Abstract
Background
Sleep loss may influence subsequent physical performance. Quantifying the impact of sleep loss on physical performance is critical for individuals involved in athletic pursuits.
Design
Systematic review and meta-analysis.
Search and Inclusion
Studies were identified via the Web of Science, Scopus, and PsycINFO online databases. Investigations measuring exercise performance under ‘control’ (i.e., normal sleep, > 6 h in any 24 h period) and ‘intervention’ (i.e., sleep loss, ≤ 6 h sleep in any 24 h period) conditions were included. Performance tasks were classified into different exercise categories (anaerobic power, speed/power endurance, high-intensity interval exercise (HIIE), strength, endurance, strength-endurance, and skill). Multi-level random-effects meta-analyses and meta-regression analyses were conducted, including subgroup analyses to explore the influence of sleep-loss protocol (e.g., deprivation, restriction, early [delayed sleep onset] and late restriction [earlier than normal waking]), time of day the exercise task was performed (AM vs. PM) and body limb strength (upper vs. lower body).
Results
Overall, 227 outcome measures (anaerobic power: n = 58; speed/power endurance: n = 32; HIIE: n = 27; strength: n = 66; endurance: n = 22; strength-endurance: n = 9; skill: n = 13) derived from 69 publications were included. Results indicated a negative impact of sleep loss on the percentage change (%Δ) in exercise performance (n = 959 [89%] male; mean %Δ = − 7.56%, 95% CI − 11.9 to − 3.13, p = 0.001, I2 = 98.1%). Effects were significant for all exercise categories. Subgroup analyses indicated that the pattern of sleep loss (i.e., deprivation, early and late restriction) preceding exercise is an important factor, with consistent negative effects only observed with deprivation and late-restriction protocols. A significant positive relationship was observed between time awake prior to the exercise task and %Δ in performance for both deprivation and late-restriction protocols (~ 0.4% decrease for every hour awake prior to exercise). The negative effects of sleep loss on different exercise tasks performed in the PM were consistent, while tasks performed in the AM were largely unaffected.
Conclusions
Sleep loss appears to have a negative impact on exercise performance. If sleep loss is anticipated and unavoidable, individuals should avoid situations that lead to experiencing deprivation or late restriction, and prioritise morning exercise in an effort to maintain performance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40279-022-01706-y.
Key Points
| Acute sleep loss negatively impacts next-day exercise performance |
| The magnitude and significance of the impact are dependent on the sleep-loss protocol preceding exercise, with sleep deprivation and late-restriction (earlier than normal waking) protocols demonstrating a consistent negative influence |
| The time awake prior to performing exercise was found to be an influential factor |
| Exercise tasks performed in the PM were consistently negatively affected by sleep loss, while tasks performed in the AM were largely unaffected |
Introduction
Sleep is essential to maintain physical and mental health. It has been shown to promote memory [1], regulate emotions [2], enhance metabolic functions [3], improve energy balance, and moderate the immune system [4], and may play a pivotal role in the stress–recovery balance, via its influence on the activity of the hypothalamic–pituitary–adrenal axis [5]. Despite this knowledge, ~ 45% of the Western adult population fail to obtain the recommended 7–9 h of sleep each night [6]. Sleep loss is often driven by lifestyle choices that reduce available sleep time, such as evening social activities [7], exposure to artificial light prior to sleep [8], consumption of caffeinated beverages [9], and smoking [10]. Stress and anxiety [11], medical conditions/illness [12], and genetic traits [13, 14] can also contribute to sleep loss. Certain populations, including professional athletes [15–24], shift workers [25] and military personnel [26], appear particularly susceptible to sleep loss. For athletes, sleep loss may be exacerbated by early morning training sessions [27, 28], training or competing at altitude (> 2000 m) [29], travel (late night and early morning departures) [30, 31], and the use of caffeine as an ergogenic aid [32].
Insufficient sleep can result in a significant personal and societal burden, including adverse effects on wellbeing [33], productivity [34] and safety [35]. For those who are physically active or involved in athletic pursuits, sleep loss may also influence acute training adaptations and exercise performance outcomes [17, 36, 37]. The consequences of sleep loss (e.g., altered training adaptations, increased workplace accidents [38, 39]) are likely to have multiple aetiologies. Negative consequences may result from a decrease in muscular strength [40] and/or endurance [41], change in mood (e.g., decreased motivation) [42], an increase in perceived effort [43, 44], changes to cognitive processing ability (e.g., decision making, executive function) and/or a reduction in fine motor skills [45], or a combination of these factors.
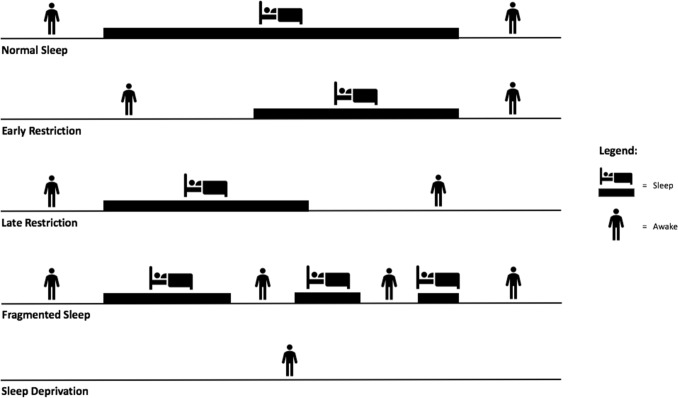
Sleep has two distinct dimensions: quantity and quality. However, sleep loss is more often measured in terms of duration, given the challenges associated with accurately determining sleep quality in most situations [46]. Sleep deprivation is a general term used to describe a period of extended wakefulness, often related to circumstances when an individual is unable to obtain any sleep across a period of ≥ 24 h [47]. Restricted sleep (also referred to as ‘partial sleep deprivation’) occurs when an individual has the opportunity to sleep, but this is limited in duration from their normal sleep habit [47] and is often a result of delayed sleep onset (sometimes termed ‘early restriction’), earlier than normal waking (sometimes termed ‘late restriction’), or fragmented sleep, which is when one or more nocturnal awakenings occur [48] (Fig. 1 depicts the different types of sleep loss). The amount (e.g., deprivation/restriction) and type (e.g., early restriction/late restriction) of sleep loss incurred may have some influence on the magnitude of effect that insufficient sleep has on physical performance [49–52].
Fig. 1.
Types of sleep loss encountered
The influence of sleep loss on physical performance has received considerable scientific attention. Studies have investigated the effects of sleep loss on performance in different exercise tasks (based on predominant physical attributes), including strength [41, 53–55], anaerobic power/capacity [56–62], endurance [41, 57], and those requiring a high level of precision (e.g., skill activities [45, 63]). The influence of contextual factors has also been explored, including the timing of exercise following sleep loss (e.g., morning vs. evening exercise) [45, 53, 62, 64]; duration of sleep loss [65]; early- vs. late-sleep restriction protocols [66, 67]; and exercise characteristics themselves (acute, chronic, type and timing) [64, 68, 69]. While several reviews have summarised these effects [36, 70, 71], only one employed meta-analytical techniques to synthesise the outcomes [72]. However, this particular review was conducted over two decades ago, and many studies conducted since have improved our understanding of sleep loss and its impact on physical performance.
Therefore, the aim of this systematic review was to summarise the available literature investigating the effects of acute sleep loss (≤ 6 h sleep in any 24 h period) on exercise performance and quantify the magnitude of effects using meta-analytical techniques. The influence of certain contextual factors (e.g., exercise type, time of day, sleep-loss duration) was also explored.
Methods
The methodology of this review was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols 2015 statement [73] and registered at the International Prospective Register of Systematic Reviews (PROSPERO; identification code: CRD42020211824).
Literature Search
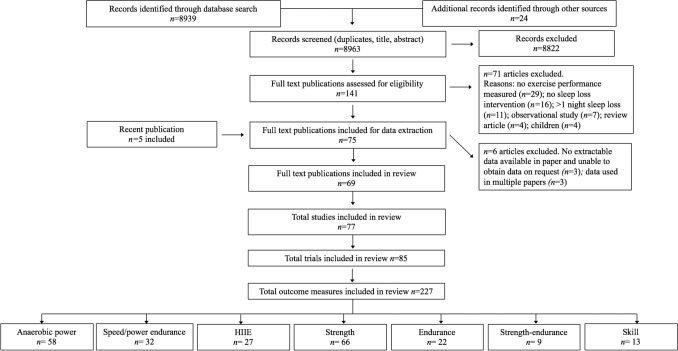
Studies were identified by searching the Web of Science (via Thomas Reuters), Scopus, and PsycINFO online databases from inception until September 2020 using the Boolean expression: (sleep restriction OR sleep deprivation OR sleep loss OR wakefulness) AND (exercis* OR performance AND NOT animal* OR rat* OR mice). The star symbol (*) was used to capture derivatives (by suffixation) of the search terms. Two investigators (JC and CI) independently screened the potential publications to identify relevant texts. Initially, all irrelevant titles were discarded. The remaining publications were then systematically screened for eligibility by abstract and full text. The decision to include or discard potential publications was made between two investigators (JC and CI) and any discrepancies were resolved in consultation with a third investigator (DM). One investigator (JC) also hand-searched the reference lists of included publications and performed a forward citation search of two previous systematic reviews [36, 71] to ensure all relevant publications were captured. An updated search was also conducted on 31 December 2021 to capture the most recent publications. Full details of the screening process are illustrated in Fig. 2.
Fig. 2.
PRISMA flow chart (study selection methodology). Some publications contained multiple participant pools. In these instances, the individual participant pools were termed ‘studies’. Some studies investigated the influence of more than one sleep-loss protocol (i.e., deprivation, early or late restriction). In these instances, the separate study arms were treated as individual investigations, and termed ‘trials’. Each individual task from a given trial was termed ‘outcome measure’. ESM Table S1 provides the original search breakdown; ESM Table S2 provides the origin of included publications; and ESM Table S3 provides the reference and reason for exclusion of full-text publications. HIIE high-intensity interval exercise, ESM electronic supplementary material
Inclusion and Exclusion Criteria
Original research studies that met the following criteria were included in this review: (1) full-text original articles written in English; (2) controlled trials employing repeated measures experimental designs; (3) human studies on adult (≥ 18 years of age) men and women with no known medical conditions and comorbidities; and (4) measured performance on an exercise/physical task (e.g., Wingate test, squat jump) under ‘sleep loss’ (i.e., ≤ 6 h sleep in any 24 h period) and ‘control’ (i.e., normal sleep, considered as > 6 h in any 24 h period) conditions.
Studies were excluded from the review if (1) a between-subject experimental design was employed and no baseline measurements were performed following ‘normal sleep’; (2) sleep-loss protocol was not ‘acute’ (i.e., it was sustained over multiple nights); (3) stimulants or sedatives were administered (e.g., caffeine, L-tryptophan, or modafinil) during the trial; (4) exercise prior to sleep intervention was not matched across conditions;1 (5) participants reported abnormal sleep behaviours (e.g., sleep disorder, shift-worker); (6) participants reported recent international travel with experience of jetlag; (7) exercise performance was measured after a period of recovery sleep (sleep latency tests were not considered ‘recovery sleep’); and (8) exercise performance data were not adequately reported (i.e., mean ± standard deviation [SD] was not reported or could not be derived).
In the event that data were not adequately reported, the corresponding author was contacted via email in an attempt to retrieve the missing data. Where data were presented in graphical format only, a web-based tool (‘WebPlotDigitizer’, https://apps.automeris.io/wpd/) was used to extract numeric values.
Several publications identified in the literature search contained more than one intervention and control comparison that was eligible for inclusion. Some publications contained multiple participant pools. In these instances, the individual participant pools were termed ‘studies’. Some studies investigated the influence of more than one sleep-loss protocol (a combination of either deprivation, early or late restriction). In these instances, the separate study arms were treated as individual investigations, and termed ‘trials’. As single trials sometimes measured serial performance (i.e., multiple times across the trial) and/or used several tasks that generated multiple outcomes, each one could contribute multiple effect estimates to the review (note, multilevel models were used to account for dependency of effect estimates in statistical analyses [74]; refer to Sect. 2.7 ‘Statistical Analyses’). In these instances, each individual effect estimate from a given trial was termed an ‘outcome measure’.
Exercise Task Classifications
Each exercise task was reviewed by two investigators (JC and PB) and allocated into one of the following seven categories: anaerobic power, speed/power endurance, high-intensity interval exercise (HIIE), strength, endurance, strength-endurance, and skill. The allocation criteria are defined in Table 1. All discrepancies were resolved in consultation with a third investigator (SS).
Table 1.
Exercise task categories
| Exercise task category | Description of exercise task | Example task |
|---|---|---|
| Anaerobic power | Duration ≤ 6 s performed at maximum effort | Wingate (peak power); CMJ; squat jump; 20 m sprint |
| Speed/power endurance | Maximal continuous exercise—duration > 6 s but < 90 s | 30 s Wingate test (mean power); 5 m multiple shuttle test (peak distance); TTE at predetermined workload; repeated CMJ—mean jump height |
| HIIE | Requires near-maximal effort (~ 45 s) with brief periods of recovery (≤ 4.5 min) | Yo-Yo intermittent recovery test level 1; 5 m multiple shuttle test (total distance) |
| Endurance | Continuous exercise ≥ 120 s | TTE for incremental exercise test; peak power output at exhaustion during incremental exercise test; 3 km TT |
| Strength | Maximum force development during a single effort | 1RM; MVC (e.g., hand-grip strength test, knee extension) |
| Strength-endurance | Resistance task ≥ 2 repetitions or > 5 s sustained contraction | Number of repetitions performed at 85% of 1RM; 30 s MVC; knee-extension fatiguing task |
| Skill | Task that requires high cognitive component for execution | Tennis serving; rugby passing; free-throw shooting (basketball); shooting |
HIIE high-intensity interval exercise, TT time trial, RM repetition maximum, MVC maximum voluntary contraction, TTE time to exhaustion, CMJ counter-movement jump
Some studies included in this meta-analysis assessed the influence of sleep loss on more than one performance task, either belonging to the same category [40, 41, 53, 58, 59, 63, 64, 75–84], or different categories [41, 43, 44, 56–59, 61, 62, 64, 65, 75, 77–81, 85–102]. For example, Souissi et al. [78] measured anaerobic power in two separate tasks (i.e., squat jump and Wingate test). In these cases, effect estimates were derived for all eligible tasks.
Measures of residual muscular fatigue (i.e., those obtained within minutes of completing an initial performance test) [64, 89] were not included in the review. However, serial measures were accepted if the preceding test was deemed unlikely to have influenced performance on the subsequent test. For example, if the preceding test used different muscle groups (e.g., knee extensor maximum voluntary contraction (MVC) and knee flexion MVC) [53, 76, 82, 83] or a contralateral muscle group [82], or if the same test was repeated at separate times of the day (e.g., once at 0600 h, then at 1700 h) [43, 53, 62, 64, 79–81, 90], then each measurement was included and considered as a separate ‘outcome measure’.
Primary and Secondary Research Outcomes
The primary outcome in this investigation was the percentage change in exercise performance (%ΔEP) following sleep loss (i.e., sleep restriction or sleep deprivation), calculated using the following formula:
where ‘EP sleep loss’ was exercise performance measured following sleep loss and ‘EP normal’ was exercise performance measured under control conditions.
Data Extraction
Data were extracted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions Checklist of Items to Consider in Data Collection or Data Extraction [103] and entered into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, WA, USA). Extracted data included (1) participant characteristics (e.g., training status, age, body mass, sex, aerobic power [O2peak]); (2) pretrial standardisation procedures; (3) the sleep-loss protocol (e.g., deprivation, restriction); (4) sleep location (e.g., laboratory, home); (5) the instrument used to monitor and record hours slept; (6) protocol used to assess exercise performance; (7) timing of tests relative to the sleep/wake cycle; and (8) whether or not participants were fed/fasted prior to performance testing.
Quality Assessment
The included publications were assessed based on their methodological quality using the Rosendal Scale (see Table 2 in the article by van Rosendal et al. [104]). This scale, which combines the Jadad scoring system [105], PEDro scale [106] and Delphi List [107], assesses a number of factors associated with the minimisation of experimental bias (e.g., blinding, participant selection, randomisation, data reporting). Excellent methodological quality is indicated by a Rosendal score ≥ 60% [105]. Scoring was determined by dividing the number of ‘yes’ responses by the total number of applicable items. Scores were compared between two investigators (JC and CI) conducting the assessments and any discrepancies were resolved (with a third investigator consulted [DM] if agreement could not be reached). As such, the final score is an agreed rating for each publication.
Table 2.
Meta-analysis results for effect of sleep loss on exercise performance
| Exercise category | Outcomes, n | Exercise performance percentage change | Heterogeneity | ||
|---|---|---|---|---|---|
| Mean (95% CI) | p value | I2 value | p value | ||
| All categories | |||||
| Overall | 227 | − 7.56 (− 11.9 to − 3.13) | 0.001 | 98.1 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 97 | − 5.25 (− 8.01 to − 2.48) | < 0.001 | 96.5 | < 0.001 |
| Restriction | 130 | − 8.59 (− 13.6 to − 3.61) | 0.001 | 98.3 | < 0.001 |
| Early restriction | 62 | − 5.85 (− 13.4 to 1.66) | 0.125 | 93.5 | < 0.001 |
| Late restriction | 60 | − 7.39 (− 10.1 to − 4.66) | < 0.001 | 98.3 | < 0.001 |
| Anaerobic power | |||||
| Overall | 58 | − 6.26 (− 9.10 to − 3.41) | < 0.001 | 98.1 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 25 | − 6.39 (− 11.7 to − 1.09) | 0.020 | 99.2 | < 0.001 |
| Restriction | 33 | − 5.99 (− 9.22 to − 2.77) | 0.001 | 94.1 | < 0.001 |
| Early restriction | 11 | − 0.50 (− 2.00 to 1.00) | 0.477 | 0.04 | 0.770 |
| Late restriction | 22 | − 7.47 (− 11.1 to − 3.85) | < 0.001 | 95.4 | < 0.001 |
| Speed/power endurance | |||||
| Overall | 32 | − 2.90 (− 4.97 to − 0.82) | 0.008 | 96.3 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 12 | − 2.93 (− 8.05 to 2.18) | 0.233 | 91.1 | < 0.001 |
| Restriction | 20 | − 3.23 (− 5.94 to − 0.53) | 0.022 | 96.4 | < 0.001 |
| Early restriction | 7 | 0.49 (− 2.05 to 3.04) | 0.652 | 24.4 | 0.366 |
| Late restriction | 13 | − 4.38 (− 7.15 to − 1.62) | 0.005 | 97.3 | < 0.001 |
| HIIE | |||||
| Overall | 27 | − 6.15 (− 10.5 to − 1.77) | 0.008 | 98.9 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 9 | − 2.38 (− 12.1 to 7.32) | 0.587 | 99.0 | < 0.001 |
| Restriction | 18 | − 8.77 (− 13.3 to − 4.27) | 0.001 | 98.3 | < 0.001 |
| Early restriction | 8 | − 3.15 (− 9.68 to 3.37) | 0.291 | 73.2 | 0.001 |
| Late restriction | 10a | − 11.5 (− 16.3 to − 6.71) | < 0.001 | 99.2 | < 0.001 |
| Strength | |||||
| Overall | 66 | − 2.85 (− 4.47 to − 1.23) | < 0.001 | 62.2 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 29 | − 3.00 (− 4.52 to − 1.48) | < 0.001 | 49.2 | < 0.001 |
| Restriction | 37 | − 2.77 (− 6.75 to 1.21) | 0.167 | 74.9 | < 0.001 |
| Early restriction | 26 | − 1.16 (− 2.57 to 0.25) | 0.102 | 0.02 | 0.952 |
| Late restriction | 11 | − 4.45 (− 9.30 to 0.41) | 0.068 | 83.7 | < 0.001 |
| Endurance | |||||
| Overall | 22 | − 5.55 (− 8.12 to − 2.99) | < 0.001 | 86.5 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 14a | − 6.75 (− 10.3 to − 3.25) | < 0.001 | 91.2 | < 0.001 |
| Restriction | 8 | − 3.27 (− 5.06 to − 1.47) | 0.004 | < 0.001 | 0.914 |
| Early restriction | 2a | − 5.28 (− 9.17 to − 1.39) | 0.008 | 0.00 | 0.798 |
| Late restriction | 3a | − 3.72 (− 6.96 to − 0.47) | 0.025 | < 0.001 | 0.620 |
| Strength-endurance | |||||
| Overall | 9 | − 9.85 (− 19.6 to − 0.13) | 0.048 | 85.4 | < 0.001 |
| Deprivation | 6 | − 6.06 (− 14.9 to 2.80) | 0.139 | 45.6 | 0.255 |
| Restriction | 3 | − 18.3 (− 35.6 to − 0.96) | 0.045 | 88.3 | 0.001 |
| Skill | |||||
| Overall | 13 | − 20.9 (− 27.0 to − 14.9) | < 0.001 | 94.1 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation | 2a | − 20.9 (− 23.6 to − 18.2) | < 0.001 | 0.00 | 0.342 |
| Restriction | 11 | − 21.0 (− 29.1 to − 12.9) | < 0.001 | 95.4 | < 0.001 |
| Early restriction | 8 | − 23.9 (− 33.6 to − 14.2) | < 0.001 | 94.8 | < 0.001 |
| Late restriction | 1 | ||||
A negative effect estimate indicates a decrease in performance under the intervention condition (‘sleep loss’)
Deprivation: participants did not sleep for an extended period of time (i.e., whole night); restriction: total sleep time ≤ 6 h in any 24 h period—this category is a combination of early restriction, late restriction, fragmented sleep and non-specified sleep restriction protocols; early restriction: participants delayed sleep (i.e., went to sleep at a later time); late restriction; participants awakened earlier than normal
HIIE high-intensity interval exercise, CI confidence interval
aAll outcomes were from independent studies and the meta-analysis was run without dependency levels (i.e., simple meta-analysis)
Statistical Analyses
A series of multilevel meta-analyses and meta-regression analyses were performed using R Studio (version 4.0.1) with the metafor-package [108] and syntax adapted from Assink and Wibbelink [74]. A two-level meta-analysis is equivalent to a traditional random-effects analysis in which there is only one random effect. For the meta-analysis and meta-regression analysis when all seven exercise categories were combined, we added random effects at two additional levels to account for dependency among effect estimates derived from the (1) same studies; and (2) same exercise categories. Therefore, the four sources of variance modelled were: (Level 1) the sampling variance for the observed effect estimates; (Level 2) the variance between effect estimates derived from the same studies; (Level 3) the variance between effect estimates derived from the same exercise categories; and (Level 4) the variance between studies. The subgroup analyses (described in Sect. 2.7.1) accounted for dependency among effect estimates derived from the same studies only. An example of the accompanying R script (for the combined exercise category analysis) is available in electronic supplementary material [ESM] Appendix S1.
Weighted Mean Effect
Meta-analyses were performed to determine the influence of sleep loss (vs. control) on overall exercise performance (all exercise categories combined) and each respective exercise category (i.e., anaerobic power, speed/power endurance, HIIE, strength, endurance, strength-endurance, skill). Individual effect estimates were calculated as the %Δ EP (as described in Sect. 2.4), where a negative effect estimate indicates a decrease in exercise performance under the intervention condition (‘sleep loss’). As the current review used the %Δ EP (i.e., rather than the net difference), the SD of exercise performance change could not be determined via standard methods. Instead, t statistics (or p values) derived from paired t tests were used to calculate the SD of the percentage change in exercise performance (SDΔ). Where an exact value was quoted [45, 76, 88, 92, 109–111], the calculation was performed using the following formula [112]:
| 1 |
where SDΔ is the SD of the percentage change in exercise performance and n is the number of participants. Where only p > x or p < 0.05 was reported (and raw exercise performance data could not be retrieved), the missing t-statistic was imputed using the following formula:
| 2 |
where SDΔ is the SD of the net exercise performance change and R is the correlation coefficient. R was approximated (0.71) as the mean correlation coefficient calculated using raw exercise performance data from nine outcome measurements derived from seven publications [45, 76, 88, 92, 109–111], as indicated by Higgins and Green [112]. Sensitivity analyses were performed using R = 0.30 and 0.80 to test the robustness of the analysis to the imputed value. In addition, outcome measures were individually excluded (i.e., one-out method) to examine their influence on the weighted mean effect estimate. The imputed SDΔ (net change) used to derive the t-statistic was calculated using the following formula:
| 3 |
Effect estimates were weighted by the inverse variance of the performance change and statistical significance was attained if the 95% confidence interval (CI) did not include zero. Heterogeneity was assessed using Cochran’s Q, the I2 index and the within- and between-cluster variance components (i.e., σ2). Significant heterogeneity was indicated by a p value < 0.05 for Cochran’s Q [113]. Subgroup analyses were performed to investigate the influence of (1) the sleep-loss protocol implemented (e.g., sleep deprivation, sleep restriction [i.e., the combination of early and late restriction, fragmented sleep and non-specified sleep restriction protocols]), and early and late restriction; (2) the timing of exercise following sleep loss (ante meridiem [AM] vs. post meridiem [PM]); and (3) body limb strength (upper- vs. lower-body strength), on %ΔEP. The time that body limb strength tasks were conducted following sleep loss and its impact on %ΔEP were also explored for each sleep-loss protocol implemented.
Meta-Regression Analysis
Restricted maximum likelihood (REML) multilevel simple meta-regression analyses were performed to determine whether the %ΔEP between treatments was influenced by the time awake prior to the exercise task (i.e., the number of hours from their last waking to the start of the exercise task). Regression analyses were examined for influential cases and outliers (i.e., studentised residuals, Cook’s distance and centred leverage values). Statistical significance was accepted as p < 0.05.
Results
Overview of the Included Studies and Study Quality
Seventy-five publications met the inclusion criteria; however six had to be excluded because data (1) could not be extracted (or retrieved) [114–116]; or (2) were reported in another included publication [117–119]. Therefore, 69 publications remained for analysis. These publications provided 77 individual ‘studies’ (i.e., eight additional participant pools [82, 90, 120–123]). Ten studies investigated the influence of more than one sleep-loss protocol (a combination of either deprivation, early or late restriction) [44, 57, 64–67, 81, 87, 124, 125]. This resulted in 85 trials, in which 14 measured the same exercise task(s) multiple times (twice, e.g., once at 0600 h, then at 1800 h [43, 53, 62, 64, 79–81, 90] or more than two times [45, 54, 55]). Thirty-six trials (derived from 23 studies) reported only one outcome measure [45, 49, 50, 54, 55, 66, 67, 110, 111, 116, 120–132], with the remaining trials yielding multiple outcome measures. This resulted in 227 separate outcome measures being included in the overall analysis. These outcome measures were further classified into their respective exercise categories (anaerobic power: n = 58; speed/power endurance: n = 32; HIIE: n = 27; strength: n = 66; endurance: n = 22; strength-endurance: n = 9; skill: n = 13). The location of the sleep protocol (i.e., slept in the laboratory or at home) and the method used to monitor sleep parameters (i.e., duration/quality) for each outcome measure are provided in ESM Table S4. Participant characteristics, mode of exercise, and timing of the exercise task are outlined in ESM Table S5; an overview of each included study is provided in ESM Appendix S2. Methodological quality assessment yielded an average Rosendal score of 67 ± 9%, with all but one publication [83] scoring ≥ 50%. Results of the quality assessment are shown in ESM Table S6.
Overall Exercise Performance (All Exercise Categories)
Seventy-seven studies (n = 959; 89% male), providing 227 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on exercise performance (mean %Δ = − 7.56%, 95% CI − 11.9 to − 3.13, p = 0.001, I2 = 98.1%) [ESM Fig. S1]. The magnitude and significance of this effect was stable during one-out (%ΔEP range = − 7.91 to − 7.28% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S7).
Subgroup analyses demonstrated that exercise performance was negatively affected by sleep deprivation, sleep restriction and late restriction, but not early restriction (Table 2). Results indicated that sleep loss had a consistent negative influence on performance when tasks were performed in both the AM and PM; however, the magnitude of the effect was larger for PM (Table 3).
Table 3.
Meta-analysis results for the effect of sleep loss on exercise tasks performed in the AM or PM
| Exercise category | Outcomes, n | Exercise performance percentage change | Heterogeneity | ||
|---|---|---|---|---|---|
| Mean (95% CI) | p value | I2 value | p value | ||
| All categories | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 115 | − 5.42 (− 9.66 to − 1.17) | 0.013 | 93.5 | < 0.001 |
| Overall (exercise PM) | 106 | − 8.31 (− 13.2 to − 3.37) | 0.001 | 98.9 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 59 | − 3.48 (− 5.89 to − 1.08) | 0.005 | 94.2 | < 0.001 |
| Deprivation (exercise PM) | 35 | − 6.85 (− 11.3 to − 2.39) | 0.004 | 97.5 | < 0.001 |
| Restriction (exercise AM) | 56 | − 5.96 (− 11.5 to − 0.43) | 0.035 | 90.7 | < 0.001 |
| Restriction (exercise PM) | 71 | − 9.50 (− 14.9 to − 4.12) | 0.001 | 99.0 | < 0.001 |
| Early restriction (exercise AM) | 27 | − 1.55 (− 4.66 to 1.56) | 0.315 | 55.1 | 0.437 |
| Early restriction (exercise PM) | 35 | − 6.23 (− 13.9 to 1.44) | 0.108 | 94.7 | < 0.001 |
| Late restriction (exercise AM) | 23 | − 2.48 (− 4.36 to − 0.60) | 0.012 | 46.1 | 0.048 |
| Late restriction (exercise PM) | 34 | − 9.67 (− 13.1 to − 6.24) | < 0.001 | 99.1 | < 0.001 |
| Anaerobic power | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 27 | − 4.58 (− 9.14 to − 0.24) | 0.049 | 97.0 | < 0.001 |
| Overall (exercise PM) | 30 | − 7.37 (− 10.3 to − 4.40) | < 0.001 | 97.8 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 14 | − 6.38 (− 14.5 to 1.73) | 0.113 | 98.5 | < 0.001 |
| Deprivation (exercise PM) | 11 | − 5.49 (− 7.93 to − 3.04) | < 0.001 | 93.5 | < 0.001 |
| Restriction (exercise AM) | 13 | − 2.77 (− 5.77 to 0.23) | 0.067 | 72.3 | 0.010 |
| Restriction (exercise PM) | 19 | − 8.35 (− 13.1 to − 3.56) | 0.002 | 96.0 | < 0.001 |
| Early restriction (exercise AM) | 5 | 0.25 (− 2.55 to 3.05) | 0.817 | < 0.01 | 0.988 |
| Early restriction (exercise PM) | 6 | − 1.10 (− 3.42 to 1.23) | 0.280 | 0.05 | 0.391 |
| Late restriction (exercise AM) | 8 | − 3.46 (− 7.22 to 0.31) | 0.067 | 78.1 | 0.002 |
| Late restriction (exercise PM) | 13 | − 10.1 (− 14.9 to − 5.13) | 0.001 | 96.3 | < 0.001 |
| Speed/power endurance | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 14 | 0.11 (− 0.94 to 1.16) | 0.823 | < 0.001 | 0.721 |
| Overall (exercise PM) | 15 | − 6.78 (− 10.8 to − 2.80) | 0.003 | 98.5 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 5a | 0.90 (− 0.89 to 2.69) | 0.323 | 14.0 | 0.203 |
| Deprivation (exercise PM) | 5a | − 7.11 (− 14.4 to 0.20) | 0.057 | 93.9 | < 0.001 |
| Restriction (exercise AM) | 9 | − 0.36 (− 1.84 to 1.12) | 0.588 | < 0.001 | 0.964 |
| Restriction (exercise PM) | 10 | − 5.58 (− 10.4 to − 0.76) | 0.028 | 98.8 | < 0.001 |
| Early restriction (exercise AM) | 4a | 0.62 (− 1.55 to 2.79) | 0.575 | 0.00 | 0.902 |
| Early restriction (exercise PM) | 3a | 0.43 (− 3.51 to 4.37) | 0.832 | 68.3 | 0.056 |
| Late restriction (exercise AM) | 5a | − 0.86 (− 2.40 to 0.68) | 0.275 | 0.00 | 0.954 |
| Late restriction (exercise PM) | 7a | − 7.17 (− 10.7 to − 3.66) | < 0.001 | 98.5 | < 0.001 |
| HIIE | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 11 | − 1.51 (− 10.4 to 7.42) | 0.714 | 97.7 | < 0.001 |
| Overall (exercise PM) | 16 | − 8.34 (− 12.2 to − 4.47) | 0.001 | 98.5 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 5a | − 2.06 (− 14.1 to 10.0) | 0.737 | 99.3 | < 0.001 |
| Deprivation (exercise PM) | 4a | − 4.13 (− 6.50 to − 1.76) | < 0.001 | 81.2 | 0.013 |
| Restriction (exercise AM) | 6 | − 3.39 (− 13.3 to 6.55) | 0.421 | 66.5 | 0.074 |
| Restriction (exercise PM) | 12 | − 10.2 (− 15.4 to − 4.97) | 0.001 | 99.0 | < 0.001 |
| Early restriction (exercise AM) | 5 | − 1.10 (− 10.7 to 8.53) | 0.767 | 57.2 | 0.176 |
| Early restriction (exercise PM) | 3a | − 4.79 (− 13.6 to 3.98) | 0.284 | 83.6 | < 0.001 |
| Late restriction (exercise AM) | 1 | ||||
| Late restriction (exercise PM) | 9a | − 11.5 (− 16.7 to − 6.24) | < 0.001 | 99.4 | < 0.001 |
| Strength | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 39 | − 1.78 (− 3.22 to − 0.33) | 0.017 | 17.6 | 0.570 |
| Overall (exercise PM) | 26 | − 4.58 (− 7.59 to − 1.58) | 0.004 | 79.5 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 21 | − 2.43 (− 4.47 to − 0.38) | 0.022 | 31.8 | 0.115 |
| Deprivation (exercise PM) | 8 | − 3.79 (− 7.27 to − 0.32) | 0.037 | 71.5 | < 0.001 |
| Restriction (exercise AM) | 18 | − 0.43 (− 2.41 to 1.54) | 0.650 | < 0.001 | 0.999 |
| Restriction (exercise PM) | 18 | − 5.20 (− 11.0 to 0.59) | 0.075 | 82.3 | < 0.001 |
| Early restriction (exercise AM) | 12 | − 0.55 (− 3.21 to 2.11) | 0.659 | < 0.001 | 0.985 |
| Early restriction (exercise PM) | 14 | − 1.51 (− 4.41 to 1.39) | 0.281 | 27.1 | 0.631 |
| Late restriction (exercise AM) | 6 | − 0.26 (− 4.06 to 3.54) | 0.867 | < 0.001 | 0.989 |
| Late restriction (exercise PM) | 4 | − 10.5 (− 20.6 to − 0.39) | 0.046 | 84.0 | < 0.001 |
| Endurance | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 12 | − 6.50 (− 11.1 to − 1.86) | 0.010 | 88.6 | < 0.001 |
| Overall (exercise PM) | 9 | − 3.56 (− 4.67 to − 2.45) | < 0.001 | < 0.001 | 0.976 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 9a | − 7.83 (− 12.9 to − 2.72) | 0.003 | 88.5 | < 0.001 |
| Deprivation (exercise PM) | 4a | − 3.45 (− 4.48 to − 2.42) | < 0.001 | 0.00 | 0.905 |
| Restriction (exercise AM) | 3a | − 2.67 (− 4.62 to − 0.73) | 0.007 | 0.00 | 0.794 |
| Restriction (exercise PM) | 5 | − 4.11 (− 7.40 to − 0.82) | 0.026 | < 0.001 | 0.855 |
| Early restriction (exercise AM) | 0 | ||||
| Early restriction (exercise PM) | 2a | − 5.28 (− 9.17 to − 1.39) | 0.008 | 0.00 | 0.798 |
| Late restriction (exercise AM) | 2a | − 2.92 (− 6.83 to 0.98) | 0.143 | 0.00 | 0.507 |
| Late restriction (exercise PM) | 0 | ||||
| Strength-endurance | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 8 | − 11.2 (− 23.3 to 0.85) | 0.064 | 87.0 | < 0.001 |
| Overall (exercise PM) | 1 | ||||
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 5 | − 7.06 (-22.9 to 8.82) | 0.285 | 60.5 | 0.172 |
| Deprivation (exercise PM) | 1 | ||||
| Restriction (exercise AM) | 3 | − 18.3 (− 35.6 to − 0.96) | 0.045 | 88.3 | 0.001 |
| Restriction (exercise PM) | 0 | ||||
| Skill | |||||
| AM vs. PM | |||||
| Overall (exercise AM) | 4 | − 14.2 (− 26.7 to − 1.68) | 0.037 | 87.0 | < 0.001 |
| Overall (exercise PM) | 9 | − 22.9 (− 29.7 to − 16.0) | < 0.001 | 93.8 | < 0.001 |
| Sleep-loss condition | |||||
| Deprivation (exercise AM) | 0 | ||||
| Deprivation (exercise PM) | 2a | − 20.9 (− 23.6 to − 18.2) | < 0.001 | 0.00 | 0.342 |
| Restriction (exercise AM) | 4 | − 14.2 (− 26.7 to − 1.67) | 0.037 | 87.0 | < 0.001 |
| Restriction (exercise PM) | 7 | − 23.7 (− 34.0 to − 13.4) | 0.001 | 95.5 | < 0.001 |
| Early restriction (exercise AM) | 1 | ||||
| Early restriction (exercise PM) | 7 | − 23.7 (− 34.0 to − 13.4) | 0.001 | 95.5 | < 0.001 |
| Late restriction (exercise AM) | 1 | ||||
| Late restriction (exercise PM) | 0 | ||||
A negative effect estimate indicates a decrease in performance under the intervention condition (‘sleep loss’)
Deprivation: participants did not sleep for an extended period of time (i.e., whole night); restriction: total sleep time ≤ 6 h in any 24 h period – this category is a combination of early restriction, late restriction, fragmented sleep and sleep restriction protocols not specified; early restriction: participants delayed sleep (i.e., went to sleep at a later time); late restriction; participants awakened earlier than normal
HIIE high-intensity interval exercise, AM ante meridiem, PM post meridiem, CI confidence interval
aAll outcomes were from independent studies and the meta-analysis was run without dependency levels (i.e., simple meta-analysis)
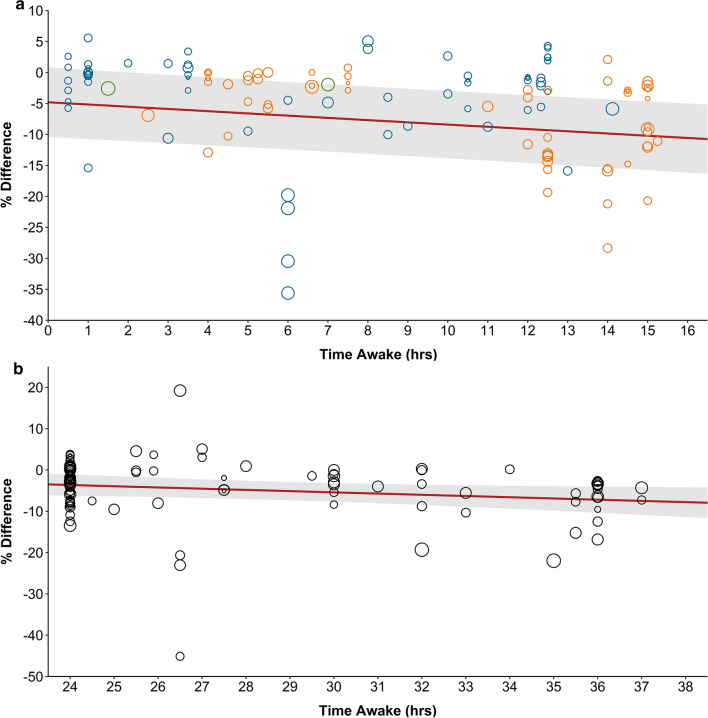
Meta-regression analyses (Fig. 3 and Table 4) identified significant relationships between time awake prior to completing the exercise task and %ΔEP for sleep restriction (mean %Δ = − 0.36, 95% CI − 0.52 to − 0.19, p < 0.001), and late restriction (mean %Δ = − 0.55, 95% CI − 0.82 to − 0.28, p < 0.001), but not sleep deprivation (mean %Δ = − 0.30, 95% CI − 0.59 to 0.01, p = 0.051), early restriction (mean %Δ = − 0.10, 95% CI − 0.27 to 0.09, p = 0.323) or when all sleep protocols were combined (mean %Δ = − 0.09, 95% CI − 0.19 to 0.01, p = 0.095). However, it is important to note that for sleep deprivation, an outcome measure from Arazi et al. [85] was identified as an influential outlier (based on Cook’s distance). When performing one-out analysis, removal of this outcome measure yielded a significant result (mean %Δ = − 0.27, 95% CI − 0.48 to − 0.05, p = 0.015).
Fig. 3.
Relationship between time awake and the mean percentage change (95% CIs shown by the grey shaded area) in exercise performance for all tasks (combined exercise categories). Circle diameter corresponds to the weight of the outcome measure from each trial. a Sleep restriction (n = 121): mean %Δ = − 0.36, 95% CI − 0.52 to − 0.19; p < 0.001. Green circles represent ‘sleep restriction’ (not further defined); blue circles represent ‘early restriction’; and orange circles represent ‘late restriction’. b Sleep deprivation (n = 97): mean %Δ = − 0.30, 95% CI − 0.59 to 0.01; p = 0.051. Deprivation: participants did not sleep for an extended period of time (i.e., whole night); early restriction: participants delayed sleep (i.e., went to sleep at a later time); late restriction: participants awakened earlier than normal. CIs confidence intervals
Table 4.
Meta-regression relationship between time awake and percentage change in exercise performance
| Exercise category | Sleep-loss condition | Outcomes, n | Exercise performance percentage change | |
|---|---|---|---|---|
| Coefficient (95% CI) | P value | |||
| All categories | Overall | 218 | –0.09 (− 0.19 to 0.01) | 0.095 |
| Deprivation | 97 | − 0.30 (− 0.59 to 0.01) | 0.051 | |
| Restriction | 121 | − 0.36 (− 0.52 to − 0.19) | < 0.001 | |
| Early restriction | 62 | − 0.10 (− 0.27 to 0.09) | 0.323 | |
| Late restriction | 57 | − 0.55 (− 0.82 to − 0.28) | < 0.001 | |
| Anaerobic power | Overall | 57 | − 0.20 (− 0.37 to − 0.04) | 0.016 |
| Deprivation | 25 | − 0.19 (− 0.49 to 0.12) | 0.213 | |
| Restriction | 32 | − 0.47 (− 0.79 to − 0.14) | 0.007 | |
| Early restriction | 11 | 0.16 (− 0.47 to 0.15) | 0.266 | |
| Late restriction | 21 | − 0.56 (− 1.09 to − 0.02) | 0.043 | |
| Speed/power endurance | Overall | 31 | − 0.19 (− 0.39 to 0.01) | 0.063 |
| Deprivation | 12 | − 1.00 (− 1.80 to − 0.21) | 0.018 | |
| Restriction | 19 | − 0.46 (− 0.71 to − 0.22) | < 0.001 | |
| Early restriction | 7 | − 0.18 (− 0.62 to 0.26) | 0.344 | |
| Late restriction | 12 | − 0.57 (− 0.98 to − 0.15) | 0.012 | |
| HIIE | Overall | 27 | − 0.05 (− 0.21 to 0.11) | 0.532 |
| Deprivation | 9 | − 0.05 (− 0.26 to 0.16) | 0.605 | |
| Restriction | 18 | − 0.79 (− 1.59 to 0.02) | 0.056 | |
| Early restriction | 8 | − 0.41 (− 2.14 to 1.32) | 0.586 | |
| Late restriction | 10 | 0.59 (− 1.56 to 2.74) | 0.546 | |
| Strength | Overall | 65 | − 0.08 (− 0.23 to 0.07) | 0.315 |
| Deprivation | 29 | − 0.11 (− 0.46 to 0.25) | 0.538 | |
| Restriction | 36 | − 0.23 (− 0.52 to 0.05) | 0.108 | |
| Early restriction | 26 | − 0.04 (− 0.33 to 0.25) | 0.792 | |
| Late restriction | 10 | − 1.07 (− 2.05 to − 0.10) | 0.035 | |
| Endurance | Overall | 21 | − 0.12 (− 0.39 to 0.14) | 0.345 |
| Deprivation | 14 | 0.77 (− 0.55 to 2.09) | 0.253 | |
| Restriction | 7 | − 0.24 (− 0.77 to 0.29) | 0.290 | |
| Early restriction | 2 | |||
| Late restriction | 3 | − 0.46 (− 1.97 to 1.05) | 0.548 | |
| Skill | Overall | 11 | − 0.03 (− 0.57 to 0.51) | 0.896 |
| Sleep deprivation | 2 | |||
| Restriction | 9 | − 0.15 (− 1.39 to 1.09) | 0.782 | |
| Early restriction | 8 | − 0.02 (− 1.28 to 1.25) | 0.975 | |
| Late restriction | 1 | |||
Deprivation: participants did not sleep for an extended period of time (i.e., whole night); restriction: total sleep time ≤ 6 h in any 24 h period—this category is a combination of early restriction, late restriction, fragmented sleep and sleep restriction protocols not specified; early restriction: participants delayed sleep (i.e., went to sleep at a later time); late restriction; participants awakened earlier than normal
HIIE high-intensity interval exercise, CI confidence interval
Anaerobic Power
Thirty-two studies (n = 368; 92% male), providing 58 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on anaerobic power (mean %Δ = − 6.26%, 95% CI − 9.10 to − 3.41, p < 0.001, I2 = 98.1%) [ESM Fig. S2]. The magnitude and statistical significance of the effect were stable during one-out (mean %Δ range = − 6.59 to − 5.24% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S8).
Subgroup analyses showed that anaerobic power was negatively affected by sleep deprivation, sleep restriction and late restriction, but not early restriction (Table 2). Results were consistent for anaerobic power tasks performed in the PM, while performance in the AM tended to be unaffected, with the exception of analysis for all sleep-loss protocols combined (Table 3).
Meta-regression analyses identified significant relationships between time awake prior to completing the exercise task and %Δ in anaerobic power when all sleep-loss protocols were included (mean %Δ = − 0.20, 95% CI − 0.37 to − 0.04, p = 0.016), when both sleep restriction protocols (i.e., early and late restriction) were combined (mean %Δ = − 0.47, 95% CI − 0.79 to − 0.14, p = 0.007) and late restriction (mean %Δ = − 0.56, 95% CI − 1.09 to − 0.02, p = 0.043). No significant relationships were detected for the other sleep-loss protocols (Table 4).
Speed/Power Endurance
Twenty studies (n = 261; 97% male), providing 32 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on speed/power endurance (mean %Δ = − 2.90%, 95% CI − 4.97 to − 0.82, p = 0.008, I2 = 96.3%) [ESM Fig. S3). The magnitude and significance of the effect were stable during one-out (mean %Δ range = − 3.72 to − 2.43% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S9).
Subgroup analyses showed that speed/power endurance was negatively affected by sleep restriction and late-restriction protocols, but not sleep deprivation or early restriction (Table 2). However, when the trial from Abedelmalek et al. [62] was removed (during one-out analyses), the effect on sleep restriction was no longer significant (ESM Table S9). Results indicated that sleep loss had a consistent negative influence on speed/power endurance when analysis was isolated to tasks performed in the PM, while tasks performed in the AM were unaffected.
Meta-regression analyses (Table 4) detected significant relationships between time awake prior to completing the exercise task and the %Δ in speed/power endurance following sleep deprivation (mean %Δ = − 1.00, 95% CI − 1.80 to − 0.21, p = 0.018), sleep restriction (mean %Δ = − 0.46, 95% CI − 0.71 to − 0.22, p < 0.001) and late restriction (mean %Δ = − 0.57, 95% CI − 0.98 to − 0.15, p = 0.012). No significant relationships were detected for the other sleep-loss protocols (Table 4).
High-Intensity Interval Exercise
Eighteen studies (n = 207; 88% male), providing 27 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on HIIE (mean %Δ = − 6.15%, 95% CI − 10.5 to − 1.77, p = 0.008, I2 = 98.9%) [ESM Fig. S4]. The magnitude and statistical significance of the effect were stable during one-out (mean %Δ range = − 7.54 to − 5.57% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S10).
Subgroup analyses indicated that HIIE performance was negatively affected following sleep restriction and late restriction, but not sleep deprivation or early restriction (Table 2). However, when the study by Arazi et al. [85] was removed (during one-out analyses) the effect for sleep deprivation was significant (mean %Δ = − 4.21%, 95% CI − 6.45 to − 1.97, p = 0.003). Results indicated that sleep loss had a consistent negative influence on HIIE when analysis was conducted on tasks performed in the PM (except for early restriction), while tasks performed in the AM were unaffected.
No significant relationships between time awake prior to completing the task and %Δ in HIIE were identified for meta-regression analysis with any of the sleep-loss protocols (Table 4).
Strength
Twenty-five studies (n = 289; 74% male), providing 66 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on strength (mean %Δ = − 2.85%, 95% CI − 4.47 to − 1.23, p < 0.001, I2 = 62.2%) [ESM Fig. S5]. The magnitude and statistical significance of the effect were stable during one-out (mean %Δ range = − 3.20 to − 2.27% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S11).
In subgroup analyses, a significant negative influence was only observed for sleep deprivation (mean %Δ = − 3.00%, 95% CI − 4.52 to − 1.48, p < 0.001, I2 = 49.2%) (Table 2). Results indicated that sleep loss had a consistent negative influence on strength when analysis was isolated to tasks performed in the PM, while tasks performed in the AM were generally unaffected (Table 3).
The effects of sleep loss were also conditional on body–limb categorisation, with tasks involving lower-body strength demonstrating a negative influence on performance, while tasks requiring upper-body strength were unaffected (Table 5; limb strength AM vs. PM comparison in ESM Table S12).
Table 5.
Influence of sleep loss on body–limb strength
| Exercise category | Outcomes, n | Exercise performance percentage change | Heterogeneity | ||
|---|---|---|---|---|---|
| Mean (95% CI) | p value | I2 value | p value | ||
| Upper- vs. lower-body strength | |||||
| Overall upper body | 18 | − 1.63 (− 3.30 to 0.04) | 0.056 | 32.7 | 0.069 |
| Overall lower body | 46 | − 3.42 (− 5.54 to − 1.31) | 0.002 | 65.6 | < 0.001 |
| Sleep-loss condition | |||||
| Upper body | |||||
| Deprivation | 6 | − 3.18 (− 9.13 to 2.77) | 0.228 | 58.9 | 0.104 |
| Restriction | 12 | − 0.73 (− 2.67 to 1.22) | 0.428 | 11.5 | 0.186 |
| Early restriction | 6 | − 1.21 (− 3.71 to 1.29) | 0.268 | < 0.001 | 0.752 |
| Late restriction | 6 | − 1.13 (− 6.07 to 3.81) | 0.583 | 61.6 | 0.035 |
| Lower-body | |||||
| Deprivation | 21 | − 3.25 (− 5.09 to − 1.41) | 0.002 | 54.7 | < 0.001 |
| Restriction | 25 | − 4.50 (− 10.2 to 1.17) | 0.114 | 72.8 | < 0.001 |
| Early restriction | 20 | − 1.36 (− 3.81 to 1.09) | 0.259 | 6.97 | 0.892 |
| Late restriction | 5 | − 8.26 (− 20.4 to 3.90) | 0.132 | 81.7 | < 0.001 |
Deprivation: participants did not sleep for an extended period of time (i.e., whole night); restriction: total sleep time ≤ 6 h in any 24 h period—this category is a combination of early restriction, late restriction, fragmented sleep and sleep restriction protocols not specified; early restriction: participants delayed sleep (i.e., went to sleep at a later time); late restriction; participants awakened earlier than normal
CI confidence interval
Meta-regression analyses (Table 4) detected a significant relationship between time awake prior to completing the exercise task and %Δ in strength, but only following late restriction (mean %Δ = − 1.07, 95% CI − 2.05 to − 0.10, p = 0.035). No significant relationships were detected for the other sleep-loss protocols (Table 4).
Endurance
Twenty studies (n = 237; 91% male), providing 22 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on endurance (mean %Δ = − 5.55%, 95% CI − 8.12 to − 2.99, p < 0.001, I2 = 86.5%) [ESM Fig. S6]. The magnitude and statistical significance of the effect were stable during one-out (mean %Δ range = − 5.94 to − 3.72% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S13).
Subgroup analyses showed that all sleep protocols were negatively affected by sleep loss (Table 2); however, there were only two outcome measures available for early restriction. Endurance performance tended to be affected (Table 3) by sleep loss, irrespective of the time of day exercise tasks were performed (AM or PM).
No significant relationships between time awake prior to completing the exercise task and the %Δ in endurance performance were identified in meta-regression analyses with any of the sleep-loss protocols (Table 4).
Strength-Endurance
Five studies (n = 62; 100% male), providing nine outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on strength-endurance (mean %Δ = − 9.85%, 95% CI − 19.6 to − 0.13, p = 0.048, I2 = 85.4%) [ESM Fig. S7]. However, the magnitude and statistical significance of the effect was unstable during one-out analyses (mean %Δ range = − 11.2 to − 8.71% and 95% CIs did not include zero except when outcome measures from six trials were sequentially removed [60, 75, 84, 86]). Findings were comparable with alternative correlation coefficients (ESM Table S14).
Subgroup analyses showed that strength-endurance was negatively affected by sleep restriction, but not sleep deprivation (Table 2). Note, however, that the three outcome measures analysed for sleep restriction were derived from one study [84]. There were no outcome measures to conduct analysis for either early- or late-restriction sleep protocols.
There were insufficient outcome measures to conduct meta-regression analyses on this exercise category.
Skill
Nine studies (n = 146; 80% male), providing 13 outcome measures, were included in this analysis. The overall weighted mean effect estimate (Table 2) indicated a negative influence of sleep loss on skill (mean %Δ = − 20.9%, 95% CI − 27.0 to − 14.9, p < 0.001, I2 = 94.1%) [ESM Fig. S8). The magnitude and statistical significance of the effect were stable during one-out (mean %Δ range = − 22.6 to − 19.2% and 95% CIs did not include zero) and sensitivity analyses (ESM Table S15).
Subgroup analyses showed that skill performance was negatively affected irrespective of the sleep-loss protocol (Table 2) or whether tasks were performed in the AM or PM. Note, there were insufficient outcome measures to conduct meta-analysis for late restriction.
No significant relationships between time awake prior to completing the task and %Δ in skill performance were identified in meta-regression analyses for any of the sleep-loss protocols (Table 4).
Discussion
The present systematic review and meta-analysis aimed to characterise the effects of acute sleep loss on exercise performance. We explored the influence of various contextual factors, including the type of exercise task(s) performed, pattern of sleep loss incurred before exercise, time of day (AM or PM) the exercise task was performed, and length of time awake prior to undertaking the exercise task. Overall, our results indicate that acute sleep loss negatively impacts next-day exercise performance; however, the magnitude of the impact depends on the type of exercise performed, as well as which sleep-loss pattern precedes exercise. Total sleep loss (deprivation) and late restriction (early awakening) appear to have a larger effect on exercise performance than early restriction (delayed sleep). Results also suggest that exercise performed in the PM is more likely to be affected by sleep loss than exercise performed in the AM, and that the length of time awake prior to exercise is an influential factor.
Influence of Acute Sleep Loss on Exercise Performance
When all sleep-loss protocols (i.e., deprivation, restriction, early restriction, late restriction) were consolidated, our meta-analyses showed that acute sleep loss has a negative impact on all exercise categories (Table 2).
Tasks requiring a skill component appear to be particularly sensitive to the effects of sleep loss (mean %Δ = − 20.9, 95% CI − 27.0 to − 14.9) (Table 2). This may be attributed to the higher cognitive demand required to undertake skill performance tasks [133]. Sleep loss has been shown to alter discrete cognitive functions, including reaction time [99, 134], alertness [58], attention [134], memory [135], decision making [136, 137] and learning [138]. Thus, physical tasks that are also cognitively demanding are likely to be most affected by acute sleep loss.
A number of investigations have attempted to identify mechanisms explaining the relationship between sleep loss and impaired exercise performance. Studies have explored changes to cardiorespiratory variables (e.g., O2peak [49, 50, 120, 126, 132], ventilation [41, 49, 93, 110, 120, 126, 132], heart rate [41, 49, 50, 52, 91, 110, 120, 124, 126, 128, 132, 139], blood pressure [50]); perceived effort (measured via rating of perceived exertion) [41, 43, 44, 51, 52, 56, 57, 75, 86, 89–92, 95, 100, 110, 124, 132, 139]; muscle glycogen [91]; lactate [49, 67, 77, 91, 93, 95, 98, 124, 128, 139]; catecholamines [67, 121, 126]; hormones (cortisol [43, 55, 63, 67, 75, 84, 127], testosterone [63, 75, 84, 127], growth hormone [67], prolactin [67], melatonin [55], hepcidin [54], insulin [61]); body temperature (oral temperature [43, 45, 78, 79, 81, 90, 94, 95, 116] and core temperature [53, 80, 91, 110]); immune function [44, 50, 54, 62, 127]; and neural drive [60, 76, 86, 92]. However, it was not the intention of the present study to explore these mechanisms; rather our aim was to quantify the magnitude of effects that acute sleep loss has on exercise performance. As such, the reader is referred to the comprehensive review by Fullagar et al. [36] on sleep and athletic performance for further details on the physiological responses associated with sleep loss.
Pattern of Sleep Loss
Another important finding in our study was the difference in the magnitude of change in performance when different types of sleep loss were analysed (i.e., deprivation, restriction, early and late restriction). We observed no change in exercise performance when early restriction sleep-loss protocols were isolated, except for skill and endurance tasks. For these two categories, the timing of the task should be considered (the influence of time of day is discussed in more detail in Sect. 4.3). For skill tasks, seven of eight outcome measures were performed in the PM. There were also only two outcome measures for endurance tasks and both were performed in the PM. Thus, individuals performing all other tasks (anaerobic power, speed/power endurance, HIIE, strength, and strength-endurance) appear able to maintain their performance under conditions of early sleep restriction.
In contrast to results identified with early-restriction protocols, the detrimental effects observed with sleep deprivation and late-restriction protocols appear to be more consistent and similar in magnitude. This may be a result of greater changes to one or more of the aforementioned mechanisms (highlighted in Sect. 4.1) underpinning exercise performance. Indeed, it may provide more opportunity to accrue particular aspects (e.g., fatigue [140]) when participants are kept awake or awoken early from sleep (i.e., in late-restriction protocols) until when the performance task is completed [36]. Given these results, when sleep loss is unavoidable and individuals have some level of control over timing, early restriction would appear preferable to late restriction. From a practical perspective, should an athlete need to travel, it would be reasonable for a health professional to recommend that it is better to do so the night before and sleep locally (even if that results in delayed sleep onset), rather than wake early for travel.
Our meta-regression analyses identified a significant negative relationship between the time awake prior to completing the exercise task and the %Δ EP for both sleep deprivation and late-restriction protocols (note, our interpretation of results for sleep deprivation is based on the removal of the influential outlier [85]). Specifically, we found that on average, exercise performance declined by ~ 0.4% per hour following sleep loss (note, this result is not inflated by the skill category because 8/13 of these tasks were performed following an early-restriction protocol). For example, if an individual rises early (e.g., ~ 0300 h) and performs a task 12 h later (~ 1500 h), then a ~ 5% decrease in performance may be anticipated. Overall, these results suggest that if exercise is to be performed after a period of sleep loss, it should be done as soon as practically possible.
Influence of Time of Day Exercise is Performed
Results of the current study suggest that exercise performed in the PM is likely to be more adversely affected by sleep loss than exercise performed in the AM (Table 3). The influence time of day has on exercise performance (without sleep loss) is well documented [70]. Evidence suggests that exercise performance may improve throughout the day for a number of tasks (skill [141–143], strength [144, 145], anaerobic power [81, 146–148], swimming [149, 150], and endurance tasks [151–153]), and this may be a consequence of physiological changes that occur with shifts in the circadian cycle (e.g., core temperature) [70, 154]. On this basis, one might anticipate that the negative impact of sleep loss may be offset when tasks are performed in the afternoon or evening. However, our results suggest that performing exercise in the PM (hence inducing a greater period between the start of sleep loss and the commencement of the task) appears to be a more significant moderator of exercise performance than changes associated with normal circadian rhythms. Therefore, in the setting of acute sleep loss, exercise should be scheduled to be performed soon after waking, before performance is potentially compromised by training in the PM.
Limitations and Future Direction
At present, we are unable to explore the relationship between sleep quality and next-day exercise performance. The majority (~ 98%) of included outcomes were obtained from studies that only assessed sleep ‘quantity’ (i.e., time spent asleep—more often reflected by ‘time in bed’). Polysomnography (PSG) is considered the ‘gold standard’ sleep assessment technique, and can provide important information on sleep architecture (e.g., time spent in non-rapid eye movement and rapid eye movement sleep stages [155]). As such, future studies should employ PSG for monitoring sleep, which will permit further exploration of the relationship between sleep quality and next-day exercise performance.
In the present review, we were unable to determine the influence of fragmented sleep (i.e., one or more nocturnal awakenings [48]) on next-day performance. To our knowledge only one investigation has been conducted on fragmented sleep [49], despite reports suggesting this is something athletes often experience [20, 24]; thus, further research targeting this specific sleep pattern is warranted. We also dichotomised time of day for task completion as AM or PM, which prevented exploration of effects at more specific times (e.g., early- vs. mid-morning and afternoon vs. evening). Furthermore, only 8/227 outcomes were measured later than 1800 h [45, 50, 86, 97, 121, 128, 132]. Given that many sporting events are carried out during the evening, future research should investigate the influence of sleep loss on tasks performed after 1800 h.
The influence of sleep loss on performance in the present study was based on discrete task categories. However, in reality, many sports require using concurrent physical/cognitive attributes (e.g., soccer, football), where certain skill activities (e.g., shot at goal/target) are frequently performed following short maximal sprint efforts or brief spurts of maximal effort interspersed with short recovery periods (e.g., HIIE). As such, future studies should explore the influence of sleep loss on performance tasks that involve a combination of physical/cognitive attributes to enhance translation and ecological validity with respect to team sports.
Finally, we were unable to explore the influence of certain factors in our analyses, often because insufficient data were available. For example, only a small number of female participants were included in studies (~ 11%), precluding exploration of sex as a variable. Furthermore, we could not investigate the impact of consecutive days/nights of sleep loss on exercise performance, nor explore the influence of participant training status. These present as opportunities for future research to further our understanding of potential factors that may influence the effects of sleep loss on exercise performance.
Conclusion
Acute sleep loss appears to have a negative impact on next-day exercise performance. The magnitude of the effect may be greater when individuals experience either sleep deprivation or late restriction, and when performance tasks are conducted in the PM. Individuals can anticipate a ~ 0.4% decline in performance for every hour spent awake following acute sleep loss. Thus, incorporating lifestyle behaviours/strategies that limit the likelihood of experiencing sleep loss must be emphasised. However, if acute sleep loss is anticipated and unavoidable, individuals should, where possible, endeavour to mimic early-restriction sleep patterns rather than deprivation or late restriction, and prioritise exercise to the morning in an effort to maintain performance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the corresponding authors of the included studies who provided raw data upon request.
Author contributions
All authors were involved in the conception and design of this review. JC and CI were responsible for collating manuscripts, retrieving information, and data analysis. All authors contributed to drafting and revising of the article, and read and approved the final manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No sources of funding were received for the preparation of this article.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
Jonathan Craven, Danielle McCartney, Ben Desbrow, Surendran Sabapathy, Phillip Bellinger, Llion Roberts and Christopher Irwin have no conflicts of interest to declare that are directly relevant to the contents of this article.
Footnotes
Goh et al. [55] undertook two additional hand-grip strength measurements during the sleep deprivation arm of the study that were not matched in the control arm; this was deemed likely to not have an influence, and was thus included in the analysis.
References
- 1.Leong RLF, Cheng GH, Chee MWL, Lo JC. The effects of sleep on prospective memory: a systematic review and meta-analysis. Sleep Med Rev. 2019;47:18–27. doi: 10.1016/j.smrv.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–238. doi: 10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch. 2012;463(1):139–160. doi: 10.1007/s00424-011-1053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dalfsen JH, Markus CR. The influence of sleep on human hypothalamic-pituitary-adrenal (HPA) axis reactivity: a systematic review. Sleep Med Rev. 2018;39:187–194. doi: 10.1016/j.smrv.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Adams RJ, Appleton SL, Taylor AW, Gill TK, Lang C, McEvoy RD, et al. Sleep health of Australian adults in 2016: results of the 2016 Sleep Health Foundation national survey. Sleep Health. 2017;3(1):35–42. doi: 10.1016/j.sleh.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Walsh NP, Halson SL, Sargent C, Roach GD, Nedelec M, Gupta L, et al. Sleep and the athlete: narrative review and 2021 expert consensus recommendations. Br J Sports Med. 2020;55:356–368. doi: 10.1136/bjsports-2020-102025. [DOI] [PubMed] [Google Scholar]
- 8.Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015;32(9):1294–1310. doi: 10.3109/07420528.2015.1073158. [DOI] [PubMed] [Google Scholar]
- 9.Lohsoonthorn V, Khidir H, Casillas G, Lertmaharit S, Tadesse MG, Pensuksan WC, et al. Sleep quality and sleep patterns in relation to consumption of energy drinks, caffeinated beverages, and other stimulants among Thai college students. Sleep Breath. 2013;17(3):1017–1028. doi: 10.1007/s11325-012-0792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetter DW, Young TB. The relation between cigarette smoking and sleep disturbance. Prev Med. 1994;23:328–334. doi: 10.1006/pmed.1994.1046. [DOI] [PubMed] [Google Scholar]
- 11.Fuller KH, Waters WF, Binks PG, Anderson T. Generalized anxiety and sleep architecture—a polysomnographic investigation. Sleep. 1997;20(5):370–376. doi: 10.1093/sleep/20.5.370. [DOI] [PubMed] [Google Scholar]
- 12.Prather AA, Carroll JE. Associations between sleep duration, shift work, and infectious illness in the United States: Data from the National Health Interview Survey. Sleep Health. 2021;7(5):638–643. doi: 10.1016/j.sleh.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146(2):194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Gennaro L, Marzano C, Fratello F, Moroni F, Pellicciari MC, Ferlazzo F, et al. The electroencephalographic fingerprint of sleep is genetically determined: a twin study. Ann Neurol. 2008;64(4):455–460. doi: 10.1002/ana.21434. [DOI] [PubMed] [Google Scholar]
- 15.Sargent C, Lastella M, Halson SL, Roach GD. How much sleep does an elite athlete need? Int J Sports Physiol Perform. 2021;16(12):1746–1757. doi: 10.1123/ijspp.2020-0896. [DOI] [PubMed] [Google Scholar]
- 16.Hoshikawa M, Uchida S, Hirano Y. A subjective assessment of the prevalence and factors associated with poor sleep quality amongst elite Japanese athletes. Sports Med Open. 2018;4(1):10. doi: 10.1186/s40798-018-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrade A, Bevilacqua GG, Casagrande PO, Brandt R, Coimbra D. Prevalence of poor sleep quality in athletes before competition. Physician Sportsmed. 2021;49(2):137–142. doi: 10.1080/00913847.2020.1784688. [DOI] [PubMed] [Google Scholar]
- 18.Drew M, Vlahovich N, Hughes D, Appaneal R, Burke LM, Lundy B, et al. Prevalence of illness, poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games. Br J Sports Med. 2018;52(1):47–53. doi: 10.1136/bjsports-2017-098208. [DOI] [PubMed] [Google Scholar]
- 19.Swinbourne R, Gill N, Vaile J, Smart D. Prevalence of poor sleep quality, sleepiness and obstructive sleep apnoea risk factors in athletes. Eur J Sport Sci. 2016;16(7):850–858. doi: 10.1080/17461391.2015.1120781. [DOI] [PubMed] [Google Scholar]
- 20.Leeder J, Glaister M, Pizzoferro K, Dawson J, Pedlar C. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J Sports Sci. 2012;30(6):541–545. doi: 10.1080/02640414.2012.660188. [DOI] [PubMed] [Google Scholar]
- 21.de Souza Bleyer FT, Barbosa DG, Andrade RD, Teixeira CS, Felden EPG. Sleep and musculoskeletal complaints among elite athletes of Santa Catarina. Rev Dor. 2015;16(2):102–108. [Google Scholar]
- 22.George CFP, Kab V, Kab P, Villa JJ, Levy AM. Sleep and breathing in professional football players. Sleep Med. 2003;4(4):317–325. doi: 10.1016/S1389-9457(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 23.Gupta L, Morgan K, Gilchrist S. Does elite sport degrade sleep quality? A systematic review. Sports Med. 2017;47(7):1317–1333. doi: 10.1007/s40279-016-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juliff LE, Halson SL, Peiffer JJ. Understanding sleep disturbance in athletes prior to important competitions. J Sci Med Sport. 2015;18(1):13–18. doi: 10.1016/j.jsams.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210. [DOI] [PubMed] [Google Scholar]
- 26.Grandou C, Wallace L, Fullagar HHK, Duffield R, Burley S. The effects of sleep loss on military physical performance. Sports Med. 2019;49(8):1159–1172. doi: 10.1007/s40279-019-01123-8. [DOI] [PubMed] [Google Scholar]
- 27.Sargent C, Lastella M, Halson SL, Roach GD. The impact of training schedules on the sleep and fatigue of elite athletes. Chronobiol Int. 2014;31(10):1160–1168. doi: 10.3109/07420528.2014.957306. [DOI] [PubMed] [Google Scholar]
- 28.Sargent C, Halson S, Roach GD. Sleep or swim? Early-morning training severely restricts the amount of sleep obtained by elite swimmers. Eur J Sport Sci. 2014;14:S310–S315. doi: 10.1080/17461391.2012.696711. [DOI] [PubMed] [Google Scholar]
- 29.Buguet A. Sleep under extreme environments: effects of heat and cold exposure, altitude, hyperbaric pressure and microgravity in space. J Neurol Sci. 2007;262(1–2):145–152. doi: 10.1016/j.jns.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 30.Fowler P, Duffield R, Howle K, Waterson A, Vaile J. Effects of northbound long-haul international air travel on sleep quantity and subjective jet lag and wellness in professional Australian soccer players. Int J Sports Physiol Perform. 2015;10(5):648–654. doi: 10.1123/ijspp.2014-0490. [DOI] [PubMed] [Google Scholar]
- 31.Fullagar HHK, Duffield R, Skorski S, White D, Bloomfield J, Kolling S, et al. Sleep, travel, and recovery responses of national footballers during and after long-haul international air travel. Int J Sports Physiol Perform. 2016;11(1):86–95. doi: 10.1123/ijspp.2015-0012. [DOI] [PubMed] [Google Scholar]
- 32.Pickering C, Kiely J. What should we do about habitual caffeine use in athletes? Sports Med. 2019;49(6):833–842. doi: 10.1007/s40279-018-0980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119(1–3):56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Gingerich SB, Seaverson ELD, Anderson DR. Association between sleep and productivity loss among 598 676 employees from multiple industries. Am J Health Promot. 2018;32(4):1091–1094. doi: 10.1177/0890117117722517. [DOI] [PubMed] [Google Scholar]
- 35.Engle-Friedman M. The effects of sleep loss on capacity and effort. Sleep Sci. 2014;7(4):213–224. doi: 10.1016/j.slsci.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fullagar HHK, Skorski S, Duffield R, Hammes D, Coutts AJ, Meyer T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015;45(2):161–186. doi: 10.1007/s40279-014-0260-0. [DOI] [PubMed] [Google Scholar]
- 37.Jones JJ, Kirschen GW, Kancharla S, Hale L. Association between late-night tweeting and next-day game performance among professional basketball players. Sleep Health. 2019;5(1):68–71. doi: 10.1016/j.sleh.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Dinges DF. An overview of sleepiness and accidents. J Sleep Res. 1995;4(2):4–14. doi: 10.1111/j.1365-2869.1995.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 39.Mullins HM, Cortina JM, Drake CL, Dalal RS. Sleepiness at work: a review and framework of how the physiology of sleepiness impacts the workplace. J Appl Psychol. 2014;99(6):1096–1112. doi: 10.1037/a0037885. [DOI] [PubMed] [Google Scholar]
- 40.Reilly T, Piercy M. The effect of partial sleep deprivation on weight-lifting performance. Ergonomics. 1994;37(1):107–115. doi: 10.1080/00140139408963628. [DOI] [PubMed] [Google Scholar]
- 41.Chase JD, Roberson PA, Saunders MJ, Hargens TA, Womack CJ, Luden ND. One night of sleep restriction following heavy exercise impairs 3-km cycling time-trial performance in the morning. Appl Physiol Nutr Metab. 2017;42(9):909–915. doi: 10.1139/apnm-2016-0698. [DOI] [PubMed] [Google Scholar]
- 42.Axelsson J, Ingre M, Kecklund G, Lekander M, Wright KP, Sundelin T. Sleepiness as motivation: a potential mechanism for how sleep deprivation affects behavior. Sleep. 2020;43(6):1–6. doi: 10.1093/sleep/zsz291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khemila S, Abedelmalek S, Romdhani M, Souissi A, Chtourou H, Souissi N. Listening to motivational music during warming-up attenuates the negative effects of partial sleep deprivation on cognitive and short-term maximal performance: effect of time of day. Chronobiol Int. 2021;38(7):1052–1063. doi: 10.1080/07420528.2021.1904971. [DOI] [PubMed] [Google Scholar]
- 44.Romdhani M, Hammouda O, Chaabouni Y, Mahdouani K, Driss T, Chamari K, et al. Sleep deprivation affects post-lunch dip performances, biomarkers of muscle damage and antioxidant status. Biol Sport. 2019;36(1):55–65. doi: 10.5114/biolsport.2018.78907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards BJ, Waterhouse J. Effects of one night of partial sleep deprivation upon diurnal rhythms of accuracy and consistency in throwing darts. Chronobiol. 2009;26(4):756–768. doi: 10.1080/07420520902929037. [DOI] [PubMed] [Google Scholar]
- 46.Kosmadopoulos A, Sargent C, Darwent D, Zhou X, Roach GD. Alternatives to polysomnography (PSG): a validation of wrist actigraphy and a partial-PSG system. Behav Res Methods. 2014;46(4):1032–1041. doi: 10.3758/s13428-013-0438-7. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. [DOI] [PubMed] [Google Scholar]
- 48.Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86(3):1134–1139. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- 49.Mougin F. Effects of sleep disturbances on subsequent physical performance. Eur J Appl Physiol. 1991;63:77–82. doi: 10.1007/BF00235173. [DOI] [PubMed] [Google Scholar]
- 50.Rae DE, Chin T, Dikgomo K, Hill L, McKune AJ, Kohn TA, et al. One night of partial sleep deprivation impairs recovery from a single exercise training session. Eur J Appl Physiol. 2017;117(4):699–712. doi: 10.1007/s00421-017-3565-5. [DOI] [PubMed] [Google Scholar]
- 51.Roberts SSH, Teo WP, Aisbett B, Warmington SA. Extended sleep maintains endurance performance better than normal or restricted sleep. Med Sci Sports Exerc. 2019;51(12):2516–2523. doi: 10.1249/MSS.0000000000002071. [DOI] [PubMed] [Google Scholar]
- 52.Roberts SSH, Teo WP, Aisbett B, Warmington SA. Effects of total sleep deprivation on endurance cycling performance and heart rate indices used for monitoring athlete readiness. J Sports Sci. 2019;37(23):2691–2701. doi: 10.1080/02640414.2019.1661561. [DOI] [PubMed] [Google Scholar]
- 53.Bambaeichi E, Reilly T, Cable NT, Giacomoni M. The influence of time of day and partial sleep loss on muscle strength in eumenorrheic females. Ergonomics. 2005;48(11–14):1499–1511. doi: 10.1080/00140130500101437. [DOI] [PubMed] [Google Scholar]
- 54.Goto K, Mamiya A, Ito H, Maruyama T, Hayashi N, Badenhorst CE. Partial sleep deprivation after an acute exercise session does not augment hepcidin levels the following day. Physiol Rep. 2020;8(10). [DOI] [PMC free article] [PubMed]
- 55.Goh VHH, Tong TYY, Lim CL, Low ECT, Lee LKH. Effects of one night of sleep deprivation on hormone profiles and performance efficiency. Mil Med. 2001;166(5):427–431. doi: 10.1093/milmed/166.5.427. [DOI] [PubMed] [Google Scholar]
- 56.Ajjimaporn A, Ramyarangsi P, Siripornpanich V. Effects of a 20-min nap after sleep deprivation on brain activity and soccer performance. Int J Sports Med. 2020;41(14):1009–1016. doi: 10.1055/a-1192-6187. [DOI] [PubMed] [Google Scholar]
- 57.Cullen T, Thomas G, Wadley AJ, Myers T. The effects of a single night of complete and partial sleep deprivation on physical and cognitive performance: a Bayesian analysis. J Sports Sci. 2019;37(23):2726–2734. doi: 10.1080/02640414.2019.1662539. [DOI] [PubMed] [Google Scholar]
- 58.Daaloul H, Souissi N, Davenne D. Effects of napping on alertness, cognitive, and physical outcomes of karate athletes. Med Sci Sports Exerc. 2019;51(2):338–345. doi: 10.1249/MSS.0000000000001786. [DOI] [PubMed] [Google Scholar]
- 59.Moore J, McDonald C, McIntyre A, Carmody K, Donne B. Effects of acute sleep deprivation and caffeine supplementation on anaerobic performance. Sleep Sci. 2018;11(1):2–7. doi: 10.5935/1984-0063.20180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skurvydas A, Zlibinaite L, Solianik R, Brazaitis M, Valanciene D, Baranauskiene N, et al. One night of sleep deprivation impairs executive function but does not affect psychomotor or motor performance. Biol Sport. 2020;37(1):7–14. doi: 10.5114/biolsport.2020.89936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sweeney EL, Peart DJ, Kyza I, Harkes T, Ellis JG, Walshe IH. Impaired insulin profiles following a single night of sleep restriction: the impact of acute sprint interval exercise. Int J Sport Nutr Exerc Metab. 2020;30(2):139–144. doi: 10.1123/ijsnem.2019-0235. [DOI] [PubMed] [Google Scholar]
- 62.Abedelmalek S, Chtourou H, Aloui A, Aouichaoui C, Souissi N, Tabka Z. Effect of time of day and partial sleep deprivation on plasma concentrations of IL-6 during a short-term maximal performance. Eur J Appl Physiol. 2013;113(1):241–248. doi: 10.1007/s00421-012-2432-7. [DOI] [PubMed] [Google Scholar]
- 63.Cook CJ, Crewther BT, Kilduff LP, Drawer S, Gaviglio CM. Skill execution and sleep deprivation: effects of acute caffeine or creatine supplementation—a randomized placebo-controlled trial. J Int Soc Sports Nutr. 2011;8(2). [DOI] [PMC free article] [PubMed]
- 64.Souissi N, Chtourou H, Aloui A, Hammouda O, Dogui M, Chaouachi A, et al. Effects of time-of-day and partial sleep deprivation on short-term maximal performances of judo competitors. J Strength Cond Res. 2013;27(9):2473–2480. doi: 10.1519/JSC.0b013e31827f4792. [DOI] [PubMed] [Google Scholar]
- 65.Vardar SA, Öztürk L, Kurt C, Bulut E, Sut N, Vardar E. Sleep deprivation induced anxiety and anaerobic performance. J Sports Sci Med. 2007;6:532–537. [PMC free article] [PubMed] [Google Scholar]
- 66.Mejri MA, Hammouda O, Zouaoui K, Chaouachi A, Chamari K, Rayana MCB, et al. Effect of two types of partial sleep deprivation on Taekwondo players’ performance during intermittent exercise. Biol Rhythm Res. 2013;45(1):17–26. doi: 10.1080/09291016.2013.787686. [DOI] [Google Scholar]
- 67.Mougin F, Bourdin H, Simon-Rigaud ML, Nguyen NU, Kantelip JP, Davenne D. Hormonal responses to exercise after partial sleep deprivation and after a hypnotic drug-induced sleep. J Sports Sci. 2001;19(2):89–97. doi: 10.1080/026404101300036253. [DOI] [PubMed] [Google Scholar]
- 68.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38(3):427–449. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 69.Thomas C, Jones H, Whitworth-Turner C, Louis J. High-intensity exercise in the evening does not disrupt sleep in endurance runners. Eur J Appl Physiol. 2020;120(2):359–368. doi: 10.1007/s00421-019-04280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thun E, Bjorvatn B, Flo E, Harris A, Pallesen S. Sleep, circadian rhythms, and athletic performance. Sleep Med Rev. 2015;23:1–9. doi: 10.1016/j.smrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Knowles OE, Drinkwater EJ, Urwin CS, Lamon S, Aisbett B. Inadequate sleep and muscle strength: Implications for resistance training. J Sci Med Sport. 2018;21(9):959–968. doi: 10.1016/j.jsams.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: a meta-analysis. Sleep. 1996;4:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 73.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Assink M, Wibbelink CJM. Fitting three-level meta-analytic models in R—a step-by-step tutorial. Tutor Quant Methods Psychol. 2016;12(3):154–174. doi: 10.20982/tqmp.12.3.p154. [DOI] [Google Scholar]
- 75.Blumert PA, Crum AJ, Ernsting M, Volek JS, Hollander DB, Haff EE, et al. The acute effects of twenty-four hours of sleep loss on the performance of national-caliber male collegiate weightlifters. J Strength Cond Res. 2007;21(4):1146–1154. doi: 10.1519/R-21606.1. [DOI] [PubMed] [Google Scholar]
- 76.Gonçalves A, Teodosio C, Pezarat-Correia P, Vila-Chã C, Mendonca VG. Effects of acute sleep deprivation on H reflex and V wave. J Sleep Res. 2021;30(6):e13118. doi: 10.1111/jsr.13118. [DOI] [PubMed] [Google Scholar]
- 77.Souissi M, Abedelmalek S, Dhiba DB, Nikolaidis PT, Ben Awicha H, Chtourou H, et al. Morning caffeine ingestion increases cognitive function and short-term maximal performance in footballer players after partial sleep deprivation. Biol Rhythm Res. 2015;46(5):617–629. doi: 10.1080/09291016.2015.1034975. [DOI] [Google Scholar]
- 78.Souissi M, Chtourou H, Abedelmalek S, Ben Ghozlane I, Sahnoun Z. The effects of caffeine ingestion on the reaction time and short-term maximal performance after 36 h of sleep deprivation. Physiol Behav. 2014;131:1–6. doi: 10.1016/j.physbeh.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Souissi M, Souissi Y, Mseddi E, Sahnoun Z. The effects of caffeine on the diurnal variation of the reaction time and short-term maximal performance after one night of sleep deprivation. Biol Rhythm Res. 2019;52(10):1–16. [Google Scholar]
- 80.Souissi N, Sesboue B, Gauthier A, Larue J, Davenne D. Effects of one night's sleep deprivation on anaerobic performance the following day. Eur J Appl Physiol. 2003;89:359–366. doi: 10.1007/s00421-003-0793-7. [DOI] [PubMed] [Google Scholar]
- 81.Souissi N, Souissi M, Souissi H, Chamari K, Tabka Z, Dogui M, et al. Effect of time of day and partial sleep deprivation on short-term. High-Power Output Chronobiol Int. 2008;25(6):1062–1076. doi: 10.1080/07420520802551568. [DOI] [PubMed] [Google Scholar]
- 82.Kujawa K, Olpinska-Lischka M, Maciaszek J. The influence of 24-hour sleep deprivation on the strength of lower limb muscles in young and physically fit women and men. Sustainability. 2020;12(7).
- 83.Bulbulian R, Heaney JH, Leake CN, Sucec AA, Sjoholm NT. The effect of sleep deprivation and exercise load on isokinetic leg strength and endurance. Eur J Appl Physiol Occup Physiol. 1996;73(3–4):273–277. doi: 10.1007/BF02425487. [DOI] [PubMed] [Google Scholar]
- 84.Cook C, Beaven CM, Kilduff LP, Drawer S. Acute caffeine ingestion's increase of voluntarily chosen resistance-training load after limited sleep. Int J Sport Nutr Exerc Metab. 2012;22(3):157–164. doi: 10.1123/ijsnem.22.3.157. [DOI] [PubMed] [Google Scholar]
- 85.Arazi H, Mehrabani J, Irandoost M, Khaleghimamaghani E. Effects of overnight sleep deprivation on appetite and physical performance in elite female soccer players. J Turk Sleep Med. 2019;6(3):93–97. doi: 10.4274/jtsm.galenos.2019.19480. [DOI] [Google Scholar]
- 86.Arnal PJ, Lapole T, Erblang M, Guillard M, Bourrilhon C, Leger D, et al. Sleep extension before sleep loss: effects on performance and neuromuscular function. Med Sci Sports Exerc. 2016;48(8):1595–1603. doi: 10.1249/MSS.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 87.Baati H, Chtourou H, Moalla W, Jarraya M, Nikolaidis PT, Rosemann T, et al. Effect of angle of view and partial sleep deprivation on distance perception. Front Psychol. 2020;11:201. doi: 10.3389/fpsyg.2020.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baati H, Hmani MS, Jarraya M, Chtourou H, Masmoudi L, Trabelsi K, et al. Effect of total sleep deprivation on egocentric distance estimation following a fatiguing task. Biol Rhythm Res. 2015;46(2):265–274. doi: 10.1080/09291016.2014.985003. [DOI] [Google Scholar]
- 89.HajSalem M, Chtourou H, Aloui A, Hammouda O, Souissi N. Effects of partial sleep deprivation at the end of the night on anaerobic performances in judokas. Biol Rhythm Res. 2013;44(5):815–821. doi: 10.1080/09291016.2012.756282. [DOI] [Google Scholar]
- 90.Romdhani M, Hammouda O, Smari K, Chaabouni Y, Mahdouani K, Driss T, et al. Total sleep deprivation and recovery sleep affect the diurnal variation of agility performance- the gender differences. J Strength Cond Res. 2021;35(1):132–140. doi: 10.1519/JSC.0000000000002614. [DOI] [PubMed] [Google Scholar]
- 91.Skein M, Duffield R, Edge J, Short MJ, Mundel T. Intermittent-sprint performance and muscle glycogen after 30 h of sleep deprivation. Med Sci Sports Exerc. 2011;43(7):1301–1311. doi: 10.1249/MSS.0b013e31820abc5a. [DOI] [PubMed] [Google Scholar]
- 92.Temesi J, Arnal PJ, Davranche K, Bonnefoy R, Levy P, Verges S, et al. Does central fatigue explain reduced cycling after complete sleep deprivation? Med Sci Sports Exerc. 2013;45(12):2243–2253. doi: 10.1249/MSS.0b013e31829ce379. [DOI] [PubMed] [Google Scholar]
- 93.Mougin F, Bourdin H, Simonrigaud ML, Didier JM, Toubin G, Kantelip JP. Effects of a selective sleep deprivation on subsequent anaerobic performance. Int J Sports Med. 1996;17(2):115–119. doi: 10.1055/s-2007-972818. [DOI] [PubMed] [Google Scholar]
- 94.Ozturk L, Bulut E, Vardar SA, Uzun C. Effects of sleep deprivation on anaerobic exercise-induced changes in auditory brainstem evoked potentials. Clin Physiol Funct Imaging. 2007;27(5):263–267. doi: 10.1111/j.1475-097X.2007.00746.x. [DOI] [PubMed] [Google Scholar]
- 95.Romdhani M, Souissi N, Chaabouni Y, Mahdouani K, Driss T, Chamari K, et al. Improved physical performance and decreased muscular and oxidative damage with postlunch napping after partial sleep deprivation in athletes. Int J Sports Physiol Perform. 2020;15(6):874–883. doi: 10.1123/ijspp.2019-0308. [DOI] [PubMed] [Google Scholar]
- 96.Skein M, Duffield R, Minett GM, Snape A, Murphy A. The effect of overnight sleep deprivation after competitive rugby league matches on postmatch physiological and perceptual recovery. Int J Sports Physiol Perform. 2013;8(5):556–564. doi: 10.1123/ijspp.8.5.556. [DOI] [PubMed] [Google Scholar]
- 97.Souissi M, Chikh N, Affes H, Sahnoun Z. Caffeine reversal of sleep deprivation effects on alertness, mood and repeated sprint performances in physical education students. Biol Rhythm Res. 2018;49(5):746–760. doi: 10.1080/09291016.2017.1413765. [DOI] [Google Scholar]
- 98.Souissi M, Souissi Y, Bayoudh A, Knechtle B, Nikolaidis PT, Chtourou H. Effects of a 30-min nap opportunity on cognitive and short-duration high-intensity performances and mood states after a partial sleep deprivation night. J Sports Sci. 2020;38(22):2553–2561. doi: 10.1080/02640414.2020.1793651. [DOI] [PubMed] [Google Scholar]
- 99.Taheri M, Arabameri E. The effect of sleep deprivation on choice reaction time and anaerobic power of college student athletes. Asian J Sports Med. 2012;3(1):15–20. doi: 10.5812/asjsm.34719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Daviaux Y, Mignardot JB, Cornu C, Deschamps T. Effects of total sleep deprivation on the perception of action capabilities. Exp Brain Res. 2014;232(7):2243–2253. doi: 10.1007/s00221-014-3915-z. [DOI] [PubMed] [Google Scholar]
- 101.Rodrigues R, de Azevedo FR, Teixeira B, Macedo R, Lopes A, Diefenthaeler F, et al. Combined and isolated effects of alcohol consumption and sleep deprivation on maximal strength, muscle endurance and aerobic exercise performance in healthy men: a cross-over randomized controlled trial. Sleep Biol Rhythms. 2021;19(4):433–441. doi: 10.1007/s41105-021-00333-w. [DOI] [Google Scholar]
- 102.Romdhani M, Souissi N, Moussa-Chamari I, Chaabouni Y, Mahdouani K, Sahnoun Z, et al. Caffeine use or napping to enhance repeated sprint performance after partial sleep deprivation: why not both? Int J Sports Physiol Perform. 2021;16(5):711–718. doi: 10.1123/ijspp.2019-0792. [DOI] [PubMed] [Google Scholar]
- 103.Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. Oxford: Wiley; 2019. [Google Scholar]
- 104.van Rosendal SP, Osborne MA, Fassett RG, Coombes JS. Guidelines for glycerol use in hyperhydration and rehydration associated with exercise. Sports Med. 2010;40(2):113–129. doi: 10.2165/11530760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 105.Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials—is blinding necessary? Controll Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 106.Maher CG, Moseley AM, Sherrington C, Elkins MR, Herbert RD. A description of the trials, reviews, and practice guidelines indexed in the PEDro database. Phys Ther. 2008;88(9):1068–1077. doi: 10.2522/ptj.20080002. [DOI] [PubMed] [Google Scholar]
- 107.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241. doi: 10.1016/S0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 108.Viechtbauer W. The metafor Package: A Meta-Analysis Package for R. 2021 [cited 24 Aug 2021]. https://www.metafor-project.org/doku.php
- 109.Filipas L, Ferioli D, Banfi G, La Torre A, Vitale JA. Single and combined effect of acute sleep restriction and mental fatigue on basketball free-throw performance. Int J Sports Physiol Perform. 2021;16(3):415–420. doi: 10.1123/ijspp.2020-0142. [DOI] [PubMed] [Google Scholar]
- 110.Oliver SJ, Costa RJ, Laing SJ, Bilzon JL, Walsh NP. One night of sleep deprivation decreases treadmill endurance performance. Eur J Appl Physiol. 2009;107(2):155–161. doi: 10.1007/s00421-009-1103-9. [DOI] [PubMed] [Google Scholar]
- 111.Holland GJ. Effects of limited sleep deprivation on performance of selected motor tasks. Res Q. 1968;39(2):285–294. [PubMed] [Google Scholar]
- 112.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions; 2011.
- 113.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. Oxford: Wiley; 2011. [Google Scholar]
- 114.Pickett GF, Morris AF. Effects of acute sleep and food deprivation on total body response time and cardiovascular performance. J Sports Med Phys Fitness. 1975;15(1):49–56. [PubMed] [Google Scholar]
- 115.Reilly T, Deykin T. Effects of partial sleep loss on subjective states, psychomotor and physical performance tests. J Hum Mov Stud. 1983;9(4):157–170. [Google Scholar]
- 116.Abedelmalek S, Boussetta N, Chtourou H, Souissi N, Tabka Z. Effect of partial sleep deprivation and racial variation on short-term maximal performance. Biol Rhythm Res. 2014;45(5):699–708. [Google Scholar]
- 117.Ricardo JSC, Cartner L, Oliver SJ, Laing SJ, Walters R, Bilzon JLJ, et al. No effect of a 30-h period of sleep deprivation on leukocyte trafficking, neutrophil degranulation and saliva IgA responses to exercise. Eur J Appl Physiol. 2009;105(3):499–504. doi: 10.1007/s00421-008-0931-3. [DOI] [PubMed] [Google Scholar]
- 118.Roberson PA, Chase JD, Bigman MB, Saunders MJ, Luden ND, Womack CJ. Time of day, but not sleep restriction, affects markers of hemostasis following heavy exercise. Appl Physiol Nutr Metab. 2019;44(2):148–152. doi: 10.1139/apnm-2018-0147. [DOI] [PubMed] [Google Scholar]
- 119.Cullen T, Thomas G, Wadley AJ. Sleep deprivation: cytokine and neuroendocrine effects on perception of effort. Med Sci Sports Exerc. 2020;52(4):909–918. doi: 10.1249/MSS.0000000000002207. [DOI] [PubMed] [Google Scholar]
- 120.Azboy O, Kaygisiz Z. Effects of sleep deprivation on cardiorespiratory functions of the runners and volleyball players during rest and exercise. Acta Physiol Hung. 2009;96(1):29–36. doi: 10.1556/APhysiol.96.2009.1.3. [DOI] [PubMed] [Google Scholar]
- 121.Froberg JE, Karlsson C, Levi L, Lidberg L. Circadian rhythms of catecholamine excretion, shooting range performance and self-ratings of fatigue during sleep deprivation. Biol Psychol. 1975;2:175–188. doi: 10.1016/0301-0511(75)90018-6. [DOI] [PubMed] [Google Scholar]
- 122.Hill DW, Borden DO, Darnaby KM, Hendricks DN. Aerobic and anaerobic contributions to exhaustive high-intensity exercise after sleep deprivation. J Sports Sci. 1994;12(5):455–461. doi: 10.1080/02640419408732195. [DOI] [PubMed] [Google Scholar]
- 123.Reyner LA, Horne JA. Sleep restriction and serving accuracy in performance tennis players, and effects of caffeine. Physiol Behav. 2013;120:93–96. doi: 10.1016/j.physbeh.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 124.Mejri MA, Yousfi N, Mhenni T, Tayech A, Hammouda O, Driss T, et al. Does one night of partial sleep deprivation affect the evening performance during intermittent exercise in Taekwondo players? J Exerc Rehabil. 2016;12(1):47–53. doi: 10.12965/jer.150256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paryab N, Taheri M, H'Mida C, Irandoust K, Mirmoezzi M, Trabelsi K, et al. Melatonin supplementation improves psychomotor and physical performance in collegiate student-athletes following a sleep deprivation night. Chronobiol Int. 2021;38(5):753–761. doi: 10.1080/07420528.2021.1889578. [DOI] [PubMed] [Google Scholar]
- 126.Chen H. Effects of 30-h sleep loss on cardiorespiratory functions at rest and in exercise. Med Sci Sports Exerc. 1991;23(2):193–198. doi: 10.1249/00005768-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 127.Dáttilo M, Antunes HKM, Galbes NMN, Mônico-Neto M, De Sá SH, Dos Santos Quaresma MVL, et al. Effects of sleep deprivation on acute skeletal muscle recovery after exercise. Med Sci Sports Exerc. 2020;52(2):507–514. doi: 10.1249/MSS.0000000000002137. [DOI] [PubMed] [Google Scholar]
- 128.Omiya K, Akashi YJ, Yoneyama K, Osada N, Tanabe K, Miyake F. Heart-rate response to sympathetic nervous stimulation, exercise, and magnesium concentration in various sleep conditions. Int J Sport Nutr Exerc Metab. 2009;19:127–135. doi: 10.1123/ijsnem.19.2.127. [DOI] [PubMed] [Google Scholar]
- 129.Racinais S, Hue O, Blonc S, Le Gallais D. Effect of sleep deprivation on shuttle run score in middle-aged amateur athletes. Influence of initial score. J Sports Med Phys Fitness. 2004;44(3):246–8. [PubMed]
- 130.Brodan V, Kuhn E. Physical performance in man during sleep deprivation. J Sports Med Phys Fitness. 1967;7(1):28–30. [PubMed] [Google Scholar]
- 131.Khcharem A, Souissi M, Atheymen R, Ben Mahmoud L, Sahnoun Z. Effects of caffeine ingestion on 8-km run performance and cognitive function after 26 hours of sleep deprivation. Biol Rhythm Res. 2022;53(6).
- 132.Martin BJ. Effect of sleep-deprivation on tolerance of prolonged exercise. Eur J Appl Physiol Occup Physiol. 1981;47(4):345–354. doi: 10.1007/BF02332962. [DOI] [PubMed] [Google Scholar]
- 133.Dumer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 134.Jarraya M, Jarraya S, Chtourou H, Souissi N, Chamari K. The effect of partial sleep deprivation on the reaction time and the attentional capacities of the handball goalkeeper. Biol Rhythm Res. 2013;44(3):503–510. doi: 10.1080/09291016.2012.721589. [DOI] [Google Scholar]
- 135.Smith ME, McEvoy LK, Gevins A. The impact of moderate sleep loss on neurophysiologic signals during working- memory task performance. Sleep. 2002;25(7):56–66. doi: 10.1093/sleep/25.7.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6(3):236–249. doi: 10.1037/1076-898X.6.3.236. [DOI] [PubMed] [Google Scholar]
- 137.Harrison Y, Horne JA. One night of sleep loss impairs innovative thinking and flexible decision making. Organ Behav Hum Decis Process. 1999;78(2):128–145. doi: 10.1006/obhd.1999.2827. [DOI] [PubMed] [Google Scholar]
- 138.Walker MP. Cognitive consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S29–S34. doi: 10.1016/S1389-9457(08)70014-5. [DOI] [PubMed] [Google Scholar]
- 139.Mejri MA, Hammouda O, Zouaoui K, Chaouachi A, Chamari K, Rayana MCB, et al. Effect of two types of partial sleep deprivation on Taekwondo players’ performance during intermittent exercise. Biol Rhythm Res. 2014;45(1):17–26. doi: 10.1080/09291016.2013.787686. [DOI] [Google Scholar]
- 140.Edwards B, Waterhouse J, Reilly T. The effects of circadian rhythmicity and time-awake on a simple motor task. Chronobiol Int. 2007;24(6):1109–1124. doi: 10.1080/07420520701795316. [DOI] [PubMed] [Google Scholar]
- 141.Deschodt VJ, Arsac LM. Morning vs. evening maximal cycle power and technical swimming ability. J Strength Cond Res. 2004;18(1):149–154. doi: 10.1519/1533-4287(2004)018<0149:mvemcp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 142.Reilly T, Atkinson G, Edwards B, Waterhouse J, Farrelly K, Fairhurst E. Diurnal variation in temperature, mental and physical performance, and tasks specifically related to football (soccer) Chronobiol Int. 2007;24(3):507–519. doi: 10.1080/07420520701420709. [DOI] [PubMed] [Google Scholar]
- 143.Rahnama N, Sajjadi N, Bambaeichi E, Sadeghipour HR, Daneshjoo H, Nazary B. Diurnal variation on the performance of soccer-specific skills. World J Sport Sci. 2009;2(1):27–30. [Google Scholar]
- 144.Coldwells A, Atkinson G, Reilly T. Sources of variation in back and leg dynamometry. Ergonomics. 1994;37(1):79–86. doi: 10.1080/00140139408963625. [DOI] [PubMed] [Google Scholar]
- 145.Squarcini CF, Pires ML, Lopes C, Benedito-Silva AA, Esteves AM, Cornelissen-Guillaume G, et al. Free-running circadian rhythms of muscle strength, reaction time, and body temperature in totally blind people. Eur J Appl Physiol. 2013;113(1):157–165. doi: 10.1007/s00421-012-2415-8. [DOI] [PubMed] [Google Scholar]
- 146.Reilly T, Down A. Circadian variation in the standing broad jump. Percept Mot Skills. 1986;62:830. doi: 10.2466/pms.1986.62.3.830. [DOI] [Google Scholar]
- 147.Bernard T, Giacomoni M, Gavarry O, Seymat M, Falgairette G. Time-of-day effects in maximal anaerobic leg exercise. Eur J Appl Physiol. 1998;77:133–138. doi: 10.1007/s004210050311. [DOI] [PubMed] [Google Scholar]
- 148.Souissi N, Gauthier A, Sesboue B, Larue J, Davenne D. Circadian rhythms in two types of anaerobic cycle leg exercise: force-velocity and 30-s wingate tests. Int J Sports Med. 2004;25:14–19. doi: 10.1055/s-2003-45226. [DOI] [PubMed] [Google Scholar]
- 149.Baxter C, Reilly T. Influence of time of day on all-out swimming. Br J Sports Med. 1983;17:122–127. doi: 10.1136/bjsm.17.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kline CE, Durstine JL, Davis JM, Moore TA, Devlin TM, Zielinski MR, et al. Circadian variation in swim performance. J Appl Physiol. 2007;102(2):641–649. doi: 10.1152/japplphysiol.00910.2006. [DOI] [PubMed] [Google Scholar]
- 151.Reilly T, Baxter C. Influence of time of day on reactions to cycling at a fixed high intensity. Br J Sports Med. 1983;17:128–130. doi: 10.1136/bjsm.17.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hill DW. Effect of time of day on aerobic power in exhaustive high-intensity exercise. J Sports Med Phys Fitness. 1996;36(3):155–160. [PubMed] [Google Scholar]
- 153.Bessot N, Nicolas A, Moussay S, Gauthier A, Sesboue B, Davenne D. The effect of pedal rate and time of day on the time to exhaustion from high-intensity exercise. Chronobiol Int. 2006;23(5):1009–1024. doi: 10.1080/07420520600920726. [DOI] [PubMed] [Google Scholar]
- 154.Shibata S, Tahara Y. Circadian rhythm and exercise. J Sports Med Phys Fitness. 2014;3(1):65–72. doi: 10.7600/jpfsm.3.65. [DOI] [Google Scholar]
- 155.Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:166. doi: 10.3389/fnagi.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.