Abstract
Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) cause two of the most prevalent debilitating viral infections. HIV appears to induce a skewing toward a Th2 response, while in HCV infection a Th1 response appears to dominate. Regeneration and tolerance factor (RTF) may participate in driving or sustaining a Th2 cytokine response. The expression of RTF on CD3+ T cells of HIV-seropositive (HIV+) individuals is increased. The purpose of this study was to compare the expression of RTF during HIV infections with that during HCV infections. Three-color flow-cytometric analysis of peripheral blood collected from HIV+ HCV-seropositive (HCV+), HIV- and HCV-seropositive (HIV+ HCV+), and HIV- and HCV-seronegative (HIV− HCV−) individuals was performed. Levels of RTF expression on T-lymphocyte subsets from these groups were compared, as were levels of RTF expression on activated T cells expressing CD38 and HLA-DR, to determine the relationship of RTF expression to these infections. We demonstrated that the expression of RTF on surfaces of T cells from HIV+ individuals is upregulated and that its expression on T cells from HCV+ individuals is downregulated. A twofold increase in the mean channel fluorescence of RTF on CD3+ T cells was seen in both HIV+ and HIV+ HCV+ individuals compared to HIV− HCV− individuals. HCV+ individuals had lower levels of RTF expression than HIV− HCV− individuals (P < 0.005 for CD4+; P < 0.0005 for CD8+). In terms of percentages of T cells expressing RTF, the groups were ranked as follows: HIV+ > HIV+ HCV+ > HIV− HCV− > HCV+. The results indicate that RTF expression correlates with HIV-associated immune activation and may be associated with Th2-type responses.
Both hepatitis C virus (HCV) and human immunodeficiency virus (HIV) cause persistent infections which result in significant morbidity and mortality (3, 21, 23). HCV infects hepatocytes, while HIV infects a variety of cells, most importantly CD4+ lymphocytes. The overall natures of the immune responses to these two viruses appear to be opposite one another (5, 6, 17). HIV appears to induce a skewing toward a T-helper type 2 (Th2) cytokine response, while in HCV infection a T-helper type 1 (Th1) cytokine response appears to dominate. Several studies of the cytokine profiles of hosts with each disease support these observations (5, 6, 8).
T cells and immunoregulatory cytokines play important roles in the host response to viral infections. Th1 cytokines (interleukin-2 [IL-2] and gamma interferon [IFN-γ]) are used in host antiviral defense for activation of cytotoxic T cells and natural killer cells (15, 16), while Th2 cytokines (IL-4, -5, -6, and -10) mediate humoral responses and also provide protection against extracellular pathogens, including multicellular eukaryotes and parasites (14, 16). Additionally, Th2 cytokines inhibit the development of Th1 effector mechanisms by inhibiting Th1 cell proliferation and shutting down IL-2 or IFN-γ production (14, 16). The Th1 and Th2 cytokine responses are the regulatory processes that guide the immune system in the induction of cellular and/or humoral immune responses. Distinguishing early in infection whether the immune system is producing a Th1 or Th2 cytokine response could prove vital in terms of deciding on the appropriate treatment. It also will increase our understanding of the mechanisms involved in the process by which the immune system “decides” whether to produce Th1 or Th2 cytokines.
One potential early marker of a Th2 response is regeneration and tolerance factor (RTF). RTF is a 70-kDa protein, encoded by the gene TJ6, with potent immunomodulating properties (1, 18, 20). Previously we have shown that RTF may participate in the Th2 cytokine responses to both HIV infection and pregnancy (2, 7, 9). The establishment of a successful pregnancy and peripheral tolerance as well as HIV infection is characterized by a shift from Th1 cytokine to Th2 cytokine production (6, 11, 22). Initial studies have shown that the expression of RTF on CD3+ T cells of HIV-seropositive (HIV+) individuals is significantly increased when compared to that of uninfected individuals (9). The purpose of this study was to compare the expression of RTF in HIV infection to that during HCV infection. In these studies, three-color flow-cytometric analyses were performed on peripheral blood from HIV+, HCV-seropositive (HCV+), HIV- and HCV-seropositive (HIV+ HCV+), and HIV- and HCV-seronegative (HIV− HCV−) individuals. The groups were also compared with regard to levels of RTF expression on T-lymphocyte subsets as well as activated T cells expressing CD38 and HLA-DR in order to determine the relationship of RTF expression to these infections.
MATERIALS AND METHODS
Study subjects.
Peripheral blood obtained from 25 HIV+ individuals at Mt. Sinai Hospital, Chicago, Ill., and from 24 HCV+ and 15 HIV+ HCV+ individuals at the Veterans Administration Hospital in North Chicago, Ill., was collected into tubes containing sodium heparin anticoagulant. Peripheral blood samples (n = 30) were obtained from HIV− HCV− male and female individuals at Finch University of Health Sciences/The Chicago Medical School and studied. The appropriate institutional review boards approved this study.
Anti-RTF MAb.
A monoclonal antibody (MAb) against the membrane portion of RTF was generated, purified, and conjugated to fluorescein isothiocyanate (FITC) as previously described (9, 13, 20). Briefly, the hybridoma cell line 2C1 was generated with a synthetic peptide (Clontech, Palo Alto, Calif.) representing amino acids 488 to 514 of the RTF gene sequence. For ascites fluid production, hybridoma 2C1 was grown in vitro and injected into the peritoneal cavities of pristane-primed BALB/c mice. Ascites fluid containing anti-RTF MAb was collected 14 to 21 days after injection, purified by HiTrap protein G column chromatography (Pharmacia Biotech, Piscataway, N.J.), and conjugated to FITC. Following conjugation, the FITC (495 nm)-to-protein (280 nm) ratio of the anti-RTF MAb was determined spectrophotometrically to be 10.1. Protein concentrations were also adjusted to 0.96 mg/ml. The specificity of the anti-RTF MAb was evaluated by enzyme-linked immunosorbent assay. Preincubation of the antibody with the peptide abolished membrane binding, confirming the reactivity of the purified antibody to a synthetic peptide representing amino acids 488 to 514 of the RTF gene sequence. Also, the specificity of the anti-RTF was measured by comparison to fluorescence obtained by labeling with mouse immunoglobulin G2a (IgG2a; Ortho Diagnostics, Raritan, N.J.) and immunoglobulin G1 (Becton Dickinson, San Jose, Calif.) isotype control antibodies conjugated to FITC and phycoerythrin (PE) (9).
Flow-cytometric analysis.
Three-color flow-cytometric analysis was performed on peripheral venous blood collected into tubes containing sodium heparin anticoagulant, using FITC-, PE-, and PE-cyanin 5.1 (PC5)-conjugated monoclonal antibodies. Within 24 h after specimen collection, 100-μl aliquots of blood were incubated for 15 min in the dark with 10 μl of a solution of antibodies (Coulter, Miami, Fla.) to either CD45-FITC–CD14-PE, CD3-PC5, CD4-PC5, or CD8-PC5 and 20 μl of a solution of antibodies to either CD38-PE (Immunotech, Westbrook, Maine) or HLA-DR-PE (Pharmingen, San Diego, Calif.). Next, 23-μl volumes of a solution of an FITC-conjugated monoclonal antibody (2C1 clone) against the membrane portion of RTF were added to tubes containing antibody to CD3 and to tubes containing CD4-CD38, CD4–HLA-DR, CD8-CD38, or CD8–HLA-DR. IgG1-FITC–IgG1-PE–IgG1-PC5 (Immunotech) was used as the isotype control. After labeling, samples were washed, lysed by the use of the Coulter Clone Immuno-Lyse reagents (Coulter), and immediately analyzed with a Coulter Epics XL-MCL flow cytometer after fixation.
Data from 5,000 events gated on PC5 fluorescence for CD4+ or CD8+ T cells were acquired and analyzed, using histogram and dot plot profiles of PE and FITC fluorescence. Lymphocytes were first identified based on forward- and side-scatter parameters. Antibodies to CD45-FITC–CD14-PE were used to validate the established lymphocyte gate. CD4+ or CD8+ cells (PC5 fluorescence) were gated on and subsequently analyzed for RTF expression (FITC fluorescence) and activation markers HLA-DR or CD38 (PE fluorescence) by using a two-parameter dot plot. Results are expressed as either the mean channel fluorescence (MCF) of RTF or the percentage of CD3+, CD4+, or CD8+ T cells expressing RTF.
Statistical analysis.
The data were analyzed by using the software Microsoft Excel (Microsoft Corporation, Redmond, Wash.) and Sigma Plot (SPSS Inc., Chicago, Ill.). Descriptive statistics are expressed as mean values ± standard errors. Comparisons between HIV+, HIV+ HCV+, HCV+, and HIV− HCV− individuals were performed with the unpaired t test (two sided). Significance was defined as a P value of <0.05.
RESULTS
RTF expression on CD3+ T cells.
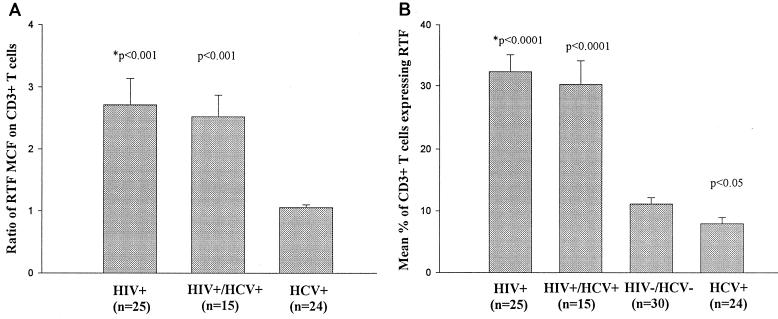
The first set of experiments measured the expression of RTF on CD3+ T cells from HIV+ and HCV+ individuals. The MCF values of CD3+ RTF+ T cells from HIV− HCV− individuals were first averaged as a normal control, and the ratio of the MCF for each individual of the HIV+, HIV+ HCV+, and HCV+ groups to the average control MCF was calculated; the values for each group were averaged and are presented in Fig. 1A. The average MCF ratio ± standard error for RTF expressed on CD3+ T cells was higher in HIV+ (2.7 ± 0.4) than in HCV+ (1.05 ± 0.04) individuals (P < 0.001) (Fig. 1A). The mean percentages of CD3+ T cells expressing RTF were 32% for HIV+ individuals and 7.4% for HCV+ individuals, compared to 11% for HIV− HCV− individuals (Fig. 1B).
FIG. 1.
Flow-cytometric analysis of RTF expression on CD3+ T cells from peripheral blood of HIV+, HIV+ HCV+, and HCV+ individuals, expressed as MCF ratios (A) and as percentage of cells positive for RTF (B). (A) ∗, a P value of <0.001 indicates that the difference between the ratio for that group is statistically significantly different from that of the HCV+ group. (B) ∗, significance is defined as a P value of <0.05, versus the HIV− HCV− group value. Means and standard errors (bars) are shown.
A third group of individuals that were infected with both HIV and HCV (HIV+ HCV+) was studied. The CD3+ T cells in this group had an RTF MCF ratio of 2.5 ± 0.3 (P < 0.001), versus the HCV+ group (Fig. 1A). The mean percentage of CD3+ cells expressing RTF was 30.2% (P < 0.001) for HIV+ HCV+ individuals, compared to 11% for the HIV− HCV− group (Fig. 1B).
In general, the mean percentages of RTF expression on CD3+ T cells ranked as follows: HIV+ > HIV+ HCV+ > HIV− HCV− > HCV+. Statistical analysis indicated that there were significant differences between the HIV− HCV− individuals and the HIV+ (P < 0.0001), HIV+ HCV+ (P < 0.0001), and HCV+ (P < 0.05) groups with regard to RTF expression on CD3+ T cells.
RTF expression on CD4+ and CD8+ T cells.
We next investigated which T-lymphocyte subsets expressed RTF. The data showed that both CD4+ and CD8+ T cells expressed RTF. The average RTF ± standard error of MCF for CD4+ T cells from HIV+ individuals was 1.9 ± 0.2, while it was 1.2 ± 0.2 HIV+ HCV+ individuals, 0.7 ± 0.05 for HIV− HCV− individuals, and 0.5 ± 0.03 for HCV+ individuals. Statistical analysis of the RTF MCF for CD4+ T cells showed that the HIV+ (P < 0.0001) and HIV+ HCV+ (P < 0.005) groups had significantly higher levels of RTF expression than the HIV− HCV− group.
The average RTF MCF ± standard error for CD8+ T cells was 2.3 ± 0.1 in HIV+ individuals, 1.6 ± 0.2 in HIV+ HCV+ individuals, 1.2 ± 0.1 in HIV− HCV− individuals, and 0.7 ± 0.05 in HCV+ individuals (Fig. 2A). Statistical analysis of the RTF MCF for CD8+ T cells showed significant differences between HIV− HCV− individuals and each of the other groups (HIV+ [P < 0.0001], HIV+ HCV+ [P < 0.0005], and HCV+ [P < 0.0005]).
FIG. 2.
Flow-cytometric analysis of RTF expression on CD4+ and CD8+ T cells from HIV+, HIV+ HCV+, HCV+, and HIV− HCV− individuals, expressed as MCF of RTF+ cells (A) and as percentage of cells expressing RTF (B). Means and standard errors are shown. ∗, significance is defined as a P value of <0.05 (versus HIV− HCV− group value).
The percentages of lymphocyte subsets (CD4+ or CD8+) expressing RTF were also determined. Flow-cytometric analysis showed that the average percentages (± standard errors) of CD4+ T cells expressing RTF were 24.2 ± 2.6 for HIV+ individuals, 22.4 ± 4.3 for HIV+ HCV+ individuals, 5.8 ± 0.5 for HCV+ individuals, and 8.6 ± 0.8 for HIV− HCV− individuals. Statistical analysis of the percentages of CD4+ T cells expressing RTF showed that the values for the HIV+ (P < 0.0001), HIV+ HCV+ (P < 0.005), and HCV+ (P < 0.01) groups were all significantly different from that of the HIV− HCV− group (Fig. 2A). Similarly, for the CD8+ T cells, the mean percentages (± standard errors) of cells expressing RTF were 33.4 ± 3.2 for HIV+ (P < 0.0001), 34.3 ± 3.9 for HIV+ HCV+ (P < 0.005), and 10.6 ± 1.5 for HCV+ (P < 0.005) individuals, compared to 17.4 ± 2.0 for HIV− HCV− individuals (Fig. 2B). The percentages of CD4+ (or CD8+) cells expressing RTF among these groups were all significantly different from those of the HIV− HCV− group. The percentage of CD8+ T cells expressing RTF was always higher than that for CD4+ T cells across all the groups (paired t test; P < 0.05 for HIV+, P < 0.05 for HIV+ HCV+, P < 0.0001 for HIV− HCV−, and P < 0.005 for HCV+). Representative flow-cytometric dot plots of CD4+ (Fig. 3A, C, E, and G) and CD8+ (Fig. 3B, D, F, and H) T cells from each group are presented.
FIG. 3.
Flow-cytometric dot plots of CD4+ T cells (A, C, E, and G) and CD8+ T cells (B, D, F, and H) from an HIV+ (A and B), HIV+ HCV+ (C and D), HIV− HCV− (E and F), or HCV+ (G and H) individual. A gate was drawn on the CD4+ or CD8+ cells, and the percentage of double-positive (RTF+ CD4+ or RTF+ CD8+) cells was determined by measuring FITC and PC5 fluorescence. Percentages indicate the proportions of cells expressing RTF.
Comparison of levels of expression of RTF on activated T cells (CD38+ or HLA-DR+) in HIV+ subjects and HCV+ individuals.
Previous work (10) had shown that T cells from HIV+ individuals expressing the activation markers CD38 and HLA-DR also expressed RTF. In the present study, we extended these findings by including HIV+ HCV+ and HCV+ individuals (Table 1). The percentages of CD4+ T cells expressing both RTF and CD38 in the HIV+ (18%; P < 0.0001) and HIV+ HCV+ (14%; P < 0.0005) groups, but not the HCV+ group (2.6%), were higher than that of the HIV− HCV− controls (3.7%). Similar findings were obtained for HLA-DR+ CD4+ T cells; however, no statistically significant difference between the percentages for HCV+ and HIV− HCV− individuals was noted (Table 1).
TABLE 1.
RTF expression in HIV+ individuals on T-cell subsets is upregulated and downregulated in HCV+ individuals
| Group | % of cells expressing RTF
|
|||
|---|---|---|---|---|
| CD4+ T cells
|
CD8+ T cells
|
|||
| CD38+ | HLA-DR+ | CD38+ | HLA-DR+ | |
| HIV+ | 18.6 ± 2.6 (P < 0.0001) | 11.5 ± 3.2 (P < 0.005) | 28.3 ± 2.9 (P < 0.0001) | 16.9 ± 2.7 (P < 0.005) |
| HIV+ HCV+ | 14.6 ± 4.4 (P < 0.0001) | 11.4 ± 4.1(P < 0.005) | 5.4 ± 1.2 (NSb) | 11.9 ± 2.8 (P < 0.05) |
| HIV− HCV− | 3.7 ± 0.3 | 2.2 ± 0.3 | 6.9 ± 1.4 | 5.4 ± 1.0 |
| HCV+ | 2.6 ± 0.4 (P = 0.042) | 1.7 ± 0.3 (NS) | 1.3 ± 0.2 (P < 0.005) | 2.4 ± 0.5 (P < 0.05) |
P values were determined versus those of HIV− HCV− individuals by unpaired t test.
NS, difference is not statistically significant.
Statistical analysis of the percentages of CD8+ T cells coexpressing RTF and CD38 led to the following ranking: HIV+ (28%; P < 0.0001) > HIV− HCV− controls (6.9%) > HCV+ (1.3%; P < 0.005) (Table 1). However, HIV+ HCV+ individuals (5.4%) did not have increased RTF expression on activated (CD38+) CD8+ T cells like HIV+ individuals. In addition, compared to HIV− HCV− individuals (5.4%), a significantly higher percentage of CD8+ T cells with dual expression of RTF and HLA-DR was seen in the HIV+ group (16.9%; P < 0.005) than in the HIV+ HCV+ group (11.9%; P < 0.05). RTF expression on HLA-DR+ CD8+ T cells of HCV+ individuals was decreased (2.4%; P < 0.05) compared to that of HIV− HCV− individuals.
DISCUSSION
These results demonstrate that surface expression of RTF on T cells is significantly increased in HIV-infected individuals and decreased in HCV+ individuals compared to that of HIV− HCV− individuals, as determined by flow-cytometric analysis. Over a twofold increase in the RTF MCF for CD3+ T cells was seen in both HIV+ and HIV+ HCV+ individuals compared to HIV− HCV− individuals. Interestingly, RTF expression was no higher in HCV+ individuals than in HIV− HCV− individuals. The percentages of T cells (CD3+, CD4+, or CD8+) expressing RTF in the different groups ranked as follows: HIV+ > HIV+ HCV+ > HIV− HCV− > HCV+. Furthermore, the percentage of CD8+ T cells expressing RTF was always higher than that for CD4+ T cells across all the groups.
A previous study (10) showed that RTF expression correlated with T-cell activation (CD38+ HLA-DR+) in HIV+ individuals. In the present study, we also demonstrated that the increased RTF expression evident for some subsets of activated T cells from HIV+ individuals was also evident in HIV+ HCV+ individuals, but not in those seropositive for HCV alone. This result indicates that there may be a correlation between RTF expression and HIV-associated immune activation. Interestingly, as illustrated in Table 1, the percentage of CD8+ T cell subsets with CD38 that expressed RTF was significantly lower in HIV+ HCV+ individuals than in HIV+ individuals. Thus, this may be a useful marker for distinguishing those seropositive for HIV alone from HIV+ individuals coinfected with HCV or having another secondary infection.
Our present findings raise an interesting question regarding the immunological role of RTF expression during HIV and/or HCV infection. In our in vitro studies, activation of Jurkat human T cells by anti-CD3 antibodies and phorbol myristate acetate upregulated RTF expression (unpublished data). It is also possible that there is a correlation between RTF expression and the states of immune activation that exist during certain, but not all, viral or bacterial infections. Infection with HIV is known to result in persistent activation of T-cell subsets. However, a study of T-cell subsets coexpressing activation markers (HLA-DR, CD38, and CD25) showed that unlike HIV+ individuals, HCV+ individuals did not exhibit lymphocyte subset alterations indicative of the immune activation caused by a chronic viral infection (19).
Several studies suggest that a Th1-Th2 switch is a critical step in the etiology of HIV infection (5, 6). The progression from HIV infection to AIDS is also associated with a shift from a Th1- to a Th2-type response (5, 6). The imbalance in the Th1- and Th2-type responses contributes to the immune dysregulation associated with HIV infection, based on the following findings: (i) progression to AIDS is characterized by increases in IL-4 and IL-10 production; (ii) several HIV-exposed individuals who were still HIV− had Th1-type dominant responses to HIV antigens; and (iii) preferential depletion of CD4+ Th1-type cells in HIV infection may result from dominant Th2-type cytokine-induced programmed cell death.
In contrast, HCV infection is believed to be associated with Th1-type responses. Th1 cytokine responses activate T cells and macrophages important for host antiviral defense. Several studies have demonstrated increased soluble IL-2 receptor and IFN-γ levels in the sera of patients with HCV infections (8, 12). Progressive liver injury in HCV infection correlates with increased intrahepatic expression of Th1-type cytokines (17). In this study, we demonstrated that RTF expression is significantly higher in HIV+ HCV+ individuals than in HCV+ individuals, suggesting that the Th2 response in patients with HIV dominates in cases of coinfection with HCV.
Based on these and other findings, we suggest that RTF may be associated with Th2-type responses and also may be responsible for maintaining a Th2 immune response while downregulating a Th1 immune response. We have shown previously that RTF plays an important role in successful pregnancy outcomes (2, 7). Pregnancy is also associated with Th2-type cytokines (4, 6, 11). Thus, RTF may be a potentially useful immunological marker for distinguishing between Th1 and Th2 responses. Long-term evaluation of HIV- and HCV-infected individuals is necessary to determine whether RTF expression changes with clinical status. Also, future studies will focus on examining RTF expression in other viral or bacterial infections to determine whether the immune response is of the Th2 type and/or is immune activation associated.
ACKNOWLEDGMENTS
We thank Gail Hoppe at The Chicago Medical School, North Chicago, Ill., for clerical assistance. We also thank Sondra Allen at Mount Sinai Hospital for assistance with accessing patients' medical records.
REFERENCES
- 1.Beaman K D, Angkachatchai V, Gilman-Sachs A. TJ6: the pregnancy-associated cytokine. Am J Reprod Immunol. 1996;35:338–341. doi: 10.1111/j.1600-0897.1996.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 2.Beaman K D, Hoversland R C. Induction of abortion in mice with a monoclonal antibody specific for suppressor T-lymphocyte molecules. J Reprod Fertil. 1988;82:691–696. doi: 10.1530/jrf.0.0820691. [DOI] [PubMed] [Google Scholar]
- 3.Beld M, Penning M, Lukashov V, McMorrow M, Roos M, Pakker N, van den Hoek A, Goudsmit J. Evidence that both HIV and HIV-induced immunodeficiency enhance HCV replication among HCV seroconverters. Virology. 1998;244:504–512. doi: 10.1006/viro.1998.9130. [DOI] [PubMed] [Google Scholar]
- 4.Chaouat G, Meliani A A, Martal J, Raghupathy A, Elliot J, Mosmann T, Wegmann T G. IL-10 prevents naturally occurring fetal loss in the CBA × DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-γ. J Immunol. 1995;154:4261–4268. [PubMed] [Google Scholar]
- 5.Clerici M, Shearer G M. The Th1-Th2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–110. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 6.Clerici M, Shearer G M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 7.Coulam C B, Beaman K D. Reciprocal alteration in circulating TJ6+ CD19+ and TJ6+ CD56+ leukocytes in early pregnancy predicts success or miscarriage. Am J Reprod Immunol. 1995;34:219–224. doi: 10.1111/j.1600-0897.1995.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 8.Cribier B, Schmitt C, Rey D, Lang J M, Kirn A, Stoll-Keller F. Production of cytokines in patients infected by hepatitis C virus. J Med Virol. 1998;55:89–91. doi: 10.1002/(sici)1096-9071(199806)55:2<89::aid-jmv1>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.DuChateau B K, Lee G W, Westerman M P, Beaman K D. Increased expression of regeneration and tolerance factor in individuals with human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1999;6:193–198. doi: 10.1128/cdli.6.2.193-198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Givens T S, DuChateau B K, Boomer J S, Westerman M P, Gilman-Sachs A, Beaman K D. Regeneration and tolerance factor: a correlate of human immunodeficiency virus-associated T-cell activation. Clin Diagn Lab Immunol. 1999;6:872–877. doi: 10.1128/cdli.6.6.872-877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H, Mosmann T R, Guilbert L, Tuntipopipat S, Wegmann T G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151:4562–4573. [PubMed] [Google Scholar]
- 12.Makris M, Preston F E, Ralph S. Increased soluble IL-2 receptor levels in HCV-infected haemophiliacs. Br J Hematol. 1994;87:419–421. doi: 10.1111/j.1365-2141.1994.tb04936.x. [DOI] [PubMed] [Google Scholar]
- 13.Mandal M, Beaman K. Purification and characterization of a pregnancy-associated protein: TJ6s. Am J Reprod Immunol. 1995;33:60–67. doi: 10.1111/j.1600-0897.1995.tb01139.x. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T R. Properties and functions of interleukin-10. Adv Immunol. 1994;56:1–26. [PubMed] [Google Scholar]
- 15.Mosmann T R, Coffman R L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2, and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 17.Napoli J, Bishop G A, McGuinness P H, Painter D M, McCaughan G W. Progressive liver injury in chronic hepatitis C infection correlates with increased intrahepatic expression of Th1-associated cytokines. Hepatology. 1996;24:759–765. doi: 10.1002/hep.510240402. [DOI] [PubMed] [Google Scholar]
- 18.Nichols T C, Kang J A, Angkachatchai V, Beer A E, Beaman K D. Expression of a membrane form of the pregnancy-associate protein TJ6 on lymphocytes. Cell Immunol. 1994;155:219–229. doi: 10.1006/cimm.1994.1114. [DOI] [PubMed] [Google Scholar]
- 19.Prince H E, Fang C T. Unaltered lymphocyte subsets in hepatitis C virus-seropositive blood donors. Transfusion. 1992;32:166–168. doi: 10.1046/j.1537-2995.1992.32292180148.x. [DOI] [PubMed] [Google Scholar]
- 20.Ribbing S L, Hoversland R C, Beaman K D. T-cell suppressor factors play an integral role in preventing fetal rejection. J Reprod Immunol. 1988;14:83–95. doi: 10.1016/0165-0378(88)90038-1. [DOI] [PubMed] [Google Scholar]
- 21.Rockstroh J K, Woitas R P, Spengler U. Human immunodeficiency virus and hepatitis C virus coinfection. Eur J Med Res. 1998;3:269–277. [PubMed] [Google Scholar]
- 22.Wegmann T G, Lin H, Guilbert L, Mosmann T R. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 23.Zylberberg H, Pol S. Reciprocal interactions between human immunodeficiency virus and hepatitis C virus infections. Clin Infect Dis. 1996;23:1117–1125. doi: 10.1093/clinids/23.5.1117. [DOI] [PubMed] [Google Scholar]