Abstract
A quote attributed to Mark Twain states, “What gets us into trouble is not what we don’t know. It’s what we know for sure that just ain’t so.” The growing focus on patient centricity has revealed a misalignment between what patients report as important to them about their disease and/or treatment, and the data collected in research and care. Decisions across healthcare are made using an evidence base most stakeholders acknowledge is inadequate. Patients might report that what is important to them are everyday life impacts, concepts that can be very different from the more typical clinical outcomes we often track. In this paper, we encourage expanding current thinking to all “impacts,” not only health outcomes, but also the other equally (and sometimes more important) concerns patients report as important to them. We propose that a patient-centered core impact set be developed for each disease or condition of interest, and/or subpopulation of patients. A patient-centered core impact set begins with gathering from patients and caregivers an inventory of all impacts disease and treatments have on a patient’s (and carers’ and families’) life. Then, through a formal prioritization process, a core set of impacts is derived, inclusive of but extending beyond relevant health outcomes. We offer several recommendations on how to move the goal of a patient-centered core impact set forward through collaboration, leadership, and establishment of a patient-centered core impact set development blueprint with supporting tools.
Key Points for Decision Makers
| Healthcare decisions are made using an evidence base most stakeholders acknowledge is inadequate, focused heavily on clinical health outcomes, often not cognizant of or ignoring the other, everyday impacts disease and treatments have on the patient, carer, and family’s lives. |
| A patient-centered core impact set (PC-CIS) is a new concept offered to help address this problem. It include all impacts patients report as important to them, such as financial or caregiver stresses, that include but go beyond typically recognized health outcomes. |
| This paper offers recommendations on how to move PC-CIS forward through collaboration and leadership, and by establishing a PC-CIS development blueprint with supporting tools. |
The Expanding Role of the Patient Voice
A quote attributed to Mark Twain states, “What gets us into trouble is not what we don't know. It’s what we know for sure that just ain’t so.” Similarly, data collected in research and care have been predicated on what research and clinical communities thought was important. Yet, numerous examples globally reveal differences in what patients versus clinicians prioritize [1]. The growing focus on patient centricity reveals a misalignment between what patients report as important to them about their disease and/or treatment, and data collected. Patients often report everyday life impacts as important, such as financial or caregiver stresses, concepts very different from the clinical data typically tracked [2–5]. In the Patient-Centered Outcomes Research Institute-funded PROSPER study, researchers compared stroke prevention strategies for those who experienced stroke. Patients, however, articulated that along with preventing stroke, a key outcome was “days out of the hospital” [6]. Similar divergence between life impacts and clinical outcomes appeared in Juvenile Idiopathic Arthritis Patient-Focused Drug Development (PFDD) where patients and families emphasized “inability to plan” and disruptions to work/school [7].
Patient centricity is a pervasive mantra in research, policy, care delivery, regulatory decision making, health technology assessment, and elsewhere [8–15]. It is an intentional effort in which patients play a central and active role as meaningfully engaged participants [16–18]. The goal is optimizing use of information patients provide to improve a process, program, or decision, to achieve the outcomes most important to patients [17]. It means engaging WITH patients as partners, rather than doing it ON BEHALF of patients [14, 19–21]. Groups like Myeloma Patients Europe are dedicated to identifying and collecting key patient-defined and family-defined impacts such as caregiver burden and ability to work [22]. These impacts became particularly important during the COVID pandemic; it was an international patient-led research collaborative that first collected data on the impact of long COVID, including the inability of patients to return to work or resume normal family and social function [23].
In tandem over the last decade, encouragement to companies from regulatory agencies to conduct PFDD has helped move research and health systems in a patient-centered direction [2, 24–29]. For example, US legislation directed the Food and Drug Administration (FDA) to conduct meetings with patient communities to collect patient views on their illness and treatments [26]. For each meeting, a Voice-of-the-Patient report describes impacts that matter most to patients, perspectives on treatments, and other themes [27–31]. Legislation also requires the FDA to develop a series of PFDD guidance documents on collecting and using “patient experience data”, defined as disease symptoms, natural history, ability to function, well-being, views on medications and procedures, outcomes, and preferences for outcomes and treatments [13, 24, 32].
Similarly in Europe, regulations went into effect at the end of 2021 requiring researchers conducting a clinical trial in a European Union country to disclose how patients were involved in designing the protocol [33]. Multi-year public-private investments, including IMI-PREFER and IMI-PARADIGM, further demonstrate interest and action [27, 28].
The shift toward patient-centered medical-product development cannot be overlooked. While the efforts are intended to enhance PFDD, they drive home the need to capture the entire patient experience to inform disease characterization and have stimulated efforts to collect patient experience data by patient groups, treatment developers, and others [7, 31, 34, 35]. In addition, data collected often inform numerous downstream care decisions.
Thus, many other stakeholders are interested in capturing and incorporating the patient voice. For example, value and health technology assessment (V/HTA) organizations want their assessments, cost analyses, and models to reflect patient experiences [8, 14, 15, 20, 21, 36–38]. The inclusion of the patient voice can often fall short in V/HTA, with a lack of availability of patient-centered data as a primary barrier. While some organizations engage patients directly, the clinical evidence V/HTA often relies on rarely includes data on what patients report as important [39, 40]. If data are available, it often was captured inconsistently, hindering comparisons across treatments [11, 41–49].
Challenges posed by the misalignment between data collected and decision-maker evidence needs also have been cited by others, including clinical-practice guideline developers, quality-assessment organizations, and individual patients [13, 50–55]. Despite significant investment in research, decisions across healthcare are made using an evidence base most stakeholders acknowledge is inadequate [8–12, 56, 57]. There is a need for broader agreement on and standardization of which patient-centered impacts (from disease and treatment) are most important and should be measured across studies and care.
Patient-Centered Core Impact Sets (PC-CIS)
We propose expanding current thinking to a focus on “impacts,” including health outcomes, but also all other meaningful concepts patients might report. The range of concerns patients report is wide and diverse, including a variety of life impacts, such as symptoms, function, survival, biomarkers, out-of-pocket costs, family stresses, and much more. While some might be captured in specific research, others are unlikely to be, despite the importance to patients and other decision makers [8, 10, 50, 58].
We further propose development and use of patient-centered core impact sets (PC-CIS), a patient-derived and patient-prioritized list of impacts a disease and/or its treatments have on a patient (and/or their family and caregivers). Intentionally broad and inclusive, the term “impacts” includes short-term and long-term health outcomes and any other related implications (e.g., carer/family stresses, economic burden, career loss). The objectives of this paper are to introduce the concept of PC-CIS, describe what they are and why they are needed, and outline a pathway for achieving them.
A Foundation to Build Upon
Patient-centered core impact set development does not begin as a blank slate. There is past recognition that core outcome sets (COS), are needed to guide outcome selection in research, especially clinical trials [59–62]. However, typically, a COS was not intended to be comprehensive of the patient experience. Rather, it represents the minimum set of health outcomes that should be measured across studies, derived by gathering information on a broad range of outcomes and prioritized to arrive at those of highest priority, the “core” [59, 61, 62]. Some diseases have a COS available [61–64]. However, methods used, intended use, and participants included in the processes have implications for whether a COS is patient centered, focused on outcomes important to patients [65]. For example, many older COS efforts did not begin with patient input or may not have included patients at all [66–68]. If a set was derived only from peer-reviewed literature and clinician input, it likely omits many impacts patients identify as most important [69, 70]. Other COS efforts may report patient involvement, but the nature of it may not be detailed enough to understand if the patient-engagement effort was meaningful or cursory [71–73].
The FDA recently initiated a pilot program to support the development of publicly available core sets of clinical outcome assessments (COAs) and related endpoints for specific indications[74]. Clinical outcome assessments capture how patients feel or function, and are reported by patients as important [75]. The COA umbrella includes patient-reported, clinician-reported, and observer-reported, as well as performance outcomes. Thus, the FDA’s COA core sets capture a narrower range of outcomes than what might be found in a COS, which are not limited by measure type. Core outcome sets could include survival, biomarkers, and economic impacts, but COA cores sets exclude them. The FDA effort begins by identifying legacy COA measures; however, those measures may have been developed with little to no patient engagement and require confirmation the concepts covered are important to patients [76].
More recent efforts to try to implement individualized patient-reported outcome measures in healthcare settings face many challenges and are far from broad acceptance [77–79]. A PC-CIS can be used to inform decisions about which concepts should be addressed in standardized patient-reported outcome measures and can factor into discussions about what can/should be addressed in individualized patient-reported outcome measures. Both approaches, traditional COS (that may/may not include COAs) and/or FDA sets of only COAs, could improve outcome comparisons across studies. However, a focus on only health outcomes, (i.e., changes in health due to care [80]) or COAs is too narrow in terms of patient centricity and breadth of everyday-life impacts. However, these past efforts are foundational in terms of the established methods and approaches they use that can be leveraged in broader patient-centered efforts.
Opening Our Eyes to Broader Impacts from the Patient Perspective
To demonstrate the diversity of impacts patients might report, we abstracted data from an FDA-led Voice-of-the-Patient report on chronic fatigue syndrome [81]. Patients reported disease-related impacts such as post-exertional malaise, weakness, muscle and joint pain, unrefreshing sleep, social isolation, and feelings of hopelessness. Treatment-related impacts included willingness to accept significant new treatment risks to alleviate or cure the condition. Financial-related impacts included loss of career, harsh financial difficulties from decreased or lost employment income, and high treatment costs, often because off-label treatments are not covered by insurance. Finally, examples of family/caregiver-related impacts included stresses on family life and individual family members.
Many researchers and COS developers previously captured some of these life impacts, but it has not happened consistently and was often from the perspective of another stakeholder, not patients and families. For example, productivity, measured as absenteeism or days/hours lost from work, might be collected because another stakeholder was interested, not because patients, carers, or families reported those subjects as meaningful.
We prefer the term “impacts” to “outcomes,” not to add confusion, but to encourage broader thinking about and acceptance of the wide range of potential concepts patients might report as important, which include, but extend beyond the most proximal health outcomes. Further, we recommend a PC-CIS be developed for each disease of interest, and/or patient population. A PC-CIS would begin with gathering from patients, carers, and families an inventory of all impacts a disease and/or treatment have on a patient and a family’s life. Then, through a formal process, a core set can be derived that keeps patient inputs at the forefront.
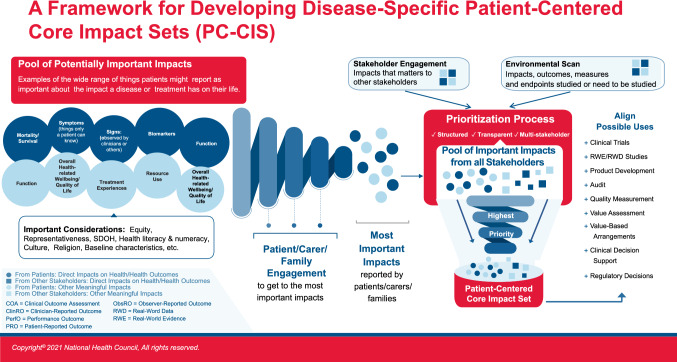
Figure 1, which was co-developed by an National Health Council multi-stakeholder advisory group on PC-CIS, depicts the envisioned framework for how a PC-CIS could be developed and used. As shown, it begins with gathering information from patients, carers, and families about what is important, and deriving from them what is most important. For example, the large circles represent the larger pool of what might be reported, the smaller circles represent those reported as most important.
Fig. 1.
Disease-specific patient-centered core impact sets
The figure also depicts a PC-CIS as an important starting point, discerning those prioritized impacts to be considered for measurement across studies and applied to multiple decision uses. It can inform: disease understanding and care delivery; measure development and endpoint selection for product-development clinical-trial programs; streamline V/HTA-model development and value-element selection for economic analyses, and improve care design and delivery through patient-informed quality-of-care assessment and audit. To capture what patients, carers, and families report, the core impacts must be translated into concepts of interest and sound measures. Each use would likely not depend on the exact same measures and endpoints in application. We will need to ensure subsequent measures and endpoints are fit for purpose for each intended use. However, all measures would originate from the core set. The PC-CIS helps ensure that across uses, measure developers aim at the same targets, though they may require different but related tools.
How Do You Move an Elephant?
There are several challenges deriving PC-CIS to adequately address all disease states for which such are needed. While not an exhaustive list, a few of the greatest challenges are discussed below.
The volume of diseases is a significant barrier as it is estimated there are over 10,000 known diseases, with 7000 being rare diseases [82, 83]. Significant time and resources will be needed to address this broad field and to maintain a learning environment to improve PC-CIS over time. Taking an incremental view, a more achievable approach to building an initial PC-CIS for a disease might include starting with concepts outlined in available data, such as a Voice-of-the-Patient Report, adding over time patient-group registry and survey data, and additional patient-centered data collected by researchers, companies, and others.
In addition to the volume of diseases, a more complex challenge is that for any one disease, a PC-CIS might vary by country, region of the world, and social determinants of health (as depicted in Fig. 1). Like past internationally collaborative COS efforts to identify what should be measured in multi-country trials, a PC-CIS also requires international collaboration. However, we cannot assume one-size-fits-all around the world. Research will be required to determine what is “core” where. Collection of patient experience data in various locations and populations will inform the transferability of PC-CIS. It is practical to think multiple sets might be required for a disease to accommodate differences among regions, but it is also assumed there will be similarities across those sets. There are also challenges related to collecting patient experience data for the large volume of rare diseases [84, 85].
The breadth of this barrier also highlights a second barrier, a lack of standardized best practices and approaches to construct PC-CIS [86]. While there are established practices used by leading COS developers [59–62], approaches differ. We need to leverage and build upon this existing solid foundation to establish standardized best practices for building and maintaining PC-CIS, with a primary focus on patient centricity, and flexibility for innovation and evolution of the set(s). It will take consensus among various stakeholders and mean continuous quality improvement as methods evolve.
The third barrier is responsibility for building and maintaining the core impact sets [87]. Development must be a multi-stakeholder effort involving patient groups, patients, researchers from industry and academia, clinicians, payers, and government bodies. All must feed data into a process and repository that grows, solidifies, and sustains a disease-specific set. The work also is best done with a non-competitive mindset. No one company or organization should “own” a set, nor withhold data that could improve a set. A PC-CIS can only be a common resource if approached collaboratively.
However, the question remains, who leads? We suggest, whenever possible, PC-CIS development for a disease be led by the patient community. For example, this might lend itself to a model whereby one disease-specific patient group convenes a consortium of patient groups, patients, life-science companies, clinicians, researchers, payers, employers, and other experts to develop a PC-CIS for that disease.
Moving the Elephant, One Inch At a Time …
The charge seems enormous. To move forward in establishing useful accessible PC-CIS, we need to overcome the barriers incrementally. We offer several recommendations on how to move forward through collaboration, leadership, and establishing a PC-CIS development blueprint with supporting tools.
No one organization can accomplish all the work that needs to be done. This effort requires multi-stakeholder collaboration, with all resulting work being open access. It must include patients, patient groups, industry, clinicians, employers, and government and quasi-governmental bodies (e.g., FDA, European Medicines Agency, and the Patient-Centered Outcomes Research Institute).
International collaboration is also needed as the sets will likely need fine tuning by region and there are already international efforts to build upon [59, 60, 62]. All must contribute to and support the end goal of a standard that makes the output usable, useful, and (most important of all) used.
While we encourage patient-group leadership, other stakeholder champions are needed. The FDA has been a champion of core sets for COAs. An effort to produce PC-CIS will require more. For example, in the USA, the coming PDUFA VII reauthorization could be a vehicle for an important FDA role. Not to take on long-term, disease-specific PC-CIS production and maintenance, but to champion the way (as it did for PFDD) and work in collaboration with other stakeholders, such as patient groups. This is the model that resulted in externally led PFDD meetings [31]. The Patient-Centered Outcomes Research Institute is another natural champion given its reauthorization charge to capture the broader range of healthcare impacts [88]. The Innovative Medicines Initiative, a public-private partnership that has invested in several patient-centered initiatives to date could become a key stakeholder in Europe.
Collaboration can also avoid unnecessary duplication of work. For example, COMET, based at the University of Liverpool, houses a database of published COS. Inclusion of PC-CIS could assist researchers in finding them.
Last, which we believe is the most important, is the production of a common blueprint, leveraging and expanding upon existing approaches to standardize methods guiding PC-CIS development. Such a process could follow the pathway outlined in Fig. 1 and clarify needed terminology and best practices. This would ensure sound methods are followed, patient-centered core impact sets are aligned with one another, and all involved speak the same language. Moreover, it allows contributors and users to have confidence a PC-CIS is a valid useful tool for complex decision making. The blueprint will need supporting tools and resources, such as a common taxonomy to capture the range of impacts patients report. Such a taxonomy does not currently exist but is an essential building block for tangible progress.
We believe PC-CIS are an important tool for the future of patient-centered healthcare and research, and that patient groups should lead PC-CIS development to ensure the patient voice is central. The National Health Council is focusing on bringing together its members, partners, and other interested parties to move the PC-CIS vision forward. We invite the broader healthcare community to engage with us as we delve further into this important work.
Acknowledgements
The authors would like to acknowledge the following members of the PC-CIS Advisory Committee for their contributions to the development of Figure 1: Upal Basu Roy, LUNGevity; Jennifer Bright, Innovation and Value Initiative; Nicholas Brooke, Patient Focused Medicines Development; Laurie Burke, LORA Group, LLC; Tim Coetzee, National Multiple Sclerosis Society; Maarten de Wit, OMERACT; J. Samantha Dougherty, PhRMA; Ryan Fischer, Parent Project Muscular Dystrophy; Dan Ignaszewski, Amputee Coalition; Annie Kennedy, EveryLife Foundation for Rare Diseases; LaTasha H. Lee, National Minority Quality Forum; Barry Liden, Edwards Lifesciences; Donna Messner, Center for Medical Technology Policy; John Schall, Caregiver Action Network; Patrick Wildman, Lupus Foundation of America; Paula Williamson, COMET; Leonard Valentino, National Hemophilia Foundation; Susan Vallow, Novartis Pharmaceuticals Corporation; Yvette Venable, Institute for Clinical and Economic Review.
Declarations
Funding
The development of this paper was supported with funding from the Innovation and Value Initiative and the EveryLife Foundation.
Conflicts of interest
Eleanor M. Perfetto is a Professor at the University of Maryland School of Pharmacy. At the time this paper was prepared, Eleanor M. Perfetto was an employee of the National Health Council, a nonprofit membership organization that receives dues, sponsorships, and grants. For a complete list of members, sponsors, and funders, see: https://www.nationalhealthhcouncil.org. In the past several years, Eleanor M. Perfetto has received funding unrelated to this paper at the University of Maryland from the Food and Drug Administration, Excerpta Medica, PhRMA Foundation, PhRMA, Merck, and Pfizer. Elisabeth M. Oehrlein is an employee of the National Health Council, a nonprofit, membership organization that receives dues, sponsorships, and grants. For a complete list of members, sponsors, and funders, see: https://www.nationalhealthhcouncil.org. T. Rosie Love is a Ph.D. candidate at the University of Maryland School of Pharmacy. She has received consulting fees unrelated to this paper from the National Health Council, a nonprofit, membership organization that receives dues, sponsorships, and grants. For a complete list of members, sponsors, and funders, see: https://www.nationalhealthhcouncil.org. Unrelated to this paper, T. Rosie Love is also an active Voice of Patient Volunteer with NephCure Kidney International, a 501 (c)(3) public charity. Silke Schoch is an employee of the National Health Council, a nonprofit membership organization that receives dues, sponsorships, and grants. For a complete list of members, sponsors, and funders, see https://www.nationalhealthhcouncil.org. Annie Kennedy has nothing to disclose. Jennifer Bright receives consulting fees from IVI, a membership organization that receives dues, sponsorships, and grants from individuals, organizations, and grant makers (see https://www.thevalueinitiative.org). In the past several years, Jennifer Bright has received additional funding at Momentum Health Strategies from FasterCures, the National Quality Forum, the National Council for Behavioral Health, and the Cancer Support Community.
Author contributions
EMP contributed conceptualization, methodology, writing the original draft, writing, review and editing, supervision, and funding acquisition. EMO contributed conceptualization, methodology, validation, investigation, writing original draft, writing, review and editing, and visualization. TRL contributed to the investigation, data curation, writing original draft, conceptualization, methodology, validation, investigation, writing, review and editing, and is the corresponding author. SS contributed to writing, review and editing, and project administration. AK contributed conceptualization and writing, review and editing. JB contributed conceptualization, writing, and review and editing.
References
- 1.Mühlbacher AC, Nübling M. Analysis of physicians’ perspectives versus patients’ preferences: direct assessment and discrete choice experiments in the therapy of multiple myeloma. Eur J Health Econ. 2011;12:193–203. doi: 10.1007/s10198-010-0218-6. [DOI] [PubMed] [Google Scholar]
- 2.Mavris M, Furia Helms A, Bere N. Engaging patients in medicines regulation: a tale of two agencies. Nat Rev Drug Discov. 2019;18:885–886. doi: 10.1038/d41573-019-00164-y. [DOI] [PubMed] [Google Scholar]
- 3.Smith H, Horobin A, Fackrell K, Colley V, Thacker B, Hall DA. Defining and evaluating novel procedures for involving patients in core outcome set research: creating a meaningful long list of candidate outcome domains. Res Involv Engagem. 2018;4:1–12. doi: 10.1186/s40900-018-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagotto E, Burgués VA, Fung A. A Taxonomy to Engage Patients: Objectives, Design, and Patient Activation. NEJM Catalyst. Massachusetts Medical Society; 2019;5. https://catalyst.nejm.org/doi/abs/10.1056/CAT.19.0626.
- 5.Vaughn Y, Richmond A, Simpson C, Israel T, Boone L. Comparing methods to make research more patient-centered. 2017. 10.25302/12.2019.ME.130603342. Accessed 5 Apr 2021.
- 6.Patient-Centered Outcomes Research Institute (PCORI). Finding the keys to a longer, better life after stroke. 2017. https://www.pcori.org/research-results/pcori-stories/finding-keys-longer-better-life-after-stroke. Accessed 15 Feb 2022.
- 7.Arthritis Foundation. Externally-led juvenile idiopathic arthritis patient: focused drug development meeting report. 2019. https://www.arthritis.org/getmedia/25118249-ea68-45b5-bfe4-c20904ddc32c/FINAL-JIA-PFDD.pdf. Accessed 15 Feb 2022.
- 8.Innovation and Value Initiative. IVI methods summit: defining needs and progress toward improving methods in value assessment: convening proceedings report. 2020. https://www.thevalueinitiative.org/wp-content/uploads/2020/05/Methods-Summit-Report_FINAL_Digital.pdf. Accessed 17 Feb 2022.
- 9.Linthicum MT, dosReis S, Slejko JF, Mattingly TJ, Bright JL. The importance of collaboration in pursuit of patient-centered value assessment. Patient. 2021;14:381–384. doi: 10.1007/s40271-020-00446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Facey K. Patient involvement in HTA: what added value? Pharm Policy Law. 2011;13:245–251. [Google Scholar]
- 11.Berglas S, Jutai L, MacKean G, Weeks L. Patients’ perspectives can be integrated in health technology assessments: an exploratory analysis of CADTH common drug review. Res Involv Engagem. 2016;2:1–13. doi: 10.1186/s40900-016-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaby V, Ali AA, Montero AJ. Value assessment frameworks in the United States: a call for patient engagement. Pharmacoecon Open. 2019;3(1):1–3. doi: 10.1007/s41669-018-0094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Patient-focused drug development: collecting comprehensive and representative input guidance for industry, Food and Drug Administration staff, and other stakeholders. 2020. pp. 4–5. https://www.fda.gov/media/139088/download. Accessed 30 Apr 2022.
- 14.National Institute for Health and Care Excellence. NICE health technology evaluations: the manual. 2022. https://www.nice.org.uk/process/pmg36/resources/nice-health-technology-evaluations-the-manual-pdf-72286779244741. Accessed 30 Apr 2022.
- 15.National Institute for Health and Care Excellence. Patient and public involvement policy. 2013. https://www.nice.org.uk/media/default/About/NICE-Communities/Public-involvement/Patient-and-public-involvement-policy/Patient-and-public-involvement-policy-November-2013.pdf. Accessed 17 Feb 2022.
- 16.Patient-Centered Outcomes Research Institute (PCORI). Patient-centered outcomes research. 2014. https://www.pcori.org/research-results/about-our-research/patient-centered-outcomes-research. Accessed 3 Aug 2020.
- 17.National Health Council. The National Health Council rubric to capture the patient voice: a guide to incorporating the patient voice into the health ecosystem. 2019. https://www.nationalhealthcouncil.org/sites/default/files/NHC_Patient_Engagement_Rubric.pdf. Accessed 30 Apr 2022.
- 18.Edwards HA, Huang J, Jansky L, Mullins CD. What works when: mapping patient and stakeholder engagement methods along the ten-step continuum framework. J Comp Eff Res. 2021. https://www.futuremedicine.com/doi/full/10.2217/cer-2021-0043. Accessed 28 Jun 2021. [DOI] [PubMed]
- 19.National Health Council. Glossary of patient engagement terms. 2019. https://nationalhealthcouncil.org/glossary-of-patient-engagement-terms/. Accessed 4 Aug 2021.
- 20.Health Technology Assessment iNternational (HTAi). Resources & materials. 2022. https://htai.org/interest-groups/pcig/resources/. Accessed 17 Feb 2022.
- 21.Canadian Agency for Drugs and Technologies in Health (CADTH). Patient input and feedback. 2021. https://www.cadth.ca/patient-input-and-feedback. Accessed 17 Feb 2022.
- 22.Myeloma Patients Europe. Evidence Generation Unit (EGU). 2020. https://www.mpeurope.org/evidence-generation-unit-egu/. Accessed 15 Feb 2022.
- 23.McCorkell L, Assaf G, Davis H, Wei H, Akrami A. Patient-led research for COVID-19: embedding patients in the long COVID narrative. OSF Preprints. 2020. [DOI] [PMC free article] [PubMed]
- 24.Public Law No: 115-52-H.R.2430-FDA Reauthorization Act of 2017. 301. Sect. 605, 115–52 2017. pp. 102–3. https://www.congress.gov/bill/115th-congress/house-bill/2430. Accessed 30 Apr 2022.
- 25.Public Law No: 114-255-H.R.34-21st Century Cures Act. 201, 114–255 2016, pp. 1000–18001. https://www.congress.gov/bill/114th-congress/house-bill/34?q=%7B%22search%22%3A%5B%22To+accelerate+the+discovery%2C+development%2C+and+delivery+of+21st+century+cures%2C+and+for+other+purposes.%22%5D%7D&r=4. Accessed 30 Apr 2022.
- 26.Public Law No: 112-144-H.R.5651-Food and Drug Administration Reform Act of 2012. 301. Sect. 901, 112–144 2013, p. 103. https://www.congress.gov/bill/112th-congress/senate-bill/3187/text?q=%7B%22search%22%3A%5B%22S.3187%22%2C%22S.3187%22%5D%7D&r=1&s=5. Accessed 30 Apr 2022.
- 27.Innovative Medicines Initiative. Patients Active in Research and Dialogues for an Improved Generation of Medicines (PARADIGM). 2021. https://imi-paradigm.eu/. Accessed 17 Feb 2022.
- 28.Innovative Medicines Initiative. The patient preferences in benefit-risk assessments during the drug life cycle (PREFER). Uppsala University, Sweden; 2021. https://www.imi-prefer.eu/. Accessed 17 Feb 2022.
- 29.International Council for Harmonisation. ICH reflection paper: proposed ICH guideline work to advance patient focused drug development. 2021. https://admin.ich.org/sites/default/files/2021-06/ICH_ReflectionPaper_PFDD_FinalRevisedPostConsultation_2021_0602.pdf. Accessed 17 Feb 2022.
- 30.US Food and Drug Administration. The voice of the patient: a series of reports from the U.S. Food and Drug Administration’s (FDA’s) patient-focused drug development initiative: breast cancer. 2015. https://www.fda.gov/media/93924/download. Accessed 21 May 2019.
- 31.US Food and Drug Administration. Externally-led patient-focused drug development meetings. Externally-led patient-focus. Drug development meeting. FDA. 2020. https://www.fda.gov/industry/prescription-drug-user-fee-amendments/externally-led-patient-focused-drug-development-meetings. Accessed 23 Nov 2020.
- 32.Patient Focused Medicines Development. Highlighting recent trends in the fast-evolving patient engagement & patient experience data landscape. 2021. https://patientfocusedmedicine.org/docs/Trends-in-the-Fast-Evolving-PE-PED-Landscape.pdf. Accessed 21 Feb 2022.
- 33.The European Parliament and the Council of the European Union. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC. 2014. https://ec.europa.eu/health/system/files/2016-11/reg_2014_536_en_0.pdf. Accessed 17 Feb 2022.
- 34.Myotonic Dystrophy Foundation. Voice of the patient report myotonic dystrophy externally-led patient-focused drug development meeting. 2017. https://www.myotonic.org/sites/default/files/MDFVoicePatientReportMay2017.pdf. Accessed 16 Feb 2020.
- 35.Medicines and Healthcare Products Regulatory Agency. Patient involvement strategy 2021–25. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1022370/Patient_involvement_strategy.pdf. Accessed 21 Feb 2022.
- 36.Institute for Clinical and Economic Review. 2020–2023 value assessment framework. 2020. http://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf. Accessed 11 Jan 2021.
- 37.Innovation and Value Initiative. Open-source value project model for major depressive disorder health economic module: draft protocol. 2021. https://www.thevalueinitiative.org/wp-content/uploads/2021/12/MDD-Model-Protocol-Draft.pdf. Accessed 24 Feb 2022.
- 38.Canadian Agency for Drugs and Technologies in Health (CADTH). Procedures for CADTH reimbursement reviews. 2021. https://www.cadth.ca/sites/default/files/Drug_Review_Process/CADTH_Drug_Reimbursement_Review_Procedures.pdf. Accessed 17 Feb 2022. [PubMed]
- 39.Institute for Clinical and Economic Review. Targeted immune modulators for rheumatoid arthritis: effectiveness & value. 2017. http://icerorg.wpengine.com/wp-content/uploads/2020/10/NE_CEPAC_RA_Evidence_Report_FINAL_040717.pdf. Accessed 30 Apr 2022.
- 40.Institute for Clinical and Economic Review. Modulator treatments for cystic fibrosis: effectiveness and value: final evidence report and meeting summary. 2020. https://icer.org/wp-content/uploads/2020/08/ICER_CF_Final_Report_092320.pdf. Accessed 17 Feb 2022.
- 41.Institute for Clinical and Economic Review. Disease-modifying therapies for relapsing remitting and primary-progressive multiple sclerosis: effectiveness and value. 2017. https://icer.org/wp-content/uploads/2020/10/CTAF_MS_Final_Report_030617.pdf. Accessed 4 Apr 2021.
- 42.Whittal A, Meregaglia M, Nicod E. The use of patient-reported outcome measures in rare diseases and implications for health technology assessment. Patient. 2021;14:485–503. doi: 10.1007/s40271-020-00493-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Single A, Facey K. Beyond guidelines: tools to support patient involvement in health technology assessment. Health Technology Assessment international. 2021. p. 17. https://g-i-n.net/wp-content/uploads/2021/06/HTA-final-for-online-publication-.pdf. Accessed 30 Apr 2022.
- 44.Sacristán JA, Aguarón A, Avendaño-Solá C, Garrido P, Carrión J, Gutiérrez A, et al. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence. 2016;10:631. doi: 10.2147/PPA.S104259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18:1–7. doi: 10.1186/s13063-017-1870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rahimi K, Malhotra A, Banning AP, Jenkinson C. Outcome selection and role of patient reported outcomes in contemporary cardiovascular trials: systematic review. BMJ. 2010;341:c5707. doi: 10.1136/bmj.c5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353. doi: 10.2147/PROM.S156279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wieseler B, Wolfram N, McGauran N, Kerekes MF, Vervölgyi V, Kohlepp P, et al. Completeness of reporting of patient-relevant clinical trial outcomes: comparison of unpublished clinical study reports with publicly available data. PLoS Med. 2013;10:e1001526. doi: 10.1371/journal.pmed.1001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercieca-Bebber R, Friedlander M, Calvert M, Stockler M, Kyte D, Kok P-S, et al. A systematic evaluation of compliance and reporting of patient-reported outcome endpoints in ovarian cancer randomised controlled trials: implications for generalisability and clinical practice. J Patient-Rep Outcomes. 2017;1:5. doi: 10.1186/s41687-017-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Food and Drug Administration. The voice of the patient: a series of reports from FDA’s patient-focused drug development initiative. 2018. https://www.fda.gov/ForIndustry/UserFees/PrescriptionDrugUserFee/ucm368342.htm. Accessed 30 Apr 2022.
- 51.Young B, Bagley H. Including patients in core outcome set development: issues to consider based on three workshops with around 100 international delegates. Res Involv Engagem. 2016;2:25. doi: 10.1186/s40900-016-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pharmacy Quality Alliance. The patient engagement in quality measurement rubric: a guide to patient partnership in the quality measure lifecycle. 2019. https://www.pqaalliance.org/assets/PQA-Patient-Engagement-Rubric.pdf. Accessed 23 Oct 2019.
- 53.O’Kane M, Agrawal S, Binder L, Dzau V, Gandhi TK, Harrington R et al. An equity agenda for the field of health care quality improvement. NAM Perspect. 2021;2021. [DOI] [PMC free article] [PubMed]
- 54.Armstrong MJ, Mullins CD, Gronseth GS, Gagliardi AR. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implement Sci. 2018;13:55. doi: 10.1186/s13012-018-0745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Addario BJ, Fadich A, Fox J, Krebs L, Maskens D, Oliver K, et al. Patient value: perspectives from the advocacy community. Health Expect. 2018;21:57–63. doi: 10.1111/hex.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heneghan C, Goldacre B, Mahtani KR. Why clinical trial outcomes fail to translate into benefits for patients. Trials. 2017;18:122. doi: 10.1186/s13063-017-1870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.International Society for Pharmacoeconomics and Outcomes Research. ISPOR science strategy. 2021. https://www.ispor.org/strategic-initiatives/science-strategy. Accessed 30 Apr 2022.
- 58.Perfetto EM, Oehrlein EM, Boutin M, Reid S, Gascho E. Value to whom? The patient voice in the value discussion. Value Health. 2017;20:286–291. doi: 10.1016/j.jval.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET handbook: version 1.0. Trials. 2017;18:280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International Consortium for Health Outcomes Measurement (ICHOM). ICHOM standard sets: view our collection. https://www.ichom.org/standard-sets/. Accessed 28 Jul 2020.
- 61.Center for Medical Technology Policy (CMTP). coreHEM: developing comparative effectiveness outcomes for gene therapy in hemophilia. 2018. http://www.cmtpnet.org/docs/resources/coreHEM_Final_Report_21_MAY_2018.pdf. Accessed 26 Apr 2019.
- 62.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d’Agostino M-A, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67:745–753. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 63.Dodd S, Harman N, Taske N, Minchin M, Tan T, Williamson PR. Core outcome sets through the healthcare ecosystem: the case of type 2 diabetes mellitus. Trials. 2020;21:1–7. doi: 10.1186/s13063-020-04403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tong A, Manns B, Wang AYM, Hemmelgarn B, Wheeler DC, Gill J, et al. Implementing core outcomes in kidney disease: report of the Standardized Outcomes in Nephrology (SONG) implementation workshop. Kidney Int. 2018;94:1053–1068. doi: 10.1016/j.kint.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clearfield E, Tambor E, Janssen EM, Messner DA. Increasing the patient-centeredness of health economics and outcomes research through patient engagement in core outcome set development. Patient. 2021;14(4):413–420. doi: 10.1007/s40271-020-00424-9. [DOI] [PubMed] [Google Scholar]
- 66.O’Brien N, Chi Y-L, Krause KR. Measuring health outcomes in HIV: time to bring in the patient experience. Ann Glob Health. 2021;87(1):2. doi: 10.5334/aogh.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alioum A, Dabis F, Dequae-Merchadou L, Haverkamp G, Hudgens M, Hughes J, et al. Estimating the efficacy of interventions to prevent mother-to-child transmission of HIV in breast-feeding populations: development of a consensus methodology. Stat Med. 2001;20:3539–3556. doi: 10.1002/sim.1076. [DOI] [PubMed] [Google Scholar]
- 68.Land L, Nixon S, Ross J. Patient-derived outcome measures for HIV services in the developed world: a systematic review. Int J STD AIDS. 2011;22:430–435. doi: 10.1258/ijsa.2011.010450. [DOI] [PubMed] [Google Scholar]
- 69.Kloppenburg M, Bøyesen P, Smeets W, Haugen I, Liu R, Visser W, et al. Report from the OMERACT Hand Osteoarthritis Special Interest Group: advances and future research priorities. J Rheumatol. 2014;41:810–818. doi: 10.3899/jrheum.131253. [DOI] [PubMed] [Google Scholar]
- 70.Jimenez-Moreno AC, Nikolenko N, Kierkegaard M, Blain AP, Newman J, Massey C, et al. Analysis of the functional capacity outcome measures for myotonic dystrophy. Ann Clin Transl Neurol. 2019;6:1487–1497. doi: 10.1002/acn3.50845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gagnon C, Heatwole C, Hébert LJ, Hogrel J-Y, Laberge L, Leone M, et al. Report of the third outcome measures in myotonic dystrophy type 1 (OMMYD-3) international workshop Paris, France, June 8, 2015. J Neuromuscul Dis. 2018;5:523–537. doi: 10.3233/JND-180329. [DOI] [PubMed] [Google Scholar]
- 72.Gagnon C, Meola G, Hébert LJ, Laberge L, Leone M, Heatwole C. Report of the second outcome measures in myotonic dystrophy type 1 (OMMYD-2) international workshop San Sebastian, Spain, October 16, 2013. Neuromuscul Disord. 2015;25:603–616. doi: 10.1016/j.nmd.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Gagnon C, Meola G, Hébert LJ, Puymirat J, Laberge L, Leone M. Report of the first outcome measures in myotonic dystrophy type 1 (OMMYD-1) international workshop: Clearwater, Florida, November 30, 2011. Neuromuscul Disord. 2013;23:1056–1068. doi: 10.1016/j.nmd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 74.US Food and Drug Administration. CDER pilot grant program: standard core clinical outcome assessments (COAs) and their related endpoints. FDA. 2019. https://www.fda.gov/drugs/development-approval-process-drugs/cder-pilot-grant-program-standard-core-clinical-outcome-assessments-coas-and-their-related-endpoints. Accessed 1 Sep 2020.
- 75.Walton MK, Powers JH, III, Hobart J, Patrick D, Marquis P, Vamvakas S, et al. Clinical outcome assessments: conceptual foundation: report of the ISPOR clinical outcomes assessment: emerging good practices for Outcomes Research Task Force. Value Health. 2015;18:741–752. doi: 10.1016/j.jval.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oehrlein EM, Perfetto EM, Love TR, Chung Y, Ghafoori P. Patient-reported outcome measures in the Food and Drug Administration pilot compendium: meeting today’s standards for patient engagement in development? Value Health. 2018;21:967–972. doi: 10.1016/j.jval.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Bansback N, Trenaman L, MacDonald KV, Hawker G, Johnson JA, Stacey D, et al. An individualized patient-reported outcome measure (PROM) based patient decision aid and surgeon report for patients considering total knee arthroplasty: protocol for a pragmatic randomized controlled trial. BMC Musculoskelet Disord. 2019;20:1–10. doi: 10.1186/s12891-019-2434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Greenhalgh J, Gooding K, Gibbons E, Dalkin S, Wright J, Valderas J, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? A realist synthesis. J Patient Rep Outcomes. 2018;2:1–28. doi: 10.1186/s41687-018-0061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Porter I, Gonçalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5:507–519. doi: 10.2217/cer-2015-0014. [DOI] [PubMed] [Google Scholar]
- 80.Donabedian A. The quality of care: how can it be assessed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.1988.03410120089033. [DOI] [PubMed] [Google Scholar]
- 81.US Food and Drug Administration. The voice of the patient: chronic fatigue syndrome and myalgic encephalomyelitis. 2013. https://www.fda.gov/media/86879/download. Accessed 3 Aug 2020.
- 82.National Institutes of Health. FAQs about rare diseases. Genetic and Rare Diseases Information Center (GARD): an NCATS Program. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases. Accessed 29 Jun 2021.
- 83.FasterCures. FasterCures mobilizes for next phase of work to save lives by improving the global medical R&D system, launches health data initiative. Milken Institute. 2018. https://milkeninstitute.org/article/fastercures-mobilizes-next-phase-work-save-lives-improving-global-medical-rd-system. Accessed 15 Feb 2022.
- 84.Morel T, Cano SJ. Measuring what matters to rare disease patients: reflections on the work by the IRDiRC taskforce on patient-centered outcome measures. Orphanet J Rare Dis. 2017;12:1–13. doi: 10.1186/s13023-017-0718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lanar S, Acquadro C, Seaton J, Savre I, Arnould B. To what degree are orphan drugs patient-centered? A review of the current state of clinical research in rare diseases. Orphanet J Rare Dis. 2020;15:1–18. doi: 10.1186/s13023-020-01400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.iConquerMS. The Accelerated Cure Project and the Italian Multiple Sclerosis Society collaborate to advance patient-reported outcomes in MS research, patient care and product development. 2018. https://www.iconquerms.org/accelerated-cure-project-and-italian-multiple-sclerosis-society-collaborate-advance-patient-reported. Accessed 3 Dec 2020.
- 87.Nowinski CJ, Miller DM, Cella D. Evolution of patient-reported outcomes and their role in multiple sclerosis clinical trials. Neurotherapeutics. 2017;14:934–944. doi: 10.1007/s13311-017-0571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pascrell B. H.R.1865-Further Consolidated Appropriations Act, 2020. Public Law No 116-94. Sect. 104, H.R. 1865 Dec 20, 2019. https://www.congress.gov/bill/116th-congress/house-bill/1865/text. Accessed 30 Apr 2022.