | n (%) |
|---|---|
| Neutropenia (<1,000 cell/mm3) | 0 |
| Lymphopenia (<1,500 cell/mm3), n (%) | 20 (38) |
| IgG < 700 mg/dL, n (%) | 4 (8) |
| IgA < 68 mg/dL, n (%) | 2 (4) |
| IgM < 40 mg/dL, n (%) | 8 (16) |
| CD4+ count (cell/mm3), median (IQR) | 585 (478–797) |
| ≥600 cells/mm3, n (%) | 20 (49) |
| 400–600 cells/mm3, n (%) | 14 (34) |
| <400 cells/mm3, n (%) | 7 (17) |
| CD8+ count (cells/mm3), median (IQR) | 358 (258–494) |
| <420 cells/mm3, n (%) | 24 (61.5) |
| CD19+ count (cells/mm3), median (IQR) | 216 (159–308) |
| <90 cells/mm3, n (%) | 1 (3) |
SARS-CoV-2 infection
Over the study period, 21 patients (35%) (median 28 years; range: 18–51 years; females 48%) were diagnosed with SARS-CoV-2 infection (Figure 1). The median duration of quantitative reverse transcriptase–polymerase chain reaction positivity was 10 days (IQR 10–16.5). Twenty patients (95%) did not require hospital admission. The severity of infection was staged as asymptomatic in 2 patients (9.5%), mild in 18 patients (85.7%), and severe in one patient (4.8%). The most common symptom was fever (57%), followed by cough (33%), asthenia (29%), and nasal discharge (19%).
Infected patients were analyzed during different periods of the pandemic (Figure 1 and Table III ). Three patients were diagnosed in the pre-vaccination period, from February 2020 to March 2021 (main circulating variant of concern [VOC] Wuhan). A 50-year-old man affected by diabetes mellitus type 2, hypertension, and obesity was diagnosed with SARS-CoV-2 infection 7 days after being hospitalized for acute kidney failure with nephrosis and secondary hypogammaglobulinemia. He developed COVID-19 acute respiratory distress syndrome and he was admitted to the intensive care unit to receive invasive mechanical ventilation, steroids, heparin, and continuous renal replacement therapy. Ten days after the COVID-19 diagnosis, the patient died. The other 2 unvaccinated patients had a mild course of infection and did not require specific treatment. In March 2021, vaccination against SARS-CoV-2 became available in Italy. In the following months (post-full immunization period), we recorded 6 cases of SARS-CoV-2 infection. In detail, from March to mid-July 2021 (main circulating VOC Alpha) 2 immunized patients (2 doses) became infected, having an asymptomatic/mild course despite their risk factors. From mid-July to October 2021 (main circulating VOC Delta), 4 immunized patients (2 doses) were infected, all with a mild course despite having risk factors for severe COVID-19.
TABLE III.
Individual data of 21 patients infected by SARS-CoV-2 during the study time
| Period | Sex | Age range (y) | Lymphocytes (cell/mm3) | CD4+ count (cell/mm3) | Immunoglobulin defect | Risk factors for severe COVID-19 | Dose(s) of vaccine at SARS-CoV-2 infection | COVID-19 course | Treatment | Days of qRT-PCR positivity | Outcome | Cardiological outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preimmunization | M | 50–29 | 1,000 | 380 | IgG | DMT2, obesity, kidney failure, mood disorders | None | Severe (ARDS) | Oxygen therapy, dialysis | NAp | Death | NAp |
| Preimmunization | F | 20–29 | 1,290 | 438 | No | Developmental disabilities, schizophrenia spectrum disorders | None | Mild | None | 21 | Recovery | No echocardiographic or ECG changes |
| Preimmunization | F | 40–49 | 1,500 | 580 | No | None | Mild | Antibiotic | 10 | Recovery | No echocardiographic or ECG changes | |
| Post-full immunization | M | 20–29 | 820 | 190 | No | Corrected subaortic VSD, multiple VSDs in natural hystory, schizophrenia spectrum disorders, developmental disabilities | 2 doses | Asymptomatic | None | 10 | Recovery | No echocardiographic or ECG changes |
| Post-full immunization | F | 30–39 | 1,230 | 488 | IgG, IgM, and IgA defect | Corrected ToF, overweight, dyslipidemic, developmental disabilities, schizophrenia spectrum disorders | 2 doses | Mild | None | 10 | Recovery | NA |
| Post-full immunization | F | 20–20 | 1,810 | NA | No | Small subaortic VSD in natural history | 2 doses | Mild | NSAID | 17 | Recovery | No echocardiographic or ECG changes |
| Post-full immunization | M | 30–39 | 1,180 | 400 | No | Small subaortic VSD in natural history, overweight, schizophrenia spectrum disorders | 2 doses | Mild | None | 39 | Recovery | No echocardiographic or ECG changes, arterial hypertension diagnosis |
| Post-full immunization | F | 40–49 | 760 | 270 | IgG | Obesity, developmental disabilities, schizophrenia spectrum disorders | 2 doses | Mild | None | 16 | Recovery | No echocardiographic or ECG changes |
| Post-full immunization | F | 20–29 | 1,690 | 662 | No | Developmental disabilities, | 2 doses | Mild | None | 31 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | F | 30–39 | 900 | 390 | No | Developmental disabilities | 3 doses | Mild | Paxlovid | 10 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | F | 18–19 | 1,755 | 720 | No | Overweight, developmental disabilities, mood disorders | 3 doses | Mild | None | 10 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | M | 20–29 | 1,180 | 554 | No | Corrected ToF, overweight, developmental disabilities, schizophrenia spectrum disorders |
3 doses | Asymptomatic | None | 10 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | F | 20–29 | 1,000 | 400 | No | Obesity, developmental disabilities, schizophrenia spectrum disorders | 3 doses | Asymptomatic | Sotrovimab | 7 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | M | 30–29 | 2,030 | 875 | No | Overweight, developmental disabilities, schizophrenia spectrum disorders | 3 doses | Mild | None | 13 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | M | 20–29 | 2,600 | 1,089 | IgM defect | Corrected IAA, overweight, developmental disabilities | 3 doses | Mild | Sotrovimab | 7 | Recovery | NA |
| Post-booster dose | F | 20–29 | 1,131 | 585 | IgG/IgA defect | Corrected ToF, corticosteroid medications | 3 doses | Mild | Molnupiravir | 10 | Recovery | Hypertension during infection. No echocardiographic or ECG changes |
| Post-booster dose | M | 50–59 | 1,650 | 650 | IgM defect | Corrected hemitruncus, DMT2, obesity | 4 doses | Mild | None (refused) | 63 | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | M | 20–29 | 1,530 | NA | No | Obesity, developmental disabilities, schizophrenia spectrum disorders | 3 doses | Mild | Sotrovimab | 15 | Recovery | NA |
| Post-booster dose | M | 30–39 | 3,700 | NA | No | Obesity | 3 doses | Mild | Paxlovid | 7 | Recovery | NA |
| Post-booster dose | M | 20–29 | 2,340 | 550 | No | Developmental disabilities, schizophrenia spectrum disorders | 3 doses | Mild | None | NA | Recovery | No echocardiographic or ECG changes |
| Post-booster dose | M | 20–29 | 1,500 | NA | No | Developmental disabilities, schizophrenia spectrum disorders | 3 doses | Mild | Molnupiravir | 10 | Recovery | NA |
| Post-booster dose | F | 20–29 | 1,000 | 400 | No | Obesity, developmental disabilities, schizophrenia spectrum disorders | 3 doses | Mild | Antibiotic | 10 | Recovery | No echocardiographic or ECG changes |
ARDS, Adult respiratory distress syndrome; DMT2, diabetes mellitus type 2; ECG electrocardiogram; IAA, interrupted aortic arch; NA, not available; NAp, not applicable; NSAIDs, nonsteroidal anti-inflammatory drugs; qRT-PCR, quantitative reverse transcriptase–polymerase chain reaction.
In October 2021, immunization with the booster dose began. In the following months, with the spread of the Omicron variant (post-booster dose period), 12 patients were infected, 10 with mild symptoms and 2 asymptomatic. Four months after recovery, one patient was reinfected by SARS-CoV-2 with a mild course. At the time of the SARS-CoV-2 infection, 11 patients were immunized with 3 doses of vaccine, and 1 patient with 4 doses (available from January 2022). Seven patients received anti–COVID-19 therapies (Table III), showing a shorter duration of infection in comparison with untreated patients (10 days [IQR 10–10] vs 14.5 days [IQR 10–28.5]; P = .0109).
Compared with uninfected patients, those infected with SARS-CoV-2 had a more severe degree of intellectual disability (moderate/severe intellectual disability 62% vs 21%; P = .0020). However, the groups did not differ in age, gender, major CHD, or psychiatric issues.
Moreover, infected patients had a lower pre-infection lymphocytes count (1,260 cells/mm3, IQR 1,003–1,796 vs 1,867 cell/mm3, IQR 1,392–2,308; P = .0055) and lower CD4+ cells count (488 cell/mm3, IQR 389–656.5 vs 720 cell/mm3, IQR 539–1,000; P = .0051) (Table IV ).
TABLE IV.
Clinical and demographic characteristics and preinfection lymphocytes in 22q11.2DS patients SARS-CoV-2–positive and SARS-CoV-2–negative
| Patients' characteristics | SARS-CoV-2–positive (n = 21) | SARS-CoV-2–negative (n = 39) | P value |
|---|---|---|---|
| Age, median (IQR) | 28 (25.5–35.5) | 27 (27–34) | .8025 |
| Female, n (%) | 10 (48) | 15 (38) | .5865 |
| Major CHD, n (%) | 8 (38) | 16 (41) | 1.0000 |
| Intellectual disability (moderate/severe), n (%) | 13 (620) | 8 (21) | .0020 |
| Psychiatric disease, n (%) | 14 (67) | 25 (64) | 1.0000 |
| Immunological data | |||
| Lymphocytes, median (IQR) | 1,260 (1,003–1,796) | 1,867 (1,392–2,308) | .0055 |
| CD4+, median (IQR) | 488 (1,003–1,796) | 720 (539–1,000) | .0063 |
| Immunoglobulin defect, n (%) | 7 (39) | 5 (16) | .0942 |
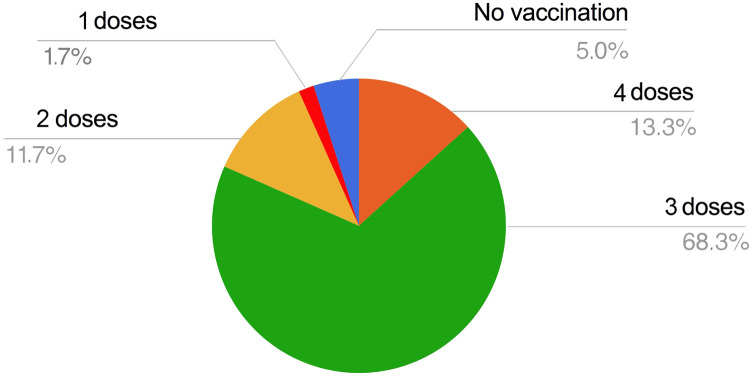
A simple logistic regression confirmed lymphopenia (<1,500 cell/mm3) as a predictor of SARS-CoV-2 infection (OD 15.7 (95% CI 3.6–68.9). At the end of the study, 68.3% of patients had been vaccinated with 3 doses, 13.3% with 4 doses, 11.7% with 2 doses, and one patient (1,7%) with only one dose. Three patients (5%) were not vaccinated, including the patient who died before vaccine availability (Figure 2 ).
FIGURE 2.
Vaccination coverage rate in the enrolled cohort of 60 adults with 22q11.2DS at the end of the study period.
Impact of SARS-CoV-2 infection on cardiovascular diseases
Forty percent of patients who recovered from SARS-CoV-2 infection had a major CHD. During SARS-CoV-2 infection, neither patients with CHDs nor those without CHDs displayed COVID-19–related cardiovascular manifestations, including myocarditis. Only one patient with corrected ToF had transient hypertension. After SARS-CoV-2 recovery, 75% of infected patients underwent transthoracic Doppler echocardiography and ECG at rest (Table III). None showed echocardiographic or heart rhythm changes compared with pre–SARS-CoV-2 infection. We recorded arterial hypertension in one overweight patient with isolated VSD. Thus, except for the latter patient, no changes in consolidated treatment for cardiovascular conditions were prescribed after recovery.
Immune response to SARS-CoV-2 vaccination
Sixteen patients (age 27 years [IQR 24–36]; females 6 [60%]) were analyzed for humoral and cellular response to immunization with the mRNA BNT162b2 (Table E1; available in this article’s Online Repository at www.jaci-inpractice.org). To note, 2 patients with autoimmunity on immunosuppressive treatment were also enrolled: one with rheumatoid arthritis treated by Tocilizumab and one with autoimmune cytopenia receiving steroids. The second patient was also receiving IgG replacement treatment owing to hypogammaglobulinemia.
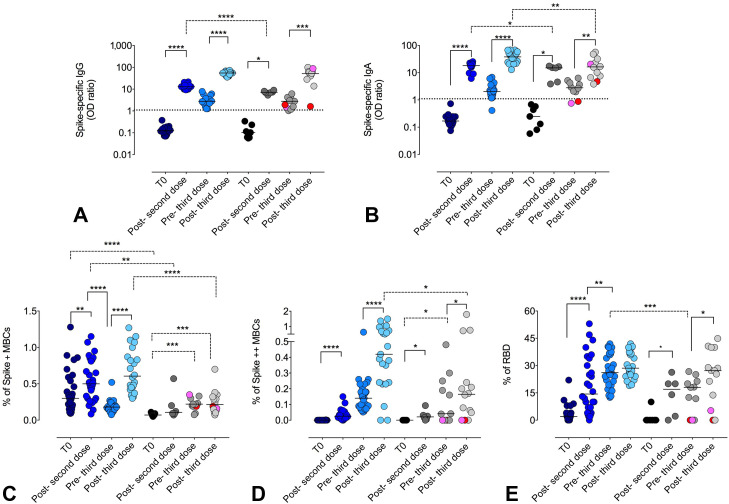
Following the second vaccine dose, anti-Spike (S1) IgG and IgA increased, even if 22q11.2DS patients showed lower antibody levels than HD (S1-IgG P < .0001; S1-IgA P = .0224). In both groups, anti-S1 antibodies decreased over time and were boosted by the third immunization (S1-IgG HD P < .0001 and 22q11.2DS P = .0020; S1-IgA HD P < .0001 and 22q11.2DS P = .0020). Differently from S1-IgG, 22q11.2DS patients reached lower S1-IgA serum levels than those in HDs (P = .0058) (Figure 3 , A, B).
FIGURE 3.
Specific antibody and B-cell responses after immunization with 2 and 3 doses of mRNA BNT162b2 vaccine. (A) Spike-specific IgG, (B) Spike-specific IgA antibodies, (C) S+ MBCs, (D) S++ MBCs, and (E) RBD-positive MBCs in HD (blue circles) and 22q11.2DS patients (gray circles) before T0, 1 week after the second dose (post-second dose), 6 months after the second dose (pre-third dose), and 1 week after the third dose (post-third dose) of BNT162b2 vaccine. The MBCs subset was defined as CD19 + CD24 + CD27 + CD38–. For Spike-specific IgG and IgA the positive cut-off value was settled at 1.0 OD ratio. For each group, the median is shown as a bar. Continuous lines represented paired Wilcoxon test, and dashed lines represented unpaired Mann U Whitney test. ∗P ≤ .05; ∗∗P ≤ .01; ∗∗∗P ≤ .001; ∗∗∗∗P < .0001.
As previously reported,5 we identified low-affinity and high-affinity MBCs that were detectable respectively positive for PE (S+) or double positive (S++) for PE and BUV395.
At T0 low-affinity MBCs (S+) were already detectable in HDs and, with lower frequency (P < .0001), in 22q11.2DS patients (Figure 3, C). In HDs, the frequency of S+ MBCs increased after the second dose (P = .0072), returned to the preimmunization levels before the third dose (P < .0001) and then augmented after the third dose (P < .0001). In 22q11.2DS, patients the frequency of S+ MBCs was lower than in HDs both after the second (P = .0034) and the third dose (P < .0001) (Figure 3, C). We have previously shown that S+ MBCs are mostly of IgM-isotype.37 In accordance with our findings, in 22q11.2DS patients, the amount of S+ MBCs was directly related with the concentration of serum IgM (R = 0.53; P = .0113).
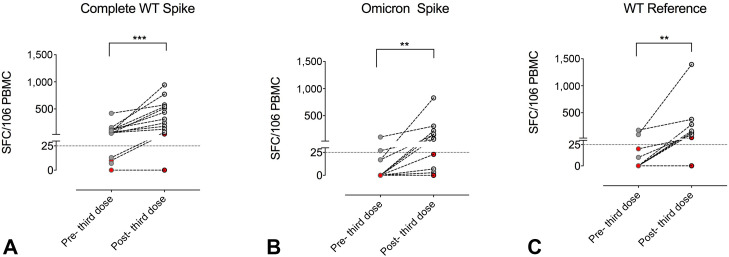
The S++ MBCs were absent before immunization both in HDs and 22q11.2DS (Figure 3, D). After the second dose, the frequency of S++ MBCs increased both in HDs (P < .0001) and in patients (P = .0312). The S++ MBCs increased after the third dose in HDs (P < .0001) and in 22q11.2DS (P = .0391), although their frequency was lower in patients (P = .0161) (Figure 3, D). Among total Spike-specific MBCs (S+ plus S++), we also identified RBD-specific MBCs (Figure 3, E), a minority of the MBCs generated by vaccination that produce most of the neutralizing antibodies.5 In HDs, RBD+ cells increased after the second dose (P < .0001) and even more in the following months (pre-third P = .0090). In 22q11.2DS, RBD+ cells expanded after the second (P = .0489) and the third doses (P = .0472), with the frequency of RBD+ and MBC S++ cells being directly related (R = 0.34; P = .0371). To note, one patient with hypogammaglobulinemia under IgRT and steroids responded to the booster dose with S+ MBC only and did not generate anti–S1 IgG, S++ MBCs, and RBD+ cells. Moreover, the patient treated with tocilizumab developed S+ MBCs but not S++ MBCs and RBD+ cells, possibly owing to impaired germinal center reaction caused by defective interleukin-6 (IL-6) release.38 We further analyzed the specific T-cell–mediated immune responses in 16 patients before and one week after the third vaccine dose by ELIspot assay to quantify IFN-γ–producing antigen-specific T cells. When stimulated by the full-length Spike WT protein, all patients developed a T-cell response, except for the one treated with steroids (Figure 4 , A). Degrees of response were variable, being directly related to the number of total peripheral lymphocytes (R = 0.43; P = .0310) and CD4+ T cells (R = 0.44; P = .0185). When stimulated by the mutated Spike epitope leading to the Omicron VOC, only 50% of patients responded (Figure 4, B). When compared with micron Spike responders, nonresponders showed a lower count of lymphocytes (2,330 cells/mm3 [IQR 1,790–3,570) vs 1,131 cells/mm3 [IQR 890–2,010]; P = .0300). Differently, when stimulated with the same epitope in the WT configuration, all but one patient showed detectable T response (Figure 4, C). This patient was the same who did not respond to the Spike WT protein.
FIGURE 4.
The SARS-CoV-2–specific T-cell responses in 22q11.2DS patients before (6 months after the second dose) and after the third dose of mRNA (BNT162b2) vaccine. The cumulative IFN-γ–positive T-cell responses (total SFC against (A) complete WT Spike, (B) Omicron Spike, and (C) Omicron WT antigens) were evaluated by the T-SPOT Discovery SARS-CoV-2 ELISpot assay in 16 virus-naive participants. Statistical analyses were performed using the Wilcoxon matched-pairs signed-rank test. ∗∗P < .01; ∗∗∗P < .001. SFC, spot forming cells.
Discussion
The 22q11.2DS is the most common chromosomal microdeletion reported in humans.39 , 40 Besides CHDs, palatal abnormalities, learning difficulties, and neuropsychiatric disorders,41, 42, 43 22q11.2DS is characterized by the absence or underdevelopment of the thymus with impaired immune functions.22 , 44 The immunological impairment is highly heterogeneous, ranging from a severe combined immunodeficiency phenotype, characterized by profound impairment of T- and B-cell responses and life-threatening infections, or more commonly, less-severe immune defect with a mild to moderate reduction of T cells and autoimmunity.21, 22, 23 , 44 Antibody deficiencies are also increasingly recognized.24
Currently, data on the course of SARS-CoV-2 infection in 22q11.2DS patients are scarce and limited to pediatric case reports9, 10, 11, 13, 14, 15, 12 or self-assessment for adults,16 with about 50 cases being reported. Moreover, descriptions of SARS-CoV-2 infections in adults did not include data on infection with the Omicron strain.16 Of note, data on immunological response to immunization are lacking.
Published reports of SARS-CoV-2 infection among people with 22q11.2DS revealed that both children and adults did surprisingly well, despite their underlying comorbidities (Table V ). In our cohort, the course of SARS-CoV-2 infection was free from complications in all but one unvaccinated patient with severe concomitant comorbidities. The symptoms commonly reported were comparable with those experienced by the general population.45 No new cardiovascular impairment during acute SARS-CoV-2 infection or in the post–COVID-19 period nor worsening prior cardiological status was observed except for 2 patients who developed arterial hypertension. In addition, transthoracic color Doppler echocardiography and ECG at rest performed postrecovery did not detect any new echocardiographic and heart rhythm alterations in 22q11.2–infected individuals. These data were entirely unexpected because the European Society of Cardiology has identified adults with CHD as an increased risk population for complications with COVID-19. In particular, cyanotic CHDs, which are frequent cardiological features of people with 22q11.2DS,29 were associated with the high-risk group.46 Moreover, comorbidities commonly found in 22q11.2DS, such as diabetes, hypertension, or being overweight,47 further increase the risk of developing more severe symptoms.47 Cardiological involvement during COVID-19 has also been shown in subjects without heart conditions, with new acute coronary syndromes, arterial and venous thrombosis, acute heart failure, arrhythmias, and myocarditis frequently observed.32 , 48 , 49 Moreover, the risk for cardiovascular impairment was also increased early after recovery in those with either symptomatic or asymptomatic SARS-CoV-2 infection.50 Compared with uninfected patients, SARS-CoV-2 22q11.21–positive patients had a higher degree of intellectual disability, possibly causing difficulties in keeping social distance and isolation from infected caregivers. Low preinfection lymphocytes count and reduced CD4+ cells were also found to be associated with a higher risk for infection. It was remarkable that, despite having persistent T lymphopenia, these patients experienced full clinical resolution of SARS-CoV-2 infection.
TABLE V.
Published reports of SARS-CoV-2 infection in 22q11.2DS
| Reference | Study design | Infection period considered | Sex/age | Comorbidities | COVID-19 symptoms | Hospital admission | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 11 | Cross-sectional study (prevalence study) in June 2020 (end of first wave) including 65 moderate/severe IEIs patients | February 2020–Jun 2020 | M/17 y | Moderate/severe lymphopenia | Asymptomatic | No | No | Recovered |
| M/5 y | Moderate/severe lymphopenia | Mild (cough) | No | No | Recovered | |||
| 13 | Retrospective survey on 20 IEIs patients | February 2020–September 2020 | M/1.5 y | Bronchiectasis, low TRECs level, hypogammaglobulinemia | Asymptomatic | No | No | Recovered |
| 1 | A retrospective study was undertaken by a Web-based survey, including 94 patients with an underlying IEIs and infected by SARS-CoV-2 | March 2020–June 2020 | M/age group 0–2 y | Lung disease, tracheostomy with chronic ventilation | Severe (fever, dyspnea) | Yes | Convalescent plasma, oxygen support, NIV | Recovered |
| 18 | Case series of 60 individuals with IEIs National Registry, data collection proformas were sent to all U.K. pediatric and adult immunologists by email; 100 IEIs patients included | March 2020–July 2020 | M/>18y | NA | NA | Yes | NA | Recovered |
| 11 | Cross-sectional, multicenter study, involving 121 patients with IEIs | March 2020–December 2020 | M/0.7 y | Arterial hypertension, corrected congenital cardiopathy, hypogamma | Severe (fever, cough, dyspnea, severe diarrhea) | Yes (ICU) | NA | Recovered |
| 14 | Retrospective multicenter survey, 114 IEIs patients included | March 2020–April 2021 | F/13.8 y | Autoimmune hypothyroidism | Mild (Fever) | No | NA | Recovered |
| F/7 y | None | Mild (Fever) | No | NA | Recovered | |||
| F/23 y | CHD, allergy, bronchiectasis | Mild (running nose/sore throat) | No | NA | Recovered | |||
| F/1.5 y | History of interventricular defect and supraventricular paroxysmal tachycardia | Mild (fever) | No | NA | Recovered | |||
| F/17 y | Autoimmune thyroiditis, interventricular defect | Asymptomatic | No | NA | Recovered | |||
| F/9.25 y | Congenital heart disease (IAA, interatrial defect, interventricular defect) | Mild (fever, asthenia) | No | NA | Recovered | |||
| M/18.4 y | Autoimmune thyroiditis, mild thrombocytopenia, hyperbilirubinemia, vitamin D deficiency | Mild (runny nose) | No | NA | Recovered | |||
| F/11.5 y | Obesity, cognitive disability | Moderate (cough, headache, dyspnea, asthenia, nausea, arthralgia) | No | NA | Recovered | |||
| M/10.6 y | Left aortic arch, developmental delay | NA | No | NA | Recovered | |||
| M/1.6 y | Hypoparathyroidism, hypocalcemia, congenital hypothyroidism, bicuspid aorta | Asymptomatic | No | NA | Recovered | |||
| F/15.2 y | Hyperthyroidism, intellectual disability, congenital heart disease | NA | No | NA | Recovered | |||
| M/1 y | Minimal left to right interatrial shunt, nephrocalcinosis, hypoparathyroidism, areas of parenchymal lung consolidation, hypogammaglobulinemia | Severe (fever) | Yes | NA | Recovered | |||
| 3 | National Registry, data collection proformas were sent and 310 patients included | March 2020–July 2021 | Two males (18 and 21 y) | NA | Severe course (1 patient) | Yes (1) | NA | Recovered |
| 10 | Cross-sectional, multicenter study involving 99 IEIs patients | June 2020–June 2021 | M/1.5 y | NA | Mild (anxiety, bradycardia) | NA | NA | Recovered |
| 12 | Case report | NA (published on February 2021) | F/12 y | Hypogammaglobulinemia, T-cell lymphocytopenia, congenital heart disease, and VP shunt | Mild (headache, emesis) | NA | No | Recovered |
| NA (published on February 2021) | M/13 y | Obesity, congenital heart disease, low IgM | Asymptomatic | NA | NA | Recovered | ||
| 15 | Multinational survey on 152 patients with 22q11.2DS | July 2021–December 2021 | 25 patients, (range 2–36 y) | Overweight 21%, immunoglobulin infusions 4%, previous heart surgery 43%, hypertension 4%, psychiatric problems 11%, arthritis 11%, asthma 29% | Fatigue (63%), headache (54%), cough (51%), rhinorrhea (45%) | 1 of 25 hospitalized for 1 d | None | 25 of 25 recovered |
IAA, Interrupted aortic arch; ICU, intensive care unit; IEIs, inborn errors of immunity; NA, not available; NIV, noninvasive ventilation; TRECs, T-cell receptor excision circles; VP, ventriculoperitoneal shunt.
The main contributor to the good outcome might be the high immunization coverage recorded in our cohort, with greater than 95% of patients being immunized with at least 3 doses. This hypothesis is supported by the prospective study on immunological response to SARS-CoV-2 immunization. In 22q11.2DS patients, the IgG responses were comparable with those found in HDs, whereas Spike-specific IgA levels were lower. Moreover, the generation of Spike-specific MBCs and RBD B-cells was slightly impaired as expected, owing to the known defect of switched memory in 22q11.2DS subjects.51 , 52 Specific T-cell responses were related to total lymphocytes and CD4+ T cell counts. Recently, low pre–SARS-CoV-2 infection lymphocyte count was confirmed to be an independent risk factor for mortality in a heterogeneous U.K. cohort of patients with primary and secondary immunodeficiencies.3 In 22q11.2DS patients, mild to moderate lymphopenia represents the primary manifestation of thymic hypoplasia53 and is more common in infancy than in adulthood.25 However, although patients have reduced T-cell numbers, their repertoire is normal.54 This was confirmed by the observation that lymphopenia does not seem to correlate with the severity and recurrence of infections.20
In conclusion, our data suggest that vaccination should be encouraged in individuals with 22q11.2DS because the mRNA vaccine was able to induce the B-/T-cell responses and a robust IgG-specific response. A limitation of the study is the short follow-up time postimmunization. For now, we know that, shortly after completing the third dose of vaccine, Spike-specific MBCs response reached lower frequencies than reported in HDs, with a subgroup of patients not being able to generate high-affinity specific-MBC, then suggesting possible incapability of B cells to undergo affinity maturation in the germinal center. Previous studies exploring the immunogenicity of vaccinations with live viruses and influenza virus showed that, despite the robust seroconversion recorded soon after immunization,26, 27, 28 patients with 22q11.2DS have difficulty in sustaining long-term protective antibodies.28 These data together suggest that patients with 22q11.2DS should be periodically reassessed to identify those needing additional recall vaccine dose administration.
Acknowledgments
We thank the Jeffrey Modell Foundation and the AIDEL22 Association, patients, and their families.
Footnotes
This work was supported by the Italian Ministry of Health RF2013-02358960 grant; Italian Ministry of Health COVID-2020-12371817 grant.
Conflicts of interest: The authors declare that they have no relevant conflicts of interest.
Online Repository
FIGURE E1.
Gating strategy. FACS plots depict the gating strategy for the identification of (A) total memory B cells (CD19+CD24+CD27+) and (B) inside memory B cells with low and (Spike-PE, S+) high binding capacity (Spike-PE and Spike-BUV395, S++) and (C) receptor binding domain–specific memory B cells. RBD, receptor-binding domain.
TABLE E1.
Characteristics of 16 22q11.2DS patients enrolled in the immunization study
| Characteristic | Value |
|---|---|
| Age (y), median (IQR) | 27 (24–36) |
| Sex, n (%) | 6 (60) |
| IgG (mg/dL), median (IQR) | 1,331 (1,110–1,427) |
| Under IgG replacement treatment, n (%) | 1 (6) |
| IgA (mg/dL), median (IQR) | 269 (196–314) |
| Selective IgA deficiency, n (%) | 1 (6) |
| IgM (mg/dL), median (IQR) | 69 (47–137) |
| Selective IgM deficiency, n (%) | 3 (20) |
| Lymphocytes, cells/mm3, median (IQR) | 1,835 (1,212–3,280) |
| CD4+ T cells/mm3, median (IQR) | 716 (576–979) |
| CD8+ T cell/mm3, median (IQR) | 395 (304–449) |
| CD19+ B cells/mm3, median (IQR) | 276 (149–337) |
| Switched MBC % of IgM-MBCs, median (IQR) | 32 (21.6–39.3) |
| Clinical-associated conditions, n (%) | |
| Previous heart surgery | 8 (53) |
| CHD | 7 (47) |
| Hypertension | 4 (27) |
| DMT | 2 (13) |
| Dyslipidemia | 3 (20) |
| Psychiatric problems | 3 (33) |
| Neurological problems | 2 (13) |
| Learning issues | 9 (60) |
| Autoimmunity | 7 (47) |
| ITP/AIHA | 1 (7) |
| Arthritis | 1 (7) |
| Thyroid disease | 4 (29) |
| Immunosuppressive treatment | 2 (12) |
CHD, Congenital heart disease; DMT, diabetes mellitus; IgG, -A, -M, immunoglobulin G, A, M; IQR, interquartile range; ITP/AIHA, idiopathic thrombocytopenic purpura/autoimmune haemolytic anemia; MBC, memory B cell.
References
- 1.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147:520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milito C., Lougaris V., Giardino G., Punziano A., Vultaggio A., Carrabba M., et al. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract. 2021;9 doi: 10.1016/j.jaip.2021.04.017. 2904-6.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shields A.M., Anantharachagan A., Arumugakani G., Baker K., Bahal S., Baxendale H., et al. Outcomes following SARS-CoV-2 infection in patients with primary and secondary immunodeficiency in the UK. Clin Exp Immunol. 2022;209:247–258. doi: 10.1093/cei/uxac008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho H.E., Mathew S., Peluso M.J., Cunningham-Rundles C. Clinical outcomes and features of COVID-19 in patients with primary immunodeficiencies in New York City. J Allergy Clin Immunol Pract. 2021;9:490–493.e2. doi: 10.1016/j.jaip.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salinas A.F., Mortari E.P., Terreri S., Quintarelli C., Pulvirenti F., Di Cecca S G., et al. SARS-CoV-2 vaccine induced atypical immune responses in antibody defects: everybody does their best. J Clin Immunol. 2021;41:1709–1722. doi: 10.1007/s10875-021-01133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmonte O.M., Bergerson J.R.E., Burbelo P.D., Durkee-Shock J.R., Dobbs K., Bosticardo M., et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148:1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagin D., Freund T., Navon M., Halperin T., Adir D., Marom R.,L., et al. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J Allergy Clin Immunol. 2021;148:739–749. doi: 10.1016/j.jaci.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Squire J., Joshi A. Seroconversion after coronavirus disease 2019 vaccination in patients with immune deficiency. Ann Allergy Asthma Immunol. 2021;127:383–384. doi: 10.1016/j.anai.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyà-Martínez A., García-García A., Gonzalez-Navarro E.A., Yiyi L., Vlagea A., Jordan I., et al. COVID-19 in children and young adults with moderate/severe inborn errors of immunity in a high burden area in pre-vaccine era. Clin Immunol. 2021;230 doi: 10.1016/j.clim.2021.108821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pieniawska-Śmiech K., Kuraszewicz A., Sado J., Śmiech K., Lewandowicz-Uszyńska A. Assessment of COVID-19 incidence and the ability to synthesise anti-SARS-CoV-2 antibodies of paediatric patients with primary immunodeficiency. J Clin Med. 2021;10:5111. doi: 10.3390/jcm10215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goudouris E.S., Pinto-Mariz F., Mendonça L.O., Aranda C.S., Guimarães R.R., Kokron C., et al. Outcome of SARS-CoV-2 infection in 121 patients with inborn errors of immunity: a cross-sectional study. J Clin Immunol. 2021;41:1479–1489. doi: 10.1007/s10875-021-01066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallatah E., Chang Y., Calderon J., Hernandez-Trujillo V. DiGeorge syndrome and COVID-19 in two pediatric patients. J Allergy Clin Immunol. 2021;147 AB66-AB66. [Google Scholar]
- 13.Marcus N., Frizinsky S., Hagin D., Ovadia A., Hanna S., Farkash M., et al. Minor clinical impact of COVID-19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol. 2021;11 doi: 10.3389/fimmu.2020.614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giardino G., Milito C., Lougaris V., Punziano A., Carrabba M., Cinetto F., et al. The impact of SARS-CoV-2 infection in patients with inborn errors of immunity: the experience of the Italian Primary Immunodeficiencies Network (IPINet) J Clin Immunol. 2022;42:935–946. doi: 10.1007/s10875-022-01264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowley T.B., McGinn D.M., Sullivan K.E. International q11.2 COVID group report. 22q11.2 deletion and duplication syndromes and COVID-19. J Clin Immunol. 2022;42:746–748. doi: 10.1007/s10875-022-01246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald-McGinn D.M., Kirschner R., Goldmuntz E., Sullivan K., Eicher P., Gerdes M., et al. The Philadelphia story: the 22q11.2 deletion: report on 250 patients. Genet Couns. 1999;10:11–24. [PubMed] [Google Scholar]
- 17.Ryan A.K., Goodship J.A., Wilson D.I., Philip N., Levy A., Seidel H., et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997;34:798–804. doi: 10.1136/jmg.34.10.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vantrappen G., Devriendt K., Swillen A., Rommel N., Vogels A., Eyskens B., et al. Presenting symptoms and clinical features in 130 patients with the velo-cardio-facial syndrome. The Leuven experience. Genet Couns. 1999;10:3–9. [PubMed] [Google Scholar]
- 19.DiGeorge A.M. Congenital absence of the thymus and its immunological consequences: concurrence with congenital hypo-thyroidism. Birth Defects. 1968;4:116–121. [Google Scholar]
- 20.Sullivan K.E. DiGeorge syndrome/chromosome 22q11.2 deletion syndrome. Curr Allergy Asthma Res. 2001;1:438–444. doi: 10.1007/s11882-001-0029-z. [DOI] [PubMed] [Google Scholar]
- 21.Jawad A.F., McDonald-Mcginn D.M., Zackai E., Sullivan K.E. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) J Pediatr. 2001;139:715–723. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- 22.Piliero L.M., Sanford A.N., McDonald-McGinn D.M., Zackai E.H., Sullivan K.E. T-cell homeostasis in humans with thymic hypoplasia due to chromosome 22q11.2 deletion syndrome. Blood. 2004;103:1020–1025. doi: 10.1182/blood-2003-08-2824. [DOI] [PubMed] [Google Scholar]
- 23.Pierdominici M., Mazzetta F., Caprini E., Marziali M., Digilio M.C., Marino B., et al. Biased T-cell receptor repertoires in patients with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Clin Exp Immunol. 2003;132:323–331. doi: 10.1046/j.1365-2249.2003.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel K., Akhter J., Kobrynski L., Benjamin Gathmann M.A., Davis O., Sullivan K.E., et al. Immunoglobulin deficiencies: the B-lymphocyte side of DiGeorge syndrome. J Pediatr. 2012;161:950–953. doi: 10.1016/j.jpeds.2012.06.018. Erratum in: J Pediatr 2013;162:658. [DOI] [PubMed] [Google Scholar]
- 25.Cancrini C., Puliafito P., Digilio M.C., Soresina A., Martino S., Rondelli R., et al. Clinical features and follow-up in patients with 22q11.2 deletion syndrome. J Pediatr. 2014;164 doi: 10.1016/j.jpeds.2014.01.056. 1475-80.e2. [DOI] [PubMed] [Google Scholar]
- 26.Azzari C., Gambineri E., Resti M., Moriondo M., Betti L., Saldias L.R., et al. Safety and immunogenicity of measles-mumps-rubella vaccine in children with congenital immunodeficiency (DiGeorge syndrome) Vaccine. 2005;23:1668–1671. doi: 10.1016/j.vaccine.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sukaiti N., Reid B., Lavi S., Al-Zaharani D., Atkinson A., Roifman C.M., et al. Safety and efficacy of measles, mumps, and rubella vaccine in patients with DiGeorge syndrome. J Allergy Clin Immunol. 2010;126:868–869. doi: 10.1016/j.jaci.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Jawad A.F., Prak E.L., Boyer J., McDonald-McGinn D.M., Zackai E., McDonald K., et al. A prospective study of influenza vaccination and a comparison of immunologic parameters in children and adults with chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) J Clin Immunol. 2011;31:927–935. doi: 10.1007/s10875-011-9569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marino B., Digilio M.C., Toscano A., Anaclerio S., Giannotti A., Feltri C., et al. Anatomic patterns of conotruncal defects associated with deletion 22q11. Genet Med. 2001;3:45–48. doi: 10.1097/00125817-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Azevedo R.B., Botelho B.G., Hollanda J.V.G., Ferreira L.V.L., Junqueira de Andrade L.Z., Oei S.S.M.L., et al. COVID-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35:4–11. doi: 10.1038/s41371-020-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehwerhemuepha L., Roth B., Patel A.K., Heutlinger O., Heffernan C., Arrieta A.C., et al. Association of congenital and acquired cardiovascular conditions with COVID-19 severity among pediatric patients in the US. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.11967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louis D.W., Saad M., Vijayakumar S., Ilyas S., Kokkirala A., Aronow H.D. The cardiovascular manifestations of COVID-19. Cardiol Clin. 2022;40:277–285. doi: 10.1016/j.ccl.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO) Living guidance for clinical management of COVID-19. https://apps.who.int/iris/bitstream/handle/10665/349321/WHO-2019-nCoV-clinical-2021.2-eng.pdf
- 34.Centers for Disease Control and Prevention Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html [PubMed]
- 35.Komissarov A.A., Dolzhikova I.V., Efimov G.A., Logunov D.Y., Mityaeva O., Molodtsov I.A., et al. Boosting of the SARS-CoV-2-specific immune response after vaccination with single-dose Sputnik light vaccine. J Immunol. 2022;208:1139–1145. doi: 10.4049/jimmunol.2101052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassaniti I., Bergami F., Percivalle E., Gabanti E., Sammartino J.C., Ferrari A., et al. Humoral and cell-mediated response against SARS-CoV-2 variants elicited by mRNA vaccine BNT162b2 in healthcare workers: a longitudinal observational study. Clin Microbiol Infect. 2022;28:301.e1–301.e8. doi: 10.1016/j.cmi.2021.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piano Mortari E., Russo C., Vinci M.R., Terreri S., Fernandez Salinas A., Piccioni L., et al. Highly specific memory B cells generation after the 2nd dose of BNT162b2 vaccine compensate for the decline of serum antibodies and absence of mucosal IgA. Cells. 2021;10:2541. doi: 10.3390/cells10102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roll P., Muhammad K., Schumann M., Kleinert S., Einsele H., Dörner T., Tony H.P. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63:1255–1264. doi: 10.1002/art.30242. [DOI] [PubMed] [Google Scholar]
- 39.Botto L.D., May K., Fernhoff P.M., Correa A., Coleman K., Rasmussen S.A., et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112:101–107. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- 40.McDonald-McGinn D.M., Sullivan K.E., Marino B., Philip N., Swillen A., Vorstman J.A., et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly D., Goldberg R., Wilson D., Lindsay E., Carey A., Goodship J., et al. Confirmation that the velo-cardio-facial syndrome is associated with haplo-insufficiency of genes at chromosome 22q11. Am J Med Genet. 1993;45:308–312. doi: 10.1002/ajmg.1320450306. [DOI] [PubMed] [Google Scholar]
- 42.Driscoll D.A., Budarf M.L., Emanuel B.S. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. Am J Hum Genet. 1992;50:924–933. [PMC free article] [PubMed] [Google Scholar]
- 43.Lischner H.W., Huff D.S. T-cell deficiency in DiGeorge syndrome. Birth Defects Orig Artic Ser. 1975;11:16–21. [PubMed] [Google Scholar]
- 44.Kornfeld S.J., Zeffren B., Christodoulou C.S., Day N.K., Cawkwell G., Good R.A. DiGeorge anomaly: a comparative study of the clinical and immunologic characteristics of patients positive and negative by fluorescence in situ hybridization. J Allergy Clin Immunol. 2000;105 doi: 10.1067/mai.2000.105527. 983-70. [DOI] [PubMed] [Google Scholar]
- 45.Ramos-Casals M., Brito-Zerón P., Mariette X. Systemic and organ-specific immune-related manifestations of COVID-19. Nat Rev Rheumatol. 2021;17:315–332. doi: 10.1038/s41584-021-00608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diller G.P., Gatzoulis M.A., Broberg C.S., Aboulhosn J., Brida M., Schwerzmann M., et al. Coronavirus disease 2019 in adults with congenital heart disease: a position paper from the ESC working group of adult congenital heart disease, and the International Society for Adult Congenital Heart Disease. Eur Heart J. 2021;42:1858–1865. doi: 10.1093/eurheartj/ehaa960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald-McGinn D.M., Sullivan K.E., Marino B., Philip N., Swillen A., Vorstman J.A., et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldmuntz E. 22q11.2 deletion syndrome and congenital heart disease. Am J Med Genet C Semin Med Genet. 2020;184:64–72. doi: 10.1002/ajmg.c.31774. [DOI] [PubMed] [Google Scholar]
- 49.Rathore S.S., Rojas G.A., Sondhi M., Pothuru S., Pydi R., Kancherla N., et al. Myocarditis associated with COVID-19 disease: a systematic review of published case reports and case series. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14470. [DOI] [PubMed] [Google Scholar]
- 50.Tereshchenko L.G., Bishop A., Fisher-Campbell N., Levene J., Morris C.C., Patel H., et al. Risk of cardiovascular events after COVID-19. Am J Cardiol. 2022;179:102–109. doi: 10.1016/j.amjcard.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klocperk A., Mejstříková E., Kayserová J., Kalina T., Šedivá A. Low marginal zone-like B lymphocytes and natural antibodies characterize skewed B-lymphocyte subpopulations in del22q11 DiGeorge patients. Clin Immunol. 2015;161:144–149. doi: 10.1016/j.clim.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Finocchi A., Di Cesare S., Romiti M.L., Capponi C., Rossi P., Carsetti R., et al. Humoral immune responses and CD27+ B cells in children with DiGeorge syndrome (22q11.2 deletion syndrome) Pediatr Allergy Immunol. 2006;17:382–388. doi: 10.1111/j.1399-3038.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 53.Barrett D.J., Ammann A.J., Wara D.W., Cowan M.J., Fisher T.J., Stiehm E.R. Clinical and immunologic spectrum of the DiGeorge syndrome. J Clin Lab Immunol. 1981;6:1–6. [PubMed] [Google Scholar]
- 54.Pierdominici M., Marziali M., Giovannetti A., Oliva A., Rosso R., Marino B., et al. T cell receptor repertoire and function in patients with DiGeorge syndrome and velocardiofacial syndrome. Clin Exp Immunol. 2000;121:127–132. doi: 10.1046/j.1365-2249.2000.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]