Abstract
Background
This work aimed to shed light on the notorious debate over the role of an educational/cognitive/behavioral or psychological approach in the reduction of interdialytic weight gain (IDWG) in patients on chronic hemodialysis.
Methods
Searches were run from 1975 to January 2022 on Medline, PubMed, Web of Science, and the Cochrane Library. The search terms included “hemodialysis/haemodialysis” AND “adherence” AND (“fluid intake” OR “water intake”) AND (“weight gain” OR “interdialytic weight gain” OR “IDWG”) AND “patient-level interventions. Randomized controlled studies were eligible if they were in English, published in a peer-reviewed journal and regarded adults patients with on chronic hemodialysis for at least 6 months; compared educational/cognitive and/or counseling/behavioral or psychological interventions to no intervention on interdialytic weight gain. Outcome of interest was interdialytic weight gain. The review was registered on the International Prospective Register of Systematic Reviews in Health and Social Care (PROSPERO, ID number CRD42022332401).
Results
Eighteen studies (1759 patients) were included in the analysis. Compared to the untreated group, educational/cognitive and/or counseling/behavioral interventions significantly reduced interdialytic weight gain with a pooled mean difference of − 0.15 kg (95% CI − 0.26, 30–0.05; P = 0.004). On the other hand, psychological/affective interventions reduced interdialytic weight gain with a pooled mean difference of − 0.26 kg (95% CI − 0.48, − 0.04; P = 0.020).
Conclusions
Educational/cognitive, counseling/behavioral or psychological/affective interventions significantly reduced the interdialytic weight gain in patients on chronic hemodialysis, although such reduction did not appear to be clinically relevant on hard outcomes.
Graphical abstract
Keywords: Hemodialysis, Interdialytic weight gain, Psychological, Educational/cognitive, Counseling/behavioral, Interventions
Introduction
Interdialytic weight gain (IDWG) should be lower than 4.0 to 4.5% of dry weight [1]. Unfortunately, many patients have an IDWG higher than this value [2, 3]. High IDWG is associated with greater risk of all-cause and cardiovascular mortality and increased morbidity, such as ventricular hypertrophy and major adverse cardiac and cerebrovascular events [2, 4–7]. In addition, it leads to supplementary dialysis sessions with consequent reduction of the quality of life and a significant increase in costs.
High IDWG is essentially due to an excessive intake of fluids and/or foods. Non-adherence to both diet and fluid restrictions is very frequent, exceeding 60% of evaluations [8]. Numerous factors have been shown to determine failure to adherence to diet and fluid restrictions [9–18]. Among these, an important role is played by loss of motivation and lack of self-assessment, defined as the inability to correctly define fluid status and salt and fluid intakes [9–18].
In routine clinical practice, improving adherence to restricted fluid intake in patients on chronic hemodialysis is difficult [19, 20]. Among the various strategies that have been attempted to increase adherence to fluid restriction in chronic hemodialysis patients, particular attention has been paid to patient-level interventions that have been categorized according to De Bleser et al. [21] as educational/cognitive (which conveys information or knowledge, individually or in a group setting, and delivers it in a verbal, written, and/or audio-visual form), counseling/behavioral (which targets, shapes and/or reinforces behavior, empowers patients to participate in their care, positively changes a patient’s skill level or normal routine), and psychological/affective (which appeals to the feelings and emotions or social relationships and social supports of the patient).
The present systematic review and meta-analysis aims to evaluate the efficacy of different categories of patient-level interventions in an effort to improve adherence and to limit IDWG in patients on chronic hemodialysis.
According to PICOS criteria, we analyzed: Population = end-stage renal disease patients on chronic hemodialysis; Intervention: educational/cognitive/behavioral treatment; psychological treatment Comparison = no intervention; Outcome = IDWG; Study = systematic review and meta-analysis. The primary outcome of the review is to determine the difference between educational/cognitive or counseling/behavioral or psychological/affective interventions and no interventions in IDGW.
Methods
This analysis was prospectively registered on the International Prospective Register of Systematic Reviews in Health and Social Care (PROSPERO, ID number CRD42022332401).
Search strategy
Searches were run from 1975 to January 2022. The following databases were searched for relevant studies: Medline, PubMed, Web of Science, and the Cochrane Library. The search terms and mesh headings included “hemodialysis/haemodialysis” AND “adherence” AND (“fluid intake” OR “water intake”) AND (“weight gain” OR “interdialytic weight gain” OR “IDWG”) AND “patient-level interventions” as the search terms. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Eligibility criteria
Studies were eligible for inclusion if they were English language papers published in a peer-reviewed journal and met the following inclusion criteria: (1) primary research studies in adult patients (over 18 years of age), (2) patients with end-stage renal disease on chronic hemodialysis for at least 6 months; (3) compared educational/cognitive and/or counseling/behavioral interventions to no interventions in terms of intradialytic weight gain; (4) compared psychological interventions to no interventions in terms of interdialytic weight gain; (5) included interdialytic weight gain as one of the outcomes of interest. We excluded studies on pediatric patients, pre-dialysis CKD patients, acute kidney injury patients, ESRD patients with other renal replacement therapy modalities such as peritoneal dialysis, and transplantation.
Data extraction
Two authors (MA and GP) independently reviewed the manuscripts considering the eligibility criteria and quality assessment tools. Two authors (M.B. and G.P.) independently reviewed titles and abstracts, and full texts of potential studies were retrieved for further appraisal. In case of disagreement between the two authors, a third author (EDS) was consulted. We also performed a manual search for eligible studies by checking the reference lists of relevant original and review articles. Conference abstracts and literature reviews were excluded. Similarly, studies not comparing standard/low sodium dialysate concentration were excluded. Any discrepancies were resolved by consensus upon discussion with another co-author (EDS). A data extraction table (Table 1) was compiled to record study characteristics and participant characteristics.
Table 1.
Studies on comparison of educational/cognitive interventions and/or counseling/behavioral interventions to no intervention: effect on IDWG
| Country | Type of study | Number of patients | Intervention | Interventionist | Duration | Outcome/Limits | |
|---|---|---|---|---|---|---|---|
| Cummings et al., 1981 [27] | USA | Randomized, controlled | 116 |
Group A: control Group B: behavioral contract Group C: behavioral contract with a family member or friend Group D: weekly telephone contact |
Nurses | 3 months |
At 3 months, IDWG did not differ among group A (2.6 ± 0.2 kg), group B (2.5 ± 0.2 kg), group C (2.3 ± 0.2), and group D (2.3 ± 0.2 kg) (P = NS)* Demographics of the sample somewhat limit its generalizability |
| Tanner et al., 1998 [28] | USA | Randomized, controlled | 40 |
Group A: control Group B: patient self-monitoring and behavioral contracting upon adherence |
Nurses | 6 months | Number of sessions with acceptable IDWG was similar in the two groups |
| Christensen et al., 2002 [29] | USA | Randomized, controlled | 20 | Group administered behavioral self-regulation intervention | Nurses | 7 weeks |
Mean IDWG increased in controls (from 3.12 to 3.3 kg) and decreased in treated patients (from 3.12 to 2.9 kg) (P = 0.06) Short study duration; small sample |
| Molaison and Yadrick, 2003 [30] | USA | Comparison of patients at two centers (one as treatment and one as control) | 316 | Group education sessions based on transtheoretical model (states of change) | Multiple | 3 months | Mean IDWG increased both in controls (from 3.44 ± 1.27 kg to 3.57 ± 1.21 kg) and in treated patients (from 3.24 ± 1.19 to 3.41 ± 1.14 kg) (P = NS)* |
| Tsay, 2003 [31] | Taiwan | Randomized, controlled | 62 | Self-efficacy training | Nurses | 3 weeks |
Mean IDWG decreased in controls from 2.6 ± 0.7 to 2.5 ± 0.9 kg and in treated patients from 3.3 ± 0.7 to 2.5 ± 0.7 kg (P = 0.006)* Short study duration |
| Sharp et al., 2005 [32] | UK | Randomized, controlled | 56 | Group-based cognitive behavioral intervention | Psychologist | 1 month | Small but significant decrease in IDWG in the treatment group: from 3.56 ± 0.91 at baseline to 2.96 ± 1.09 kg at 4 weeks* |
| Kauric-Klein et al., 2012 [33] | USA | Randomized, controlled | 118 | Supportive nursing intervention incorporating monitoring, goal setting, and reinforcement | Nurses | 4 months | No differences in IDWG |
| Cho et al., 2013 [34] | Korea | Randomized, controlled | 43 | Health contract intervention based on the goal attainment theory | Nurses | 1 month |
IDWG lower in the experimental group (P = 0.017) Short study duration |
| Welch et al., 2013 [35] | USA | Randomized, controlled | 44 | Electronic self-monitoring intervention based on social cognitive theory | Nurses | 6 weeks |
No differences in IDWG Short study duration |
| Cukor et al., 2014 [36] | USA | Randomized, crossover | 59 | Individual cognitive behavioral therapy | Psychologist | 3 months | Percentage of change in weight per day in controls was 3.6 at baseline and 2.5 at 3 months and in the treatment group it was 4.0 at baseline and 3.6 at 3 months (P = 0.002) |
| Griva et al., 2018 [37] | UK | Randomized, controlled | 235 | Interactive and targeted self-management training program | Multiple | 9 months | IDWG significantly (P < 0.001) lower in experimental group |
| Baser et al., 2018 [38] | Turkey | Randomized controlled | 78 | The participants in the intervention group were trained through four education sessions over 4 months, and the measurement tools were administered to them | Nurses | 4 months | Mean IDWG decreased in controls from 2.3 ± 1.4 to 2.2 ± 1.9 kg (P = 0.772) and in treated patients from 3.2 ± 1.7 to 1.8 ± 1.1 kg (P = 0.0001) |
Data are expressed as * mean ± SD
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS 22.0; SPSS Inc, Chicago, IL, United States) and Microsoft Excel. The primary outcome of the review is to determine the difference between educational/cognitive or counseling/behavioral or psychological/affective interventions and no interventions in the IDGW (mean difference); each meta-analysis (Forest plot) was built using studies enrolling one of the two therapeutic approaches compared to untreated subjects, and the mean difference (random effect), weight of the single study and heterogeneity parameters (Tau, Chi2, p, I2 and Z and p for overall effect) are reported. Statistical heterogeneity among studies was quantified with Higgins I2 statistic. Publication bias was assessed graphically using funnel plots.
Results
Search results
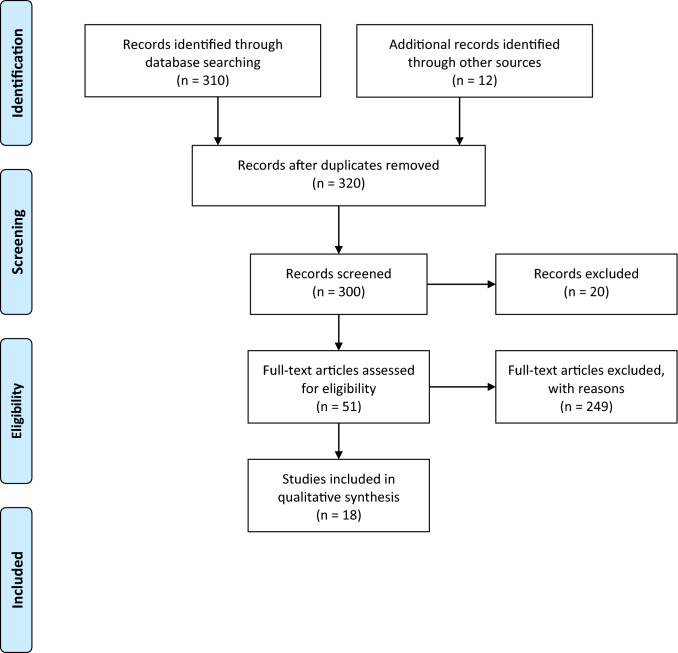
A total of 320 publications were identified via electronic databases. After screening titles, abstracts, and full texts, 18 studies meeting the inclusion criteria were included for analysis. The PRISMA flowchart is shown in Fig. 1. All studies were randomized and controlled. We divided the analysis into two sections: (1) Comparison of educational/cognitive/counseling/behavioral interventions versus no intervention, in terms of IDWG (12 studies); (2) Comparison of psychological/affective interventions versus no intervention in terms of IDWG (6 studies).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart of our analysis
Study characteristics
Educational/cognitive interventions and/or counseling/behavioral interventions
Overall, 1187 patients were included. The number of patients in each individual study ranged from 20 to 316. The length of the studies ranged from 3 weeks to 9 months and a description is presented in Table 1 [27–38]. In the majority of studies, educational/cognitive interventions were performed in combination with counseling/behavioral interventions [30–33, 35, 36, 38], whereas in four studies, counseling/behavioral interventions were delivered [27–29, 34] as the sole strategy. Moreover, educational/cognitive and counseling/behavioral interventions were generally performed through an individual format, except in two studies where a group format was used [32, 37].
Psychological/affective interventions
Overall, 572 patients were included. The number of patients in each individual study ranged from 67 to 119. The length of studies ranged from 5 weeks to 12 months and a description is presented in Table 2 [39–44]. In most studies, psychological/affective interventions were performed through an individual format, except in one study where a group format was used [42].
Table 2.
Studies on comparison of psychological/affective interventions to no intervention: effect on IDWG
| Country | Type of study | Number of patients | Intervention | Interventionist | Duration | Outcome/Limits | |
|---|---|---|---|---|---|---|---|
| Hou et al., 2010 [39] | China | Randomized, controlled | 92 | Rational emotive therapy based on ABC theory | Psychologist | 3 months |
Significant decrease in IDWG at 3 months in the treatment group Relatively small sample size |
| Pasyar et al., 2015 [40] | Iran | Randomized, controlled | 86 | Relaxation technique | Professional relaxation therapist | 2 months |
Small but significant decrease in IDWG at 4 months in the treatment group Exclusion of patients with unstable hypertension, angina, arrhythmia, congestive heart failure, acute cerebrovascular accident, hepatic failure Relatively small sample size |
| Bellomo et al., 2015 [41] | Italy | Randomized, controlled | 117 | Group sessions held once a week | Psychologist | 5 weeks |
IDWG decreased from baseline to 6 months: in controls, from 1.31 ± 0.33 to 1.32 ± 0.32 kg (P = 0.57); in treatment group, from 1.33 ± 0.33 to 1.2 ± 0.28 kg (P < 0.001) * Short study duration |
| Howren et al., 2016 [42] | USA | Randomized, controlled | 119 | Behavioral self-regulation intervention | Psychologist | 7 weeks |
No differences between groups in mean IDWG Short study duration Information regarding patient expectations or motivation were not collected or reported here |
|
Wileman et al., 2016 [43] |
UK | Randomized, controlled | 91 | Self-affirmation theory to reduce resistance to health-risk information | Psychologist | 12 months |
Small but significant decrease in IDWG at 12 months in the treatment group Relatively small sample size Residual kidney function not assessed |
| Valsaray et al., 2021 [44] | India | Randomized, controlled | 67 | Cognitive behavior therapy | Nurse | 6 months |
IDWG changed from baseline to 6 months: in controls, from 4.3 ± 0.7 to 4.6 ± 0.4 kg (P = NS); in treatment group, from 4.4 ± 0.9 to 3.2 ± 0.6 kg (P = 0.001) Relatively small sample size |
Data are expressed as * mean ± SD
Efficacy of interventions on IDWG
Educational/cognitive interventions and/or counseling/behavioral interventions
Nine of the 12 studies comparing educational/cognitive interventions and/or counseling/behavioral interventions to no intervention were included in the meta-analysis.
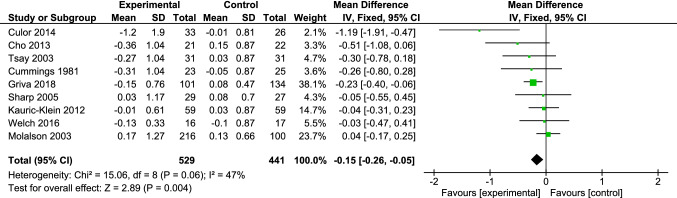
As shown in Fig. 2, compared to no intervention, educational/cognitive/counseling/behavioral interventions reduced IDWG, with a pooled mean difference (MD) of − 0.15 kg (95% CI − 0.26, − 0.05; P = 0.004). As no significant heterogeneity was observed (Chi2 = 15.06; I2 = 47%; P = 0.06), the pooled analysis was performed using a fixed-effect model.
Fig. 2.
Forest plot of studies comparing educational/cognitive interventions and/or counseling/ behavioral interventions to no intervention with regard to change in interdialytic weight gain (kg)
Three studies were not included in the meta-analysis because IDWG was not expressed as mean (SD) difference between pre- and post-treatment values [10, 28, 38]. In the study by Tanner et al., the number of sessions with acceptable IDWG was similar in the two groups [28]. In the study by Christensen et al., mean IDWG increased in controls (from 3.12 to 3.3 kg) and decreased in treated patients (from 3.12 to 2.9 kg), but the difference was not statistically significant (P = 0.06) [29]. In the study by Baser et al., mean IDWG decreased in controls from 2.3 ± 1.4 to 2.2 ± 1.9 kg (P = 0.772) and in treated patients from 3.2 ± 1.7 to 1.8 ± 1.1 kg (P = 0.0001) [38].
Psychological/affective interventions
Four of the 6 studies comparing psychological/affective interventions to no intervention were included in the meta-analysis.
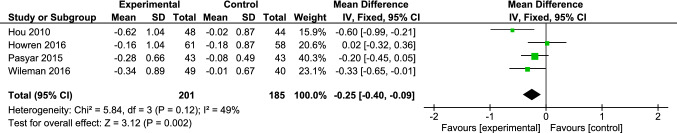
As shown in Fig. 3, compared to no intervention, psychological/affective interventions reduced IDWG with a pooled MD of − 0.26 kg (95% CI − 0.48, − 0.04; P = 0.002). As no significant heterogeneity was observed (Chi2 = 5.84; I2 = 49%; P = 0.12), the pooled analysis was performed using a fixed-effect model.
Fig. 3.
Forest plot of studies comparing psychological/affective interventions to no interventions with regard to change in interdialytic weight gain (kg)
Two studies were not included in the meta-analysis because IDWG was not expressed as mean (SD) difference between pre- and post-treatment values [21, 44]. In the study by Bellomo et al., IDWG changed from baseline to 6 months; in controls it ranged from 1.31 ± 0.33 to 1.32 ± 0.32 kg (P = 0.57) and in the treatment group it decreased from 1.33 ± 0.33 to 1.2 ± 0.28 kg (P < 0.001) [41]. In the study by Valsaray et al., the IDWG changed from baseline to 6 months; in controls it went from 4.3 ± 0.7 to 4.6 ± 0.4 kg (P = 0.856) and in the treatment group it dropped from 4.4 ± 0.9 to 3.2 ± 0.6 kg (P = 0.001) [44].
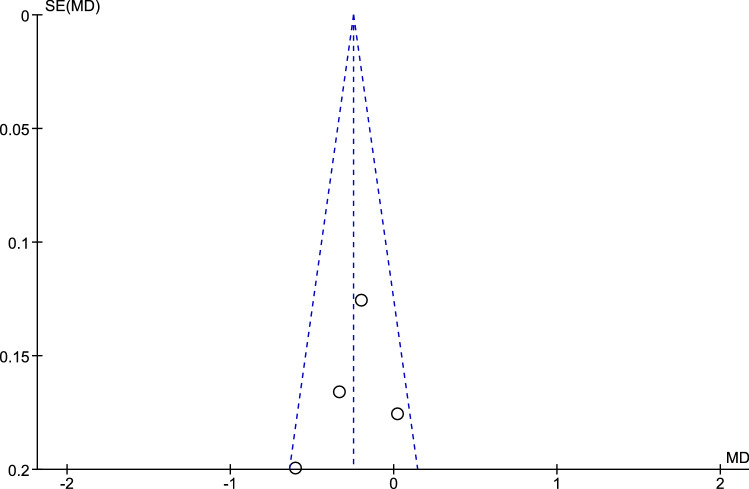
Publication bias
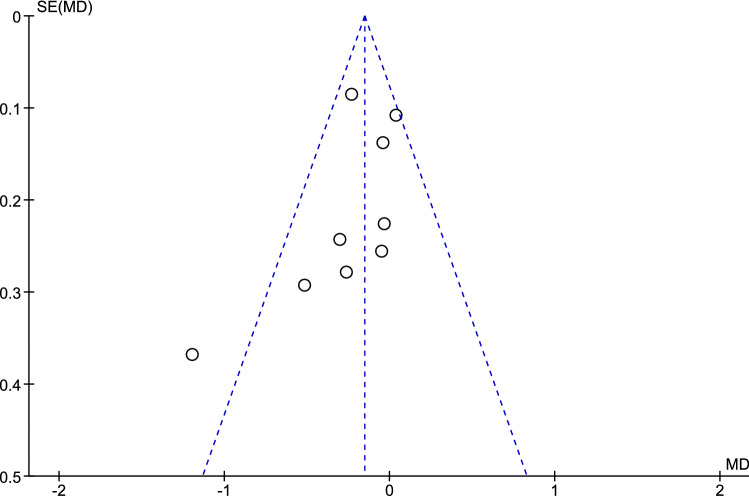
Funnel plots were generated to assess publication bias in the included studies. No obvious asymmetry, which indicated no clear evidence of publication bias, was observed either in the studies comparing educational/cognitive interventions and/or counseling/behavioral interventions to no intervention (Fig. 4) or in the studies comparing psychological/affective interventions to no interventions (Fig. 5).
Fig. 4.
Funnel plot of studies comparing educational/cognitive interventions and/or counseling/behavioral interventions to no intervention
Fig. 5.
Funnel plot of studies comparing psychological/affective interventions to no interventions
Discussion
The meta-analysis of 9 and 4 randomized studies shows that educational/cognitive interventions and/or counseling/behavioral interventions or psychological/affective interventions are both effective in significantly reducing IDWG in patients on chronic hemodialysis; the pooled mean difference of IDWG was reduced by − 0.15 and − 0.26 kg, respectively. In addition, the review of studies not included in the meta-analysis reveals conflicting results in both treatment approaches.
The results of the present meta-analysis are in agreement with the recent work by Murali et al. in which IDWG was significantly reduced as the effect of patient-level or health system-related interventions, with a pooled IDWG reduction of − 0.20 [− 0.32 to − 0.081]; it is important to note that in Murali’s study, patient-level interventions were considered as an individual entity, without distinguishing among educational/cognitive, counseling/behavioral, and psychological/affective interventions [46].
However, it could be questioned whether such differences in weight actually reflect a clinically relevant effect. Interestingly, other interventions have led to better results in terms of reduction of IDWG. In fact, a recent systematic review and meta-analysis showed that the use of a low dialysate sodium concentration significantly reduced the IDWG in prevalent patients on chronic hemodialysis, with a pooled mean difference of − 0.42 kg (P < 0.00001) [47]. In addition, the large study by Marshall et al. showed that the use of low dialysate sodium concentration led to a sustained decrease in IDWG (− 0.56 kg and − 0.61% of pre-dialysis body weight) accompanied by an early decrease in extracellular fluid volume [48]. Interestingly, during a 10-year period, between 2004 and 2014, in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [2], both absolute and relative IDWG decline were − 0.29 kg and − 0.5% of post-HD weight in the United States, − 0.25 kg and − 0.8% of post-HD weight in Canada, and − 0.22 kg and − 0.5% of post-HD weight in Europe, respectively. The DOPPS study also demonstrated that the dialysate sodium concentration accounted for 0.13 greater relative IDWG per 1-mEq/L greater dialysate sodium concentration, suggesting that it played a relatively important role in explaining the decline in IDWG [48].
Low dialysate sodium concentration results in greater diffusive sodium removal during dialysis with consequent lower total body sodium content by the end of treatment, which might therefore lessen thirst and water intake in the interdialytic period. This in turn might reduce extracellular fluid overload, hypertension, and ultimately, left ventricular hypertrophy and CV death. In daily clinical practice, dialysate sodium concentration may be fixed (low or high) or variable (individualized). High dialysate sodium concentrations provide hemodynamic benefits and prevent hypotensive episodes, but reduce the loss of sodium and consequently, thirst is stimulated and the weight gain increases. However, it should be kept in mind that the use of a low dialysate sodium concentration may be in some cases associated with adverse events such as muscle cramps and intra-dialytic or post-dialytic hypotension, which are the result of intradialytic hemodynamic instability [2]. Thus, the final decision to adopt a low dialysate sodium concentration depends on the clinical evaluation and assessment of costs and benefits.
Patient-level interventions are expensive, time consuming and require the not always achievable cooperation of patients [20, 21, 27, 28]. Educational/cognitive, counseling/behavioral or psychological/affective interventions often need multiple sessions over time [20, 21]. Educational/cognitive interventions require videos, posters, and presentations to improve patient education. In addition, the educational content of such interventions is complex and includes information about the nature of the renal disease and the consequences of renal insufficiency, the physiology of thirst, and the consequences of high salt and excessive fluid intake [20, 21]. Finally, the acquisition of knowledge is not necessarily associated with behavioral changes. Counseling/behavioral interventions require continuous reinforcements, directly or via phone call, regular feed-back and contacts at home [20, 21, 29–32]. Often, once adherence has been achieved, there is a risk of recurrence of non-adherence [20, 21, 27–32]. Psychological/affective interventions are based on the intervention of a psychologist. Unfortunately, this action is not available in many hemodialysis units.
Finally, it is unclear whether patient-level interventions need to be continued at length in order to have a clinically meaningful effect [27–42]. Indeed, the duration of the studies included in the present review ranged between 3 weeks and 12 months. In addition, it remains unknown if the reduction of IDWG persists after the interruption of the patient-level interventions. In fact, none of the studies reported the long-term impact of patient-level interventions after their cessation. This is a key point that needs to be clarified by adequate, randomized, controlled studies in the near future.
In light of these considerations, and of the clinically irrelevant IDWG reduction, the role of patient-level interventions aimed at reducing weight gain in patients on chronic hemodialysis should be questioned. The issue remains whether, it is worth continuing these interventions in routine clinical practice in an attempt to limit IDWG in patients on chronic hemodialysis. Overall, it seems that further, large, randomized controlled studies are warranted to reach a definitive result.
However, the present review highlights some interesting observations. It focuses on fluid adherence, a fundamental aspect of patients on chronic hemodialysis, as measured by IDWG, and considers only patient-level interventions categorized as educational/cognitive/counseling/behavioral interventions and psychological/affective interventions.
The present review has some limitations. First, the sample size of many of the included trials was small. Second, the length of the studies was extremely varied and short, ranging from one month to twelve months. Third, the residual urine volume was not reported in all studies. Finally, data collected from the literature could be affected by some biases (different operators, substantially non-numerable parameters, other national health systems).
In conclusion, the present meta-analysis shows that educational/cognitive interventions and/or counseling/behavioral interventions or psychological/affective interventions are effective in reducing IDWG in patients on chronic hemodialysis. However, the absolute IDWG reduction associated with these interventions seems to be of limited relevance in the clinical setting. Thus, more studies are warranted to improve the efficacy of educational/cognitive, counseling/behavioral or psychological/affective interventions in reducing IDWG.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement. None.
Declarations
Conflict of interest
None of the authors has conflict of interest.
Ethical approval
Considering that no individual patient data are involved in the analysis, there was no need for ethical approval or individual patient consent according to the Catholic University Review Board.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NKF Kdoqi GUIDELINES Clinical Practice Guidelines for Hemodialysis Adequacy, Update 2006. Am J Kidney Dis. 2006;48:S2–S90. doi: 10.1053/j.ajkd.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 2.Wong MM, McCullough KP, Bieber BA, et al. Interdialytic weight gain: trends, predictors, and associated outcomes in the international dialysis outcomes and practice patterns study (DOPPS) Am J Kidney Dis. 2017;69:367–379. doi: 10.1053/j.ajkd.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Bossola M, Pepe G, Vulpio C. The Frustrating Attempt to Limit the Interdialytic Weight Gain in Patients on Chronic Hemodialysis: New Insights Into an Old Problem. J Ren Nutr. 2018;28:293–301. doi: 10.1053/j.jrn.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Kurita N, Hayashino Y, Yamazaki S, Akizawa T, Akiba T, Saito A, Fukuhara S. Revisiting interdialytic weight gain and mortality association with serum albumin interactions: the Japanese dialysis outcomes and practice pattern study. J Ren Nutr. 2017;27:421–429. doi: 10.1053/j.jrn.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kimmel PL, Varela MP, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Amarashinge A, Mishkin GJ, Cruz I, Veis JH. Interdialytic weight gain and survival in hemodialysis patients: effects of duration of ESRD and diabetes mellitus. Kidney Int. 2000;57:1141–1151. doi: 10.1046/j.1523-1755.2000.00941.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee MJ, Doh FM, Kim CH, Koo HM, Oh HJ, Park JT, Han SH, Yoo TH, Kim YL, Kim YS, Yang CW, Kim NH, Kang SW. Interdialytic weight gain and cardiovascular outcome in incident hemodialysis patients. Am J Nephrol. 2014;39:427–435. doi: 10.1159/000362743. [DOI] [PubMed] [Google Scholar]
- 7.Saran R, Bragg-Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, Kurokawa K, Piera L, Saito A, Fukuhara S, Young EW, Held PJ, Port FK. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64:254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 8.Vr V, Kaur Kang H. (2021) The Worldwide Prevalence of Nonadherence to Diet and Fluid Restrictions Among Hemodialysis Patients: A Systematic Review and Meta-analysis. J Ren Nutr. 17:S1051–2276(21)00296-X. doi: 10.1053/j.jrn.2021.11.007 [DOI] [PubMed]
- 9.Friend R, Hatchett L, Schneider MS, Wadhwa NK. A comparison of attributions, health beliefs, and negative emotions as predictors of fluid adherence in renal dialysis patients: a prospective analysis. Ann Behav Med. 1997;19:344–347. doi: 10.1007/BF02895152. [DOI] [PubMed] [Google Scholar]
- 10.Christensen AJ, Moran PJ, Lawton WJ, Stallman D, Voigts AL. Monitoring attentional style and medical regimen adherence in hemodialysis patients. Health Psychol. 1997;16:256–262. doi: 10.1037/0278-6133.16.3.256. [DOI] [PubMed] [Google Scholar]
- 11.Ifudu O, Uribarri J, Rajwani I, et al. Relation between interdialytic weight gain, body weight and nutrition in hemodialysis patients. Am J Nephrol. 2002;22:363–368. doi: 10.1159/000065228. [DOI] [PubMed] [Google Scholar]
- 12.Schneider MS, Friend R, Whitaker P, Wadhwa NK. Fluid noncompliance and symptomatology in end-stage renal disease: cognitive and emotional variables. Health Psychol. 1991;10:209–215. doi: 10.1037/0278-6133.10.3.209. [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Coston M, Glock K, Elasy TA, Wallston KA, Ikizler TA, Cavanaugh KL. Patient perspectives on fluid management in chronic hemodialysis. J Ren Nutr. 2010;20:334–341. doi: 10.1053/j.jrn.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson EL, Held I, Khawnekar D, Rutherford P. Differences in knowledge, stress, sensation seeking, and locus of control linked to dietary adherence in hemodialysis patients. Front Psychol. 2016;7:1864. doi: 10.3389/fpsyg.2016.01864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahrari S, Moshki M, Bahrami M. The Relationship between social support and adherence of dietary and fluids restrictions among hemodialysis patients in Iran. J Caring Sci. 2014;3:11–19. doi: 10.5681/jcs.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh E, Lehane E. An exploration of the relationship between adherence with dietary sodium restrictions and health beliefs regarding these restrictions in Irish patients receiving haemodialysis for end-stage renal disease. J Clin Nurs. 2011;20:331–340. doi: 10.1111/j.1365-2702.2010.03348.x. [DOI] [PubMed] [Google Scholar]
- 17.Nerbass FB, Morais JG, dos Santos RG, Kruger TS, Sczip AC, da Luz Filho HA. Factors associated to salt intake in chronic hemodialysis patients. J Bras Nefrol. 2013;35:87–92. doi: 10.5935/0101-2800.20130015. [DOI] [PubMed] [Google Scholar]
- 18.Gebrie MH, Ford J. Depressive symptoms and dietary non-adherence among end stage renal disease patients undergoing hemodialysis therapy: systematic review. BMC Nephrol. 2019;20(1):429. doi: 10.1186/s12882-019-1622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15:351. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 20.Murali KM, Lonergan M. Breaking the adherence barriers: strategies to improve treatment adherence in dialysis patients. Semin Dial. 2020;33:475–485. doi: 10.1111/sdi.12925. [DOI] [PubMed] [Google Scholar]
- 21.De Bleser L, Matteson M, Dobbels F, Russell C, De Geest S. Interventions to improve medication-adherence after transplantation: a systematic review. Transpl Int. 2009;22:780–797. doi: 10.1111/j.1432-2277.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 22.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.University of York Centre for Reviews and Dissemination . Systematic Reviews:CRD's Guidance for Undertaking Reviews in Health Care. CRD: University of York, York; 2009. [Google Scholar]
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 27.Cummings KM, Becker MH, Kirscht JP, Levin NW. Intervention strategies to improve compliance with medical regimens by ambulatory hemodialysis patients. J Behav Med. 1981;4:111–127. doi: 10.1007/BF00844851. [DOI] [PubMed] [Google Scholar]
- 28.Tanner JL, Craig CB, Bartolucci AA, et al. The effect of a self-monitoring tool on self-efficacy, health beliefs, and adherence in patients receiving hemodialysis. J Ren Nutr. 1998;8:203–211. doi: 10.1016/S1051-2276(98)90019-X. [DOI] [PubMed] [Google Scholar]
- 29.Christensen AJ, Moran PJ, Wiebe JS, Ehlers SL, Lawton WJ. Effect of a behavioral self-regulation intervention on patient adherence in hemodialysis. Health Psychol. 2002;21:393–397. doi: 10.1037/0278-6133.21.4.393. [DOI] [PubMed] [Google Scholar]
- 30.Molaison EF, Yadrick MK. Stages of change and fluid intake in dialysis patients. Patient Educ Couns. 2003;49:5–12. doi: 10.1016/s0738-3991(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 31.Tsay SL. Self-efficacy training for patients with end-stage renal disease. J Adv Nurs. 2003;43:370–375. doi: 10.1046/j.1365-2648.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- 32.Sharp J, Wild MR, Gumley AI, Deighan CJ. A cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: a randomized controlled trial. Am J Kidney Dis. 2005;45:1046–1057. doi: 10.1053/j.ajkd.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Kauric-Klein Z. Improving blood pressure control in end stage renal disease through a supportive educative nursing intervention. Nephrol Nurs J. 2012;39:217–228. [PubMed] [Google Scholar]
- 34.Cho MK. Effect of health contract intervention on renal dialysis patients in Korea. Nurs Health Sci. 2013;15:86–93. doi: 10.1111/nhs.12003. [DOI] [PubMed] [Google Scholar]
- 35.Welch JL, Astroth KS, Perkins SM, Johnson CS, Connelly K, Siek KA, Jones J, Scott LL. Using a mobile application to self-monitor diet and fluid intake among adults receiving hemodialysis. Res Nurs Health. 2013;36:284–298. doi: 10.1002/nur.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cukor D, Ver Halen N, Asher DR, Coplan JD, Weedon J, Wyka KE, Saggi SJ, Kimmel PL. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol. 2014;25:196–206. doi: 10.1681/ASN.2012111134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griva K, Nandakumar M, Ng JH, Lam KFY, McBain H, Newman SP. Hemodialysis Self-management intervention randomized trial (HED-SMART): A practical low-intensity intervention to improve adherence and clinical markers in patients receiving hemodialysis. Am J Kidney Dis. 2018;71:371–381. doi: 10.1053/j.ajkd.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Başer E, Mollaoğlu M. The effect of a hemodialysis patient education program on fluid control and dietary compliance. Hemodial Int. 2019;23:392–401. doi: 10.1111/hdi.12744. [DOI] [PubMed] [Google Scholar]
- 39.Hou YM, Hu PC, Liang YP, Mo ZY (2010) Effects of rational-emotive therapy on adherence to fluid restrictions of patients maintained on hemodialysis prior to and after kidney transplantation Journal of Clinical Rehabilitative Tissue Engineering Research. 31: 5869-5872
- 40.Pasyar N, Rambod M, Sharif F, Rafii F, Pourali-Mohammadi N. Improving adherence and biomedical markers in hemodialysis patients: the effects of relaxation therapy. Complement Ther Med. 2015;23:38–45. doi: 10.1016/j.ctim.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Bellomo G, Coccetta P, Pasticci F, Rossi D, Selvi A. The effect of psychological intervention on thirst and interdialytic weight gain in patients on chronic hemodialysis: a randomized controlled trial. J Ren Nutr. 2015;25:426–432. doi: 10.1053/j.jrn.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Howren MB, Kellerman QD, Hillis SL, Cvengros J, Lawton W, Christensen AJ. Effect of a Behavioral Self-Regulation Intervention on Patient Adherence to Fluid-Intake Restrictions in Hemodialysis: a Randomized Controlled Trial. Ann Behav Med. 2016;50:167–176. doi: 10.1007/s12160-015-9741-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wileman V, Chilcot J, Armitage CJ, Farrington K, Wellsted DM, Norton S, Davenport A, Franklin G, Da Silva GM, Horne R, Almond M. Evidence of improved fluid management in patients receiving haemodialysis following a self-affirmation theory-based intervention: A randomised controlled trial. Psychol Health. 2016;31:100–114. doi: 10.1080/08870446.2015.1073729. [DOI] [PubMed] [Google Scholar]
- 44.Valsaraj BP, Bhat SM, Prabhu R, Kamath A. Follow-Up Study on the Effect of Cognitive Behaviour Therapy on Haemodialysis Adherence: A randomised controlled trial. Sultan Qaboos Univ Med J. 2021;21:e58–e65. doi: 10.18295/squmj.2021.21.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geng X, Song Y, Hou B, Ma Y, Wang Y. The efficacy and safety of low dialysate sodium levels for patients with maintenance haemodialysis: A systematic review and meta-analysis. Int J Surg. 2020;79:332–339. doi: 10.1016/j.ijsu.2020.05.027. [DOI] [PubMed] [Google Scholar]
- 46.Murali KM, Mullan J, Roodenrys S, Hassan HC, Lambert K, Lonergan M. Strategies to improve dietary, fluid, dialysis or medication adherence in patients with end stage kidney disease on dialysis: A systematic review and meta-analysis of randomized intervention trials. PLoS ONE. 2019;14:e0211479. doi: 10.1371/journal.pone.0211479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bossola M, Di stasio E. Interdialytic weight gain and low dialysate sodium concentration in patients on chronic hemodialysis. A systematic review and Meta-analysis. Sem Dial. In press [DOI] [PMC free article] [PubMed]
- 48.Marshall MR, Vandal AC, de Zoysa JR, et al. Effect of Low-Sodium versus Conventional Sodium Dialysate on Left Ventricular Mass in Home and Self-Care Satellite Facility Hemodialysis Patients: A Randomized Clinical Trial. J Am Soc Nephrol. 2020;31:1078–1091. doi: 10.1681/ASN.2019090877. [DOI] [PMC free article] [PubMed] [Google Scholar]